Abstract
Methanogenesis in wetlands is dependent on intermediary substrates derived from the degradation of biopolymers. Formate is one such substrate and is stimulatory to methanogenesis and acetogenesis in anoxic microcosms of soil from the fen Schlöppnerbrunnen. Formate dissimilation also yields CO2 as a potential secondary substrate. The objective of this study was to resolve potential differences between anaerobic formate- and CO2-utilizing prokaryotes of this fen by stable isotope probing. Anoxic soil microcosms were pulsed daily with low concentrations of [13C]formate or 13CO2 (i.e., [13C]bicarbonate). Taxa were evaluated by assessment of 16S rRNA genes, mcrA (encoding the alpha-subunit of methyl-coenzyme M reductase), and fhs (encoding formyltetrahydrofolate synthetase). Methanogens, acetogens, and formate-hydrogen lyase-containing taxa appeared to compete for formate. Genes affiliated with Methanocellaceae, Methanobacteriaceae, Acetobacteraceae, and Rhodospirillaceae were 13C enriched (i.e., labeled) in [13C]formate treatments, whereas genes affiliated with Methanosarcinaceae, Conexibacteraceae, and Solirubrobacteraceae were labeled in 13CO2 treatments. [13C]acetate was enriched in [13C]formate treatments, but labeling of known acetogenic taxa was not detected. However, several phylotypes were affiliated with acetogen-containing taxa (e.g., Sporomusa). Methanosaetaceae-affiliated methanogens appeared to participate in the consumption of acetate. Twelve and 58 family-level archaeal and bacterial 16S rRNA phylotypes, respectively, were detected, approximately half of which had no isolated representatives. Crenarchaeota constituted half of the detected archaeal 16S rRNA phylotypes. The results highlight the unresolved microbial diversity of the fen Schlöppnerbrunnen, suggest that differing taxa competed for the same substrate, and indicate that Methanocellaceae, Methanobacteriaceae, Methanosarcinaceae, and Methanosaetaceae were linked to the production of methane, but they do not clearly resolve the taxa responsible for the apparent conversion of formate to acetate.
INTRODUCTION
Methane is the second most important greenhouse gas (76), and its atmospheric concentration has increased to approximately 1,775 ppb (39) (evaluation from 2005). Wetlands contribute 27 to 53% (i.e., 160 to 314 Tg of methane) to the global emission of methane (39), underscoring the importance of understanding microbial-mediated processes that are linked to methanogenesis in wetlands. Methanogens have a very limited substrate range (34, 95), and their in situ activities are linked to “intermediary ecosystem metabolism,” i.e., a complex food web of interconnected microorganisms that catalyze essential intermediary processes that ultimately drive methanogenesis (19, 64, 94). Thus, methane production in many ecosystems including wetlands is dependent on intermediary substrates formed during the degradation of plant-derived polymers. Fifty to 80% of plant-derived organic matter consists of lignocelluloses (2), polymers that can be degraded by fungi and bacteria to glucose, xylose, and aromatic compounds (82). Chitin, a biopolymer of N-acetylglucosamine, is another potentially important source of organic carbon in various ecosystems (75). Primary fermenters (e.g., Aeromonadaceae and Clostridiaceae) in wetland soils can produce organic acids, alcohols, molecular hydrogen (H2), and carbon dioxide (CO2) from potential breakdown products of biopolymers (i.e., glucose, xylose, and N-acetylglucosamine) (32, 90). Organic acids and alcohols are further metabolized to H2 and CO2 by secondary fermenters (e.g., Syntrophobacteraceae [6]). The terminal stage of the methanogenic food web is catalyzed by methanogens (e.g., Methanomicrobiaceae and Methanosarcinaceae) that collectively convert formate, acetate, methanol, and H2-CO2 to methane (1, 19, 34, 64, 95).
Several studies have demonstrated that wetland soils contain complex prokaryotic communities (10, 15, 40, 43), a finding consistent with the aforementioned network of trophically linked processes that yield methane. The methanogenic community of the fen Schlöppnerbrunnen in southeast Germany is composed of Methanobacteriaceae, Methanomicrobiaceae, Methanosaetaceae, and Methanosarcinaceae (32, 90). Formate is a significant driver of methanogenesis under experimental conditions and might be derived from the fermentation of monosaccharides such as glucose and N-acetylglucosamine; it also stimulates acetogenesis (i.e., the reductive synthesis of acetate from CO2 via the acetyl-coenzyme A [CoA] Wood-Ljungdahl pathway [21]) (32, 90). The periodic occurrence of up to 0.65 mM formate in fen pore water (47) reinforces the likelihood that formate is a relevant in situ substrate for fen methanogens and other competing prokaryotic taxa. Dissimilation of formate also yields CO2, which could subsequently be utilized as a secondary source of carbon. The main objective of the present study was to resolve potential differences between anaerobic formate- and CO2-utilizing prokaryotic taxa in soil from the fen Schlöppnerbrunnen by stable isotope probing.
MATERIALS AND METHODS
Sampling site.
Fens are specialized mires (29), and the moderately acidic, methane-emitting fen Schlöppnerbrunnen is located 700 m above sea level (50°07′53″N, 11°52′51″E) in the Lehstenbach catchment of the Fichtelgebirge (translates as Spruce Mountains) in southeast Germany (for site description, see references 32 and 71). The pH of fen pore water approximates 4.5, and formate concentrations in fen pore water can range from 0 to 0.65 mM (47). Three soil cores of 0- to 20-cm depth were taken in July 2008 (4 to 5 m apart) with a soil corer, transported in airtight sterile plastic bags, and stored on ice until processed within 6 h of sampling.
Anoxic microcosms.
The three soil cores were homogenized together; the homogenized fen soil had an 83.5% water content. Thirty-five grams (fresh weight) of homogenized soil was placed in sterile 500-ml infusion flasks (Merck ABS, Dietikon, Switzerland) and diluted with 125 ml of anoxic mineral solution (pH 4.8) containing mineral salts, trace metals, and vitamins (90). The infusion flasks were closed with rubber stoppers and crimp seals and flushed with sterile N2 (100%). Anoxic solutions were prepared by using modified Hungate techniques (14).
A 15-day anoxic preincubation of soil slurry microcosms was used to ensure that endogenous nitrate, sulfate, and iron(III) were reduced (19, 32, 90). Formate-treated soil slurry microcosms were then pulsed daily with approximately 64 μmol of formate per microcosm, yielding approximately 0.3 to 0.6 mM formate in the aqueous phase (i.e., 9.7 to 17.4 μmol of formate g soildw−1 [where dw is dry weight]) during the incubation, a variation due to the daily sampling that yielded a changing volume. The formate that was pulsed was from a filter-sterilized solution of either sodium [13C]formate (99 atom% 13C) or sodium [12C]formate (i.e., unlabeled formate with a natural abundance of 13C). Additional microcosms were pulsed daily with approximately 160 μmol of CO2 (from a filter-sterilized solution of sodium [13C]bicarbonate or sodium [12C]bicarbonate) per microcosm, yielding approximately 1.0 to 3.1 mM CO2 in the combined aqueous and gas phases (i.e., 28.9 to 87.1 μmol of CO2 g soildw−1) during the incubation. The CO2 that was pulsed was from a filter-sterilized solution of either sodium [13C]bicarbonate (99 atom% 13C) or sodium [12C]bicarbonate (i.e., unlabeled bicarbonate with a natural abundance of 13C). Control microcosms lacked supplemental formate or CO2. The purpose of the 13CO2 treatment was twofold: (i) to control for potential cross-feeding (i.e., labeling of microorganisms by assimilation of [13C]formate-derived 13CO2) and (ii) to assess taxa capable of utilizing CO2 (i.e., assimilating CO2 at the expense of endogenous reductant). Two additional safeguards against CO2 cross-feeding were taken: (i) formate treatments were pulsed daily with 192 μmol of 12CO2 (i.e., sodium [12C]bicarbonate, yielding 1.9 to 4.4 mM CO2 in the combined aqueous and the gas phases, equivalent to 53.7 to 122.0 μmol of CO2 g soildw−1) per microcosm, and (ii) the gas phases of microcosms were exchanged with sterile N2 (100%) before substrate pulsing was initiated and every subsequent fourth day. For exchanging the gas phase with N2, microcosms were evacuated under sterile conditions for 30 min at approximately −800 mbar, after which the gas phase was replaced with sterile N2 (100%). This procedure was repeated after 15 min. Microcosms were then flushed with sterile N2 (100%) for 20 min. The pH was adjusted every fourth day to approximately pH 4.5 with anoxic sterile 5 M HCl. Soil slurries were incubated horizontally in the dark at 15°C and were exposed to light only during analyses. The gas and liquid phases of soil slurries were sampled with sterile syringes. Liquid samples were stored at −20°C for chemical analyses or at −80°C for molecular analyses.
Nucleic acid extraction.
Nucleic acids were extracted by bead-beating lysis, organic solvent extraction, and precipitation (30). DNA was purified and separated from RNA with Qiagen RNA/DNA mini-kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol.
Density gradient centrifugation.
DNA stable isotope probing was performed by published protocol (68). DNA was added to a gradient solution (buoyant density of 1.725 g ml−1) containing a cesium chloride solution (buoyant density of 1.881 g ml−1; 80.8% of total) and gradient buffer (pH 8; 100 mM Tris, 100 mM KCl, 1 mM EDTA; 19.2% of total) and filled into OptiSeal tubes (Beckman Coulter, Fullerton, CA). Differences within the gradient density could cause differences in the gene libraries prepared from gradient fractions, thus resulting in inconsistencies in determining which microorganisms are labeled. This problem was minimized by preparing all gradients with the same gradient solution. DNA was subjected to isopycnic centrifugation (177,200 × g [44,100 rpm] at 20°C for 40 h [Vti 65.2 vertical rotor; Beckman Coulter, Fullerton, CA) and fractionated. The buoyant densities of the gradient solution and fractions (see Fig. S1A in the supplemental material) were determined by weighing gradient solutions and fractions at 20°C. DNA was precipitated with glycogen and polyethylene glycol 6000, and DNA concentrations in gradients (see Fig. S1A) were measured with a Quanti-iT PicoGreen Assay Kit (Invitrogen, Karlsruhe, Germany).
