Abstract
The change in hormonal milieu associated with perimenopause and menopause can lead to a variety of symptoms that can affect a woman's quality of life. Postmenopausal hormone therapy (HT) is an effective, well-tolerated treatment for these symptoms. However, combined HT consisting of conjugated equine estrogen and medroxyprogesterone acetate has been associated with an increased number of health risks when compared with conjugated equine estrogen alone or placebo. As a result, some women are turning to alternative hormonal formulations known as compounded bioidentical HT because they perceive them to be a safer alternative. This article defines compounded bioidentical HT and explores the similarities and differences between it and US Food and Drug Administration–approved HT. We will examine the major claims made by proponents of compounded bioidentical HT and recommend strategies for management of patients who request bioidentical HT from physicians.
BHT = bioidentical HT; bi-est = bi-estrogen; CBHT = compounded bioidentical HT; CEE = conjugated equine estrogen; E+P = estrogen plus progestin; FDA = US Food and Drug Administration; HT = hormone therapy; MPA = medroxyprogesterone acetate; tri-est = tri-estrogen; WHI = Women's Health Initiative
Menopause, the permanent cessation of menstruation that results from loss of ovarian function, can occur naturally, surgically, or as the result of medical intervention.1 The change in hormonal milieu associated with perimenopause and menopause can lead to a wide variety of symptoms that may negatively affect a woman's quality of life. The most common symptoms include hot flashes, night sweats, emotional lability, poor concentration, and sleep disturbance; these can range from mild to severe. Postmenopausal hormone therapy (HT) is an effective, well-tolerated treatment for menopausal symptoms. In the United States, a number of US Food and Drug Administration (FDA)–approved hormone preparations are available for treatment of women with menopausal symptoms.2 In 2002, results from the estrogen plus progestin (E+P) arm of the Women's Health Initiative (WHI) revealed an increased risk of breast cancer, cardiovascular disease, stroke, and thromboembolic events in women taking conjugated equine estrogen (CEE) and medroxyprogesterone acetate (MPA) compared with those in the placebo group.3 These findings prompted many women to discontinue HT or to seek a safer alternative to FDA-approved HT for treatment of menopausal symptoms. As a result of the WHI, many women ask their physicians for non–FDA-approved compounded bioidentical HT (CBHT), which is also known as natural HT, believing that it is safer than FDA-approved therapy.3,4 It is estimated that CBHT is a multibillion-dollar industry, possibly affecting millions of women.5
WHAT WE LEARNED FROM THE WHI
The findings of the E+P arm (CEE and MPA) of the WHI that were published in 2002 dramatically changed the prescribing practices of physicians in the United States.6 Before the trial demonstrated adverse cardiovascular disease events and a 26% increased risk of breast cancer in female participants, the number of women for whom E+P was prescribed had been steadily increasing, from 58 million in 1995 to 90 million in 1999.6 From 1999 to 2002, the numbers stabilized; however, within 3 months of publication of the WHI findings, prescriptions for E+P decreased by 63%. Many women stopped HT and some sought out alternative therapies for treatment of symptoms associated with menopause.6 In addition to their effect on physician-prescribing patterns, the WHI findings changed the perceptions of patients about the trustworthiness of the advice dispensed by physicians. A small survey of 97 women conducted in 2005 demonstrated that all the participants had heard of the WHI; a substantial number expressed a loss of trust in the information about HT that they received from their physicians.7
This sequence of events, combined with celebrity endorsements, media coverage, and the growing number of women who discontinued HT and experienced a return of menopausal symptoms, coalesced to set the stage for the growth and expansion of the CBHT industry worldwide. Our research on resources available to patients yielded more than 70 books listed on Amazon.com, numerous blogs and Web sites, online pharmacies, and an Internet company endorsing affiliated physicians as experts in the use of CBHT.8-10
UNDERSTANDING TERMINOLOGY
The human steroid hormones are divided into the following 5 major classes: estrogens, progestogens, androgens, mineralocorticoids, and glucocorticoids. The most common steroid hormones prescribed for the treatment of menopausal symptoms are the estrogens and progestogens. Estrogens and progestogens are available in a wide variety of FDA-approved and non–FDA-approved formulations for the treatment of perimenopausal and menopausal symptoms.
