Abstract
Antimicrobial prophylaxis is commonly used by clinicians for the prevention of numerous infectious diseases, including herpes simplex infection, rheumatic fever, recurrent cellulitis, meningococcal disease, recurrent uncomplicated urinary tract infections in women, spontaneous bacterial peritonitis in patients with cirrhosis, influenza, infective endocarditis, pertussis, and acute necrotizing pancreatitis, as well as infections associated with open fractures, recent prosthetic joint placement, and bite wounds. Perioperative antimicrobial prophylaxis is recommended for various surgical procedures to prevent surgical site infections. Optimal antimicrobial agents for prophylaxis should be bactericidal, nontoxic, inexpensive, and active against the typical pathogens that can cause surgical site infection postoperatively. To maximize its effectiveness, intravenous perioperative prophylaxis should be administered within 30 to 60 minutes before the surgical incision. Antimicrobial prophylaxis should be of short duration to decrease toxicity and antimicrobial resistance and to reduce cost.
AAOS = American Association of Orthopedic Surgeons; ADA = American Dental Association; ANP = acute necrotizing pancreatitis; AP = antimicrobial prophylaxis; AUA = American Urological Association; CP = chemoprophylaxis; FDA = US Food and Drug Administration; HIV = human immunodeficiency virus; IDSA = Infectious Diseases Society of America; IE = infective endocarditis; IS = Information Statement; MRSA = methicillin-resistant Staphylococcus aureus; PJI = prosthetic joint infection; PJR = prosthetic joint replacement; RF = rheumatic fever; SBP = spontaneous bacterial peritonitis; SCIP = Surgical Care Improvement Project; Tdap = tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine, adsorbed; UGI = upper gastrointestinal; UTI = urinary tract infection
Antimicrobial prophylaxis (AP) can be used effectively to prevent infection, but its use should be limited to specific, well-accepted indications to avoid excess cost, toxicity, and antimicrobial resistance. Antimicrobial prophylaxis may be considered primary (prevention of an initial infection) or secondary (prevention of the recurrence or reactivation of an infection), or it may also be administered to prevent infection by eliminating a colonizing organism. This article reviews widely accepted indications for AP in nonsurgical and surgical patients and is an update of a previously published review of this topic.1 In selected situations, vaccination may be recommended as part of a prophylaxis regimen. This article is meant to be a point-of-care overview topic for the busy clinician. Many of these recommendations are based on expert opinion rather than on prospective clinical trials. Most of the recommended antimicrobial agents are not approved by the US Food and Drug Administration (FDA) for prophylaxis. Current full prescribing information available in the package insert of each drug should be consulted before prescribing any product. Detailed information on individual topics can be found in the cited references.
The potential risks and benefits of AP should be discussed in detail with the patient. Potential risks include allergic reactions that may be severe or life-threatening as well as Clostridium difficile colitis with the use of antibacterial agents.2 Patients taking fluoroquinolones should be warned of the risk of developing tendinitis, including Achilles tendon rupture.3 For all antibiotic dosing recommended in this article, normal hepatic and renal function are assumed.
NONSURGICAL AP
Rheumatic Fever
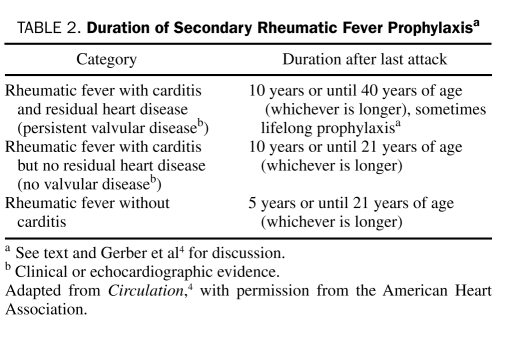
Rheumatic fever (RF), which is associated with tonsillopharyngitis caused by the group A β-hemolytic streptococci, may result in carditis with or without valvulopathy. Primary prevention of RF involves prompt and appropriate antibiotic treatment of group A β-hemolytic streptococcal pharyngitis with a penicillin (drug of choice) or alternative antibiotic.4 Continuous secondary AP prevents recurrent episodes of RF, which could otherwise lead to worsening of the severity of rheumatic heart disease that developed after the initial attack or the development of rheumatic carditis in those who did not develop carditis with the initial RF episode. Guidelines for secondary AP of RF have recently been updated (recommendations for AP regimens are summarized in Table 1).4 Penicillins are the antibiotics of choice for secondary prophylaxis for RF, and intramuscular penicillin is superior to oral penicillins.25 Macrolides (eg, erythromycin, clarithromycin, azithromycin) should be reserved for patients who are allergic to both penicillin and sulfa antibiotics. The duration of secondary prophylaxis for RF is reviewed in detail elsewhere and is summarized in Table 2.4 Physicians should tailor the duration of secondary prophylaxis to the individual patient, taking into account the patient's risk factors for RF recurrence, such as exposure to young children and the presence of carditis with or without underlying valvular disease. Antimicrobial prophylaxis should be considered for at least 10 years or until age 40 years (whichever is longer) for patients with carditis with persistent valvular disease. Prophylaxis should be continued in patients even after prosthetic valve replacement surgery. Antibiotic suppression for the prevention of RF is not adequate for infective endocarditis (IE) prophylaxis before dental procedures.
TABLE 1.
Selected Nonsurgical Antimicrobial Prophylaxis Regimens for Adultsa,b
TABLE 2.
Duration of Secondary Rheumatic Fever Prophylaxisa
Recurrent Cellulitis
Patients with lymphedema or severe venous insufficiency of their extremities are at increased risk of recurring β-streptococcal cellulitis. Common scenarios for recurrent cellulitis of the lower extremity include patients with venous insufficiency after saphenous vein graft harvesting or pelvic lymphadenectomy. Recurrent cellulitis has been observed in the upper extremity after lymphadenectomy performed at the time of mastectomy for breast cancer. Antimicrobial prophylaxis may be a useful addition to the control of lymphedema with local measures and treatment of concurrent tinea pedis in the prevention of recurrent cellulitis. However, this recommendation is based on small, uncontrolled studies.26-28 Typically, more than 2 or 3 episodes per year should occur before AP is initiated. Recommended prophylactic antibiotics for recurrent cellulitis are summarized in Table 1. Oral penicillin V (phenoxymethylpenicillin) is a reasonable first choice, but optimal dosing of this agent is not well established.5-7 Although monthly administration of 1.2 MU of intramuscular benzathine penicillin is recommended as an alternative to oral penicillin V, this dosing regimen was shown to be effective only in those patients not at risk of cellulitis recurrence.28 Some experts recommend intramuscular administration of benzathine penicillin every 2 to 3 weeks for individuals who break through once-monthly intramuscular benzathine penicillin regimens.5
Recurrent pyogenic skin infections caused by Staphylococcus aureus, including methicillin-resistant S aureus (MRSA), may be managed by encouraging good personal hygiene, the avoidance of shared personal items, and the diligent cleaning of high-touch environmental surfaces. If a patient is found to be colonized by S aureus, nasal decolonization with mupirocin for 5 to 10 days with or without a topical body decolonization with a skin antiseptic solution such as 4% chlorhexidine for 5 to 14 days may be reasonable in an attempt to decolonize the patient.8 Antimicrobial prophylaxis options are listed in Table 1 for recurrent methicillin-susceptible S aureus skin infections.9,29 Long-term oral AP of recurrent MRSA skin infections is not well studied, and formal recommendations for this situation were not included in recently published MRSA treatment guidelines.8
Meningococcal Disease
Antimicrobial prophylaxis for meningococcal diseases should be offered to close contacts of sporadic cases of Neisseria meningitidis infection (Table 1). Close contacts include household members, day care center staff, and any person directly exposed to an infected person's oral secretions (for example, through kissing, mouth-to-mouth resuscitation, endotracheal intubation, or endotracheal tube management).11 Public health authorities may recommend population-based prophylaxis in the event of an outbreak. Prophylaxis should be offered as soon as possible. Close contacts should be offered meningococcal vaccination if the outbreak strain is one that is contained in the currently available meningococcal tetravalent conjugate vaccine.30
Asplenic Patients
Penicillin prophylaxis is recommended in children during the first few years after splenectomy to prevent overwhelming Streptococcus pneumoniae sepsis.31 French and American authorities have advocated this form of prophylaxis (eg, 250 mg of oral penicillin V or amoxicillin twice daily) in adults for 1 to 2 years after splenectomy, although data showing the efficacy of this approach are lacking.31-33 Haemophilus influenzae type B, meningococcal, and pneumococcal vaccinations should be current in asplenic adults.