PCR conditions and cloning.
cDNA was amplified with the following primer sets: mcrAf (5′-TAYGAYCARATHTGGYT-3′) and mcrAr (5′-ACRTTCATNGCRTARTT-3′) for mcrA (83), FTHFSf (5′-TTYACWGGHGAYTTCCATGC-3′) and FTHFSr (5′-GTATTGDGTYTTRGCCATACA-3′) for fhs (53), 27f (5′-AGAGTTTGATCMTGGCTC-3′) and 907r (5′-CCGTCAATTCMTTTRAGT-3′) for bacterial 16S rRNA genes (52), and Arc21Fa (5′-TCCGGTTGATCCYGSCRG-3′) (38) and Arc915 (5′-GTGCTCCCCCGCCAATTCCT-3′) (74) for archaeal 16S rRNA genes. Amplification of mcrA was as described previously (59) with the following modifications: initial denaturation at 94°C for 5 min, followed by 35 cycles of denaturation at 94°C for 45 s, annealing at 50°C for 45 s, and elongation at 72°C for 45 s, with a terminal elongation step at 72°C for 5 min. fhs was amplified as follows: initial denaturation at 94°C for 5 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 45 s, and elongation at 72°C for 70 s, with a terminal elongation step at 72°C for 5 min. Bacterial 16S rRNA genes were amplified as follows: initial denaturation at 95°C for 5 min, followed by five precycles of denaturation at 95°C at 60 s, annealing at 40°C for 60 s, and elongation at 72°C for 60 s and then by 30 subsequent cycles of denaturation at 95°C for 30 s, annealing at 43°C for 30 s, and elongation at 72°C for 5 s, with terminal elongation step at 72°C for 5 min. Archaeal 16S rRNA genes were amplified as described previously (32) with the following modifications: initial denaturation at 95°C for 5 min, followed by 33 cycles of denaturation at 95°C for 50 s, annealing at 55°C for 50 s, and elongation at 72°C for 110 s, with a terminal elongation step at 72°C for 5 min. Each PCR assay was facilitated with 5 Prime Mastermix (5 Prime; Hamburg, Germany). Final concentrations of PCR reagents were 0.4 mg bovine serum albumin ml−1, 4 μM (mcrA and fhs) or 0.6 μM (16S rRNA genes) of each primer, 0.6 U of Taq DNA polymerase, 0.2 mM each deoxynucleoside triphosphate (dNTP), and 2.6 mM (mcrA) or 3.6 mM (fhs or 16S rRNA genes) MgCl2.
PCR products for cloning were ligated into pGEM-T vector plasmids (Promega, Mannheim, Germany). Competent cells of Escherichia coli JM109 (Promega) (protocol as per manufacturer's instructions) were transformed with ligated pGEM-T vector plasmid. Clones were randomly picked, and the correct insert was determined by M13 PCR (primer set M13f/M13r) according to a previously published protocol (65) and selected for sequencing at Macrogen (Seoul, South Korea).
Analysis of DNA.
The DNA in fractions 3 to 9 from density gradients prepared from [13C]formate-supplemented fen microcosms was evaluated with terminal restriction fragment length polymorphism (TRFLP) analysis. PCR was performed with fluorescently labeled primers mcrAf-DY681 and mcrAr (Biomers GmbH, Ulm, Germany) (83). Gel-purified DNA (Montage DNA Gel Extraction Kit, Millipore Corp., Bedford, MA) was digested with mung bean endonuclease (New England BioLabs, Frankfurt/Main, Germany) according to the manufacturer's protocol to minimize the occurrence of pseudo-terminal restriction fragments (22). DNA was then cleaved at specific restriction sites with 2 units of each restriction enzyme, MspI and RsaI (New England BioLabs, Frankfurt/Main, Germany). Remaining DNA was quantified with a Quanti-iT PicoGreen Assay Kit, and TRFLP analysis was performed as described previously (32).
The 210-bp terminal restriction fragments of heavy fractions increased in relative intensity (see Fig. S1B in the supplemental material), indicating a greater abundance of mcrA genes in the heavier fractions. Heavy fraction 4 contained enough DNA to obtain a clear PCR signal. DNA of heavy fraction 4 was used for establishing gene libraries of mcrA and 16S rRNA genes from [13C]formate-, [12C]formate-, 13CO2-, and 12CO2-supplemented microcosms to identify active consumers of formate and CO2. Adequate PCR signals for fhs were not detected in fraction 4 but were detected in fraction 5. Fraction 7 also yielded an adequate fhs PCR signal and was therefore analyzed for fhs to increase the detection of the overall diversity of this gene. Thus, fhs was analyzed from DNA of fraction 5 and fraction 7 of [13C]formate-supplemented microcosms and fraction 5 of [12C]formate-supplemented microcosms after 23 days of incubation with formate.
Sequence analyses and identification of phylotypes.
All sequences were analyzed with Mega (85) and ARB software (58). MegaBLAST was used to compare sequences to those in public databases (67). Chimeric sequences of 16S rRNA gene sequences were identified by the Greengenes tool Bellerophon (16) and excluded from further analyses. Phylotypes of 16S rRNA genes were determined with the Ribosomal Database Project (RDP) Classifier at a confidence threshold of 80% (88), aligned with SINA Webaligner, and merged with the latest 16S rRNA gene database from the SILVA homepage (www.arb-silva.de) (72). Sequences of 16S rRNA genes were assigned to novel family-level phylotypes if they had <87.5% similarity to the next cultured taxon (93). Sequences of mcrA and fhs were translated in silico and aligned with reference sequences obtained from MegaBLAST using the ClustalW algorithm implemented in ARB software. Coverages were calculated by a previously published protocol (81).
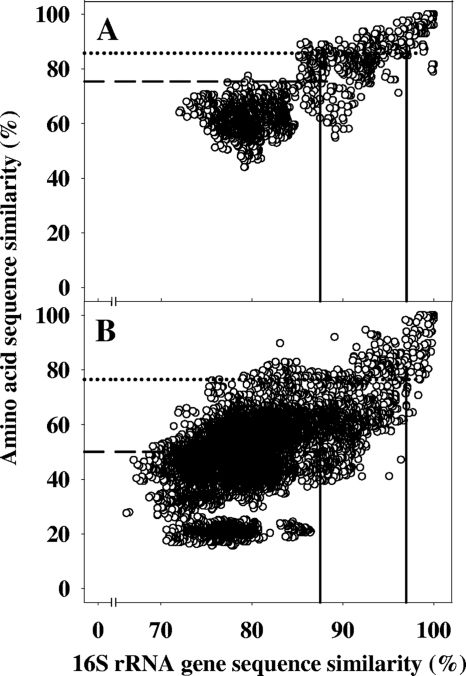
Phylogenic correlation plots (70, 73) of 16S rRNA gene sequence similarities and amino acid sequence similarities of mcrA or fhs were prepared with the following filters: for mcrA, 100% similarity filter and 131 valid amino acids between positions 98 and 227 of mcrA of Methanocella paludicola strain SANAE; for fhs, 100% similarity filter and 351 valid amino acids between positions 134 and 486 of fhs of Clostridium difficile strain 630. Assignment of mcrA and fhs sequences to taxonomic hierarchic phylotypes was based on correlations between amino acid sequences of the translated structural gene to the 16S rRNA gene sequences of cultured organisms (Fig. 1). 16S rRNA gene sequence similarities of 97.0% and 87.5% are conservative threshold values for determining species- and family-level differences, respectively, between organisms (93). These values yielded species- and family-level thresholds for mcrA-encoded amino acid sequences of 85.7% and 75.4%, respectively, and bacterial fhs-encoded amino acid sequences of 76.4% and 50.0%, respectively (Fig. 1).
Fig. 1.
Phylogenic correlation plots of 16S rRNA gene sequence similarities and amino acid sequence similarities of mcrA (A) and fhs (B). Seventy-nine mcrA and 238 fhs sequences are plotted. The vertical solid lines that intersect the horizontal axes at 97% and 87.5% 16S rRNA gene sequence similarities identify species- and family-level phylotype thresholds, respectively. The horizontal dotted and dashed lines that intersect the left vertical axes represent the 90% quantile of pairwise comparisons of mcrA-encoded (A) or fhs-encoded (B) amino acid sequence similarity and 16S rRNA gene sequence similarity.
Phylogenic trees of mcrA and fhs were calculated with neighbor-joining (Dayhoff correction) (77), maximum-likelihood (Jukes-Cantor or Dayhoff correction), and maximum-parsimony methods. mcrA trees used a 100% similarity filter and 131 valid amino acid positions between 98 and 227 of mcrA of Methanocella paludicola SANAE. fhs trees used either a 100% similarity filter and 197 valid amino acids between positions 138 and 335 of fhs of C. difficile 630 or a 100% similarity filter and 175 valid amino acids between positions 292 and 468 of fhs of C. difficile 630. Phylogenetic trees of 16S rRNA genes were calculated with neighbor-joining (Felsenstein correction) (77), AxML, and maximum-parsimony methods. Archaeal 16S rRNA gene sequence trees used a 100% similarity filter and 759 valid nucleotide positions between positions 103 and 894 of the 16S rRNA sequence of E. coli ATCC 11775. Bacterial 16S rRNA gene sequence trees used a 100% similarity filter and 412 valid nucleotide positions between positions 311 and 745 of the 16S rRNA sequence of E. coli ATCC 11775. Calculated trees contained a bootstrap test with 100 to 10,000 replicates (24).
A species or family was considered labeled when its relative abundance in the gene library from heavy fraction 4 of the 13C treatment was higher than its relative abundance in the gene library from heavy fraction 4 of the 12C treatment (79).
Sequencing of mcrA sequence of Methanoplanus limicola DSM 2279.
M. limicola was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ; (Braunschweig, Germany). mcrA of M. limicola was amplified, sequenced, and used as a reference sequence for tree calculations.
Calculations of Gibbs free energy.
The calculated Gibbs free energies (ΔG) are based on the concentrations of reactants and products in the liquid phase of soil microcosms at 15°C and the pH of the liquid phase (87). ΔG° values were calculated from the standard Gibbs energies of formation (ΔGf°) at the pH of the liquid phase (that varied from pH 4.2 to 6.3) and utilized to calculate ΔG values for the following reactions: 4 HCOO− + 4 H+ → CH4 + 3 CO2 + 2 H2O (ΔG° varied from −161 to −208 kJ mol−1 CH4); CH3COO− + H+ → CH4 + CO2 (ΔG° varied from −44 to −50 kJ mol−1 CH4); 4 HCOO− + 3 H+ → CH3COO− + 2 CO2 + 2 H2O (ΔG° varied from −121 to −157 kJ mol−1 acetate). Estimated concentrations of H+ and estimated ΔGf° for H+ are based on the pH of the liquid phase (i.e., the ΔGf° for H+ at pH 4.2 to 6.3 was −23.9 to −35.8 kJ mol−1, respectively [61]).
Analytical techniques.
