Abstract
Xanthomonas oryzae pv. oryzicola, the causative agent of bacterial leaf streak, injects a plethora of effectors through the type III secretion system (T3SS) into rice cells to cause disease. The T3SS, encoded by the hrp genes, is essential for the pathogen to elicit the hypersensitive response (HR) in nonhost tobacco and for pathogenicity in host rice. Whether or not a putative lytic transglycosylase, Hpa2, interacts with a translocon protein, HrpF, to facilitate bacterial pathogenicity remains unknown. Here we demonstrated that both the hpa2 and hrpF genes are required for the pathogenicity of X. oryzae pv. oryzicola strain RS105 in rice but not for HR induction in tobacco. The expression of hpa2 was positively regulated by HrpG and HrpD6 but not by HrpX. In vivo secretion and subcellular localization analyses confirmed that Hpa2 secretion is dependent on HpaB (a T3SS exit protein) and that Hpa2 binds to the host cell membrane. Protein-protein assays demonstrated that Hpa2 interacts with HrpF. In planta translocation of AvrXa10 indicated that the mutation in hpa2 and hrpF inhibits the injection of the HpaB-dependent transcriptional activator-like (TAL) effector into rice. These findings suggest that Hpa2 and HrpF form a complex to translocate T3S effectors into plant cells for pathogenesis in host rice.
INTRODUCTION
Xanthomonas oryzae pv. oryzicola, the causative agent of bacterial leaf streak disease in rice, is one of the model organisms for studying the molecular mechanisms of plant-pathogen pathosystems (41, 59). The bacterial ability to trigger the hypersensitive response (HR), a rapid and localized programmed cell death in nonhosts or in resistant hosts, and to be pathogenic in host plants depends on a type III secretion system (T3SS) encoded by a 27-kb hrp cluster containing 10 hrp, 9 hrc (hrp-conserved), and 8 hpa (hrp-associated) genes according to the homologous regions in other Xanthomonas species (9, 41, 59). Some of the hrp-hrc-hpa gene products comprise a pedestal-like T3SS structure that traverses the two bacterial membranes (21, 24), a pilus-like secretion channel (HrpE) outside HrcC (52), and a translocon protein (HrpF) in the eukaryotic host membrane (3, 4, 6, 21, 48). As a whole, the T3SS apparatus injects a number of effectors into the apoplast and cytosol of eukaryotic host cells, including harpins, which elicit HR induction in nonhost apoplasts (4, 45, 58), and transcriptional activator-like (TAL) effectors, which lead to disease susceptibility in hosts or trigger disease resistance in nonhosts upon interaction with a specific R gene product surveillance system (4, 11, 38, 39, 40, 49, 56). The virulence of Xanthomonas oryzae pv. oryzae is markedly reduced when hrpF is mutated (48). However, hrpF mutation has not been investigated in X. oryzae pv. oryzicola.
The hpa genes contribute to virulence, but strains with mutations in hpa genes generally do not exhibit phenotypic changes in disease symptoms of the same severity as those with other hrp-hrc gene mutations (9, 25, 29). Some Hpa proteins, such as HpaB and HpaC from Xanthomonas campestris pv. vesicatoria and T3SS exit proteins that promote the secretion of a large set of effectors and prevent the delivery of noneffectors into the plant cell, are indispensable for plant-pathogen interactions (5, 19). In X. oryzae pv. oryzae, diverse phenotypes displayed in rice by hpa gene mutants suggest that Hpa proteins have distinct individual functions (9).
hpa2 is the first gene upstream of the core hrp cluster, beyond the hrpA operon of X. oryzae pv. oryzicola (59), and similar gene arrangements are also found in the genomes of X. oryzae pv. oryzae (57), X. campestris pv. vesicatoria (43), and Xanthomonas axonopodis pv. glycines (29). Unfortunately, the roles of the hpa2 homologs of these Xanthomonas species in their proliferation in host tissues, virulence in host plants, and HR induction in nonhosts are inconsistent with each other (7, 29, 43, 57, 58). The predicted Hpa2 protein, which belongs to the lysozyme-like family of proteins, is proposed to dissolve or disrupt the bacterial cell wall (31, 57) and is homologous to VirB1 in Agrobacterium tumefaciens, which promotes the formation of the type IV secretion system (T4SS) (39). However, there is no evidence to demonstrate either that Hpa2 is secreted through the T3SS, where it localizes within the plant, or with which proteins it interacts.
The expression of hrp genes is induced in planta and in the hrp-inducing medium XOM3 (55) and is controlled by two key regulatory genes, hrpG and hrpX, located outside the hrp gene cluster (59). Commonly, the expression of the hrpA operon and hrpX is activated by an OmpR family member, HrpG (42, 54). HrpX, an AraC-type transcriptional activator, controls the expression of operons hrpB to hrpF, which are located downstream of genes encoding T3S effectors (53). Many HrpX regulons possess a plant-inducible promoter (PIP) box 30 to 32 bp upstream of a conserved −10 box-like sequence (13, 14, 50). Since there is an imperfect PIP box in the hpa2 promoter region of X. oryzae pv. oryzicola (59), it is not clear whether hpa2 is regulated by HrpX.
Having investigated the questions discussed above, we adduce evidence that the loss of pathogenicity of an X. oryzae pv. oryzicola hpa2 hrpF double mutant in susceptible rice is consistent with a model in which a lack of Hpa2-HrpF complex formation prevents the translocation of HpaB-dependent TAL effectors into plants.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table S1 in the supplemental material. The wild-type X. oryzae pv. oryzicola strain RS105, X. oryzae pv. oryzae PXO99A, and other Xanthomonas strains were grown on NA (0.5% peptone, 0.1% yeast, 1% sucrose, 0.3% beef extract, and 1.5% agar) or NB (NA without agar) medium at 28°C. Escherichia coli and Agrobacterium tumefaciens strains were grown in Luria-Bertani medium at 37°C and 28°C, respectively (36). The hrp-inducing medium for X. oryzae strains is XOM3 (d-xylose,1.8 g/liter; d,l-methionine, 670 μM; sodium l-glutamate, 10 mM; NaFe2+-EDTA, 240 μM; MgCl2, 5 mM; KH2PO4, 14.7 mM; MnSO4, 40 μM [pH 6.0]) (55). Yeast strains were grown in YPD medium (Clontech), and the positive yeast clones for the yeast two-hybrid (Y2H) system were screened on selective dextrose (SD) medium lacking adenine, leucine, tryptophan, and histidine (SD/-Ade/-Leu/-Trp/-His) and on SD/-Leu/-Trp/-His at 30°C. Onion tissue was cultured on 1/2 MS medium (0.22% Murashige and Skoog basal medium, 0.05% morpholineethanesulfonic acid [MES], and 1.5% agar) at 28°C in the dark. Antibiotics were used at the following concentrations: ampicillin (Ap), 100 μg/ml; kanamycin (Km), 50 μg/ml; rifampin (Rif), 50 μg/ml; spectinomycin (Sp), 100 μg/ml.
DNA manipulation and plasmid construction.
DNA isolation, restriction enzyme digestion, subcloning, electrotransformation, PCR, Southern blotting, and Western immunoblotting were performed according to standard procedures (47). The PCR primers for the genes discussed in this report are listed in Table S2 in the supplemental material. The PCR products were first cloned into the pMD18-T vector (TaKaRa, Dalian, China) and were then verified by sequencing. DNA sequences were analyzed with VECTOR NTI software (Invitrogen).
To construct an hpa2 promoter–β-glucuronidase (GUS) fusion construct, the entire promoter region (bp −1 to −216) upstream of the hpa2 open reading frame (ORF) was amplified from the genomic DNA of X. oryzae pv. oryzicola RS105 with primers phpa2-F and phpa2-R (see Table S2 in the supplemental material), and the product was then fused with the gusA gene (37), which was amplified with primers gusA-F and gusA-R (see Table S2). The fusion was then cloned into pUFR034 (10) at the EcoRI site, giving phpa2GUS (see Table S1).
To generate Hpa2 with a c-Myc tag, hpa2 with its native promoter (−1 to −216 bp upstream) was amplified from the genomic DNA of X. oryzae pv. oryzicola RS105 by PCR with the Hpa2-F/Hpa2Myc-R primer set (see Table S2 in the supplemental material). The amplified PCR product was then cloned into pUFR034 at the EcoRI and KpnI sites in frame with a c-Myc epitope-encoding sequence, generating pHpa2-c-Myc (see Table S1).