Urinary Tract Infection
Several prophylactic antibiotic options are available to non-pregnant women with recurrent (≥3 per year), uncomplicated urinary tract infections (UTIs)13 (Table 1). Continuous low-dose AP and patient-initiated treatment after onset of symptoms are both effective.13,14 During AP, monthly urine cultures should be performed to monitor for bacteriuria and the development of antibiotic resistance.34 Structural abnormality of the urinary tract, renal involvement with infection, or chronic prostatitis (in men) should be considered in the setting of recurrent UTIs. Methenamine hippurate (dosage, 1 g twice daily) has been approved by the FDA for UTI prophylaxis. A recent Cochrane review concluded that methenamine hippurate may be effective for short-term prophylaxis (≤1 week) in patients without known renal tract abnormalities.35 The typical duration of an initial trial of continuous AP is 6 months. Patients with prolonged exposure to nitrofurantoin should be counseled about the rare but serious complications associated with this agent, including hepatitis, pulmonary reactions, and neuropathy. Cranberries contain 2 substances that prevent fimbriated Escherichia coli from adhering to uroepithelial cells.36 Clinical studies have shown that cranberry juice and cranberry products may reduce the recurrence of UTIs in women. A recent Cochrane review noted limitations in these studies, including variable cranberry products and dosing used in the various studies, as well as high study participant dropout rates.37 Other patients who may be considered for prophylaxis of frequent UTIs include pregnant women, persons with spinal cord injuries, persons with neurogenic bladders, renal transplant recipients, and men with chronic bacterial prostatitis.13,34 Postcoital regimens may be appropriate for female patients with UTIs temporally related to sexual intercourse.15,38 Patients who use postcoital regimens should be informed that only 1 dose per day is recommended, regardless of the frequency of intercourse. Postcoital AP in pregnancy can be managed with a single dose of either cephalexin (250 mg) or nitrofurantoin (50 mg).34 Tetracyclines and fluoroquinolones should be avoided during pregnancy, and sulfonamides should be avoided during the last weeks of gestation to minimize the risk of hyperbilirubinemia and kernicterus in the newborn. Topical vaginal estrogen therapy has been shown to reduce the risk of recurrent UTIs in postmenopausal women; it may be a consideration for postmenopausal women who are not receiving estrogen replacement therapy and who have no contraindications to estrogen therapy.39
Spontaneous Bacterial Peritonitis
Spontaneous bacterial peritonitis (SBP) in patients with cirrhosis is associated with increased morbidity and mortality. Aerobic gram-negative organisms and streptococci are the most frequent causes of this infection. In a recent Cochrane review of 12 treatment trials, empirical oral or parenteral antimicrobial treatment of patients with cirrhosis and upper gastrointestinal (UGI) bleeding reduced the incidence of bacterial infections and was associated with shortened hospital stays and reduced rates of overall mortality, mortality from bacterial infections, and rebleeding.40 No one antibiotic regimen or route of administration was found to be superior. On the basis of these data, 7 days of empirical antibiotics are recommended for patients with ascites and UGI bleeding16 (Table 1). In prospective randomized clinical trials, primary prophylaxis in high-risk patients and secondary prophylaxis after an initial episode of SBP have been shown to be effective in preventing SBP.41-44 A recent Cochrane review of 7 trials of empirical AP to prevent SBP in cirrhotic patients with ascites without UGI bleeding revealed a pooled reduction in SBP and mortality but noted issues with trial methodology and findings suggestive of systematic bias in publication and design.45 A 1998 analysis concluded that prophylaxis in high-risk patients (serum bilirubin level >2.5 mg/dL [to convert to μmol/L, multiply by 17.104]; ascitic fluid protein level, <1 g/dL) is cost-effective.46 The American Association for the Study of Liver Diseases has published guidelines that recommend long-term daily AP for patients with previous SBP and for primary prophylaxis in those with an ascitic fluid protein level of less than 1.5 g/dL and at least 1 of the following criteria: a serum creatinine level of 1.2 mg/dL or higher (to convert to μmol/L, multiply by 88.4), a blood urea nitrogen level of 25 mg/dL or higher (to convert to mmol/L, multiply by 0.357), a serum sodium level of 130 mEq/L or less (to convert to mmol/L, multiply by 1), or a Child-Pugh score of 9 points or higher with a bilirubin level of 3 mg/dL or higher16 (Table 1). Before initiation of AP, SBP should be ruled out in all patients with ascites at hospital admission and in cirrhotic patients with ascites with signs, symptoms, or laboratory abnormalities suggestive of infection.16
Acute Necrotizing Pancreatitis
Severe pancreatitis with necrosis is associated with an overall mortality rate of 17% and a mortality rate of 25% to 30% with infected necrosis. Debate is ongoing as to whether AP in the setting of acute necrotizing pancreatitis (ANP) leads to improved outcomes (some consider the use of antibiotics in this setting preemptive).47 A recent Cochrane database review of 7 randomized studies concluded that patients randomized to receive AP for ANP had no statistically significant reduction in infections.48 Recent practice guidelines published by the American College of Gastroenterology do not recommend AP for ANP.49 If AP is initiated, a broad-spectrum β-lactam such as imipenem-cilastatin is often recommended and should be limited to computed tomography–documented pancreatic necrosis involving 30% or more of the pancreas for 14 days or less.50
Bite Wound Infection
Five percent of dog bites and 30% of cat bites become secondarily infected because these wounds are highly contaminated by microorganisms present in the oral cavity of these animals. These infections can lead to septic arthritis, tenosynovitis, severe soft tissue infection, or sepsis.51 The microbiology of dog and cat bite infections is typically polymicrobial and includes Pasteurella species as the most common isolate, followed by staphylococci, streptococci, and anaerobes.52 Although AP for animal bites remains controversial, a meta-analysis of 8 clinical trials by Cummings53 found that AP significantly protects against subsequent wound infection. Antimicrobial prophylaxis of a contaminated wound may be more accurately considered expectant therapy to prevent the development of a wound infection in a contaminated but not yet infected wound. No clinical trials have shown superiority of one antibiotic regimen over another; choices should be based on the likely microbiology of dog and cat bite infections.54 Antimicrobial prophylaxis for bite wounds has recently been reviewed and should be offered to all patients who are thought to have an increased risk of infection17 (Table 1). High-risk situations include, but are not limited to, bites to body areas where deeper structures (tendons and bones) can become easily injured, bites to the hand(s) or close to a bone or joint, crush injuries, puncture wounds (difficult to clean), bites in which treatment is delayed more than 8 to 10 hours, wounds requiring closure, bites in compromised persons (diabetic patients, persons with no spleen, immunocompromised patients), bites in persons with indwelling prosthetic devices, and all cat bites.17,18 Consideration for hospitalization and intravenous antibiotics may be reasonable for patients in the setting of fever, sepsis, spread of cellulitis, significant edema or crush injury, loss of function, compromised immunity, or patient nonadherence to treatment.19 All dog and cat bites should be appropriately irrigated and débrided, and rabies prophylaxis should be administered, if indicated. Delayed primary closure of heavily contaminated wounds should be considered to decrease the risk of wound infection.
Human bite wounds, including clenched fist injuries, are considered to be at high-risk of infection with organisms such as Streptococcus anginosus, S aureus, Eikenella corrodens, and anaerobes. Recommended AP is similar to that for animal bite wounds17,55 (Table 1). Patients who have sustained human bites should be assessed for human immunodeficiency virus (HIV) and hepatitis B infection risk, and prophylaxis should be offered as indicated according to published guidelines. Tetanus immune globulin and tetanus toxoid should be administered to patients who have not been immunized or tetanus toxoid alone to any patient who has not received a tetanus booster within the past 5 years.
Pertussis
Pertussis (whooping cough), an upper respiratory tract infection caused by Bordetella pertussis, is associated with prolonged bouts of coughing that may last 1 to 6 weeks. Numerous pertussis outbreaks have occurred in the United States during the past 6 years among adolescents and adults as immunity from childhood vaccination has waned. Because pertussis is spread by aerosolized respiratory droplets, it is recommended that all household and other close contacts of infected patients who did not use respiratory precautions while in contact with an infected patient receive AP, regardless of age or immunization status20 (Table 1).
