Abstract
The number and proportion of CTX-M positive Escherichia coli organisms were determined in feces from cattle, chickens, and pigs in the United Kingdom to provide a better understanding of the risk of the dissemination of extended-spectrum β-lactamase (ESBL) bacteria to humans from food animal sources. Samples of bovine (n = 35) and swine (n = 20) feces were collected from farms, and chicken cecal contents (n = 32) were collected from abattoirs. There was wide variation in the number of CTX-M-positive E. coli organisms detected; the median (range) CFU/g were 100 (100 × 106 to 1 × 106), 5,350 (100 × 106 to 3.1 × 106), and 2,800 (100 × 105 to 4.7 × 105) for cattle, chickens, and pigs, respectively. The percentages of E. coli isolates that were CTX-M positive also varied widely; median (range) values were 0.013% (0.001 to 1%) for cattle, 0.0197% (0.00001 to 28.18%) for chickens, and 0.121% (0.0002 to 5.88%) for pigs. The proportion of animals designated high-density shedders (≥1 × 104 CFU/g) of CTX-M E. coli was 3/35, 15/32, and 8/20 for cattle, chickens, and pigs, respectively. We postulate that high levels of CTX-M E. coli in feces facilitate the dissemination of blaCTX-M genes during the rearing of animals for food, and that the absolute numbers of CTX-M bacteria should be given greater consideration in epidemiological studies when assessing the risks of food-borne transmission.
INTRODUCTION
The emergence and spread of extended-spectrum beta-lactamases (ESBLs), particularly among Enterobacteriaceae, is well recognized as a threat to the efficacy of extended-spectrum cephalosporins for the treatment of serious infections (8, 11, 14, 31, 35). During the last 5 years, ESBLs belonging to the CTX-M family of enzymes have been reported from many countries from a variety of different food-producing animals, including cattle, chickens, and pigs (17, 21, 22, 25), and these animals are recognized as reservoirs of extended-spectrum β-lactamase producers (12). The presence of CTX-M ESBLs in United Kingdom cattle was first reported in 2005 and 2006 (20, 37). More recently, CTX-M ESBL-positive Escherichia coli has also been detected in United Kingdom poultry flocks (32).
Epidemiological studies often use selective agar to ascertain and report the presence or absence of CTX-M-producing bacteria in samples; however, the number of bacteria present in samples is usually not reported. Fecal carriage is an important factor for the spread of CTX-M ESBL bacteria among both human communities (34) and animals (1, 12, 15, 24) and will be influenced by factors such as previous treatment with antimicrobials (2, 4, 6). The dissemination of CTX-M E. coli in food production units may occur via fecal cross-contamination between groups of animals (or individuals), and the contamination of food derived from animals may occur during processing in the abattoir. Previous studies have shown that the number of E. coli O157 organisms shed in feces is an important factor for dissemination during slaughter and carcass processing (29). Therefore, the aim of this study was to quantify the number and percentage of E. coli organisms that contain blaCTX-M genes in fecal samples from cattle and pigs and in cecal contents from broiler chickens to provide information on the potential for dissemination during food production.
MATERIALS AND METHODS
CHROMagar ECC (selective for all E. coli) and CHROMagar CTX (selective mainly for CTX-M-positive E. coli [33]) media were obtained from M-Tech Diagnostics Ltd., United Kingdom.
Sample screening.
