Abstract
Aromatic dioxygenase genes have long been of interest for bioremediation and aromatic carbon cycling studies. To date, 115 primers and probes have been designed and used to analyze dioxygenase gene diversities in environmental samples. Here we analyze those primers' specificities, coverages, and PCR product lengths compared to known aromatic dioxygenase genes based on in silico analysis as well as summarize their differing advantages or effectiveness from over 50 reported experimental studies. We also provide some guidance for primer use in future studies.
TEXT
Aromatic compounds, such as polycyclic aromatic hydrocarbons (PAHs), biphenyls, and dioxins, are widespread contaminants due to human activities as well as from natural events (6, 10). Aromatic dioxygenases, which initiate the aerobic degradation of these compounds, have been of considerable interest for bioremediation. Furthermore, these aromatic hydrocarbons have basic structures similar to some plant and soil aromatic compounds, and hence these and similar dioxygenase genes are likely involved in the natural carbon cycle. Improved understanding of the microbial contribution to terrestrial carbon turnover, especially of its more resistant aromatic components, is of increasing importance for predicting and potentially influencing the carbon cycle, and hence affect climate change (1).
The α-subunits of these multicomponent dioxygenases have a well-known, common structure central to their electron transfer and catalysis, called the Rieske center, and mononuclear iron and are called Rieske nonheme iron oxygenases (8). These dioxygenases have been divided into four groups based on the substrates metabolized, which also correspond to their phylogeny: toluene/biphenyl (T/B), naphthalene (or PAH), benzoate, and phthalate dioxygenases (8). The first two groups have been the most studied, and 115 primers and probes have been reported for their study (see Table S1 in the supplemental material). As more recent studies have revealed much greater diversity of these genes in nature (3, 11, 13, 16, 25), an evaluation of the current primers is timely for informing future studies. Primer coverage and specificity, which are important factors in choosing a primer set appropriate for experimental purposes, have never been compared and discussed. In this review, we compare the 115 primer sets in terms of specificity, coverage, and length of the products by using in silico analysis and by summarizing their performance from published studies.
BIPHENYL AND PAH DIOXYGENASE GENES
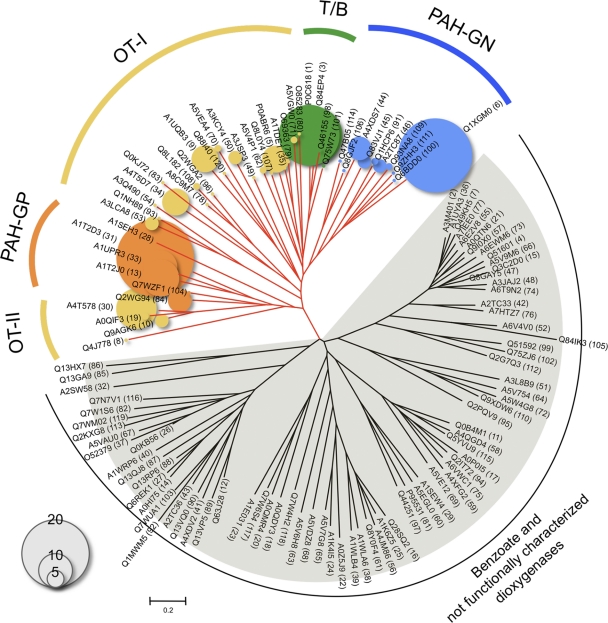
As reference sequences, we retrieved 464 Rieske nonheme iron dioxygenase genes from the Pfam protein family database (7) that are more than 350 amino acids in length and have both the Rieske family domain (Pfam PF00355) (Rieske center) and the Ring_hydroxyl_A family domain (Pfam PF00848) (iron binding site). Retrieved protein sequences were aligned using MUSCLE (4), and dissimilarity matrices were calculated and used in DOTUR (19) for complete linkage clustering. A distance cutoff of 0.2 produced 120 clusters. The middle distance sequence in each cluster was selected as a representative sequence for the cluster. The representative sequences were used for constructing a phylogenetic tree by using the neighbor-joining method and Phylip 3.67 software (5) (Fig. 1). Of the retrieved genes, the aromatic dioxygenase genes, including well-characterized toluene/biphenyl and PAH dioxygenase genes, are located in red branches in Fig. 1. The rest of the gene clusters located in the bottom half of the tree are benzoate dioxygenases or are not functionally characterized Rieske-type dioxygenases. Since aromatic dioxygenase genes usually indicate genes in the red-branched clade and thus most of the primers were designed for those genes, we used the 44 clusters that represent 204 dioxygenase genes in this large clade as reference sequences in this study. We grouped those reference genes into five subclades: PAH dioxygenases from Gram-negative bacteria (PAH-GN; blue circles), toluene/biphenyl dioxygenases (T/B; green circles), other dioxygenases I and II (OT-I and -II; yellow circles), and PAH dioxygenases from Gram-positive bacteria (PAH-GP; orange circles) (Fig. 1).
