Abstract
To determine whether the pathogenic Lyme disease spirochete Borrelia spielmanii is adapted exclusively to garden dormice, we compared the reservoir competence of various rodent species for this spirochete, including sympatric and peridomestic rodents. The different kinds of rodents varied in their attractiveness to nymphal ticks and their level of susceptibility to tick-borne B. spielmanii infection, but only the edible dormouse appeared to be refractory. Although hazel dormice and Norway rats became infectious to ticks somewhat later than did garden dormice, they remained infectious for a longer period of time. During the course of a tick season, garden and hazel dormice contributed theoretically more than twice as many B. spielmanii-infected ticks than the somewhat less susceptible Norway rats and wood or yellow-necked mice. Hazel dormice appeared to be extraordinarily competent as reservoir hosts for B. spielmanii. Because peridomestic rodents proved to be reservoir competent, urban foci of transmission of B. spielmanii may affect the health of townspeople.
INTRODUCTION
Borrelia spielmanii, a pathogenic Lyme disease spirochete transmitted by central European Ixodes ricinus ticks (5, 10, 28, 33, 34), seems closely associated with garden and hazel dormice (26). Particular Lyme disease genospecies appear to be more host specific than others. B. lusitaniae, for example, appears to thrive mainly in lizards (3, 24), whereas B. garinii and B. valaisiana are associated with birds. Rodents most commonly are infected by B. afzelii. Hosts that are reservoir competent for one Lyme disease genospecies may be zooprophylactic to another, because a tick that has acquired B. afzelii from a rodent during its larval blood meal may be cleared of these spirochetes when feeding as a nymph on a bird (11). B. burgdorferi sensu stricto, on the other hand, appears to be a generalist, as both birds and rodents serve as reservoir hosts (23, 25). The various Lyme disease genospecies differ in their host associations.
The geographical distribution of B. spielmanii appears to correspond to that of garden dormice. The range of these rodents may extend as far north as a latitude of 52° (30). In sites where garden dormice thrive, they may contribute numerous infected ticks to the vector population (15). They are abundant in each of the regions where B. spielmanii spirochetes were initially isolated: strain A14S from a patient in The Netherlands (33, 34), the type strain PC-Eq17N5 from the Petite Camargue Alsacienne in France (26, 28), strain I-77 from the Czech Republic (2), and DK35 from a Danish patient (28). The distribution of B. spielmanii is not ubiquitous in these regions but appears to be focal (4). Occasionally, B. spielmanii has been detected in ticks questing in sites where garden or hazel dormice are not expected to thrive, such as an inner-city park in Munich (4). However, in a transmission focus of B. spielmanii, this spirochete was found solely in ticks that had been feeding on dormice and not in those feeding on other sympatric rodents (26). It seems paradoxical that B. spielmanii is detected in ticks questing in an urban habitat that does not appear to be suitable for garden or hazel dormice.
To determine whether B. spielmanii is adapted exclusively to garden dormice, we compared the reservoir competence of various rodent species for this spirochete, including sympatric rodents and those that are most abundant in urban sites. Accordingly, we examined their relative susceptibility to B. spielmanii, the intrinsic incubation time, and the degree and duration of their infectivity to larval ticks. In addition, we observed whether replete ticks detach from garden dormice when they are active or at rest. Finally, we compared the theoretical efficiency with which they may contribute B. spielmanii-infected ticks.
(Portions of this research were conducted by D. B. Schlee in partial fulfillment of the requirements for a doctoral degree from the Freie Universität Berlin, Berlin, Germany.)
MATERIALS AND METHODS
All rodents were laboratory bred, except edible dormice, which were captured as juveniles. Garden and hazel dormice were first-generation descendants, and Norway rats and wood and yellow-necked mice were descendants between the fourth and sixth generation of animals that originally had been captured in the wild. House mice of the SKH-1 strain were used. All rodents were maintained at 21°C with a photophase commencing at 7:00 a.m. and a scotophase at 8:00 p.m. without twilight. They were fed standard laboratory chow; dormice and rats were provided with fruits every other day. The animal experiment was carried out in accordance with the guidelines approved by the Animal Care and Usage Committee of the city of Berlin, Germany.