Samples of bovine feces (fecal pat samples) were collected from three epidemiologically distinct farms, and samples of swine feces (fecal pat samples) were collected from seven farms, six of which were epidemiologically linked as a network of breeding, growing, and finishing units. The cattle and pig farms were epidemiologically independent of each other, and none of the farms provided both cattle and pig samples. All of these farms were subject to investigation for outbreaks of CTX-M E. coli. Samples of chicken cecal contents were collected from 13 different poultry abattoirs as part of a survey for CTX-M E. coli in broilers in the United Kingdom (18). All samples were screened initially for CTX-M E. coli by preparing a 10% suspension of feces in buffered peptone water (BPW) and were then incubated aerobically at 37°C for approximately 18 h to enrich the total bacterial population. A 10-μl aliquot of these enriched cultures was then plated onto CHROMagar CTX medium (33) and incubated as described above to identify samples containing presumptive CTX-M E. coli. Only samples positive for growth at the screening stage were selected for further analysis (n = 35 for cattle feces, n = 32 for chicken cecal samples, and n = 20 for pig feces) to determine the fraction of CTX-M-positive E. coli with respect to the total E. coli population (from growth on CHROMagar ECC). The total number (CFU/g) of presumptive E. coli organisms expressing blaCTX-M genes was estimated by using a serial dilution method based on that previously described by Miles and Misra (27). Briefly, fresh portions of the original sample material were suspended in 0.1 M phosphate-buffered saline (PBS) (1g/10 ml), and further dilutions (10−3, 10−5, and 10−7) were prepared in 0.1 M PBS and inoculated (100 μl) onto CHROMagar ECC or CHROMagar CTX. The number of CFU/g was calculated from the colony counts and by correcting for the dilutions used, and these values were used to calculate the percentage of presumptive CTX-M-positive E. coli cells in the samples.
Real-time PCR determination of CTX-M group.
The presence of a blaCTX-M gene was confirmed for up to four colonies from each plate by using a real-time PCR system (LightCycler 2.0 system and the LightCycler Fast Start DNA master SYBR green I kit; Roche) and by using the CTX-M universal forward (5′-CGA TGT GCA GCA CCA GTA A-3′) and reverse (5′-TAA GTG ACC AGA ATC AGC GG-3′) primers. Positive controls for CTX-M groups 1, 2, and 9 were included in each assay to aid in the identification of blaCTX-M genes belonging to these groups by melting curve analysis.
blaCTX-M gene sequencing.
A selection of isolates from cattle, pig, and chicken samples (n = 5 each) was analyzed to determine the blaCTX-M gene sequence type. Briefly, a PCR amplicon was produced using adjacent flanking primers for blaCTX-M genes from either group 1 (forward, 5′-CCC ATG GTT AAA AAA CAC TGC-3′; reverse, 5′-CAG CGC TTT TGC CGT CTA AG-3′) or group 9 (forward, 5′-GTG ACA AAG AGA GTG CAA CGG-3′; reverse, 5′-ATG ATT CTC GCC GCT GAA GCC-3′). The PCR product was then sequenced using an ABI 3730 DNA analyzer (Applied Biosystems, Warrington, United Kingdom). The CTX-M sequence type was then determined by the use of the ABI SeqScape program, which compared unknown sequences to known CTX-M sequence types.
Species confirmation by MALDI-TOF mass spectrometry.
Bacterial species identity was confirmed by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (Autoflex II; Bruker Daltonics Ltd., United Kingdom) by using the manufacturer's Biotyper database identification software (23).
CTX-M-14 ESBL E. coli calf challenge study.
A small-scale infection experiment was conducted to obtain further data on the extent of shedding and colonization (defined as the presence and growth of bacteria on host tissues without tissue invasion or damage) of CTX-M E. coli under more controlled conditions and in the absence of antibiotic treatment. These animal studies were performed in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986 and were approved by the Veterinary Laboratories Agency (VLA) ethics committee. Ten-week-old, conventionally reared Holstein-Friesian calves (n = 10) were obtained from VLA-approved suppliers (details have been withheld for confidentiality). Rectal fecal samples were collected from all calves 7 days before the inoculation of the bacterial test strain, and these samples were screened using the protocol outlined above to ensure that the calves were not carrying any ESBL E. coli isolates.
At 12 weeks of age, each calf was given a single oral dose of a CTX-M-14-positive E. coli bacteria (serotype O33) that previously had been isolated from cattle (20, 37). The inocula contained 1 × 1010 CFU in 20 ml of PBS and was delivered directly to the pharynx by using a worming gun. Rectal fecal samples were then collected at 24-h intervals for the following 19 days. The proportion of CTX-M ESBL E. coli from the rectal fecal samples was determined by plating serial dilutions (10−1 to 10−8) of BPW-vortexed fecal samples onto CHROMagar CTX medium as described above. For each sample, up to four colonies isolated were selected to check for the recovery of the inoculum strain by phenotyping (serum agglutination to confirm the detection of E. coli) and by molecular typing (PCR) to confirm the presence of the blaCTX-M gene. At the end of the study, all calves were euthanized and four were selected at random for postmortem examination.
