Abstract
Deep brain stimulation (DBS) has developed during the past 20 years as a remarkable treatment option for several different disorders. Advances in technology and surgical techniques have essentially replaced ablative procedures for most of these conditions. Stimulation of the ventralis intermedius nucleus of the thalamus has clearly been shown to markedly improve tremor control in patients with essential tremor and tremor related to Parkinson disease. Symptoms of bradykinesia, tremor, gait disturbance, and rigidity can be significantly improved in patients with Parkinson disease. Because of these improvements, a decrease in medication can be instrumental in reducing the disabling features of dyskinesias in such patients. Primary dystonia has been shown to respond well to DBS of the globus pallidus internus. The success of these procedures has led to application of these techniques to multiple other debilitating conditions such as neuropsychiatric disorders, intractable pain, epilepsy, camptocormia, headache, restless legs syndrome, and Alzheimer disease. The literature analysis was performed using a MEDLINE search from 1980 through 2010 with the term deep brain stimulation, and several double-blind and larger case series were chosen for inclusion in this review. The exact mechanism of DBS is not fully understood. This review summarizes many of the current and potential future clinical applications of this technology.
AD = Alzheimer disease; AN = anterior nucleus; CM = centromedian; DBS = deep brain stimulation; ET = essential tremor; GPi = globus pallidus internus; GTS = Gilles de la Tourette syndrome; IPG = implantable pulse generator; MCS = minimally conscious state; NAc = nucleus accumbens; NBIA = neurodegeneration with brain iron accumulation; OCD = obsessive-compulsive disorder; PAG = periaqueductal gray; PD = Parkinson disease; PVG = periventricular gray; PVS = persistent vegetative state; RLS = restless legs syndrome; STN = subthalamic nucleus; SUNCT = short-lasting unilateral neuralgiform headache with conjunctival injection and tearing; Y-BOCS = Yale-Brown Obsessive-Compulsive Scale
The spectrum of disease to which deep brain stimulation (DBS) surgery has been applied during the past decade continues to expand. Since the initial observation of tremor control with stimulation of the thalamus, investigators have been exploring options to expand stimulation to a variety of disorders and diseases. Trials to elicit the mechanisms of action of DBS are ongoing. Meanwhile, clinical investigators continue studying the effects of DBS in these disorders and defining optimal targets. For some conditions, such as essential tremor and Parkinson disease (PD), well-established studies have confirmed the positive effects of DBS. For other conditions, such as neuropsychiatric disorders, epilepsy, and pain, long-term results and universally agreed on optimal targets are less well defined. The history of psychosurgery is a cautionary tale to all those who want to apply stimulation procedures to progressive and debilitating diseases.
For this review, the pertinent MEDLINE literature from 1980 through 2010 was analyzed using the search term deep brain stimulation with a focus on the best-designed randomized double-blind trials and case series. Several of the current clinical applications of DBS and potential future development are highlighted. Functional imaging and neuroelectrophysiological data will be essential to the development of targets, trials, and unbiased assessment of clinical response. For the newer applications of DBS, more well-controlled prospective clinical trials are necessary to accurately assess the efficacy and, most importantly, the safety of DBS. The major conditions and deep brain nuclei targeted for DBS are summarized in Table 1.
TABLE 1.
Major Conditions Currently Being Treated With Deep Brain Stimulation
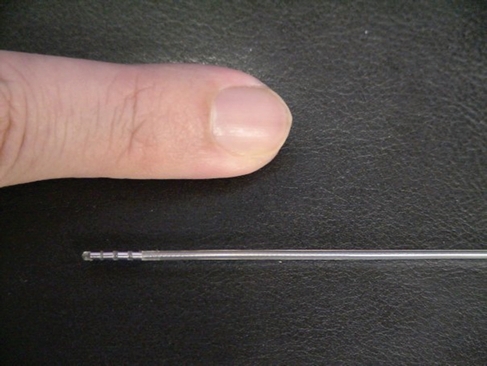
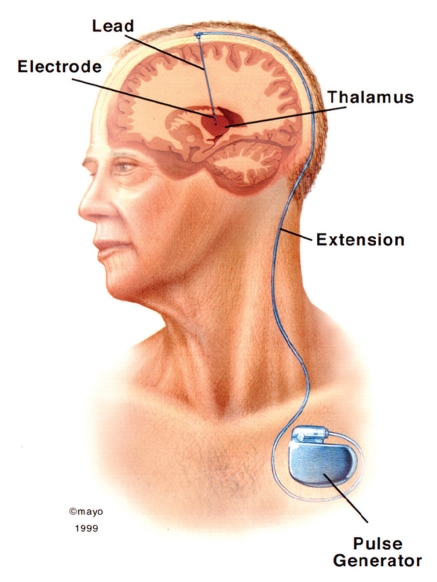
The surgical procedure of DBS is generally performed with the patient awake and use of a stereotactic localizing system. Midline anatomical structures, such as the anterior and posterior commissures, are often used as reliable landmarks for target planning. After local anesthesia of the scalp, a bur hole is made in the skull. Identification of the deep nuclei is based on a combination of magnetic resonance imaging or computed tomography, stereotactic atlases, and microelectrode recordings. Although not essential, microelectrode recordings allow for stimulation of the target area and can aid in placement of the permanent electrode (Figure 1). After electrode placement, lead extensions and the pulse generator are surgically implanted (Figure 2). The device is programmed via a transdermal programming unit that allows for innumerable therapeutic options (Figure 3). In addition, the programming feature permits ongoing adjustments given the dynamic nature of the central nervous system and progression of disease. The major risks of DBS are hemorrhage; transient confusion; infection; and fracture, misplacement, or migration of the lead. The mean morbidity rate for DBS surgery is 3% to 4%.1 During the past 2 decades, these risks have continued to decline as experience has grown due to more than 75,000 procedures performed.
FIGURE 1.
Permanent deep brain stimulation electrode. Note 4 contacts at distal end of lead, each 1.5 mm in length.
FIGURE 2.
Drawing depicting the deep brain stimulation lead, lead extension, and infraclavicular location on implanted pulse generator.
FIGURE 3.
Transcutaneous programming unit.
Article Highlights
Deep brain stimulation surgery is a safe and effective treatment for many disorders
Correct preoperative diagnosis is essential
Microelectrode recording and nuclear mapping are helpful, but not essential, for optimal electrode placement
Multiple deep brain nuclei targets and diseases are currently being investigated
Multiple programmable options allow for adaptation to the electrophysiologic changes that develop in the neuronal circuitry in these patients
PARKINSON DISEASE
Parkinson disease is thought to affect at least 100 persons in every 100,000. The cardinal symptoms of tremor, bradykinesia, postural instability, and rigor result in substantial disability for patients with PD. During the course of the disease, up to 50% of patients will have symptoms refractory to medication and will experience drug-induced dyskinesias. Overactivity of the globus pallidus internus (GPi) and the subthalamic nucleus (STN) is believed to be part of the pathophysiologic mechanism of PD. In 1994, Benabid et al2 and Siegfried and Lippitz3 reported successful treatment of patients with PD who underwent DBS of the STN and of the GPi, respectively. Since those reports, thousands of patients with PD have undergone successful DBS surgery worldwide.
Multiple series have reported on the long-term efficacy of DBS for PD. The motor symptoms of PD respond well to bilateral DBS of the STN4-7 and bilateral DBS of the GPi.8,9 Weaver et al10 conducted a large meta-analysis and found that, although response was better for motor symptoms in patients who underwent STN DBS vs those who underwent GPi DBS, the difference was not statistically significant. In many patients, medication-induced dyskinesias can be as debilitating as symptoms experienced when they are not taking medication. Both STN DBS and GPi DBS can result in reduction of dyskinesias.5-9 Because GPi is thought to act directly on l-dopa–induced dyskinesias, neurostimulation is more independent of medication reduction,11 whereas medication reduction is necessary to decrease dyskinesias in patients undergoing STN DBS.5 One study reported significant reductions in dyskinesias with bilateral GPi DBS.10 Although there is some evidence that neurocognitive complications and programming adjustments with bilateral STN DBS are higher than with GPi DBS, many investigators continue to favor the STN over the GPi for PD.12 The mechanism of the stimulation effect on PD is not fully understood but thought to likely be related to modulation of neuronal activity. The elegant work by Agnesi et al13 using their wireless instantaneous neurotransmitter concentration system, which allows for in vivo measurements of real-time dopamine release, is an area ripe for ongoing and future research in DBS. Deep brain stimulation has become part of the standard treatment of advanced PD.
