Abstract
Recurrent treatments with Bacillus thuringiensis subsp. israelensis are required to control the floodwater mosquito Aedes vexans that breeds in large numbers in the wetlands of the Bolle di Magadino Reserve in Canton Ticino, Switzerland. Interventions have been carried out since 1988. In the present study, the spatial distribution of resting B. thuringiensis subsp. israelensis spores in the soil was measured. The B. thuringiensis subsp. israelensis concentration was determined in soil samples collected along six transects covering different elevations within the periodically flooded zones. A total of 258 samples were processed and analyzed by quantitative PCR that targeted an identical fragment of 159 bp for the B. thuringiensis subsp. israelensis cry4Aa and cry4Ba genes. B. thuringiensis subsp. israelensis spores were found to persist in soils of the wetland reserve at concentrations of up to 6.8 log per gram of soil. Continuous accumulation due to regular treatments could be excluded, as the decrease in spores amounted to 95.8% (95% confidence interval, 93.9 to 97.7%). The distribution of spores was correlated to the number of B. thuringiensis subsp. israelensis treatments, the elevation of the sampling point, and the duration of the flooding periods. The number of B. thuringiensis subsp. israelensis treatments was the major factor influencing the distribution of spores in the different topographic zones (P < 0.0001). These findings indicated that B. thuringiensis subsp. israelensis spores are rather immobile after their introduction into the environment.
INTRODUCTION
Biopesticides based on Bacillus thuringiensis are used world wide to control pest insects. Formulations containing B. thuringiensis subsp. israelensis (19) are applied on a large scale for the control of mosquito and black fly larvae (5, 10, 23, 24). The entomopathogenic effect of B. thuringiensis subsp. israelensis is based on highly specific toxic proteins (Cry4Aa, Cry4Ba, Cry10Aa, Cry11Aa, Cyt1Aa, and Cyt2Ba) that are produced during spore formation (8, 11) and deposited within the parasporal body.
Bolle di Magadino is a natural wetland reserve of approximately 660 ha, located in Canton Ticino, Southern Switzerland. This area is periodically flooded as a consequence of the rising level of Verbano Lake caused by heavy precipitation. Each flooding triggers synchronous hatching of a large number of the floodwater mosquitoes Aedes vexans and Ochlerotatus sticticus. To limit the number of mosquitoes, treatments with the granular B. thuringiensis subsp. israelensis formulation Vectobac-G (200 international toxic units [ITU] mg−1; Valent BioSciences Corporation, Libertyville, IL) have been carried out regularly in this area since 1988. Larval breeding sites are treated with Vectobac-G by helicopter, spreading about 14 kg of product per hectare at each treatment. The frequency of B. thuringiensis subsp. israelensis applications depends on the degree of flooding and on the number of mosquito larvae developing in the area. On average, 2 to 3 applications are required per year. After more than 20 years of B. thuringiensis subsp. israelensis application, Bolle di Magadino represents an ideal model for a microbiological survey aiming at tracing the fate of B. thuringiensis subsp. israelensis released into the environment and to investigate possible long-term effects on target and nontarget organisms. The persistence and accumulation of allochthonous B. thuringiensis subsp. israelensis in the environment as a consequence of these regular treatments, although not proven to have a negative impact on the ecosystem, may, however, result in the acquisition of resistance by mosquito larvae after prolonged exposure to this biopesticide (17, 40).
Bacillus thuringiensis is widely applied for biological pest control, but only general, nonquantitative information about its fate in the environment is available (30). Regular B. thuringiensis subsp. israelensis applications are usually required in larval breeding sites because the active component does not exhibit residual activity (6, 18, 42). The activity of the delta-endotoxin crystals seems to disappear quite fast from the feeding zones of the target insects. They either attach to organic solid matter, are inactivated by sunlight, or reach the sediment and bind to soil particles (17, 29). It has also been reported that B. thuringiensis subsp. israelensis delta-endotoxins bind to humic acids and clays of acidic soils and are thus protected from microbial degradation but retain their biological activity (37, 38). In contrast, the B. thuringiensis subsp. israelensis spores, applied together with the protein crystals, show high longevity. Like any other bacterial spores, they can persist in the environment for years (16, 17, 43).
Limited information is available on the persistence and distribution of B. thuringiensis subsp. israelensis spores in the Bolle di Magadino Reserve. De Respinis et al. (15) showed that within selected spots, the number of B. thuringiensis subsp. israelensis spores decreased between two Vectobac-G applications, although a given level always persisted. They concluded that a proliferation of B. thuringiensis subsp. israelensis between Vectobac-G treatments could be excluded. In their study, however, due to the laborious methodology of quantification, samples were collected only from a limited number of sites, each covering 1 m2. Thus, their data could not be extrapolated to the whole area and did not provide information on the overall distribution and the fate of the biopesticide in the soil. A large-scale analysis of the area using a faster quantitative method could help to determine the overall distribution of B. thuringiensis subsp. israelensis spores in the soil and to analyze factors influencing their distribution. The main aim of this study was to assess the quantitative distribution of Bacillus thuringiensis subsp. israelensis spores in the Bolle di Magadino natural reserve. The project encompassed the influence of the topography, the water flooding dynamics, and the frequency of the Vectobac-G applications on the B. thuringiensis subsp. israelensis spore distribution. The concentrations of B. thuringiensis subsp. israelensis spores were determined along transects covering different zones of various elevations flooded at different frequencies.