To detect the interaction between the HrpF and Hpa2 proteins by the Y2H system, the hpa2 open reading frame was amplified from the genomic DNA of strain RS105 with the hpa2-F1/hpa2-R1 primer set (see Table S2 in the supplemental material) and was ligated into pGADT-7 and pGBDT-7 at the EcoRI and BamHI sites, giving pAHpa2 and pBHpa2 (see Table S1), respectively. Similarly, the hrpF gene was amplified with the hrpF-F1/hrpF-R1 primer set (see Table S2) and was cloned into pGADT-7 and pGBDT-7 at the NdeI sites, giving pAHrpF and pBHrpF (see Table S1), respectively.
To express the c-Myc-tagged HrpF protein, the hrpF open reading frame was c-Myc tagged at the 3′ terminus by PCR amplification from the genomic DNA of strain RS105 with the HrpFMyc-F/HrpFMyc-R primer set (see Table S2 in the supplemental material). The product was then cloned into the pET30a (+) vector at the NdeI and EcoRV sites, giving pHrpF-c-Myc (see Table S1). To express the glutathione S-transferase (GST)-tagged Hpa2 protein, the entire hpa2 ORF without its native stop codon was PCR amplified from RS105 genomic DNA with the hpa2-F2/hpa2-R2 primer set (see Table S2) and was then cloned into the pET41a (+) vector at BamHI and XhoI, giving pGST-Hpa2 (see Table S1).
To construct the binary vector expressing the Hpa2-YN fusion, we first used PCR with primers hpa2-F3 and hpa2-R3 (see Table S2 in the supplemental material) to amplify the full-length Hpa2 coding sequence without the native stop codon and then fused this sequence upstream of the N terminus of the yellow fluorescent protein (YFP) sequence, which encodes the N-terminal portion of YFP (amino acids [aa] 1 to 155), at the BamHI and KpnI sites, giving pHpa2-YN (see Table S1). Similarly, to construct the binary vector expressing the HrpF-YC fusion, the full-length HrpF coding sequence without the stop codon was amplified with the hrpF-F2/hrpF-R2 primer set (see Table S2) and was fused upstream of the C terminus of the YFP sequence, which encodes the C-terminal portion of YFP (aa 156 to 239), at the XbaI and KpnI sites, giving pHrpF-YC (see Table S1).
To express Hpa2 in onion epidermal cells, the entire hpa2 gene was amplified with the hpa2-F4/hpa2-R4 primer set and was cloned into the pA-GFP vector with XhoI and SpeI, giving pHpa2-GFP. All plasmid constructs were verified by sequencing.
Mutagenesis of the hpa2 and hrpF genes.
To generate nonpolar mutations in the hpa2 and hrpF genes of X. oryzae pv. oryzicola strain RS105, upstream and downstream flanking fragments of hpa2 were amplified from RS105 genomic DNA with the hpa2I-F/hpa2I-R and hpa2II-F/hpa2II-R primer sets (see Table S2 in the supplemental material), respectively, and were then cloned into pMD18-T vectors (TaKaRa, Dalian, China). After the upstream and downstream fragments were digested by BamHI and XbaI and by XbaI and SalI, respectively, the two fragments were cloned into the suicide vector pKMS1 (27) at the BamHI and SalI sites, giving pKΔhpa2 (see Table S1). Correspondingly, the upstream and downstream flanking fragments of hrpF were PCR amplified with the hrpFI-F/hrpFI-R and hrpFII-F/hrpFII-R primer sets (see Table S2), respectively, and were then cloned into pKMS1 at the BamHI and SphI sites, resulting in pKΔhrpF (see Table S1). These constructs were introduced into X. oryzae pv. oryzicola RS105, and then single mutants of hpa2 and hrpF were isolated according to the procedure described by Jiang et al. in 2009 (27). Then pKΔhpa2 was transformed into the RΔhrpF mutant for the construction of a double mutant, RΔhpa2ΔhrpF, by following the same procedure. The mutants were verified by PCR amplification with the hpa2I-F/hpa2II-R and hrpFI-F/hrpFII-R primer sets (see Table S2) and by Southern blotting with the probes of the hpa2 and hrpF genes, respectively. By following the same procedure, the single and double mutants of hpa2 and hrpF in strain PXO99A (22) of X. oryzae pv. oryzae, the causal agent of rice bacterial blight, were generated and named PΔhpa2, PΔhrpF, and PΔhpa2ΔhrpF, respectively (see Table S1).
Pathogenicity and HR assays.
Hypersensitive response and pathogenicity assays were performed as described previously (59). X. oryzae and its derivatives were assessed for their abilities to cause disease symptoms and to multiply in IR24 and IRBB10 rice (Oryza sativa subsp. indica) plants by inoculation of rice seedlings (2 weeks old) by use of needleless syringes and of adult rice (2 months old) by leaf needling for X. oryzae pv. oryzicola strains and by leaf clipping for X. oryzae pv. oryzae strains with bacterial suspensions that were adjusted to a concentration of 3 × 108 CFU/ml. IR24 is susceptible to RS105 and PXO99A. IRBB10, containing the Xa10 gene, is resistant to X. oryzae pv. oryzae strains if they carry a matching avrXa10 gene (28, 56). Xanthomonad strains were tested for their abilities to elicit an HR on Nicotiana benthamiana by infiltration of plant tissue using needleless syringes with strains adjusted to a concentration of 3 × 108 CFU/ml. Plant responses were scored 24 h postinoculation for the HR in tobacco, 3 days postinoculation (dpi) for water-soaking symptoms in rice seedlings, and 14 dpi for lesion lengths. Leaves that became brown in the infiltrated area instead of water soaked indicated HR in rice (56). All plants were grown in growth chambers at 25°C with a 12-h photoperiod. Experiments were repeated at least three times.
Measurement of bacterial growth ability in rice.
Cell suspensions of RS105 strains adjusted to a concentration of 3 × 108 CFU/ml were infiltrated into recently expanded leaves of 2-week-old IR24 rice with needleless syringes at three spots on each leaf. Three 0.8-cm-diameter leaf discs were harvested with a cork borer from each area after infiltration. After sterilization in 70% ethanol and 30% hypochlorite, the discs were ground with a sterile mortar and pestle into 1 ml of distilled water, diluted, and plated to determine the CFU/cm2. Serial dilutions were spotted in triplicate onto NA plates with appropriate antibiotics. Plates were incubated at 28°C for 3 to 4 days until single colonies could be counted. The number of bacterial CFU per square centimeter of leaf area was then estimated, and the standard deviation was calculated using colony counts from the three triplicate spots from each of the three samples per time point per inoculum. Experiments were repeated at least three times.
Promoter activity assays and reverse transcription-PCR (RT-PCR) analysis.
For GUS activity assays, X. oryzae pv. oryzicola strain RS105 and hrp mutants were cultured in XOM3 to an optical density at 600 nm (OD600) of 0.5. Bacterial cells were diluted and disrupted in sonic buffer (20 mM Tris-HCl [pH 7.0], 10 mM 2-mercaptoethanol, 5 mM EDTA, and 1% Triton X-100). GUS activities were determined at 30-min intervals for 3 h by measuring absorbance at 415 nm (A415) using p-nitrophenyl-d-glucuronide as the substrate (26). One unit was defined as 1 nmol of 4-methyl-umbelliferone produced per min per bacterium.
For RT-PCR analysis, the bacteria were cultured as described for the GUS activity assay. Total RNA was extracted by using the Trizol reagent according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Total RNAs were quantified by measuring the OD260/OD280 ratio and were analyzed by gel electrophoresis. Before synthesis of the first strands, total RNAs were treated with RNase-free DNase I (TaKaRa, Dalian, China) to remove potential traces of genomic DNAs. To confirm the removal of contaminating DNA, extracted RNAs were used as templates to amplify the target genes discussed in this report with the primers listed in Table S2 in the supplemental material. cDNA synthesis and PCR were conducted with AMV and Ex Taq DNA polymerases (both from TaKaRa, Dalian, China) with primers hpa2-F and hpa2-R. PCR was performed with a cycler using the following cycle parameters: 36 cycles of 94°C for 35 s, 52°C for 35 s, and 72°C for 20 s. The resulting amplification products were analyzed in 1.2% agarose gels. The 16S rRNA gene of X. oryzae pv. oryzicola was used as the internal control to verify the absence of significant variation at the cDNA level in three samples.
Type III secretion assays.