Bioidentical HT
The term bioidentical hormone does not have a standardized definition and thus often confuses patients and practitioners. Women who request bioidentical HT (BHT) from their physicians may have differing expectations. Depending on the circumstances, it can mean natural (not artificial), compounded, plant derived, or chemically identical to the human hormone structure. The Endocrine Society has defined bioidentical hormones as “compounds that have exactly the same chemical and molecular structure as hormones that are produced in the human body.”11 This broad definition does not address the manufacturing, source, or delivery methods of the products and thus can include non–FDA-approved custom-compounded products as well as FDA-approved formulations.
FDA-Approved HT
Numerous FDA-approved hormone preparations are available for the treatment of menopausal symptoms. These include those that fulfill the definition of bioidentical and those that are clearly not bioidentical. Products can contain only estrogen (synthetic conjugated estrogens; natural, nonhuman conjugated estrogens; or plant-derived bioidentical estrogens), only progestogens (synthetic progestin or bioidentical progesterone), or a combination of estrogen and progestin. Those that contain synthetic conjugated estrogens, conjugated estrogens (derived from the urine of pregnant mares), or progestins are not bioidentical to the endogenous human sex steroid hormones.12
FDA-Approved BHT
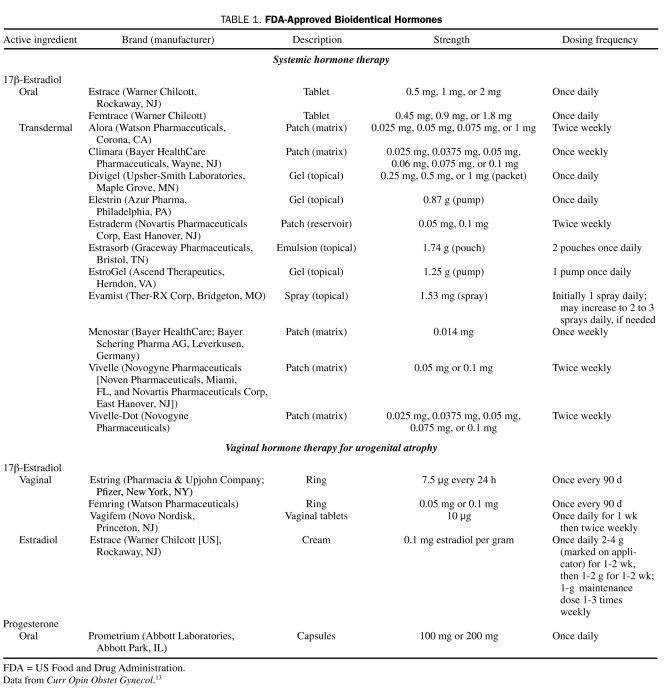
Currently, FDA-approved products containing bioidentical estrogen and progesterone are available. Bioidentical estrogen derived from plant sources (17β-estradiol) is available in pills, patches, sprays, creams, gels, and vaginal tablets. These preparations differ from custom CBHT preparations in that they are carefully controlled and regulated formulations (eg, oral, transdermal, and vaginal preparations), they are manufactured under strict standards, and their effects are subjected to scientific scrutiny.13 Numerous peer-reviewed publications have documented the beneficial effects of various doses of FDA-approved estrogen products on vasomotor symptoms, hot flashes, bone density, urogenital atrophy, and fracture prevention.14 In contrast, large-scale, randomized, controlled studies have not been conducted for custom CBHT.15
Progesterone bioidentical to that found in humans is currently available in certain FDA-approved preparations (as oral micronized progesterone in oil or as vaginal progesterone gel) (Table 1).
TABLE 1.