Dry weight of soil was determined by weighing soil before and after drying at 60°C for 72 h. Iron(II) was determined photometrically (86). Nitrate and sulfate were analyzed with a Dx500 ion chromatograph equipped with an ED 40 detector and AS 4A-SC column (Dionex Corporation, Sunnyvale, CA) at the Center for Analytical Chemistry (Bayreuth Centre of Ecological and Environmental Research, University of Bayreuth, Bayreuth, Germany). The mobile phase was 1.8 mM sodium carbonate and 1.7 mM sodium bicarbonate at a flow rate of 2 ml min−1. The column temperature was 35°C. pH was measured with an InLab R422 pH electrode (InLab Semi-Micro; Mettler Toledo, Giessen, Germany). Organic acids were determined by high-performance liquid chromatography (1090 series II with UV detector; Hewlett Packard, Palo Alto, CA) (91). H2 was measured with a gas chromatograph (5890 series II with a thermal conductivity detector; Hewlett-Packard, Palo Alto, CA) (48). CO2 and methane were separated with a HayeSep-D column (2m by 1/8 in; SRI Instruments, Torrance, CA) and analyzed with a flame ionization detector (SRI Instruments, Torrance, CA). The carrier gas was helium at a flow rate of 40 ml min−1, injector and column temperatures were 60°C, and detector temperature was 380°C. Units of concentration are per gram of soil (dry weight) (g soildw−1). Concentrations of gases are combined concentrations from gas and liquid phases and were calculated from the ideal gas law, taking into consideration the actual pressure, temperature, pH, and volume of gas and liquid phases in incubation flasks (5, 44). The 13C content of acetate was determined by liquid chromatography coupled to isotope ratio mass spectrometry (Finnigan LC IsoLink; Thermo Fisher Scientific Inc., Waltham, MA) (46). Acetate was separated from other organic compounds by high-performance liquid chromatography, oxidation and acid/catalyst reagents (ammonium peroxodisulfate, phosphoric acid, and silver nitrate) were added, and organic compounds were oxidized to CO2 in an oxidation reactor at 100°C (46). CO2 of the liquid phase was degassed by a helium counter-flow, which was then dried in an on-line gas-drying unit and injected into the mass spectrometer (46). Sodium [13C]formate and sodium [13C]bicarbonate were obtained from Campro Scientific (Berlin, Germany). In this study, no distinction is made between CO2 and its carbonate forms.
Nucleotide sequence accession numbers.
The sequences obtained in this study are available from the EMBL nucleotide sequence database under accession numbers FR725451 to FR725861 (mcrA), FR725862 to FR725930 (fhs), FR732102 to FR732501 (bacterial 16S rRNA genes), FR744942 to FR745247 (archaeal 16S rRNA genes), and FR745248 (mcrA from M. limicola DSM 2279).
RESULTS
Effect of supplemental formate and CO2 on product profiles.
Alternative electron acceptors [i.e., approximately 103 μmol of iron(III) and 15 μmol of sulfate g soildw−1] in fen microcosms were reduced during 15 days of anoxic preincubation prior to supplementation of substrates. Sulfate was not detected, and iron(II) reached a stable end concentration at the end of the preincubation period. Nitrate was not detected during the preincubation period (detection limit was 0.13 μmol of nitrate g soildw−1). Concentrations of methane, acetate, and CO2 approximated 2.5, 4, and 85 μmol g soildw−1, respectively, at the end of the preincubation period. A total of approximately 25 μmol methane g soildw−1, 5 μmol acetate g soildw−1, and 2 μmol propionate g soildw−1 were produced in the subsequent 23 days in unsupplemented controls (Fig. 2). Formate and H2 remained below 1 μmol g soildw−1 in unsupplemented controls. Traces of butyrate, isobutyrate, and methyl butyrate were detected after 15 days in all treatments and controls (data not shown).
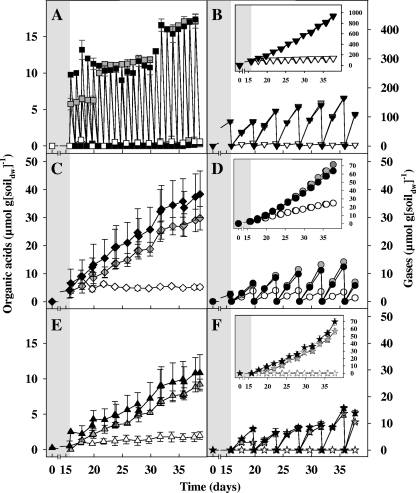
Fig. 2.
Effect of formate on the production of organic acids and gases in anoxic fen soil microcosms at 15°C. Data are for formate (A), CO2 (B), acetate (C), methane (D), propionate (E), and H2 (F). In panel B, CO2 in formate treatments represents the combined CO2 from the bicarbonate pulses and CO2 derived from the apparent conversion of formate to H2 and CO2. Symbols: empty symbols, unsupplemented controls; gray symbols, [12C]formate treatments; black symbols, [13C]formate treatments. Insets show cumulative gas concentrations. Values are the means of triplicate microcosms, and the error bars indicate standard deviations.
Each formate pulse was essentially consumed within 24 h (Fig. 2A). In total, an additional 63 μmol of H2 g soildw−1, 43 μmol of methane g soildw−1, 29 μmol of acetate g soildw−1, and 8 μmol of propionate g soildw−1 were detected in formate treatments compared to unsupplemented controls, indicating that formate stimulated the production of these compounds. The apparent formate-dependent stimulation of the production of H2 suggested that formate-hydrogen lyase-containing taxa were active in formate treatments. Approximately 17 atom% and 1 atom% of acetate-derived carbon was enriched with 13C in the [13C]formate and [12C]formate treatments, respectively, reinforcing the likelihood that acetogens participated in the synthesis of acetate in formate treatments. Formate-derived carbon is incorporated preferentially into the methyl carbon of acetate during acetogenesis (i.e., formate preferentially enters the methyl branch of the acetyl-CoA pathway [54, 63]), and it is therefore possible that the 13C enrichment of [13C]formate-derived acetate was mostly in the methyl carbon.
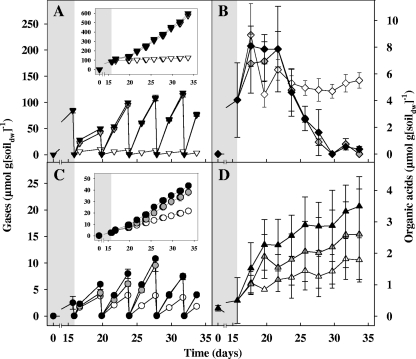
An additional 20 μmol of methane g soildw−1 and 1.7 μmol of propionate g soildw−1 were detected in CO2 treatments compared to unsupplemented controls (Fig. 3), indicating that CO2 stimulated the production of these compounds. Supplemental CO2 also appeared to stimulate the consumption of endogenously produced acetate (i.e., acetate produced before addition of CO2), and the production of methane increased during the disappearance of acetate (Fig. 3B and C).
Fig. 3.
Effect of CO2 on the production of organic acids and gases in anoxic fen microcosms at 15°C. Data are for CO2 (A), acetate (B), methane (C), and propionate (D). Symbols: empty symbols, unsupplemented controls; gray symbols, 12CO2 treatments; black symbols, 13CO2 treatments. Insets show cumulative gas concentrations. Values are the means of triplicate microcosms and the error bars indicate standard deviations.
Product profiles of 13C and 12C treatments (Fig. 2 and 3) were very similar, indicating that similar microbial activities occurred in these treatments. For example, at 23 days postsupplementation, based on the amount of product per gram of soildw (values in parentheses are the percentage of reductant theoretically recovered from formate-derived reductant), approximately 39 μmol of methane (54%), 33 μmol of acetate (46%), 70 μmol of H2 (24%), and 9 μmol of propionate (22%) were produced from 287 μmol of [13C]formate, whereas approximately 47 μmol of methane (70%), 25 μmol of acetate (38%), 57 μmol of H2 (21%), and 7 μmol of propionate (18%) were produced from 267 μmol of [12C]formate (values have been corrected by values from unsupplemented controls). These values indicated that most reducing equivalents from supplemental formate were recovered in methane and acetate. As shown above, recovery of supplemental formate-derived reductant exceeded 100% in both 13C and 12C treatments. A recovery of greater than 100% suggested that supplemental substrate enhanced the use of endogenous substrates, a priming effect observed in other studies (25, 31, 79).
Bioenergetics.
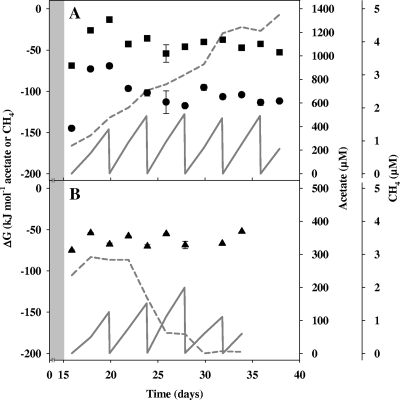
The estimated Gibb's free energies of the apparent formate-dependent methanogenesis and apparent formate-dependent acetogenesis in [13C]formate-supplemented microcosms averaged −104 kJ mol−1 CH4 and −42 kJ mol−1 acetate, respectively (Fig. 4A). The estimated Gibb's free energy of the apparent acetoclastic methanogenesis in 13CO2-supplemented microcosms averaged −64 kJ mol−1 CH4 (Fig. 4B).
Fig. 4.
Estimated Gibbs free energies (ΔG) in the [13C]formate (A) and 13CO2 (B) treatments shown in Fig. 2 and Fig. 3, respectively. Values are the means of triplicate microcosms, and the error bars indicate standard deviations. Symbols: filled circles, ΔG for formate-dependent methanogenesis; filled squares, ΔG for formate-dependent acetogenesis; filled triangles, ΔG for acetate-dependent methanogenesis; dashed line, acetate; solid line, methane in the aqueous phase.
Formate- and CO2-consuming methanogens.
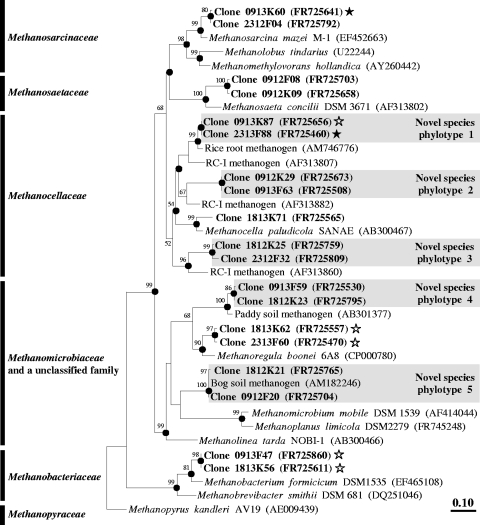
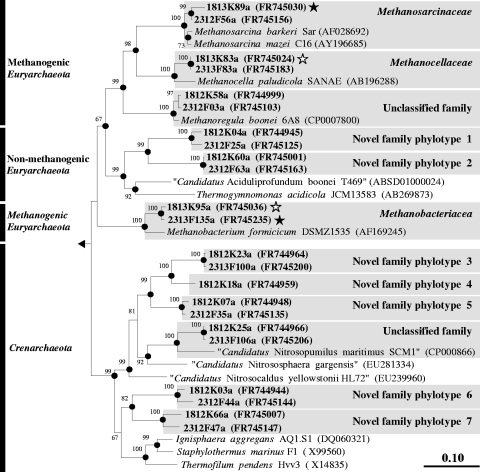
A total of 365 mcrA sequences and 306 archaeal 16S rRNA gene sequences were analyzed from [13C]formate-, [12C]formate-, 13CO2-, and 12CO2-supplemented microcosms. The percentage of methanogens and nonmethanogens in the 16S rRNA gene library approximated 67% and 33%, respectively. Family-level coverage of mcrA and archaeal 16S rRNA genes was >99%. mcrA sequences were affiliated with the Methanocellaceae (48% of total), Methanosarcinaceae (17% of total), Methanomicrobiaceae (15% of total), Methanosaetaceae (4% of total), and Methanobacteriaceae (3% of total) families and with an unclassified family (13% of total, all of which were affiliated with Methanoregula boonei) (Table 1), whereas archaeal 16S rRNA gene sequences were affiliated with Methanocellaceae (27% of total), Methanosarcinaceae (22% of total), Methanobacteriaceae (16% of total), two unclassified families (11% and 1% of total were affiliated with “Candidatus Nitrosopumilus maritimus” and Methanoregula boonei, respectively), and seven novel family-level phylotypes (23% of total) (Table 2). At 9 days postsupplementation, higher relative abundances of Methanobacterium formicicum-affiliated mcrA sequences and novel species-level mcrA phylotype 1 (most closely related to Methanocella paludicola) were obtained from heavy fractions of [13C]formate-supplemented microcosms than from those of [12C]formate-supplemented microcosms (Table 1), indicating that organisms of these phylotypes were early assimilators of formate. In contrast, at this same time interval, higher relative abundances of Methanosarcina mazei-affiliated mcrA sequences and novel phylotype 1 were obtained from heavy fractions of 13CO2-supplemented microcosms than from those of 12CO2-supplemented microcosms (Table 1), indicating that organisms of these phylotype were early assimilators of CO2.