Fig. 1.
Phylogenetic analysis of 120 Rieske nonheme iron dioxygenase clusters representing 464 genes. The middle distance sequence in each cluster was used to build a tree as a representative sequence for the cluster. Numbers in parentheses identify assigned cluster numbers to be used to cross-reference with Table S2. The red branches highlight the clades and their reference genes used in this study. Clusters were grouped into five subclades (coded by color in all figures): PAH-GP, PAH dioxygenases from Gram-positive bacteria; T/B, toluene/biphenyl dioxygenases; OT-I, other dioxygenases I; PAH-GN, PAH dioxygenases from Gram-negative bacteria; OT-II, other dioxygenases II. The sizes of circles correspond to the number of members in each reference gene cluster (see Table S2 in the supplemental material for a complete list of members in each cluster).
PRIMER SELECTION: IMPORTANCE OF PRIMER COVERAGE, SPECIFICITY, AND PCR PRODUCT LENGTH
In addition to basic primer design strategies described elsewhere (18), three criteria should be considered for dioxygenase primer selection: coverage, specificity, and PCR product length. Primer coverage, which is the number of alleles of the target gene that should be amplified by the primer set during PCR as estimated from known sequences, is a key parameter because it suggests the possible diversity of the sequences that will be recovered from natural samples. The coverage range depends on the conservation of the primer region, degeneracy of the primer, and PCR conditions that may allow some primer-template mismatches. Second, specificity, or the extent to which the primers align with the correct genes while not aligning with other undesired genes, is also critical. Specificity is counter to coverage. Higher degeneracy is often used to increase coverage to more alleles, but this may result in lower specificity. Primer coverage-specificity relationships are especially critical when using primers for deep sequencing methodologies such as pyrosequencing: less specificity increases the chances of obtaining many nontarget sequences. Thus, an appropriate balance of coverage and specificity for each primer should be examined. If a highly degenerate primer is selected for one end of the amplicon, a low-degeneracy one could be chosen for the other end to increase the specificity of the primer set. Another important consideration is that different samples have different dioxygenase gene contents. Therefore, it is always important to test multiple annealing temperatures on the particular samples to determine the specificity of the primer set. Third, PCR product length, or the distance between forward and reverse primers, is an important consideration since the currently available instruments provide sequence for only partial gene lengths. For traditional Sanger sequencing, up to around 700 bp can be sequenced. For pyrosequencing using the current Genome Sequencer FLX Titanium system (454 Life Sciences, Branford, CT), sequences up to 400 to 500 bp can be obtained from PCR product sizes of 200 to 600 bp. By using Genome Analyzer (Illumina, San Diego, CA), 150 bp is currently the longest obtained sequence. Longer sequences of course lead to a better understanding of the gene, which is the ultimate goal, but the higher capacities of the 454 and Illumina technologies provide a much greater sampling of nature's gene diversity, also is an important goal.
ROLE OF PRIMER DESIGN: LESSONS FROM PREVIOUS STUDIES
Considerable research has taken place in trying to understand the aromatic degradation potentials of bacteria in terms of diversity of dioxygenases in the environment and for the type of dioxygenase contained within a bacterial isolate(s) with known aromatic degradation capacity. As noted, 115 primers have been reported for environmental studies (see Table S1 in the supplemental material). The questions for future studies are which primers do I use and are further improvements possible? We considered the role of target gene specificity, clade coverage, amplicon length, and reference sequences used in primer design as the parameters for judging quality of primers, and then we related this to the experimental outcomes from use of these primer sets.