FDA-Approved Bioidentical Hormones
Non–FDA-Approved HT
The term CBHT refers to hormone preparations that (1) have exactly the same chemical and molecular structure as the estrogens and progesterone produced within the human body, (2) are plant derived, and (3) are specifically compounded for an individual patient. Custom CBHT is not FDA-approved for treatment of menopausal symptoms.16
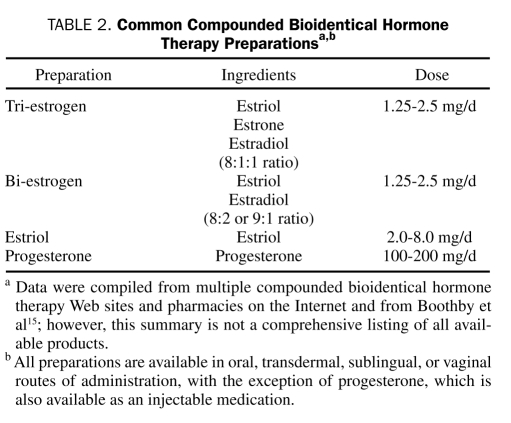
The FDA defines compounding as: “the combining or altering of ingredients by a pharmacist, in response to a licensed practitioner's prescription, to produce a drug tailored to an individual patient's special medical needs”.12 The most common compounded hormones include combinations of the endogenous estrogens (17β-estradiol, estrone, estriol) and progesterone. Although testosterone, dehydroepiandrosterone, and pregnenolone are sometimes added to CBHT preparations, the main components are usually estrogen and progesterone.15 Custom CBHT is available only at select pharmacies. Proponents of custom CBHT preparations claim that they offer improved safety, efficacy, and tolerability because of the individualization of the formulas, the source of the hormones, and the routes of delivery.15
ESTROGENS AND PROGESTOGENS
Estrogen Metabolism and Estrogen Receptors
A basic understanding of sex hormone metabolism is useful when discussing and prescribing HT. The endogenous estrogens found in humans include 17β-estradiol, estriol, estrone, and their conjugates.17 The human ovary produces 17β-estradiol and estrone, whereas estriol is formed through 16α-hydroxylation of estrone and estradiol.1 Before menopause, the predominant estrogen in circulation is 17β-estradiol, which is mainly secreted by the ovaries. Estrone is found in highest concentration after menopause and is converted in adipose tissue from estradiol and adrenal androstenedione.1,18 Estriol is short acting and is the least potent endogenous estrogen. It is found in very low concentrations (10 pg/mL) in the serum of nonpregnant women but is produced in high quantity by the placenta and, unlike estrone, is not converted to estradiol.17
The activity of all forms of estrogen is related to their affinity for 1 of the 2 estrogen receptors, α or β, which are found in different tissues. In addition, activity is determined by which type of estrogen binds to and subsequently causes a conformational change that produces an estrogen effect specific to the site of the receptor and modulated by other activating or repressing molecules.1 For example, the estrogen receptor α is located in the endometrium, breast cancer cells, and the ovary, whereas the β receptor is found in bone, kidney, lung, and endothelial cells, as well as in several other tissues.1 The binding affinity for the receptor is not strictly proportional to the potency of any given estrogen: 17β-estradiol has the highest affinity for both α and β receptors; estrone is midrange in affinity but predominantly binds to estrogen receptor α; and estriol has the weakest binding affinity for either receptor.17 Hormone effects are not dependent only on affinity or dose, as evidenced by the fact that a similar biological response can be achieved with low concentrations of estrogens for a prolonged period or by the short-term presence of high concentrations of estrogen.17
Compounded BHT: Estrogens
The compounded estrogen formulations most frequently prescribed contain 2 or 3 forms of estrogen that are “identical” to those found in humans: estradiol, estrone, and estriol in varying percentages. Common CBHT preparations are listed in Table 2. Although the compounded drug bi-estrogen (bi-est) is composed primarily (80%) of estriol, 17β-estradiol accounts for most of its estrogenic activity.15 It is the most physiologically active form of estrogen and is the predominant circulating estrogen before menopause, with 80 times the activity of estriol but making up only 10% to 20% of the formulation. In addition to 17β-estradiol and estriol, the compounded drug tri-estrogen (tri-est) contains estrone (at a ratio of 8:1:1) and is the predominant circulating, active form of estrogen in postmenopausal women. These 2 formulations (bi-est and tri-est) are generally available in oral, transdermal, and vaginal preparations. They must be compounded and usually are labeled with doses in milligrams, which can be misleading to physicians with little experience in this arena. For example, a typical formulation of bi-est will be labeled as a 2.5-mg dose. This does not reflect a single component but rather the total of the doses in milligrams of estradiol and estriol combined. If a particular formulation of bi-est is composed of 80% estriol, then that formulation would contain 2.0 mg of estriol and 0.5 mg of estradiol.15 Estimating a dose that is bioequivalent to conventional HT is difficult but is currently under investigation in a phase 1 clinical trial.19
TABLE 2.