Table 1.
Taxonomic identities and relative abundances of mcrA sequences
| Taxonomic identity (class, family, and genus and species) | Relative abundance of sequence (%) by treatment and time posttreatmenta |
|||||||
|---|---|---|---|---|---|---|---|---|
| Labeled-CO2 treatment |
Labeled-formate treatment |
|||||||
| 9 days |
18 days |
9 days |
23 days |
|||||
| 12C | 13C | 12C | 13C | 12C | 13C | 12C | 13C | |
| Methanobacteria | ||||||||
| Methanobacteriaceae | ||||||||
| Methanobacterium formicicum | — | — | — | 2.1 | 2.3 | 8.7 | 6.3 | 6.5 |
| Methanomicrobia | ||||||||
| Methanocellaceae | ||||||||
| Methanocella paludicola | 2.2 | — | — | 2.1 | — | — | — | — |
| Novel species phylotype 1b | 33.3 | 43.2 | 44.4 | 45.8 | 32.6 | 56.5 | 35.4 | 65.2 |
| Novel species phylotype 2b | 2.2 | — | — | — | 9.3 | 4.3 | — | — |
| Novel species phylotype 3b | — | — | 2.2 | — | 2.3 | — | 2.1 | — |
| Methanomicrobiaceae | ||||||||
| Novel species phylotype 4b | 6.7 | — | 6.7 | 2.1 | 4.7 | 6.5 | 6.3 | 2.2 |
| Novel species phylotype 5b | 11.1 | 6.8 | 15.6 | 14.6 | 11.6 | 8.7 | 10.4 | 8.7 |
| Methanosaetaceae | ||||||||
| Methanosaeta concilii | 11.1 | 2.3 | 6.7 | — | 7.0 | — | 2.1 | — |
| Methanosarcinaceae | ||||||||
| Methanosarcina mazei | 17.8 | 38.6 | 15.6 | 20.8 | 11.6 | 2.2 | 27.1 | 2.2 |
| Unclassified family | ||||||||
| Methanoregula boonei | 15.6 | 9.1 | 8.9 | 12.5 | 18.6 | 13.0 | 10.4 | 15.2 |
| Total no. of sequences | 45 | 44 | 45 | 48 | 43 | 46 | 48 | 46 |
—, not detected.
Sequences were considered to be novel at the species level when the mcrA sequence was <85.7% identical to that of the next cultured species (Fig. 1A).
Table 2.
Class-and family-level identities and relative abundances of archaeal 16S rRNA gene sequences
| Taxonomic identity (class and family) | Relative abundance of sequence (%) by treatment (time postsupplementation) |
|||
|---|---|---|---|---|
| Labeled-CO2 treatment (18 days) |
Labeled-formate treatment (23 days) |
|||
| 12C | 13C | 12C | 13C | |
| Methanobacteria | ||||
| Methanobacteriaceae | 2.4 | 12.8 | 14.9 | 33.8 |
| Methanomicrobia | ||||
| Methanocellaceae | 9.6 | 16.7 | 41.9 | 40.8 |
| Methanosarcinaceae | 15.7 | 56.4 | 10.8 | 5.6 |
| Unclassified familya | 1.2 | 1.3 | 1.4 | 1.4 |
| Unclassified Euryarchaeotab | ||||
| Novel family phylotype 1c | 4.8 | —d | 2.7 | — |
| Novel family phylotype 2c | 1.2 | — | 1.4 | — |
| Unclassified Crenarchaeotab | ||||
| Unclassified familye | 26.5 | — | 8.1 | 8.5 |
| Novel family phylotype 3c | 22.9 | 1.3 | 4.1 | 5.6 |
| Novel family phylotype 4c | 2.4 | — | — | — |
| Novel family phylotype 5c | 2.4 | — | 4.1 | — |
| Novel family phylotype 6c | 1.2 | — | 2.7 | 4.2 |
| Novel family phylotype 7c | 9.6 | 11.5 | 8.1 | — |
| Total no. of archaeal sequences: | 83 | 74 | 78 | 71 |
Closest related cultivated species, Methanoregula boonei (CP0007800), with 95 to 98% 16S rRNA gene similarity.
Family-level phylotypes listed underneath do not necessarily belong to the same class.
Sequences were considered to be novel at the family level when the 16S rRNA gene sequence was <87.5% identical to that of the next cultured species (92).
—, not detected.
Closest related cultivated species, “Candidatus Nitrosopumilus maritimus” (CP000866), with 88 to 89% 16S rRNA gene similarity.
At 23 days postsupplementation, higher relative abundances of Methanocellaceae-affiliated mcrA sequences and Methanobacteriaceae-affiliated 16S rRNA gene sequences were obtained from heavy fractions of [13C]formate-supplemented microcosms than from those of [12C]formate-supplemented microcosms (Tables 1 and 2). In contrast, at 18 days postsupplementation, higher relative abundances of Methanobacteriaceae-, Methanocellaceae-, and Methanosarcinaceae-affiliated 16S rRNA gene sequences were obtained from heavy fractions of 13CO2-supplemented microcosms than from those of 12CO2-supplemented microcosms (Table 2). Relative abundances of Methanosarcinaceae-affiliated mcrA sequences were also higher at 18 days postsupplementation in heavy fractions of 13CO2-supplemented microcosms than in those of 12CO2-supplemented microcosms (Table 1). At the end of incubation, Methanoregula-affiliated mcrA sequences were marginally higher in heavy fractions of 13CO2- and [13C]formate-supplemented microcosms than in those of 12CO2- and [12C]formate-supplemented microcosms (Table 1). Methanocella paludicola, Methanosarcina mazei, Methanosarcina barkeri, Methanobacterium formicicum, and Methanoregula boonei were the cultivated species most closely related to labeled mcrA and 16S rRNA gene phylotypes (Fig. 5 and 6).
Fig. 5.
Phylogenic neighbor-joining tree of representative species-level amino acid sequences encoded by mcrA retrieved from formate and CO2 treatments and of reference sequences. Values next to the branches represent the percentages of replicate trees (>50%) in which the associated taxa clustered together in the bootstrap test (1,000 bootstraps). Dots at nodes indicate the confirmation of tree topology by maximum-likelihood and maximum-parsimony calculations with the same data set. Bar indicates a 0.1 estimated change per amino acid. Clones are identified, in order, by the number of days postsupplementation (09, 18, or 23) the type of treatment (12K, 12CO2 treatment; 12F, [12C]formate treatment; 13K, 13CO2 treatment; 13F, [13C]formate treatment) and clone number. Accession numbers are given in parentheses. Symbols: filled stars, labeled phylotypes; empty stars, marginally labeled phylotypes.
Fig. 6.
Phylogenic neighbor-joining tree of representative family-level archaeal 16S rRNA gene sequences retrieved from formate and CO2 treatments and reference sequences. Values next to the branches represent the percentages of replicate trees (>50%) in which the associated taxa clustered together in the bootstrap test (10,000 bootstraps). Dots at nodes indicate the confirmation of tree topology by maximum-likelihood and maximum-parsimony calculations with the same data set. Quotation marks indicate provisional taxa (J. P. Euzéby, List of Prokaryotic Names with Standing in Nomenclature [http://www.bacterio.cict.fr/number.html]). Bar indicates a 0.1 estimated change per amino acid. E. coli (X80725) was used as an outgroup. Clones are identified, in order, by the number of days postsupplementation (18 or 23) the type of treatment (12K, 12CO2 treatment; 12F, [12C]formate treatment; 13K, 13CO2 treatment; 13F, [13C]formate treatment) and clone number, followed by “a” to indicate archaeal sequence. Accession numbers are given in parentheses. Symbols: filled stars, labeled phylotypes; empty stars, marginally labeled phylotypes.
Bacterial diversity.
A total of 393 bacterial 16S rRNA gene sequences and 69 fhs sequences were analyzed. Family-level coverage of 16S rRNA gene sequences and fhs approximated 94% and 93%, respectively. Twenty-eight of the 58 detected bacterial 16S rRNA family-level phylotypes did not have cultured representatives (see Fig. S2 in the supplemental material). Bacterial 16S rRNA gene sequences were affiliated with the phyla Proteobacteria (32% of total), Acidobacteria (28% of total), Actinobacteria (27% of total), Firmicutes (1% of total), Planctomycetes (1% of total), Verrucomicrobia (1% of total), Bacteroidetes (<1% of total), Chloroflexi (<1% of total), Spirochaetes (<1% of total), and unclassified taxa (8% of total) (Table 3; see also Table S1 and Fig. S2 in the supplemental material).
Table 3.