ESSENTIAL TREMOR
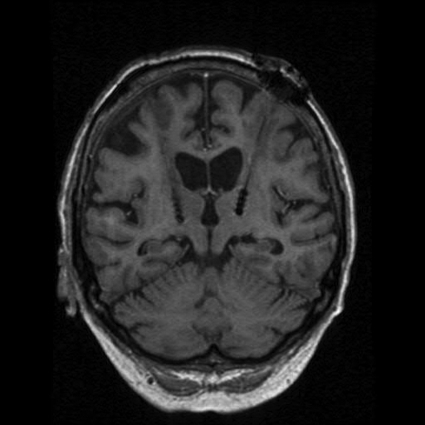
Essential tremor (ET) is the most common form of pathologic tremor. It most frequently affects the hands but can also involve the head, voice, tongue, and lower extremities. The prevalence of ET increases with age. Many patients will have a family history of ET consistent with a Mendelian dominant genetic pattern. Essential tremor can be effectively treated with propranolol and primidone, and alcohol can markedly diminish the tremor in many patients. Stereotactic thalamotomy has been largely replaced by thalamic DBS as the surgical treatment of choice. The thalamus is a large nucleus with several subnuclear divisions. Some centers still prefer radiosurgical ablative treatment for ET and have reported good long-term results.14 The ventralis intermedius nucleus of the thalamus is the most widely agreed on target (Figure 4). Most series report 70% to 90% tremor control in patients undergoing thalamic DBS for ET.15,16 Treatment of head and voice tremor with thalamic DBS is less effective, and generally these types of tremor require bilateral stimulation for optimal results.16,17 Other investigators have recently suggested that the STN, zona incerta, or the prelemniscal radiation may be a more effective target in some patients.18,19 Nonetheless, DBS for tremor control is effective and safe.
FIGURE 4.
Coronal T2-weighted magnetic resonance image demonstrating bilateral electrode placement in the thalamus.
The association of upper extremity ET and several types of dystonias, including spasmodic dysphonia, has been reported. Schweinfurth et al20 found a well-defined association between spasmodic dysphonia and ET with a 79% female preponderance. Spasmodic dysphonia with vocal tremor has been reported to respond to bilateral thalamic DBS.21 Orthostatic tremor is the result of rhythmic muscle discharges of the lower extremities. Medical treatment is often ineffective and the condition disabling. Recently, orthostatic tremor was reported to be responsive to DBS.22 However, further study is required because of the limited number of reports.
DYSTONIA
Primary Dystonia
Medical treatment of dystonia does not always produce adequate symptom control and often leads to intolerable adverse effects. Initially, ablative procedures of either the thalamus or the GPi demonstrated symptomatic improvement in patients with dystonia.23 Several reports of DBS for intractable dystonia have targeted the ventral intermedius nucleus of the thalamus24 and the GPi.25-28 In general, responses have been favorable with both targets. Double-blind prospective trials of bilateral GPi DBS for primary dystonia have documented therapeutic response.29 Although clinical trials comparing the targets have not yet been performed, the generally accepted target is currently the GPi. Results of DBS for secondary dystonias have been mixed.
Neurodegenerative Dystonias
Deep brain stimulation for other forms of dystonia, including posttraumatic, postanoxic, dystonia-plus syndromes, and tardive dystonias, has been reported in small series or case reports with generally favorable results.25,27 Kurtis et al30 found significant clinical and neurophysiological improvement in a patient who underwent bilateral GPi DBS for myoclonus-dystonia secondary to a mutation in the epsilon-sarcoglyan gene. Neurodegeneration with brain iron accumulation (NBIA) represents a rare group of neurodegenerative disorders characterized by iron accumulation in the brain. Severe generalized dystonia is a prominent symptom manifested by speech and swallowing difficulties as well as pain and gait and respiratory compromise. Timmermann et al31 conducted a multicenter retrospective study in patients with dystonia secondary to NBIA treated with bilateral pallidal stimulation. Two-thirds of the patients had improvement in their dystonia severity score of 20% or more, and more than 30% had improvement in their disability impairment. This patient cohort confirmed that GPi DBS may be an effective treatment of NBIA-induced dystonia.
Meige Syndrome
Idiopathic cranial cervical dystonia is an adult-onset movement disorder that results in segmental dystonia. Blake, Wood, Brueghel, and Meige syndromes are other terms for this disorder, the most common of which is Meige syndrome.32-40 Patients with Meige syndrome have blepharospasm, cervical dystonia, and facial oromandibular dystonia. In 5 of 7 cases of Meige syndrome, thalamic and/or basal ganglia lesions have been detected on single positron emission computed tomography and functional magnetic resonance imaging.41 Although the underlying cause of Meige syndrome is unknown, it is primarily considered a variant of idiopathic torsion dystonia; however, autopsy reports have not provided specific details.33,40 Stereotactic surgical ablation of the thalamus and the GPi has been associated with mixed results.34,36 Recent reports have described the efficacy of GPi DBS in selected patients with Meige syndrome32,34,38,39; however, no definitive conclusions can yet be made regarding DBS for Meige syndrome, and further study is needed. Nonetheless, bilateral GPi DBS may be effective in patients with medically refractory Meige syndrome.
HEADACHE
Cluster Headache
Cluster headache is a rare condition that results in severe headaches occurring cyclically and can last for weeks or months at a time. In as many as 20% of patients, cluster headaches are considered medically refractory.42 Positron emission tomography has identified focal increase in blood flow in the ipsilateral hypothalamus during a cluster headache attack.43 In 2001, Leone et al44 reported the first successful DBS of the posterior hypothalamus for the treatment of refractory cluster headache. Since then, more than 50 cases have been reported worldwide of hypothalamic DBS for cluster headache.45-47 In a recent study of 10 patients who had undergone hypothalamic DBS for cluster headache, positron emission tomography showed both activation and deactivation in cerebral structures known to be activated during cluster headache attacks.48 These findings suggest that, rather than inhibiting ipsilateral activity in the presumed generator, hypothalamic DBS may result in functional modulation of the pain neural matrix. Several other targets, including the periaqueductal gray (PAG) region, anterior hypothalamus, and subcommisural targets, are being studied for cluster headache.
Short-lasting Unilateral Neuralgiform Headache With Conjunctival Injection and Tearing
Short-lasting unilateral neuralgiform headache with conjunctival injection and tearing (SUNCT) is a rare primary headache disorder often refractory to treatment. Patients with SUNCT experience excruciating paroxysms of strictly unilateral orbitotemporal headache that persist for seconds to minutes and recur up to 200 times per day. Topiramate, lamotrigine, intravenous lidocaine, and gabapentin are the only medications that have been shown to have some effectiveness in isolated cases of SUNCT. On the basis of successful results for the treatment of cluster headache, Leone et al44,45 developed a surgical option for patients with medically refractory SUNCT. The posterior inferior hypothalamus was targeted in part because functional magnetic resonance imaging demonstrated activation in this area during headache attacks, similar to that seen during cluster headache attacks.43-49 Lyons et al50 reported similar results in a patient with a history of SUNCT who underwent ipsilateral hypothalamic DBS. Although this procedure is not curative, it could be effective for medically resistant SUNCT.