The first tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine, adsorbed (Tdap) licensed for adults was approved by the FDA in 2005 (ADACEL; Sanofi Pasteur; Swiftwater, PA [US Headquarters]; Lyon, France [Global Headquarters]) as a single-dose booster vaccine for persons aged 11 to 64 years to provide protection against tetanus, diphtheria, and pertussis. Tdap was initially recommended to replace the next adult booster dose of tetanus- and diphtheria-toxoid vaccines in patients whose last tetanus booster was 10 years or more earlier. The interval between the most recent tetanus vaccination and Tdap for persons with contact with infants, child care providers, or health care professionals with direct patient contact could be as short as 2 years or less.56 Given the poor adult pertussis vaccine coverage (5.9% in 200857), and in the setting of increasing numbers of pertussis cases in the United States (16,858 cases in 2009, including 14 infant deaths58), the Pertussis Vaccine Working Group of the Advisory Committee on Immunization Practices59 recommends the administration of a single Tdap (either ADACEL or BOOSTRIX [GlaxoSmithKline Biologicals; Morrisville, NC]), when indicated, for any adult, at any interval since the previous tetanus-diphtheria vaccination. A single Tdap should be considered for adults 65 years or older who have or anticipate having close contact with an infant younger than 12 months as well as for children aged 7 through 10 years who are not fully vaccinated against pertussis. Tdap is not licensed for revaccination. A provisional recommendation from the Advisory Committee on Immunization Practices (February 23, 2011) states that the data on the need for postexposure AP for Tdap-vaccinated health care professionals are inconclusive.60 In view of this, Tdap-vaccinated health care professionals may still be at risk of acquiring pertussis and should be considered for chemoprophylaxis (CP) after a significant pertussis exposure, particularly if they are likely to be exposed to a patient at risk of severe pertussis, such as hospitalized neonates and pregnant women.
Infective Endocarditis
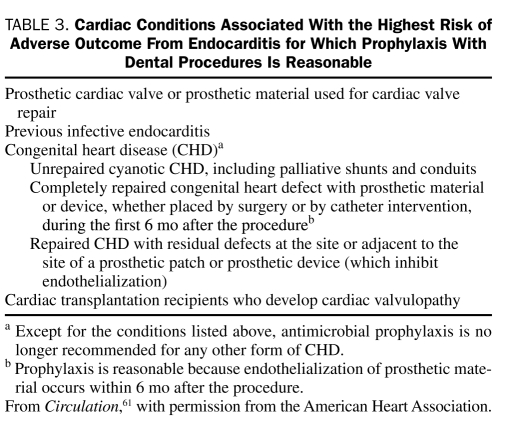
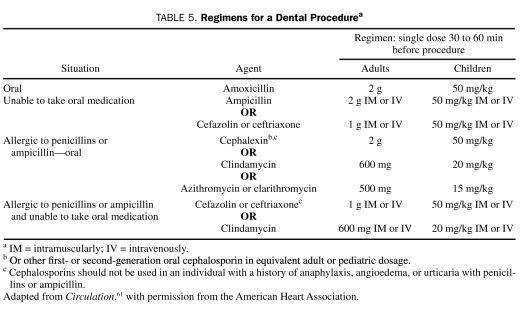
Infective endocarditis is a relatively rare endocardial infection that can lead to catastrophic complications and death. Guidelines for the prevention of IE have been published by the American Heart Association for more than 50 years. The first 9 guidelines (1955-1997) were based on low-level evidence; more recently, guidelines have been stratified according to the lifetime risk of IE. The recommendations of the most recent (2007) guidelines reflected a new reticence about using AP for IE based on the following premises: (1) cumulative bacteremia risk is much greater with daily activities than dental procedures; (2) antibiotics do not eliminate bacteremia or clearly reduce IE risk; (3) there are no prospective, placebo-controlled AP trials; and (4) even if 100% effective, antibiotics would prevent only rare cases of IE.61 The 2007 AP guidelines for IE from the American Heart Association and the Infectious Diseases Society of America (IDSA) recommend AP only for patients at highest risk of complications of IE (Table 3) and only for selected dental procedures (Table 4). Administration of prophylactic antibiotics is no longer stratified according to lifetime IE risk. The antibiotics that are recommended for IE prophylaxis before dental procedures are listed in Table 5. Patients receiving a penicillin for RF prophylaxis should not receive a penicillin for IE dental prophylaxis.
TABLE 3.
Cardiac Conditions Associated With the Highest Risk of Adverse Outcome From Endocarditis for Which Prophylaxis With Dental Procedures Is Reasonable
TABLE 4.
Dental Procedures for Which Endocarditis Prophylaxis Is Reasonable for Patients in Table 3
TABLE 5.
Regimens for a Dental Procedurea
Prophylaxis is no longer recommended for uncomplicated gastrointestinal bronchoscopy without incision of the respiratory mucosa and for urinary procedures. If the urine is colonized or infected before an elective cystoscopy, antibiotic therapy to eradicate the infection before the urologic manipulation is recommended. If an urgent cystoscopy is to be performed in the setting of colonized or infected urine, then an antibiotic with activity against enterococci should be administered. Ampicillin or amoxicillin are the preferred agents in this setting; vancomycin should be used in the setting of severe penicillin intolerance. Urinary tract colonization or infection with enterococci known or suspected to be resistant (including those resistant to vancomycin) may require a consultation with an infectious diseases expert.61
Although many respiratory tract procedures reportedly cause bacteremia involving a wide variety of microorganisms, no published data conclusively demonstrate a link between these procedures and IE. Antimicrobial prophylaxis (for regimens, see Table 5) is thought to be reasonable for patients at highest risk of complications from IE (Table 3) who undergo invasive procedures of the respiratory tract that involve incision or biopsy of the respiratory mucosa (eg, tonsillectomy, adenoidectomy). Patients at highest risk of complications from IE who undergo an invasive respiratory tract procedure to treat an established infection, such as drainage of an abscess or empyema, should receive an antibiotic that is active against the viridans group streptococci. If an infection is known or suspected to be caused by S aureus, the antibiotic regimen should contain an antistaphylococcal penicillin or a cephalosporin for patients who are unable to tolerate a penicillin. Vancomycin should be used in those in whom an infection is known or suspected to be caused by a methicillin-resistant strain of S aureus or in those who have a history of a severe reaction to β-lactam antibiotics.61
Prosthetic Joint Infections
By 2030, an estimated 4 million total knee or hip arthroplasties will be performed annually in the United States.62 Prosthetic joint infections (PJIs), which are rare but serious complications of prosthetic joint replacements (PJRs), occur in 0.3% to 1.0% of patients after primary total hip replacement and 1.0% to 2.0% of patients after primary total knee replacements, with the greatest risk occurring during the first 2 postoperative years (6.5, 3.2, and 1.4 infections per 1000 patient-years during the first year, second year, and after the second year, respectively).63,64 These infections may be associated with devastating financial and personal consequences. Most PJIs are acquired in the operating room as a result of colonization of the prosthesis at the time of implantation or airborne contamination of the wound.63 Infection of a prosthesis via hematogenous seeding is a less common cause of PJI. Among PJIs occurring via the hematogenous route, most are the result of S aureus bacteremia, skin infections, or urosepsis.65-67 The development of a PJI due to hematogenous seeding after dental procedures is thought to be a rare event. According to a recent literature review, this occurred in 0.04% to 0.20% of reported PJR case series; many of these infections were seen in patients with dental disease.68 Pins, plates, and screws not within the synovial joint are not thought to be at increased risk of hematogenous seeding by microorganisms. No studies have shown that AP before dental procedures prevents PJI.69 A recently published prospective case-control study concluded that dental procedures were not risk factors for subsequent total hip or knee infection. Additionally, the use of AP before dental procedures did not decrease the risk of subsequent total hip or knee infection.70
Despite the lack of data supporting AP before dental procedures, many surveys of health care professionals have shown that a substantial number of them recommend AP before dental procedures in patients with a PJR.71,72 Antimicrobial prophylaxis for patients with a prosthetic joint undergoing a dental procedure or other invasive medical procedure has been controversial for decades.67,71,73-75 Consensus guidelines for this practice were initially published in 1997 and affirmed in 2003 by the American Dental Association (ADA) and the American Association of Orthopedic Surgeons (AAOS) on the basis of low-level evidence.69,76 It was proposed that AP be administered before dental procedures thought most likely to be associated with bacteremia for patients who were considered to be at highest risk of bacteremia-associated PJI. High-risk patients are thought to include all patients during the first 2 years after joint replacement, immunocompromised or immunosuppressed patients, patients with comorbid conditions (eg, diabetes, obesity, HIV infection, smoking), and patients with inflammatory arthropathies (eg, rheumatoid arthritis), systemic lupus erythematosus, medication- or radiation-induced immunosuppression, previous PJI, malnourishment, hemophilia, HIV infection, insulin-dependent (type 1) diabetes, megaprosthesis, or malignancy. More recently (February 2009), the Patient Safety Committee of the AAOS posted an Information Statement (IS) advising that “clinicians consider antibiotic prophylaxis for...all total joint replacement patients prior to any invasive procedure that may cause bacteremia.”77 The ADA no longer supports the 2003 AAOS/ADA Guidelines and refers patients and health care professionals to the AAOS IS (Karen London, American Dental Association, written communication, March 28, 2011).77 Although specific dental procedures that may cause bacteremia are not listed in the AAOS IS, the ADA lists the dental procedures that may cause bacteremia in the AAOS/ADA 2003 guidelines.76,77 The antibiotics recommended in the AAOS IS to be administered to patients with PJR before dental procedures include 2 g of oral cephalexin, cephradine, or amoxicillin 1 hour before dental procedures. The AAOS IS makes no mention of parenteral antibiotic options or antibiotic alternatives for penicillin-allergic patients. The 2003 AAOS/ADA advisory statement recommended 1 g of intravenous cefazolin or ampicillin as parenteral antibiotic alternatives or 600 mg of clindamycin (intravenous or oral) for penicillin-allergic patients, to be administered 1 hour before the dental procedure; in our opinion, these remain valid antibiotic alternatives.76
A panel that included representatives from the ADA, AAOS, and IDSA was recently convened with the goal of producing an evidence-based antimicrobial guideline for patients with PJR before dental procedures (D.R.O. is a member of the working group). It is hoped that this will lead to a simpler consensus guideline for patients and health care professionals. Good dental health before and after total joint replacement and prompt treatment of active oral infection should be encouraged for all patients with PJR.
