Abstract
Naturally occurring CD4+CD25+Foxp3+ T regulatory cells (nTregs) regulate lung allergic responses through production of IL-10 and TGF-β. nTregs from CD8−/− mice failed to suppress lung allergic responses and were characterized by reduced levels of Foxp3, IL-10, and TGF-β, and high levels of IL-6. Administration of anti–IL-6 or anti–IL-6R to wild-type recipients prior to transfer of CD8−/− nTregs restored suppression. nTregs from IL-6−/− mice were suppressive, but lost this capability if incubated with IL-6 prior to transfer. The importance of CD8 in regulating the production of IL-6 in nTregs was demonstrated by the loss of suppression and increases in IL-6 following transfer of nTregs from wild-type donors depleted of CD8+ cells. Transfer of nTregs from CD8−/− donors reconstituted with CD8+ T cells was suppressive, and accordingly, IL-6 levels were reduced. These data identify the critical role of CD8–T regulatory cell interactions in regulating the suppressive phenotype of nTregs through control of IL-6 production.
Naturally occurring CD4+CD25+Foxp3+ T regulatory cells (nTregs) are essential for maintaining self-tolerance and immune homeostasis (1). Continuous and high levels of Foxp3 appear necessary for sustaining the T regulatory cell (Treg) phenotype and function (2, 3). In mice (4) or humans (5, 6) expressing a nonfunctional allele of Foxp3, a fatal, early-onset autoimmune syndrome develops. In the lung, immune homeostasis is achieved by balancing the levels of proinflammatory and protective cytokines. IL-10 is one such anti-inflammatory cytokine produced by a variety of cell types, including CD4+CD25+Foxp3+ Tregs (7). Depletion of these Tregs enhanced the severity of both lung inflammation and the development of airway hyperresponsiveness (AHR) (8). We and others have shown that adoptive transfer of Ag-specific or naturally occurring Tregs can suppress the full spectrum of lung allergic responses, including AHR, airway inflammation, and local Th2 cytokine production (9–12). nTregs can suppress lung allergic responses through the endogenous production of IL-10 and TGF-β (10, 13, 14) and in an Ag-independent manner (15).
Although the importance of nTregs in the control of autoimmunity and allergic lung inflammation is well established, it is unclear how stable the suppressive phenotype of nTregs is in vivo. A number of studies have suggested that Tregs are indeed unstable and that the suppressive phenotype can in fact be subverted by a variety of experimental conditions, including manipulation of Foxp3 expression in vitro (3), ligation of GITR in vitro with GITR ligand (12), or neutralizing GITR ligand in vivo (12). Using a Foxp3 reporter lineage-marker system, the loss of Foxp3 in a subset of Foxp3-expressing cells could be demonstrated, with acquisition of a pathogenic effector cell phenotype (16). In a similar manner, nTregs could be subverted to an enhancing (pathogenic) phenotype when transferred into CD8−/− recipients (12). These latter findings not only confirmed the instability of nTregs in vivo under certain experimental conditions, but identified a critical role for CD8 in maintaining the suppressive phenotype of nTregs.
Among the factors that may contribute to the instability of Tregs is IL-6. IL-6 inhibits Treg function (17) and Treg expansion (18), and IL-6 production by spleen dendritic cells has been shown to enhance effector T cell responses by neutralizing CD4+CD25+ Treg suppression (17). In conjunction with IL-1, IL-6 downregulates Foxp3 in a STAT 3-dependent manner (19). Signaling through IL-6 may result in remethylation of a critical Foxp3 CpG motif and suppress Foxp3 expression (20). Previous studies demonstrated that in patients with allergic asthma, soluble IL-6R levels were increased (21). Furthermore, blockade of the membrane-bound IL-6R resulted in the expansion of CD4+CD25+Foxp3+ Tregs and increased immunosuppression in a mouse model of asthma (21). Together, these results identify the potential for IL-6 to serve as a major regulator of the balance between effector T cells and Tregs in the lungs of sensitized and challenged mice.
Given the increasing evidence for the instability of Tregs and their conversion to a pathogenic phenotype, it is important to identify those factors that may limit or terminate Foxp3 expression and attenuate suppression. Based on our earlier findings of the functional differences of nTreg activity related to CD8 expression (11, 12), we have examined the activities of nTregs isolated from CD8+/+ and CD8−/− mice in their regulation of lung allergic responses. nTregs from CD8−/− mice not only exhibited marked differences in surface receptor expression and levels of Foxp3, IL-10, and TGF-β, but they were strong producers of IL-6. Manipulation of CD8 or IL-6 levels or blockade of the IL-6R had profound effects on the outcome of nTreg-mediated suppression of lung allergic responses.
Materials and Methods
Animals
Pathogen-free, 6- to 8-wk-old female CD8α−/− and IL-6−/− mice and wild-type (WT) C57BL/6 littermates were obtained from The Jackson Laboratory (Bar Harbor, ME) and bred at National Jewish Health. All mice were maintained on an OVA-free diet. All protocols were approved by the Institutional Animal Care and Use Committee of National Jewish Health.
Sensitization and challenge
Sensitization was carried out by i.p. injection of 20 µg OVA (Sigma Aldrich, St. Louis, MO) emulsified in 2.25 mg alum hydroxide (AlumImject; Pierce, Rockford, IL) in a total volume of 100 µl on days 1 and 14. Sensitized and challenged mice, denoted OVA/OVA, and nonsensitized, but challenged littermates (PBS/OVA) received aerosol challenges for 20 min each day on 3 consecutive days (days 26, 27, and 28) with 1% OVA in PBS using an ultrasonic nebulizer (Omron, Vernon Hills, IL) (10).
