Abstract
Enterovirus environmental surveillance on sewage from the city of Jinan, Shandong Province, China, was initiated in 2008. Thirty echovirus 6 (E6) strains—1 in 2008 and 29 in 2010—were isolated and identified. Most E6 isolates (n = 21) came from the sewage collected on August 2010, revealing high local E6 activity at that time. Interestingly, the VP1 sequences of most isolates, even from the same sewage, were not identical. Phylogenetic analysis of VP1 sequences revealed two lineages for these isolates, with 78.0 to 80.0% nucleotide identities with one another, 94.8 to 100.0% identity within the major lineage, and 92.7 to 98.5% identity within the minor one. The VP1 sequences of environmental isolates, clinical isolates from 1998 to 2010, and global E6 were subjected to evolutionary analysis using Bayesian phylodynamic methods. The inferred E6 VP1 ancestral sequence dated back to 1901 (range, 1873 to 1928) and evolved with 7.047 × 10−3 substitutions per site per year. Shandong E6 segregated into three clusters, and the two environmental lineages belonged to clusters A and C, which originated in 2003 and 1992, respectively. The antigenicity analysis via neutralization assay confirmed great antigenic differences between Shandong isolates and a prototype strain. These findings underscore the value of continuous environmental surveillance and genetic analysis to monitor circulating enteroviruses in the population and give further insight into E6 evolution.
INTRODUCTION
Human enteroviruses (HEVs) belong to the genus Enterovirus, family Picornaviridae. Their infection is known to be generally asymptomatic, but they sometimes may cause illnesses such as summer colds, aseptic meningitis (AM), acute myocarditis, acute flaccid paralysis (AFP), acute hemorrhagic conjunctivitis (AHC), hand, foot, and mouth disease (HFMD), etc.
Enteroviruses were traditionally typed by their antigenic properties via a neutralization test (NT), and the introduction of the molecular typing method, which suggested that enteroviruses should be classified into the same serotype if they have >75% nucleotide similarity in the VP1 coding sequence (>85% amino acid sequence similarity) and into different serotypes if they have <70% nucleotide similarity (<85% amino acid similarity) (18), has been broadly used for typing enteroviruses and for molecular epidemiology investigations (19, 20, 22). HEV comprises more than 90 serotypes, which are classified into four species, HEV-A to HEV-D (6, 28).
Echovirus 6 (E6) is a member of HEV-B. Children less than 1 year old were the most commonly affected by E6 in the period from 1970 to 2005 in the United States (13). For children with E6 infection requiring hospitalization, AM was the most common manifestation, followed by meningismus, upper respiratory tract infection, pneumonia, and herpangina (15). Previously, many outbreaks of AM caused by E6 had been reported throughout the world, including an outbreak that occurred in Anhui Province, China, in 2005 (1, 7, 16, 17). However, there is limited information about other E6 isolates from other regions of China. Whether the genetic characterization of other Chinese E6 isolates is similar to that of Anhui isolates needs further investigation.
There has been to date no specialized enterovirus surveillance system in China. AFP surveillance, developed for a polio eradication program, can obtain non-polio enterovirus (NPEV) isolates as a side benefit and produce baseline data of local NPEV distribution and a genetic overview (3). But the NPEVs isolated from AFP surveillance cannot reflect the current prevalent virus in circulation in a given region due to the low incidence of AFP. Nevertheless, environmental surveillance has been demonstrated to be a sensitive method to detect silently circulating viruses (24, 25). Therefore, the results from environmental surveillance not only reflect the periodic trend of NPEV distribution and variation but also possess a unique advantage for investigating silently circulating viruses in the corresponding area.
Surveillance for enteroviruses in environmental samples has been conducted in the city of Jinan, Shandong Province, China, since 2008 in order to monitor poliovirus (30) and NPEV circulation in the population of the city. Here, we investigated the genetic characterization of E6 isolated from sewage collected from February 2008 to December 2010 and phylogenetically analyzed the Shandong E6 isolates from both the environmental surveillance samples and clinical cases to assess the capability of environmental surveillance to monitor the prevalent enteroviruses in the population and to present a genetic overview of E6 strains isolated in Shandong Province.
MATERIALS AND METHODS
Shandong Province and Jinan City.
Shandong Province is located in the eastern part of China, with an area of 156,700 km2 and a population of 94.7 million. Jinan is the capital city of Shandong Province. Its metropolitan area and population are 296 km2 and 2.6 million, respectively. There are two sewage treatment plants located in the metropolitan area of Jinan city, and the number 2 plant treats the domestic sewage from the western part of the city, with a daily sewage treatment capacity of 200,000 tons.
Sampling.
Sewage samples were collected monthly from the number 2 sewage treatment plant in Jinan from February 2008 to December 2010. The samples were collected from the inlet collector canal by the grab sampling method in the afternoon between 1400 and 1500 h. A stainless plastic bucket was lowered into the flowing water to collect approximately 0.5 to 1 liter of sewage sample. A cold temperature (approximately 4°C) was maintained during sample transport to the laboratory, storage (<24 h), and processing.