Common Compounded Bioidentical Hormone Therapy Preparationsa,b
In summary, the common CBHT formulations contain US Pharmacopeia–grade, plant-derived estrogens and chemically converted estrogens (as already noted), but they have only a small percentage of the most active estrogen, 17β-estradiol.
Progesterone Metabolism, Progestins, and Progesterone Receptors
The term progestogen refers to both progesterone and synthetic compounds that have progestogenic activity similar to that of progesterone. The human ovaries and adrenal glands are responsible for progesterone production in the nonpregnant female.1 Oral progestogens are used in HT to prevent the development of endometrial hyperplasia or neoplasia as a result of estrogen administration.20 All progestogens cause a change from proliferative to secretory histology of the endometrium (that has been primed with estrogen).21 Progesterone is poorly absorbed and is rapidly metabolized, whereas progestins have high oral bioavailability. The development of the process of micronization allowed for improved absorption of oral progesterone. Micronized progesterone in peanut oil was approved by the FDA in December 1998; before then, micronized oral progesterone was available only in custom compounded products.22
Different types of synthetic progestins may have differing affinities for, and effects on, the progesterone receptor, and they may also activate non–progesterone receptor steroid receptors in different tissues.23
Evidence is not yet sufficient to fully support the claim that progesterone is safer than synthetic progestins, but 1 observational study reported a lower risk of breast cancer in patients receiving progesterone than in patients receiving synthetic progestins.24 Further study is needed. The only FDA-approved oral progesterone is micronized in peanut oil.
Compounded BHT: Progesterone
Before FDA approval of micronized progesterone in peanut oil, custom compounded products were the only source of micronized oral progesterone.22 For patients with peanut or other nut allergy, non-FDA–approved custom-compounded formulations can provide the option to prescribe oral progesterone micronized in other oils.25 Progesterone preparations can also be custom compounded into topical preparations, but these have not been shown to exert adequate activity to protect the endometrium from the effects of estrogen.
CLAIMS ABOUT CBHT
Claim 1: Compounded BHT Is Safer Than FDA-Approved HT
Proponents of CBHT claim that compounded products containing plant-derived hormones that are bioidentical to those found in the human ovary offer better safety, efficacy, and tolerability than noncompounded, FDA-approved HT.23 Unfortunately, these claims have led to confusion among patients, who often look to their physicians for direction. Therefore, physicians who care for women seeking relief from menopausal symptoms need to be familiar with the claims made about custom CBHT products and the evidence in the medical literature on their effectiveness or the lack thereof.
Objective evidence and scientific studies to substantiate these claims are lacking, and the use of custom CBHT vs FDA-approved HT remains a topic of ongoing debate in the lay press, in alternative medicine literature, and in the popular media.15 The claim that the safety profile of bioidentical compounds is better than that of FDA-approved HT15 belies the complexities of the topic. Both CBHT and FDA-approved HT are available in various dosages, combinations, preparations, and routes of delivery that may have differing effects on risk to an individual patient.26
Despite the contention by proponents that CBHT has been found to be safer, more efficacious, or less likely to cause breast or uterine cancer than FDA-approved HT, no reports published in peer-reviewed journals support this claim.14 Observational data suggest that estradiol in combination with progesterone may confer a lower risk of breast cancer than that seen with the CEE+MPA combination used in the WHI, but further study is needed to confirm this finding.27
Claim 2: Compounding Provides Improved Delivery and Tolerability of HT
Compounded medications require a written prescription from a licensed physician. Prescriptions filled in a compounding pharmacy are prepared, mixed, and assembled according to the specifications of the prescriber. Compounding plays an important role in providing drugs to meet the individualized needs of patients that cannot be met by a commercially available FDA-approved preparation. It may provide a different strength, dose, or delivery system, or it may allow a medication that is otherwise poorly tolerated to be essentially reformulated and delivered in an alternative form (eg, capsules, vaginal creams or troches, suppositories, nasal sprays, sublingual drops, topical gels, rapidly dissolving tablets, or transdermal gels).28,29 Compounded hormone preparations are not required to undergo the rigorous safety and efficacy studies required of FDA-approved HT and can demonstrate wide variation in active and inactive ingredients.5
