Abstract
Bacterioplankton plays a central role in the microbial functioning of lacustrine ecosystems; however, factors that constrain its structural variation are still poorly understood. Here we evaluated the driving forces exerted by a large set of environmental and biological parameters on the temporal and spatial dynamics of free-living bacterial community structures (BCS) in two neighboring perialpine lakes, Lake Bourget and Lake Annecy, which differ in trophic status. We analyzed monthly data from a 1-year sampling period at two depths situated in the epi- and hypolimnia for each lake. Overall, denaturing gradient gel electrophoresis (DGGE) revealed significant differences in the BCS in the two lakes, characterized by a higher number of bands in the oligotrophic ecosystem (i.e., Lake Annecy). The temporal dynamics of BCS differed greatly between depths and lakes, with temporal scale patterns being much longer in the mesotrophic Lake Bourget. Direct-gradient multivariate ordination analyses showed that a complex array of biogeochemical parameters was the driving force behind BCS shifts in both lakes. Our results indicated that 60 to 80% of the variance was explained only by the bottom-up factors in both lakes, indicating the importance of nutrients and organic matter from autotrophic origin in controlling the BCS. Top-down regulation by flagellates together with ciliates or viruses was found only in the hypolimnion and not in the epilimnion for both lakes and explained less than 18% of the bacterial community changes during the year. Our study suggests that the temporal dynamics of the free-living bacterial community structure in deep perialpine lakes are dependent mainly on bottom-up factors and to a lesser extent on top-down factors, whatever the specific environmental conditions of these lakes.
INTRODUCTION
With concentrations ranging from 104 to 106 cells ml−1 and an estimated 20 to 30% of primary productivity channeled through bacterioplankton, bacteria are the most important biological component in the transformation and mineralization of the organic matter in aquatic systems (1, 8, 11). Temperature, nutrient resources, predation by protists, and viral lysis have been demonstrated to be the main factors controlling the spatial and/or temporal dynamics of bacterial communities (21, 48, 59). It has been also suggested that bacterial growth is more controlled by top-down factors in nutrient-poor environments whereas bottom-up control is favored in nutrient-rich systems (21). While bacterial composition is an important variable controlling the rates and patterns of organic matter remineralization (42), little information is available on the relative importance of bottom-up and top-down factors in controlling this bacterial community composition. Thus, knowledge on the composition of bacterial communities, how their structures vary over space and time, and how they can be related to biological, chemical, and physical parameters are key issues for understanding the role of bacterioplankton in lacustrine systems.
Some experimental studies carried out in micro- or mesocosms have suggested that organic matter, in terms of both quality and concentration, can regulate the structure of the bacterioplankton community (30). Inorganic nutrients, grazing by both protozoan and metazoan organisms, and viral lysis have also been identified as important driving forces for the changes in bacterial community structure (BCS) (26, 31, 50). Other studies aimed to identify factors influencing the BCS by comparing in situ temporal shifts in community composition/structure and correlating these patterns with spatial and temporal conditions of aquatic systems (32, 36, 56). It is noteworthy, however, that very few studies have used the strategy of comparing temporal patterns of bacterial community changes among different systems (33, 36, 61). In most cases, dissimilarity in the BCS appeared to be mainly due to differences in the composition of biota, which in turn may have been determined by lake conditions (33, 36). Furthermore, to the best of our knowledge, studies about temporal annual changes in the BCS have dealt mainly with a limited number of parameters and, above all, have focused only on surface waters (28, 36, 55, 61). Thus, the relationships linking BCS to the vertical physicochemical and biological properties of the water column remain little examined, and this issue still leaves open fundamental questions concerning the relative importance of top-down and bottom-up factors in driving the BCS for the whole freshwater ecosystem. Such a complex ecological question may be addressed by robust statistical analysis such as direct-gradient analysis (e.g., canonical correspondence analysis [CCA]) in combination with high-throughput molecular technologies.
In this study, we collected a large data set, including physical, chemical, and biological parameters, in two neighboring (<50 km apart) large and deep perialpine lakes (Lake Annecy and Lake Bourget, France) located in a similar ecoregion, through monthly sampling over a complete year (2007) and at two depths (situated in the epi- and hypolimnia), to unravel the main driving forces of temporal changes in the structures of the free-living bacterial communities in both lakes. Specifically, this work examines whether (i) the patterns of detected changes in the free-living BCS are the same in lakes with different characteristics, (ii) the detected temporal patterns of change are the same among the epilimnion and the hypolimnion, and (iii) the relative contributions of bottom-up and top-down factors are the same among depths and among lakes.
MATERIALS AND METHODS
Study site and sampling strategy.
Water samples were collected from Lakes Bourget and Annecy. Lake Bourget is situated on the western edge of the Alps (45°44′N, 05°51′W; 231-m altitude). It is an elongated and north-south-oriented lake (length, 18 km; width, 3.5 km; area, 44 km2; volume, 3.5 × 109 m3; maximum depth, 147 m; mean depth, 80 m; residence time, ∼8.5 years). Its characteristics make it the largest natural French lake. It is a mesotrophic ecosystem with a total phosphorus concentration varying between 20 and 25 μg liter−1 (25), and a bloom of the filamentous cyanobacterium Planktothrix rubescens has been regularly observed since 1998, at least during the summer and autumn periods (25). Lake Annecy is the second-largest French lake, with an area of 28 km2, a width of 3.2 km, a length of 14.6 km, a maximum depth of 65 m, and a volume of 1.2 × 109 m3, at an altitude of 447 m. Also located in the eastern part of France, this lake has been reported to be oligotrophic since the late 1960s, with total phosphorus concentrations lower than 8 μg liter−1 (23).
Samplings were carried out once a month from January to December 2007, from the surface (at 2 or 3 m, i.e., in the upper epilimnion) and “deeper water” (at 45 or 50 m, i.e., in the upper hypolimnion). Samples were collected at the reference sampling station of each lake located above the deepest parts of the lakes. Water was sampled in sterile polycarbonate bottles and kept in the dark at 4°C until being processed immediately on return to the laboratory (i.e., within the next 3 h). Due to technical problems, samples from August and November were not available for Lake Annecy.
Physicochemical variables.
Total organic carbon (TOC) and nutrient concentrations (total nitrogen, NH4+, NO3−, SiO2, PO43−, and total phosphorus) were measured at each station and date, according to the standard French protocols AFNOR (details are available at http://www.thonon.inra.chimie.net/page/public/analyses.asp). A conductivity-temperature-depth measuring device (CTD Seabird SAB 19 Seacat profiler) and a chlorophyll (Chl) fluorescence fluoroprobe (BBE Moaldenke, Germany) were used to obtain vertical profiles of water temperature, conductivity, dissolved oxygen concentration, and chlorophyll a fluorescence.
Assessment of in situ microbial community dynamics.