MATERIALS AND METHODS
Sampling design and collection methods.
Samples were collected in December 2009, 135 days after the last B. thuringiensis subsp. israelensis treatment in that year. Six east-west transects measuring from 287 m to 439 m were selected in two representative areas of the reserve (see Fig. S1 in the supplemental material). The transects included zones of various elevations flooded at different frequencies over the past 22 years (Table 1). A total of 93 sampling points along the transects were chosen. The exact elevation and coordinate reference were determined for each sample point using a Global Positioning System (GPS) device (GPS Leica System 1200+ using the Swipos-GIS/GEO positioning system; Leica Geosystems AG, Switzerland, and Swisstopo, respectively) with an accuracy of ±2 cm. Seven sampling points with a measurement error higher than 4 cm were excluded from the analysis. At each point, triplicate samples were collected using a stainless-steel soil corer with an internal diameter of 2.5 cm (Eijkelkamp Agrisearch Equipment BV, Giesbeek, Netherlands). After having removed the upper 2 cm, containing loose litter of fresh decaying leaf and grass debris, cores of soil down to a depth of 7 to 8 cm were sampled. B. thuringiensis (Bt) spores are normally not found below a depth of 6 to 10 cm (43). To avoid contamination, the soil corer was cleaned with 95% ethanol between each sampling. The probes were stored at 4°C until processed.
Table 1.
Treatments, flooding events, and number of samples analyzed at the different elevation levels
| Elevation interval (m a.s.l.) | Total no. of treatmentsa | Mean annual flooding period (days)b | No. of samples analyzed |
|---|---|---|---|
| 193.50–193.75 | 11 | 105 | 9 |
| 193.75–194.00 | 24 | 92 | 45 |
| 194.00–194.25 | 25 | 78 | 72 |
| 194.25–194.50 | 14 | 14 | 60 |
| 194.50–194.75 | 8 | 5 | 27 |
| 194.75–195.00 | 3 | 2 | 27 |
| 195.00–195.50 | 1 | 0 | 18 |
Total treatments from 1990 to 2009.
The determination of mean annual flooding periods was based on data for 15 April to 31 July of 1989 to 2009.
Analytical methods.
Before DNA extraction, the soil samples were dried overnight at 60°C, homogenized, and sieved (2-mm-mesh sieve).
Nucleic acid extractions and quantitative PCR were performed as previously described by Guidi et al. (20), with some modifications. Briefly, an aliquot of 250 mg from each soil sample was submitted to DNase treatment (200 U/ml DNase I; Roche Diagnostic AG, Rotkreuz, Switzerland) to digest free Bacillus DNA, followed by a spore germination step to facilitate cell disruption. DNA was then extracted using a FastDNA spin kit for soil (MP Biomedicals, Illkirch, France) (20). DNA templates were analyzed in duplicate by quantitative PCR. The methodology developed by Guidi et al. (20) was slightly modified to amplify shorter fragments (159 bp) of the B. thuringiensis subsp. israelensis cry4Aa and cry4Ba genes and to optimize the reaction by reducing the number of PCR cycles needed for detection. A new TaqMan probe (Cry4Probe) and a reverse primer (Cry4R) were designed (Table 2), and their sequences were checked for specificity in the GenBank database (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Cry4Probe was labeled with the fluorescent FAM (6-carboxyfluorescein) reporter dye at the 5′ end and with the TAMRA (6-carboxytetramethylrhodamine) quencher dye at the 3′ end (Microsynth AG, Balgach, Switzerland). An internal positive control (TaqMan exogenous internal positive control [Exo IPC]-VIC probe; Applied Biosystems, Rotkreuz, Switzerland) was added to the reaction mixture to distinguish true-negative samples from PCR inhibition due to coextracted contaminants. Each reaction was performed in a 20-μl final volume containing 10 μl of TaqMan environmental master mix 2.0 (Applied Biosystems, Rotkreuz, Switzerland), 0.9 μM Un4(d) forward primer (7), 0.9 μM Cry4R reverse primer, 0.2 μM Cry4Probe, 2 μl 10× Exo IPC mix, 0.4 μl 50× Exo IPC DNA, and 2 μl template DNA. The reactions were carried out in a 7000 real-time PCR system instrument (Applied Biosystems, Rotkreuz, Switzerland). The thermal cycling conditions were 2 min at 50°C and 10 min at 95°C, followed by 45 cycles of 15 s at 95°C and 1 min at 60°C.