The oversight of accredited compounding pharmacies falls to state pharmacy boards, which are to direct the method of preparation and enforce standards for compounding as dictated by the US Pharmacopeia. Thus, most compounded products do not have scientifically rigorous clinical testing for either safety or efficacy.5,30 A limited FDA survey in 2001 analyzed 29 product samples from 12 compounding pharmacies. None of the products failed identity testing; however, 10 (34%) of the 29 failed 1 or more quality test, and 9 (90%) of the 10 failing products also failed potency or assay testing. For FDA-approved therapies, the usual comparison failure rate is less than 2%.28
The FDA has been under increasing pressure to take enforcement action against compounding pharmacies since a citizens' petition was filed in October 2005 by Wyeth Pharmaceuticals (now part of Pfizer, New York, NY), the manufacturer of Prempro (conjugated estrogens/MPA) and other HT formulations, in which the company claimed that the compounding pharmacies producing CBHT often flagrantly violate the law.5 The petition resulted in a heightened level of concern about claims made by pharmacies that compound BHT, and in January 2008, the FDA censured 7 pharmacies involved in the Internet marketing and compounding of BHT by sending them letters of warning for making false and misleading claims regarding the safety and effectiveness of their advertised bioidentical hormone preparations.5 In January 2008, the FDA ruled that pharmacies could not compound drugs containing estriol without first obtaining an investigational new drug authorization. Estriol, a weak estrogen common to most CBHT formulations, has not been shown to be safe and effective for the uses for which it is being prescribed (eg, bone loss). Currently, estriol is not a component of any FDA-approved drug.31 This action generated considerable controversy because estriol is a component of tri-est, which is a common CBHT.
Claim 3: Custom CBHT Contains Safer Ingredients Than FDA-Approved HT
The production of US Pharmacopeia ingredients begins with the extraction of diosgenin from plants such as soy and yams.32 A chemical conversion is then required to produce progesterone, which is the precursor to the estrogens (estradiol, estrone, and estriol) and the androgens (progesterone and testosterone) that are used to formulate the final compounded product.32 This derivation and production process is similar to that of many commercially available, FDA-approved BHT products for which safety and efficacy data are available.
Claim 4: Salivary Testing Should Be Used to Provide “Personalized Therapy”
With the publication of the results of the WHI HT trial in 2002, the risks and benefits attributed to a specific formulation of estrogen and progestin were defined. The stark contrast between the observational data and the findings of the randomized, controlled clinical trial led to the recommendation that, in addition to advising that women be informed of the risks and benefits of HT, the lowest possible dose of hormone(s) should be used to treat symptoms for the shortest period (FDA black box change).33 These findings were thought to be generalizable to all available hormone preparations.
Proponents of CBHT claim that customized compounded formulations provide the best means of “tailoring or personalizing” therapy. They recommend salivary hormone testing to guide therapeutic decisions for the determination of dosages. In its recent position statement on HT, the North American Menopause Society endorsed the use of the clinical end point of symptom relief to guide dosing.34
Easily collected, saliva is similar to an ultrafiltrate of blood. Proponents of salivary testing suggest that measured hormone levels are reflective of the levels of free or bioavailable hormones in the serum of any given patient. The science supporting this type of testing for sex hormones has demonstrated poor reproducibility and large collection and assay variability even for a given individual, depending on the time of day, diet, salivary flow, and hormone being tested.35,36 Further, the idea that salivary hormone levels are related to the menopausal symptoms experienced by women has no scientific foundation.30 Even with standard FDA-approved HT for menopausal women, measurement of serum hormone levels can be potentially misleading because hormone levels do not always predict therapeutic effect (thus the recommendation to use symptoms as the clinical end point for dosing).17,34 The FDA has stated that no scientific evidence supports the use of salivary testing to titrate hormone dosages or monitor hormone levels.31
Individualization in dosing and delivery methods is also possible with FDA-approved BHT and nonbioidentical HT because many dosages and delivery methods are available.