Other Headache Syndromes
Franzini et al51 reported their experience in targeting the posterior hypothalamus for trigeminal neuropathy and multiple sclerosis–induced trigeminal neuralgia. In patients with trigeminal neuropathy, DBS was ineffective; however, patients with refractory trigeminal neuralgia due to multiple sclerosis showed significant improvement in V1 distribution attacks. Moreover, Walcott et al52 reported a single case of chronic paroxysmal hemicrania that responded to ipsilateral posterior hypothalamic DBS. Matharu et al53 noted a similar observation in their patient. Thus, DBS may be effective for certain cases of refractory headache disorders.
CHRONIC PAIN
Treatment of a variety of pain syndromes using DBS initially focused on the sensory nucleus of the thalamus for neuropathic pain. The ventral posterolateral and ventroposteromedial nuclei were the most commonly targeted areas.54 Subsequent trials found that chronic stimulation of the PAG region and periventricular gray (PVG) region at the level of the third ventricle was also effective.55 The PAG/PVG region is generally targeted for nociceptive pain, whereas the ventral posterolateral and ventro-posteromedial thalamic subnuclear area have been used more often for neuropathic pain.56 Several recent international studies have reported successful treatment of differing chronic pain syndromes with DBS. Hamani et al57 performed DBS of the ventralis caudalis nucleus of the thalamus or PAG/PVG region in 21 patients with chronic pain, 13 of whom underwent permanent implantation; only 5 patients had long-term relief, and implantation was primarily in the thalamic subnuclei.57 Conversely, Bittar et al58 reported that PAG/PVG stimulation was more effective for phantom limb pain. Katayama et al59 found that DBS of the posterior nucleus ovalis of the thalamus was much more effective for long-term relief of neuropathic pain after cerebrovascular accident compared with DBS of the ventralis caudalis nucleus of the thalamus or internal capsule.
NEUROPSYCHIATRIC DISORDERS
Tourette Syndrome
Gilles de la Tourette syndrome (GTS) is a neuropsychiatric disorder that occurs most commonly in childhood and is characterized by phonic, vocal, and motor tics; pathophysiology is poorly understood. Among patients with GTS, severity of symptoms and responsiveness to treatment vary substantially. Nearly 1% of children worldwide reportedly have GTS.60 Many of these children have psychological comorbidities, including obsessive-compulsive disorder (OCD), anxiety, depression, attention deficit disorder, and self-mutilation.60 Most patients exhibit a self-limiting form of the disorder and, after the peak of tic severity during prepubescent years, note a significant decline in symptoms by the age of 20 years. In most patients, symptoms respond to pharmacological treatment with alpha2-adrenergic agonists or neuroleptics. Since the mid-1950s, ablative neurosurgical procedures have been used for patients with refractory GTS. The thalamus, limbic system, frontal lobes, and cerebellum have all been targeted. Results have generally been poor with serious complications.61,62 Several recent series have reported on the effectiveness of DBS for GTS,63-71 and most have described a decrease in or termination of behavioral symptoms.63,66-71
The first report of DBS for GTS by Vandewalle et al65 targeted the centromedian (CM) and ventral oralis internus nuclei of the thalamus. Since then, other targets, including the thalamus, GPi, nucleus accumbens (NAc), and anterior limb of the internal capsule, have been used.64,70,71 In a study by Servello et al69 in 2008, 15 of the 18 patients who underwent bilateral thalamic DBS had symptomatic improvement. A prospective, randomized, double-blind study by Maciunas et al67 demonstrated marked improvement in 3 of 5 adult patients who underwent thalamic DBS. In a controlled double-blind, randomized, crossover trial, Welter et al68 implanted bilateral thalamic and GPi electrodes in 3 patients and reported significantly better outcomes with GPi stimulation. Although DBS surgery is considered to have a relatively low risk of morbidity and mortality, the optimal target has yet to be determined. Systematic study of this condition and the optimal target is necessary.
Aggressive Behavior
Impulsive and aggressive behavior unresponsive to maximal medical management can be extremely challenging. Lesional therapies involving the hypothalamus have been successful in improving behavior. Recently, investigators have reported on a small number of patients with severe aggressive and violent behavioral disorders who underwent posterior hypothalamic stimulation.72-74 Bilateral medial hypothalamic stimulation in a young male with medically refractory aggressiveness and cognitive impairment resulted in sustained clinical improvement at 18-month follow-up.72 Kuhn et al73 demonstrated complete resolution of self-mutilation behavior in a 22-year-old woman after bilateral hypothalamic DBS. In their series of 6 patients who underwent hypothalamic DBS for violent and aggressive behavior, Franzini et al74 noted that 5 of the patients experienced significant improvement. The initial anecdotal experience of DBS in patients with aggressive behavioral disorders is promising, but a substantial amount of work still needs to be done.
Depression
Major depression is the most common psychiatric disorder worldwide. Despite neuropharmocological agents, electroconvulsant therapies, and neuroablative procedures, depression in nearly 20% of patients is refractory to all interventions. In 2005, Mayberg et al75 reported their experience in 6 patients with depression who underwent bilateral DBS of the subgenu of the corpus callosum. This target was selected on the basis of positron emission tomographic findings of a decrease in the subgenual cingulate activity in patients whose symptoms had initially responded favorably to treatments. At 6-month follow-up, 4 of their 6 patients had sustained improvement, as measured by the Hamilton Depression Rating Scale. In a study by Schlaepfer et al,76 3 patients who underwent bilateral DBS of the ventral striatum experienced improvement. Interestingly, after blinded withdrawal of stimulation, patient scores worsened, suggesting that the improvement was not due to placebo effect.
As with all DBS applications to psychosurgery, care must be taken to ensure adequate patient protection. The exhaustive analysis of the literature by Voon et al77 regarding the neuropsychological effects of DBS in patients with PD is a cautionary tale of the importance of unintended adverse effects. Several different targets have been studied, and there appears to be overlap of these targets in Tourette syndrome, OCD, aggressive behavior, and depression. Information on the application of DBS techniques for these disorders is preliminary. The hope for potential “cure” of these devastating disorders among patients, the media, and health care professionals is substantial, and thus cautious interpretation of these early results is paramount for patient safety.
Obsessive-Compulsive Disorder
Obsessive-compulsive disorder is a psychiatric disorder manifested by thoughts and impulses that produce anxiety and result in patients performing repetitive rituals. Treatment generally consists of cognitive behavioral intervention and serotonin reuptake inhibitors. Up to 40% of patients will have functional impairment that significantly affects their quality of life.78 Neuroablative procedures, including cingulotomy and anterior capsulotomy, have been used during the past half century, with reports of 30% to 70% of patients responding.78-80 Although the precise pathophysiologic mechanism of OCD is unknown, it appears that abnormal functioning of the cortico-striato-thalamo-cortical circuitry plays an important role.81
Several small series of DBS for OCD have been reported during the past 10 years, but an optimal target has yet to be be defined.82-86 Improvement in symptoms is commonly assessed with the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS). Mallet et al86 and Fontaine et al82 noted good responses targeting the STN. The anterior limb of the internal capsule was one of the original targets based on the successful results from the original ablation techniques in anterior capsulotomy.87 More recently, the ventral capsule in combination with the ventral striatum, which contains the NAc, has become a target of interest in several studies.83,84 Long-term improvement in the Y-BOCS scores of 10 patients who underwent targeting of the right NAc was not apparent.84 The inferior thalamic peduncle, which links the orbitofrontal cortex with the thalamus, has also been a target of recent interest in the treatment of OCD. This target has been used in only one study: Jiménez-Ponce et al85 treated 5 patients with bilateral DBS for OCD. Although their study patients were not stringently controlled, the authors found reductions in the Y-BOCS scores of at least 35% in all patients. These preliminary studies indicate that DBS for severe, refractory OCD may be a promising treatment option. Clearly, the optimal target has yet to be defined, and further well-controlled studies are needed. Suicidal ideation and hypomania are potential serious complications in patients who have undergone DBS for OCD. Multidisciplinary treatment is essential for such patients.