Table 2.
Primers and probe used for quantitative PCR in this study
| Primer or probe | Oligonucleotide sequence (5′–3′) | Oligonucleotide positiona (gene amplified) | Reference or source |
|---|---|---|---|
| Un4(d) | GCATATGATGTAGCGAAACAAGCC | 589–612 (cry4Aa) | 7 |
| 2800–2823 (cry4Ba) | |||
| Cry4R | ACCTGGAACATCTGACAACCAATC | 454–477 (cry4Aa) | This study |
| 2935–2958 (cry4Ba) | |||
| Cry4Probe | FAM-ACGACACTCGCTCAAATTCAGTACGCT-TAMRA | 514–540 (cry4Aa) | This study |
| 2872–2898 (cry4Ba) |
The inclusivity and exclusivity (32) of the new PCR conditions were checked as described by Guidi et al. (20) using pure cultures of the following set of Bacillus reference strains: B. amyloliquefaciens (ATCC 23350T), B. brevis (ATCC 8246T), B. cereus (ATCC 10876a, ATCC 7004, LMG 8950, LMG 9679, GP7, ATCC 11778, and ATCC 14579T), B. circulans (ATCC 11033 and ATCC 24T), B. licheniformis (ATCC 14580T), B. megaterium (ATCC 14581T, LMG 12253, and DSM 90), B. mycoides (ATCC 6462T, LMG 9680, LMG 12256, and LMG 12410), B. polymyxa (ATCC 842T), B. sphaericus (ATCC 14577T), B. subtilis (ATCC 6051T and ATCC 7003), B. subtilis subsp. niger (ATCC 9372), B. thuringiensis (NRRL B4039), B. thuringiensis subsp. berliner (ATCC 10792T), B. thuringiensis H3a (LMG 12268), B. thuringiensis H4ab (LMG 12266), B. thuringiensis H5ab (LMG 12265), B. thuringiensis H9 (LMG 12269), B. thuringiensis subsp. aizawai (DSM 6099), B. thuringiensis subsp. israelensis (DSM 5724, Vectobac-G strain, and IP 4444), B. thuringiensis subsp. kurstaki (DSM 5725), and B. thuringiensis subsp. morrisoni (DSM 6112 and DSM 6113). In addition to DNA templates, every PCR assay included a no-template sample as a negative control and standard samples for the quantification of spores. Standard templates were obtained from 10-fold dilutions of pure spore suspensions of known concentrations of B. thuringiensis subsp. israelensis (20). The spore concentrations per gram of soil were extrapolated from a standard curve generated by processing each extract in triplicate. The data were analyzed with the 7000 System SDS software, version 1.2.3f2 (Applied Biosystems, Rotkreuz, Switzerland), with the automatic settings used for the baseline and a threshold set at 0.2 ΔRn (i.e., the magnitude of the signal generated by the specified set of PCR conditions).
Determination of the number of B. thuringiensis subsp. israelensis spores in Vectobac-G.
To obtain baseline data, the number of spores per milligram of Vectobac-G was determined by quantitative PCR to calculate the amount of B. thuringiensis subsp. israelensis spores released at each sampling point during the past 2 decades. One granule of Vectobac-G was weighed, transferred to a test tube, and suspended in 1 ml of sterile water, followed by overnight incubation at room temperature to release the spores. The spore suspension was transferred to a clean Eppendorf tube. The extraction of spores from the granule was repeated, and duplicate DNA extraction from each spore suspension was performed as previously described (20). Quantitative PCR was carried out in duplicate for each DNA extract. The whole analysis was done in duplicate.
Data analysis.
The B. thuringiensis subsp. israelensis spore concentrations per gram of soil obtained with quantitative PCR analysis were log transformed, and the logs of the spore concentrations of the three duplicate samples from each sampling point along the transects were averaged.
The elevations of the sample points, ranging from 193.50 to 195.50 m above sea level (a.s.l.), were grouped into 25-cm intervals. The elevations between 195.00 and 195.50 m a.s.l. were merged into a single interval because only one sampling point was higher than 195.25 m a.s.l. For each elevation interval, the mean annual flooding period from 1989 to 2009 was calculated. The annual period selected for the determination of flooding dynamics ranged from 15 April to 31 July, which corresponds to the main hatching period of mosquito larvae and to the months during which treatments are generally required. We determined how many times a given elevation (interval) was treated with B. thuringiensis subsp. israelensis over the past 20 years. Since the B. thuringiensis subsp. israelensis product distributed by the spreader attached to the helicopter also reaches the adjacent waterfront, we considered the area corresponding to the level of the lake ± 20 cm of elevation at the time of treatment to be B. thuringiensis subsp. israelensis treated. Data regarding B. thuringiensis subsp. israelensis treatments for the years 1988 and 1989 were lacking, as well as data for flooding events during the year 1988.