Statistical analysis.
Software programs from the R Foundation for Statistical Computing (http://www.r-project.org/) and GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego California) were used to analyze the data. The level for statistical significance was predetermined to be a probability of P < 0.05. Normality tests were obtained using the Kolmogorov-Smirnov (KS) test as implemented by GraphPad Prism version 4.00 for Windows. Bartlett's test was used to test for equal variances between species, both for the absolute levels of bacteria present (CFU/g) and for percentages of CTX-M-positive E. coli. Welch's test was used to test for differences between means when it was not possible to assume equal variance in the data sets. The power of this test to discern significant differences in means was calculated based on the average sample size, variance of the means of each group, and the mean variance within each group. A chi-square test was used to investigate whether there were significant differences in the proportion of animals with low, medium, or high shedding densities between cattle, chickens, and pigs.
RESULTS
Prevalence of CTX-M-positive samples from farms.
The proportions of all samples collected from cattle and pig farms that produced growth on CHROMagar CTX medium are shown in Table 1.
Table 1.
Summary of numbers of animal samples per farm and the proportion of samples with growth on CHROMagar CTX
| Farm code | Species | Total no. of samples collected | Proportion of samples that had growth on CHROMagar CTX medium (%) |
|---|---|---|---|
| A | Cattle | 674 | 274/674 (41) |
| B | Cattle | 61 | 41/61 (67) |
| C | Cattle | 96 | 53/96 (55) |
| D | Pigs | 340 | 337/340 (99) |
| E | Pigs | 20 | 10/20 (50) |
| F | Pigs | 20 | 2/20 (10) |
| G | Pigs | 20 | 4/20 (20) |
| H | Pigs | 20 | 3/20 (15) |
| I | Pigs | 32 | 32/32 (100) |
| J | Pigs | 52 | 50/52 (96) |
Quantification of CTX-M ESBL E. coli isolates selected for enumeration studies.
Since the majority of the presumptive CTX-M E. coli isolates that were used for enumeration studies were found to carry a blaCTX-M gene when tested by real-time PCR (RT-PCR) (47 out of 51 tested; 92%), the assumption was made that those not tested by PCR but found growing on this highly selective agar (33) also were highly likely to carry the blaCTX-M gene. The total numbers of presumptive E. coli organisms and the numbers of presumptive CTX-M-positive E. coli organisms (CFU/g) isolated from CHROMagar ECC and CHROMagar CTX, respectively, were highly variable in samples from cattle, chickens, and pigs (Table 2). These sets of data did not have normal distributions, which prevented direct statistical analysis. The log transformation of the data produced normal distributions for chicken and pigs but not for cattle (data not shown).
Table 2.
Summary statistics of the numbers of presumptive E. coli organisms isolated from different animal species from CHROMagar ECC and CHROMagar CTX agar medium
| Statistic | No. of E. coli organisms (CFU/g) isolated from: |
|||||
|---|---|---|---|---|---|---|
| Cattle feces (n = 35) |
Chicken cecal contents (n = 32) |
Pig feces (n = 20) |
||||
| ECCa | CTXb | ECC | CTX | ECC | CTX | |
| Minimum | 6 × 104 | 100 | 8 × 104 | 100 | 1 × 106 | 100 |
| 25th percentile | 9 × 105 | 100 | 4.2 × 106 | 250 | 3 × 106 | 100 |
| Mean | 2.8 × 108 | 8.6 × 104 | 1.2 × 108 | 2.1 × 105 | 1.1 × 107 | 1.0 × 105 |
| Median | 1.8 × 106 | 100 | 3.5 × 107 | 5,350 | 7.5 × 106 | 2,800 |
| 75th percentile | 7 × 106 | 1,000 | 8.05 × 107 | 1.75 × 105 | 1 × 107 | 1.95 × 105 |
| Maximum | 4.9 × 109 | 1 × 106 | 1 × 109 | 3.1 × 106 | 4.4 × 107 | 4.7 × 105 |
| SD | 1.1 × 109 | 2.8 × 105 | 2.5 × 108 | 5.7 × 105 | 1.2 × 107 | 1.6 × 105 |
ECC, CHROMagar ECC.