COVERAGE OF PREVIOUS PRIMERS
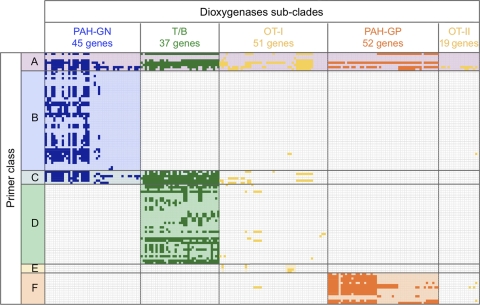
To summarize the coverage of previously reported primers, each primer was searched against 204 reference dioxygenase genes using BLAST+ (2), and perfect match genes were mapped. From the overall patterns that indicate the coverages of amplified genes (Fig. 2; shown by the densities of boxes in subclades), primers were grouped into six classes. The first class (class A) covers all five reference subclades. (DP2 or Rieske_f [17] and Nah-for [26] primers showed especially high coverage of more than 100 genes [see Fig. S1 in the supplemental material]. Those high-coverage primers have high degeneracy and were designed to target the highly conserved Rieske motifs.] The other classes are more specific to each target subclade(s): class B targets PAH-GN, class C targets PAH-GN and T/B, class D targets T/B, class E targets especially dioxin dioxygenases in OT-I, and class F targets PAH-GP. An expanded, readable map of target genes for each primer is shown in Fig. S1 of the supplemental material.
Fig. 2.
Primer coverage pattern against 204 reference dioxygenase genes. Each box indicates a perfect match between the gene and the primer. Primers can be classified into the six classes noted on the left, based on their coverages: class A targets all five reference subclades; class B targets PAH-GN; class C targets PAH-GN and T/B; class D targets T/B; class E targets dioxin dioxygenases in OT-I; class F targets PAH-GP. Figure S1 in the supplemental material expands this figure to show each primer name, its computed degeneracies, and the number of perfect matches to the corresponding gene's nucleic acid and protein identification number.
While the published studies used various techniques to accomplish different goals, in terms of coverage of primers, Lozada et al. (15) highlighted a comparison of a broad coverage primer set with a narrow coverage primer set. The Ac114F/Ac596R primer set has been used frequently (9, 12, 20, 21, 23) with fairly consistent success. In the Lozada et al. study (15), clone libraries were constructed using the Ac114F/Ac596R primer set, yielding seven distinct sequence clusters, five of them novel (only 58% to 68% identical to known amino acid sequences). Using the narrow-coverage Cyc372F/Cyc854R primer set, which targets phnA1 from Burkholderia sp. strain RP007, all sequences showed high amino acid identity (98.6 to 100%) to Cycloclasticus isolates. Those authors were able to detect broad diversity with the Ac144F/Ac596R primer set and then find specific genes, possibly specific to Cycloclasticus, with the second primer set. Primers specific to other phylotypes were attempted for this second step, but they did not result in amplification.
Primer set P8073/P9047 is also an instructive example. This primer set was designed with one reference sequence, phnAc (14), and our BLASTn results also showed that it is the only sequence with a perfect match (see Fig. S1 in the supplemental material). In multiple studies, this primer set was more specific to apparent PAH dioxygenases contained in uncultured bacteria. In two studies, strains isolated by PAH enrichment did not produce amplicons using these primers, yet the primer did produce amplicons in soils and a biofilm contaminated with phenanthrene (14, 20). In two other studies, this primer set was able to produce amplicons from PAH-degrading isolates, while primer sets targeting the nahAc gene were less successful (22, 24). In the latter study, the authors postulated that the phnAc gene was horizontally transferred, because identical copies of the gene were detected in phylogenetically diverse isolates (24). Apparently, the primer set targets a broad set of genes that may be dissimilar to those currently known. These examples indicate that previously known genes are only part of nature's diverse gene pool and that in silico analysis based on previous sequence data is a helpful first step in estimating primer coverage but is not (and should not) be expected to be completely accurate.