To detect the secretion of T3S effectors through the type III system, X. oryzae pv. oryzicola strains were preincubated in NB medium to logarithmic phase. Bacterial cells were harvested, adjusted to an OD600 of 2.0 with sterilized water, and washed twice. Then 40 μl of the bacterial suspension was inoculated into 1 ml of modified XOM3 (55) and was incubated at 28°C for 16 h. Cell and supernatant fractions were separated by centrifugation, and the protein in the supernatant fraction was precipitated with 12.5% trichloroacetic acid (33). Proteins were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and were transferred to membranes for immunoblotting using anti-c-Myc primary antibodies (Genescript, Nanjing, China). Primary antibodies were recognized by anti-rabbit secondary antibodies (Genescript, Nanjing, China) and were visualized on autoradiographs with the Western-Light chemiluminescence system (Transgene, Beijing, China).
Y2H and β-gal assays.
Yeast two hybrid assays were conducted according to the manufacturer's instructions (Clontech, CA). Construct pairs consisting of pAHpa2 and pBHrpF or of pBHpa2 and pAHrpF (see Table S1 in the supplemental material), either in a prey vector or in a bait vector, were transformed into yeast strain AH109 in order to examine whether Hpa2 interacts with HrpF. The positive clones on SD/-Ade/-Leu/-Trp/-His/ were confirmed by a β-galactosidase (β-gal) assay based on the manufacturer's (Clontech, CA) manual (12).
GST pulldown assays.
GST pulldown assays were performed as described previously (5). pGST-Hpa2 and pHrpF-c-Myc were expressed in E. coli BL21(DE3). Similarly, GST was expressed from the pET41a (+) vector (Novagen, WI). Bacterial cells from 20 ml of cultures were resuspended in 2 ml phosphate-buffered saline (PBS) and were broken by sonication. Insoluble cell debris was removed by centrifugation, and soluble proteins were immobilized on a glutathione resin according to the manufacturer's instructions (Genescript, Nanjing, China). A total of 30 μl of GST lysate and 400 μl of GST-Hpa2 lysate were incubated with 50 μl of resin according to protein stabilities and/or expression levels. Unbound E. coli proteins were removed by two washes with PBS. After incubation with 600 μl of the HrpF-c-Myc lysate for 1 h at 37°C, unbound proteins were removed by centrifugation, and the resin was washed four times with PBS containing 1% Triton X-100. HrpF-c-Myc was eluted with 30 μl of 10 mM reduced glutathione at room temperature for 2 h. Three microliters of total-protein lysates and 10 μl of a solution of eluted proteins were analyzed by SDS-PAGE and Western blotting using anti-c-Myc and anti-GST antibodies.
BiFC assays.
For bimolecular fluorescence complementation (BiFC) assays, plasmids were transferred into Agrobacterium tumefaciens GV3101 by a freeze-thaw method (23). For infiltration, 50-ml cultures of Agrobacterium grown in LB broth supplemented with 10 mM MES and 20 mM acetosyringone were harvested, washed, and resuspended in a solution containing 10 mM MgCl2, 10 mM MES, and 100 mM acetosyringone. Tobacco (N. benthamiana) leaves from 3-month-old plants were used for infiltration. Five plants (two leaves per plant) were infiltrated per experiment. After 12 to 16 h, Bisbenzimide H was infiltrated into tobacco leaves. Four hours later, YFP fluorescence in tobacco leaves was observed and imaged under a fluorescence microscope (Olympus IX71). The excitation wavelength used for YFP was 488 nm, and the emission filter wavelength was 520 to 550 nm. For visualization, tobacco leaf pieces were mounted directly onto glass slides in a drop of water. For each experiment, at least 10 different samples were examined under the microscope. Experiments were repeated three times.
Subcellular localization of the Hpa2-GFP fusion protein.
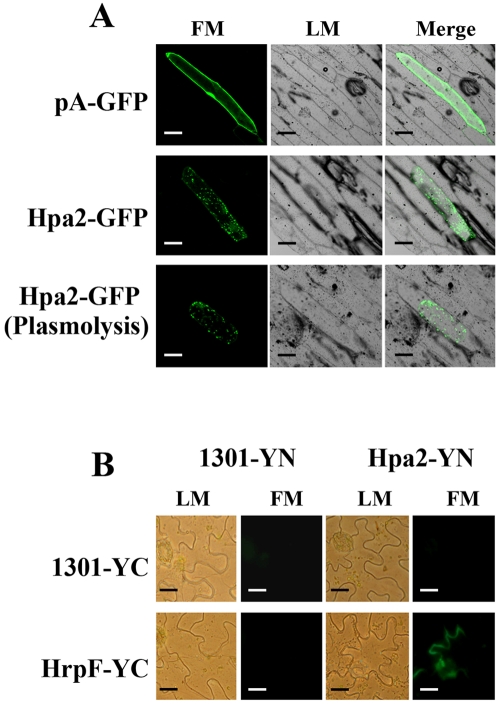
For subcellular localization, the full-length Hpa2 coding region was inserted into a pA-GFP vector, which generated a C-terminal fusion with the green fluorescent protein (GFP) gene under the control of the double cauliflower mosaic virus (CaMV) 35S promoter. pA-GFP (control) and pHpa2-GFP were transiently expressed in onion epidermal cells following transformation with a biolistic particle delivery system (PDS-1000; Bio-Rad), using gold particles coated with column-purified (Axygen) plasmid DNA, according to the manufacturer's instructions. Onion cells were cultured for 48 h on 1/2 MS medium and were subsequently bombarded under a slight vacuum using a helium pressure of 1,100 lb/in2. After bombardment, plates were incubated at 28°C for 24 h. The subcellular localization of the Hpa2-GFP fusion protein was observed using an MRC-1024 confocal laser scanning microscope (Bio-Rad, CA).
RESULTS
Hpa2, together with HrpF, is essential for the pathogenicity of X. oryzae pv. oryzicola in rice but not for the HR in tobacco.
The predicted Hpa2 protein in X. oryzae pv. oryzicola RS105 contains an N-terminal signal peptide of 34 amino acids and is almost identical to Hpa2 from X. oryzae pv. oryzae (98% identity; 97% similarity) (59) and to HpaH (91% identity; 89% similarity) from X. campestris pv. vesicatoria (43). These proteins belong to the lytic transglycosylase family, which includes VirB1 in A. tumefaciens, which promotes T4SS formation (39), and HrpH and HopP1 in Pseudomonas syringae pv. tomato DC3000; the latter suppresses pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) in plants (44). It has been shown that the formation of a T3SS and efficient translocation of XopF1 are affected by the lysozyme-like HpaH in X. campestris pv. vesicatoria (7), and X. oryzae pv. oryzae Hpa2 has lytic activity against bacterial cell walls (57). These reports prompted us to investigate whether Hpa2 may work together with the translocator HrpF for bacterial pathogenicity in rice. To determine the role of hpa2 in the pathogenesis of X. oryzae pv. oryzicola in rice, we deleted hpa2, hrpF, or both the hpa2 and hrpF genes in the wild-type strain RS105 to generate the single mutants RΔhpa2 and RΔhrpF and the double mutant RΔhpa2ΔhrpF, respectively (see Table S1 in the supplemental material).
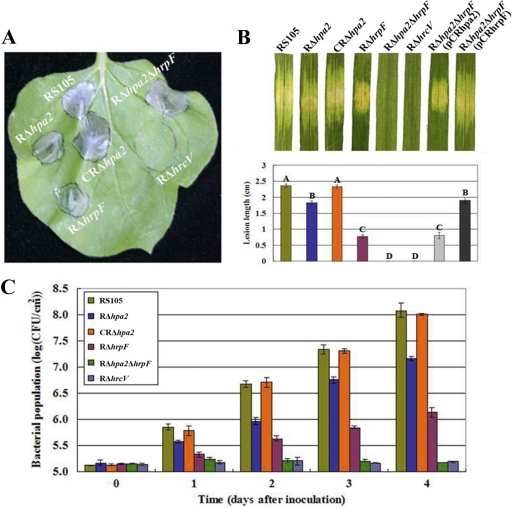
To analyze HR induction, nonhost tobacco (N. benthamiana) leaves were inoculated by infiltrating bacterial suspensions adjusted to a concentration of 3 × 108 CFU/ml. The results of the HR assay showed that RΔhpa2, RΔhrpF, and RΔhpa2ΔhrpF elicited HR symptoms similar to those induced by the wild-type strain, while a T3SS-deficient mutant, RΔhrcV (see Table S1 in the supplemental material), used as a control, did not trigger the HR (Fig. 1A). This result demonstrated that Hpa2 and HrpF are not involved in HR induction in nonhost tobacco and that the secretion of the HR-inducing factor(s) is affected in the hrcV mutant, but not in the single hpa2 and hrpF mutants or in the hpa2 hrpF double mutant.
Fig. 1.