CTX, CHROMagar CTX.
For each species, the animals could be divided arbitrarily into three shedding density groups: low (1 to 199 CFU/g), medium (200 to 9,999 CFU/g), and high (≥1 × 104 CFU/g) (Table 3). Chi-square analysis indicated that there were significant differences (P = 0.0062) in the proportions of animals with low, medium, or high shedding densities between species.
Table 3.
Percentage of animals shedding CTX-M E. coli at three different shedding levels
| Shedding level (CFU/g) | % (Fraction positive) of animals shedding CTX-M |
||
|---|---|---|---|
| Cattle | Chicken | Pigs | |
| Low (1–199) | 54.3 (19/35) | 21.9 (7/32) | 35.0 (7/20) |
| Mediuma (200–9,999) | 37.1 (13/35) | 31.3 (10/32) | 25.0 (5/20) |
| Highb (≥1 × 104) | 8.6 (3/35) | 46.9 (15/32) | 40.0 (8/20) |
Equivalent to a high-density shedder (3).
Equivalent to a supershedder (3).
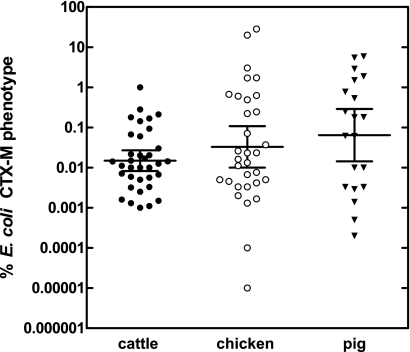
There was also a wide range of values for the percentage of E. coli organisms containing blaCTX-M genes from the cattle, chicken, and pig samples tested, which were all non-Gaussian in distribution and had unequal variances (Fig. 1). The median values for the percentage of E. coli organisms containing blaCTX-M genes and expressing extended-spectrum cephalosporin resistance in cattle feces, chicken cecal contents, and pig feces were 0.013, 0.0197, and 0.121%, respectively. Welch's test suggested that there was no statistically significant difference in mean values between species (P = 0.1302); however, this interpretation must be considered with caution, since the power of this test to discern significant differences given the sample size and variance was only 38%. The number of samples in which the proportion of CTX-M-positive E. coli organisms was >1% was 1, 5, and 5 for cattle, chickens, and pigs, respectively.
Fig. 1.
Estimates for the percentages of the E. coli population in cattle feces (n = 35), chicken cecal contents (n = 32), and pig feces (n = 20) with a CTX-M phenotype. Bars represent geometric means with 95% confidence intervals.
blaCTX-M gene sequence results.
Gene sequence analysis of a small number of representative samples from each animal species showed that blaCTX-M-1 genes were present in E. coli isolates from both pigs and chickens (n = 5 each), whereas the E. coli isolates from cattle contained either blaCTX-M-15 (n = 2) or blaCTX-M-14 (n = 3). Subsequent serotyping confirmed that the two cattle isolates of E. coli carrying blaCTX-M-15 were not the human O25-ST131 pandemic strain.
CTX-M-14 ESBL E. coli calf challenge study.
The 7-day predosing samples confirmed that the 10 calves did not harbor CTX-M-positive E. coli, including the CTX-M-14 test strain used for the dosing challenge. The shedding density of the inoculated strain was maximal 24 h postchallenge and then declined steadily to minimum levels (≈200 CFU/g) after 16 days (results not shown). At the end of the experiment, postmortem examinations of four calves indicated that the CTX-M E. coli test strain was associated at low levels with tissue samples from the colon (≈330 CFU/g; one of four calves), cecum (≈390 CFU/g; four of four calves), rectum (≈360 CFU/g; four of four calves), and recto-anal junction (≈200 CFU/g; three of four calves). The test strain was not detected in tissue samples from the duodenum, jejunum, Peyer's patches, or ileum.
DISCUSSION
The extent of CTX-M-positive E. coli fecal contamination in pat samples from pigs, cattle, and chicken cecal contents varied widely both in terms of absolute numbers and the percentage of the total E. coli population. The variability for total E. coli numbers was consistent with previously published data for these animal species (5, 13, 28).