To infect rodents with B. spielmanii and determine their infectivity for ticks, we permitted infected nymphal ticks that had engorged originally as larvae on experimentally infected garden dormice to feed on each host. Hosts were individually caged and exposed to ticks as previously described (17). For xenodiagnosis, noninfected larval ticks were permitted to attach to each animal at 2- or 4-week intervals. Resulting nymphs were tested for the presence of spirochetes by the amplification of a fragment of the gene encoding the outer surface protein A (OspA) (26) or by direct immunofluorescence using an affinity-purified fluorescein isothiocyanate (FITC)-labeled goat anti-Borrelia burgdorferi antibody (BacTrace; Kirkegaard & Perry Laboratories, Gaithersburg, MD).
Ticks used for xenodiagnosis were derived from laboratory-bred adult Ixodes ricinus ticks. Subadult and adult ticks were reared by feeding them on spirochete-free jirds (Meriones unguiculatus) and on rabbits (Oryctolagus cuniculus), respectively. These ticks were in their third generation of continuous laboratory rearing and had never been exposed to infected hosts. Because another Ixodes-borne spirochete, B. miyamotoi, may be transmitted transovarially (29), a portion of each larval cohort was routinely examined for the presence of spirochetes by the amplification of a fragment of the 16S rRNA gene (27).
To determine whether replete larval ticks detach from garden dormice when their hosts are active or at rest, we placed a grid underneath their experimental drop-off cages as described previously (12). For the detachment experiments, commercially available standard Macrolon cages (type III) were modified. A stainless steel wire box was suspended within each cage, and food and water were provided in separate stainless steel cups placed within the box. A white plastic grid subdivided the 750-cm2 floor of the cage into 350 open cubes, each measuring 13 mm on a side. Water was placed in each cage to a depth of 8 to 10 mm to immobilize detached ticks within the cube. A resting chamber was placed in the center of each wire box. Garden dormice were supplied with an aluminum resting chamber measuring 13 cm in length, 10 cm in width, and 8 cm in height and with an entrance hole of 45 mm in diameter. The base of the chamber covered 63 (7 by 9) cubes of the underlying plastic grid. The undersurface of each resting chamber was open, thereby permitting detached ticks to drop into the cubes of the grid. The experimental photophase was the same as that used for the maintenance of the rodents.
RESULTS
We first compared the relative attractiveness of various rodents for nymphal ticks and determined whether garden dormice (Eliomys quercinus) become more readily infected by tick-borne B. spielmanii spirochetes than do sympatric and peridomestic rodents. Nymphal ticks infected by B. spielmanii were permitted to attach to each of the various rodents. Based on previous experience, we chose the number of ticks according to the host species to ensure attachment and successful engorgement. Eight nymphs were applied per host in the case of house mice (Mus musculus) and as many as 45 nymphal ticks per host in the case of yellow-necked mice (Apodemus flavicollis) (Table 1). The infection rates of cohorts of infecting nymphs used had been determined beforehand and ranged between 78 and 95%. Virtually all nymphs attached to hazel dormice (Muscardinus avellanarius) and house mice and engorged successfully. Less than half of the nymphs attached to and fed to repletion on garden dormice and on Norway rats (Rattus norvegicus). Only a quarter of such ticks fed on wood mice (Apodemus sylvaticus), and few replete nymphs were recovered from edible dormice and yellow-necked mice. At least one infected tick engorged on each of the rodents, as verified by subsequent PCR. All garden and hazel dormice as well as all house mice exposed to tick-borne B. spielmanii subsequently were able to infect xenodiagnostic ticks. All but one wood mouse and two-thirds of the Norway rats acquired B. spielmanii spirochetes and became infectious for xenodiagnostic ticks, but only half of the yellow-necked mice did so. No edible dormice (Glis glis) appeared to infect xenodiagnostic ticks, and no spirochetal DNA was detected in skin, heart, liver, spleen, kidney, and urinary bladder obtained from them (data not shown). The different kinds of rodents varied in their attractiveness to nymphal ticks and their level of susceptibility to tick-borne B. spielmanii infection, but only the edible dormouse appeared to be refractory.
Table 1.