Abundances of virus-like particles (VLP), heterotrophic prokaryotes (mostly bacteria) (13), and picocyanobacteria were measured by flow cytometry (FCM). Briefly, VLP and heterotrophic prokaryotes were fixed with 0.2 μm-filtered glutaraldehyde (0.5% final concentration) (grade I; Merck) for 30 min in the dark until being counted with a FACSCalibur (Becton Dickinson) flow cytometer, using the same protocol as described by Personnic et al. (41). To explore picocyanobacterial community dynamics, samples were analyzed without addition of any fixative or dye (41).
Glutaraldehyde (1% final concentration) was used to fix flagellates. Samples were filtered (pressure, <100 mm Hg) on black polycarbonate membranes (diameter, 25 mm; pore size, 0.8 μm) and then stained with primuline (6) and stored for at most a few days at −20°C until analysis. Slides were examined using epifluorescence microscopy under UV light to count the heterotrophic nanoflagellates (HNF) and under blue light to count the pigmented nanoflagellates (PNF) at a magnification of ×1,250.
Ciliates were preserved with mercuric bichloride (25%) and identified and counted (within 15 days of sampling) according to the method of Sime-Ngando et al. (51) using an inverted light microscope (Olympus; magnification, ×500).
Nucleic acid extraction, PCR, and DGGE.
Analysis of the free-living bacterial community structure (BCS) was done using denaturing gradient gel electrophoresis (DGGE). Bacteria were harvested from approximately 250 ml water onto 47-mm-diameter, 0.2-μm-pore-size, polycarbonate white membrane filters (Nuclepore) after a prefiltration step through 2-μm-pore-size polycarbonate membrane filters (Nuclepore) to eliminate large eukaryotes and filamentous cyanobacteria. The filters were then stored at −80°C until nucleic acid extraction. Nucleic acid extraction was performed as described by Dorigo et al. (18). Molecular weight distribution and purity of the DNA were assessed by 1% agarose gel electrophoresis and quantified by both visual comparison with molecular weight markers in ethidium bromide-stained agarose gels (rough estimate) and optical density measurements using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific). Such material was then stored at −20°C until PCR amplification.
PCRs were carried out using the eubacterium-specific primer 358-GC (37) and the universal primer 907rM (44), which amplify the variable V3 region of the 16S rRNA gene and yield a DNA fragment of ca. 550 bp. All PCR amplifications were carried out using about 30 ng of extracted DNA in a 50-μl reaction mix containing 10× Taq reaction buffer (Eurobio), 1.5 mM MgCl2, 120 μM each deoxynucleotide, 1 μM each primer, bovine serum albumin (Sigma; 0.5 mg ml−1 final concentration), and 1.25 U Taq DNA polymerase (Eurobluetaq; Eurobio). PCR amplification consisted of an initial denaturation step of 94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 52°C for 1 min, and extension at 72°C for 1 min and a final elongation step at 72°C for 5 min, using a PTC100 thermocycler (MJ Research). Correct sizes (ca. 500-bp length) of PCR products were determined by 2% agarose gel electrophoresis with a DNA size standard (low DNA mass ladder; GIBCO BRL).
DGGE analysis was performed on PCR fragments as described by Dorigo et al. (18), using Ingenyphor U-2 (Ingeny International) and a 40 to 80% gradient. Digital images of the gels were obtained using a Kodak DC290 camera and were then saved for further analysis using the Microsoft photo editor software. The DGGE banding patterns were analyzed using the GelCompare II software package (Applied Maths, Kortrijk, Belgium) after digitalization of the DGGE gels. Briefly, banding patterns were first standardized with a reference pattern included in all gels. Each band was described by its position (Y, in pixels on the image file) and its relative intensity in the profiles (Pi), which could be described as the ratio between the surface of the peak (ni) and the sum of the surfaces for all the peaks within the profile (N).
Statistical analysis.
Comparative analysis of DGGE profiles based on both the presence and relative abundance of bands was carried out with the PRIMER 5 software (PRIMER-E, Ltd., United Kingdom) after transfer of GelCompare II data. Ordination of Bray-Curtis similarities among normalized sample profiles was performed by hierarchical agglomerative clustering using the unweighted pair group method with arithmetic averages (UPGMA). To test the null hypothesis that there was no difference between bacterial communities at the two depths, we conducted an analysis of similarities with the subroutine ANOSIM of PRIMER. ANOSIM is a nonparametric technique designed to allow statistical comparisons for multivariate data sets in a manner similar to that for univariate techniques (analysis of variance [ANOVA]) (10). ANOSIM first calculates the R statistic, which displays the degree of separation between groups. Complete separation is indicated by R = 1, whereas R = 0 suggests no separation. Having determined R, ANOSIM randomly assigns samples to different groups to generate a null distribution of R (Monte Carlo test) to test whether within-group samples are more closely related to each other than would be expected by chance.
To investigate the relationships between BCS and environmental parameters, we used a direct-gradient approach, i.e., a canonical correspondence analysis (CCA) using the software package CANOCO, version 4.5 for Windows (54). This method was chosen after initial analysis by detrended correspondence analysis (DCA) revealed that the data exhibited a unimodal rather than a linear response to the environmental variables. We first imported operational taxonomic unit (OTU) abundance data from spreadsheets using the WCanoImp program within the CANOCO package. We then used the CANOCO program to perform CCA with species scaling on intersample distances so that samples and environmental variables formed a biplot. Explanatory variables were added until further addition of variables failed to contribute significantly (P < 0.05) to a substantial improvement to the model's explanatory power. To statistically evaluate the significance of the first canonical axis and of all canonical axes together, we used a Monte Carlo permutation full-model test with 999 unrestricted permutations. Finally, to represent biplots, we used the program CANODRAW within the CANOCO package. Spearman's rank pairwise correlations between the different sets of tested environmental variables mentioned above were performed before CCA in order to remove covarying variables.
Variation partitioning was used to evaluate whether bottom-up variables affected the bacterial community independently of the effect of top-down variables. Explanatory variables were divided into three groups. First, we separated the “pure bottom-up” effect (A), referring to the control by resources including nutrients (nitrate, nitrite, ammonium, phosphate, total nitrogen, and phosphorus). Second, we separated “other physicochemical variables” (B) (temperature, O2, SiO2, and Chl a) from the pure bottom-up variables. Third, we generated a set of variables related to top-down regulation, referring to the control by predators (C) (abundance of viruses, HNF, PNF, and ciliates). Each group of explanatory variables was tested independently as well as in combination for variation partitioning analysis, as described by Peres-Neto et al. (40). Spearman's rank pairwise correlations between the different sets of tested environmental variables mentioned above were performed before CCA in order to remove covarying variables. Explanatory variables were added until further addition of variables failed to contribute significantly (P < 0.01) to a substantial improvement to the model's explanatory power. We called “all physicochemical variables” the compilation of pure bottom-up and other physicochemical variables (A + B). The indirect effect of all physicochemical variables on grazers allowed distinguishing between the true and apparent top-down effects on bacterial community composition. “Pure top-down” effect referred to analysis based on abundance of predators only (C), and “apparent top-down effect” referred to the difference between the variance explained by taking into account the pure top-down effect in the full set of explanatory variables or not [(A + B + C) − (A + B)].