EPILEPSY
Epilepsy is one of the most prevalent and disabling disorders across all age groups. Nearly 1% of adults and up to 5% of children are diagnosed as having epilepsy; more than 30% of cases are refractory to treatment. A study in the early 1970s by Cooper et al88 demonstrated significant seizure reduction in more than 50% of their patients with intractable epilepsy who had undergone cerebellar electrical stimulation; improvements in visual, verbal, and memory function were noted. Salcman et al89 described 5 patients who underwent cerebellar cortical stimulation for intractable epilepsy. Histopathologic analysis performed at the time of electrode implantation revealed marked degeneration of the Purkinje cell layer in all patients; the authors concluded that neuronal damage in patients with epilepsy may be related to the cumulative effects of the frequency and chronicity of the disease. Davis and Emmonds90 reported that most of their patients who had undergone cerebellar stimulation were either seizure-free or had significant reduction in seizure frequency during an average stimulation time frame of 8 years; in 65% of the patients, anticonvulsant medication requirements were reduced. However, a double-blind prospective clinical trial of cerebellar stimulation in 12 patients with various types of medically refractory epilepsy found no decrease in the severity or frequency of seizures.91 In a double-blind trial of cerebellar stimulation in 5 patients with generalized seizures, Velasco et al92 reported a 33% reduction in seizure frequency.
Most investigators studying cerebellar stimulation for seizure control place the electrodes via a bur hole approach. Despite postoperative imaging to confirm location, such placement may lead to variability of the exact structures being stimulated, and this may partially explain the variability in some of the reports. Larger double-blind trials with defined cohorts are necessary to fully evaluate the potential benefits of cerebellar stimulation for epilepsy.
The CM and the anterior nucleus (AN) of the thalamus have been proposed as targets for DBS treatment of epilepsy.93-96 Andrade et al96 described 8 patients with intractable seizures who underwent bilateral DBS of the AN (6 patients) or of the CM (2 patients) of the thalamus; 3 patients had a generalized seizure disorder, and 5 had partial complex seizures. During a follow-up period of 2 to 7 years, all patients experienced a reduction in seizures; however, the 2 patients who underwent CM DBS did not have a clear benefit in overall control of their seizures. Of the 6 patients who underwent AN DBS, 5 had a greater than 50% reduction in seizure frequency, although not with initial stimulator activation. These findings led the authors to postulate that seizure reduction may initially be due to the postsurgical microthalamotomy effect and that longer term improvement may be due to long-term stimulation. Placebo effect cannot be completely excluded when interpreting these findings. McIntrye et al97 noted improvement in seizure frequency after discontinuation of stimulation.
The Stimulation of the Anterior Nucleus of the Thalamus in Epilepsy (SANTE) trial, a double-blind trial of AN DBS for refractory seizures, has suggested that targeting the AN of the thalamus is effective for refractory epilepsy. On the basis of the recently published results of the SANTE trial, the European Union has approved this strategy for treating epilepsy; however, the US Food and Drug Administration has not granted approval in the United States.98 The AN is a relatively large area, and the precise target within that subnucleus has yet to be clarified. Chkhenkeli et al99 demonstrated improvement in seizure activity with low frequency stimulation of the inferior caudate nucleus. Although their study consisted of 57 patients, the severity of seizures and the evaluation protocols varied substantially, and several patients had undergone previous resective surgeries. A study of a small number of patients with refractory seizures reported benefit with STN stimulation.100 The hippocampus has also been a target; its appeal is the potential for being a treatment for patients who have bilateral seizure activity for which bilateral temporal lobectomy is rarely an option. Initial results from Velasco et al101 showed variable but consistent reductions in seizures in 85% of patients (N=15) undergoing hippocampal stimulation. In a long-term follow-up study, Boon et al102 reported that their 10 patients did not experience significant improvement after unilateral hippocampal stimulation ipsilateral to the seizure focus.
A randomized, double-blind multicenter sham stimulation trial of the responsive neurostimulator is currently under way in the United States. The responsive neurostimulator system is an implanted device designed to detect abnormal activity in the brain and respond, similar to an implantable cardiac defibrillator, by delivering electrical stimulation to suppress development of seizure activity. Electrodes rest on the surface of the brain connected to the programmable neurostimulator, which is implanted in the skull. More randomized, double-blind, controlled multicenter trials are necessary to establish the future role of DBS in patients with epilepsy. However, this renewed interest will undoubtedly spawn further investigations into the potential of this treatment option.
CAMPTOCORMIA
Camptocormia, a posture abnormality, is characterized by involuntary truncal flexion induced by standing or sitting and has been found to be associated with other neurologic disorders, including idiopathic PD.103,104 Nandi et al103 reported a case of a young man who did not have PD but who underwent bilateral GPi DBS for disabling camptocormia secondary to adverse effects of neuroleptic medication. Micheli et al104 targeted the GPi bilaterally in a patient with PD and camptocormia; at 14 months postoperatively, the patient had near-complete resolution of his truncal flexion deformity. In the largest series of PD patients with camptocormia who underwent DBS surgery, Sako et al105 targeted the STN bilaterally; all 6 of their patients experienced substantial improvement in their camptocormia and motor symptoms. Reports of success with STN or GPi stimulation in controlling axial posturing in patients with camptocormia support the notion that the basal ganglia plays an important role in maintenance of posture. These reports suggest that bilateral stimulation benefits camptocormia in patients with PD.
RESTLESS LEGS SYNDROME
Restless legs syndrome (RLS) can affect up to 25% of the adult population, and the percentage of patients with PD who also have RLS may be even higher.106 Although the pathophysiology is unknown, it might be related to impaired central dopaminergic transmission. Single photon emission computed tomography has revealed reduced striatal dopamine D2-receptor binding in patients with RLS; thus, central striatal dopaminergic dysfunction is a possibility.107
Functional magnetic resonance imaging has shown that activation of the thalamus is associated with RLS sensory symptoms.108 The effect of striatal dopaminergic dysfunction on basal ganglia and thalamic neuronal activity in RLS is unknown. High-frequency STN stimulation results in increased substantia nigra pars compacta neuronal firing, without an appreciable increase in central dopamine levels.109 Therefore, it is unlikely that STN DBS improves RLS through alteration of central dopamine levels. Stimulation may result in neuronal firing in the basal ganglia with effects on the thalamus and diencephalon-spinal dopamine pathway.
Reports on the effect of DBS surgery on RLS symptoms are limited. Kedia et al110 noted emergence of RLS after bilateral STN DBS surgery for PD. Conversely, in a study by Driver-Dunckley et al111 of 6 patients who underwent bilateral STN DBS for PD with concomitant RLS, 3 patients had complete resolution, and 3 had near-total resolution of their symptoms. Bilateral STN DBS surgery can improve RLS in patients with advanced PD. More prospective studies should be undertaken to elucidate further the possible mechanisms whereby DBS improves RLS symptoms.
OBESITY AND ADDICTIONS
Obesity is an increasingly important health problem, and DBS has been used in obese patients.112-115 The lateral hypothalamus and ventromedial hypothalamus are the appetite and satiety centers of the brain, respectively. More recent efforts have been directed toward the reward center of the brain, the NAc.112 Current reports of chronic stimulation of the NAc suggest that modulation of the reward sensation may affect dietary preferences. Additional analysis has concluded that DBS for obesity needs to achieve a success rate of 83% to be comparable to current bariatric surgical procedures.113 Interestingly, however, obesity has developed in patients with PD who underwent STN DBS.114 Other addictions, including smoking and alcoholism, have been reported to improve after NAc DBS.116,117
DISORDERS Of CONSCIOUSNESS
Traumatic brain injury, a leading cause of persistent vegetative state (PVS) or minimally conscious state (MCS), has been a recent, albeit sparse, area of study of the effects of DBS. Reports of brain stimulation for PVS/MCS have been published as early as 1950. In 2010, Yamamoto et al118 described their experience in 21 traumatic and nontraumatic brain–injured patients who were in either a PVS or a MCS and who underwent DBS targeting primarily the thalamic CM parafascicularis complex. Eight to 19 months postoperatively, the authors noted improvement in cognitive functioning in 8 of the patients. The recent reviews by Sen et al119 and Lancioni et al120 concluded that DBS for PVS or MCS may be an effective and viable option for future research and clinical trials.