Classes and relative abundances of bacterial 16S rRNA gene sequences
| Class | Relative abundance of sequence (%) by treatment (time postsupplementation)a |
|||
|---|---|---|---|---|
| Labeled-CO2 treatment (18 days) |
Labeled-formate treatment (23 days) |
|||
| 12C | 13C | 12C | 13C | |
| Acidobacteria | 35.2 | 23.5 | 26.9 | 18.2 |
| Actinobacteria | 21.6 | 38.2 | 26.9 | 20.2 |
| Alphaproteobacteria | 22.7 | 19.6 | 16.3 | 40.4 |
| Bacteroidia | — | — | — | 1.0 |
| Betaproteobacteria | 3.4 | — | 10.6 | 5.1 |
| Clostridia | 1.1 | 1.0 | — | 2.0 |
| Deltaproteobacteria | 3.4 | 2.9 | 1.0 | 1.0 |
| Gammaproteobacteria | — | — | — | 2.0 |
| Holophagae | 2.3 | 2.0 | 1.9 | 4.0 |
| Ktedonobacteria | — | — | 1.0 | 1.0 |
| Opitutae | 1.1 | — | — | — |
| Planctomycea | 1.1 | 1.0 | 1.0 | 1.0 |
| Sphingobacteria | — | — | 1.0 | — |
| Spirochaetes | — | 2.0 | — | — |
| Verrucomicrobiae | 1.1 | — | 1.9 | — |
| Unclassifiedb | 6.8 | 9.8 | 11.5 | 4.0 |
| Total no. of bacterial sequences | 88 | 102 | 104 | 99 |
Detailed analyses of bacterial sequences are provided in Table S1 in the supplemental material. —, not detected.
16S rRNA gene sequences were assigned to unclassified taxa when the sequence was <78.4% identical to that of the next cultured species (92). Thus, these novel phylotypes could represent novel phyla.
Fifteen species-level fhs phylotypes were detected and affiliated with the families Phyllobacteriaceae (36% of total), Acetobacteraceae (34% of total), Rhodobacteraceae (10% of total), Verrucomicrobiaceae (9% of total), Oceanospirillaceae (3% of total), Hyphomicrobiaceae (1% of total), Hyphomonadaceae (1% of total), Thermoanaerobacteraceae (1% of total), Veillonellaceae (1% of total), one novel family (1% of total), and one unclassified family (3% of total, all of which were affiliated with “Candidatus Pelagibacter sp.”) (see Fig. S3 and Table S2 in the supplemental material). None of the 15 detected species-level fhs phylotypes had cultured representatives. fhs sequences affiliated with Phyllobacteriaceae (36% of total) and Acetobacteraceae (34% of total) were the most abundant fhs phylotypes obtained from both [13C]formate and [12C]formate treatments. These fhs phylotypes were most closely related to Mesorhizobium loti (67 to 83% fhs amino acid similarity) and Granulibacter bethesdensis (67 to 82% fhs amino acid similarity) (see Fig. S3). Two fhs phylotypes detected in formate-pulsed microcosms were related to Sporomusa ovata (72% fhs amino acid similarity) and Moorella thermoacetica (74% fhs amino acid similarity) (see Fig. S3), indicating that the fen soil harbors organisms belonging to the monophyletic acetogenic genera Moorella and Sporomusa (17, 18, 21, 89).
Formate- and CO2-consuming Bacteria.
Higher relative abundances of Acetobacteraceae- and Rhodospirillaceae-affiliated 16S rRNA gene sequences (Alphaproteobacteria) were obtained from heavy fractions of [13C]formate-supplemented microcosms than from those of [12C]formate-supplemented microcosms (see Table S1 in the supplemental material), indicating that organisms of these phylotypes assimilated formate. The relative abundances of Acidimicrobiaceae-affiliated 16S rRNA gene sequences (Actinobacteria) was marginally higher in heavy fractions of [13C]formate-supplemented microcosms than in those of [12C]formate-supplemented microcosms (see Table S1 in the supplemental material), indicating that organisms of this phylotype could have assimilated a marginal amount of formate. In contrast, higher relative abundances of Conexibacteraceae- and Solirubrobacteraceae-affiliated 16S rRNA gene sequences (Actinobacteria) were obtained from heavy fractions of 13CO2-supplemented microcosms than from those of 12CO2-supplemented microcosms (see Table S1), indicating that these phylotypes assimilated CO2. The relative abundance of Thermomonosporaceae-affiliated 16S rRNA gene sequences (Actinobacteria) was marginally higher in heavy fractions of 13CO2-supplemented microcosms than in those of 12CO2-supplemented microcosms (see Table S1), indicating that this phylotype could have assimilated a marginal amount of CO2. None of the labeled taxa are known to contain acetogens. The cultivated species most closely related to the labeled bacterial 16S rRNA phylotype were Rhodovastum atsumiense (90 to 97% 16S rRNA gene similarity), Acidimicrobium ferrooxidans (87 to 91% 16S rRNA gene similarity), Rhodocista centenaria (88 to 93% 16S rRNA gene similarity), Conexibacter woesei (84 to 94% 16S rRNA gene similarity), Solirubrobacter soli (85 to 95% 16S rRNA gene similarity), and Actinomadura formosensis (85 to 99% 16S rRNA gene similarity) (see Fig. S2).
DISCUSSION
Formate and CO2 constitute potential trophic links to both methanogenesis and acetogenesis (21, 34, 95). Both formate and CO2 experimentally stimulated methanogenesis, whereas only formate stimulated acetate synthesis. CO2-dependent stimulation of methanogenesis suggests that endogenously available CO2 limited methane production under the experimental conditions of this study. Whether such limitation occurs in situ is unknown, but the findings underscore the importance that CO2 availability might have for optimal methanogenesis. The availability of CO2 can affect the metabolic capacities of certain acetogens (21). That CO2 did not appear to stimulate acetate production suggests that the endogenous reductant available for acetogenesis was limiting. However, this speculation must be qualified since (i) endogenously produced acetate was likely not restricted to acetogenesis (i.e., acetate can be produced by nonacetogens [21]) and (ii) acetoclastic methanogenesis appeared to be stimulated in CO2 treatments, thus resulting in a consumption of acetate that might mask acetate production.
Taxa associated with formate- and CO2-enhanced methanogenesis.
The collective labeling data indicated that Methanobacteriaceae and Methanocellaceae assimilated formate-derived carbon and that Methanobacteriaceae, Methanocellaceae, and Methanosarcinaceae assimilated CO2-derived carbon. The labeling of the same taxa in both formate and CO2 treatments is consistent with the capacity of many methanogens to utilize both formate and CO2 (34).
Methanobacterium formicicum was the most closely related cultured species to labeled Methanobacteriaceae-affiliated phylotypes (89 to 95% mcrA amino acid similarity and 89 to 93% 16S rRNA gene similarity) (Fig. 5 and 6). Certain members of the family Methanobacteriaceae can utilize CO2, H2, and formate for methane production (9). [13C]-formate yielded labeling of Methanobacteriaceae-affiliated phylotypes whereas 13CO2 yielded marginal labeling of these phylotypes (Tables 1 and 2), indicating that growth of fen Methanobacteriaceae-related species was more robust with supplemental formate than with supplemental CO2 and endogenous reductant.
Methanocella paludicola was the most closely related cultured species to labeled Methanocellaceae-affiliated phylotypes in both formate and CO2 treatments (79 to 83% mcrA amino acid similarity and 86 to 97% 16S rRNA gene similarity) (Fig. 5 and 6). M. paludicola was isolated from rice paddy soil as the first cultured species within rice cluster I is a mesophilic methanogen capable of utilizing formate, CO2, and H2 and can use acetate as a source of carbon (78). The detection of novel labeled Methanocella-related mcrA and 16S rRNA gene sequences in formate and CO2 treatments (Tables 1 and 2) indicated that novel species of Methanocella used formate and CO2 for methane production and possibly acetate as a source of carbon in anoxic fen microcosms.
Methanosarcina mazei (88 to 96% mcrA amino acid similarity, 93 to 98% 16S rRNA gene similarity) and Methanosarcina barkeri (87 to 96% mcrA amino acid similarity, 94 to 98% 16S rRNA gene similarity) were the most closely related cultured species to labeled Methanosarcinaceae-affiliated phylotypes (Fig. 5 and 6). M. mazei and M. barkeri utilize acetate and H2-CO2 but do not utilize formate (34). Formate is not known to be utilized by species of Methanosarcina (34). Methanosarcina-related phylotypes were labeled in 13CO2-supplemented microcosms in which the disappearance of acetate was concomitant to the production of methane but were not labeled in [13C]formate-supplemented microcosms in which 13C-enriched acetate accumulated (Tables 1 and 2). Certain Methanosarcina species may not be able to utilize acetate under certain conditions (51, 62). Although Methanosarcina-affiliated phylotypes could have theoretically assimilated 13CO2-produced [13C]acetate in the 13CO2 treatment, there was no evidence for the stimulation of acetate production in CO2 treatments, and the [13C]formate treatment in which labeled acetate was produced did not yield labeling of Methanosarcina-affiliated phylotypes. It thus seems likely that Methanosarcina-related phylotypes used CO2 for methane production at the expense of endogenous reductant.
Methanoregula boonei was the most closely related cultured species to the marginally labeled unclassified methanogenic family (81 to 90% mcrA amino acid similarity, 95 to 98% 16S rRNA gene similarity) (Fig. 5 and 6). M. boonei was isolated from an acidic bog, grows by hydrogenotrophic methanogenesis, requires small amounts of acetate as a source of carbon, and cannot utilize formate (7). On the other hand, Methanoregula formicica can use formate and H2 for methanogenesis (92). The late marginal labeling (Table 1) of M. boonei-related phylotypes might have occurred by assimilation of [13C]-formate, [13C]-formate-derived 13CO2 and/or [13C]-acetate formed by acetogens.
Acetoclastic methanogenesis.
Although supplemental acetate does not stimulate methanogenesis in anoxic Schlöppnerbrunnen fen microcosms (38, 90), methane production was concomitant to the disappearance of acetate in CO2-supplemented microcosms (Fig. 3). Acetate can be used as a source of carbon by hydrogenotrophic methanogens such as M. paludicola (78), utilized by acetoclastic methanogens like Methanosaetaceae (34), or oxidized in a syntrophic partnership (33). The greatest production of methane in CO2 treatments was coincident with the disappearance of acetate (Fig. 3). The increased concentration of CO2 or the periodic increase in pH (i.e., up to approximately 5) could have enhanced acetoclastic methanogenesis in CO2 treatments. For example, acetoclastic methanogenesis by M. barkeri MS does not occur at low pH (e.g., pH 4.5) (62). Furthermore, the estimated Gibbs free energy for the apparent acetoclastic methanogenesis in the CO2 treatments was exergonic (Fig. 4B), and mcrA sequences related to Methanosaetaceae (a taxon that can only use acetate for methanogenesis [34]) were more abundant when acetate was being consumed (Fig. 3 and Table 1). Although the syntrophic oxidation of acetate is well documented for high-temperature habitats, its occurrence at low temperatures is less well understood (33, 69). Acetoclastic methanogenesis is thermodynamically more favorable than syntrophic acetate oxidation at 15°C in sediments of Lake Kinneret (Israel) (69). Thus, it is likely that the consumption of acetate was at least partially linked to acetoclastic methanogenesis and that Methanosaetaceae-affiliated methanogens were participants in this consumption. The inability of supplemental acetate to stimulate methanogenesis (38, 90) suggests that acetoclastic methanogens cannot utilize substantially more acetate than that produced endogenously or that supplemental acetate is toxic (60).
Formate-dependent acetogenesis.