Pearson's correlation analysis, multifactorial analysis of variance (ANOVA, α set to 0.05), and multiple linear regression analysis with the number of B. thuringiensis subsp. israelensis spores per gram of soil, the elevation, the mean flooding period, and the number of treatments as variables were carried out using R (version 2.9.1).
For each sampling point, the total number of B. thuringiensis subsp. israelensis spores applied during the treatments was estimated, assuming that the helicopter releases about 14 kg per hectare of Vectobac-G. Thus, every sampling point, measuring 2.5 cm in diameter, received 0.7 mg of product per treatment. The percentage of reduction of the number of spores was calculated for every sampling point and averaged. ANOVA (α set to 0.05) was performed to test the influence of elevation and mean flooding period on the percentages of spores which were recovered. Natural logarithmic transformations were used to normalize the percentages of spores recovered.
RESULTS
Distribution of B. thuringiensis subsp. israelensis spores.
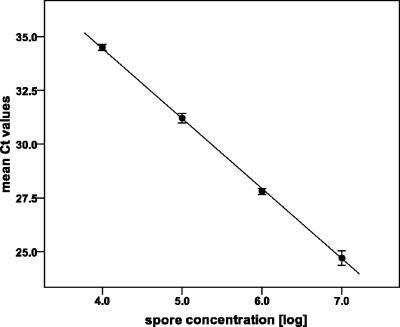
The quantitative distribution of Bacillus thuringiensis subsp. israelensis spores in the soil of the Bolle di Magadino Reserve was assessed by quantitative PCR targeting the cry4Aa and cry4Ba plasmid genes encoding insecticidal toxins. The results are based on 258 probes sampled along the six transects. The number of B. thuringiensis subsp. israelensis spores per gram of soil was established based on a standard curve constructed using a serial dilution of known amounts of spores (Fig. 1). B. thuringiensis subsp. israelensis spores were present in 99.6% of the soil samples. Only one sample was free of B. thuringiensis subsp. israelensis spores. The maximum concentration observed was 6.8 log B. thuringiensis subsp. israelensis spores g−1 soil. Amplifications of the internal positive control were neither completely inhibited nor retarded, indicating that the number of B. thuringiensis subsp. israelensis spores detected was not underestimated due to inhibition by coextracted humic substances.
Fig. 1.
Standard curve created using known amounts of B. thuringiensis subsp. israelensis spores. DNA was extracted from 400-μl amounts of 10-fold dilutions from 6.25 × 106 to 6.25 × 103 CFU ml−1, corresponding to 7 × 106 to 4 × 103 spores g−1 soil. Ct, threshold cycle. Threshold was set at 0.2 ΔRn. R2 = 1.00. Efficiency of quantitative PCR is 102% (calculated according to the method of Cordier et al. [13]). Error bars show standard deviations of the mean results from three replicates.
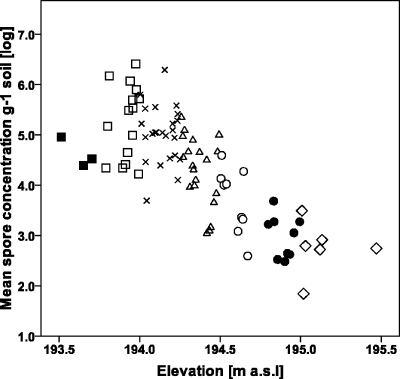
The concentration of B. thuringiensis subsp. israelensis spores in the soil correlated well with the elevation, the frequency of treatments, and the duration of the temporary flooding events during the past 21 years. The elevations of sampling points ranged from 193.50 to 195.50 m a.s.l. A decreasing trend in the concentration of spores as a function of increasing elevation was observed (Fig. 2). The mean concentration of spores and the elevation were inversely and strongly correlated (Pearson correlation coefficient, r = −0.77; P < 0.0001). A positive correlation was seen between the mean flooding periods and the spore concentrations (Pearson correlation coefficient, r = 0.71; P < 0.0001). A strong positive correlation (r = 0.79; P < 0.0001) between the total number of Vectobac-G applications from 1989 to 2009 and the concentration of B. thuringiensis subsp. israelensis spores in soil could also be established (Fig. 3).
Fig. 2.
Log concentrations of B. thuringiensis subsp. israelensis spores plotted against elevation. Each point on the graph is the mean of the results for three duplicate samples. Different symbols represent the mean annual flooding period in days of every sample point, as follows: ■, 105; □, 92; ×, 78; ▵, 14; ○, 5; ●, 2; ♢, 0.
Fig. 3.
Mean concentrations of B. thuringiensis subsp. israelensis spores plotted against the number of B. thuringiensis subsp. israelensis applications from 1990 to 2009. Spore concentrations and B. thuringiensis subsp. israelensis treatments are grouped into intervals of elevation (m a.s.l.), shown by the following symbols: ■, 193.50 to 193.75; □, 193.75 to 194.00; ×, 194.00 to 194.25; ▵, 194.25 to 194.50; ○, 194.50 to 194.75; ●, 194.75 to 195.00; ♢, 195.00 to 195.50. Bars show 95% CIs.