ALZHEIMER DISEASE
Alzheimer disease (AD) is a progressive degenerative disorder; however, recent data suggest that the disease may also represent a disorder of the integrated cortical and subcortical pathways.121 Hamani et al115 reported memory improvement in a patient who underwent fornix/hypothalamus DBS for obesity. These findings led Laxton et al122 to develop a phase 1 trial of fornix/hypothalamus DBS in 6 patients with mild AD. The researchers used positron emission tomography to measure pre- and postoperative cerebral glucose utilization as an indicator of quantitative effects of DBS. Increased glucose metabolism was observed in the temporal and parietal cortical areas at 1 month in all patients and was sustained in most of the affected areas at 1-year follow-up.122 Cognitive assessments suggested improvement or slowing of anticipated decline at 6 and 12 months after DBS. No conclusions regarding the efficacy of DBS in AD can yet be drawn from this phase 1 study. However, given the unrelenting and destructive nature of AD, any advances in treatment options should be explored.
CONCLUSION
Deep brain stimulation has provided substantial clinical improvement in patients with several different diseases and disorders. The understanding of how DBS works has advanced during the past 2 decades, but there is still much to be learned. Functional imaging studies and intraoperative electrophysiological monitoring have added greatly to the understanding of the effects of stimulation on the neurotransmitters and functional brain pathways. Ongoing trials and proposed studies to assess the safety and clinical efficacy of DBS in multiple diseases are being aggressively pursued at multiple international centers.
Footnotes
An earlier version of this article appeared Online First.
REFERENCES
- 1. Voges J, Waerzeggers Y, Maarouf M, et al. Deep-brain stimulation: long-term analysis of complications caused by hardware and surgery-experiences from a single centre. J Neurol Neurosurg Psychiatry. 2006;77(7):868-872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benabid AL, Pollak P, Gross C, et al. Acute and long-term effects of subthalamic nucleus stimulation in Parkinson's disease. Stereotact Funct Neurosurg. 1994;62(1-4):76-84 [DOI] [PubMed] [Google Scholar]
- 3. Siegfried J, Lippitz B. Bilateral continuous electrostimulation of ventroposterolateral pallidum: a new therapeutic approach for alleviating all Parkinsonian symptoms. Neurosurgery. 1994;35(6):1126-1130 [DOI] [PubMed] [Google Scholar]
- 4. Schupbach WM, Chastan N, Welter ML, et al. Stimulation of the subthalamic nucleus in Parkinson's disease: a 5-year follow-up. J Neurol Neurosurg Psychiatry. 2005;76(12):1640-1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 2003;349(20):1925-1934 [DOI] [PubMed] [Google Scholar]
- 6. Pahwa R, Wilkinson SB, Overman J, Lyons KE. Bilateral subthalamic stimulation with Parkinson's disease: long-term follow-up. J Neurosurg. 2003;99(1):71-77 [DOI] [PubMed] [Google Scholar]
- 7. Kleiner-Fisman G, Fisman DN, Sime E, Saint-Cyr JA, Lozano AM, Lang AE. Long-term follow-up of bilateral deep brain stimulation of the subthalamic nucleus in patients with advanced Parkinson's disease. J Neurosurg. 2003;99(3):489-495 [DOI] [PubMed] [Google Scholar]
- 8. Anderson VC, Burchiel KJ, Hogarth P, Farve J, Hammerstad JP. Pallidal vs. subthalamic nucleus deep brain stimulation in Parkinson's disease. Arch Neurol. 2005;62(4):554-560 [DOI] [PubMed] [Google Scholar]
- 9. Krause M, Fogel W, Heck A, et al. Deep brain stimulation for the treatment of Parkinson's disease: subthalamic nucleus versus globus pallidus internus. J Neurol Neurosurg Psychiatry. 2001;70(4):464-470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weaver F, Follet K, Hur K, Ippolito D, Stern M. Deep brain stimulation in Parkinson disease: a metaanalysis of patient outcomes. J Neurosurg. 2005;103(6):956-967 [DOI] [PubMed] [Google Scholar]
- 11. Voges J, Koulousakis A, Sturm V. Deep brain stimulation for Parkinson's disease. Acta Neurochir Suppl. 2007;97(pt 2):171-184 [DOI] [PubMed] [Google Scholar]
- 12. Saint-Cyr JA, Trepanier LL, Kumar R, Lozano AM, Lang AE. Neurophysiological consequences of chronic bilateral stimulation of the subthalamic nucleus in Parkinson's disease. Brain. 2000;123(pt 10):2091-2108 [DOI] [PubMed] [Google Scholar]
- 13. Agnesi F, Tye SJ, Bledsoe JM, et al. Wireless Instantaneous Neurotransmitter Concentration System-based amperometric detection of dopamine, adenosine, and glutamate for intraoperative neurochemical monitoring. J Neurosurg. 2009;111(4):701-711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Young RF, Vermeulen S, Meier R. Gamma knife thalamotomy for treatment of essential tremor: long-term results. J Neurosurg. 2010;112(6):1311-1317 [DOI] [PubMed] [Google Scholar]
- 15. Schuurman PR, Bosch DA, Bossuyt PM, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med. 2000;342(7):461-468 [DOI] [PubMed] [Google Scholar]
- 16. Limousin P, Speelman JD, Gielen F, Janssens M. Multicentre European study of thalamic stimulation in parkinsonian and essential tremor. J Neurol Neurosurg Psychiatry. 1999;66(3):289-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Putzke JD, Uitti RJ, Obwegeser AA, Wszolek ZK, Wharen RE. Bilateral thalamic deep brain stimulation: midline tremor control. J Neurol Neurosurg Psychiatry. 2005;76(5):684-690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murata J, Kitagawa M, Uesugi H, et al. Electrical stimulation of the posterior subthalamic area for the treatment of intractable proximal tremor. J Neurosurg. 2003;99(4):708-715 [DOI] [PubMed] [Google Scholar]
- 19. Plaha P, Patel NK, Gill SS. Stimulation of the subthalamic region for essential tremor. J Neurosurg. 2004;101(1):48-54 [DOI] [PubMed] [Google Scholar]
- 20. Schweinfurth JM, Billante M, Courey MS. Risk factors and demographics in patients with spasmodic dysphonia. Laryngoscope. 2002;112(2):220-223 [DOI] [PubMed] [Google Scholar]
- 21. Lyons MK, Boucher OK, Evidente VGH. Spasmodic dysphonia and thalamic deep brain stimulation: long-term observations, possible neurophysiologic mechanism and comparison of unilateral versus bilateral stimulation. J Neurol Neurophysiol. 2010;1(3):106 [Google Scholar]
- 22. Espay AJ, Duker AP, Chen R, et al. Deep brain stimulation of the ventral intermediate nucleus of the thalamus in medically refractory orthostatic tremor: preliminary observations. Mov Disord. 2008;23(16):2357-2362 [DOI] [PubMed] [Google Scholar]
- 23. Balas I, Kovacs N, Hollody K. Staged bilateral stereotactic pallidotomy for life-threatening dystonia in a child with Hallervorden-Spatz disease. Mov Disord. 2006;21(1):82-85 [DOI] [PubMed] [Google Scholar]