RESULTS
Environmental and biological characteristics.
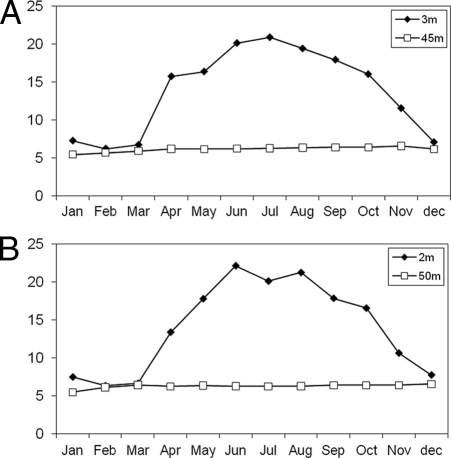
Seasonal fluctuations of temperature, shown in Fig. 1, were almost the same for both lakes, with stratification starting in early spring (March and April) and finishing in autumn (October and November). The main physicochemical and biological characteristics of the lakes in 2007 are listed in Table 1. Briefly, except for both Chl a and silicates, concentrations of the chemical variables (nitrogen and phosphorus compounds, O2, and TOC) were always higher in Lake Bourget (t test, P < 0.05; n = 12). The chlorophyll a concentration was generally below 3 μg liter−1 for both lakes. High temporal dynamics of heterotrophic prokaryote abundance, in the epilimnion, were observed in Lake Bourget in comparison to Lake Annecy (Table 1).
Fig. 1.
Temporal evolution of temperatures in Lakes Bourget (2 m versus 50 m) and Annecy (3 m versus 45 m) in 2007.
Table 1.
Environmental variables and abundances of the different biological groups considered in this study in Lake Annecy (3 versus 45 m) and Lake Bourget (2 versus 50 m) in 2007
| Parameter | Mean (minimum-maximum) value in: |
|||
|---|---|---|---|---|
| Lake Annecy |
Lake Bourget |
|||
| 3 m | 45 m | 2 m | 50 m | |
| Chl a (μg liter−1) | 2.5 (0.7–5.1) | 1.2 (0.6–2.7) | 0.9 (0.04–2.3) | 0.3 (0–1.9) |
| Dissolved oxygen (mg liter−1) | 10.1 (9.3–11.0) | 5.8 (4.3–8.3) | 10.7 (9.6–14) | 8.8 (7.6–10.6) |
| NH4 (μg liter−1) | 3 (0–8) | 2 (0–9) | 5 (0–15) | 2 (0–12) |
| Total P (μg liter−1) | 7 (2–10) | 8 (1–8) | 12 (3–45) | 11 (5–19) |
| NO3 (mg liter−1) | 0.2 (0.03–0.4) | 0.3 (0.2–0.4) | 0.35 (0.1–0.6) | 0.7 (0.5–0.8) |
| SiO2 (mg liter−1) | 1.6 (0.3–2.6) | 4.2 (3.4–5.2) | 1.2 (0.3–2.5) | 2.7 (2.4–3.2) |
| TOC (mg liter−1) | 1.9 (1.5–2.3) | 1.7 (1.3–1.9) | 2.4 (1.8–2.6) | 2.1 (1.9–2.3) |
| Picocyanobacteria (104 cells ml−1) | 4 (0.8–7) | 0.4 (0.03–1.5) | 1.1 (0.01–3.5) | 0.02 (0–0.2) |
| Heterotrophic bacteria (106 cells ml−1) | 2.2 (1.6–2.7) | 1.7 (0.9–3.2) | 2.4 (0.4–4.3) | 1.2 (0.7–1.7) |
| HNF (102 cells ml−1) | 0.6 (0.2–1.4) | 0.2 (0–0.3) | 11 (0.03–35) | 2 (0.4–8) |
| PNF (102 cells ml−1) | 0.7 (0.06–2.1) | 0.2 (0–0.7) | 31 (0.3–121) | 0.8 (0–6) |
| Viruses (107 cells ml−1) | 14 (1.9–43) | 6 (3.4–8.4) | 20 (7.4–64) | 5.4 (4–8.2) |
| Ciliates (cells ml−1) | NDa | ND | 22 (1.8–6.9) | 7 (0–16) |
ND, not determined.
Bacterial community structure in Lake Annecy.
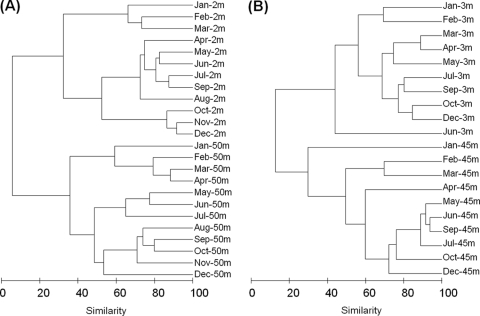
The number of Lake Annecy DGGE bands varied between 8 (January) and 19 (June) per sample (mean = 12; standard deviation [SD] = 3; n = 10) (see Fig. S1 in the supplemental material), and 15% of the bands were present in all samples taken at 3 m. By comparison, the number of bands per sample varied from 13 (January) to 21 (April) at 45 m (mean = 17; SD = 2; n = 10). Ten percent of the bands were present in all samples. The ANOSIM analysis indicated significant differences among bacterial communities from the 3-m and 45-m depth layers (r = 0.909; P < 0.001) over the entire temporal period.
Cluster analysis at 3 m showed three separate clusters (Fig. 2), the first grouping winter samples (January and February), the second grouping samples taken in spring (March to May), and the third grouping summer and autumn samples (July to December, except for June, which was separated from the other clusters). The January sample was the most divergent cluster at 45 m (30% similarity) (Fig. 2). The remaining samples divided into two clusters, one grouping winter samples (February and March) and one grouping spring, summer, and autumn samples (April to December) (Fig. 2).
Fig. 2.
Dendrogram obtained by UPGMA clustering of DGGE banding patterns from Lakes Bourget (A) and Annecy (B). Similarity is expressed as a percentage of the Bray-Curtis index.
Temporal dynamics of bacterial community structure in Lake Bourget.
In contrast to the case for Lake Annecy, a total of only 20 bands was observed for Lake Bourget at 2 m. At 50 m, this number was much higher (27 bands). The number of bands for Lake Bourget varied between 6 (June) and 13 (March) (mean = 8; SD = 2; n = 24) and between 11 (May and July) and 18 (November) (mean = 14; SD = 2; n = 24). None of the bands were present in all samples taken at either 2 or 50 m (see Fig. S1 in the supplemental material).