CONTROL OF PRIMER SPECIFICITY
Perfect match sequences are not only the products of PCR amplification. Some primers showed a lower number of perfect match genes or even no matches, although the primers were designed to target a wide range of genes. For example, the pah-rhdα primers used by Ding et al. (3) have no perfect match sequence in reference genes. Those primers were designed to use a lower annealing temperature and allow several mismatches during PCR in order to amplify a broader set of target genes. This effect could not be reflected in Fig. 2 because we were not able to estimate the effects of PCR conditions on specificity. Thus, the summary of previous studies in Table S3 of the supplemental material can help in understanding actual primer coverage, including the effects of PCR conditions.
Targeting conserved motifs within the target gene enhances primer specificity to the target gene. Ní Chadhain et al. (17), as have others, designed a primer set targeting the Rieske center of the dioxygenase, a highly conserved portion of the gene, as indicated by arrows in Fig. 3. Those investigators included degenerate bases to increase coverage but relied on the conserved positions to maintain specificity. Clone libraries were constructed in two studies (17, 25), and in both studies the amplicons formed many clusters. Some clusters were distantly related to reference sequences, indicating that the primer amplified a gene similar to those from both cultured and uncultured bacteria.
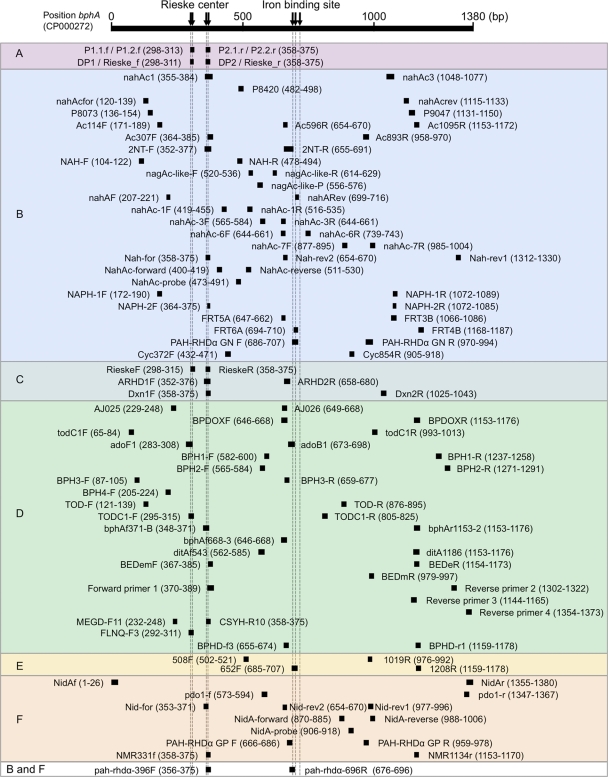
Fig. 3.
Primer annealing positions. Positions are based on bphA from Burkholderia xenovorans LB400. If the primer annealing position did not exist on the bphA gene because of gaps, the closest position number was used. Arrows indicate the positions of well-known conserved structures: the Rieske center and iron binding site.
Another method of ensuring specificity is to use a large number of reference sequences with which to find consensus sequences within the gene for use in primer design. Recently we designed the BPHD-f3/BPHD-r1 primer set with 31 reference sequences to ensure that the positions selected as primers were conserved between many strains. This allowed known conserved motifs to be contained within the amplicon to use as a quality filter during pyrosequencing data analysis. As a result, we found that the bphA1 genes in the environment were mainly novel, with only a few similar to known sequences (11). This result suggests that the current knowledge severely underrepresents bphA1sequences in nature, which may be the case for many organic carbon-degrading genes, and indicates that new strategies are needed for assessing nature's gene repertoire.
PCR PRODUCT LENGTH OF DIOXYGENASE PRIMERS
Since PCR product length is limited for certain sequencing technologies, we have summarized the positions of each primer for easy calculation of estimated product length with different combinations of primers. By using MUSCLE alignment of 204 dioxygenase genes, primer annealing positions were calculated based on bphA from Burkholderia xenovorans LB400 (GenBank accession number M86348) (Fig. 3). The length of the PCR product in each primer pair varies from less than 100 bp to the entire α-subunit. Although two of the primer sets in class A target genes from all subclades, the amplified product lengths of both are 78 bp, which limits classification and diversity assessment of these sequenced PCR products. The primers in Fig. 3 could be tried in different combinations after consideration of similar annealing temperatures and the coverage of subclades, depending on the purpose of the study or the location of conserved motifs within the gene.