Attractiveness for nymphs and susceptibility to tick-borne Borrelia spielmanii of various rodents
| Host species | No. of hosts | Infecting nymphs |
% Hosts with ≥1 infected xenodiagnostic tick | ||
|---|---|---|---|---|---|
| No. permitted to attach | % Infecteda | % Recovered | |||
| Garden dormouse | 4 | 64 | 86.3 | 40.6 | 100.0 |
| Hazel dormouse | 6 | 76 | 91.7 | 96.1 | 100.0 |
| Edible dormouse | 5 | 127 | 78.0 | 14.2 | 0.0 |
| Wood mouse | 6 | 130 | 90.8 | 24.6 | 83.3 |
| Yellow-necked mouse | 4 | 180 | 80.0 | 7.8 | 50.0 |
| Norway rat | 6 | 72 | 95.0 | 41.7 | 66.6 |
| House mouse | 4 | 32 | 80.0 | 81.3 | 100.0 |
As determined for the cohort that was used for infection of rodent hosts.
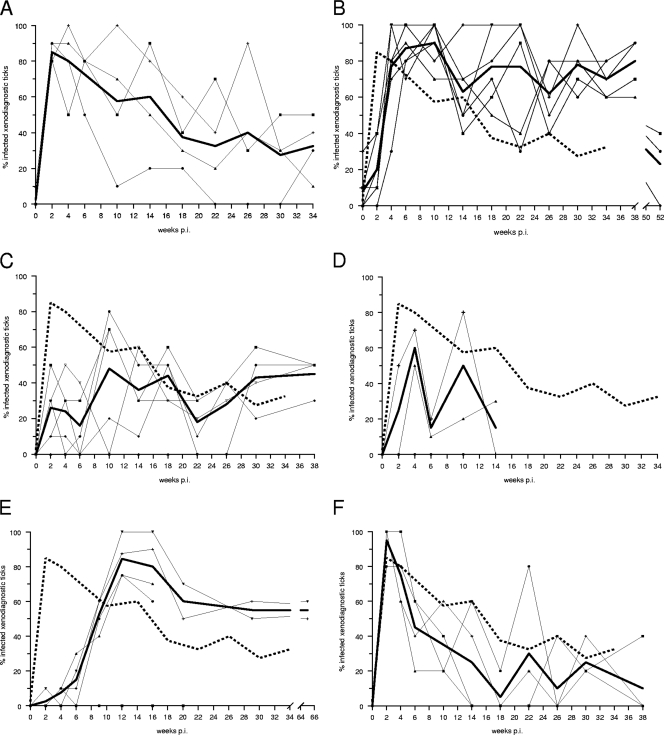
We then determined the relative degree and duration of infectivity of each rodent species susceptible to B. spielmanii infection during a period of several months by repeated xenodiagnosis and compared them to those of garden dormice. Generally, xenodiagnoses with noninfected larvae were performed biweekly until week 6 postinfection (p.i.) and thereafter in monthly intervals, except for xenodiagnoses of rats. For each individual and each xenodiagnosis, 10 resulting nymphs were analyzed for the presence of spirochetes, at least half of which were examined by OspA-PCR and the remaining ticks by immunofluorescence. For garden and hazel dormice, the first xenodiagnosis was performed as early as 3 days after exposure to infected nymphs. At this time, only a few larvae acquired spirochetes from either kind of host (Fig. 1A and B). Garden dormice reached their peak infectivity at the second xenodiagnosis, performed 2 weeks p.i. (Fig. 1A). An average of 85 and 80% of the larvae engorging 2 and 4 weeks p.i., respectively, acquired spirochetes. Thereafter, the infectivity of garden dormice waned gradually; however, at week 34 p.i. they still infected a third of the larvae feeding on them. Although hazel dormice infected only a fifth of the xenodiagnostic ticks at 2 weeks p.i. (Fig. 1B), their infectivity increased sharply by week 4 p.i. and reached a peak of 90% at week 10 p.i. Hazel dormice continued to infect a large proportion of xenodiagnostic ticks until week 38 p.i. One year after infection, these hosts still infected as many as a third of the ticks feeding on them. Virtually all larvae acquired B. spielmanii spirochetes in additional xenodiagnoses that were performed on one of the infected hazel dormice as late as 32 to 34 months p.i. (data not shown). Hazel dormice become highly infectious to ticks 2 weeks later than garden dormice (P = 0.0001; Fisher exact test), but they remain infectious far longer than garden dormice (P = 0.0006; Fisher exact test). Similarly to hazel dormice, wood mice infected only a quarter of the ticks feeding on them at 2 weeks p.i. (Fig. 1C). Wood mice reached their peak infectivity at week 10 p.i., when about half of the ticks acquired infection. Individual infectivity varied greatly over time, but the average infectivity remained between one- and two-fifths throughout the course of 10 xenodiagnoses until the last at week 38 p.i. Compared to that of garden