Measurement of airway responsiveness
Airway responsiveness was assessed 48 h following the last challenge as a change in airway function to increasing concentrations of aerosolized methacholine administered for 10 s (60 breaths/min, 500 µl tidal volume) (22). Lung resistance (RL) was continuously computed (Labview; National Instruments, Austin, TX) by fitting flow, volume, and pressure to an equation of motion. Maximum values of RL were taken and expressed as a percentage change from baseline following PBS aerosol.
Bronchoalveolar lavage
Immediately following measurement of AHR, lungs were lavaged. Total leukocyte numbers were counted (Coulter counter; Coulter, Hialeah, FL), and differential cell counts were performed in a blinded manner under light microscopy by counting at least 200 cells on cytocentrifuged preparations (Cytospin 2; Shandon, Runcorn, Cheshire, United Kingdom), stained with Leukostat (Fisher Diagnostics, Middletown, VA), and differentiated by standard hematological procedures.
Isolation of nTregs
CD4+CD25+ T cells from naive C57BL/6, CD8−/−, and IL-6−/− donors were isolated by collagenase digestion from lungs and enriched using nylon wool columns, as described previously (10). Lymphocytes were further purified by CD4+CD25+ Treg MACS beads (Miltenyi Biotec, Bergisch-Gladbach, Germany), resulting in a purity of >95% CD4+CD25+, and following cell sorting, provided a purified population of >99% CD4+ CD25+ T cells. The majority of these cells (>95%) expressed Foxp3.
Adoptive transfer of nTregs
Recipient mice received 5 × 105 isolated lung CD4+CD25+ T cells intratracheally in 50 µl PBS prior to the first allergen challenge. Where indicated, prior to transfer in some experiments, CD4+CD25+ T cells from IL-6−/− mice were treated with rIL-6 (1 µg/ml; eBiosciences, San Diego, CA) in vitro for 1 h and washed extensively with PBS before transfer.
Antibodies
Control Abs, anti–IL-6, or anti–IL-6Rα (50 µg; eBiosciences) were administered by microspray (Penn-Century, Philadelphia, PA) intratracheally prior to intratracheal transfer of CD4+CD25+ T cells.
In vitro culture of nTregs
Isolated lung nTregs (1 × 106/well) were cultured in complete medium in the presence or absence of PMA (100 ng/ml) and ionomycin (2 µg/ml; Sigma-Aldrich). Supernates were harvested after 72 h and analyzed for cytokine levels, as described below.
Measurement of cytokine levels
Cytokine levels in the bronchoalveolar lavage (BAL) fluid and supernatants of in vitro cultured lung cells were measured by ELISA (IL-4, IL-5, IL-10, IFN-γ, TGF-β from BD Biosciences, Minneapolis, MN), and IL-6, IL-13, and IL-17 from eBioscience. ELISAs were performed according to the manufacturers’ directions. The limits of detection were 4 pg/ml for IL-4 and IL-5, 10 pg/ml for IL-10 and IFN-γ, 8 pg/ml for IL-6 and IL-13, 6 pg/ml for TGF-β, and 4 pg/ml for IL-17.
Depletion of CD8 in WT mice and reconstitution of CD8 in CD8−/− mice
To deplete CD8+ cells, anti-CD8β (200 µg; eBiosciences) was given i.p. once per week, beginning at 1 wk of age and continuing to the time of nTreg harvesting (6–8 wk of age). Reconstitution of CD8−/− mice with naive spleen CD8+ T cells (or CD8− T cells) (23) was achieved by administering 2 × 106 cells each week i.p. up until the time of nTreg harvesting (6–8 wk of age).
FACS analysis
Enriched lung and BAL cells, following preincubation with naive mouse serum in staining buffer (PBS, 2% FCS, 0.2% sodium azide), were labeled with the following conjugated Abs purchased from BD Biosciences: anti-CD3 FITC, PE, PerCP, allophycocyanin (17A2); anti-CD4 FITC, PE, PerCP, allophycocyanin (L3T4); anti-CD25 FITC (7D4), PE (PC61); anti-CD8α FITC, PE, PerCP (53-6.7); PE, anti–IL-6Rα (D7715A7), CD152 (UC10-4B9), GITR (DTA-1); and CD39 (24DMS 1), anti-CD62L PE, allophycocyanin, FITC (MEL-14). For intracellular staining, cells were stimulated with PMA (100 ng/ml) and ionomycin (2 µg/ml; Sigma-Aldrich) in complete medium overnight and for 6 h in the presence of brefeldin A (10 µg/ml; Sigma-Aldrich). Cells were fixed with 4% formaldehyde in PBS, permeabilized in 0.5% saponin, and stained with anti–IL-10 PE, allophycocyanin (JES5-16E3), anti-IL-6 PE, FITC, PerCP (MP5-20F3); Foxp3 PE, allophycocyanin (FJK-16s); and TGF-β (A75-3.1) (eBioscience). Fluorochrome (FITC, PE, PerCP allophycocyanin)-labeled isotype-matched control Abs were used for background fluorescence staining. Staining was analyzed by FACSCalibur flow cytometry (BD Pharmingen) using CellQuest Pro software. Fluorescence intensity was compared with cells stained with corresponding labeled isotype-matched controls.
Immunofluorescence staining
Intracellular expression of IL-6 and Foxp3 in the same cell was analyzed by immunofluorescent staining. Sorted cells were stimulated with PMA (100 ng/ml) and ionomycin (2 µg/ml) in complete medium overnight and for 6 h in the presence of brefeldin A (10 µg/ml; Sigma-Aldrich) prior to seeding on poly(d-lysine)–coated coverslips. Cells were fixed and permeabilized for 1 h, as described previously (15), washed several times, and blocked with a Superblock solution supplemented with 2% naive rat serum for 1 h. Cells were then incubated with either control Ab, anti–IL-6 PE, or Foxp3 allophycocyanin (all from eBioscience) overnight, and then washed extensively for 1 h. The coverslips were affixed with mounting medium containing DAPI (Vector Laboratories, Burlingame, CA). Slides were analyzed using a Leica (Deerfield, IL) microscope equipped with a camera (SensiCam QE) and Slidebook analysis software (4.0.1.43). Exposure times were chosen in a manner that limited background (control Ab) staining.