The association of hpa2 with hrpF is essential for X. oryzae pv. oryzicola RS105 to grow in rice and to cause disease on host plants. (A) HR induced on nonhost plant tobacco (N. benthamiana) leaves by infiltration of plant tissue with strains adjusted to 3 × 108 CFU/ml by use of a needleless syringe. Three replications were done in each experiment, and each experiment was repeated three times. (B) Water-soaking symptoms caused by X. oryzae pv. oryzicola strains on inoculated leaves of the host plant, IR24 rice (a susceptible cultivar). Photographs were taken 3 days after inoculation into seedling rice (14 days old) by needleless syringe, and lesion length was measured 14 days after inoculation into adult rice (2 months old) by the leaf-needling method (59). Bars with the same capital letter are not significantly different. (C) Growth of bacteria in inoculated leaves. Bacteria were recovered from the inoculated leaves every day for a period of 4 days postinoculation. Data are means ± standard deviations from three replicates.
To investigate the roles of hpa2 and hrpF in the pathogenesis of X. oryzae pv. oryzicola, we infiltrated the strains at a concentration of 3 × 108 CFU/ml into rice seedling leaves (Oryza sativa cv. IR24, 2 weeks old) and monitored the plants for water-soaked symptoms and for bacterial growth in planta. Bacterial strains were also inoculated into adult rice plants (2 months old), and the lengths of the lesions formed were measured. The results revealed that the hrpF mutant, RΔhrpF, still caused weak water-soaked symptoms in rice seedlings, as did the RΔhpa2 mutant (Fig. 1B), but the RΔhrpF bacterial leaf streak lesions in adult plants were significantly shorter than those caused by RΔhpa2 (Fig. 1B). The growth of RΔhrpF in rice tissue was significantly reduced (P, 0.01 by t test) from that of the hpa2 mutant (Fig. 1C). In contrast, the double mutant RΔhpa2ΔhrpF was unable to cause water-soaked symptoms in rice seedlings (Fig. 1B) and was also deficient for pathogenicity in adult rice, like RΔhrcV. The in planta growth of RΔhpa2ΔhrpF was similar to that of RΔhrcV in rice (Fig. 1C). Complementation of RΔhpa2ΔhrpF with either hpa2 or hrpF restored water-soaked symptoms in rice seedlings, and pathogenicity and bacterial growth in adult rice tissues, to the levels caused by either RΔhpa2 or RΔhrpF, correspondingly (Fig. 1). Overall, the results discussed above demonstrated that hpa2 together with hrpF is essential for the pathogenicity of X. oryzae pv. oryzicola in rice, but not for HR induction in nonhost tobacco, suggesting that some T3S effectors contributing to HR induction are not translocated into host cells.
Expression of hpa2 is positively regulated by HrpG and HrpD6 but not by HrpX.
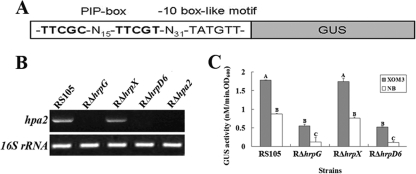
hpa2 is the first gene left of the pathogenicity island and beyond the hrpA operon of X. oryzae pv. oryzicola (59). Intriguingly, the expression profiles of the hrp-hrc-hpa mutants (10 hrp, 9 hrc, and 8 hpa genes and 2 hrp regulatory genes, hrpG and hrpX) of X. oryzae pv. oryzicola revealed that hpa2 expression was greatly reduced in the hrpD6 mutant, RΔhrpD6 (data to be published elsewhere). This prompted us to investigate the hpa2 expression pattern in this pathogen. The DNA sequence 31 bp upstream of the hpa2 locus shows that there is an imperfect PIP box (TTCGC-N15-TTCGT) and also a −10 box-like motif (TATGTT) (Fig. 2A). HrpX is known to directly bind the PIP box so as to regulate the transcription of hrp genes (30), and the presence of an imperfect PIP box upstream of hpa2 implies that its expression is positively regulated by HrpX. To determine whether the expression of hpa2 with a predicted PIP box-regulated promoter does indeed depend on HrpX, we performed an RT-PCR analysis of X. oryzae pv. oryzicola RS105, the hrpG mutant RΔhrpG, the hrpX mutant RΔhrpX, and the hrpD6 mutant RΔhrpD6 grown in the hrp-inducing medium XOM3, using RΔhpa2 as the negative control. As shown in Fig. 2B, the hpa2 transcript is highly abundant both in the wild-type strain RS105 and in RΔhrpX but not in RΔhrpG, RΔhrpD6, or RΔhpa2 (Fig. 2B). This indicates that hpa2 is not regulated by HrpX but most likely by HrpG and HrpD6.
Fig. 2.
Expression of hpa2 in X. oryzae pv. oryzicola strain RS105 when the hrpG, hrpX, or hrpD6 gene is mutated. (A) Schematic map of the promoter region containing the PIP box and −10 box-like motif of hpa2 fused with GUS. (B) Detection of hpa2 expression by RT-PCR. RS105 and the hrpG, hrpX, and hrpD6 mutant strains were incubated in the hrp-inducing medium XOM3, and semiquantitative RT-PCR was performed. PCR products were electrophoretically separated on a 1.2% agarose gel. The 16S rRNA PCR product was used as a control. (C) GUS activities of the hpa2 promoter-GUS reporter in RS105, RΔhrpG, RΔhrpX, and RΔhrpD6. Strains were cultured in either XOM3 or NB medium for 16 h, and GUS activities were then determined by measurement of the OD415 using p-nitrophenyl-β-d-glucuronide as a substrate. The experiment was repeated twice, and similar results were obtained. Statistically different data groups are indicated by different letters.
Additionally, the hpa2 promoter-driven β-glucuronidase (gusA) transcriptional fusion reporter plasmid phpa2GUS (containing a DNA fragment of the entire hpa2 ORF and its promoter region fused to the promoterless gusA gene with its ribosome binding site [RBS] in the pUFR034 vector) (see Table S1 in the supplemental material) was introduced into RΔhrpG, RΔhrpX, RΔhrpD6, and RS105 by biparental conjugation using Escherichia coli S17-1 as described previously (10, 30). Transconjugants were screened on rich NA medium supplemented with appropriate antibiotics. The resulting reporter strains were named RΔhrpG(phpa2GUS), RΔhrpX(phpa2GUS), RΔhrpD6(phpa2GUS), and RS105(phpa2GUS) (see Table S1). In X. oryzae pv. oryzae, the expression of hrp genes, including the regulators hrpG and hrpX, is induced in nutrient-deficient medium but is repressed in nutrient-rich medium (13, 14, 15, 16). Therefore, we measured the GUS activities of the reporter strains in both the minimal medium XOM3 (18, 55) and the nutrient-rich NA medium. The results showed that the GUS activities of the phpa2GUS reporter plasmid in the hrpG and hrpD6 mutant backgrounds were significantly (P, 0.01 by t test) lower than that in the hrpX mutant and in the wild-type background (Fig. 2C), indicating that hpa2 expression is induced, and its transcription is positively regulated, by HrpG and HrpD6, but not by HrpX, in X. oryzae pv. oryzicola.
The secretion of Hpa2 is T3SS dependent.
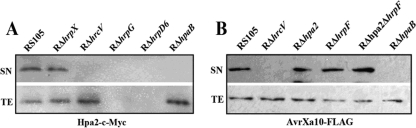
Hpa2, like HpaH in X. campestris pv. vesicatoria (7, 43), shares sequence identity with lytic transglycosylases. Information obtained from the sequence analysis prompted us to investigate whether or not there are N-terminal amino acid patterns typical of T3S substrates as described by Furutani et al. (14). In the first 50 amino acid residues at the N terminus of Hpa2, there are a total of 8 Ser and Pro residues (less than 10), only 2 Leu residues, and no Asp or Glu residues within the first 12 residues, and the fourth residue is a Ser (data not shown), indicating that Hpa2 may be secreted through the T3SS. To investigate the secretion characteristics of Hpa2 in X. oryzae pv. oryzicola RS105, hpa2 was expressed as a C-terminally c-Myc epitope tagged derivative in plasmid pHpa2-c-Myc (see Table S1 in the supplemental material) and was introduced into the wild-type strain RS105 and a T3SS-deficient mutant, RΔhrcV. Immunoblotting indicated that a functional T3SS is necessary for the secretion of Hpa2, since the Hpa2-c-Myc protein (expected size, 20 kDa for Hpa2 plus 5 kDa for the c-Myc epitope) was not detected in the culture supernatant (SN) of RΔhrcV but was present in the total-cell extract (TE) when bacteria were incubated in the secretion medium XOM3 (Fig. 3A).
Fig. 3.