dormice, the infectivity of wood mice differed mainly during the first 8 weeks after exposure to infected ticks, because at that time far fewer ticks acquired spirochetes from wood mice than from garden dormice (P = 0.0001; Fisher exact test). Only two of four yellow-necked mice were able to infect ticks feeding on them, reaching an average peak infectivity of 60% at week 4 p.i. (Fig. 1D). The infectivity of these mice ranged between 15 and 50%. Although a Norway rat became infectious as early as week 2 p.i., only one larva had acquired infection at that time. The rats' infectivity rose between week 6 and 12 p.i., reaching a peak level of 84% (Fig. 1E). These rodents continued to infect more than half of their xenodiagnostic ticks throughout the course of our study until week 65 p.i. Norway rats attained an infectivity comparable to that of garden dormice, but they attained it 2 months later than garden dormice (P = 0.0001; Fisher exact test). However, they subsequently infected a larger proportion of ticks than did garden dormice (P = 0.0001; Fisher exact test). Infected house mice reached their peak infectivity as early as 2 weeks p.i., infecting virtually all xenodiagnostic ticks feeding on them (Fig. 1F). Their infectivity, however, waned rapidly, and by week 14 p.i. only a quarter or fewer ticks, on average, acquired infection from these mice. Although hazel dormice and Norway rats became infectious to ticks somewhat later than did garden dormice, they remained infectious for a longer period of time, whereas wood, yellow-necked, and house mice never attained the level of infectivity of garden dormice.
Fig. 1.
Degree and duration of infectivity of various rodents infected with Borrelia spielmanii for xenodiagnostic larvae. Each thin line represents the infectivity of an individual host, the broad continuous line displays the mean infectivity, and the broken line displays the mean infectivity of garden dormice as a comparison. (A) Garden dormice; (B) hazel dormice; (C) wood mice; (D) yellow-necked mice; (E) Norway rats; (F) house mice.
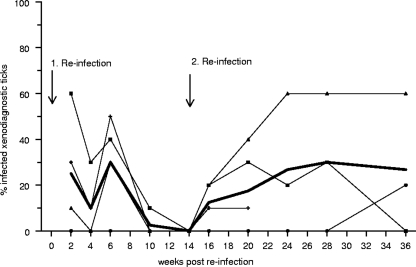
Because the infectivity of garden dormice for ticks waned over time, we examined their susceptibility to reinfection. Forty weeks after the initial exposure to infected ticks, garden dormice were exposed to tick-borne infection again. Their infectivity, however, failed to increase subsequently, as only about a quarter of xenodiagnostic ticks acquired spirochetes (Fig. 2). At 14 weeks after the second infection, garden dormice appeared to have lost their infectivity. At this point, we exposed the animals to infected ticks for a third time. They became infectious again and continued to infect about a quarter of their xenodiagnostic ticks throughout the subsequent 22 weeks. Upon reinfection, garden dormice fail to attain levels of infectivity that are comparable to those following their initial infection.
Fig. 2.
Degree and duration of infectivity of garden dormice upon two reinfections with tick-borne Borrelia spielmanii. Each thin line represents the infectivity of an individual host, and the broad line displays the mean infectivity.