Real-time PCR
RNA was isolated using RNeasy mini kit (Qiagen, Valencia, CA) and was reversed transcribed using Bio-Rad IScript cDNA synthesis kit (Hercules, CA). Real-time cDNA primers and probes for murine IL-6, Foxp3, and GAPDH were obtained from Applied Biosystems (Carlsbad, CA). Realtime PCR was performed using ABI Prism 7000 Sequence Detection System. Analysis of relative gene expression was carried out, as described (24).
In vitro suppression of cell proliferation
Isolated WT CD4+CD25− cells (2.5 × 104) were activated with bound anti-CD3 (2 µg/ml, 17A2) and soluble anti-CD28 (1 µg/ml, 37.51; both from eBioscience) and cultured with or without CD4+CD25+ T cells isolated from CD8+/+ and CD8−/− mice at a 1:1 ratio in complete medium. Cultures (72 h) were pulsed during the final 6 h with 1 µCi/well tritiated thymidine; DNA was harvested; and counts were determined.
Statistical analysis
ANOVA was used to determine statistical significance. Comparisons for all pairs were performed by Tukey-Kramer highest significant difference test. The p values for significance were set to 0.05. Values for all measurements were expressed as the mean ± SEM.
Results
CD8−/−CD4+CD25+ T cells fail to suppress OVA-induced AHR and airway inflammation
The aim of these experiments was to compare the regulatory capacity of lung nTregs isolated from naive CD8+/+ and CD8−/− donors on airway responsiveness, airway inflammation, and cytokine levels induced following sensitization and challenge with OVA. As previously reported (25), following sensitization and airway challenge with allergen, C57BL/6 mice developed significantly increased AHR (p < 0.05), measured as a change in RL in response to increasing doses of inhaled methacholine. In contrast to the transfer of WT CD4+CD25+ T cells, intratracheal transfer of CD4+CD25+ T cells from CD8−/− mice failed to suppress AHR (Fig. 1A).
FIGURE 1.
Functional activity of nTregs from naive CD8−/− and CD8+/+ donors following transfer into sensitized and challenged WT (C57BL/6) recipients. Sensitized C57BL/6 mice received nTregs intratracheally prior to challenge. A, AHR; B, BAL fluid inflammatory cell composition; C, BAL cytokine levels; D, phenotypic characterization of isolated CD4+CD25+ cells from CD8−/− and CD8+/+ mice; E, cytokine levels recovered in supernates of cultured nTregs from CD8−/− and CD8+/+ donors; F, intracytoplasmic staining of a typical nTreg froma CD8−/− and CD8+/+ mouse: blue IL-6, red Foxp3, green CD25, white DAPI staining of the nucleus (original magnification × 100); and G, in vitro proliferation of WTCD4+CD25− T cells. Shown are the means ± SEM from three independent experiments (4 mice/group, n = 12 mice analyzed for each group). *p < 0.05 comparing nTregs from CD8−/− and CD8+/+ donors.
Associated with the increases in AHR, the numbers of airway eosinophils were significantly increased in sensitized and challenged WT mice (p < 0.05), peaking 48 h following the last challenge, as shown in Fig. 1B. Airway eosinophilia was significantly reduced in the recipients of WT CD4+CD25+, but not in the recipients of CD8−/−CD4+CD25+ T cells.
Transfer of CD8−/−CD4+CD25+ T cells results in high IL-6 levels
Following sensitization and challenge with OVA in WT mice, a skewing of Th2 cytokine levels in BAL fluid was detected. Significant increases in levels of IL-4, IL-5, and IL-13 were detected (p < 0.05), and levels of IL-10 and IFN-γ were decreased (p < 0.05) in sensitized and challenged mice; levels of TGF-β were modestly increased (Fig. 1C). Intratracheal transfer of CD8+/+ nTregs decreased the levels of IL-5 and IL-13 (p < 0.05), whereas IL-10 and TGF-β levels were increased (p < 0.05). Little change was seen in levels of IL-4, IL-17, and IFN-γ. Transfer of CD8−/− nTregs failed to significantly reduce the levels of IL-5 and IL-13, and the levels of IL-4, IL-10, TGF-β, IL-17, and IFN-γ were similar to the levels in sensitized and challenged mice that did not receive any cells (Fig. 1C). Most strikingly, significant increases in the levels of IL-6 were detected in the BAL fluid of recipients of CD8−/−, but not in the recipients of WT nTregs.
Phenotype of CD8−/− nTregs
Previously, we showed that the suppressive activities of nTregs were critically dependent on the engagement/interaction between MHC I on nTregs and CD8 in the lungs of C57BL/6 mice (11), as well as in BALB/c mice (15). Compared with WT (CD8+/+) nTregs, CD8−/− nTregs expressed higher GITR and lower CD152 (CTLA-4) mean fluorescence intensity levels, but similar mean fluorescence intensities for CD25, CD39, CD62L, and the IL-6R (Fig. 1D). We also detected significantly lower intracellular expression levels of IL-10 and TGF-β as well as Foxp3 in CD8−/− nTregs (Fig. 1D).