Antimicrobial prophylaxis in patients undergoing invasive gastrointestinal procedures is not recommended by the American Society of Colon and Rectal Surgeons78 or the American Society for Gastrointestinal Endoscopy.79 If clinicians elect to recommend AP for the prevention of hematogenous PJI in these patients, they should discuss with them the possibility of life-threatening adverse reactions (rare) and the more common drug toxicities. If used, antimicrobial agents should be chosen on the basis of the expected flora at the site of the procedure.
The American Urological Association (AUA) and the AAOS first published consensus- and expert opinion–based AP guidelines in 2003 for patients with total joint replacement who were undergoing urologic procedures.80 Antimicrobial prophylaxis is recommended for patients at increased risk of hematogenous PJI who undergo urologic procedures associated with an increased risk of bacteremia. The details of these recommendations can be found in the 2007 AUA Best Practice Policy Statement on Urologic Surgery Antimicrobial Prophylaxis, which is available on the AUA Web site.80,81 The guidelines assume that the urine is sterile preoperatively. If bacteriuria is present, it should be treated with appropriate antibacterial agents before manipulation of the urinary tract.
Travelers' Diarrhea
Antibacterial agents have been shown to decrease the risk of travelers' diarrhea by up to 84%.82-84 Antimicrobial agents are not routinely recommended for the prevention of travelers' diarrhea because antibiotic self-treatment is so rapidly effective. The traveler may be instructed to carry a supply of an antibiotic (often a 1- to 3-day course of a fluoroquinolone for travel to Central or South America or Africa or of azithromycin when traveling to Asia or the Indian subcontinent) to be taken on an as-needed basis.12 In certain circumstances (risk-averse travelers, athletes, persons taking antacids, or persons with diabetes, an elevated gastric pH, or inflammatory bowel disease), a daily oral antibiotic regimen may be considered on a short-term basis (ideally <2-3 weeks) to prevent travelers' diarrhea. Fluoroquinolones may be less effective in areas with quinolone-resistant Campylobacter species infections (eg, India, Southeast Asia), so an agent such as azithromycin (250 mg once daily) may be considered, although this has not been studied. In a 14-day study among travelers to Mexico, rifaximin (200 mg 1-3 times daily) was 72% effective in preventing travelers' diarrhea.23 Bismuth subsalicylate prophylaxis (Pepto-Bismol [Proctor & Gamble; Cincinnati, OH]: two 262-mg chewable tablets 4 times daily, with meals and once in the evening) is less effective (62%-65% effective) than antibiotics, is inconvenient to take, contains a salicylate (to be avoided if receiving anticoagulant therapy or high-dose salicylates), causes a black tongue, and may interfere with the absorption of medications such as doxycycline.12 Probiotics containing Lactobacillus GG or Saccharomyces boulardii are of limited efficacy (0%-60% effective) in the prevention of travelers' diarrhea and generally are not recommended for this purpose.85,86
Open Fractures
Open fractures, particularly Gustilo grade 3 fractures, are at an increased risk of infection.87 The key to infection avoidance of open class III fractures is wound irrigation, surgical débridement of devitalized tissue, and delayed wound closure. A recent Surgical Infection Society Guideline recommended AP with a first-generation cephalosporin after open fracture until 24 to 48 hours after wound closure.88 Some groups recommend adding gram-negative coverage for class III open fractures.89
Herpes Simplex Viral Infection
Frequent recurrent genital herpes simplex viral infections (>5-6 episodes per year) are amenable to prophylaxis with continuous acyclovir (400 mg twice daily), famciclovir (250 mg twice daily), or valacyclovir (500-1000 mg once daily).90,91 Famciclovir may be less effective for suppression of viral shedding, and 500 mg of valacyclovir once daily might be less effective than other valacyclovir or acyclovir dosing regimens in patients who have very frequent recurrences (ie, ≥10 episodes per year).91 Patients should be counseled regarding consistent condom use and avoidance of sexual activity during recurrences in addition to suppressive antiviral therapy.
Influenza
Chemoprophylaxis of influenza A and B infection with a neuraminidase inhibitor (zanamivir [inhaled] or oseltamivir [oral]) is 70% to 90% effective92,93 (Table 1). These agents are particularly useful for prophylaxis after exposure in unvaccinated high-risk patients and unvaccinated health care professionals in an outbreak setting in a medical institution or community. Chemoprophylaxis is recommended for persons who are at high risk of influenza complications (Table 6) and those who are hospitalized or have severe, complicated, or progressive illness.94 Low-risk, healthy persons who are not in contact with high-risk patients do not typically require CP. Adults for whom antiviral CP should be considered during periods of increased influenza activity in the community are listed in Table 7. Zanamivir and oseltamivir are classified as category C (risk cannot be ruled out) for use during pregnancy. Influenza CP should be considered as an adjunct to influenza vaccination. Chemoprophylaxis should not be administered 48 hours before or 2 weeks after administration of the intranasal live-attenuated FluMist influenza vaccine (MedImmune, Gaithersburg, MD); CP has no effect on the inactivated influenza vaccine.21 Chemoprophylaxis may be stopped 10 days after exposure for household contacts and 7 days after other exposures.94 For control of outbreaks in long-term care facilities and hospitals, the Centers for Disease Control and Prevention recommends CP for a minimum of 2 weeks, even for vaccinated persons, up to 1 week after the last known case was identified.22,94 In patients who are unable to receive influenza vaccination and who are at high risk of complications, treatment should be continued for the duration of the influenza season in the community. Oseltamivir- and zanamivir-resistant influenza A strains have been reported; one should monitor the Centers for Disease Control and Prevention influenza Web site (http://www.cdc.gov/flu) for seasonal updates. The adamantanes (amantadine and rimantadine) are active only against influenza A; with the emergence of adamantane resistance in most seasonal A H3N2 and pandemic 2009-2010 A H1N1 strains, these agents are no longer recommended for CP.
TABLE 6.
Persons at High Risk of Influenza Complicationsa,b,c
TABLE 7.