As observed in Lake Annecy, the ANOSIM analysis indicated significant differences among bacterial communities from the 2-m and 50-m depth layers (r = 0.986; P < 0.001) over the entire temporal period. In Lake Bourget, three clusters could be distinguished at both depths (Fig. 2). At 2 m, the first cluster grouped winter samples (January to March), the second grouped spring and summer samples (April to September), and the third grouped autumn samples (October to December). At 50 m, one cluster grouped winter samples (January to March) together with April, another grouped spring and summer samples (except August), and the third grouped August and autumn samples (September to December).
Multivariate analysis.
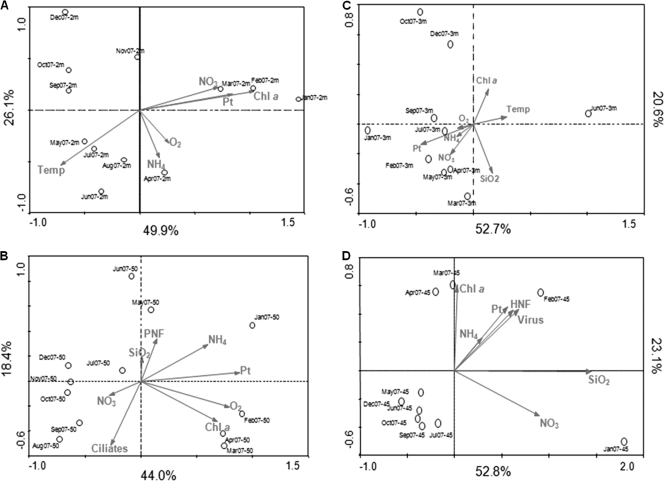
For the entire data set (Lakes Annecy and Bourget for the two depths), a strong Spearman's rank pairwise correlation between NO3 and total N (r = 0.8;, P < 0.01) and between PO4 and total P (r = 0.5, P < 0.01) allowed us to use NO3 as a proxy of total N and total P as a proxy of PO4 to perform CCA together with the rest of the physicochemical parameters. Monte Carlo tests for the first axis and all canonical axes were highly significant (P < 0.01), indicating that the parameters selected were good explanatory variables of the BCS. According to the CCA, the cumulative percent variance of the species-environment relationship indicated that the first and second canonical axes accounted for 52.7% and 20.6% of this variance at 3 m and for 52.8% and 23.1% of this variance at 45 m in Lake Annecy. In Lake Bourget, the first two axes accounted for 49.9% and 26.1% of this variance at 2 m and for 44.0% and 18.4% of this variance at 50 m, respectively (Table 2; Fig. 3). Subsequent axes accounted for less than 13.0% of the variance each and were not considered further. At 3 m, in Lake Annecy, the first and second canonical axes were highly positively correlated to temperature and Chl a, respectively, but negatively correlated to total P and SiO2 (Fig. 3). At 45 m, the first and second canonical axes were highly positively correlated to SiO2 and Chl a, respectively. In Lake Bourget, the first canonical axis was highly positively correlated to Chl a, total P, and NO3 at 2 m and to only total P at 50 m (Fig. 3), while the second axis was highly negatively correlated to NH4 at 2 m and to ciliates at 50 m (Fig. 3).
Table 2.
Summary of results from canonical correspondence analyses of the bacterioplankton community structure data when constrained by physicochemical and predators variables at 2 and 50 m in Lake Bourget and at 3 and 45 m in Lake Annecya
| Lake, depth, and environmental variable | Total inertia | Sum of all canonical eigenvalues | Eigenvalue |
Species-environment correlation |
Cumulative % variance of: |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Axis 1 | Axis 2 | Axis 1 | Axis 2 | Species data |
Species-environment relation |
|||||
| Axis 1 | Axis 2 | Axis 1 | Axis 2 | |||||||
| Bourget | ||||||||||
| 2 m | ||||||||||
| Physicochemical | 1.346 | 0.932 | 0.465 | 0.243 | 0.936 | 0.899 | 34.6 | 52.7 | 49.9 | 76.0 |
| Nutrients | 1.346 | 0.544 | 0.340 | 0.145 | 0.837 | 0.780 | 25.3 | 36.0 | 61.3 | 87.5 |
| 50 m | ||||||||||
| Physicochemical | 1.065 | 0.832 | 0.416 | 0.138 | 0.995 | 0.936 | 39.1 | 52.1 | 50.0 | 66.6 |
| Nutrients | 1.065 | 0.592 | 0.359 | 0.119 | 0.941 | 0.947 | 33.7 | 45.0 | 60.7 | 80.9 |
| Physicochemical + predators | 1.065 | 0.955 | 0.420 | 0.176 | 0.999 | 0.978 | 39.5 | 56.0 | 44.0 | 62.4 |
| Annecy | ||||||||||
| 3 m | ||||||||||
| Physicochemical | 0.786 | 0.626 | 0.329 | 0.129 | 0.964 | 0.930 | 41.9 | 58.3 | 52.7 | 73.3 |
| Nutrients | 0.786 | 0.512 | 0.308 | 0.126 | 0.930 | 0.938 | 39.2 | 55.2 | 60.2 | 84.7 |
| 45 m | ||||||||||
| Physicochemical | 0.832 | 0.595 | 0.363 | 0.116 | 0.945 | 0.894 | 43.6 | 57.5 | 60.9 | 80.4 |
| Nutrients | 0.832 | 0.494 | 0.350 | 0.092 | 0.935 | 0.770 | 42.1 | 53.2 | 70.9 | 89.7 |
| Physicochemical + predators | 0.832 | 0.766 | 0.405 | 0.177 | 0.993 | 0.999 | 48.6 | 69.9 | 52.8 | 75.9 |
In Lake Bourget, the physicochemical variables were temperature, nitrate, ammonium, total phosphorus, dissolved oxygen, and chlorophyll a for 2-m samples and nitrate, ammonium, total phosphorus, silicates, dissolved oxygen, and chlorophyll a for 50-m samples. Predators were ciliates and PNF at 50 m. In Lake Annecy, the physicochemical variables were temperature, nitrate, ammonium, total phosphorus, silicates, dissolved oxygen, and chlorophyll a for 3-m samples and nitrate, ammonium, total phosphorus, and silicates for 45-m samples. Predators were HNF and viruses at 45 m. The total variance explained corresponded to the sum of all canonical eigenvalues divided by total inertia.
Fig. 3.
Canonical correspondence analysis of the bacterioplankton community structure from samples in Lake Bourget (A and B) and Lake Annecy (C and D) at 2 m (A) versus 50 m (B) and at 3 m (C) versus 45 m (D), respectively, using physicochemical and biological parameters. Arrows point in the direction of increasing values of each variable. The length of the arrow indicates the degree of correlation with the represented axes. The position of samples relative to arrows is interpreted by projecting the points on the arrow and indicates the extent to which a sample bacterial community structure is influenced by the environmental parameter represented by that arrow. Chl a, chlorophyll a; Temp, Temperature; O2, dissolved oxygen; NH4, ammonium; NO3, nitrates; Pt, total phosphorus; SiO2, silicates; PNF, pigmented nanoflagellates; HNF, heterotrophic nanoflagellates.