CTX-M gene sequence analysis of a small number of E. coli isolates from samples of chicken cecal contents and pig feces (n = 5 each) showed that they all contained blaCTX-M-1 genes. This finding is consistent with a recently completed survey of broiler chickens for E. coli carrying extended-spectrum β-lactamases in Great Britain (32) that indicated that the predominant sequence type in United Kingdom broiler chickens was CTX-M-1. This is also the sequence type found in broilers in The Netherlands (16). CTX-M-1-producing E. coli strains have also been isolated from pigs in Demark (1, 13, 19, 39) and Spain (7, 9); however, the present study appears to be the first report of CTX-M-1-producing E. coli isolated from pigs in the United Kingdom.
The E. coli isolates from the cattle samples tested contained either CTX-M-14 (n = 3) or CTX-M-15 (n = 2). These results are consistent with previous reports for E. coli carrying CTX-M-14 in United Kingdom cattle (20, 37). However, to date there have been relatively few reports of animal sources of E. coli CTX-M-15 producers, despite the widespread prevalence of these bacteria among human populations (12). E. coli carrying CTX-M-15 was isolated from a urinary sample from cattle in France (25). To our knowledge, this is the first report of United Kingdom cattle carrying CTX-M-15-positive E. coli. Based on serotyping these isolates, they were not the human pandemic CTX-M-15 O25-ST131 E. coli strain.
Our data indicate that of the samples studied, the median levels of CTX-M E. coli were higher in chicken cecal contents and pig feces than in samples from cattle feces (Table 2). There were a number of cattle, pigs, and chickens designated (arbitrarily) in this study as being high-density shedders (≥1 × 104 CFU/g) of CTX-M E. coli (Table 3). This high shedding level is equivalent to that described for so-called supershedders of E. coli O157:H7 in beef cattle that was associated with an increased risk of contamination of animals hides (3). Also, for cattle shedding high levels of E. coli O157 (>1 × 104 CFU/g), it has been recognized that such animals pose an increased risk of contamination of the food chain when they are presented for slaughter at abattoirs (29). We suggest that food-producing animals that are also high-density shedders of CTX-M E. coli pose similar risks of the contamination of the food chain if they reach the abattoir. The samples from broiler chickens were collected at slaughter, and therefore the results from these samples indicate the potential for the contamination of food products from these birds. However, the samples from pigs and cattle were collected from animals in the rearing and/or breeding phases of production, and while providing an indication of the level of contamination at that stage of production, they may not reflect the values that could be obtained from animals at or immediately prior to slaughter and therefore are less likely to reflect the potential for the contamination of food products.
The factors responsible for the high levels of CTX-M E. coli shedding in the cattle, chickens, and pigs used in this study are unknown. However, one of the acknowledged risk factors for the selection of ESBL-producing bacteria is the use of antibiotics (10, 30). “Third-generation” (i.e., expanded-spectrum antibiotics) and “fourth-generation” (broad-spectrum antibiotics with enhanced activity against Gram-positive bacteria and β-lactamase stability) cephalosporin antibiotics are not licensed for use in poultry in the United Kingdom but can be used under the cascade system (38). Ceftiofur is licensed in the United Kingdom for the treatment of cattle and pigs with respiratory disease and for cattle with foot rot and metritis, whereas cefquinome and cefoperazone are used in veterinary medicines for the treatment and prophylaxis of bovine mastitis (http://www.noahcompendium.co.uk/Compendium/Overview/-21789.html). Cefquinome injection also may be used as a treatment for respiratory disease in pigs (http://www.noahcompendium.co.uk/Compendium/Overview/-21789.html). The presampling antimicrobial therapeutic history of the cattle and broilers used for this study is unknown, hence it is not possible to link the usage of antimicrobials with high levels of CTX-M-positive E. coli from these animals. However, the pigs recently had been treated with florfenicol in feed to treat respiratory disease as well as amoxicillin for diarrhea. The coselection of β-lactamase resistance by the use of florfenicol has been reported recently for French cattle in which florfenicol use in the production stage facilitated the isolation of ceftiofur-resistant E. coli (26). The use of cephalosporin antibiotics directly would be expected to increase the proportion of E. coli with blaCTX-M genes in fecal samples, as has been shown for pigs treated with ceftiofur or cefquinome (13, 19).