To compare the relative role that each of the rodent species may have in contributing infected ticks to the population of questing ticks, we averaged the ratio of infected to noninfected ticks obtained throughout the several xenodiagnoses during a course of 26 weeks, roughly equaling the season of tick activity in central Europe, ranging from April/May through September/October. Because the susceptibility of the host to tick-borne infection factors in, data from animals that failed to become infectious for xenodiagnostic ticks were included in this analysis. Whereas more than half of the ticks feeding on garden dormice during the course of 6 months acquired B. spielmanii, hazel dormice infected about two-thirds of their ticks during this time span (Table 2). Somewhat more than a quarter of ticks acquired spirochetes when feeding on wood mice and Norway rats, and one-fifth of the ticks became infected when feeding on yellow-necked mice. Because each of the house mice became infectious, they infected almost half of the ticks feeding on them during the course of half a year. Only edible dormice appeared to fail to produce any infected ticks. Theoretically, garden and hazel dormice, but not edible dormice, contribute more than twice as many B. spielmanii-infected ticks as do wood or yellow-necked mice or Norway rats.
Table 2.
Efficiency of various rodents to infect larval Ixodes ricinus ticks with Borrelia spielmanii from week 2 through 26 p.i.
| Host species | No. of hosts | Xenodiagnostic ticks |
|
|---|---|---|---|
| No. examined | % Infected | ||
| Garden dormouse | 4 | 320 | 58.1 |
| Hazel dormouseb | 6 | 470 | 69.1 |
| Edible dormousea | 5 | 190 | 0.0 |
| Wood mousec | 6 | 450 | 26.2 |
| Yellow-necked mousea,d | 4 | 160 | 20.6 |
| Norway rate | 6 | 396 | 28.5 |
| House mouse | 4 | 160 | 40.0 |
Xenodiagnoses for edible dormice and yellow-necked mice were performed only until week 14 p.i.
Only five hazel dormice at week 26 p.i.
Only five wood mice at week 22 p.i. and only four mice at week 26 p.i.
Only two yellow-necked mice at week 14 p.i.
Only four Norway rats at week 20 p.i. and 2 rats at week 26 p.i.
To determine whether larval ticks feeding on garden dormice detach from them while their hosts are at rest within their nests or during their host's activity phase, we maintained each of eight garden dormice during a xenodiagnosis in a specially designed drop-off cage. Any tick detaching was immediately immobilized within one of the small water-filled cubes formed by a plastic grid underneath the drop-off cage. Of 879 detached larvae, 80% ± 7% larvae were recovered from the cubes directly underneath the dormouse's nest. Thus, most of the ticks feeding on dormice detached when and where their hosts were at rest.
DISCUSSION
A competent reservoir host must readily acquire spirochetes from an infected tick bite, it must maintain them for an extended period, and it must become infectious for ticks subsequently feeding on it (23). Reservoir competence must, therefore, be determined by transmission experiments in the laboratory. First, a reservoir host has to be attractive for nymphal ticks to allow for an infective tick bite. Because virtually all nymphal ticks applied to hazel dormice attached and engorged successfully, spirochetal input appears twice as likely for these hosts as for garden dormice. The outstanding attractiveness of hazel dormice for nymphal ticks seems surprising, as we had previously observed that the likelihood of nymphal attachment generally increases with the mammalian host's size (14). Hazel dormice weighing no more than 30 g are the smallest dormice tested in our study, whereas garden and edible dormice weigh as much as 180 and 280 g, respectively (30). In addition, hazel dormice infected with B. spielmanii also become more infectious, i.e., infect a larger proportion of larval ticks feeding on them, than do other rodents. Hazel dormice remain highly infectious for several years and possibly throughout their lives. Hazel dormice appear to be extraordinarily competent as reservoir hosts for B. spielmanii.
In contrast to hazel dormice, the infectivity of B. spielmanii-infected garden dormice decreases over time. After a tick season of about half a year, they infect only half the number of ticks that they infected when they first became infectious. Although garden dormice were susceptible to reinfection with the same spirochetes, attempts to boost their infectivity failed. This observation of a standardized experiment suggests that garden dormice are most infectious early in their lives when exposed to tick-borne B. spielmanii for the first time. Because larval ticks detach from garden dormice while they rest in their nest (15 and this study), their offspring may be exposed to infected ticks before they are old enough to leave the nest. In field observations, garden dormice infected virtually all larvae feeding on them with B. spielmanii, regardless of whether they had been captured or recaptured as late as 25 weeks later (26 and unpublished results). In nature, continuous exposure to tick-borne infections in an established transmission focus of B. spielmanii may result in a higher infectivity of these hosts.