In vitro culture of nTregs
Following in vitro culture of isolated nTregs in the presence or absence of PMA/ionomycin, CD8−/− nTregs released significantly higher amounts of IL-6 and lower amounts of IL-10 and TGF-β compared with similarly cultured CD8+/+ nTregs (Fig. 1E). Negligible levels of IL-17 were detected under any condition. These findings were confirmed by intracellular cytokine staining (Fig. 1D, 1F). As shown in Fig. 1F, CD8−/− nTregs demonstrated a prominent blue fluorescence, indicating IL-6 staining, whereas little or no blue signal was seen in WT nTregs. As well, Foxp3 staining (red) was very prominent in CD8+/+ nTregs and much lower in the CD8−/− nTregs. Overall, 56 ± 4.8% of CD4+CD25+ T cells from CD8−/− mice were positive for IL-6, whereas only 4.5 ± 2.4% of CD4+CD25+ T cells from WT mice were positive. In parallel to the absence of in vivo suppression, CD8−/− nTregs were significantly less effective, compared with nTregs from WT mice, in suppressing the in vitro proliferation of CD4+CD25− T cells activated with anti-CD3 and anti-CD28 (Fig. 1G).
Taken together, these data demonstrate that nTregs from CD8−/− were phenotypically and functionally different from the nTregs obtained from CD8+/+ mice; they expressed different levels of surface markers implicated in regulatory activity (CD152, GITR) and markedly reduced levels of Foxp3. In addition, they produced low levels of IL-10 and TGF-β, but released high levels of IL-6, and failed to suppress lung allergic responses when adoptively transferred into sensitized and challenged WT recipients.
Anti–IL-6 or anti–IL-6R restores the regulatory function of transferred CD8−/−nTregs in WT recipients, and IL-6 treatment of nTregs from IL-6−/− donors attenuates suppression
To further examine the association of IL-6 and the failure of nTregs from CD8−/− mice to suppress lung allergic responses, we examined the effects of administering either anti–IL-6 or anti–IL-6R to WT recipients prior to the intratracheal transfer of CD8−/− nTregs. In vivo administration of either of these Abs restored suppressive activity of the CD8−/− nTregs, resulting in significant decreases in AHR and eosinophilic inflammation (Fig. 2A, 2B), comparable to levels seen in the untreated recipients of WT nTregs (Fig. 1). The administration of anti–IL-6 to WT recipients of the CD8−/− nTregs was associated with significantly reduced levels of IL-6 in the BAL fluid, whereas the levels were increased in recipients of anti–IL-6R and highest in mice that received anti–IL-6R Abs and CD8−/− nTregs (Fig. 2C). Administration of either Ab prior to CD8−/− nTreg transfer was also associated with increased BAL levels of IL-10, TGF-β, and IFN-γ with corresponding decreases in IL-4, IL-5, and IL-13 levels. These data indicated that in vivo neutralization of IL-6 or blockade of the IL-6R enabled the transferred CD8−/− nTregs to exhibit a suppressive phenotype on lung allergic responses.
FIGURE 2.
Effect of in vivo treatment of WT recipients of CD8−/− nTregs with anti–IL-6 or anti–IL-6R prior to cell transfer. A, AHR; B, BAL cell composition; and C, BAL cytokine levels. Results are shown as means ± SEM from three independent experiments, n = 12. *p < 0.05, comparing the suppressive activities of CD8−/− nTregs in recipients given anti–IL-6 or anti–IL-6R to recipients given the Abs alone.
Suppressive activity of IL-6−/− nTregs is attenuated following treatment with IL-6
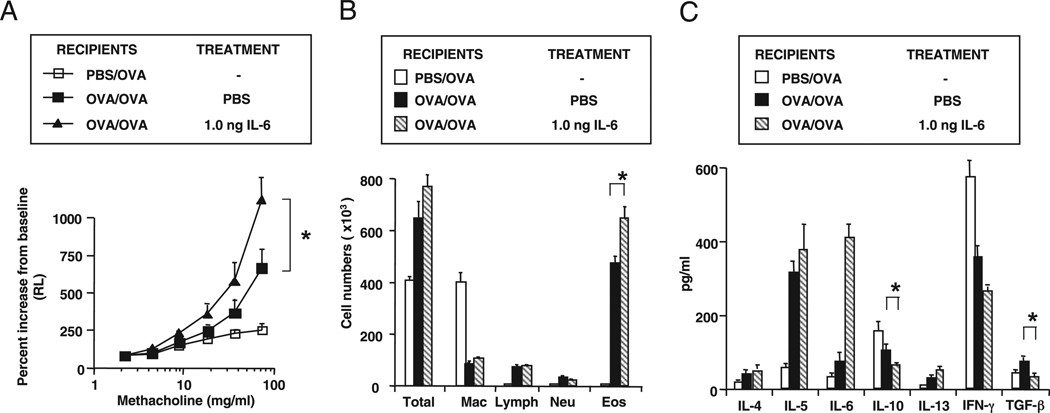
Administration of either Ab in the absence of transferred nTregs did not further enhance the level of lung allergic responses in sensitized and challenged WT mice (Fig. 2), suggesting that these Abs alone did not alter the intrinsic balance of effector cells and Tregs in a demonstrable way under these conditions. This is in keeping with the relatively low levels of IL-6 seen in the BAL of sensitized and challenged WT mice. However, administration of IL-6 to sensitized mice prior to allergen challenge did enhance lung allergic responses correlating with enhanced Th2 cytokine production and decreased levels of IL-10 and TGF-β in BAL fluid (Fig. 3).
FIGURE 3.
Effect of IL-6 administration into WT recipients on lung allergic responses. Mouse rIL-6 was administered intratracheally 4 h prior to the first allergen challenge. A, AHR; B, BAL cell composition; C, BAL cytokine levels. Results represent means ± SEM from three independent experiments, n = 12. *p < 0.05 comparing recipients of IL-6 with those that received PBS.