Claim 5: Compounded BHT Has a Lower Breast Cancer Risk Than FDA-Approved HT
Observational studies and the WHI HT trial have established an association between HT (E+P) and an increased risk of breast cancer.3,37 Advocates for CBHT have claimed that estriol, given its decreased estrogenic activity, is not only safer but also associated with a decreased breast cancer risk. Some sources found on Internet sites go so far as to claim that it protects against cancer, yet no controlled trials substantiate this claim. Moreover, concerns have been raised about the continued use of estriol in high doses and its potential risk for stimulating breast parenchyma.38 It is unknown whether the increased risk of breast cancer in the E+P arm of the WHI vs the estrogen-only arm was the result of the addition of a progestin, the specific combination and dosage of E+P that was used in the trial, or the specific type of progestin (MPA) that was used.27
For many clinicians, the preferred progesterone formulation has been Prometrium (Abbott Laboratories, Abbott Park, IL), the FDA-approved, micronized formulation of oral progesterone, because it is identical to endogenous progesterone and has improved bioavailability.38 In an observational study, a decreased risk of histology- and hormone receptor–defined invasive breast cancer was noted with use of a combination of micronized progesterone and estrogen vs the use of synthetic progestogens; however, further study is needed to establish long-term safety.26 There is a lack of published studies in peer-reviewed journals about the safety and efficacy of CBHT in decreasing breast cancer risk. Patients need to be well informed about the risks and benefits, and controlled clinical trials are necessary to provide evidence of long-term safety.
Claim 6: Compounded BHT Has a Better Effect on Bones Than FDA-Approved HT
Among the claims in favor of CBHT for better bone health are the scientifically based observations about the benefits of estrogen and testosterone in preventing bone loss, as well as the suggestion of progesterone as an agent that can “assist” in rebuilding lost bone. These claims are an extrapolation of data derived from many peer-reviewed studies using standard doses of manufactured estrogen and testosterone that have shown a beneficial effect of estrogen in preventing postmenopausal bone loss.39 The WHI is the only randomized, controlled study demonstrating reduced hip fracture risk with E+P therapy.40 None of these claims has been substantiated directly with CBHT or correlated with the wide variety of CBHT preparations in use. Although credible by inference with the class of therapy (estrogen, for example), the claims that CBHT prevents bone loss or rebuilds lost bone have not been proven.
APPROACH TO PATIENT CARE
With all the interest in CBHT generated by the popular media, women often turn for definitive information about CBHT to their primary care physicians, who are expected to refill existing prescriptions or write new prescriptions for compounded products. We have found that it is important to acknowledge the use of the bioidentical formulations without appearing to be judgmental or dismissive. Many patients are well versed in the topic and have often conducted an exhaustive online investigation before they present with questions. Prescriptions from other physicians for compounded formulations should not be refilled unless the physician is in full agreement about the need for the formulations, their dosage, and indication and is familiar with the compounded product. An attempt should be undertaken to educate the patient about currently available FDA-approved BHT products (Table 1). Patients are often surprised to learn that FDA-approved products identical to those in the compounded products are available via a number of delivery methods. In accordance with the latest recommendations from the North American Menopause Society, the objective of HT in women who have gone through natural menopause should be the relief of symptoms that a woman finds disruptive to her life. The lowest efficacious dose should be prescribed, with the goal of using it for the shortest period. For women who have undergone an early surgical menopause, replacement should continue at least until the average age of menopause (52 years).1 We recommend annual evaluation of all HT in the context of new data or changes in the patient's health.
CONCLUSION
No evidence currently suggests that custom CBHT formulations offer clinically relevant benefit over the FDA-approved products available to treat the symptoms of menopause. Because of their wide array of formulations, dosages, and delivery systems, FDA-approved HT products can be used to individualize therapy and tailor it to meet the needs and expectations of patients desiring relief of menopausal symptoms. Custom CBHT formulations provide practitioners the option to prescribe HT for women who cannot tolerate FDA-approved products or the nonhormonal ingredients contained in them.31 Practitioners should discuss risks and benefits of the proposed therapy with each patient and should prescribe only the products with which they are familiar and experienced.17,34
CME Questions About Bioidentical Hormone Therapy
-
Which one of the following estrogens is the predominant circulating agent before menopause?