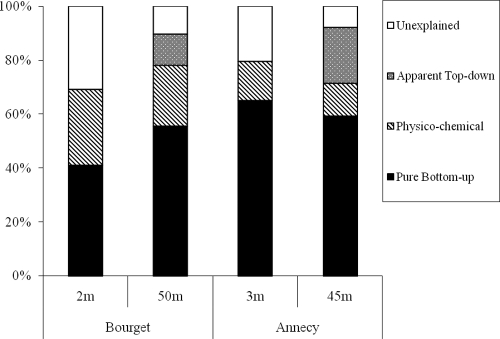
According to variation partitioning analysis, physicochemical parameters explained between 69.2% and 79.0% of the total variance in the diversity of the four fitted samples (2 depths × 2 lakes). Between five and seven variables were included in the set of physicochemical parameters selected by the forward selection (Fig. 4). Pure bottom-up control (nutrients) explained a large fraction of the variation (between 41.0% and 65.0%). None of the models including top-down parameters explained significantly the variation in the epilimnion in both Lakes Annecy and Bourget, while these parameters explained 20.5% of the total variance at 45 m in Lake Annecy and 11.5% at 50 m in Lake Bourget. Nevertheless, when physicochemistry-related variation was removed from the data from each depth of both lakes, no significant variation related to pure top-down control was related to the BCS temporal dynamics (Fig. 4).
Fig. 4.
Variation partitioning analysis of 16S rRNA gene data sets from Lake Bourget (A and B) and Lake Annecy (C and D) at 2 m (A) versus 50 m (B) and at 3 m (C) versus 45 m (D), respectively. Pure bottom-up variables were nitrate, ammonium, total phosphorus, and silicates. In Lake Bourget, physicochemical parameters were temperature, nitrate, ammonium, and total phosphorus for 2-m samples and nitrate, ammonium, total phosphorus, silicate, dissolved oxygen, and chlorophyll a for 50-m samples. Apparent top-down variables were ciliate and HNF abundances at 50 m. In Lake Annecy, physicochemical factors were nitrate, total phosphorus, silicates, dissolved oxygen, and chlorophyll a for 3-m samples and temperature, silicate, nitrate, ammonium, total phosphorus, dissolved oxygen, and chlorophyll a for 45-m samples. Pure bottom-up factors were PNF and viruses at 3 m and HNF and viruses at 45 m. For each data set, P < 0.01.
DISCUSSION
Our study revealed significant differences in the bacterial community structure with depth, in both lakes, regardless of whether the water column was stratified or mixed, suggesting that sensitive community responses to depth differences in environmental conditions could be more important than season succession (38, 47).
Moreover, our results showed a striking temporal variability in BCS within and among lakes, revealing the highly dynamic nature of the bacterioplanktonic communities. Such temporal dynamic of BCS in lacustrine systems has been reported elsewhere (33, 57, 61). However, for most of these studies, the temporal patterns of changes were different between the lakes. Yannarell et al. (61) observed some similar general patterns in the BCSs in three temperate lakes with different trophic statuses, characterized by a relative stability in spring and autumn and a strong variability in summer. In a more recent study, Kent et al. (28) also observed that the bacterial community displayed concordant dynamics among six temperate humic lakes, despite clear lake-specific differences. These differences in the temporal patterns of changes may be due to the sampling time scale of each study, distinct lake communities, sensitivity of the molecular fingerprinting method, or distinct environmental constraints.
Although several studies described the temporal variations of the BCS, only a few attempted to determine the driving factors controlling their changes (4, 35, 36). Here we used direct-gradient multivariate approaches to explore relationships between the BCSs as obtained by PCR-DGGE with respect to concomitant measurements of physicochemical and biological parameters. We are aware that some biases regarding nucleic acid extraction, PCR amplification (60), and 16S rRNA gene copy number variability (19) may have led to distorted ecological relationships in the data set. Indeed, a main drawback of PCR-DGGE fingerprinting is that it gives only partial information about microbial diversity, since the number of bands is related to the number of populations that may account for more than 0.3 to 0.4% of the total cell counts (7). This is due to comigration of PCR products from different species within the same band, as depicted by several authors (46, 60). Also, Schauer et al. (45) mentioned the subjectivity in deciding whether a very weak DGGE band is a real band or a background artifact. In our study, this problem was at least partly overcome by taking into account the presence or absence of individual peaks and the relative contribution of each peak to the total surface area of the pattern/profile. The advantage of analyzing fingerprinting data in this way has been previously highlighted by numerical simulation (36). By simulating different sources of experimental errors, Muylaert et al. (36) found that errors introduced by presence-absence data transformations were the most likely to obscure relationships between species and environment. In addition, a recent numerical simulation reported by Loisel et al. (34) underlined that fingerprinting patterns contain extractable data about diversity although not on the basis of a number of bands, as it is generally assumed to be the case, and can be considered an “image” of the whole microbial ecosystem free of inventory (cloning) limitation. On the other hand, the use of adequate numerical and statistical tools overcomes the difficulty of analyzing complex data sets.
Keeping this in mind, the important finding of this study was to statistically demonstrate that physicochemical variables explained the majority of the detected variations in the epilimnion and in the hypolimnion (between 62% and 79% of the total inertia) and that pure bottom-up control explained a large fraction of the variation (between 41% and 65% of the total inertia) in both Lake Bourget and Lake Annecy. The study by Jardillier et al. (26) is among the rare studies which also evaluated the relative importance of bottom-up and top-down factors in the temporal change of bacterial community structure and composition in lacustrine systems. Those authors found that bottom-up values represent 40.2% in the oligotrophic Sep Reservoir and 44.2% in the eutrophic Lake Aydat. They considered that bottom-up control of the bacterial community composition is much stronger than top-down control in lakes. Our results are consistent with their results.
Nutrient concentrations may directly influence bacterial biomass (9) as well as community structure (26) through effects on growth (36). However, a significant relationship between BCS and nutrients may also arise from covariation of nutrient concentrations with phytoplankton. Some studies have indeed shown interactions between phytoplankton and BCS (28, 39, 55, 58). Our results revealed a clear relationship between BCS and the concentration of Chl a in both lakes. Nevertheless, this relationship was much stronger in Lake Bourget than in Lake Annecy. The different compositions of phytoplankton in Lake Bourget and Lake Annecy could explain such results according to the notion of specific association between bacteria and autotrophic organisms (24, 39). In Lake Bourget, 21.3% of the total phytoplankton abundance was represented by the filamentous cyanobacterium Planktothrix rubescens, while Chlorophyceae, Chrysophyceae, Diatomophyceae, and Cryptophyceae represented 23.7%, 18.4%, 18%, and 14.8%, respectively (25). Comparatively, Diatomophyceae dominated at up to 55% of the total phytoplankton in Lake Annecy, followed by Cryptophyceae (22.7%), Chlorophyceae (19%), and Chrysophyceae (13%), and cyanobacteria represented less than 5% of the phytoplankton community (23). It is highly probable that such a difference in phytoplanktonic composition was a key factor in explaining the strong relationship between silicates and BCS observed only in Lake Annecy and thus the importance of the Diatomyceae in structuring the bacterial community in this oligotrophic ecosystem.