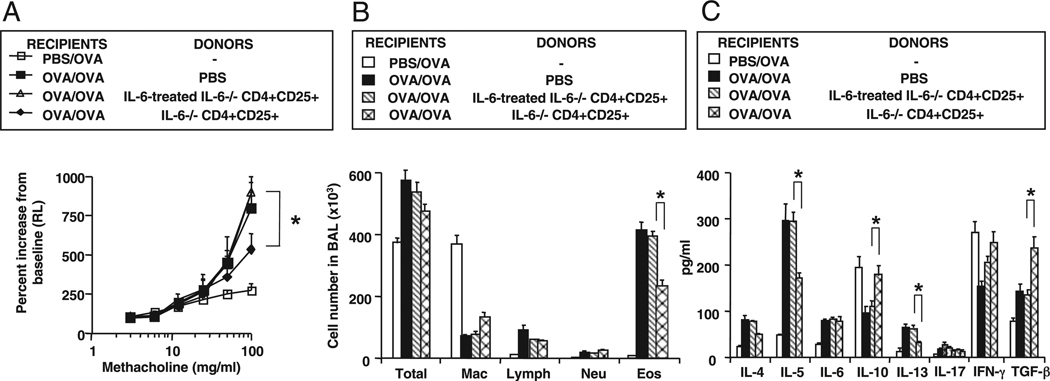
To further define the relationship between IL-6 and nTreg activity, we determined the function of nTregs harvested from IL-6+/+ and IL-6−/− mice. As shown in Fig. 4A, nTregs from IL-6−/− mice suppressed AHR, similar to the nTregs from WT (IL-6+/+) mice (Fig. 1). Following a 1-h incubation of nTregs from IL-6−/− donors with rIL-6 and then adoptive transfer into WT recipients, the cells were no longer capable of suppressing AHR (Fig. 4A), airway eosinophilia (Fig. 4B), or IL-5 and IL-13 production (Fig. 4C). In parallel, levels of IL-10 and TGF-β were significantly reduced (Fig. 4C). These data established that suppression of lung allergic responses was maintained in nTregs that were incapable of synthesizing IL-6, but when activated by IL-6, suppression of the full spectrum of lung allergic responses was attenuated.
FIGURE 4.
Effect of in vitro IL-6 treatment of nTregs isolated from IL-6−/− donors. nTregs from IL-6−/− donors were treated with IL-6 (1 µg for 1 h) prior to transfer into sensitized and challenged WT recipients. A, AHR; B, BAL cell composition; and C, BAL cytokine levels. Results represent means ± SEM from three independent experiments, n = 12. *p < 0.05 comparing the suppressive activities in recipients of untreated IL-6−/− nTregs with IL-6–treated IL-6−/− nTregs.
nTregs isolated from CD8-depleted WT mice fail to suppress lung allergic responses
To further define the roles of CD8 and IL-6 in maintaining or attenuating the regulatory function of nTregs, we examined the consequences of depleting/blocking CD8 in WTand IL-6−/− donor mice and assessed their activity following transfer into WT recipients. Control Ab or anti-CD8 Ab was initially given i.p. to WT or IL-6−/− mice soon after birth and continued weekly to the isolation of nTregs (6–8 wk of age), at which point <0.2% CD8+ T cells were detected in the spleen. In sensitized and challenged WT recipients, transfer of nTregs from rat IgG-treated WT donors reduced AHR, eosinophilic inflammation, and levels of IL-4, IL-5, and IL-13 (Fig. 5A–C). In contrast, nTregs obtained from anti-CD8–treated WT donor mice failed to suppress AHR, eosinophilic inflammation, or Th2 cytokine levels in sensitized and challenged WT recipients. Interestingly, nTregs obtained after anti-CD8 treatment of IL-6−/− mice still maintained their suppressive properties (Fig. 5A–C), implying that IL-6 production by nTregs was involved in the loss of suppressive activity in the absence of CD8–nTreg interactions.
FIGURE 5.
Functional activity of nTregs isolated from WT or IL-6−/− donors following treatment with anti-CD8 or of nTregs from CD8−/− mice reconstituted with CD8+ T cells. WT or IL-6−/− donors were treated weekly with control Ab or anti-CD8 from birth to 6–8 wk when lung nTregs were harvested and transferred into sensitized and challenged WT recipients. Alternatively, CD8−/− mice received no cells or CD8+ T cells from birth prior to harvesting nTregs and then transferred into sensitized and challenged WT recipients. A, AHR; B, BAL cell composition; C, BAL cytokine levels; D, cytokine levels in supernates of in vitro cultured nTregs in the presence or absence of PMA/ionomycin; and E, relative levels of IL-6 and Foxp3 gene expression. Results represent means ± SEM from three independent experiments, n = 12. A–D, *p < 0.05 comparing the activities in WT recipients of nTregs obtained from WT or IL-6−/− mice treated with control rat IgG or anti-CD8 or WT recipients of nTregs obtained from CD8−/− mice that received no cells or CD8+ T cells. #p < 0.05 comparing WT recipients of nTregs from anti-CD8–treated IL-6+/+ or IL-6−/− donors. E, *p < 0.05 comparing WT recipients of nTregs from CD8−/− mice that did or did not receive CD8+ T cells or WT recipients of nTregs from CD8+/+ mice that were or were not treated with anti-CD8.
The attenuation of suppression seen with nTregs obtained from anti-CD8–treated WT mice, similar to the transfer of nTregs from CD8−/− donors (Fig. 1), was associated with lower levels of IL-10 and TGF-β, but increased IL-6 in the BAL fluid of recipient mice (Fig. 5C). When these nTregs were cultured in vitro, the levels of cytokine production paralleled the in vivo findings in BAL fluid. nTregs obtained from anti-CD8–treated WT donor mice released lower levels of IL-10 and TGF-β, but higher levels of IL-6 (Fig. 5D, left panel); IL-17 levels were essentially unchanged. Similarly, levels of IL-6 gene expression were markedly increased in nTregs from anti-CD8–treated WT mice, similar to nTregs from CD8−/− mice. In parallel, Foxp3 gene expression was reduced (Fig. 5E, left panel).