- 24. Kuncel AM, Turner DA, Ozelius LJ, Greene PE, Grill WM, Stacy MA. Myoclonus and tremor response to thalamic deep brain stimulation parameters in a patient with inherited myoclonus–dystonia syndrome. Clin Neurol Neurosurg. 2009;111(3):303-306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sako W, Goto S, Shimazu H, et al. Bilateral deep brain stimulation of the globus pallidus internus on tardive dystonia. Mov Disord. 2008;23(13):1929-1931 [DOI] [PubMed] [Google Scholar]
- 26. Trottenberg T, Volkmann J, Deuschl G, et al. Treatment of severe tardive dystonia with pallidal deep brain stimulation. Neurology. 2005;64(2):344-346 [DOI] [PubMed] [Google Scholar]
- 27. Loher TJ, Hasdemir MG, Burgunder JM, Krauss JK. Long-term follow-up study of chronic globus pallidus internur stimulation for posttraumatic hemidystonia. J Neurosurg. 2000;92(3):457-460 [DOI] [PubMed] [Google Scholar]
- 28. Magarinos-Ascone CM, Regidor I, Martinez-Castrillo JC, Gomez-Galan M, Figuerias-Mendez R. Pallidal stimulation relieves myoclonus–dystonia syndrome. J Neurol Neurosurg Psychiatry. 2005;76(7):989-991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kupsch A, Benecke R, Muller J, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006;355(19):1978-1990 [DOI] [PubMed] [Google Scholar]
- 30. Kurtis MM, San Luciano M, Yu Q, et al. Clinical and neurophysiological improvement of SGCE myoclonus-dystonia with GPi deep brain stimulation. Clin Neurol Neurosurg. 2010;112(2):149-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Timmermann L, Pauls KA, Wieland K, et al. Dystonia in neurodegeneration with brain iron accumulation: outcome of bilateral pallidal stimulation. Brain. 2010133(pt 3):701-712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blomstedt P, Tisch S, Hariz MI. Pallidal deep brain stimulation in the treatment of Meige syndrome. Acta Neurol Scand. 2008;118(3):198-202 [DOI] [PubMed] [Google Scholar]
- 33. Houser M, Waltz T. Meige syndrome and pallidal deep brain stimulation. Mov Disord. 2005;20(9):1203-1205 [DOI] [PubMed] [Google Scholar]
- 34. Opherk C, Gruber C, Steude U, Dichgans M, Botzel K. Successful bilateral pallidal stimulation for Meige syndrome and spasmodic torticollis. Neurology. 2006;66(4):E14 [DOI] [PubMed] [Google Scholar]
- 35. Andrew J, Fowler CJ, Harrison MJG. Stereotaxic thalamotomy in 55 cases of dystonia. Brain. 1983;106(pt 4):981-1000 [DOI] [PubMed] [Google Scholar]
- 36. Capelle HH, Weigel R, Krauss JK. Bilateral pallidal stimulation for blepharospasm-oromandibular dystonia (Meige syndrome). Neurology. 2003;60(12):2017-2018 [DOI] [PubMed] [Google Scholar]
- 37. Kumar R, Dagher A, Hutchison WD, Lang AE, Lozano AM. Globus pallidus deep brain stimulation for generalized dystonia: clinical and PET investigation. Neurology. 1999;53(4):871-874 [DOI] [PubMed] [Google Scholar]
- 38. Ostrem JL, Marks WJ, Volz MM, et al. Pallidal deep brain stimulation in patients with cranial-cervical dystonia (Meige syndrome). Mov Disord. 2007;22(13):1885-1891 [DOI] [PubMed] [Google Scholar]
- 39. Lyons MK, Birch BD, Hillman RA, Boucher OK, Evidente VGH. Long-term follow-up of deep brain stimulation for Meige syndrome. Neurosurg Focus. 2010;29(2):E5 [DOI] [PubMed] [Google Scholar]
- 40. Tolosa E, Kulisevsky J, Fahn S. Meige syndrome: primary and secondary forms. Adv Neurol. 1988;50:509-515 [PubMed] [Google Scholar]
- 41. Sakai T, Shikishima K, Kawai K, Kitahara K. Meige syndrome associated with basal ganglia and thalamic functional disorders [article in Japanese]. Nippon Ganka Gakkai Zasshi. 1998;102(11):764-770 [PubMed] [Google Scholar]
- 42. Russell MB. Epidemiology and genetics of cluster headache. Lancet Neurol. 2004;3(5):279-283 [DOI] [PubMed] [Google Scholar]
- 43. May A, Bahra A, Buchel C, Frackowiak RS, Goadsby PJ. PET and MRA findings in cluster headache and MRA in experimental pain. Neurology. 2000;55(9):1328-1335 [DOI] [PubMed] [Google Scholar]
- 44. Leone M, Franzini A, Bussone G. Stereotactic stimulation of posterior hypothalamic gray matter for intractable cluster headache. N Engl J Med. 2001;345(19):1428-1429 [DOI] [PubMed] [Google Scholar]
- 45. Leone M, Franzini A, Broggi G, May A, Bussone G. Long-term follow up of bilateral hypothalamic stimulation for intractable cluster headache. Brain. 2004;127(pt 10):2259-2264 [DOI] [PubMed] [Google Scholar]
- 46. Starr PA, Barbaro NM, Raskin NH, Rashin NH, Ostrem JL. Chronic stimulation of the posterior hypothalamic region for cluster headache: technique and 1-year results in four patients. J Neurosurg. 2007;106(6):999-1005 [DOI] [PubMed] [Google Scholar]
- 47. Bartsch T, Pinsker MO, Rasche D, et al. Hypothalamic deep brain stimulation for cluster headache: experience from multicase series. Cephalgia. 2008;28(3):285-295 [DOI] [PubMed] [Google Scholar]
- 48. May A, Leone M, Boecker H, et al. Hypothalamic deep brain stimulation in positron emission tomography. J Neurosci. 2006;26(13):3589-3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. May A, Bahra A, Buchel C, Turner R, Goadsby PJ. Functional magnetic resonance imaging in spontaneous attacks of SUNCT: short-lasting unilateral neuralgiform headache with conjunctival injection and tearing. Ann Neurol. 1999;46(5):791-793 [DOI] [PubMed] [Google Scholar]
- 50. Lyons MK, Dodick DW, Evidente VGH. Responsiveness of short-lasting unilateral neuralgiform headache with conjunctival injection and tearing to hypothalamic deep brain stimulation. J Neurosurg. 2009;110(2):279-281 [DOI] [PubMed] [Google Scholar]
- 51. Franzini A, Messina G, Cordella R, Marras C, Broggi G. Deep brain stimulation of the posteromedial hypothalamus: indications, long-term results, and neurophysiological considerations. Neurosurg Focus. 2010;29(2):E13 [DOI] [PubMed] [Google Scholar]
- 52. Walcott BP, Bamber NI, Anderson DE. Successful treatment of chronic paroxysmal hemicrania with posterior hypothalamic stimulation: technical case report. Neurosurgery. 2009;65(5):E997 [DOI] [PubMed] [Google Scholar]
- 53. Matharu MS, Cohen AS, Frackowiak RS, Goadsby PJ. Posterior hypothalamic activation in paroxysmal hemicrania. Ann Neurol. 2006;59(3):535-545 [DOI] [PubMed] [Google Scholar]
- 54. Turnbull IM, Shulman R, Woodhurst WB. Thalamic stimulation for neuropathic pain. J Neurosurg. 1980;52(4):486-493 [DOI] [PubMed] [Google Scholar]
- 55. Richardson DE, Akil H. Long term results of periventricular gray self stimulation. Neurosurgery. 1977;1(2):199-202 [DOI] [PubMed] [Google Scholar]
- 56. Levy RM, Lamb S, Adams JE. Treatment of chronic pain by deep brain stimulation: long-term follow-up and review of the literature. Neurosurgery. 1987;21(6):885-893 [DOI] [PubMed] [Google Scholar]
- 57. Hamani C, Schwalb JM, Rezai AR, Dostrovsky JO, Davis KD, Lozano AM. Deep brain stimulation for chronic neuropathic pain: long-term outcome and the incidence of insertional effect. Pain. 2006;125(1-2):188-196 [DOI] [PubMed] [Google Scholar]