A multifactorial ANOVA was carried out to test for significance of the mean flooding periods, the frequency of treatments, and the elevation following the concentration of B. thuringiensis subsp. israelensis spores. In the model, each variable was analyzed individually and in relation to the other variables to check for interaction. As interactions lacked statistical significance, only single variables were maintained in the model. Among the three variables, only the frequency of treatments and the elevation were statistically significant (P < 0.0001); the mean flooding periods apparently had no influence (P = 0.44) on the quantitative distribution of B. thuringiensis subsp. israelensis spores, perhaps due to horizontal transport. Thus, a multiple linear regression model including only significant variables from multifactorial ANOVA for the concentration of B. thuringiensis subsp. israelensis spores could be expressed by the equation logSC = 202.58 − 1.02 E + 0.06 T (where logSC is log spore concentration, E is elevation, and T is the number of B. thuringiensis subsp. israelensis treatments), with a determination coefficient R2 of 0.67 and a P value of <0.0001.
Persistence of B. thuringiensis subsp. israelensis spores.
The number of B. thuringiensis subsp. israelensis spores per mg of Vectobac-G, determined by quantitative PCR, was 5.4 log, which is in agreement with the work of Snarski (36), who found 2 · 108 to 4 · 108 CFU g−1. We calculated that every sampling point received about 0.7 mg of product per treatment. Thus, it was possible to estimate the average number of spores reaching the soil during a B. thuringiensis subsp. israelensis application, i.e., 1.8 · 105 spores. Knowing the number of B. thuringiensis subsp. israelensis applications since 1989, we calculated for every sampling point the total amount of B. thuringiensis subsp. israelensis spores introduced since the beginning of treatments and the percentages of spores that had disappeared after 22 years. The average spore loss after 21 years of treatments was 95.8% (95% confidence interval [CI], 93.9 to 97.7%). Accordingly, the percentage of spores recovered was 4.2% (95% CI, 2.3 to 6.1%) of the total number applied since 1989. According to the results of the ANOVA, the percentages of spores in soils were higher at lower elevations (P < 0.05) and decreased with increasing elevations (Fig. 4). Thus, the elevation apparently plays a role in the persistence of B. thuringiensis subsp. israelensis spores (ANOVA, P < 0.0001). In contrast, the influence of mean flooding periods on the percentage of spores after 21 years of treatments was not significant (ANOVA, P = 0.096).
Fig. 4.
Percentages of spores recovered plotted against elevation. The percentages of spores were naturally log transformed to obtain normally distributed data. Each point on the graph is the mean of the results for three duplicate samples. The dashed line represents the linear regression, with an R2 value of 0.34.
DISCUSSION
In the present study, it was found that the spores of Bacillus thuringiensis subsp. israelensis were not homogeneously dispersed in the natural wetland of the Bolle di Magadino Reserve, where B. thuringiensis subsp. israelensis treatments have been carried out since 1988. The number of B. thuringiensis subsp. israelensis applications and the topography of the larval breeding sites are reflected in the distribution and the concentration of B. thuringiensis subsp. israelensis spores in the soil. It could be demonstrated that the spore concentration clearly depends on the number of treatments carried out over the past 20 years. Moreover, it could be shown that the spores are not displaced by water to other locations on the site after their introduction into the environment. Accordingly, flooding of the area outside the mosquito season, which is frequently observed between fall and early spring, seems not to displace the spores to nontreated areas. These findings are in agreement with the results obtained in previous studies, pointing out that B. thuringiensis subsp. israelensis is relatively immobile in the soil, both horizontally and vertically (1, 14, 22, 27).
As expected, B. thuringiensis subsp. israelensis spores were found to show a considerable persistence in the soil, but their number decreases over time. After 135 days from the last treatment, the number of spores that could be detected was approximately 6.8 log spores g−1 soil. The possibility that all these spores originate from the last treatments can be excluded. In fact, in several sampling points, the number of spores detected was higher than the total number of spores applied in 2009 (data not shown). Spores were detected, even if at low concentrations, in areas that received only one treatment, in 1997.