The reservoir competence of dormice varies greatly among the three species thriving in Germany. B. spielmanii-infected hazel and garden dormice infect the majority of larval ticks feeding on them, whereas edible dormice exposed to tick-borne B. spielmanii appear not to contribute any such infected ticks. Recently, molecular phylogenetic analysis placed hazel dormice in the same subfamily as garden dormice, which is separated from edible dormice (7, 19, 22). Although the physiologic factors influencing reservoir competence for Lyme disease spirochetes are unknown, our observation appears to reflect the phylogenetic relationship of these dormice. Edible dormice, however, are not reservoir incompetent for all genospecies, because they proved highly competent for Lyme disease spirochetes in earlier experimental and field observations (13). At that time, Lyme disease spirochetes had not been differentiated into several genospecies, and thus it remains unknown which genospecies had infected these edible dormice. Of 13 edible dormice captured in southern Germany, about half were infected by spirochetes as determined by nested PCR amplifying a fragment of the 16S RNA gene (27) on skin samples and/or ticks feeding on them; B. afzelii accounted for more than 90% of the infections besides a single B. valaisiana and a single B. miyamotoi infection. Similarly, only B. afzelii spirochetes were isolated from urinary bladders obtained from Croatian edible dormice (32). Whereas garden dormice perpetuate both B. spielmanii and B. afzelii (26), only B. spielmanii has been associated with hazel dormice and only B. afzelii with edible dormice. Dormice appear to differ in their reservoir competence for particular spirochetal genospecies.
Except edible dormice, all rodents examined proved to be reservoir competent for B. spielmanii. Our experimental observation that wood and yellow-necked mice develop a low level of reservoir competence for B. spielmanii seems to contrast with earlier field observations (24), in which no such sympatric mice infected any larvae with this spirochete. In nature, only about 10 nymphs feed on an Apodemus mouse during the course of a year (13). Considering that approximately 3% of the questing nymphs in our field site harbored B. spielmanii, an Apodemus mouse theoretically would come into contact with a B. spielmanii-infected nymph only once in 3 years, which greatly exceeds their life span (20, 21). Because as many as 18 nymphs can infest a Norway rat at any one time (16), these rodents would receive a weekly to biweekly inoculation of B. spielmanii in a transmission focus, where every 30th tick is infected. Although peridomestic rodents, such as Norway rats and house mice, appear to be somewhat less efficient reservoir hosts than are hazel and garden dormice, their attractiveness to nymphs may be sufficient to perpetuate B. spielmanii in an urban environment.
Inner-city habitats that allow Ixodes ricinus ticks to thrive, such as parks, are often limited in size and may constitute ecological islands surrounded by built-up areas. Although the diversity of potential host species in an urban habitat is poorer than that present in a rural habitat, the population densities of synurbanized animals exceeds that of rural animals (9). Biodiversity and the loss thereof strongly influence pathogen transmission (8). We suspect that the composition of host animals in a particular urban setting supports the transmission of B. spielmanii, although these peridomestic hosts may not be as reservoir competent as are other reservoir hosts perpetuating this spirochete in rural sites. In addition, the vagility of Norway rats (1), bridging rural and urban environments, may help to establish urban foci of transmission of Lyme disease spirochetes (16) and particularly of B. spielmanii. In an inner-city park in Munich, B. spielmanii was detected as frequently as in every sixth infected adult tick (4). This spirochete was similarly prevalent in questing ticks in recreational areas in the town of Hannover (31). Recently, we even detected B. spielmanii in ticks questing in the backyard of an apartment building in downtown Berlin (unpublished data). Because B. spielmanii has been associated with erythema migrans in numerous patients across central Europe and is resistant to complement-mediated lysis, its pathogenicity for people is undisputed (5, 6, 10, 18, 33, 34). Because peridomestic rodents proved to be reservoir competent, urban foci of transmission of B. spielmanii may affect the health of townspeople.
ACKNOWLEDGMENTS
This study was supported by grants Ma 942/10-1 and 942/10-2 from the Deutsche Forschungsgemeinschaft.
We thank Rainer Allgöwer for providing laboratory-bred hazel and garden dormice and for capturing edible dormice.
Footnotes
Published ahead of print on 1 April 2011.