Detection of the secretion of Hpa2 and the hpaB-dependent T3S effector AvrXa10 in X. oryzae pv. oryzicola by immunoblotting. (A) Strains RS105, RΔhrcV, RΔhrpG, RΔhrpX, RΔhrpD6, and RΔhpaB expressing pHpa2-c-Myc were incubated in secretion medium. Total-protein extracts (TE) and culture supernatants (SN) were analyzed by immunoblotting using anti-c-Myc antibodies. (B) Strains RS105, RΔhrcV, RΔhpa2, RΔhrpF, RΔhpa2ΔhrpF, and RΔhpaB harboring AvrXa10-FLAG were incubated in secretion medium. TEs and SNs were analyzed by immunoblotting using anti-FLAG antibodies.
Since HpaB is an exit protein for the T3SS, we sought to investigate whether the Hpa2 secretion via the T3SS is affected by a mutation in hpaB. Thus, plasmid pHpa2-c-Myc was introduced into the hpaB deletion mutant RΔhpaB and the wild-type strain RS105 (see Table S1 in the supplemental material). After incubation in the secretion medium XOM3, the TEs and SNs were analyzed by immunoblotting using an anti-c-Myc antibody. Hpa2-c-Myc was detected only in the TE of the RΔhpaB culture, not in the SN, while it was detectable in both the TE and the SN of strain RS105 (Fig. 3A), suggesting that Hpa2 secretion is HpaB dependent.
The fact that hpa2 expression is positively regulated by HrpG and HrpD6, but not by HrpX, prompted us to investigate whether hpa2 is secreted in the hrpG, hrpX, and hrpD6 mutants. The Hpa2-c-Myc fusion protein was expressed in RΔhrpG, RΔhrpX, and RΔhrpD6 (see Table S1 in the supplemental material). After incubation in an hrp-inducing medium for 16 h, a protein of the expected size was detected by a c-Myc epitope-specific antibody in the TEs and SNs of the bacterial strains tested. The results showed that the Hpa2-c-Myc fusion protein was detected in the SNs of the wild-type strain RS105 and the RΔhrpX mutant only, not in RΔhrpG, RΔhrcV, and RΔhrpD6 (Fig. 3A). Comparing these results with the hpa2 expression profiles of the hrpG, hrpX, and hrpD6 mutants (Fig. 2), we conclude that Hpa2 is a member of the HrpD6 and HrpG regulon secreted through the T3SS but is not a member of the HrpX regulon.
Hpa2 interacts with HrpF in the plasma membranes of plant cells.
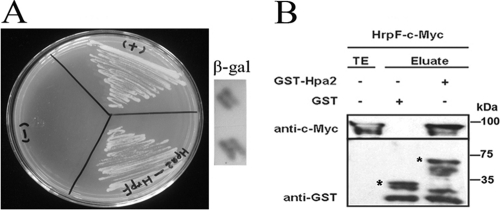
In X. campestris pv. vesicatoria, the injection of T3S effectors into plant cells is mediated by a translocon protein, HrpF, which is the last extracellular component of the T3SS appendage to penetrate the membrane of plant cells (6, 7, 46). The fact that the hpa2 and hrpF double mutant RΔhpa2ΔhrpF lost its pathogenicity in rice (Fig. 1) suggests that HrpF requires Hpa2 to form a complex for the translocation of T3S effectors into plant cells. To test this hypothesis, we utilized the yeast two-hybrid (Y2H) system to determine whether Hpa2 interacts with HrpF. Our Y2H assay showed that Hpa2 interacts with HrpF (Fig. 4A), and this was true no matter which protein (Hpa2 or HrpF) was used as the bait (data not shown). To confirm the interaction, we expressed a pGST-Hpa2 chimera in E. coli, immobilized it on glutathione Sepharose, and added an E. coli lysate containing pHrpF-c-Myc (Fig. 4B). The result showed that HrpF-c-Myc specifically bound to GST-Hpa2 but not to GST alone (Fig. 4B), which strongly suggested that Hpa2 and HrpF interact with each other. To evaluate whether Hpa2 and HrpF self-interact, we also tested whether Hpa2-c-Myc specifically bound GST-Hpa2 and whether HrpF-c-Myc coeluted with GST-HrpF. Indeed, no such self-interactions occurred (data not shown).
Fig. 4.
Protein-protein assays to detect interaction of Hpa2 with HrpF of X. oryzae pv. oryzicola. (A) Interactions of Hpa2 and HrpF were evaluated using full-length proteins by Y2H and β-gal assays. The reactions of pGADT7-T with pGBKT7-53 and of pGADT7-T with pGBKT-Lam were regarded as the positive (+) and negative (−) controls, respectively. Y2H and β-gal assays were performed according to standard procedures (Clontech). For the image shown, Hpa2 was used as the bait. Similar results were seen when HrpF was used as the bait. (B) Hpa2 interacts with HrpF by a pulldown assay. GST and GST-Hpa2 were immobilized on glutathione Sepharose and were incubated with HrpF-c-Myc. Total-cell lysates (TE) and eluted proteins (Eluate) were analyzed by immunoblotting using antibodies directed against the c-Myc epitope and GST, respectively. Bands corresponding to GST and GST chimeras are marked by asterisks. Arrows indicate c-Myc epitope-tagged proteins.
Since X. campestris pv. vesicatoria HrpF is a translocator that binds to the membranes of host cells (6), the interaction between Hpa2 and HrpF prompted us to determine the cellular localization of Hpa2 once it is secreted via the T3SS. GFP-tagged Hpa2 was transiently expressed in onion epidermal cells, and the localization was examined by fluorescence microscopy. Hpa2 exhibited a plasma membrane localization that is distinguishable from that of GFP (Fig. 5A). Interestingly, we observed unique punctate staining of Hpa2 in the membranes of onion cells before or after plasmolysis, and Hpa2-GFP did not colocalize with the cell wall in the plasmolyzed cell (Fig. 5A), suggesting that Hpa2 binds the membranes of plant cells. To further confirm that the interaction of Hpa2 with HrpF occurs in the plasma membrane of the host, we performed a bimolecular fluorescence complementation (BiFC) technique (51) to visualize Hpa2-HrpF association in plant cells. For BiFC, the nonfluorescent N-terminal domain of YFP (YN) and the nonfluorescent C-terminal domain of YFP (YC) were fused to the N- and C-terminal ends of the test proteins (i.e., Hpa2 and HrpF), respectively. Interactions between the respective fusion proteins were then tested in N. benthamiana using the Agrobacterium-mediated transient expression assay. Representative BiFC confocal microscopy images showing the Hpa2-HrpF interaction are presented in Fig. 5B. Coexpression of pHpa2-YN and pHrpF-YC resulted in bright fluorescence near the plant cell membrane (Fig. 5B). Taken together, the BiFC studies show that Hpa2 interacts with HrpF and that localization occurs in the plant cell membrane.
Fig. 5.
Fluorescence assays to detect the localization of Hpa2 with HrpF in plant cells. (A) Subcellular localization of Hpa2-GFP in onion epidermal cells. Particle bombardment was used to transfect onion cells with the pA-GFP (control) or Hpa2-GFP vector. Expression was driven by the CaMV 35S promoter. For confocal laser scanning microscopy, samples were taken 24 h postinoculation. The plasmolysis of cells expressing Hpa2-GFP was implemented in 30% (wt/vol) sucrose solution for 15 min. Representative fluorescent images of cells expressing pHpa2-GFP are shown. Cytosolic GFP bombardment was performed as the control. (B) BiFC visualization of Hpa2 and HrpF association in a stably transformed N. benthamiana cell membrane. A. tumefaciens GV3101 harboring p1301-YN or pHpa2-YN coinfiltrated with p1301-YC or pHrpF-YC, respectively. Representative images from microscopy show reconstituted YFP fluorescence. Bars, 10 μm. LM, light microscopy; FM, fluorescence microscopy.
Hpa2 and HrpF are involved in the translocation of TAL effectors into host cells.