Including all treatments since 1989, on average, only 4.2% (95% CI, 2.3 to 6.1%) of the spores remained in the breeding sites. They disappeared gradually from the sites, persisting at levels that depended on the number of B. thuringiensis subsp. israelensis applications. The percentage of spore loss was shown to be related to the elevation, whereby the decrease in spore number was less in lower-elevation zones. Spore reduction is thus dependent on the cumulative total amount applied. Although spores may persist in the soil at low frequencies, a continuous accumulation due to regular Vectobac-G treatments was not observed. Our results are in line with the findings of De Respinis et al. (15), who reported a B. thuringiensis subsp. israelensis concentration of 104 spores g−1 soil after 220 days from the treatments, thereby showing that an accumulation of B. thuringiensis subsp. israelensis spores after multiple Vectobac-G applications could be excluded. In the Rhine Valley (Germany), where B. thuringiensis subsp. israelensis had been sprayed for 23 years, Becker (4) could detect 7.0 · 105 to 4.4 · 107 spores g−1 soil. Smith and Barry (35) found that Bt spores persist and remain viable (105 spores g−1 soil) in a forest soil (Wasatch, UT) for at least 24 months after aerial application. On the other hand, Hajaij et al. (21) reported a low persistence of B. thuringiensis subsp. israelensis spores in soil of a salt marsh ecosystem. They did not detect any spores in soil 3 months after two B. thuringiensis subsp. israelensis treatments in an area that was never previously treated. Thus, it can be concluded that the type of the ecosystem plays an important role in the reduction dynamics of applied B. thuringiensis subsp. israelensis spores.
Our findings do not discriminate whether a fraction of the spores detected in soil samples resulted from on-site multiplication or whether they had been all introduced with treatments and have persisted in soil without germination and multiplication. According to the literature, Bt spores grow and sporulate in soils supplemented with additional nutrients or in previously sterilized soil (34, 41). Other authors demonstrated that Bt spores were not able to germinate in the soil under natural conditions (2, 31). There is evidence that B. thuringiensis subsp. israelensis spores can germinate and proliferate in cadavers of mosquito larvae (3, 25), where an exchange of plasmids may occur (39). Recent studies also showed the germination of Bacillus spores outside their host: Bacillus anthracis was found to multiply in the rhizosphere of grass plants (33), B. thuringiensis subsp. kurstaki was able to proliferate in the phylloplane of clover (9) and in the digestive tract of earthworms (22), and germinating B. thuringiensis subsp. israelensis cells were detected in excreted food vacuoles of protozoa (26). Nevertheless, even if germination and proliferation of spores occur in the environment, we may assume that this is only a very minor proportion, as their total number shows a general decrease. It is likely that the impact of abiotic factors, such as UV irradiation (28), on the spore concentration outweigh the impact of germination and cell multiplication.
The methodology used in the present study is based on the quantification of spores carrying the cry4A and cry4B genes, located on the pBtoxis plasmid. Since transfer of the plasmid to Bacillus cereus or another closely related species requires germination of spores, multiplication, and a large number of vegetative cells in close contact to allow conjugation, we can assume that the spores detected in soil by our method are B. thuringiensis subsp. israelensis spores. This is supported by a previous study carried out in the Bolle di Magadino Reserve, where it was shown that the percentage of B. thuringiensis subsp. israelensis spores within the Bacillus cereus morphotypes isolated from soil and sediment samples was 3.5% (12). By ribotyping, Chappuis (12) confirmed that all of the 76 cry4-positive colonies were B. thuringiensis subsp. israelensis, excluding any plasmid transfer between B. thuringiensis subsp. israelensis and other closely related Bacillus isolates.
In conclusion, this study contributes to a better knowledge of the dispersion, distribution, and persistence of B. thuringiensis subsp. israelensis spores released in the environment of a natural wetland reserve following large-scale treatments against mosquito larvae. We showed that the distribution of spores is mainly influenced by the number of B. thuringiensis subsp. israelensis treatments in the different topographic zones. Moreover, the lack of spore dispersion and continuous accumulation after 2 decades of B. thuringiensis subsp. israelensis applications confirms the environmental safety of this biopesticide.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Swiss Expert Committee for Biosafety (SECB, Bern, Switzerland).
We are grateful to Paolo Salmina and Claudio Saldarini (Ufficio della Misurazione Ufficiale e della Geoinformazione, Bellinzona, Switzerland) for GPS measurements, Sasha Maftej (Bolle di Magadino Foundation, Magadino, Switzerland) for elaborating the data on mean flooding periods and the map, and the Ufficio dei Corsi d'Acqua della Repubblica e Cantone Ticino for providing us altimetry data. We thank Nicola Storelli for help in sample collection. We gratefully acknowledge Nicole Strepparava for help in statistical analysis and Sophie De Respinis, Cinzia Benagli Goll, and Orlando Petrini for helpful advice.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 15 April 2011.