Adults for Whom Antiviral Chemoprophylaxis Should Be Considered During Periods of Increased Influenza Activity in the Communitya,b,c
SURGICAL AP
Surgical site infections account for 14% to 18% of all health care infections and are the third most frequently reported nosocomial infection.96,97 Factors that may increase the risk of surgical site infection include those related to the patient (age, nutritional status, diabetes, smoking status, obesity, coexisting infections at a remote site, colonization with a pathogenic microorganism, altered immune response, and length of preoperative stay) and the operative procedure (duration of surgical scrub, skin antisepsis, preoperative shaving, preoperative skin preparation, duration of operation, AP, operating room ventilation, inadequate sterilization of instruments, foreign material at the surgical site, surgical drains, and surgical technique).98 The risk of surgical site infection also depends on whether the surgical procedure is clean, clean-contaminated, contaminated, or dirty-infected based on standard definitions of these terms.98 Improvements in operating room ventilation, sterilization methods, barriers, and surgical technique as well as the use of perioperative topical, oral, and intravenous AP have been important in decreasing the incidence of surgical site infection.98,99
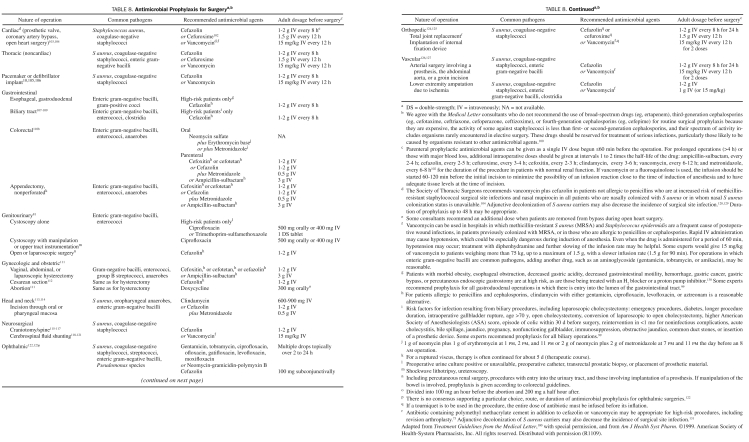
Perioperative antimicrobial surgical prophylaxis is recommended for operative procedures that have a high rate of postoperative wound infection, when foreign material is implanted, or when the wound infection rate is low but the development of a wound infection results in a disastrous event.98-100 Prophylactic antimicrobial agents should be bactericidal, nontoxic, and inexpensive and have in vitro activity against the common organisms that cause postoperative wound infection after a specific surgical procedure. Consensus panels most often recommend cefazolin and other cephalosporins because they meet the aforementioned criteria.98,100 Broad-spectrum antibiotics (eg, ertapenem) should be avoided for surgical prophylaxis.100,101 Perioperative antimicrobial surgical prophylaxis regimens for various surgical procedures adapted from the published recommendations of 2 consensus panels are summarized in Table 8.99,100,102 The use of vancomycin for prophylaxis is appropriate in the event of true type I hypersensitivity or other serious reaction to penicillin or when the incidence of surgical site infection is high due to methicillin-resistant staphylococci.132 Adherence to this practice will help to avoid the emergence of vancomycin-resistant organisms and vancomycin-related toxicity.133-136 Prophylactic antimicrobial agents should be administered not more than 30 to 60 minutes before surgery, including cesarian sections.100,112,137,138 Exceptions to this include oral administration of antimicrobial agents before colonic and urologic procedures (Table 8). Infusions should be completed before the tourniquet is placed with orthopedic surgeries. Vancomycin and fluoroquinolone infusions should be started 90 to 120 minutes before surgical incision because these require at least 1 hour to infuse. Therapeutic concentrations of antimicrobial agents should be present in the tissue throughout the period that the wound is open. Additional antibiotic doses may need to be administered intraoperatively for prolonged procedures or with antimicrobial agents with short half-lives.102,139 Initiating intravenous antimicrobial therapy before the perioperative period provides no benefit. Prolonged postoperative AP should be discouraged because of the possibility of added antimicrobial toxicity, selection of resistant organisms, and unnecessary expense. The duration of AP for most procedures should not exceed 24 hours, with the exception of cardiac surgeries, in which antibiotics may be continued for up to 48 hours.99,100,102,103,140 The duration of antibiotic therapy for ophthalmic procedures has not been established. An advisory statement for AP in dermatologic surgery has been published recently.141 The IDSA, American Society of Health-System Pharmacists (ASHP), Society for Healthcare Epidemiology of America, and Surgical Infection Society are currently in the process of revising the 1999 ASHP Antimicrobial Prophylaxis in Surgery Guideline.99
TABLE 8.
Antimicrobial Prophylaxis for Surgerya,b
In 2002, the Center for Medicaid and Medicare Services implemented a quality initiative project, currently entitled the Surgical Care Improvement Project (SCIP), in an attempt to decrease postoperative surgical site infections.140 As part of the SCIP, medical institutions are being graded on 3 surgical AP performance measures with cardiothoracic, vascular, colon, hip/knee, and vaginal or abdominal hysterectomy surgeries: (1) the proportion of patients who have parenteral AP initiated within 1 hour before surgical incision, (2) the proportion of patients who are provided an antibiotic agent that is consistent with currently published guidelines, and (3) the proportion of patients whose prophylactic antibiotic is discontinued within 24 hours after the end of the operation (48 hours for cardiothoracic surgery). The most up-to-date list of approved antibiotics for various surgeries is posted on the SCIP Web site.140
CONCLUSION
The use of AP has led to the prevention of a large number and variety of infections and to substantial declines in surgical site infections. Antimicrobial prophylaxis should be limited to specific, well-accepted indications to avoid excess cost, toxicity, and antimicrobial resistance. Patients should understand the potential risks and benefits of any AP regimen. Although some AP practices are evidence-based, many are based on low-level evidence or expert opinion. More studies in the area of AP are needed.
Supplementary Material
Footnotes
On completion of this article, readers should be able to: (1) identify common surgical and nonsurgical indications for the use of antimicrobial prophylaxis in adults, (2) formulate selected surgical and nonsurgical antimicrobial prophylaxis regimens for adults, and (3) summarize the arguments for and against the use of antimicrobial prophylaxis in adults.
REFERENCES
- 1. Osmon DR. Antimicrobial prophylaxis in adults. Mayo Clin Proc. 2000;75:98-109 [DOI] [PubMed] [Google Scholar]
- 2. Crabtree TD, Pelletier SJ, Gleason TG, Pruett TL, Sawyer RG. Clinical characteristics and antibiotic utilization in surgical patients with Clostridium difficile-associated diarrhea. Am Surg. 1999;65(6):507-511 [PubMed] [Google Scholar]
- 3. US Food and Drug Administration (FDA) Information for healthcare professionals: fluoroquinolone antimicrobial drugs [ciprofloxacin (marketed as Cipro and generic ciprofloxacin), ciprofloxacin extended-release (marketed as Cipro XR and Proquin XR), gemifloxacin (marketed as Factive), levofloxacin (marketed as Levaquin), moxifloxacin (marketed as Avelox), norfloxacin (marketed as Noroxin), and ofloxacin (marketed as Floxin)]: tendonitis risk black box warning, 7/8/2008; 2008. http://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm089652.htm Accessed May 20, 2011
- 4. Gerber MA, Baltimore R, Eaton C, et al. Prevention of rheumatic fever and diagnosis and treatment of acute streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Diseases in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research. Circulation. 2009;119:1541-1551 [DOI] [PubMed] [Google Scholar]
- 5. Baddour LM. Cellulitis and erysipelas. In: Baron EL, ed. UpToDate; 2008. [Google Scholar]
- 6. Pasternack MS, Swartz MN. Cellulitis, necrotizing fasciitis, and subcutaneous tissue infections. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practices of Infectious Diseases. Vol 1 7th ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2010:1289-1312 [Google Scholar]
- 7. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406 [DOI] [PubMed] [Google Scholar]
- 8. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant S aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e8-e55 [DOI] [PubMed] [Google Scholar]
- 9. Klempner MS, Styrt B. Prevention of recurrent staphylococcal skin infections with low-dose oral clindamycin therapy. JAMA. 1988;260:2682-2685 [PubMed] [Google Scholar]