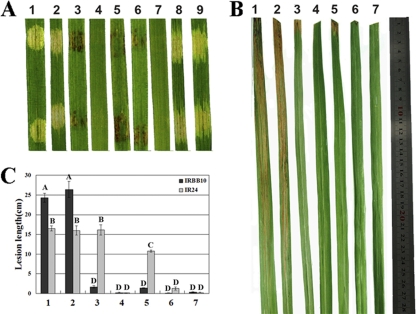
It has been demonstrated that X. oryzae pv. oryzae AvrXa10 is a TAL effector that triggers the HR in a T3SS-dependent manner (48) on Oryza sativa cv. IRBB10 (containing the corresponding R gene Xa10), and this avr-R gene-mediated response is inhibited by T3S effectors of X. oryzae pv. oryzicola (35). To investigate the roles of Hpa2 and HrpF in HR inhibition, we employed the hrp system of X. oryzae pv. oryzae as the reporter. The avrXa10 gene was introduced into the hpa2 and hrpF single mutants of X. oryzae pv. oryzae strain PXO99A (PΔhpa2 and PΔhrpF), the hpa2 and hrpF double mutant (PΔhpa2ΔhrpF), and the T3S mutant PΔhrcV (see Table S1 in the supplemental material). Three days after rice seedlings (2 weeks old) were syringe-infiltrated with Xanthomonas strains at 3 × 108 CFU/ml, the innate AvrXa10 in the wild-type X. oryzae pv. oryzae strain PXO99A, PΔhpa2, or PΔhrpF could trigger a typical HR (e.g., the infiltrated area became brown) in IRBB10 rice, which contains the Xa10 R gene, but did not elicit the HR response when expressed in the double mutant PΔhpa2ΔhrpF or in the T3SS mutant PΔhrcV (Fig. 6A). Furthermore, expression of N-terminally truncated AvrXa10 (AvrXa1029-1201) or full-length AvrXa10 in wild-type X. oryzae pv. oryzicola RS105 did not stimulate the HR in IRBB10 rice (Fig. 6A). To confirm these results, the strains listed above, except for the transconjugants of wild-type RS105 with either the empty vector pURF034 or plasmid pAvrXa10, were inoculated at 3 × 108 CFU/ml into adult rice leaves (IRBB10) by the leaf-clipping method. Fourteen days after inoculation, we observed that the avrXa10 gene had transformed the wild-type PXO99A, PΔhpa2, and PΔhrpF strains from compatibility to incompatibility with IRBB10 rice, with lesion lengths less than 1.5 cm (Fig. 6B and C), but N-terminally truncated AvrXa10 did not alter the compatibility of the wild-type strain PXO99A with IRBB10 rice. In addition, no lesions were formed in IRBB10 rice by the double mutant PΔhpa2ΔhrpF or the T3SS mutant PΔhrcV, even though they harbored the innate avrXa10 gene (Fig. 6B and C). In contrast, the avrXa10 gene did not alter the virulence of the wild-type strain PXO99A, PΔhpa2, or PΔhrpF in IR24 rice, lacking the matching Xa10 gene (Fig. 6C). These data indicate that a single mutation in either hpa2 or hrpF does not inhibit the translocation of AvrXa10 into plant cells but that a mutant lacking both hpa2 and hrpF is unable to translocate AvrXa10, which implies that both HrpF and Hpa2 contribute to the translocation of T3S effectors into plant cells.
Fig. 6.
The translocation of effector protein AvrXa10 was obstructed by Hpa2 and HrpF. (A) The X. oryzae pv. oryzae-rice pathosystem was used to check AvrXa10 secretion and translocation, since the AvrXa10-triggered HR is inhibited in X. oryzae pv. oryzicola (35). Strains at 3 × 108 CFU/ml containing the fusion gene in pAvrXa10 were infiltrated into seedling leaves of IRBB10 rice (2 weeks old) with needleless syringes. The response in rice was photographed 3 days after inoculation in three independent experiments. (B) Strains containing pAvrXa10 were inoculated into IRBB10 rice (2 months old) at 3 × 108 CFU/ml by leaf clipping. Photographs were taken 14 days postinoculation. (C) Lesion lengths in IRBB10 and IR24 rice, scored 14 days postinoculation. Data are means ± standard deviations from three repeats, each using 10 leaves. Bars with the same capital letter are not significantly different. Lane or image numbers are as follows: 1, X. oryzae pv. oryzae PXO99A(pUFR034); 2, PXO99A(pΔ28AvrXa10); 3, PXO99A(pAvrXa10); 4, PΔhrcV(pAvrXa10); 5, PΔhpa2(pAvrXa10); 6, PΔhrpF(pAvrXa10); 7, PΔhpa2ΔhrpF(pAvrXa10); 8, RS105(pUFR034); 9, RS105(pAvrXa10).
To verify that the secretion of the TAL effector AvrXa10 via the T3SS is not affected by the absence of Hpa2 and HrpF, AvrXa10 was expressed as a C-terminally FLAG epitope tagged derivative in plasmid pAvrXa10 (see Table S1 in the supplemental material), and this plasmid was introduced into strains RΔhpa2, RΔhrpF, RΔhpa2ΔhrpF, and RS105. When the bacteria were incubated in XOM3 secretion medium, AvrXa10-FLAG was detected by a FLAG epitope-specific antibody in both the TEs and the SNs. The results showed that AvrXa10-FLAG was detectable in the TEs of all the strains tested (Fig. 3B), indicating that the protein is expressed. However, the fusion protein was undetectable only in the SNs of RΔhrcV and RΔhpaB but was detectable in RΔhpa2, RΔhrpF, and RΔhpa2ΔhrpF (Fig. 3B), indicating that the AvrXa10 TAL effector is an HpaB-dependent T3SS protein and that neither Hpa2 nor HrpF plays a role in the secretion of AvrXa10 via the T3SS but that both are involved in the translocation of the effector into plant cells, as demonstrated above.
DISCUSSION
In this study, we showed that Hpa2 works as a helper for HrpF to form a translocon complex on the plant membrane in order to facilitate the translocation of X. oryzae T3S effectors into plant cells.
Hpa2 shares sequence identity with the lytic transglycosylase family members Hpa2 in X. oryzae pv. oryzae (57), HpaH in X. campestris pv. vesicatoria (7), HrpH, HopP1, and HopAJ1 in P. syringae pv. tomato DC3000 (44), and VirB1 in A. tumefaciens (60). The members of this predicted lytic transglycosylase family do not contribute significantly to bacterial pathogenicity in Shigella flexneri (1), P. syringae pv. tomato DC3000 (44), or X. oryzae pv. oryzae (57) but enhance virulence in X. campestris pv. vesicatoria and X. axonopodis pv. glycines (29, 43). The efficient T3SS secretion and translocation of AvrBs3 in X. campestris pv. vesicatoria depends not only on the global T3S chaperone HpaB but also on HpaH (5, 7). This is consistent with our hypothesis that the translocation of TAL effectors through HrpF requires the participation of Hpa2. Possibly, after secretion through the Hrp pilus, the lytic activity of Hpa2 may also dissolve the membranes of plant cells as it disrupts plant cell walls (57) to aid in the insertion of HrpF into the plant plasma membrane, which facilitates the transportation of T3S effectors into plant cells. This hypothesis is based on our subcellular localization analysis with GFP-labeled Hpa2, which demonstrated that Hpa2 binds to the plasma membrane with unique punctate staining (Fig. 5A), suggesting that HrpF together with Hpa2 forms a translocon complex in the membranes of plant cells.
PIP box and −10 box-like motifs are characteristic features of HrpX regulons (14, 30, 50). Although there is an imperfect PIP box and a −10 box-like motif in the hpa2 promoter region of X. oryzae pv. oryzicola RS105, the expression level of hpa2 in the hrpX mutant is equivalent to that in wild-type RS105, but hpa2 expression is significantly repressed in the hrpG mutant. This demonstrates that hpa2 is regulated by HrpG rather than by HrpX or that hpa2 is not naturally a member of the HrpX regulon in X. oryzae pv. oryzicola. In addition, base substitutions in the PIP box consensus sequences (TTCGC sequences) have revealed that one or two base substitutions can considerably suppress promoter activities. For example, when the last C in the TTCGC sequence is replaced by T, promoter activity is reduced by 50% (50). Contrasting results might be obtained when base substitutions are made in PIP box sequences of different genes in various bacteria. Remarkably, hpa2 is regulated by HrpD6 in X. oryzae pv. oryzicola, suggesting that additional regulatory pathways exist among these hrp genes.
The mutation of hpa2 in X. oryzae pv. oryzae (57) and of hpaH in X. axonopodis pv. glycines (5, 7) resulted in an inability to trigger HR in plants, in contrast to the mutation of hpa2 in X. oryzae pv. oryzicola RS105. Otherwise, the X. oryzae pv. oryzicola RS105 hpa2 mutant displayed reduced pathogenicity in host rice similar to that of the hpa2 mutant of X. oryzae pv. oryzae and the hpaH mutant of X. axonopodis pv. glycines (29, 57). Collectively, these data indicated that homologous genes from different bacteria may play dissimilar roles during the plant-microbe interaction.