REFERENCES
- 1. Akiba Y. 1991. Assessment of rainwater-mediated dispersion of field-sprayed Bacillus thuringiensis in the soil. Appl. Entomol. Zool. 26:477–483 [Google Scholar]
- 2. Akiba Y. 1986. Microbial ecology of Bacillus thuringiensis. VI. Germination of Bacillus thuringiensis spores in the soil. Appl. Entomol. Zool. 21:76–80 [Google Scholar]
- 3. Aly C., Mulla M. S., Federici B. A. 1985. Sporulation and toxin production by Bacillus thuringiensis var. israelensis in cadavers of mosquito larvae (Diptera: Culicidae). J. Invertebr. Pathol. 46:251–258 [Google Scholar]
- 4. Becker N. 2002. Sterilization of Bacillus thuringiensis israelensis products by gamma radiation. J. Am. Mosq. Control Assoc. 18:57–62 [PubMed] [Google Scholar]
- 5. Becker N., Ludwig M. 1993. Investigations on possible resistance in Aedes vexans field populations after a 10-year application of Bacillus thuringiensis israelensis. J. Am. Mosq. Control Assoc. 9:221–224 [PubMed] [Google Scholar]
- 6. Beegle C. C., Dulmage H. T., Wolfenbarger D., Martinez E. 1981. Persistence of Bacillus thuringiensis Berliner insecticidal activity on cotton foliage. Environ. Entomol. 10:400–401 [Google Scholar]
- 7. Ben-Dov E., et al. 1997. Extended screening by PCR for seven cry-group genes from field-collected strains of Bacillus thuringiensis. Appl. Environ. Microbiol. 63:4883–4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berry C., et al. 2002. Complete sequence and organization of pBtoxis, the toxin-coding plasmid of Bacillus thuringiensis subsp. israelensis. Appl. Environ. Microbiol. 68:5082–5095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bizzarri M. F., Bishop A. H. 2007. Recovery of Bacillus thuringiensis in vegetative form from the phylloplane of clover (Trifolium hybridum) during a growing season. J. Invertebr. Pathol. 94:38–47 [DOI] [PubMed] [Google Scholar]
- 10. Boyer S., Tilquin M., Ravanel P. 2007. Differential sensitivity to Bacillus thuringiensis var. israelensis and temephos in field mosquito populations of Ochlerotatus cataphylla (Diptera: Culicidae): toward resistance? Environ. Toxicol. Chem. 26:157–162 [DOI] [PubMed] [Google Scholar]
- 11. Bravo A., Gill S., Soberón M. 2007. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 49:423–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chappuis S. 2002. Approche moléculaire de l'impact de Bacillus thuringiensis israelensis en tant que biopesticide. Ph.D. thesis (no. 3377) Université de Genève, Faculté des sciences, Geneva, Switzerland [Google Scholar]
- 13. Cordier C., et al. 2007. SCAR-based real time PCR to identify a biocontrol strain (T1) of Trichoderma atroviride and study its population dynamics in soils. J. Microbiol. Methods 68:60–68 [DOI] [PubMed] [Google Scholar]
- 14. DeLucca A. J. 1981. Bacillus thuringiensis distribution in soils of the United States. Can. J. Microbiol. 27:865–870 [DOI] [PubMed] [Google Scholar]
- 15. De Respinis S., et al. 2006. Molecular identification of Bacillus thuringiensis var. israelensis to trace its fate after application as a biological insecticide in wetland ecosystems. Lett. Appl. Microbiol. 43:495–501 [DOI] [PubMed] [Google Scholar]
- 16. Glare T., O'Callaghan M. 2003. Environmental impacts of bacterial biopesticides, p. 119–150 In Hokkanen H. M. T., Hajek A. E. (ed.), Environmental impacts of microbial insecticides: need and methods for risk assessment. Kluwer Academic Publishers, Dordrecht, Netherlands [Google Scholar]
- 17. Glare T. R., O'Callaghan M. 2000. Bacillus thuringiensis: biology, ecology and safety. John Wiley & Sons, Chichester, United Kingdom [Google Scholar]
- 18. Glare T. R., O'Callaghan M. 1998. Environmental and health impacts of Bacillus thuringiensis israelensis. Report prepared for the New Zealand Ministry of Health. New Zealand Ministry of Health, Wellington, New Zealand [Google Scholar]
- 19. Goldberg L., Margalit J. 1977. A bacterial spore demonstrating rapid larvicidal activity against Anopheles sergentii, Uranotaenia unguiculata, Culex univitattus, Aedes aegypti and Culex pipiens. Mosq. News 37:355–358 [Google Scholar]
- 20. Guidi V., De Respinis S., Benagli C., Lüthy P., Tonolla M. 2010. A real-time PCR method to quantify spores carrying the Bacillus thuringiensis var. israelensis cry4Aa and cry4Ba genes in soil. J. Appl. Microbiol. 109:1209–1217 [DOI] [PubMed] [Google Scholar]
- 21. Hajaij M., et al. 2005. Low persistence of Bacillus thuringiensis serovar israelensis spores in four mosquito biotopes of a salt marsh in southern France. Microb. Ecol. 50:475–487 [DOI] [PubMed] [Google Scholar]
- 22. Hendriksen N., Hansen B. 2002. Long-term survival and germination of Bacillus thuringiensis var. kurstaki in a field trial. Can. J. Microbiol. 48:256–261 [DOI] [PubMed] [Google Scholar]