The abundances of predators (pure top-down control) alone did not give any significant explanation of the temporal changes of the BCS in the epi- and hypolimnia, regardless of the lake examined. This result does not imply that pure top-down control is not of importance in determining the bacterial community composition in both lakes. From this study, we can conclude only that pure top-down control was not related directly to temporal changes in the structure of the free-living bacterial community. The influence of the apparent top-down predation was much lower in the lakes we studied than in the lakes studied by Muylaert et al. (36) and Jardillier et al. (26). The analysis procedure based on the different variables related to top-down regulation (abundance of predators in this study, biomass of predators in the study by Muylaert et al. [36], and grazing rates of predators in the study by Jardillier et al. [26]) may be the main hypothesis to explain the difference in explanatory power of bottom-up and top-down control in our study compared to the literature.
The identification of factors responsible for the temporal dynamics of the bacterial community over only 1 year could also underestimate the role of top-down controls, which could be more important over an interannual scale. Studies that have examined the seasonal dynamics of bacterial communities have reported significant temporal trajectories across seasons but incomplete or inconsistent interannual cycles (14, 29, 61), suggesting an interannual variability of controlling factors. However, both Lakes Annecy and Bourget have large and relatively predictable seasonal variations in nutrient availability, primary production, and physical properties (41), and, furthermore, according to Curtis and Sloan (15) and Fuhrman et al. (20), the degree of recurrence of an interannual dynamic of a bacterial community depends on the phylogenetic diversity of functionally suitable bacteria and the random substitution of functionally identical populations.
In both lakes, we found a significant difference in the relative contribution of apparent top-down control in the epi- versus hypolimnion layer (Fig. 4). Indeed, apparent top-down control seemed to be a significant parameter explaining the detected changes of BCS in the hypolimnion only. Given that the abundances of both predators (ciliates, PNF, and HNF) and viruses were significantly higher in the epi- than in the hypolimnion in both lakes (t test [n = 12], P < 0.01) (Table 1), the results suggested that the lack of coupling between BCS and predators/viruses could be the result of factors driving strongly the temporal dynamics of predators and viruses in the epilimnion compared to the hypolimnion. Between-depth variation in the coupling between compartments of the microbial food web has indeed been reported for other freshwater systems (53). Nutrient availability, temperature, and grazer composition are often different between these two depths (Table 1) (13, 25), and it has already been shown that various grazers generally have different feeding modes (27) and grazing efficiencies (3). Finally, the magnitude of mechanisms such as the selective removal of active bacterioplankton by protists (16) or infection by viral lysis (12) may vary among depths.
Top-down factors seemed to be represented mainly by ciliates and to a lesser extent by pigmented nanoflagellates at 50 m in Lake Bourget and by both heterotrophic flagellates and viruses at 45 m in Lake Annecy, revealing variation in the importance of these predators as bacterial mortality agents in the two lakes, with the greatest impact of viral activity and predation by HNF on the BCS under oligotrophic conditions. Studies reported the increase of both viral lysis and HNF bacterivory with decreasing aquatic system productivity (2, 49), and our results agree with these observations. Šimek et al. (49) observed pronounced changes in the BCS due to the combined effects of HNF grazing pressure and P limitation. The same conclusion has been reported by Bettarel et al. (2) concerning the impact of viral activity on the bacterial community. However, many other studies reported contrasting results (22, 26, 43), highlighting the complexity and the importance of interactions between bottom-up and top-down factors.
Flagellates and ciliates are known to be the major causes of bacterial mortality in Lake Annecy and Lake Bourget (13, 17). Grazing by flagellates and/or ciliates affects the size distribution of the bacterial community through “size-selective mortality,” which may affect strongly the BCS (13, 32, 33, 36, 52). In Lake Bourget, a previous study (13) observed that grazing by ciliates, particularly oligotrichs, induced BCS changes, with an important drop in filamentous Cytophaga-Flavobacteria and an increase in betaproteobacteria in summer. The direct impact of viral activity on the bacterial community structure and composition has also been reported in many studies, and it has been shown that this mortality agent can affect bacterial activity and induce changes in the structure of the bacterial communities (59) through selective mortality with high “host specificity” (5). However, considering the oligotrophic conditions of Lake Annecy, we propose here that the impact of viral activity was instead indirect in this lake, with the virus-induced cell lysis releasing both inorganic and organic materials that could be utilized by noninfected bacteria, thereby promoting enhanced growth and production of some bacterial groups (59).
Our study revealed that only a small fraction of parameters (bottom-up factors) could be included in conceptual and mechanistic models to predict the temporal dynamics of the BCS in French perialpine lakes. Past studies of planktonic bacteria have found some similar results (26, 55). It is noteworthy, however, that contradictory results have also been reported in the literature. For instance, Muylaert et al. (36), who also used PCR-DGGE in four shallow eutrophic lakes, found no evidence for top-down regulation of the bacterial community composition in turbid lakes, while grazing by ciliates and daphnids (Daphnia and Ceriodaphnia) was significantly related to changes in the bacterial community in clearwater lakes, suggesting that seasonality of BCS is dependent on the dominant substrate source as well as on the food web structure. More recently, Kent et al. (28) identified a combination of meteorology, biotic interactions, and local environmental variables responsible for bacterial structure variation. The differences between our study and the references cited above could be due to several aspects: differences in taxa, sensitivity to environmental variables, closed versus open nature of the systems, differences in the ages of the systems, locally unique perturbation frequencies, etc. Clearly, further study is needed to evaluate which of these possibilities best may account for the observed differences. Although seasonal changes in environmental factors are likely to generate predictable seasonality in BCS in both Lakes Annecy and Bourget, linking bacterioplankton phenology to regional meteorologic patterns, landscape process, and seasonal transition will enhance our understanding of how bacterial communities respond to environmental factors on multiple scales.
Supplementary Material
ACKNOWLEDGMENTS
We thank J. C. Hustache, P. Chifflet, P. Perney J. Lazzaroto, A. Millery, I. Domaizon, and J. N. Avriller for technical assistance with sampling and analyses and J. Kirkman for correcting and improving the English in the manuscript. We greatly acknowledge three anonymous reviewers for helping us to improve a former version of this article.
L. Berdjeb received a grant from a French-Algerian cooperation fellowship completed by INRA.
Footnotes
This study is a contribution to the ANR AQUAPHAGE project.
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 8 April 2011.