- 58. Bittar RG, Kar-Purkayastha I, Owen SL, et al. Deep brain stimulation for pain relief: a meta-analysis. J Clin Neurosci. 2005;12(5):515-519 [DOI] [PubMed] [Google Scholar]
- 59. Katayama Y, Yamamoto T, Kobayashi K, Oshima H, Fukaya C. Deep brain and motor cortex stimulation for post-stroke movement disorders and post-stroke pain. Acta Neurochir Suppl. 2003;87:121-123 [DOI] [PubMed] [Google Scholar]
- 60. Freeman RD, Fast DK, Burd L, Kerbeshian J, Robertson MM, Sandor P. An international perspective on Tourette syndrome: selected findings from 3,500 individuals in 22 countries. Dev Med Child Neurol. 2000;42(7):436-447 [DOI] [PubMed] [Google Scholar]
- 61. Temel Y, Visser-Vandewalle V. Surgery in Tourette syndrome. Mov Disord. 2004;19(1):3-14 [DOI] [PubMed] [Google Scholar]
- 62. Babel TB, Warnke PC, Ostertag CB. Immediate and long term outcome after infrathalamic and thalamic lesioning for intractable Tourette syndrome. J Neurol Neurosurg Psychiatry. 2001;70(5):666-671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bajwa RJ, de Lotbiniere AJ, King RA, et al. Deep brain stimulation in Tourette syndrome. Mov Disord. 2007;22(9):1346-1350 [DOI] [PubMed] [Google Scholar]
- 64. Dehning S, Mehrkens JH, Muller N, Botzel K. Therapy-refractory Tourette syndrome: beneficial outcome with globus pallidus internus deep brain stimulation. Mov Disord. 2008;23(9):1300-1302 [DOI] [PubMed] [Google Scholar]
- 65. Vandewalle V, van der Linden C, Groenewegen HJ, Caemaert J. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet. 1999;353(9154):724 [DOI] [PubMed] [Google Scholar]
- 66. Visser-Vandewalle V, Temel Y, Boon P, et al. Chronic bilateral thalamic stimulation: a new therapeutic approach in intractable Tourette syndrome: report of three cases. J Neurosurg. 2003;99(6):1094-1100 [DOI] [PubMed] [Google Scholar]
- 67. Maciunas RJ, Maddux BN, Riley DE, et al. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J Neurosurg. 2007;107(11):1004-1014 [DOI] [PubMed] [Google Scholar]
- 68. Welter ML, Mallet L, Houteo JL, et al. Internal pallidal and thalamic stimulation in patients with Tourette syndrome. Arch Neurol. 2008;65(7):952-957 [DOI] [PubMed] [Google Scholar]
- 69. Servello D, Porta M, Sassi M, Brambilla A, Robertson MM. Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulation. J Neurol Neurosurg Psychiatry. 2008;79(2):136-142 [DOI] [PubMed] [Google Scholar]
- 70. Neuner I, Podoll K, Lenartz D, Sturm V, Schneider F. Deep brain stimulation in the nucleus accumbens for intractable Tourette's syndrome: follow-up report of 36 months [letter]. Biol Psychiatry. 2009;65(4):e5-e6 [DOI] [PubMed] [Google Scholar]
- 71. Kuhn J, Lenartz D, Mai JK, et al. Deep brain stimulation of the nucleus accumbens and the internal capsule in therapeutically refractory Tourette syndrome. J Neurol. 2007;254(7):963-965 [DOI] [PubMed] [Google Scholar]
- 72. Hernando V, Pastor J, Pedrosa M, Pena E, Sola RG. Low-frequency bilateral hypothalamic stimulation for treatment of drug-resistant aggressiveness in a young man with mental retardation. Stereotact Funct Neurosurg. 2008;86(4):219-223 [DOI] [PubMed] [Google Scholar]
- 73. Kuhn J, Lenartz D, Mai JK, Huff W, Klosterkoetter J, Sturm V. Disappearance of self-aggressive behavior in a brain-injured patient after deep brain stimulation of the hypothalamus: technical case report. Neurosurgery. 2008;62(5):E1182 [DOI] [PubMed] [Google Scholar]
- 74. Franzini A, Messina G, Cordella R, Marras C, Broggi G. Deep brain stimulation of the posteromedial hypothalamus: indications, long-term results, and neurophysiological considerations. Neurosurg Focus. 2010;29(2):E13 [DOI] [PubMed] [Google Scholar]
- 75. Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660 [DOI] [PubMed] [Google Scholar]
- 76. Schlaepfer TE, Cohen MX, Frick C, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2007;33(2):368-377 [DOI] [PubMed] [Google Scholar]
- 77. Voon V, Kubu C, Krack P, Houeto JL, Troster AI. Deep brain stimulation: neuropsychological and neuropsychiatric issues. Mov Disord. 2006;21(suppl 14):S305-S327 [DOI] [PubMed] [Google Scholar]
- 78. Milan MK, Campos M, Sheth SA, Eskandar EN. Deep brain stimulation for obsessive-compulsive disorder: past, present, and future. Neurosurg Focus. 2010;29(2):E10 [DOI] [PubMed] [Google Scholar]
- 79. Lippitz BE, Mindus P, Meyerson BA, Kihlstrom L, Lindquist C. Lesion topography and outcome after thermocapsulotomy or gamma knife capsulotomy for obsessive-compulsive disorder: relevance of the right hemisphere. Neurosurgery. 1999;44(3):452-458 [DOI] [PubMed] [Google Scholar]
- 80. Dougherty DD, Baer L, Cosgrove GR, et al. Prospective long-term follow-up of 44 patients who received cingulotomy for treatment refractory obsessive-compulsive disorder. Am J Psychiatry. 2002;159(2):269-275 [DOI] [PubMed] [Google Scholar]
- 81. Rauch SL, Dougherty DD, Malone D, et al. A functional neuroimaging investigation of deep brain stimulation in patients with obsessive-compulsive disorder. J Neurosurg. 2006;104(4):558-565 [DOI] [PubMed] [Google Scholar]
- 82. Fontaine D, Mattei V, Borg M, et al. Effect of subthalamic nucleus stimulation on obsessive-compulsive disorder in a patient with Parkinson disease: case report. J Neurosurg. 2004;100(6):1084-1086 [DOI] [PubMed] [Google Scholar]
- 83. Goodman WK, Foote KD, Greenberg BD, et al. Deep brain stimulation for intractable obsessive compulsive disorder: pilot study using a blinded, staggered-onset design. Biol Psychiatry. 2010;67(6):535-542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Huff W, Lenartz D, Schormann M, et al. Unilateral deep brain stimulation of the nucleus accumbens in patients with treatment-resistant obsessive compulsive disorder: outcomes after one year. Clin Neurol Neurosurg. 2010;112(2):137-143 [DOI] [PubMed] [Google Scholar]
- 85. Jiménez-Ponce F, Velasco-Campos F, Castro-Farfán G, et al. Preliminary study in patients with obsessive-compulsive disorder treated with electrical stimulation in the inferior thalamic peduncle. Neurosurgery. 2009;65(6) (suppl):203-209 [DOI] [PubMed] [Google Scholar]
- 86. Mallet L, Polosan M, Jaafari N, et al. Subthalamic nucleus stimulation in severe obsessive compulsive disorder. N Engl J Med. 2008;359(20):2121-2134 [DOI] [PubMed] [Google Scholar]
- 87. Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. 1999;354(9189):1526 [DOI] [PubMed] [Google Scholar]
- 88. Cooper IS, Upton AR, Amin I. Reversibility of chronic neurologic deficits: some effects of electrical stimulation of the thalamus and internal capsule in man. Appl Neurophysiol. 1980;43(3-5):244-258 [DOI] [PubMed] [Google Scholar]
- 89. Salcman M, Defendini R, Correll J, Gilman S. Neuropathological changes in cerebellar biopsies of epileptic patients. Ann Neurol. 1978;3(1):10-19 [DOI] [PubMed] [Google Scholar]