The concomitant formate-dependent stimulation of the production of methane and acetate (Fig. 2), the enrichment of 13C in acetate in [13C]formate treatments, and the estimated exergonic Gibbs free energies for the apparent formate-driven acetogenesis and methanogenesis (Fig. 4A) suggest that acetogens and methanogens competed for supplemental formate. The most closely related cultured species to the labeled Acetobacteraceae- and Rhodospirillaceae-affiliated phylotypes and the marginally labeled Acidimicrobiaceae-affiliated phylotypes are not known to be capable of acetogenesis, but some are capable of either anaerobic phototrophic growth with organic acids or anaerobic growth via the reduction of iron (11, 26, 37). The high relative abundances of Phyllobacteriaceae- and Acetobacteraceae-affiliated sequences in fhs libraries (see Table S2 in the supplemental material) indicated that these taxa might have been involved in formate-dependent processes. fhs sequences most closely affiliated with the acetogens S. ovata and Moorella thermoacetica (17, 18, 21, 89) were detected in formate-pulsed microcosms. Although species of Sporomusa and Moorella have not been previously isolated from acidic fens, species of these genera have been isolated from various soils (17, 18, 21, 89). Spirochaetaceae and Holophagaceae contain acetogenic species (8, 56), and Spirochaetaceae- and Holophagaceae-affiliated 16S rRNA gene sequences were likewise detected. Two novel family-level phylotypes were also detected within the Clostridia, a class that contains many acetogenic clostridial species (20, 21). However, a labeling of sequences affiliated with these acetogen-containing taxa was not apparent.
The capacity of acetogens to utilize very diverse organic compounds (18, 21) might result in a very limited assimilation of formate-derived carbon, with dissimilation (i.e., the conservation of energy) being the primary purpose of formate utilization. Alternatively, the extent of replication of acetogens at the expense of formate might have been inadequate relative to detectable labeling, a possibility reinforced by the relatively poor thermodynamics of formate-coupled acetogenesis (Fig. 4A). In addition, the large uncultured diversity of detected 16S rRNA gene-based phylotypes and the broad distribution of fhs in prokaryotic taxa (35, 53) suggest that highly diverse previously uncultivated fhs-containing taxa are likely present in fen soil and therefore might not have been efficiently targeted with the fhs primers. In this regard, a recent study in which new fhs primers were developed for accessing acetogens in the rumen found that the majority of retrieved fhs sequences were affiliated with nonacetogenic taxa and identified potential acetogens that were not closely related to known acetogens (35). These results highlight the difficulty in targeting highly novel acetogens with fhs primers designed from the currently available sequences in public databases. It is thus possible that hitherto unknown acetogens were involved in the acetogenic consumption of supplemental formate and that the fhs analysis failed to detect them.
Many acetogens are nonmonophyletic; i.e., they are phylogenetically distributed with nonacetogens in the same genera (18, 20, 21), a factor complicating their assessment by standard 16S rRNA gene analysis. Several organisms originally described as nonacetogens have been later discovered to be acetogenic (e.g., Clostridium glycolicum [20, 21, 49]), thus raising the question as to whether any of the detected nonacetogenic taxa might contain heretofore unknown acetogenic capabilities. The recent isolations of taxonomically and physiologically novel acetogens such as Alkalibaculum bacchi (3) and Moorella perchloratireducens (4) illustrate the existence of hitherto unknown acetogens in various ecosystems. In addition, certain archaea (i.e., Methanosarcina acetivorans and Archaeoglobus fulgidus) are capable of carbon monoxide-dependent acetogenesis in pure culture (36, 55), suggesting that archaeal taxa might have participated in the formation of acetate. However, archaeal formate-driven acetogenesis has not been documented, and an obvious labeling of a nonmethanogenic archaea was not detected.
General diversity and additional CO2- and formate-metabolizing taxa.
A high degree of novelty was detected in the 16S rRNA gene sequence data set (Fig. 6; see also Fig. S2 in the supplemental material). Most of the detected bacterial 16S rRNA gene sequences were affiliated with the phyla Proteobacteria and Acidobacteria (Table 3), taxa that have been observed to be dominant in other boggy soils (15, 40, 43). The detection of the phyla Actinobacteria, Bacteroidetes, Chloroflexi, Firmicutes, Planctomycetes, Spirochaetes, and Verrucomicrobia is likewise consistent with the occurrence of these taxa in other fens and bogs (15, 40, 43). Previous studies have documented the occurrence of Crenarchaeota in the fen Schlöppnerbrunnen (32, 90), and half of the detected archaeal 16S rRNA phylotypes were affiliated with this phylum (Table 2). The Crenarchaeota was earlier thought to contain only obligate thermophiles (28) but has since been shown to contain mesophiles (e.g., 84), including “Ca. Nitrosopumilus maritimus” (42) to which 11% of the detected archaeal 16S rRNA gene sequences were distantly affiliated (Table 2 and Fig. 6). Additional distantly related cultured genera of the detected crenarchaeotal phylotypes grow anaerobically with peptides and sulfur (e.g., Thermofilum and Staphylothermus [28]) (Fig. 6). It is likely that Crenarchaeota-affiliated organisms have anaerobic physiological capabilities not currently represented in cultured crenarchaeotal taxa.
Certain 16S rRNA gene sequences detected in [13C]formate or [12C]formate treatments were affiliated with Acidimicrobiaceae, Sinobacteraceae, Rhodocyclaceae, and Holophagaceae, taxa capable of organic acid-coupled anaerobic growth and the reduction of electron acceptors other than CO2 (11, 12, 23, 27). The reduction of alternative electron acceptors in fen soil microcosms during the preincubation period supports previous studies that have identified different anaerobic metabolic activities in fen soil (57, 71). The nearest cultured species of labeled Conexibacteraceae and Solirubrobacteraceae (i.e., C. woesei and S. soli, respectively) and marginally labeled Thermomonosporaceae (i.e., A. formosensis) are not known to be capable of anaerobic growth (41, 45, 66), thus raising questions about the metabolic potentials these taxa might have under anoxic conditions. Rhodospirillum rubrum of the family Rhodospirillaceae contains formate-hydrogen lyase (26), and the labeling of Rhodospirillaceae-affiliated taxa in formate treatments suggest that they might have been involved in the apparent production of H2 from formate.
Approximately one-fifth of the apparent formate-derived reductant was accounted for in propionate. Propionate can be formed anaerobically by Desulfobulbus propionicus from acetate, CO2, and H2 via the reversal of syntrophic propionate oxidation (50). The reductive formation of propionate from acetate, CO2, and H2 is associated with rice roots and might be linked to organisms with physiological properties similar to D. propionicus (e.g., ethanol fermentation or sulfate reduction) or to syntrophic propionate oxidizers that can also catalyze the synthesis of propionate from acetate, CO2, and H2 (13). The ΔG values for this reaction with rice roots ranged from −15 to −38 kJ mol−1 propionate (13). A minor fraction of the 16S rRNA gene sequences from the [13C]formate treatment were closely related to the Desulfuromonadaceae-affiliated ethanol fermenter Pelobacter propionicus (96.4 to 96.9% similarity) (see Table S1 and Fig. S2 in the supplemental material), an organism with physiological properties that overlap those of D. propionicus (80). Furthermore, 16S rRNA gene sequences related to the Syntrophobacteraceae-affiliated syntrophic propionate oxidizer Syntrophobacter wolinii (94.3%) (6) were detected in CO2 treatments (see Table S1 and Fig. S2). The detection of these taxa in fen soils (32, 43, 57) and the availability of acetate, CO2, and H2 in formate treatments suggest that propionate production might have been catalyzed by microorganisms capable of reductive propionate formation.
Conclusions, limitations, and future perspectives.
Detected labeling patterns indicated that methanogens and nonmethanogens concomitantly assimilated the same substrate. Likewise, methanogens and acetogens appeared to concomitantly dissimilate formate. These observations indicate that differing taxa competed for the same substrate under experimental conditions. The daily pulsing of low concentrations of formate was designed to achieve adequate labeling without grossly exceeding in situ relevant concentrations of formate (47). However, although pH was periodically controlled, temporal fluctuations in pH due to the formation of fatty acids yielded changing conditions not fully representative of in situ conditions. Furthermore, because of potential biases introduced by stable isotope probing and the different labeling patterns of mcrA sequences and methanogen-derived 16S rRNA gene sequences, one cannot exclude the possibility that taxa that were unlabeled or only marginally labeled were more involved in substrate utilization than indicated by the labeling patterns (i.e., assimilation may not have been tightly linked to dissimilation for all dissimilating taxa). In this regard, endogenous organic carbon rather than supplemental formate or CO2 was likely a major source of cell carbon since highly oxidized one-carbon substrates are less than ideal for most taxa relative to biomass synthesis (i.e., more reduced carbonaceous substrates are preferred by heterotrophs). Nonetheless, within the constraints of these limitations, the current study resolved methanogenic taxa potentially involved in the emission of methane from the acidic fen Schlöppnerbrunnen and extended previous findings on the acetogenic potentials of this fen (90). Although acetogenic taxa were found in the fhs and 16S rRNA gene libraries, a labeling of a known acetogenic taxon was not detected. It thus remains uncertain which microbes were responsible for the apparent conversion of formate to acetate and whether dissimilation of formate to acetate was concomitant with the acetogenic assimilation of formate. That formate-derived H2 accumulated in anoxic microcosms suggested that H2 production exceeded the H2-consuming capacities of hydrogenotrophic methanogens. This speculation is consistent with the fact that cultivated H2-forming fermenters, a likely origin of the apparent formate-hydrogen lyase activity in fen soil, can outnumber cultivated H2-consuming methanogens in soil from the Schlöppnerbrunnen fen by a factor of approximately 1,000 (19, 90). The apparent concomitant occurrence of methanogenesis and formate-hydrogen lyase activity suggests that certain methanogenic taxa might have utilized formate-derived H2 for a source of reductant. Current studies focus on determining potential differences between formate- and H2-utilizing methanogenic taxa, identifying the formate-hydrogen lyase-associated taxa, and further resolving the fen acetogens, a functional group that has remained difficult to characterize at the level of the taxa.
Supplementary Material
ACKNOWLEDGMENTS
We thank Katharina Palmer for helpful suggestions, Peter Claus for assistance with the analysis of [13C]acetate, Yin Chen (University of Warwick) for assistance with the stable isotope probing analyses, Charles R. Lovell (University of South Carolina) for assistance with the fhs analysis, and Volker Müller (University of Frankfurt) for the culture of Methanosarcina mazei Gö1.
We thank the Deutsche Forschungsgemeinschaft (DR310/3-2, HO4020/2-2), the Max Planck Institute for Terrestrial Microbiology, and the University of Bayreuth for financial support.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 8 April 2011.