17β-estradiol
Estrone
Estrone sulfate
Estriol
Ethinyl estradiol
-
Which one of the following hormone therapies (HTs) is approved by the US Food and Drug Administration (FDA) for treatment of menopausal symptoms?
Transdermal testosterone
Bioidentical and nonbioidentical medications
Pregnenolone
Dehydroepiandrosterone
Estriol
-
Which one of the following best guides HT dosing decisions?
Salivary hormone testing
Blood levels
Relief of clinical symptoms
Bone density
Patient preference
-
Which one of the following is required for compounding?
The same strength and dosing for all patients
Rigorous clinical testing for safety and efficacy
A written prescription and a compounding pharmacy
Reformulation and delivery systems available only as transdermal preparations
Both salivary and serum hormone testing for personalization of therapy
-
Which one of the following best describes non–FDA-approved vs FDA-approved BHT?
Safer
More efficacious
Better studied
Assumed to have the same risks
Well tolerated
Supplementary Material
Footnotes
On completion of this article, you should be able (1) to define compounded bioidentical hormone therapy and recognize its formulations and their differences from and similarities to bioidentical hormone therapy products approved by the US Food and Drug Administration, (2) to examine the claims made by proponents of compounded bioidentical hormone therapy, and (3) to recommend a general approach to managing patients who request bioidentical hormone therapy.
An earlier version of this article appeared Online First.
REFERENCES
- 1. Speroff L, Glass RH, Kase NG, eds. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- 2. MacLennan A, Lester S, Moore V. Oral oestrogen replacement therapy versus placebo for hot flushes. Cochrane Database Syst Rev. 2001;CD002978 [DOI] [PubMed] [Google Scholar]
- 3. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321-333 [DOI] [PubMed] [Google Scholar]
- 4. Stefanick ML. Estrogens and progestins: background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. Am J Med. 2005;118(suppl 12B):64-73 [DOI] [PubMed] [Google Scholar]
- 5. Patsner B. Pharmacy compounding of bioidentical hormone replacement therapy (BHRT): a proposed new approach to justify FDA regulation of these prescription drugs. Food Drug Law J. 2008;63:459-491 [PubMed] [Google Scholar]
- 6. Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47-53 [DOI] [PubMed] [Google Scholar]
- 7. McIntosh J, Blalock SJ. Effects of media coverage of Women's Health Initiative study on attitudes and behavior of women receiving hormone replacement therapy. Am J Health Syst Pharm. 2005;62:69-74 [DOI] [PubMed] [Google Scholar]
- 8.http://www.bodylogicmd.com/ [Accessed Aug 18, 2010]. http://www.bodylogicmd.com/ BodyLogicMD.
- 9. Google Search term: bioidentical hormones. http://www.google.com/ Accessed Aug 18, 2010
- 10.Amazon.com. http://www.amazon.com/ [Accessed Jul 26, 2010]. Amazon.comhttp://www.amazon.com/
- 11. Endocrine Society Bioidentical hormones: position statement. 2006. http://www.endo-society.org/advocacy/policy/upload/BH_position_Statement_final_10_25_06_w_Header.pdf Accessed April 6, 2011
- 12. US Food and Drug Adminstration (FDA) FDA Web site Compounded menopausal hormone therapy questions and answers. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/PharmacyCompounding/ucm183088.htm Accessed April 6, 2011
- 13. Boothby LA, Doering PL. Bioidentical hormone therapy: a panacea that lacks supportive evidence. Curr Opin Obstet Gynecol. 2008;20:400-407 [DOI] [PubMed] [Google Scholar]
- 14. Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95:s1-s66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boothby LA, Doering PL, Kipersztok S. Bioidentical hormone therapy: a review. Menopause. 2004;11:356-367 [DOI] [PubMed] [Google Scholar]
- 16. Iftikhar S, Shuster LT, Johnson RE, et al. Use of bioidentical hormones for menopausal concerns: cross sectional survey in an academic menopause center. J Womens Health. 2011;20:559-565 [DOI] [PubMed] [Google Scholar]