CHOOSING APPROPRIATE PRIMER SETS
We recommend the following general steps in choosing primers for gene-targeted (amplicon) metagenomics. (i) Select the target subclade, e.g., all, PAH-GN, T/B, or PAH-GP (Fig. 1). (ii) Collect a set of candidate primers from each of the groups based on success of past studies (see Table S3 in the supplemental material) and their coverage against the desired genes (see Fig. S1 in the supplemental material). (iii) The amplicon length should be as long as possible to gain maximum information but short enough so that it can be sequenced through the reverse primer for quality control (the current 454 Titanium version of pyrosequencing is able to sequence up to 500 bp). (iv) Include conserved regions within the amplicon so that those sequences can be used as a quality filter in processing. We considered all primers as candidates for forward or reverse primers; however, it is possible that a primer will not function as well for the reverse complement, especially due to low GC content in the 3′ end (≤40% in last five bases). All primers should be tested empirically, since predictions are not reality. Amplification conditions may also need further optimization for the type of sample matrix.
From our evaluation of past work, we offer a few primer pair suggestions. For the most comprehensive targeting of dioxygenase clades (all; our category A), we suggest DP1/Rieske_f and ARHD2R. For other subclades, such as PAH-GN, T/B, and PAH-GP, we suggest (i) Ac596R (for use as a reverse complement) and NAPH-2R, (ii) adoB1 (for use as a reverse complement) and BPHD-r1, and (iii) NidA-forward and pdo1-r, respectively. We also recommend DP1/Rieske_f and Nah-for (for use as a reverse complement), which covers 92 of the reference genes from all classes, as a general primer set to determine if a novel isolate or gene contains a Rieske dioxygenase motif, but these are not generally recommended for amplicon sequencing due to their short length, 77 bp.
CONCLUSIONS
Many recent studies have revealed a much higher diversity of aromatic dioxygenases in environmental samples than previously thought, and hence these studies suggest a need for a better approach. One key is the selection of primer sets. The primer coverages, specificities, and lengths that we have summarized as well as our summary of the experimental studies will help guide experimenters in choosing the most appropriate primer sets for new studies. We also expect that the current expansion of environmental metagenome sequencing projects will provide an additional sequence resource for evaluating and improving primers, as that data will not be limited by what is in GenBank, which is biased toward easily cultured strains. For example, about 100 Gbp of soil metagenomic data contains about 1,000 biphenyl dioxygenase-like genes (J. R. Cole, personal communication). During the time that we wrote this review, soil metagenome projects have produced terabases of sequences. The next phase in primer design may entail detection and assessment of the dioxygenase-like genes in these resources for improvement in our knowledge of nature's aromatic degradation capacity.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by Superfund Basic Research Program grant P42 ES004911-20 from the U.S. National Institute of Environmental Health Sciences.
Biographies

Shoko Iwai is a postdoctoral fellow in Susan V. Lynch's lab at the University of California San Francisco, CA. She received her Ph.D. in Environmental Engineering from the University of Tokyo, Japan, where she worked on bioremediation of recalcitrant compounds and the development of a diagnostic microarray for bioremediation. Upon completion of her Ph.D. in 2007, she worked as a postdoctoral fellow with James M. Tiedje and Syed A. Hashsham at Michigan State University, MI. There, using pyrosequencing techniques, she analyzed functional genes in the environment, in particular biphenyl dioxygenase, as well as performed whole genome transcriptome analysis on dioxin-degrading bacteria. In 2009, she joined Susan Lynch's lab and currently works on describing the HIV microbiome. Her interests have now expanded to human microbiome structure and function as well as bacterium-host (human) interactions.