- 10. Baddour LM, Epstein AE, Erickson CC, et al. ; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care and Outcomes Research Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121:458-477 [DOI] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention (CDC) Control and prevention of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 1997;46(RR-5):1-10 [PubMed] [Google Scholar]
- 12. Hill DR, Ericsson CD, Pearson RD, et al. The practice of travel medicine: guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43(12):1499-1539 [DOI] [PubMed] [Google Scholar]
- 13. Stapleton A, Stamm W. Prevention of urinary tract infection. Infect Dis Clin North Am. 1997;11:719-733 [DOI] [PubMed] [Google Scholar]
- 14. Gupta K, Hooton TM, Roberts PL, Stamm WE. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9-16 [DOI] [PubMed] [Google Scholar]
- 15. Melekos MD, Asbach HW, Gerharz E, Zarakovitis IE, Weingaertner K, Naber KG. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157:935-939 [PubMed] [Google Scholar]
- 16. Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49(6):2087-2107 [DOI] [PubMed] [Google Scholar]
- 17. Moran GJ, Talan DA, Abrahamian FM. Antimicrobial prophylaxis for wounds and procedures in the emergency department. Infect Dis Clin North Am. 2008;22:117-143 [DOI] [PubMed] [Google Scholar]
- 18. Endom EE. Initial management of animal and human bites (V.18.3). UpToDate. 2010. http://www.uptodate.com/contents/initial-management-of-animal-and-human-bites?source=search_result&selectedTitle=4%7E150 Accessed May 20, 2011
- 19. Goldstein EJC. Bites. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett's Principles and Practices of Infectious Diseases. Vol 2 Philadelphia, PA: Churchill Livingstone Elsevier; 2010:3911-3915 [Google Scholar]
- 20. Centers for Disease Control and Prevention (CDC) Recommended antimicrobial agents for treatment and postexposure prophylaxis of pertussis: 2005 CDC Guidelines. MMWR Morb Mortal Wkly Rep. 2005;54:1-15 [PubMed] [Google Scholar]
- 21. Antiviral drugs for influenza. Med Lett Drugs Ther. 2009;51(1325):89-92 [PubMed] [Google Scholar]
- 22. Harper SA, Bradley JS, Englund JA, et al. Seasonal influenza in adults and children: diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(8):1003-1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DuPont HL, Jiang ZD, Okhuysen PC, et al. A randomized, double-blind, placebo-controlled trial of rifaximin to prevent travelers' diarrhea [published correction appears in Ann Intern Med. 2005;143(3):239]. Ann Intern Med. 2005;142(10):805-812 [DOI] [PubMed] [Google Scholar]
- 24. Hayden FG, de Jong MD. Emerging influenza antiviral resistance threats [editorial]. J Infect Dis. 2011;203:6-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feinstein AR, Wood HF, Epstein JA, Taranta A, Simpson R, Tursky E. A controlled study of three methods of prophylaxis against streptococcal infection in a population of rheumatic children: results of the first three years of the study, including methods for evaluating the maintenance of oral prophylaxis. N Engl J Med. 1959;260:697-702 [DOI] [PubMed] [Google Scholar]
- 26. Jorup-Rönström C, Briotton S. Recurrent erysipelas: predisposing factors and costs of prophylaxis. Infection. 1987;15(2):105-106 [DOI] [PubMed] [Google Scholar]
- 27. Sjöblom AC, Eriksson B, Jorup-Rönström C, Karkkonen K, Lindqvist M. Antibiotic prophylaxis in recurrent erysipelas. Infection. 1993;21:390-393 [DOI] [PubMed] [Google Scholar]
- 28. Wang Jh, Liu Yc, Cheng Dl, et al. Role of benzathine penicillin G in prophylaxis for recurrent streptococcal cellulitis of the lower legs. Clin Infect Dis. 1997;25:685-689 [DOI] [PubMed] [Google Scholar]
- 29. Baddour LM. Recurrent cellulitis after saphenous venectomy for coronary artery bypass graft surgery. In: Baron EL, ed. UpToDate. October 28, 2010 ed; 2011. http://www.uptodate.com/contents/recurrent-cellulitis-after-saphenous-venectomy-for-coronary-artery-bypass-graft-surgery?source=search_result&selectedTitle=3%7E10 Accessed May 20, 2011
- 30. Centers for Disease Control and Prevention (CDC) Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2005;54 (RR-7):1-10 15647722 [Google Scholar]
- 31. Price VE, Blanchette VS, Ford-Jones EL. The prevention and management of infections in children with asplenia or hyposplenia. Infect Dis Clin North Am. 2007;21(3):697-710, viii-ix [DOI] [PubMed] [Google Scholar]
- 32. American Academy of Pediatrics Committee on Infectious Diseases Policy statement: recommendations for the prevention of pneumococcal infections, including the use of pneumococcal conjugate vaccine (Prevnar), pneumococcal polysaccharide vaccine, and antibiotic prophylaxis. Pediatrics. 2000;106(2, pt 1):362-366 [DOI] [PubMed] [Google Scholar]
- 33. Montalembert M, Lenoir G. Antibiotic prevention of pneumococcal infections in asplenic hosts: admission of insufficiency. Ann Hematol. 2004;83:18-21 [DOI] [PubMed] [Google Scholar]
- 34. Sobel JD, Kaye D. Urinary tract infections. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett's Principles and Practices of Infectious Diseases. Vol 1 7th ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2010:957-985 [Google Scholar]
- 35. Lee BB, Simpson JM, Craig JC, Bhuta T. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003265 [DOI] [PubMed] [Google Scholar]
- 36. Raz R, Chazan B, Dan M. Cranberry juice and urinary tract infection. Clin Infect Dis. 2004;38:1413-1419 [DOI] [PubMed] [Google Scholar]
- 37. Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;(1):CD001321 [DOI] [PubMed] [Google Scholar]
- 38. Albert X, Huertas I, Pereiró II, Sanfélix J, Gosalbes V, Perrotta C. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;(3):CD001209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329:753-756 [DOI] [PubMed] [Google Scholar]
- 40. Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila FI, Soares-Weiser K, Uribe M. Antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding. Cochrane Database Syst Rev. 2010;(9):CD002907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gines P, Rimola A, Planas R, et al. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: results of a double-blind, placebo-controlled trial. Hepatology. 1990;12:716-724 [DOI] [PubMed] [Google Scholar]
- 42. Novella M, Sola R, Soriano G, et al. Continuous versus inpatient prophylaxis of the first episode of spontaneous bacterial peritonitis with norfloxacin. Hepatology. 1997;25:532-536 [DOI] [PubMed] [Google Scholar]
- 43. Rolachon A, Cordier L, Bacq Y, et al. Ciprofloxacin and long-term prevention of spontaneous bacterial peritonitis: results of a prospective controlled trial. Hepatology. 1995;22:1171-1174 [DOI] [PubMed] [Google Scholar]
- 44. Singh N, Gayowski T, Yu V, Wagener M. Trimethoprim-sulfamethoxazole for the prevention of spontaneous bacterial peritonitis in cirrhosis: a randomized trial. Ann Intern Med. 1995;122:595-598 [DOI] [PubMed] [Google Scholar]
- 45. Cohen MJ, Sahar T, Benenson S, Elinav E, Brezis M, Soares-Weiser K. Antibiotics for spontaneous bacterial peritonitis in cirrhotic patients with ascites, without gastro-intestinal bleeding. Cochrane Database Syst Rev. 2009;(2):CD004791 [DOI] [PubMed] [Google Scholar]
- 46. Das A. A cost analysis of long term antibiotic prophylaxis for spontaneous bacterial peritonitis in cirrhosis. Am J Gastroenterol. 1998;93:1895-1900 [DOI] [PubMed] [Google Scholar]
- 47. Baron MJ, Madoff LC. Pancreatic infection. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett's Principles and Practices of Infectious Diseases. Vol 1 Philadelphia, PA: Churchill Livingstone Elsevier; 2010:1045-1053 [Google Scholar]
- 48. Villatoro E, Mulla M, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev. 2010;(5):CD002941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379-2400 [DOI] [PubMed] [Google Scholar]
- 50. Forsmark CE, Baillie J. AGA Institute technical review on acute pancreatitis. Gastroenterology. 2007;132(5):2022-2044 [DOI] [PubMed] [Google Scholar]
- 51. Kizer K. Animal bites. In: Gorbach S, Bartlett J, Blacklow N, eds. Infectious Diseases. 2nd ed. Philadelphia, PA: Saunders; 1998:1559-1563 [Google Scholar]
- 52. Talan DA, Citron DM, Abrahamian FM, Moran GJ, Goldstein EJC. Bacteriologic analysis of infected dog and cat bites. N Engl J Med. 1999;340:85-92 [DOI] [PubMed] [Google Scholar]
- 53. Cummings P. Antibiotics to prevent infection in patients with dog bite wounds: a meta-analysis of randomized trials. Ann Emerg Med. 1994;23:535-540 [DOI] [PubMed] [Google Scholar]
- 54. Fleisher GR. The management of bite wounds. N Engl J Med. 1999;340:138-140 [DOI] [PubMed] [Google Scholar]