CD8 reconstitution of CD8−/− mice restores nTreg-suppressive activities
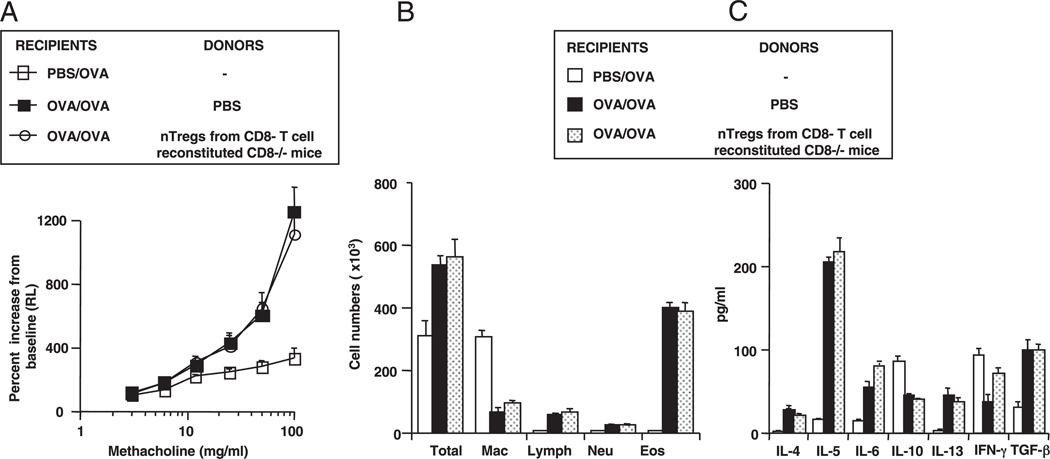
In a reciprocal manner, we investigated the effects of reconstituting CD8−/− mice with CD8+ T cells on the restoration of nTreg-suppressive activity. Negatively isolated CD8+ cells obtained from naive mice were injected i.p. into CD8−/− newborns soon after birth and continued weekly until the time of isolation of nTregs (6–8 wk of age). nTregs isolated from CD8−/− donors reconstituted with CD8+ T cells and transferred into sensitized and challenged WT recipients were suppressive, including the reduction of AHR; a decrease in BAL eosinophil numbers and IL-4, IL-5, and IL-13 levels; and a lowering of BAL IL-6 levels (Fig. 5A–C). These changes were accompanied by increases in BAL IL-10 and TGF-β levels. The cytokine pattern in the BAL fluid was reproduced when the nTregs were cultured in vitro. nTregs obtained from CD8+ T cell-reconstituted CD8−/− donor mice now produced increased levels of IL-10 and TGF-β and lower levels of IL-6 (Fig. 5D, right panel). Reconstitution of the CD8−/− donor mice with CD8− T cells failed to alter the phenotype or function of the CD8−/− nTregs (Fig. 6). As shown in Fig. 5E, right panel, CD8+ T cell reconstitution of CD8−/− mice resulted in an expression pattern for IL-6 and Foxp3 similar to that observed in CD8+/+ (WT) nTregs, with increased expression of Foxp3 and decreased expression of IL-6. Taken together, these data confirmed the interplay between CD8 and nTregs and the importance of IL-6 in dictating the suppressive activity of the nTregs.
FIGURE 6.
Functional activity of nTregs from CD8−/− mice reconstituted with CD8− T cells following adoptive transfer into WT recipients. CD8− T cells were isolated from WT donors and adoptively transferred into CD8−/− mice at 1 wk of age and on a weekly basis thereafter until nTregs were isolated at 8 wk of age, as described in Materials and Methods. nTregs were administered intratracheally just prior to the first allergen challenge. A, AHR; B, BAL cell composition; C, BAL cytokine levels. Results represent means ± SEM from three independent experiments, n = 12. Results indicate absence of suppressive activity in nTregs from CD8−/− mice reconstituted with CD8− T cells compared with the activity of nTregs from CD8−/− mice reconstituted with CD8+ T cells (Fig. 5).
Discussion
nTregs, although comprising only a small subset of T cells derived in the thymus (26), appear essential to the maintenance of immunological tolerance. In their absence and associated with the absence of Foxp3, the key transcription regulator of Treg development and function (27, 28), severe, life-threatening autoimmune and allergic diseases develop (5, 6). These Tregs have also been implicated in the maintenance of immune homeostasis in the airways, as they have been shown to regulate allergen-induced AHR and inflammation, at least in part, through the actions of IL-10 and TGF-β (10, 17). In the lung, the activation and expression of this suppressive phenotype appeared dependent on the interaction of MHC class I on nTregs with CD8; inhibition or interference with this interaction led to the loss of nTreg-suppressive activity (11). A role for CD8 in optimizing Treg-suppressive activity has been demonstrated in other situations. CD8-expressing cells were required for the optimal expansion and function of lupus-ameliorating CD4+CD25+ Tregs (29), and a subset of CD8+ splenic dendritic cells was shown to induce the differentiation of Foxp3+ Tregs (30).