The results of the small-scale in vivo study showed that the shedding density of the CTX-M-14 E. coli test strain declined steadily to low levels after 16 days. This experiment was conducted in the absence of any antimicrobial treatment of calves and suggests that the presence of high-density shedding cattle on farms is associated with unidentified environmental factors or farm management practices. The postmortem examinations indicated that the inoculated strain was associated at low levels with some gut tissues at a stage when the fecal shedding density was at minimal levels. Such host reservoirs would allow for continued low-level environmental contamination and are a risk factor for the selection of resistant strains with antimicrobial chemotherapy.
With respect to fecal contamination, it is assumed that both the absolute levels as well as the proportion of CTX-M-resistant E. coli present are likely to be important factors for environmental contamination and spread to the food chain. The relative importance of these two parameters for contributing to risks of contamination of food are not certain at present, particularly with respect to the threshold levels for significant risk for each parameter. Despite the extent of the variability in both absolute numbers and percentages of CTX-M E. coli present in feces, the similarity of the median values from cattle, pigs, and chickens suggests that at present there is a comparable risk of contamination from these sources. However, the risk of environmental contamination from these fecal sources also will be influenced by the management/rearing systems used for these species. For example, the risk of environmental contamination of surface water from runoff from cattle on the open pasture is likely to be higher than the risk from pigs and poultry that are reared in confined accommodations.
In summary, the present work extends the current understanding of CTX-M ESBL carriage in cattle, pigs, and chickens by providing data on the absolute numbers and proportions of CTX-M-positive E. coli organisms present in samples of feces and cecal contents. We suggest that greater emphasis be given to the absolute numbers of CTX-M bacteria that are shed in feces when considering risks of the contamination of the food chain. The results have potential value for informing future microbial risk assessments (36) that aim to assess the risk to human or animal health due to the dissemination of CTX-M ESBL E. coli or the blaCTX-M gene via food-borne transmission or environmental routes, such as farm waste. The factors driving the selection of CTX-M E. coli remain unidentified and require further investigation to identify effective interventions to reduce their number and to maintain the safety of food production.
ACKNOWLEDGMENTS
This work was funded by the United Kingdom government, Department of Environment, Food and Rural Affairs (project grants OD2023 and OD2028).
We acknowledge the cooperation of the farm owners and abattoir companies (details have been withheld for confidentiality) for the collection of samples. We are grateful to the VLA Animal Services Unit for assistance with the experimental calf study and also to J. Nunez-Garcia from the VLA Centre for Epidemiology and Risk Analysis for advice on the statistical analysis of the data.
Footnotes
Published ahead of print on 8 April 2011.
REFERENCES
- 1. Aarestrup F. M., et al. 2006. First description of blaCTX-M-1 carrying Escherichia coli isolates in Danish primary food production. J. Antimicrob. Chemother. 57:1258–1259 [DOI] [PubMed] [Google Scholar]
- 2. Alali W. Q., Scott H. M., Norby B., Gebreyes W., Loneragan G. H. 2009. Quantification of the BlaCMY-2 in feces from beef feedlot cattle administered three different doses of ceftiofur in a longitudinal controlled field trial. Foodborne Pathog. Dis. 6:917–924 [DOI] [PubMed] [Google Scholar]
- 3. Arthur T. M., et al. 2009. Longitudinal study of Escherichia coli O157:H7 in a beef cattle feedlot and role of high-level shedders in hide contamination. Appl. Environ. Microbiol. 75:6515–6523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Asensio A., et al. 2000. Outbreak of a multiresistant Klebsiella pneumoniae strain in an intensive care unit: antibiotic use as risk factor for colonization and infection. Clin. Infect. Dis. 30:55–60 [DOI] [PubMed] [Google Scholar]