- 55. Dellinger EP, Wertz MJ, Miller SD, Coyle MB. Hand infections: bacteriology and treatment: a prospective study. Arch Surg. 1988;123:745-750 [DOI] [PubMed] [Google Scholar]
- 56. Centers for Disease Control and Prevention (CDC) Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine; recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2006;55:1-34 16410759 [Google Scholar]
- 57. Centers for Disease Control and Prevention (CDC) Tetanus and pertussis vaccination coverage among adults aged ≥18 years — United States, 1999 and 2008. MMWR Morb Mortal Wkly Rep. 2010;59:1302-1306 [PubMed] [Google Scholar]
- 58. Centers for Disease Control and Prevention (CDC) Final 2009 reports of nationally notifiable diseases. MMWR Morb Mortal Wkly Rep. 2010;59:1025,1035 [Google Scholar]
- 59. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2011;60:13-15 [PubMed] [Google Scholar]
- 60. Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) Provisional recommendations for health care personnel on use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) and use of postexposure antimicrobial prophylaxis. http://www.cdc.gov/vaccines/recs/provisional/downloads/use-of-Tdap-in-hcp.pdf Accessed May 20, 2011
- 61. Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116:1736-1754 [DOI] [PubMed] [Google Scholar]
- 62. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785 [DOI] [PubMed] [Google Scholar]
- 63. Steckelberg JM, Osmon DR. Prosthetic joint infections. In: Waldvogel FA, Bisno AL, eds. Infections Associated With Indwelling Medical Devices. 3rd ed. Washington, DC: American Society for Microbiology; 2000:173-209 [Google Scholar]
- 64. Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645-1654 [DOI] [PubMed] [Google Scholar]
- 65. Ahlberg A, Carlsson A, Lindberg L. Hematogenous infection in total joint replacement. Clin Orthop Relat Res. 1978;137:69-75 [PubMed] [Google Scholar]
- 66. Bengtson S, Blomgren G, Knutson K, Wigren A, Lidgren L. Hematogenous infection after knee arthroplasty. Acta Orthop Scand. 1987;58:529-534 [DOI] [PubMed] [Google Scholar]
- 67. Deacon JM, Pagilaro AJ, Zelicof SB, Horowitz HW. Current concepts review: prophylactic use of antibiotics for procedures after total joint replacement. J Bone Joint Surg Am. 1996;78(11):1755-1770 [DOI] [PubMed] [Google Scholar]
- 68. Uçkay I, Pittet D, Bernard L, Lew D, Perrier A, Peter R. Antibiotic prophylaxis before invasive dental procedures in patients with arthroplasties of the hip and knee. J Bone Joint Surg Br. 2008;90:833-838 [DOI] [PubMed] [Google Scholar]
- 69. Lockhart PB, Loven B, Brennan MT, Fox PC. The evidence base for the efficacy of antibiotic prophylaxis in dental practice. J Am Dent Assoc. 2007;138:458-474 [DOI] [PubMed] [Google Scholar]
- 70. Berbari EF, Osmon DR, Carr A, et al. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case-control study. Clin Infect Dis. 2010;50:8-16 [DOI] [PubMed] [Google Scholar]
- 71. Wahl M. Myths of dental-induced prosthetic joint infections. Clin Infect Dis. 1995;20:1420-1425 [DOI] [PubMed] [Google Scholar]
- 72. Lockhart PB, Brennan MT, Fox PC, Norton HJ, Jernigan DB, Strausbaugh LJ. Decision-making on the use of antimicrobial prophylaxis for dental procedures: a survey of infectious disease consultants and review. Clin Infect Dis. 2002;34:1621-1626 [DOI] [PubMed] [Google Scholar]
- 73. Hanssen AD, Osmon DR. The use of prophylactic antimicrobial agents during and after hip arthroplasty. Clin Orthop Relat Res. 1999. December;(369):124-138 [DOI] [PubMed] [Google Scholar]
- 74. Little JW. Patients with prosthetic joints: are they at risk when receiving invasive dental procedures? Spec Care Dentist. 1997;17(5):153-160 [DOI] [PubMed] [Google Scholar]
- 75. Sandhu SS, Lowry JC, Reuben SF, Morton ME. Who decides on the need for antibiotic prophylaxis in patients with major arthroplasties requiring dental treatment: is it a joint responsibility? Ann R Coll Surg Engl. 1997;79:143-147 [PMC free article] [PubMed] [Google Scholar]
- 76. American Dental Association; American Association of Orthopedic Surgeons Antibiotic prophylaxis for dental patients with total joint replacements. J Am Dent Assoc. 2003;134(7):895-898 [DOI] [PubMed] [Google Scholar]
- 77. American Association of Orthopedic Surgeons (AAOS) Information statement: antibiotic prophylaxis for bacteremia in patients with joint replacements. http://www.aaos.org/about/papers/advistmt/1033.asp Accessed May 20, 2011 [PubMed]
- 78. Oliver G, Lowry A, Vernava A, et al. ; Standards Task Force; American Society of Colon and Rectal Surgeons Practice parameters for antibiotic prophylaxis—supporting documentation. Dis Colon Rectum. 2000;43(9):1194-1200 [DOI] [PubMed] [Google Scholar]
- 79. ASGE Standards of Practice Committee Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67(6):791-798 [DOI] [PubMed] [Google Scholar]
- 80. American Urological Association; American Academy of Orthopaedic Surgeons Antibiotic prophylaxis for urological patients with total joint replacements. J Urol. 2003;169:1796-1797 [DOI] [PubMed] [Google Scholar]
- 81. Wolf JS, Jr, Bennett C, Dmochowski R, Hollenbeck B, Pearle M, Schaeffer A. Best Practice Policy Statement on Urologic Surgery Antimicrobial Prophylaxis. Linthicum, MD: American Urological Association; 2007:1-44 http://www.auanet.org/content/media/antimicroprop08.pdf Accessed May 20, 2011 [DOI] [PubMed] [Google Scholar]
- 82. Aranda-Michel J, Giannella R. Acute diarrhea: a practical review. Am J Med. 1999;106:670-676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Caeiro JP, DuPont HL. Management of travelers' diarrhoea. Drugs. 1998;56(1):73-81 [DOI] [PubMed] [Google Scholar]
- 84. Passaro DJ, Parsonnet J. Advances in the prevention and management of traveler's diarrhea. Curr Clin Top Infect Dis. 1998;18:217-236 [PubMed] [Google Scholar]
- 85. Ansdell VE, Ericsson CD. Prevention and empiric treatment of traveler's diarrhea. Med Clin North Am. 1999;83:945-973 [PubMed] [Google Scholar]
- 86. Rendi-Wagner P, Kollaritsch H. Drug prophylaxis for travelers' diarrhea. Clin Inf Dis. 2002;34(5):628-633 [DOI] [PubMed] [Google Scholar]
- 87. Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58:453-458 [PubMed] [Google Scholar]
- 88. Hauser CJ, Adams CA, Jr, Eachempath SR. Prophylactic antibiotic use in open fractures: an evidence-based guideline. Surg Infect (Larchmt). 2006;7(4):379-405 [DOI] [PubMed] [Google Scholar]
- 89. Luchette FA, Bone LB, Born CT, et al. ; Eastern Association for the Surgery of Trauma (EAST) Working Group Practice management guidelines for prophylactic antibiotic use in open fractures. Published 1998 Updated in 2009. http://www.east.org/tpg/OpenFxUpdate.pdf Accessed May 20, 2011
- 90. ACOG Committee on Practice Guidelines—Gynecology Gynecologic Herpes simplex virus infections. Obstet Gynecol. 2004;104(5, pt 1):1111-1117 [PubMed] [Google Scholar]
- 91. Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC) Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110 [PubMed] [Google Scholar]
- 92. Hayden FG, Atmar RL, Schilling M, et al. Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N Engl J Med. 1999;341(18):1336-1343 [DOI] [PubMed] [Google Scholar]
- 93. Monto AS, Robinson DP, Herlocher ML, Hinson JM, Jr, Elliott MJ, Crisp A. Zanamivir in the prevention of influenza among healthy adults: a randomized controlled trial. JAMA. 1999;282(1):31-35 [DOI] [PubMed] [Google Scholar]
- 94. Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM; Centers for Disease Control and Prevention (CDC) Antiviral agents for the treatment and chemoprophylaxis of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(1):1-24 [PubMed] [Google Scholar]
- 95. Fiore AE, Shay DK, Broder K, et al. ; Centers for Disease Control and Prevention (CDC) Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57(RR-7):1-60 [PubMed] [Google Scholar]
- 96. Emori TG, Gaynes RP. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev. 1993;6(4):428-442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol. 2008;29(11):996-1011 [DOI] [PubMed] [Google Scholar]
- 98. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR; Hospital Infection Control Practices Advisory Committee Guidelines for the prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol. 1999;20(4):250-278 [DOI] [PubMed] [Google Scholar]
- 99. American Society of Health-System Pharmacists ASHP therapeutic guidelines on antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 1999;56(18):1839-1888 [DOI] [PubMed] [Google Scholar]