In the present investigation, we further delineated the requirement for CD8 in the maintenance of suppressive activity of nTregs. nTregs obtained from CD8−/− mice or from CD8-depleted WT mice failed to regulate lung allergic responses. In vitro, nTregs from CD8−/− mice were significantly less effective than nTregs from WT mice in suppressing the proliferation of CD4+CD25− T cells activated with anti-CD3 and anti-CD28. The phenotype of CD8−/− nTregs differed from that of nTregs from CD8+/+ (WT) mice, as characterized by higher levels of GITR expression, lower expression of CD152 (CTLA-4), lower levels of the immunosuppressive cytokines IL-10 and TGF-β, and decreased expression of Foxp3. Most strikingly, nTregs from CD8−/− mice produced large amounts of IL-6, which appeared linked to the attenuation of their suppressive activities. Intracytoplasmic staining of IL-6 and Foxp3 in the CD8−/− nTregs confirmed the unique phenotype of these cells with intense staining of IL-6 and little detection of Foxp3. This link between CD8 and IL-6 was confirmed in WT recipients of CD8−/− nTregs. If the recipients received anti–IL-6 or anti–IL-6R prior to the CD8−/− nTreg transfer, suppression of AHR and eosinophilic inflammation was restored and was associated with increased levels of IL-10 and TGF-β, but lower levels of IL-5 and IL-13 in BAL fluid. Despite the increased IL-6 levels seen with blockade of the IL-6R, expression of the suppressive phenotype was maintained. However, unlike Doganci et al. (21), when anti–IL-6R (or in the current study, anti–IL-6) was administered to sensitized and challenged WT mice in the absence of transferred nTregs, we did not observe an increase in suppressive function. However, when IL-6 was administered to WT recipients prior to challenge, we did observe significant enhancement of lung allergic responses. In agreement, nTregs from IL-6−/− mice, which retained a suppressive phenotype, lost suppressive activity when treated with rIL-6 prior to adoptive transfer into WT recipients.
IL-6 is a known proinflammatory cytokine that plays a prominent role in host defense and response to injury (31). IL-6 has been implicated in the negative regulation of Treg function (17, 18), but to date there has been no report demonstrating endogenous IL-6 production in nTregs or directly linking CD8 to the regulation of IL-6 production in these nTregs. To further establish the role of CD8 in the regulation of IL-6 and nTreg function, nTreg donor mice were manipulated in different ways. In one approach, WT donor mice were treated with anti-CD8 from birth onward. This effectively depleted all CD8+ T cells and conceivably blocked expression on other CD8-expressing cells. The nTregs from these treated mice were no longer suppressive, and BAL IL-6 levels were markedly increased, whereas levels of IL-10 and TGF-β were decreased after their adoptive transfer into sensitized and challenged WT recipients. In parallel experiments, CD8−/− mice were reconstituted with CD8+/+ T cells prior to harvesting nTregs. nTreg-suppressive activity was restored only with CD8−/− nTregs obtained from the mice reconstituted with CD8+ T cells, but not with those reconstituted with CD8− T cells, and this was accompanied by reductions in BAL IL-6 levels and increases in IL-10 and TGF-β. Furthermore, nTregs isolated from CD8-depleted WT mice showed marked increases in IL-6 gene transcription and decreased Foxp3 expression, whereas the opposite was observed in nTregs isolated from CD8−/− mice reconstituted with CD8+ T cells. Of note, nTregs from IL-6−/− mice treated with anti-CD8 remained suppressive, unlike their WT counterparts, supporting the notion that CD8 regulated nTreg function indirectly, through the production of IL-6; in the absence of CD8–nTreg interactions, IL-6 levels increased with attenuation of suppressive activity, and when CD8 was present, IL-6 levels were lower and suppressive activity was sustained.
These studies indicated that the inhibitory effects of IL-6 on nTreg function were not fixed because suppressive function could be restored in the CD8−/− nTregs by these different approaches. Several studies have now described the instability of this subset of nTregs. In these studies, regulatory function did not appear to be terminally dictated by lineage, but instead confirmed their plasticity, adopting entirely different functions through modulation of expression of endogenous Foxp3 (3, 16), cytokines present in the local environment (32), and signals generated from other cells in their environment (12, 33). Following adoptive transfer into recipient CD8−/− mice, we previously showed that not only was the function of WT nTregs subverted (loss of suppressive activity), but these cells converted to an effector (pathogenic) phenotype producing IL-13, and enhanced the development of lung allergic responses (12). A somewhat similar finding has been described in nTregs and adaptive Tregs; following transient or unstable Foxp3 expression (exFoxp3 cells), these cells became autoreactive effector cells secreting IFN-γ and IL-17 (16). As demonstrated in the CD8−/− nTregs, these exFoxp3 cells expressed lower levels of CD25 and GITR.
Collectively, the results demonstrate the inverse relationship between IL-6 and Foxp3/IL-10/TGF-β in the nTregs; when IL-6 increased, Foxp3/IL-10/TGF-β decreased and vice versa. The data also delineate the role of CD8 in the maintenance of the suppressive activities of nTregs. In the absence of CD8, changes in the phenotype and function of nTregs occurred, highlighted by the significant increases in production of IL-6, which in turn was negatively associated with the expression of Foxp3, IL-10, and TGF-β, leading to the attenuation of nTreg suppression of lung allergic responses. These findings identify an important pathway whereby CD8 maintains the suppressive function of nTregs by controlling IL-6 production in these cells. The specific signaling pathways involved are currently under investigation, but our studies suggest that manipulating the engagement of CD8 with nTregs, and hence the production of IL-6, offers novel therapeutic approaches to the maintenance or removal of nTreg-suppressive function.
Acknowledgments
We thank Dr. Joseph Lucas for critical review of the manuscript and Diana Nabighian for assistance in the preparation of this manuscript.
This work was supported by National Institutes of Health Grants AI-77609, HL-36577, and HL-61005.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Health, Lung, and Blood Institute or the National Institutes of Health.