REFERENCES
- 1. Abbanat D. R., Aceti D. J., Baron S. F., Terlesky K. C., Ferry J. C. 1989. Microbiology and biochemistry of the methanogenic archaeobacteria. Adv. Space Res. 9:101–105 [Google Scholar]
- 2. Ahmed Z., Banu H., Rahman M. M., Akhter F., Haque M. S. 2001. Microbial activity on the degradation of lignocellulosic polysaccharides. J. Biol. Sci. 1:993–997 [Google Scholar]
- 3. Allen T. D., Caldwell M. E., Lawson P. A., Huhnke R. L., Tanner R. S. 2010. Alkalibaculum bacchi gen. nov., sp. nov., a CO-oxidizing, ethanol-producing acetogen isolated from livestock-impacted soil. Int. J. Syst. Evol. Micorbiol. 60:2483–2489 [DOI] [PubMed] [Google Scholar]
- 4. Balk M., van Gelder T., Weelink S. A., Stams A. J. M. 2008. (Per)chlorate reduction by the thermophilic bacterium Moorella perchloratireducens sp. nov., isolated from underground gas storage. Appl. Environ. Microbiol. 74:403–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blachnik R. 1998. D'Ans Lax Taschenbuch für Chemiker und Physiker, 4th ed., vol. 3 Springer, Berlin, Germany [Google Scholar]
- 6. Boone D. R., Bryant M. P. 1980. Propionate-degrading bacterium, Syntrophobacter wolinii sp. nov. gen. nov., from methanogenic ecosystems. Appl. Environ. Microbiol. 40:626–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bräuer S., Cadillo-Quiroz H., Ward R. J., Yavitt J., Zinder S. 2011. Methanoregula boonei gen. nov., sp. nov., an acidiphilic methanogen isolated from an acidic peat bog. Int. J. Syst. Evol. Microbiol. 61:45–52 [DOI] [PubMed] [Google Scholar]
- 8. Breznak J. A., Leadbetter J. R. 2006. Termite gut Spirochetes, p. 318–329 In Dworkin M., Falkow S., Rosenberg E., Schleifer K.-H., Stackebrandt E. (ed.), The prokaryotes, 3rd ed., vol. 7 Springer, New York, NY [Google Scholar]
- 9. Bryant M. P., Boone D. R. 1987. Isolation and characterization of Methanobacterium formicicum MF. Int. J. Syst. Bacteriol. 37:171 [Google Scholar]
- 10. Cadillo-Quiroz H., Yashiro E., Yavitt J. B., Zinder S. H. 2008. Characterization of the archaeal community in a minerotrophic fen and terminal restriction fragment length polymorphism-directed isolation of a novel hydrogenotrophic methanogen. Appl. Environ. Microbiol. 74:2059–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clark D. A., Norris P. R. 1996. Acidimicrobium ferrooxidans gen. nov., sp. nov.: mixed-culture ferrous iron oxidation with Sulfobacillus species. Microbiology 142:785–790 [DOI] [PubMed] [Google Scholar]
- 12. Coates J. D., Ellis D. J., Gaw C. V., Lovley D. R. 1999. Geothrix fermentans gen. nov., sp. nov., a novel Fe(III)-reducing bacterium from a hydrocarbon-contaminated aquifer. Int. J. Syst. Bacteriol. 49:1615–1622 [DOI] [PubMed] [Google Scholar]
- 13. Conrad R., Klose M. 1999. Anaerobic conversion of carbon dioxide to methane, acetate and propionate on washed rice roots. FEMS Microbiol. Ecol. 30:147–155 [DOI] [PubMed] [Google Scholar]
- 14. Daniel S. L., Drake H. L. 1993. Oxalate- and glyoxylate-dependent growth and acetogenesis by Clostridium thermoaceticum. Appl. Environ. Microbiol. 59:3062–3069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dedysh S. N., Pankratov T. A., Belova S. E., Kulichevskaya I. S., Liesack W. 2006. Phylogenetic analysis and in situ identification of bacteria community composition in an acidic Sphagnum peat bog. Appl. Environ. Microbiol. 72:2110–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DeSantis T. Z., et al. 2006. NAST: a multiple sequence alignment server for comparative analysis of 16S rRNA genes. Nucleic Acids Res. 34:W394–W399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Drake H. L. 2009. Sporomusa, p. 1112–1116 In De Vos P., et al. (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 3 Springer, New York, NY [Google Scholar]
- 18. Drake H. L., Gössner A. S., Daniel S. L. 2008. Old acetogens, new light. Ann. N. Y. Acad. Sci. 1125:100–128 [DOI] [PubMed] [Google Scholar]
- 19. Drake H. L., Horn M. A., Wüst P. K. 2009. Intermediary ecosystem metabolism as a main driver of methanogenesis in acidic wetland soil. Environ. Microbiol. Rep. 1:307–318 [DOI] [PubMed] [Google Scholar]
- 20. Drake H. L., Küsel K. 2005. Acetogenic clostridia, p. 719–746 In Dürre P. (ed.), Handbook on Clostridia. CRC Press, Boca Raton, FL [Google Scholar]
- 21. Drake H. L., Küsel K., Matthies C. 2006. Acetogenic prokaryotes, p. 354–420 In Dworkin M., Falkow S., Rosenberg E., Schleifer K.-H., Stackebrandt E. (ed.), The prokaryotes, 3rd ed., vol. 2 Springer, New York, NY [Google Scholar]
- 22. Egert M., Friedrich M. W. 2003. Formation of pseudo-terminal restriction fragments, a PCR-related bias affecting terminal restriction fragment length polymorphism analyses of microbial community structure. Appl. Environ. Microbiol. 69:2555–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fahrbach M., et al. 2008. Steroidobacter denitrificans gen. nov., sp. nov., a steroidal hormone-degrading gammaproteobacterium. Int. J. Syst. Evol. Microbiol. 58:2215–2223 [DOI] [PubMed] [Google Scholar]
- 24. Felsenstein J. 1985. Confidence limits on phylogenies—an approach using the bootstrap. Evolution 39:783–791 [DOI] [PubMed] [Google Scholar]
- 25. Fontaine S., et al. 2004. Mechanisms of the priming effect in a Savannah soil amended with cellulose. Soil Sci. Soc. Am. J. 68:125–131 [Google Scholar]
- 26. Garrity G. M., Bell J. A., Lilburn T. 2005. Family I. Rhodospirillaceae, p. 1–40 In Brenner D. J., Krieg N. R., Staley J. T. (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 2 Springer, New York, NY [Google Scholar]
- 27. Garrity G. M., Bell J. A., Lilburn T. 2005. Family I. Rhodocyclaceae, p. 887–922 In Brenner D. J., Krieg N. R., Staley J. T. (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 2 Springer, New York, NY [Google Scholar]
- 28. Garrity G. M., Holt J. G. 2001. Phylum AI. Crenarchaeota phy. nov., p. 169–210 In Boone D. R., Castenholz R. W., Garrity G. M. (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 1 Springer, New York, NY [Google Scholar]
- 29. Gore A. J. P. 1983. Mires: swamp, bog, fen, and moor. Elsevier, New York, NY [Google Scholar]
- 30. Griffiths R. I., Whiteley A. S., O'Donnell A. G., Bailey M. J. 2000. Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Appl. Environ. Microbiol. 66:5488–5491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guenet B., Danger M., Abbadie L., Lacroix G. 2010. Priming effect: bridging the gap between terrestrial and aquatic ecology. Ecology 91:2850–2861 [DOI] [PubMed] [Google Scholar]
- 32. Hamberger A., Horn M. A., Dumont M. G., Murrell J. C., Drake H. L. 2008. Anaerobic consumers of monosaccharides in a moderately acidic fen. Appl. Environ. Microbiol. 74:3112–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hattori S. 2008. Syntrophic acetate-oxidizing microbes in methanogenic environments. Microbes Environ. 23:118–127 [DOI] [PubMed] [Google Scholar]
- 34. Hedderich R., Whitman W. B. 2006. Physiology and biochemistry of the methane-producing Archaea, p. 1050–1079 In Dworkin M. M., Falkow S., Rosenberg E., Schleifer K.-H., Stackebrandt E. (ed.), The prokaryotes, 3rd ed., vol. 2 Springer, New York, NY [Google Scholar]
- 35. Henderson G., Naylor G. E., Leahy S. C., Janssen P. H. 2010. Presence of novel, potentially homoacetogenic bacteria in the rumen as determined by analysis of formyltetrahydrofolate synthetase sequences from ruminants. Appl. Environ. Microbiol. 76:2058–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Henstra A. M., Dijkema C., Stams A. J. M. 2007. Archaeoglobus fulgidus couples CO oxidation to sulfate reduction and acetogenesis with transient formate accumulation. Environ. Microbiol. 9:1836–1841 [DOI] [PubMed] [Google Scholar]
- 37. Hiraishi A., Matsuzawa Y., Kanbe T., Wakao N. 2000. Acidisphaera rubrifaciens gen. nov., sp. nov., an aerobic bacteriochlorophyll-containing bacterium isolated from acidic environments. Int. J. Syst. Evol. Microbiol. 50:1539–1546 [DOI] [PubMed] [Google Scholar]
- 38. Horn M. A., Matthies C., Küsel K., Schramm A., Drake H. L. 2003. Hydrogenotrophic methanogenesis by moderately acid-tolerant methanogens of a methane-emitting acidic peat. Appl. Environ. Microbiol. 69:74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Intergovernmental Panel on Climate Change 2007. Klimaänderung 2007: Zusammenfassungen für politische Entscheidungsträger. World Meterological Organization and United Nations Environment Programme, Geneva, Switzerland: http://www.bmu.de/files/pdfs/allgemein/application/pdf/ipcc_entscheidungstraeger_gesamt.pdf [Google Scholar]
- 40. Juottonen H., et al. 2005. Methanogen communities and Bacteria along an ecohydrological gradient in a northern raised bog complex. Environ. Microbiol. 7:1547–1557 [DOI] [PubMed] [Google Scholar]
- 41. Kim M. K., et al. 2007. Solirubrobacter soli sp. nov., isolated from soil of a ginseng field. Int. J. Syst. Evol. Microbiol. 57:1453–1455 [DOI] [PubMed] [Google Scholar]
- 42. Könneke M., et al. 2005. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437:543–546 [DOI] [PubMed] [Google Scholar]
- 43. Kraigher B., et al. 2006. Microbial activity and community structure in two drained fen soils in the Ljubljana Marsh. Soil Biol. Biochem. 38:2762–2771 [Google Scholar]
- 44. Krichevsky I. R., Kasarnovsky J. S. 1935. Thermodynamical calculations of solubilities of nitrogen and hydrogen in water at high pressures. J. Am. Chem. Soc. 57:2168–2171 [Google Scholar]
- 45. Kroppenstedt R. M., Goodfellow M. 1992. The family Thermomonosporaceae, p. 1085–1114 In Balows A., Trüper H. G., Dworkin M., Harder W., Schleifer K.-H. (ed.), The prokaryotes, 2nd ed., vol. 2 Springer, New York, NY [Google Scholar]
- 46. Krummen M., et al. 2004. A new concept for isotope ratio monitoring liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 18:2260–2266 [DOI] [PubMed] [Google Scholar]
- 47. Küsel K., Blöthe M., Schulz D., Reiche M., Drake H. L. 2008. Microbial reduction of iron and porewater biogeochemistry in acidic peatlands. Biogeosciences 5:1537–1549 [Google Scholar]