The HrpF protein of X. campestris pv. vesicatoria is considered a T3SS translocon protein, which inserts a translocation channel into the eukaryotic plasma membrane (6). The pathogenicity of the X. oryzae pv. oryzicola hrpF mutant showed minimal virulence, distinguishing itself from other hrp-hrc mutants, in accordance with the reduced virulence of the hrpF mutant in X. oryzae pv. oryzae (9, 48), but not in X. axonopodis pv. glycines (29). Thus, it could be expected that the reduced pathogenicity of the hrpF mutant is due to incomplete channel formation, which may occur in other components of the translocon. Interestingly, the hpa2 hrpF double mutant of RS105 completely lost pathogenicity in host rice but did not prevent Hpa1, HrpB2 (data not shown), and the T3S effector AvrXa10 from being secreted by the T3SS. Moreover, protein-protein assays that revealed the interaction of Hpa2 with HrpF strongly support our hypothesis that Hpa2 and HrpF form a complex on the plant cell membrane to control the translocation of T3S effectors into the plant cell cytosol for bacterial pathogenicity in hosts. However, further experiments are still needed to clarify the complex mechanisms that control the translocation of T3S effectors into plant cells.
Intriguingly, we also found that the hpa2 hrpF double mutant retains the ability to trigger the HR in tobacco (Fig. 1A), implying that the HR elicitor(s) of X. oryzae pv. oryzicola is not translocated through the HrpF-Hpa2 complex into plant cells. This is consistent with the finding that some T3SS substrates are only secreted into the plant apoplast but not injected into plant cells (35). The following evidence supports our hypothesis. (i) Harpin proteins are apoplastic HR elicitors in plants, since HR induction in tobacco occurs when the purified harpins are infiltrated into tobacco leaves (59). (ii) Gram-negative plant-pathogenic bacteria have more than one HR elicitor for HR induction in nonhost plants. For example, in P. syringae pv. tomato DC3000, at least four proteins, HrpZ (20), HrpW (8), HopAK1 (32), and HopP-1 (32), were able to induce the HR in tobacco. In Ralstonia solanacearum, three HR elicitors, PopA (2), HrpW (17), and PopW (34), have been reported. (iii) Only the hpa1 gene product has been identified as a harpin in X. oryzae pv. oryzicola (59). However, the hpa1 mutation in X. oryzae pv. oryzicola does not render the pathogen unable to trigger the HR in nonhost tobacco (unpublished data), in contrast to that in X. oryzae pv. oryzae (9), suggesting that there are more than two harpins in Xanthomonas species. (iv) Hpa1 is secreted via the T3SS in an HpaB-independent manner (our unpublished data) similar to that of its homolog XopA in X. campestris pv. vesicatoria (43), but TAL effectors are HpaB dependent, which explains why the hpa2 hrpF double mutant of X. oryzae pv. oryzicola still triggers the HR in nonhost tobacco but does not cause bacterial leaf streak in rice. The information obtained from the mutations generated in hpa1, hpa2, hpaB, and hrpF will serve as clues for the identification of an additional HR elicitor(s) in X. oryzae pv. oryzicola.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Alan Collmer at Cornell University for critical suggestions and helpful discussions on the experiments in this study.
This work was supported by the State Key Basic Research and Development Project of China, the Natural Science Foundation of China (30710103902 and 31071656), and the Special Fund for Agro-Scientific Research in the Public Interest (NYHYZX07-056 and 201003067-09).
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 8 April 2011.
REFERENCES
- 1. Allaoui A., Ménard R., Sansonetti P. J., Parsot C. 1993. Characterization of the Shigella flexneri ipgD and ipgF genes, which are located in the proximal part of the mxi locus. Infect. Immun. 61:1707–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Belbahri L., et al. 2001. A local accumulation of the Ralstonia solanacearum PopA protein in transgenic tobacco renders a compatible plant-pathogen interaction incompatible. Plant J. 28:419–430 [DOI] [PubMed] [Google Scholar]
- 3. Büttner D., Bonas U. 2002. Port of entry—the type III secretion translocon. Trends Microbiol. 10:186–192 [DOI] [PubMed] [Google Scholar]
- 4. Büttner D., Bonas U. 2002. Getting across—bacterial type III effector proteins on their way to the plant cell. EMBO J. 21:5313–5322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Büttner D., Gürlebeck D., Noël L. D., Bonas U. 2004. HpaB from Xanthomonas campestris pv. vesicatoria acts as an exit control protein in type III-dependent protein secretion. Mol. Microbiol. 54:755–768 [DOI] [PubMed] [Google Scholar]
- 6. Büttner D., Nennstiel D., Klusener B., Bonus U. 2002. Functional analysis of HrpF, a putative type III translocon protein from X. campestris pv. vesicatoria. J. Bacteriol. 184:2389–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Büttner D., Noël L., Stuttmann J., Bonas U. 2007. Characterization of the nonconserved hpaB-hrpF region in the hrp pathogenicity island from Xanthomonas campestris pv. vesicatoria. Mol. Plant-Microbe Interact. 20:1063–1074 [DOI] [PubMed] [Google Scholar]
- 8. Charkowski A. O., et al. 1998. The Pseudomonas syringae pv. tomato HrpW protein has domains similar to harpins and pectate lyases and can elicit the plant hypersensitive response and bind to pectate. J. Bacteriol. 180:5211–5217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cho H. J., et al. 2008. Molecular analysis of the hrp gene cluster in Xanthomonas oryzae pathovar oryzae KACC10859. Microb. Pathog. 44:473–483 [DOI] [PubMed] [Google Scholar]
- 10. DeFeyter R., Kado C. I., Gabriel D. W. 1990. Small, stable shuttle vectors for use in Xanthomonas. Gene 88:65–72 [DOI] [PubMed] [Google Scholar]
- 11. Espinosa A., Alfano J. R. 2004. Disabling surveillance: bacterial type III secretion system effectors that suppress innate immunity. Cell. Microbiol. 6:1027–1040 [DOI] [PubMed] [Google Scholar]
- 12. Evangelista C., Lockshon D., Fields S. 1996. The yeast two-hybrid system: prospects for protein linkage maps. Trends Cell Biol. 6:196–199 [DOI] [PubMed] [Google Scholar]
- 13. Furutani A., Nakayama T., Ochiai H. 2006. Identification of novel HrpXo regulons preceded by two cis-acting elements, a plant-inducible promoter box and a −10 box-like sequence, from the genome database of Xanthomonas oryzae pv. oryzae. FEMS Microbiol. Lett. 259:133–141 [DOI] [PubMed] [Google Scholar]
- 14. Furutani A., et al. 2009. Identification of novel type III secretion effectors in Xanthomonas oryzae pv. oryzae. Mol. Plant-Microbe Interact. 22:96–106 [DOI] [PubMed] [Google Scholar]
- 15. Furutani A., Tsuge S., Ohnishi K. 2004. Evidence for HrpXo-dependent expression of type II secretory proteins in Xanthomonas oryzae pv. oryzae. J. Bacteriol. 186:1374–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Furutani A., et al. 2003. Hpa1 secretion via type III secretion system in Xanthomonas oryzae pv. oryzae. J. Gen. Plant Pathol. 69:271–275 [Google Scholar]
- 17. Gaudriault S., Brisset M. N., Barny M. A. 1998. HrpW of Erwinia amylovora, a new Hrp-secreted protein. FEBS Lett. 428:224–228 [DOI] [PubMed] [Google Scholar]
- 18. Guo X. X., Zou H. S., Li Y. R., Zou L. F., Chen G. Y. 2010. hrpD6 gene determines Xanthomonas oryzae pv. oryzae to trigger hypersensitive response in tobacco and pathogenicity in rice. Acta Microbiol. Sinica 50:1155–1163 (In Chinese.) [PubMed] [Google Scholar]
- 19. Gürlebeck D., Thieme F., Bonas U. 2006. Type III effector proteins from the plant pathogen Xanthomonas and their role in the interaction with the host plant. J. Plant Physiol. 163:233–255 [DOI] [PubMed] [Google Scholar]
- 20. He S. Y., Huang H. C., Collmer A. 1993. Pseudomonas syringae pv. syringae harpinPss: a protein that is secreted via the Hrp pathway and elicits the hypersensitive response in plants. Cell 73:1255–1266 [DOI] [PubMed] [Google Scholar]
- 21. He S. Y., Nomura K., Whittam T. S. 2004. Type III protein secretion mechanism in mammalian and plant pathogens. Biochim. Biophys. Acta 1694:181–206 [DOI] [PubMed] [Google Scholar]