Abbreviations used in this paper
- AHR
airway hyperresponsiveness
- BAL
bronchoalveolar lavage
- nTreg
naturally occurring CD4+CD25+Foxp3+ T regulatory cell
- RL
lung resistance
- Treg
T regulatory cell
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 2.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat. Immunol. 2007;8:277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 3.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 4.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 5.Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, Bowcock AM. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J. Clin. Invest. 2000;106:R75–R81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 7.Hawrylowicz CM, O’Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat. Rev. Immunol. 2005;5:271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 8.Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Köhl J, Belkaid Y, Wills-Karp M. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J. Exp. Med. 2005;202:1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J. Exp. Med. 2005;202:1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joetham A, Takeda K, Taube C, Miyahara N, Matsubara S, Koya T, Rha YH, Dakhama A, Gelfand EW. Naturally occurring lung CD4 (+)CD25(+) T cell regulation of airway allergic responses depends on IL-10 induction of TGF-β. J. Immunol. 2007;178:1433–1442. doi: 10.4049/jimmunol.178.3.1433. [DOI] [PubMed] [Google Scholar]
- 11.Joetham A, Takeda K, Miyahara N, Matsubara S, Ohnishi H, Koya T, Dakhama A, Gelfand EW. Activation of naturally occurring lung CD4(+)CD25(+) regulatory T cells requires CD8 and MHC I interaction. Proc. Natl. Acad. Sci. USA. 2007;104:15057–15062. doi: 10.1073/pnas.0706765104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joetham A, Matsubara S, Okamoto M, Takeda K, Miyahara N, Dakhama A, Gelfand EW. Plasticity of naturally occurring regulatory T cells: subversion of suppressive function and conversion to enhancement of lung allergic responses. J. Immunol. 2008;180:7117–7124. doi: 10.4049/jimmunol.180.11.7117. [DOI] [PubMed] [Google Scholar]
- 13.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 14.Presser K, Schwinge D, Wegmann M, Huber S, Schmitt S, Quaas A, Maxeiner JH, Finotto S, Lohse AW, Blessing M, Schramm C. Coexpression of TGF-β1 and IL-10 enables regulatory T cells to completely suppress airway hyperreactivity. J. Immunol. 2008;181:7751–7758. doi: 10.4049/jimmunol.181.11.7751. [DOI] [PubMed] [Google Scholar]
- 15.Joetham A, Takeda K, Okamoto M, Taube C, Matsuda H, Dakhama A, Gelfand EW. Antigen-specificity is not required for naturally occurring regulatory T cell modulation of lung allergic responses. J. Immunol. 2009;183:1821–1827. doi: 10.4049/jimmunol.0900303. [DOI] [PubMed] [Google Scholar]
- 16.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 18.Wan S, Xia C, Morel L. IL-6 produced by dendritic cells from lupus-prone mice inhibits CD4+CD25+ T cell regulatory functions. J. Immunol. 2007;178:271–279. doi: 10.4049/jimmunol.178.1.271. [DOI] [PubMed] [Google Scholar]
- 19.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lal G, Zhang N, van der Touw W, Ding Y, Ju W, Bottinger EP, Reid SP, Levy DE, Bromberg JS. Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. J. Immunol. 2009;182:259–273. doi: 10.4049/jimmunol.182.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doganci A, Eigenbrod T, Krug N, De Sanctis GT, Hausding M, Erpenbeck VJ, Haddad B, Lehr HA, Schmitt E, Bopp T, et al. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J. Clin. Invest. 2005;115:313–325. doi: 10.1172/JCI22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeda K, Hamelmann E, Joetham A, Shultz LD, Larsen GL, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J. Exp. Med. 1997;186:449–454. doi: 10.1084/jem.186.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyahara N, Takeda K, Miyahara S, Taube C, Joetham A, Koya T, Matsubara S, Dakhama A, Tager AM, Luster AD, Gelfand EW. Leukotriene B4 receptor-1 is essential for allergen-mediated recruitment of CD8+ T cells and airway hyperresponsiveness. J. Immunol. 2005;174:4979–4984. doi: 10.4049/jimmunol.174.8.4979. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto M, Takeda K, Joetham A, Ohnishi H, Matsuda H, Swasey CH, Swanson BJ, Yasutomo K, Dakhama A, Gelfand EW. Essential role of Notch signaling in effector memory CD8+ T cell-mediated airway hyperresponsiveness and inflammation. J. Exp. Med. 2008;205:1087–1097. doi: 10.1084/jem.20072200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyahara N, Ohnishi H, Matsuda H, Miyahara S, Takeda K, Koya T, Matsubara S, Okamoto M, Dakhama A, Haribabu B, Gelfand EW. Leukotriene B4 receptor 1 expression on dendritic cells is required for the development of Th2 responses and allergen-induced airway hyperresponsiveness. J. Immunol. 2008;181:1170–1178. doi: 10.4049/jimmunol.181.2.1170. [DOI] [PubMed] [Google Scholar]
- 26.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 28.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 29.Sharabi A, Mozes E. The suppression of murine lupus by a tolerogenic peptide involves foxp3-expressing CD8 cells that are required for the optimal induction and function of foxp3-expressing CD4 cells. J. Immunol. 2008;181:3243–3251. doi: 10.4049/jimmunol.181.5.3243. [DOI] [PubMed] [Google Scholar]
- 30.Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, Nussenzweig MC, Steinman RM. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J. Immunol. 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naka T, Nishimoto N, Kishimoto T. The paradigm of IL-6: from basic science to medicine. Arthritis Res. 2002;4 Suppl. 3:S233–S242. doi: 10.1186/ar565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng S, Wang GJ, Horwitz DA. Cutting edge: Foxp3+CD4 +CD25+ regulatory T cells induced by IL-2 and TGF-β are resistant to Th17 conversion by IL-6. J. Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 33.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]