- 23. Hongyu Z., Changju Y., Jingye H., Lin L. 2004. Susceptibility of field populations of Anopheles sinensis (Diptera: Culicidae) to Bacillus thuringiensis subsp. israelensis. Biocontrol Sci. Technol. 14:321–325 [Google Scholar]
- 24. Hougard B., Poudiougo P., Back C., Akpoboua L. K. B. 1993. Criteria for the selection of larvicides by the Onchocerciasis Control Programme in West Africa. Ann. Trop. Med. Parasitol. 87:435–442 [DOI] [PubMed] [Google Scholar]
- 25. Khawaled K., Ben-Dov E., Zaritsky A., Barak Z. 1990. The fate of Bacillus thuringiensis var. israelensis in B. thuringiensis var. israelensis-killed pupae of Aedes aegypti. J. Invertebr. Pathol. 56:312–316 [DOI] [PubMed] [Google Scholar]
- 26. Manasherob R., Ben-Dov E., Zaritsky A., Barak Z. 1998. Germination, growth, and sporulation of Bacillus thuringiensis subsp. israelensis in excreted food vacuoles of the protozoan Tetrahymena pyriformis. Appl. Environ. Microbiol. 64:1750–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martin W. F., Reichelderfer C. F. 1989. Bacillus thuringiensis: persistence and movement in field crops, p 25 In Abstracts of the Society for Invertebrate Pathology XXII Annual Meeting, University of Maryland, College Park, Maryland, 20 to 24 August 1989 Society for Invertebrate Pathology, Marceline, MO [Google Scholar]
- 28. Nicholson W. L. 2002. Roles of Bacillus endospores in the environment. Cell. Mol. Life Sci. 59:410–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ohana B., Margalit J., Barak Z. E. 1987. Fate of Bacillus thuringiensis subsp. israelensis under simulated field conditions. Appl. Environ. Microbiol. 53:828–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pedersen J. C., Damgaard P. H., Eilenberg J., Hansen B. M. 1995. Dispersal of Bacillus thuringiensis var. kurstaki in an experimental cabbage field. Can. J. Microbiol. 41:118–125 [Google Scholar]
- 31. Petras S. F., Casida L. E., Jr 1985. Survival of Bacillus thuringiensis spores in soil. Appl. Environ. Microbiol. 50:1496–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reekmans R., Stevens P., Vervust T., De Vos P. 2009. An alternative real-time PCR method to detect the Bacillus cereus group in naturally contaminated food gelatine: a comparison study. Lett. Appl. Microbiol. 48:97–104 [DOI] [PubMed] [Google Scholar]
- 33. Saile E., Koehler T. 2006. Bacillus anthracis multiplication, persistence, and genetic exchange in the rhizosphere of grass plants. Appl. Environ. Microbiol. 72:3168–3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saleh S. M., Harris R. F., Allen O. N. 1970. Fate of Bacillus thuringiensis in soil: effect of soil pH and organic amendment. Can. J. Microbiol. 16:677–680 [DOI] [PubMed] [Google Scholar]
- 35. Smith R., Barry J. 1998. Environmental persistence of Bacillus thuringiensis spores following aerial application. J. Invertebr. Pathol. 71:263–267 [DOI] [PubMed] [Google Scholar]
- 36. Snarski V. M. 1990. Interactions between Bacillus thuringiensis subsp. israelensis and fathead minnows, Pimephales promelas Rafinesque, under laboratory conditions. Appl. Environ. Microbiol. 56:2618–2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stotzky G. 2005. Persistence and biological activity in soil of the insecticidal proteins from Bacillus thuringiensis, especially from transgenic plants. Plant Soil 266:77–89 [Google Scholar]
- 38. Tapp H., Stotzky G. 1998. Persistence of the insecticidal toxin from Bacillus thuringiensis subsp. kurstaki in soil. Soil Biol. Biochem. 30:471–476 [Google Scholar]
- 39. Thomas D., Morgan J., Whipps J., Saunders J. 2001. Plasmid transfer between Bacillus thuringiensis subsp. israelensis strains in laboratory culture, river water, and dipteran larvae. Appl. Environ. Microbiol. 67:330–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tilquin M., et al. 2008. Long lasting persistence of Bacillus thuringiensis subsp. israelensis (Bti) in mosquito natural habitats. PLoS One 3:e3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. West A. W., Burges H. D., Dixon T. J., Wyborn C. H. 1985. Survival of Bacillus thuringiensis and Bacillus cereus spore inocula in soil: effects of pH, moisture, nutrient availability and indigenous microorganisms. Soil Biol. Biochem. 17:657–665 [Google Scholar]
- 42. West A. W., Burges H. D., White R. J., Wyborn C. H. 1984. Persistence of Bacillus thuringiensis parasporal crystal insecticidal activity in soil. J. Invertebr. Pathol. 44:128–133 [Google Scholar]
- 43. WHO 1999. Microbial pest control agent: Bacillus thuringiensis. Environmental Health Criteria 217. World Health Organization, Geneva, Switzerland [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.