- 90. Davis R, Emmonds SE. Cerebellar stimulation for seizure control: 17-year study. Stereotact Funct Neurosurg. 1992;58(1-4):200-208 [DOI] [PubMed] [Google Scholar]
- 91. Wright GDS, McLellan DL, Brice JG. A double-blind trial of chronic cerebellar stimulation in twelve patients with severe epilepsy. J Neurol Neurosurg Psychiatry. 1984;47(8):769-774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Velasco M, Velasco F, Velasco AL, Lujan M, Vasquez del Mercado J. Epileptiform EEG activities of the centromedian thalamic nuclei in patients with intractable partial motor, complex partial, and generalized seizures. Epilepsia. 1989;30(3):295-306 [DOI] [PubMed] [Google Scholar]
- 93. Fisher RS, Uematsu S, Krauss GL, et al. Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia. 1992;33(5):841-851 [DOI] [PubMed] [Google Scholar]
- 94. Kerrigan JF, Litt B, Fisher RS, et al. Electrical stimulation of the anterior nucleus of the thalamus for the treatment of intractable epilepsy. Epilepsia. 2004;45(4):346-354 [DOI] [PubMed] [Google Scholar]
- 95. Velasco F, Velasco M, Velasco AL, Jimenez F, Marquez I, Rise M. Electrical stimulation of the centromedian thalamic nucleus in control of seizures: long-term studies. Epilepsia. 1995;36(1):63-71 [DOI] [PubMed] [Google Scholar]
- 96. Andrade DM, Zumsteg D, Hamani C, et al. Long-term follow-up of patients with thalamic deep brain stimulation for epilepsy. Neurology. 2006;66(10):1571-1573 [DOI] [PubMed] [Google Scholar]
- 97. McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol. 2004;115(6):1239-1248 [DOI] [PubMed] [Google Scholar]
- 98. Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899-908 [DOI] [PubMed] [Google Scholar]
- 99. Chkhenkeli SA, Sramka M, Lortkipanidze GS, et al. Electrophysiological effects and clinical results of direct brain stimulation for intractable epilepsy. Clin Neurol Neurosurg. 2004;106(4):318-329 [DOI] [PubMed] [Google Scholar]
- 100. Handforth A, DeSalles AA, Krahl SE. Deep brain stimulation of the subthalamic nucleus as adjunct treatment for refractory epilepsy. Epilepsia. 2006;47(7):1239-1241 [DOI] [PubMed] [Google Scholar]
- 101. Velasco F, Velasco M, Velasco AL, Menez D, Rocha L. Electrical stimulation for epilepsy: stimulation of hippocampal foci. Stereotact Funct Neurosurg. 2001;77(1-4):223-227 [DOI] [PubMed] [Google Scholar]
- 102. Boon P, Vonck K, De Herdt V, et al. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia. 2007;48(8):1551-1560 [DOI] [PubMed] [Google Scholar]
- 103. Nandi D, Parkin S, Scott R, et al. Camptocormia treated with bilateral pallidal stimulation: case report. J Neurosurg. 2002;97(2):461-466 [DOI] [PubMed] [Google Scholar]
- 104. Micheli F, Cersosimo MG, Piedimonte F. Camptocormia in a patient with Parkinson's disease: beneficial effects of pallidal deep brain stimulation: case report. J Neurosurg. 2005;103(6):1081-1083 [DOI] [PubMed] [Google Scholar]
- 105. Sako W, Nishio M, Maruo T, et al. Subthalamic nucleus deep brain stimulation for camptocormia associated with Parkinson's disease. Mov Disord. 2009;24(7):1076-1079 [DOI] [PubMed] [Google Scholar]
- 106. Ondo WG, Vuong KD, Jankovic J. Exploring the relationship between Parkinson disease and restless legs syndrome. Arch Neurol. 2002;59(3):421-424 [DOI] [PubMed] [Google Scholar]
- 107. Michaud M, Soucy JP, Chabli A, Lavigne G, Montplaisir J. SPECT imaging of striatal pre- and postsynaptic dopaminergic status in restless legs syndrome with periodic leg movements of sleep. J Neurol. 2002;249(2):164-170 [DOI] [PubMed] [Google Scholar]
- 108. Bucher SF, Seelos KC, Oertel WH, Reiser M, Trenkwalder C. Cerebral generators involved in the pathogenesis of the restless legs syndrome. Ann Neurol. 1997;41(5):639-645 [DOI] [PubMed] [Google Scholar]
- 109. Strafella AP, Sadikot AF, Dagher A. Subthalamic deep brain stimulation does not induce striatal dopamine release in Parkinson's disease. Neuroreport. 2003;14(9):1287-1289 [DOI] [PubMed] [Google Scholar]
- 110. Kedia S, Moro E, Tagliati M, Lang AE, Kumar R. Emergence of restless legs syndrome during subthalamic stimulation for Parkinson disease. Neurology. 2004;63(12):2410-2412 [DOI] [PubMed] [Google Scholar]
- 111. Driver-Dunckley E, Evidente VGH, Adler CH, et al. Restless legs syndrome in Parkinson's disease patients may improve with subthalamic stimulation. Mov Disord. 2006;21(8):1287-1289 [DOI] [PubMed] [Google Scholar]
- 112. Halpern CH, Wolf JA, Bale TL, et al. Deep brain stimulation in the treatment of obesity: a review. J Neurosurg. 2008;109(10):625-634 [DOI] [PubMed] [Google Scholar]
- 113. Pisapia JM, Halpern CH, Williams NN, Wadden TA, Baltuch GH, Stein SC. Deep brain stimulation compared with bariatric surgery for the treatment of morbid obesity: a decision analysis study. Neurosurg Focus. 2010;29(2):E15 [DOI] [PubMed] [Google Scholar]
- 114. Bannier S, Montaurier C, Derost PP, et al. Overweight after deep brain stimulation of the subthalamic nucleus in Parkinson disease: long term follow-up. J Neurol Neurosurg Psychiatry. 2009;80(5):484-488 [DOI] [PubMed] [Google Scholar]
- 115. Hamani C, McAndrews MP, Cohn M, et al. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol. 2008;63(1):119-123 [DOI] [PubMed] [Google Scholar]
- 116. Mantione M, van der Brink W, Schuurman PR, Denys D. Smoking cessation and weight loss after chronic deep brain stimulation of the nucleus accumbens: therapeutic and research implications: case report. Neurosurgery. 2010;66(1):E218 [DOI] [PubMed] [Google Scholar]
- 117. Kuhn J, Lenartz D, Huff W, et al. Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: valuable therapeutic implications? J Neurol Neurosurg Psychiatry. 2007;78(10):1152-1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Yamamoto T, Katayama Y, Kobayashi K, Oshima H, Fukaya C, Tsubokawa T. Deep brain stimulation for the treatment of vegetative state. Eur J Neurosci. 2010;32(7):1145-1151 [DOI] [PubMed] [Google Scholar]
- 119. Sen AN, Campbell PG, Yalda S, Jallo J, Sharan AD. Deep brain stimulation in the management of disorders of consciousness: a review of physiology, previous reports, and ethical considerations. Neurosurg Focus. 2010;29(2):E14 [DOI] [PubMed] [Google Scholar]
- 120. Lancioni GE, Bosco A, Belardinelli MO, Singh NN, O'Reilly MK, Sigafoos J. An overview of intervention options for promoting adaptive behavior of persons with acquired brain injury and minimally conscious state. Res Dev Disabil. 2010;31(6):1121-1134 [DOI] [PubMed] [Google Scholar]
- 121. Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25(34):7709-7017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Laxton AW, Tang-Wai DF, McAndrews MP, et al. A phase 1 trial of deep brain stimulation of memory circuits in Alzheimer's disease. Ann Neurol. 2010;68(4):521-534 [DOI] [PubMed] [Google Scholar]