REFERENCES
- 1. Becker K. 1978. Rattus norvegicus (Berkenhout, 1769)-Wanderratte, p. 401–416 In Niethammer J., Krapp F. (ed.), Handbuch der Säugetiere Europas, vol. 1/I Akademische Verlagsgesellschaft, Wiesbaden, Germany [Google Scholar]
- 2. Derdáková M., Beati L., Pet'ko B., Stanko M., Fish D. 2003. Genetic variability within Borrelia burgdorferi sensu lato genospecies established by PCR-single-strand conformation polymorphism analysis of the rrfA-rrlB intergenic spacer in Ixodes ricinus ticks from the Czech Republic. Appl. Environ. Microbiol. 69:509–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dsouli N., et al. 2006. Reservoir role of lizard Psammodromus algirus in transmission cycle of Borrelia burgdorferi sensu lato (spirochaetaceae) in Tunisia. J. Med. Entomol. 43:737–742 [DOI] [PubMed] [Google Scholar]
- 4. Fingerle V., et al. 2008. Epidemiological aspects and molecular characterization of Borrelia burgdorferi sl. from southern Germany with special respect to the new species Borrelia spielmanii sp. nov. Int. J. Med. Microbiol. 298:279–290 [DOI] [PubMed] [Google Scholar]
- 5. Földvári G., Farkas R., Lakos A. 2005. Borrelia spielmanii erythema migrans, Hungary. Emerg. Infect. Dis. 11:1794–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hammerschmidt P., et al. 2007. Human pathogenic Borrelia spielmanii sp. nov. resists complement-mediated killing by direct binding of immune regulators factor H and factor H-like protein 1. Infect. Immun. 75:4817–4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holden M. E. 2005. Family Gliridae, p. 819–841 In Wilson D. E., Reeder D. M. (ed.), Mammalian species of the world: a taxonomic and geographic reference, vol. 1 Johns Hopkins University Press, Baltimore, MD [Google Scholar]
- 8. Keesing F., et al. 2010. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468:647–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luniak M. 2004. Synurbanization–adaptation of animal wildlife to urban development, p. 50–55 In Shaw W. W., Harris L. K., VanDruff L. (ed.), Proceedings of the 4th International Symposium on Urban Wildlife Conservation School of Natural Resources, College of Agriculture and Life Sciences, University of Arizona, Tucson, AZ [Google Scholar]
- 10. Maraspin V., Ruzic-Sabljic E., Strle F. 2006. Lyme borreliosis and Borrelia spielmanii. Emerg. Infect. Dis. 12:1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matuschka F.-R., Spielman A. 1992. Loss of Lyme disease spirochetes from Ixodes ricinus ticks feeding on European blackbirds. Exp. Parasitol. 74:151–158 [DOI] [PubMed] [Google Scholar]
- 12. Matuschka F.-R., Richter D., Spielman A. 1991. Differential detachment from resting hosts of replete larval and nymphal Ixodes ticks. J. Parasitol. 77:341–345 [PubMed] [Google Scholar]
- 13. Matuschka F.-R., Eiffert H., Ohlenbusch A., Spielman A. 1994. Amplifying role of edible dormice in Lyme disease transmission in central Europe. J. Infect. Dis. 170:122–127 [DOI] [PubMed] [Google Scholar]
- 14. Matuschka F.-R., Fischer P., Heiler M., Richter D., Spielman A. 1992. Capacity of European animals as reservoir hosts for the Lyme disease spirochete. J. Infect. Dis. 165:479–483 [DOI] [PubMed] [Google Scholar]
- 15. Matuschka F.-R., Allgöwer R., Spielman A., Richter D. 1999. Characteristics of garden dormice that contribute to their capacity as reservoirs for Lyme disease spirochetes. Appl. Environ. Microbiol. 65:707–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matuschka F.-R., et al. 1996. Risk of urban Lyme disease enhanced by the presence of rats. J. Infect. Dis. 174:1108–1111 [DOI] [PubMed] [Google Scholar]
- 17. Matuschka F.-R., Endepols S., Richter D., Spielman A. 1997. Competence of urban rats as reservoir hosts for Lyme disease spirochetes. J. Med. Entomol. 34:489–493 [DOI] [PubMed] [Google Scholar]