- 5. Baurhoo B., Letellier A., Zhao X., Ruiz-Feria C. A. 2007. Cecal populations of lactobacilli and bifidobacteria and Escherichia coli populations after in vivo Escherichia coli challenge in birds fed diets with purified lignin or mannanoligosaccharides. Poult. Sci. 86:2509–2516 [DOI] [PubMed] [Google Scholar]
- 6. Blake D. P., et al. 2003. Influence of tetracycline exposure on tetracycline resistance and the carriage of tetracycline resistance genes within commensal Escherichia coli populations. J. Appl. Microbiol. 94:1087–1097 [DOI] [PubMed] [Google Scholar]
- 7. Blanc V., et al. 2006. ESBL- and plasmidic class C β-lactamase-producing E. coli strains isolated from poultry, pig and rabbit farms. Vet. Microbiol. 118:299–304 [DOI] [PubMed] [Google Scholar]
- 8. Bonnet R. 2004. Growing group of extended-spectrum β-lactamases: the CTX-M enzymes. Antimicrob. Agents Chemother. 48:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Briñas L., et al. 2005. Monitoring and characterization of extended-spectrum beta-lactamases in Escherichia coli strains from healthy and sick animals in Spain in 2003. Antimicrob. Agents Chemother. 49:1262–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cantón R., Coque T. M. 2006. The CTX-M β-lactamase pandemic. Curr. Opin. Microbiol. 9:466–475 [DOI] [PubMed] [Google Scholar]
- 11. Cantón R., et al. 2008. Prevalence and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 14:144–153 [DOI] [PubMed] [Google Scholar]
- 12. Carattoli A. 2008. Animal reservoirs for extended spectrum β-lactamase producers. Clin. Microbiol. Infect. 14:117–123 [DOI] [PubMed] [Google Scholar]
- 13. Cavaco L. M., Abatih E., Aarestrup F. M., Guardabassi L. 2008. Selection and persistence of CTX-M-producing Escherichia coli in the intestinal flora of pigs treated with amoxicillin, ceftiofur, or cefquinome. Antimicrob. Agents Chemother. 52:3612–3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coque T. M., Baquero F., Canton R. 2008. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Eurosurveill. 13:pii=19044 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19044 [PubMed] [Google Scholar]
- 15. Costa D., et al. 2006. Detection of Escherichia coli harbouring extended-spectrum β-lactamases of the CTX-M, TEM and SHV classes in faecal samples of wild animals in Portugal. J. Antimicrob. Chemother. 58:1311–1312 [DOI] [PubMed] [Google Scholar]
- 16. Dierikx C., van Essen-Zandbergen A., Veldman K., Smith H., Mevius D. 2010. Increased detection of extended spectrum beta-lactamase producing Salmonella enterica and Escherichia coli isolates from poultry. Vet. Microbiol. 145:273–278 [DOI] [PubMed] [Google Scholar]
- 17. Duan R. S., et al. 2006. Escherichia coli producing CTX-M β-lactamases in food animals in Hong Kong. Microb. Drug Resist. 12:145–148 [DOI] [PubMed] [Google Scholar]
- 18. European Commission 2007. Commission decision of 19 July 2007 concerning a financial contribution from the community towards a survey on the prevalence and antimicrobial resistance of Campylobacter spp. in broiler flocks and on the prevalence of Campylobacter spp. and Salmonella spp. in broiler carcasses to be carried out in the member states [notified under document number C(2007) 3440]. 2007/516/EC. European Union Publications Office, Luxembourg City, Luxembourg: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32007D0516:EN:NOT [Google Scholar]
- 19. Jørgensen C. J., Cavaco L. M., Hasman H., Emborg H. D., Guardabassi L. 2007. Occurrence of CTX-M-1-producing Escherichia coli in pigs treated with ceftiofur. J. Antimicrob. Chemother. 59:1040–1042 [DOI] [PubMed] [Google Scholar]
- 20. Liebana E., et al. 2006. Longitudinal farm study of extended-spectrum β-lactamase-mediated resistance. J. Clin. Microbiol. 44:1630–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu J. H., et al. 2007. Detection and characterisation of CTX-M and CMY-2 β-lactamases among Escherichia coli isolates from farm animals in Guangdong Province of China. Int. J. Antimicrob. Agents 29:576–581 [DOI] [PubMed] [Google Scholar]