Timothy A. Johnson is a Ph.D. student at Michigan State University advised by James M. Tiedje and Syed A. Hashsham. He received his B.S. in Environmental Soil Science from Brigham Young University in Provo, UT. Prior to beginning graduate studies, he conducted research in soil fertility and the long-term persistence of acetylene metabolizing bacteria in soil despite the absence of the substrate with Bruce Webb and Richard Terry. His research at Michigan State has focused on molecular microbial ecology through the amplification and high-throughput sequencing of 16S rRNA and other functional genes, also known as gene-targeted metagenomics. He and his colleagues are attempting to retrieve full-length novel dioxygenases with activity toward dibenzo-p-dioxin from contaminated soils and sediments through molecular, “omic,” and culture-based methods, in which they will utilize and extend the utility of gene-targeted metagenomics.

Benli Chai is a bioinformatics specialist in Ribosomal Database Project (RDP) at Michigan State University. His expertise in bioinformatics has developed while working in the Mouse Genome Informatics group (MGI) at the Jackson Laboratory and RDP through bioinformatics analysis roles in various research projects and user support tasks. He received his Bachelor's degree in genetics and Master's degree in botany at Sichuan University, China, followed by a Ph.D. degree in molecular biology. He is also trained through the bioinformatics Master's degree program at the University of Illinois at Chicago.

Syed A. Hashsham is Professor of Environmental Engineering at Michigan State University. A native of Siddhartha Nagar, U.P., India, he studied Civil Engineering at Aligarh Muslim University, India, and completed his Masters in Environmental Engineering at IIT, Mumbai. He then moved to the United States, where he obtained his Ph.D. in Environmental Engineering from the University of Illinois at Urbana-Champaign, working on the effect of vitamin B12 addition on biotransformation of carbon tetrachloride. During his postdoctoral work at Michigan State and at Stanford University, he studied ecological principles governing bioreactor communities and on environmental genomics. In 2000, he joined Michigan State University as Assistant Professor. Since then has been working in the areas of environmental genomics, development of hand-held genetic analysis systems using low-cost microfluidic systems, and interactions of environmental contaminants to gut microbiome and human health. His research interests lie at the intersection of engineering, genomics, and microbial ecology.

James M. Tiedje is a University Distinguished Professor of Microbiology and Molecular Genetics and of Crop and Soil Sciences at Michigan State University, where he has been since completing his Ph.D. at Cornell University. He received his B.S. degree from Iowa State University. He began his career concurrent with the growth of microbial ecology as a discipline and has participated in its phases from process studies to modeling to molecular biology and now to genomics. He has applied these approaches to denitrification, biodegradation and community studies in soils, sediments, or bioreactors. Recently, he has used genomics and metagenomics to better understand ecological functions, speciation, and niche adaptation. He was President of the American Society for Microbiology and of the International Society of Microbial Ecology and is a member of the U.S. National Academy of Sciences.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 15 April 2011.
REFERENCES
- 1. Bardgett R. D., Freeman C., Ostle N. J. 2008. Microbial contributions to climate change through carbon cycle feedbacks. ISME J. 2:805–814 [DOI] [PubMed] [Google Scholar]
- 2. Camacho C., et al. 2009. BLAST+: architecture and applications. BMC Bioinformatics 10:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ding G.-C., et al. 2010. Soil type-dependent responses to phenanthrene as revealed by determining the diversity and abundance of polycyclic aromatic hydrocarbon ring-hydroxylating dioxygenase genes by using a novel PCR detection system. Appl. Environ. Microbiol. 76:4765–4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Edgar R. C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Felsenstein J. 1989. PHYLIP: Phylogeny Inference Package (version 3.2). Cladistics 5:164–166 [Google Scholar]
- 6. Field J. A., Sierra-Alvarez R. 2008. Microbial transformation and degradation of polychlorinated biphenyls. Environ. Pollut. 155:1–12 [DOI] [PubMed] [Google Scholar]