- 100. Antimicrobial Prophylaxis for Surgery. Treatment Guidelines from the Medical Letter. 2009. June; 7(82):47-52 [PubMed] [Google Scholar]
- 101. Sexton DJ. Carbapenems for surgical prophylaxis [editorial]? N Engl J Med. 2006;355(25):2693-2695 [DOI] [PubMed] [Google Scholar]
- 102. Bratzler DW, Houck PM. Antimicrobial Prophylaxis for Surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis. 2004;38:1706-1715 [DOI] [PubMed] [Google Scholar]
- 103. Edwards FH, Engelman RM, Houck P, Shahian DM, Bridges CR. The Society of Thoracic Surgeons Practice Guideline Series: antibiotic prophylaxis in cardiac surgery; Part I: duration. Ann Thorac Surg. 2006;81:397-404 [DOI] [PubMed] [Google Scholar]
- 104. Engelman R, Shahian D, Shemin R, et al. The Society of Thoracic Surgeons Practice Guideline Series: antibiotic prophylaxis in cardiac surgery; Part II: antibiotic choice. Ann Thorac Surg. 2007;83:1569-1576 [DOI] [PubMed] [Google Scholar]
- 105. de Oliveira JC, Martinelli M, Nishioka SA, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large prospective, randomized, double-blind, placebo-controlled trial. Circ Arrhythm Electrophysiol. 2009;2(1):29-34 [DOI] [PubMed] [Google Scholar]
- 106. Sohail MR, Uslan DZ, Khan AH, et al. Risk factor analysis of permanent pacemaker infection. Clin Infect Dis. 2007;45(2):166-173 [DOI] [PubMed] [Google Scholar]
- 107. Dobay KJ, Freier D, Albear P. The absent role of prophylactic antibiotics in low-risk patients undergoing laparoscopic cholecystectomy. Am Surg. 1999;65(3):226-228 [PubMed] [Google Scholar]
- 108. Higgins A, London J, Charland S, et al. Prophylactic antibiotics for elective laparoscopic cholecystectomy: are they necessary? Arch Surg. 1999;134(6):611-613 [DOI] [PubMed] [Google Scholar]
- 109. Meijer WS, Schmitz PI, Jeekel J. Meta-analysis of randomized, controlled clinical trials of antibiotic prophylaxis in biliary tract surgery. Br J Surg. 1990;77(3):282-290 [DOI] [PubMed] [Google Scholar]
- 110. Baum ML, Anish DS, Chalmers TC, Sacks HS, Smith H, Jr, Fagerstrom RM. A survey of clinical trials of antibiotic prophylaxis in colon surgery: evidence against further use of no-treatment controls. N Engl J Med. 1981;305(14):795-799 [DOI] [PubMed] [Google Scholar]
- 111. American College of Obstetricians and Gynecologists (ACOG) Committee on Practice Bulletins—Gynecology ACOG Practice Bulletin No. 104: antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2009;113(5):1180-1189 [DOI] [PubMed] [Google Scholar]
- 112. American College of Obstetricians and Gynecologists (ACOG) Committee Antimicrobial prophylaxis for cesarean delivery: timing of administration: Obstet Gynecol. 2010;116(3):791-792 [DOI] [PubMed] [Google Scholar]
- 113. Simo R, French G. The use of prophylactic antibiotics in head and neck oncological surgery. Curr Opin Otolaryngol Head Neck Surg. 2006;14:55-61 [DOI] [PubMed] [Google Scholar]
- 114. Weber RS. Wound infection in head and neck surgery: implications for perioperative antibiotic treatment. Ear Nose Throat J. 1997;76(11):790-791, 795-798 [PubMed] [Google Scholar]
- 115. Barker FG., II Efficacy of prophylactic antibiotics for craniotomy: a meta-analysis. Neurosurgery. 1994;35(3):484-490 [DOI] [PubMed] [Google Scholar]
- 116. Barker FG., II Efficacy of prophylactic antibiotics in spinal surgery: a meta-analysis. Neurosurgery. 2002;51(2):391-400 [PubMed] [Google Scholar]
- 117. Barker FG., II Efficacy of prophylactic antibiotics against meningitis after craniotomy: a meta-analysis. Neurosurgery. 2007;60(5):887-894 [DOI] [PubMed] [Google Scholar]
- 118. Infection in Neurosurgery Working Party of the British Society for Antimicrobial Chemotherapy Antimicrobial prophylaxis in neurosurgery and after head injury. Lancet. 1994;344(8936):1547-1551 [PubMed] [Google Scholar]
- 119. Haines SJ, Walters BC. Antibiotic prophylaxis for cerebrospinal fluid shunts: a metanalysis. Neurosurgery. 1994;34(1):87-92 [PubMed] [Google Scholar]
- 120. Langley JM, LeBlanc JC, Drake J, Millner R. Efficacy of antimicrobial prophylaxis in placement of cerebrospinal fluid shunts: meta-analysis. Clin Infect Dis. 1993;17(1):98-103 [DOI] [PubMed] [Google Scholar]
- 121. Ratilal B, Costa J, Sampaio C. Antibiotic prophylaxis for surgical introduction of intracranial ventricular shunts: a systematic review. J Neurosurg Pediatr. 2008;1:48-56 [DOI] [PubMed] [Google Scholar]
- 122. DeCroos FC, Afshari NA. Perioperative antibiotics and anti-inflammatory agents in cataract surgery. Curr Opin Ophthalmol. 2008;19:22-26 [DOI] [PubMed] [Google Scholar]
- 123. Liesegang TJ. Perioperative antibiotic prophylaxis in cataract surgery [published correction appears in Cornea. 2000;19(1):123]. Cornea. 1999;18(4):383-402 [DOI] [PubMed] [Google Scholar]
- 124. Gillespie WJ, Walenkamp G. Antibiotic prophylaxis for surgery for proximal femoral and other closed long bone fractures. Cochrane Database Syst Rev. 2001;(1): CD000244 [DOI] [PubMed] [Google Scholar]
- 125. Prokuski L. Prophylactic antibiotics in orthopaedic surgery. J Am Acad Orthop Surg. 2008;16:283-293 [DOI] [PubMed] [Google Scholar]
- 126. Homer-Vanniasinkam S. Surgical site and vascular infections: treatment and prophylaxis. Int J Infect Dis. 2007;11(suppl 1):S17-S22 [DOI] [PubMed] [Google Scholar]
- 127. Stewart AH, Eyers PS, Earnshaw JJ. Prevention of infection in peripheral arterial reconstruction: a systematic review and meta-analysis. J Vasc Surg. 2007;46:148-155 [DOI] [PubMed] [Google Scholar]
- 128. Kluytmans JA, Mouton JW, VandenBergh MF, et al. Reduction of surgical-site infections in cardiothoracic surgery by elimination of nasal carriage of Staphylococcus aureus. Infect Control Hosp Epidemiol. 1996;17(12):780-785 [DOI] [PubMed] [Google Scholar]
- 129. Perl TM, Cullen JJ, Wenzel RP, et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med. 2002;346:1871-1877 [DOI] [PubMed] [Google Scholar]
- 130. Rey JR, Axon A, Budzynsak A, Kruse A, Nowak A. Guidelines of the European Society of Gastrointestinal Endoscopy (ESGE) antibiotic prophylaxis for gastrointestinal endoscopy. Endoscopy. 1998;30(3):318-324 [PubMed] [Google Scholar]
- 131. Gernaat-van der Sluis AJ, Hoogenboom-Verdegaal AMM, Edixhoven PJ, Spies-van Rooijen NH. Prophylactic mupirocin could reduce orthopedic wound infections: 1,044 patients treated with mupirocin compared with 1,260 historical controls. Acta Orthop Scand. 1998;69:412-414 [DOI] [PubMed] [Google Scholar]
- 132. Centers for Disease Control and Prevention (CDC) Recommendations for preventing the spread of vancomycin resistance: recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR Recom Rep. 1995;44(RR-12):1-13 [PubMed] [Google Scholar]
- 133. Centers for Disease Control and Prevention (CDC) Interim guidelines for prevention and control of staphylococcal infection associated with reduced susceptibility to vancomycin. MMWR Morb Mortal Wkly Rep. 1997;46(27):626-628; 635 [PubMed] [Google Scholar]
- 134. Centers for Disease Control and Prevention (CDC) Reduced susceptibility of Staphylococcus aureus to vancomycin—Japan, 1996. MMWR Morb Mortal Wkly Rep. 1997;46(27):624-626 [PubMed] [Google Scholar]
- 135. Centers for Disease Control and Prevention (CDC) Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1997 [published correction appears in MMWR Morb Mortal Wkly Rep. 1997;46:851]. MMWR Morb Mortal Wkly Rep. 1997;46(33):765-766 [PubMed] [Google Scholar]
- 136. Southorn PA, Plevak DJ, Wright AJ, Wilson WR. Adverse effects of vancomycin administered in the perioperative period. Mayo Clin Proc. 1986;61:721-724 [DOI] [PubMed] [Google Scholar]
- 137. Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961;50:161-168 [PubMed] [Google Scholar]
- 138. Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical wound infection. N Engl J Med. 1992;326(5):281-286 [DOI] [PubMed] [Google Scholar]
- 139. Zanetti G, Giardina R, Platt R. Intraoperative redosing of cefazolin and risk for surgical site infection in cardiac surgery. Emerg Infect Dis. 2001;7:828-831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Surgical Care Improvement Project (SCIP) http://www.aha.org/aha/issues/Quality-and-Patient-Safety/scip.html. [Accessed May 20, 2011]. http://www.aha.org/aha/issues/Quality-and-Patient-Safety/scip.html QualityNet Web site.
- 141. Wright TI, Baddour LM, Berbari EF, et al. Antibiotic prophylaxis in dermatologic surgery: advisory statement 2008. J Am Acad Dermatol. 2008;59:464-473 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.