- 22. Hopkins C. M., White F. F., Choi S. H., Guo A., Leach J. E. 1992. A family of avirulence genes from Xanthomonas oryzae pv. oryzae. Mol. Plant-Microbe Interact. 5:451–459 [DOI] [PubMed] [Google Scholar]
- 23. Huang J. Q., Wei Z. M., An H. L., Zhu Y. X. 2001. Agrobacterium tumefaciens-mediated transformation of rice with the spider insecticidal gene conferring resistance to leaffolder and striped stem borer. Cell Res. 11:149–155 [DOI] [PubMed] [Google Scholar]
- 24. Hueck C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huguet E., Hahn K., Wengelnik K., Bonas U. 1998. hpaA mutants of Xanthomonas campestris pv. vesicatoria are affected in pathogenicity but retain the ability to induce host-specific hypersensitive reaction. Mol. Microbiol. 29:1379–1390 [DOI] [PubMed] [Google Scholar]
- 26. Jefferson R. A., Kavanagh T. A., Bevan M. W. 1987. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6:3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jiang J., Zou H. S., Li Y. R., Chen G. Y. 2009. Expression of the hrcC, hrpE and hpa3 genes is not regulated by the hrpG and hrpX genes in a rice pathogen Xanthomonas oryzae pv. oryzicola. Wei Sheng Wu Xue Bao 49:1018–1025 (In Chinese.) [PubMed] [Google Scholar]
- 28. Kauffman H. E., Reddy A. P. K., Hsieh S. P. V., Merca S. D. 1973. An improved technique for evaluating resistance of rice varieties to Xanthomonas oryzae. Plant Dis. Rep. 57:537–541 [Google Scholar]
- 29. Kim J. G., et al. 2003. Characterization of the Xanthomonas axonopodis pv. glycines Hrp pathogenicity island. J. Bacteriol. 185:3155–3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koebnik R., Krüger A., Thieme F., Urban A., Bonas U. 2006. Specific binding of the Xanthomonas campestris pv. vesicatoria AraC-type transcriptional activator HrpX to plant-inducible promoter boxes. J. Bacteriol. 188:7652–7660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koraimann G. 2003. Lytic transglycosylases in macromolecular transport systems of gram-negative bacteria. Cell. Mol. Life Sci. 60:2371–2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kvitko B. H., Ramos A. R., Morello J. E., Oh H. S., Collmer A. 2007. Identification of harpins in Pseudomonas syringae pv. tomato DC3000, which are functionally similar to HrpK1 in promoting translocation of type III secretion system effectors. J. Bacteriol. 189:8059–8072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Laemmli U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 34. Li J. G., et al. 2010. PopW of Ralstonia solanacearum, a new two-domain harpin targeting the plant cell wall. Mol. Plant Pathol. 11:371–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Makino S., Sugio A., White F. F., Bogdanove A. J. 2006. Inhibition of resistance gene-mediated defense in rice by Xanthomonas oryzae pv. oryzicola. Mol. Plant Microbe Interact. 19:240–249 [DOI] [PubMed] [Google Scholar]
- 36. Miller J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 37. Mitsuhara I., et al. 1996. Efficient promoter cassettes for enhanced expression of foreign genes in dicotyledonous and monocotyledonous plants. Plant Cell Physiol. 37:49–59 [DOI] [PubMed] [Google Scholar]
- 38. Moscou M. J., Bogdanove A. J. 2009. A simple cipher governs DNA recognition by TAL effectors. Science 326:1501. [DOI] [PubMed] [Google Scholar]
- 39. Mudgett M. B. 2005. New insights to the function of phytopathogenic bacterial type III effectors in plants. Annu. Rev. Plant Biol. 56:509–531 [DOI] [PubMed] [Google Scholar]
- 40. Mushegian A. R., Fullner K. J., Koonin E. V., Nester E. W. 1996. A family of lysozyme-like virulence factors in bacterial pathogens of plants and animals. Proc. Natl. Acad. Sci. U. S. A. 93:7321–7326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Niño-Liu D. O., Ronald P. C., Bogdanove A. J. 2006. Xanthomonas oryzae pathovars: model pathogens of a model crop. Mol. Plant Pathol. 7:303–324 [DOI] [PubMed] [Google Scholar]
- 42. Noël L., Thieme F., Nennstiel D., Bonas U. 2001. cDNA-AFLP analysis unravels a genome-wide hrpG regulon in the plant pathogen Xanthomonas campestris pv. vesicatoria. Mol. Microbiol. 41:1271–1281 [DOI] [PubMed] [Google Scholar]
- 43. Noël L., Thieme F., Nennstiel D., Bonas U. 2002. Two novel type III-secreted proteins of Xanthomonas campestris pv. vesicatoria are encoded within the hrp pathogenicity island. J. Bacteriol. 184:1340–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oh H. S., Park D. H., Collmer A. 2010. Components of the Pseudomonas syringae type III secretion system can suppress and may elicit plant innate immunity. Mol. Plant Microbe Interact. 23:727–739 [DOI] [PubMed] [Google Scholar]
- 45. Oh J., et al. 2007. Amyloidogenesis of type III-dependent harpins from plant pathogenic bacteria. J. Biol. Chem. 282:13601–13609 [DOI] [PubMed] [Google Scholar]
- 46. Rossier O., den Ackerveken G. V., Bonas U. 2000. HrpB2 and HrpF from Xanthomonas are type III-secreted proteins and essential for pathogenicity and recognition by the host plant. Mol. Microbiol. 39:828–838 [DOI] [PubMed] [Google Scholar]
- 47. Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 48. Sugio A., Yang B., White F. F. 2005. Characterization of the hrpF pathogenicity peninsula of Xanthomonas oryzae pv. oryzae. Mol. Plant-Microbe Interact. 18:546–554 [DOI] [PubMed] [Google Scholar]
- 49. Szurek B., Marois E., Bonas U., Van den Ackerveken G. 2001. Eukaryotic features of the Xanthomonas type III effector AvrBs3: protein domains involved in transcriptional activation and the interaction with nuclear import receptors from pepper. Plant J. 26:523–534 [DOI] [PubMed] [Google Scholar]
- 50. Tsuge S., et al. 2005. Effects on promoter activity of base substitutions in the cis-acting regulatory element of HrpXo regulons in Xanthomonas oryzae pv. oryzae. J. Bacteriol. 187:2308–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Walter M., et al. 2004. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40:428–438 [DOI] [PubMed] [Google Scholar]
- 52. Weber E., Koebnik R. 2005. Domain structure of HrpE, the Hrp pilus subunit of Xanthomonas campestris pv. vesicatoria. J. Bacteriol. 187:6175–6186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wengelnik K., Bonas U. 1996. HrpXv, an AraC-type regulator, activates expression of five of the six loci in the hrp cluster of Xanthomonas campestris pv. vesicatoria. J. Bacteriol. 178:3462–3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wengelnik K., Rossier O., Bonas U. 1999. Mutations in the regulatory gene hrpG of Xanthomonas campestris pv. vesicatoria result in constitutive expression of all hrp genes. J. Bacteriol. 181:6828–6831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xiao Y. L., Li Y. R., Liu Z. Y., Xiang Y., Chen G. Y. 2007. Establishment of the hrp-inducing systems for the expression of the hrp genes of Xanthomonas oryzae pv. oryzicola. Acta Microbiol. Sinica 47:396–401 (In Chinese.) [PubMed] [Google Scholar]
- 56. Yang B., White F. F. 2004. Diverse members of the AvrBs3/PthA family of type III effectors are major virulence determinants in bacterial blight disease of rice. Mol. Plant-Microbe Interact. 17:1192–1200 [DOI] [PubMed] [Google Scholar]
- 57. Zhang J. H., Wang X. Y., Zhang Y., Zhang G. Y., Wang J. S. 2008. A conserved Hpa2 protein has lytic activity against the bacterial cell wall in phytopathogenic Xanthomonas oryzae. Appl. Microbiol. Biotechnol. 79:605–616 [DOI] [PubMed] [Google Scholar]
- 58. Zhu W., Magbanua M. M., White F. F. 2000. Identification of two novel hrp-associated genes in the hrp gene cluster of Xanthomonas oryzae pv. oryzae. J. Bacteriol. 182:1844–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zou L. F., et al. 2006. Elucidation of the hrp clusters of Xanthomonas oryzae pv. oryzicola that control the hypersensitive response in nonhost tobacco and pathogenicity in susceptible host rice. Appl. Environ. Microbiol. 72:6212–6224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zupan J., Hackworth C. A., Aguilar J., Ward D., Zambryski P. 2007. VirB1 promotes T-pilus formation in the vir-type IV secretion system of Agrobacterium tumefaciens. J. Bacteriol. 189:6551–6563 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.