- 7. Finn R. D., et al. 2008. The Pfam protein families database. Nucleic Acids Res. 36:D281–D288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gibson D. T., Parales R. E. 2000. Aromatic hydrocarbon dioxygenases in environmental biotechnology. Curr. Opin. Biotechnol. 11:236–243 [DOI] [PubMed] [Google Scholar]
- 9. Gomes N. C. M., et al. 2007. Diversity of ndo genes in mangrove sediments exposed to different sources of polycyclic aromatic hydrocarbon pollution. Appl. Environ. Microbiol. 73:7392–7399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Green N. J., Hassanin A., Johnston A. E., Jones K. C. 2004. Observations on historical, contemporary, and natural PCDD/Fs. Environ. Sci. Technol. 38:715–723 [DOI] [PubMed] [Google Scholar]
- 11. Iwai S., et al. 2010. Gene-targeted-metagenomics reveals extensive diversity of aromatic dioxygenase genes in the environment. ISME J. 4:279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jeon C. O., et al. 2003. Discovery of a bacterium, with distinctive dioxygenase, that is responsible for in situ biodegradation in contaminated sediment. Proc. Natl. Acad. Sci. U. S. A. 100:13591–13596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kimura N., Kamagata Y. 2009. Impact of dibenzofuran/dibenzo-p-dioxin amendment on bacterial community from forest soil and ring-hydroxylating dioxygenase gene populations. Appl. Microbiol. Biotechnol. 84:365–373 [DOI] [PubMed] [Google Scholar]
- 14. Lloyd-Jones G., Laurie A., Hunter D. W. F., Fraser R. 1999. Analysis of catabolic genes for naphthalene and phenanthrene degradation in contaminated New Zealand soils. FEMS Microbiol. Ecol. 29:69–79 [Google Scholar]
- 15. Lozada M., et al. 2008. Novel aromatic ring-hydroxylating dioxygenase genes from coastal marine sediments of Patagonia. BMC Microbiol. 8:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marcos M. S., Lozada M., Dionisi H. M. 2009. Aromatic hydrocarbon degradation genes from chronically polluted sub-Antarctic marine sediments. Lett. Appl. Microbiol. 49:602–608 [DOI] [PubMed] [Google Scholar]
- 17. Ní Chadhain S. M., Norman R. S., Pesce K. V., Kukor J. J., Zylstra G. J. 2006. Microbial dioxygenase gene population shifts during polycyclic aromatic hydrocarbon biodegradation. Appl. Environ. Microbiol. 72:4078–4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robertson J. M., Walsh-Weller J. 1998. An introduction to PCR primer design and optimization of amplification reactions. Methods Mol. Biol. 98:121–154 [DOI] [PubMed] [Google Scholar]
- 19. Schloss P. D., Handelsman J. 2005. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl. Environ. Microbiol. 71:1501–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stach J. E. M., Burns R. G. 2002. Enrichment versus biofilm culture: a functional and phylogenetic comparison of polycyclic aromatic hydrocarbon-degrading microbial communities. Environ. Microbiol. 4:169–182 [DOI] [PubMed] [Google Scholar]
- 21. Tuomi P. M., Salminen J. M., Jørgensen K. S. 2004. The abundance of nahAc genes correlates with the 14C-naphthalene mineralization potential in petroleum hydrocarbon-contaminated oxic soil layers. FEMS Microbiol. Ecol. 51:99–107 [DOI] [PubMed] [Google Scholar]
- 22. Widada J., et al. 2002. Molecular detection and diversity of polycyclic aromatic hydrocarbon-degrading bacteria isolated from geographically diverse sites. Appl. Microbiol. Biotechnol. 58:202–209 [DOI] [PubMed] [Google Scholar]
- 23. Wilson M. S., Bakermans C., Madsen E. L. 1999. In situ, real-time catabolic gene expression: extraction and characterization of naphthalene dioxygenase mRNA transcripts from groundwater. Appl. Environ. Microbiol. 65:80–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilson M. S., Herrick J. B., Jeon C. O., Hinman D. E., Madsen E. L. 2003. Horizontal transfer of phnAc dioxygenase genes within one of two phenotypically and genotypically distinctive naphthalene-degrading guilds from adjacent soil environments. Appl. Environ. Microbiol. 69:2172–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yagi J. M., Madsen E. L. 2009. Diversity, abundance, and consistency of microbial oxygenase expression and biodegradation in a shallow contaminated aquifer. Appl. Environ. Microbiol. 75:6478–6487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou H. W., Guo C. L., Wong Y. S., Tam N. F. Y. 2006. Genetic diversity of dioxygenase genes in polycyclic aromatic hydrocarbon-degrading bacteria isolated from mangrove sediments. FEMS Microbiol. Lett. 262:148–157 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.