REFERENCES
- 1. Azam F., et al. 1983. The ecological role of water column microbes in the sea. Mar. Ecol. Prog. Ser. 10:257–263 [Google Scholar]
- 2. Bettarel Y., Sime-Ngando T., Amblard C., Dolan J. 2004. Viral activity in two contrasting lake ecosystems. Appl. Environ. Microbiol. 70:2941–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boenigk J., Arndt H. 2002. Bacterivory by heterotrophic flagellates: community structure and feeding strategies. Antonie Van Leeuwenhoek 81:465–480 [DOI] [PubMed] [Google Scholar]
- 4. Boucher D., Jardillier L., Debroas D. 2006. Succession of bacterial community composition over two consecutive years in two aquatic systems: a natural lake and a lake-reservoir. FEMS Microbiol. Ecol. 55:79–97 [DOI] [PubMed] [Google Scholar]
- 5. Bouvier T., del Giorgio P. A. 2007. Key role of selective viral-induced mortality in determining marine bacterial community composition. Environ. Microbiol. 9:287–297 [DOI] [PubMed] [Google Scholar]
- 6. Caron D. A. 1983. Technique for enumeration of heterotrophic and phototrophic nanoplankton, using epifluorescence microscopy, and comparison with other procedures. Appl. Environ. Microbiol. 46:491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Casamayor E. O., Schäfer H., Baňeras L., Pedrós-Alió C. 2000. Identification of and spatio-temporal differences between microbial assemblages from two neighboring sulfurous lakes: comparison by microscopy and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 66:499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cho B. C., Azam F. 1990. Biogeochemical significance of bacterial biomass in the ocean's euphotic zone. Mar. Ecol. Prog. Ser. 63:253–259 [Google Scholar]
- 9. Chrzanowski T. H., Sterner R. W., Elser J. J. 1995. Nutrient enrichment and nutrient regeneration stimulate bacterioplankton growth. Microb. Ecol. 29:221–230 [DOI] [PubMed] [Google Scholar]
- 10. Clarke K. R., Warwick R. W. 2001. Change in marine communities: an approach to statistical analysis and interpretation, 2nd ed. Primer-E, Plymouth, United Kingdom [Google Scholar]
- 11. Cole J. J., Findlay S., Pace M. L. 1988. Bacterial production in fresh and saltwater ecosystems: a cross-system overview. Mar. Ecol. Prog. Ser. 43:1–10 [Google Scholar]
- 12. Colombet J., et al. 2006. Depth-related gradients of viral activity in Lake Pavin. Appl. Environ. Microbio. 72:4440–4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Comte J., et al. 2006. Microbial community structure and dynamics in the largest natural French lake (Lake Bourget). Microb. Ecol. 52:72–89 [DOI] [PubMed] [Google Scholar]
- 14. Crump B. C., Kling G. W., Bahr M., Hobbie J. E. 2003. Bacterioplankton community shifts in an arctic lake correlate with seasonal changes in organic matter source. Appl. Environ. Microbiol. 69:2253–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Curtis T. P., Sloan W. T. 2004. Prokaryotic diversity and its limits: microbial community structure in nature and implications for microbial ecology. Curr. Opin. Microbiol. 7:221–226 [DOI] [PubMed] [Google Scholar]
- 16. del Giorgio P. A., et al. 1996. Bacterioplankton community structure: protists control net production and the proportion of active bacteria in a coastal marine community. Limnol. Oceanogr. 41:1169–1179 [Google Scholar]
- 17. Domaizon I., Viboud S., Fontvieille D. 2003. Taxon-specific and seasonal variations in flagellates grazing on heterotrophic bacteria in the oligotrophic Lake Annecy—importance of mixotrophy. FEMS Microbiol. Ecol. 46:317–329 [DOI] [PubMed] [Google Scholar]
- 18. Dorigo U., Fontvieille D., Humbert J. F. 2006. Spatial variability in the abundance and composition of the free-living bacterioplankton community in the pelagic zone of Lake Bourget (France). FEMS Microbiol. Ecol. 58:109–119 [DOI] [PubMed] [Google Scholar]
- 19. Farrelly V., Rainey F. A., Stackebrandt E. 1995. Effect of genome size and rrn gene copy number on PCR amplification of 16S ribosomal-RNA genes from a mixture of bacterial species. Appl. Environ. Microbiol. 61:2798–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fuhrman J. A., et al. 2006. Annually reoccurring bacterial communities are predictable from ocean conditions. Proc. Natl. Acad. Sci. U. S. A. 103:13104–13109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gasol J. M., Pedros-Alio C., Vaqué D. 2002. Regulation of bacterial assemblages in oligotrophic plankton systems: results from experimental and empirical approaches. Antonie Van Leeuwenhoek 81:435–452 [DOI] [PubMed] [Google Scholar]
- 22. Gasol J. M., et al. 2002. A transplant experiment to identify the factors controlling bacterial abundance, activity, production and community composition in a eutrophic canyon-shaped reservoir. Limnol. Oceanogr. 47:62–77 [Google Scholar]
- 23. Gerdeaux D., et al. 2008. Suivi de la qualité des eaux du lac d'Annecy pour l'année 2008. Rapport 2007. SILA, Cran-Gevrier, France [Google Scholar]
- 24. Ghiglione J. F., et al. 2008. Role of environmental factors for the vertical distribution (0–1000 m) of marine bacterial communities in the NW Mediterranean Sea. Biogeoscience 5:1751–1764 [Google Scholar]
- 25. Jacquet S., et al. 2008. Suivi de la qualité des eaux du lac du Bourget pour l'année 2007. Rapport 2008. INRA/CISALB, Thonon-les-Bains, France [Google Scholar]
- 26. Jardillier L., et al. 2005. Relative importance of nutrients and mortality factors on prokaryotic community composition in two lakes of different trophic status: microcosm experiments. FEMS Microbiol. Ecol. 53:429–443 [DOI] [PubMed] [Google Scholar]
- 27. Jones R. I. 2000. Mixotrophy in planktonic protists: an overview. Freshw. Biol. 45:219–226 [Google Scholar]
- 28. Kent A. D., Yannarell A. C., Rusak J. A., Triplett E. W., McMahon K. D. 2007. Synchrony in aquatic microbial community dynamics. ISME J. 1:38–47 [DOI] [PubMed] [Google Scholar]
- 29. Kent A. D., et al. 2004. Annual patterns in bacterioplankton community variability in a humic lake. Microb. Ecol. 48:550–560 [DOI] [PubMed] [Google Scholar]
- 30. Kritzberg E. S., Langenheder S., Lindström E. S. 2006. Influence of dissolved organic matter source on lake bacterioplankton structure and function—implications for seasonal dynamics of community composition. FEMS Microbiol. Ecol. 56:406–417 [DOI] [PubMed] [Google Scholar]
- 31. Langenheder S., Jürgens K. 2001. Regulation of bacterial biomass and community structure by metazoan and protozoan predation. Limnol. Oceanogr. 46:121–134 [Google Scholar]