- 48. Küsel K., Drake H. L. 1995. Effect of environmental parameters on the formation and turnover of acetate in forest soils. Appl. Environ. Microbiol. 61:3667–3675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Küsel K., et al. 2001. Physiological ecology of Clostridium glycolicum RD-1, an aerotolerant acetogen isolated from sea grass roots. Appl. Environ. Microbiol. 67:4734–4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Laanbroek H. J., Abee T., Voogd I. L. 1982. Alcohol conversions by Desulfobulbus propionicus Lindhorst in the presence and absence of sulfate and hydrogen. Arch. Microbiol. 133:178–184 [Google Scholar]
- 51. Lai M.-C., et al. 1999. Characterization of Methanosarcina mazei N2M9705 isolated from an aquaculture fishpond. Curr. Microbiol. 39:79–84 [DOI] [PubMed] [Google Scholar]
- 52. Lane D. J. 1991. 16S/23S rRNA sequencing, p. 115–175 In Stackebrandt E., Goodfellow M. (ed.), Nucleic acid techniques in bacterial systematics. John Wileys and Sons Ltd., Chichester, United Kingdom [Google Scholar]
- 53. Leaphart A. B., Lovell C. R. 2001. Recovery and analysis of formyltetrahydrofolate synthetase gene sequences from natural populations of acetogenic bacteria. Appl. Environ. Microbiol. 67:1392–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lentz K., Wood H. G. 1955. Synthesis of acetate from formate and carbon dioxide by Clostridium thermoaceticum. J. Biol. Chem. 215:645–654 [PubMed] [Google Scholar]
- 55. Lessner D. J., et al. 2006. An unconventional pathway for reduction of CO2 to methane in CO-grown Methanosarcina acetivorans revealed by proteomics. Proc. Natl. Acad. Sci. U. S. A. 103:17921–17926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liesack W., Bak F., Kreft J.-U., Stackebrandt E. 1994. Holophaga foetida gen. nov., sp. nov., a new, homoacetogenic bacterium degrading methoxylated aromatic compounds. Arch. Microbiol. 162:85–90 [DOI] [PubMed] [Google Scholar]
- 57. Loy A., Küsel K., Lehner A., Drake H. L., Wagner M. 2004. Microarray and functional gene analyses of sulfate-reducing prokaryotes in low-sulfate, acidic fen reveal cooccurrence of recognized genera and novel lineages. Appl. Environ. Microbiol. 70:6998–7009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ludwig W., et al. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lueders T., Chin K.-J., Conrad R., Friedrich M. W. 2001. Molecular analyses of methyl-coenzyme M reductase α-subunit (mcrA) genes in rice field soil and enrichment cultures reveal the methanogenic phenotype of a novel archaeal lineage. Environ. Microbiol. 3:194–204 [DOI] [PubMed] [Google Scholar]
- 60. Luli G. W., Strohl W. R. 1990. Comparison of growth, acetate production, and acetate inhibition of Escherichia coli strains in batch and fed-batch fermentations. Appl. Environ. Microbiol. 56:1004–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Madigan M. T., Martinko J. M. 2006. Brock Biology of microorganisms, 11th ed Pearson Prentice Hall, New York, NY [Google Scholar]
- 62. Maestrojuán G. M., Boone D. R. 1991. Characterization of Methanosarcina barkeri MST and 227, Methanosarcina mazei S-6T, and Methanosarcina vacuolata Z-761T. Int. J. Syst. Bacteriol. 41:267–274 [Google Scholar]
- 63. Martin D. R., Misra A., Drake H. L. 1985. Dissimilation of carbon monoxide to acetic acid by glucose-limited cultures of Clostridium thermoaceticum. Appl. Environ. Microbiol. 49:1412–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McInerney M. J., Bryant M. P. 1981. Basic principles of bioconversions in anaerobic digestion and methanogenesis, p. 277–296 In Sofer S., Zaborsky O. R. (ed.), Biomass conversion processes for energy and fuels. Plenum, New York, NY [Google Scholar]
- 65. Messing J. 1983. New M13 vectors for cloning. Methods Enzymol. 101:20–78 [DOI] [PubMed] [Google Scholar]
- 66. Monciardini P., Cavaletti L., Schumann P., Rohde M., Donadio S. 2003. Conexibacter woesei gen. nov., sp. nov., a novel representative of a deep evolutionary line of descent within the class Actinobacteria. Int. J. Syst. Evol. Microbiol. 53:569–576 [DOI] [PubMed] [Google Scholar]
- 67. Morgulis A., et al. 2008. Database indexing for production MegaBLAST searches. Bioinformatics 24:1757–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Neufeld J. D., et al. 2007. DNA stable-isotope probing. Nat. Protoc. 2:860–866 [DOI] [PubMed] [Google Scholar]
- 69. Nüsslein B., Chin K.-J., Eckert W., Conrad R. 2001. Evidence for anaerobic syntrophic acetate oxidation during methane production in the profundal sediment of subtropical Lake Kinneret (Israel). Environ. Microbiol. 3:460–470 [DOI] [PubMed] [Google Scholar]
- 70. Palmer K., Drake H. L., Horn M. A. 2009. Genome-derived criteria for assigning environmental narG and nosZ sequences to operational taxonomic units of nitrate reducers. Appl. Environ. Microbiol. 75:5170–5174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Paul S., Küsel K., Alewell C. 2006. Reduction processes in forest wetlands: tracking down heterogeneity of source/sink functions with a combination of methods. Soil Biol. Biochem. 38:1028–1039 [Google Scholar]
- 72. Pruesse E., et al. 2007. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 35:7188–7196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Purkhold U., et al. 2000. Phylogeny of all recognized species of ammonia oxidizers based on comparative 16S rRNA and amoA sequence analysis: implication for molecular diversity surveys. Appl. Environ. Microbiol. 66:5368–5382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Raskin L., Stromley J. M., Rittmann B. E., Stahl D. A. 1994. Group-specific 16S rRNA hybridization probes to describe natural communities of methanogens. Appl. Environ. Microbiol. 60:1232–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Reguera G., Leschine S. B. 2001. Chitin degradation by cellulolytic anaerobes and facultative aerobes from soils and sediments. FEMS Microbiol. Lett. 204:367–374 [DOI] [PubMed] [Google Scholar]
- 76. Rodhe H. 1990. A comparison of the contribution of various gases to the greenhouse effect. Science 248:1217–1219 [DOI] [PubMed] [Google Scholar]
- 77. Saitou N., Nei M. 1987. The neighbour-joining method—a new method for constructing phylogenetic trees. Mol. Biol. Evol. 4:406–425 [DOI] [PubMed] [Google Scholar]
- 78. Sakai S., et al. 2008. Methanocella paludicola gen. nov., sp. nov., a methane-producing archaeon, the first isolate of the lineage “Rice Cluster I,” and proposal of the new archaeal order Methanocellales ord. nov. Int. J. Syst. Evol. Microbiol. 58:929–936 [DOI] [PubMed] [Google Scholar]
- 79. Schellenberger S., Kolb S., Drake H. L. 2010. Metabolic responses of novel cellulolytic and saccharolytic agricultural soil Bacteria to oxygen. Environ. Microbiol. 12:845–861 [DOI] [PubMed] [Google Scholar]
- 80. Schink B. 1984. Fermentation of 2,3-butanediol by Pelobacter carbinolicus sp. nov. and Pelobacter propionicus sp. nov., and evidence for propionate formation from C2 compounds. Arch. Microbiol. 137:33–41 [Google Scholar]
- 81. Schloss P. D., Larget B. R., Handelsman J. 2004. Integration of microbial ecology and statistics: a test to compare gene libraries. Appl. Environ. Microbiol. 70:5485–5492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Schwarz W. H. 2001. The cellulosome and cellulose degradation by anaerobic bacteria. Appl. Microbiol. Biotechnol. 56:634–649 [DOI] [PubMed] [Google Scholar]
- 83. Springer E., Sachs M. S., Woese C. R., Boone D. R. 1995. Partial gene sequences for the α-subunit of methyl-coenzyme M reductase (mcrA) as a phylogenetic tool for the family Methanosarcinaceae. Int. J. Syst. Bacteriol. 45:554–559 [DOI] [PubMed] [Google Scholar]
- 84. Stopnišek N., et al. 2010. Thaumarchaeal ammonia oxidation in an acidic forest peat soil is not influenced by ammonium amendment. Appl. Environ. Microbiol. 76:7626–7634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tamura K., Dudley J., Nei M., Kumar S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 86. Tamura H., Goto K., Yotsuyanagi T., Nagayam M. 1974. Spectrophotometric determination of iron(II) with 1,10-phenantroline in the presence of large amounts of iron(III). Talanta 21:314–318 [DOI] [PubMed] [Google Scholar]
- 87. Thauer R. K., Jungermann K., Decker K. 1977. Energy-conservation in chemotropic anaerobic bacteria. Bacteriol. Rev. 41:100–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wang Q., Garrity G. M., Tiedje J. M., Cole J. R. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into new bacterial taxonomy. Appl. Environ. Microbiol. 73:5261–5267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wiegel J. 2009. Moorella, p. 1247–1253 In De Vos P., et al. (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 3 Springer, New York, NY [Google Scholar]
- 90. Wüst P. K., Horn M. A., Drake H. L. 2009. Trophic links between fermenters and methanogens in a moderately acidic fen soil. Environ. Microbiol. 11:1395–1409 [DOI] [PubMed] [Google Scholar]
- 91. Wüst P. K., Horn M. A., Drake H. L. 2009. In situ hydrogen and nitrous oxide as indicators of concomitant fermentation and denitrification in the alimentary canal of the earthworm Lumbricus terrestris. Appl. Environ. Microbiol. 75:1852–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yashiro Y., et al. 2011. Methanoregula formicica sp. nov., a methane-producing archaeon isolated from methanogenic sludge. Int. J. Syst. Evol. Microbiol. 61:53–59 [DOI] [PubMed] [Google Scholar]
- 93. Yarza P., et al. 2008. The all-species living tree project: a 16S rRNA-based phylogenetic tree of all sequenced type strains. Syst. Appl. Microbiol. 31:241–250 [DOI] [PubMed] [Google Scholar]
- 94. Zehnder J. B. 1978. Ecology of methane formation, p. 349–376 In Mitchell R. (ed.), Water pollution microbiology. John Wiley and Sons, New York, NY [Google Scholar]
- 95. Zinder S. H. 1993. Physiological ecology of methanogens, p. 128–206 In Ferry J. G. (ed.), Methanogenesis: ecology, physiology, biochemistry and genetics. Chapman and Hall Inc., New York, NY [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.