- 18. Michel H., et al. 2004. An ospA-polymerase chain reaction/restriction fragment length polymorphism-based method for sensitive detection and reliable differentiation of all European Borrelia burgdorferi sensu lato species and OspA types. Med. Microbiol. Immunol. 193:219–226 [DOI] [PubMed] [Google Scholar]
- 19. Montgelard C., Matthee C. A., Robinson T. J. 2003. Molecular systematics of dormice (Rodentia: Gliridae) and the radiation of Graphiurus in Africa. Proc. R. Soc. Lond. B 270:1947–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Niethammer J. 1978. Apodemus flavicollis (Melchior, 1834)-Gelbhalsmaus, p. 325–336 In Niethammer J., Krapp F. (ed.), Handbuch der Säugetiere Europas, vol. 1/I Akademische Verlagsgesellschaft, Wiesbaden, Germany [Google Scholar]
- 21. Niethammer J. 1978. Apodemus sylvaticus (Linnaeus, 1758)-Waldmaus, p. 337–358 In Niethammer J., Krapp F. (ed.), Handbuch der Säugetiere Europas, vol. 1/I Akademische Verlagsgesellschaft, Wiesbaden, Germany [Google Scholar]
- 22. Nunome M., Yasuda S. P., Sato J. J., Vogel P., Suzuki H. 2007. Phylogenetic relationships and divergence times among dormice (Rodentia, Gliridae) based on three nuclear genes. Zoologica Scripta 36:537–546 [Google Scholar]
- 23. Richter D., Spielman A., Komar N., Matuschka F.-R. 2000. Competence of American robins as reservoir hosts for Lyme disease spirochetes. Emerg. Infect. Dis. 6:133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Richter D., Matuschka F.-R. 2006. Perpetuation of the Lyme disease spirochete Borrelia lusitaniae by lizards. Appl. Environ. Microbiol. 72:4627–4632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Richter D., Klug B., Spielman A., Matuschka F.-R. 2004. Adaptation of diverse Lyme disease spirochetes in a natural rodent reservoir host. Infect. Immun. 72:2442–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Richter D., Schlee D. B., Allgöwer R., Matuschka F.-R. 2004. Relationships of a novel Lyme disease spirochete, Borrelia spielmani sp. nov., with its hosts in central Europe. Appl. Environ. Microbiol. 70:6414–6419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Richter D., Schlee D. B., Matuschka F.-R. 2003. Relapsing fever-like spirochetes infecting European vector tick of Lyme disease agent. Emerg. Infect. Dis. 9:697–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Richter D., et al. 2006. Delineation of Borrelia burgdorferi sensu lato species by multilocus sequence analysis and confirmation of the delineation of Borrelia spielmanii sp. nov. Int. J. Syst. Evol. Microbiol. 56:873–881 [DOI] [PubMed] [Google Scholar]
- 29. Scoles G. A., Papero M., Beati L., Fish D. 2001. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector-Borne Zoonotic Dis. 1:21–34 [DOI] [PubMed] [Google Scholar]
- 30. Storch G. 1978. Familie Gliridae Thomas, 1897-Schläfer, p. 201–280 In Niethammer J., Krapp F. (ed.), Handbuch der Säugetiere Europas. Akademische Verlagsgesellschaft, Wiesbaden, Germany [Google Scholar]
- 31. Strube C., Montenegro V. M., Epe C., Eckelt E., Schnieder T. 2010. Establishment of a minor groove binder-probe based quantitative real time PCR to detect Borrelia burgdorferi sensu lato and differentiation of Borrelia spielmanii by ospA-specific conventional PCR. Parasites Vectors 3:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Turk N., et al. 2008. The role of fat dormouse (Glis glis L.) as reservoir host for spirochete Borrelia burgdorferi sensu lato in the region of Gorski Kotar, Croatia. Eur. J. Wildl. Res. 54:117–121 [Google Scholar]
- 33. van Dam, et al. 1993. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin. Infect. Dis. 17:708–717 [DOI] [PubMed] [Google Scholar]
- 34. Wang G., van Dam A. P., Dankert J. 1999. Phenotypic and genetic characterization of a novel Borrelia burgdorferi sensu lato isolate from a patient with Lyme borreliosis. J. Clin. Microbiol. 37:3025–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]