- 32. Lindström E. S. 2001. Investigating influential factors on bacterioplankton community composition: results from a field study on five mesotrophic lakes. Microb. Ecol. 42:598–605 [DOI] [PubMed] [Google Scholar]
- 33. Lindström E. S. 2000. Bacterioplankton community composition in five lakes differing in trophic status and humic content. Microb. Ecol. 40:104–113 [DOI] [PubMed] [Google Scholar]
- 34. Loisel P., et al. 2006. Denaturing gradient electrophoresis (DGE) and single-strand conformation polymorphism (SSCP) molecular fingerprintings revisited by simulation and used as a tool to measure microbial diversity. Environ. Microb. 8:720–731 [DOI] [PubMed] [Google Scholar]
- 35. Martinez-Alonson M., et al. 2008. Spatial heterogeneity of bacterial populations in monomectic Lake Estanya (Huesca, Spain). Microb. Ecol. 55:737–750 [DOI] [PubMed] [Google Scholar]
- 36. Muylaert K., et al. 2002. Relationship between bacterial community composition and bottom-up versus top-down variables in four eutrophic shallow lakes. Appl. Environ. Microbiol. 68:4740–4750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Muyzer G., DeWaad E. C., Uitterlinden A. G. 1993. Profiling of complex microbial populations by denaturing gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nelson C. E. 2009. Phenology of high-elevation pelagic bacteria: the roles of meteorologic variability, catchment inputs and thermal stratification in structuring communities. ISME J. 3:13–30 [DOI] [PubMed] [Google Scholar]
- 39. Newton R. J., Kent A. D., Triplett E. W., McMahon K. D. 2006. Microbial community dynamics in a humic lake: differential persistence of common freshwater phylotypes. Environ. Microbiol. 8:956–970 [DOI] [PubMed] [Google Scholar]
- 40. Peres-Neto P. R., Legendre P., Dray S., Brocard D. 2006. Variation partitioning of species data matrices estimation and comparison of fractions. Ecology 87:2614–2625 [DOI] [PubMed] [Google Scholar]
- 41. Personnic S., Domaizon I., Dorigo U., Berdjeb L., Jacquet S. 2009. Seasonal and spatial variability of virio-, bacterio-, and picophytoplanktonic abundances in three peri-alpine lakes. Hydrobiology 627:99–116 [Google Scholar]
- 42. Pinhassi J., et al. 1999. Coupling between bacterioplankton species composition, population dynamics, and organic matter degradation. Aquat. Microb. Ecol. 17:13–26 [Google Scholar]
- 43. Pradeep Ram A. S., Sime-Ngando T. 2008. Functional responses of prokaryotes and viruses to graze effects and nutrient additions in freshwater microcosms. ISME J. 2:498–509 [DOI] [PubMed] [Google Scholar]
- 44. Schauer M., Balagué V., Pedrós-Alió C., Massana R. 2003. Seasonal changes in the taxonomic composition of bacterioplankton in coastal oligotrophic system. Aquat. Microb. Ecol. 31:163–174 [Google Scholar]
- 45. Schauer M., Massana R., Pedrös-Alio C. 2000. Spatial differences in bacterioplankton composition along the Catalan coast (NW Mediterranean) assessed by molecular fingerprinting. FEMS Microb. Ecol. 33:51–59 [DOI] [PubMed] [Google Scholar]
- 46. Schmalenberger A., Tebbe C. C. 2003. Bacterial diversity in maize rhizospheres: conclusions on the use of genetic profiles based on PCR-amplified partial small subunit rRNA genes in ecological studies. Mol. Ecol. 12:251–261 [DOI] [PubMed] [Google Scholar]
- 47. Shade A., Jones S. E., McMahon K. D. 2008. The influence of habitat heterogeneity on freshwater bacterial community composition and dynamics. Environ. Microbiol. 10:1057–1067 [DOI] [PubMed] [Google Scholar]
- 48. Shiah F.-K., Ducklow H. W. 1994. Temperature and substrate regulation of bacterial abundance, production and specific growth rate in Chesapeake Bay, U. S. A. Mar. Ecol. Prog. Ser. 103:297–308 [Google Scholar]
- 49. Šimek K., et al. 2003. Comparing the effects of resource enrichment and grazing on a bacterioplankton community of a meso-eutrophic reservoir. Aquat. Microb. Ecol. 31:123–135 [Google Scholar]
- 50. Šimek K., et al. 2007. Grazer and virus-induced mortality of bacterioplankton accelerates development of Flectobacillus populations in a freshwater community. Environ. Microbiol. 9:789–800 [DOI] [PubMed] [Google Scholar]
- 51. Sime-Ngando T., Bourdier G., Amblard C., Pinel-Alloul B. 1991. Short-term variations in specific biovolumes of different bacterial forms in aquatic ecosystems. Microb. Ecol. 21:211–226 [DOI] [PubMed] [Google Scholar]
- 52. Tadonléké D. R., Planas D., Lucotte M. 2005. Microbial food web in boreal humic lakes and reservoirs: ciliates as a major factor related to the dynamics of the most active bacteria. Microb. Ecol. 49:325–341 [DOI] [PubMed] [Google Scholar]
- 53. Tadonléké R. D., Pinel-Alloul B., Bourbonnais N., Pick F. R. 2004. Factors affecting the bacteria-heterotrophic nanoflagellates relationship in oligo-mesotrophic lakes. J. Plankton Res. 26:681–695 [Google Scholar]
- 54. ter Braak C. J. F., Šmilauer P. 2002. CANOCO reference manual and CanoDraw for Windows user's guide: software for canonical community ordination (version 4.5). Microcomputer Power, Ithaca, NY [Google Scholar]
- 55. Tian C., et al. 2009. Spatiotemporal transition of bacterioplankton diversity in a large shallow hypertrophic freshwater lake, as determined by denaturing gradient gel electrophoresis. J. Plankton Res. 31:885–897 [Google Scholar]
- 56. Tijdens M., et al. 2008. Population dynamics and diversity of viruses, bacteria and phytoplankton in shallow eutrophic lake. Microb. Ecol. 56:29–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Van der Gucht K., et al. 2001. Contrasting bacterioplankton community composition and seasonal dynamics in two neighbouring hypertrophic freshwater lakes. Environ. Microbiol. 3:680–690 [DOI] [PubMed] [Google Scholar]
- 58. van Hannen E. J., et al. 1999. Changes in bacterial and eukaryotic community structure after mass lysis of filamentous cyanobacteria associated with viruses. Appl. Environ. Microbiol. 65:795–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Weinbauer M. G. 2004. Ecology of prokaryotic viruses. FEMS Microbiol. Rev. 28:127–181 [DOI] [PubMed] [Google Scholar]
- 60. Wintzingerode F., Göbel U. B., Stackebrandt E. 1997. Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol. Ecol. Rev. 21:213–229 [DOI] [PubMed] [Google Scholar]
- 61. Yannarell A. C., Kent A. D., Lauster G. H., Kratz T. K., Triplett E. W. 2003. Temporal patterns in bacterial communities in three temperate lakes of different trophic status. Microb. Ecol. 46:391–405 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.