- 22. Madec J. Y., et al. 2008. Prevalence of fecal carriage of acquired expanded-spectrum cephalosporin resistance in Enterobacteriaceae strains from cattle in France. J. Clin. Microbiol. 46:1566–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mellmann A., et al. 2008. Evaluation of matrix-assisted laser desorption ionization-time-of-flight mass spectrometry in comparison to 16S rRNA gene sequencing for species identification of nonfermenting bacteria. J. Clin. Microbiol. 46:1946–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mesa R. J., et al. 2006. Extended-spectrum β-lactamase-producing Enterobacteriaceae in different environments (humans, food, animal farms and sewage). J. Antimicrob. Chemother. 58:211–215 [DOI] [PubMed] [Google Scholar]
- 25. Meunier D., Jouy E., Lazizzera C., Kobisch M., Madec J. Y. 2006. CTX-M-1- and CTX-M-15-type β-lactamases in clinical Escherichia coli isolates recovered from food-producing animals in France. Int. J. Antimicrob. Agents 28:402–407 [DOI] [PubMed] [Google Scholar]
- 26. Meunier D., et al. 2010. Plasmid-borne florfenicol and ceftiofur resistance encoded by the floR and blaCMY-2 genes in Escherichia coli isolates from diseased cattle in France. J. Med. Microbiol. 59:467–471 [DOI] [PubMed] [Google Scholar]
- 27. Miles A. A., Misra S. S. 1938. The estimation of the bactericidal power of the blood. J. Hyg. (London) 38:732–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moriarty E. M., Sinton L. W., Mackenzie M. L., Karki N., Wood D. R. 2008. A survey of enteric bacteria and protozoans in fresh bovine faeces on New Zealand dairy farms. J. Appl. Microbiol. 105:2015–2025 [DOI] [PubMed] [Google Scholar]
- 29. Omisakin F., MacRae M., Ogden I. D., Strachan N. J. C. 2003. Concentration and prevalence of Escherichia coli O157 in cattle feces at slaughter. Appl. Environ. Microbiol. 69:2444–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paterson D. L., Bonomo R. A. 2005. Extended-spectrum β-lactamases: a clinical update. Clin. Microbiol. Rev. 18:657–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perez F., Endimiani A., Hujer K. M., Bonomo R. A. 2007. The continuing challenge of ESBLs. Curr. Opin. Pharmacol. 7:459–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Randall L. P., et al. 2011. Prevalence of Escherichia coli carrying extended-spectrum β-lactamases (CTX-M and TEM-52) from broiler chickens and turkeys in Great Britain between 2006 and 2009. J. Antimicrob. Chemother. 66:86–95 [DOI] [PubMed] [Google Scholar]
- 33. Randall L. P., et al. 2009. Evaluation of CHROMagar CTX, a novel medium for isolating CTX-M-ESBL-positive Enterobacteriaceae while inhibiting AmpC-producing strains. J. Antimicrob. Chemother. 63:302–308 [DOI] [PubMed] [Google Scholar]
- 34. Rodríguez-Baño J., López-Cerero L., Navarro M. D., Díaz de Alba P., Pascual A. 2008. Faecal carriage of extended-spectrum β-lactamase-producing Escherichia coli: prevalence, risk factors and molecular epidemiology. J. Antimicrob. Chemother. 62:1142–1149 [DOI] [PubMed] [Google Scholar]
- 35. Rodríguez-Baño J., Pascual A. 2008. Clinical significance of extended-spectrum β-lactamases. Expert Rev. Anti-Infect. Ther. 6:671–683 [DOI] [PubMed] [Google Scholar]
- 36. Snary E. L., Kelly L. A., Davison H. C., Teale C. J., Wooldridge M. 2004. Antimicrobial resistance: a microbial risk assessment perspective. J. Antimicrob. Chemother. 53:906–917 [DOI] [PubMed] [Google Scholar]
- 37. Teale C. J., et al. 2005. Extended-spectrum beta-lactamase detected in E coli recovered from calves in Wales. Vet. Rec. 156:186–187 [PubMed] [Google Scholar]
- 38. VMD 2009. Guidance on the use of the cascade. Veterinary medicines guidance note 15. Veterinary Medicines Directorate, Surrey, United Kingdom: http://www.vmd.gov.uk/pdf/vmgn/VMGNote15.pdf [Google Scholar]
- 39. Wu S., et al. 2008. Detection of a single isolate of CTX-M-1-producing Escherichia coli from healthy pigs in Denmark. J. Antimicrob. Chemother. 61:747–749 [DOI] [PubMed] [Google Scholar]