- 17. Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;8(suppl 1):3-63 [DOI] [PubMed] [Google Scholar]
- 18. Coelingh Bennink HJ. Are all estrogens the same? Maturitas. 2004;47:269-275 [DOI] [PubMed] [Google Scholar]
- 19. ClinicalTrials.gov A pharmacokinetic evaluation of bioidentical compounded estrogen cream and natural progesterone (HRT). History of Changes and the ClinicalTrials.gov Archive Site Web site http://clinicaltrials.gov/ct2/archive/NCT00864214 Accessed April 6, 2011
- 20. Archer DF. The effect of the duration of progestin use on the occurrence of endometrial cancer in postmenopausal women. Menopause. 2001;8:245-251 [DOI] [PubMed] [Google Scholar]
- 21. Moyer DL, Felix JC. The effects of progesterone and progestins on endometrial proliferation. Contraception. 1998;57:399-403 [DOI] [PubMed] [Google Scholar]
- 22. The Writing Group for the PEPI Trial Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women: the Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial [published correction appears in JAMA. 1995;274:1676]. JAMA. 1995;273:199-208 [PubMed] [Google Scholar]
- 23. Holtorf K. The bioidentical hormone debate: are bioidentical hormones (estradiol, estriol, and progesterone) safer or more efficacious than commonly used synthetic versions in hormone replacement therapy? Postgrad Med. 2009;121:73-85 [DOI] [PubMed] [Google Scholar]
- 24. Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prometrium [package insert]. Marietta, GA: Solvay Pharmaceuticals, Inc; 2010. [Google Scholar]
- 26. Fournier A, Fabre A, Mesrine S, Boutron-Ruault MC, Berrino F, Clavel-Chapelon F. Use of different postmenopausal hormone therapies and risk of histology- and hormone receptor-defined invasive breast cancer. J Clin Oncol. 2008;26:1260-1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. L'Hermite M, Simoncini T, Fuller S, Genazzani AR. Could transdermal estradiol + progesterone be a safer postmenopausal HRT? A review. Maturitas. 2008;60:185-201 [DOI] [PubMed] [Google Scholar]
- 28. ACOG Committee on Gynecologic Practice ACOG Committee Opinion #322: Compounded bioidentical hormones. Obstet Gynecol. 2005;106(5, pt 1):1139-1140 [DOI] [PubMed] [Google Scholar]
- 29. Allen LV., Jr Dosage form design and development. Clin Ther. 2008;30:2102-2111 [DOI] [PubMed] [Google Scholar]
- 30. Cirigliano M. Bioidentical hormone therapy: a review of the evidence. J Womens Health (Larchmt). 2007;16:600-631 [DOI] [PubMed] [Google Scholar]
- 31. North American Menopause Society Estrogen and progestogen use in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17:242-255 [DOI] [PubMed] [Google Scholar]
- 32. Taylor M. Unconventional estrogens: estriol, biest, and triest. Clin Obstet Gynecol. 2001;44:864-879 [DOI] [PubMed] [Google Scholar]
- 33. US Food and Drug Administration (FDA) MedWatch the FDA safety information and adverse event reporting program. Prempro (conjugated estrogens/medroxyprogesterone acetate tablets). http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm153360.htm Accessed April 6, 2011
- 34. North American Menopause Society Estrogen and progestogen use in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17(2):242-255 [DOI] [PubMed] [Google Scholar]
- 35. Davison S. Salivary testing opens a Pandora's box of issues surrounding accurate measurement of testosterone in women. Menopause. 2009;16:630-631 [DOI] [PubMed] [Google Scholar]
- 36. Granger DA, Shirtcliff EA, Booth A, Kivlighan KT, Schwartz EB. The ``trouble'' with salivary testosterone. Psychoneuroendocrinology. 2004;29:1229-1240 [DOI] [PubMed] [Google Scholar]
- 37. Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047-1059 [PubMed] [Google Scholar]
- 38. Head KA. Estriol: safety and efficacy. Altern Med Rev. 1998;3:101-113 [PubMed] [Google Scholar]
- 39. Khosla S. Update on estrogens and the skeleton. J Clin Endocrinol Metab. 2010;95:3569-3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women's Health Initiative randomized trial. JAMA. 2003;290:1729-1738 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.