Clinical E6 isolates.
The specimens from AFP cases from 1998 to 2010 were collected and processed according to standard protocols recommended by the WHO (31). Cerebrospinal fluid specimens from two aseptic meningitis patients in Shandong Province in 2010 were inoculated onto RD and HEp-2 cell lines directly.
Concentration of sewage.
Sewage samples were concentrated by the method described by Iwai et al. (12). Briefly, the sewage samples were centrifuged at 3,000 × g for 30 min at 4°C; 2.5 M MgCl2 was added to the supernatant to a final concentration of 0.05 M. The pH value was adjusted to 3.5 by 0.5 M hydrochloric acid. Then, the solution was filtered through a 0.45-μm-pore-size mixed cellulose ester membrane filter (A045A142C; Advantec, Tokyo, Japan) by positive pressure pump. Absorbents on the filter were then eluted with 10 ml of 3% beef extract solution by ultrasonication three times (1 min each time), and the solution was centrifuged at 12,000 × g for 30 min. Subsequently, the supernatant was filtered through a 0.22-μm-pore-size filter and was ready for cell inoculation.
Virus isolation and serotyping.
L20B, RD, and HEp-2 cell lines were used for virus isolation. A total of 200 μl of treated solution was added to each of the cell culture tubes (18 tubes of each cell line for one sewage sample). After absorption at 36°C for 2 h, 1 ml of Eagle's minimal essential medium with 2% fetal calf serum was added, and the tubes were kept in a 36°C incubator for 7 days and examined every day. After 7 days, the tubes were frozen and thawed and repassaged in L20B, RD, and HEp-2 cell lines, and another 7-day examination was performed. If a complete cytopathic effect (CPE) was obtained in the RD or HEp-2 cell line, the cells in the tube were frozen and thawed and inoculated into L20B cells to rule out the possibility of poliovirus.
According to standard protocols recommended by the WHO (31), microneutralization assays were carried out in 96-well tissue culture plates using an antibody pool for enterovirus (National Institute for Public Health and the Environment, [RIVM], Netherlands). The antiserum-virus mixtures were incubated for 1 h at 36°C. Subsequently, a suspension fluid of RD or HEp-2 cells was added to the plate, which was subsequently examined daily for the presence of CPE. The antiserum that inhibited the development of CPE was evaluated according to the manufacturer's instructions. Isolates identified as E6 were used for further investigation.
Nucleotide sequencing and molecular typing.
Total RNA was extracted from 140 μl of the infected RD and HEp-2 cell culture using a QIAamp viral RNA minikit (Qiagen, Valencia, CA) according to the manufacturer's recommended procedure. Reverse transcription-PCR (RT-PCR) was performed using an Access RT-PCR System (Promega). Approximately 730 bp, including the 3′ end of VP3 and 480 bp of the 5′ end of a partial VP1 gene, was amplified using the primer pair 490/492 (21). Primer pair 491/493 that corresponded to the 3′ end of VP1 and 5′ end of the 2A was used to amplify a 760-bp sequence. The combination of the two sequences yielded the entire VP1 coding region.
The products were analyzed by agarose gel electrophoresis, and positive products were purified and sequenced directly with a BigDye Terminator, version 3.0, Cycle Sequencing kit (Applied Biosystems, Foster City, CA); sequences were analyzed by an ABI 3130 genetic analyzer (Applied Biosystems). The PCR products were sequenced in both directions to avoid possible ambiguous nucleotides. The VP1 sequence was compared with sequences available in GenBank using BLAST, obtained online from the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/BLAST/). Isolates showing >75% nucleotide sequence identities with the E6 D'Amori prototype strain were designated relative serotypes (18).
Homologous comparison and phylogenetic analysis.
Nucleotide sequence alignments were carried out by BioEdit software, version 7.0.5.3 (11). Phylogenetic trees were constructed by using Mega, version 4.0 (29), using the neighbor-joining method after estimation of genetic distance using the Kimura two-parameter method (14). A bootstrapping test was performed with 1,000 duplicates, and the transition/transversion rate was set at 2.0.
Evolutionary analysis based on the Bayesian MCMC method.
The evolution rate and molecular clock phylogeny of global E6 isolates were inferred using the Bayesian Markov Chain Monte Carlo (MCMC) method in BEAST, version 1.6.1 (9), and the time of the most recent common ancestor (tMRCA) with 95% highest posterior density (HPD) of global E6 and Shandong clusters was estimated. In order to reduce the computation load, sequences with high homogeneity and identical isolation years were deleted. The data were analyzed under both the Hasegawa-Kishino-Yano (HKY) and the general time reversible (GTR) nucleotide substitution models with gamma distribution of among-site rate variation. Two different models of rate variation among branches were implemented in our analysis: the strict clock and the uncorrelated log-normal distributed (UCLD) relaxed molecular clock. Both constant and exponential growth (EG) population sizes were used as tree priors. For each model, the MCMC chain was run for 30,000,000 steps and sampled every 1,000 steps. The first 3,000,000 steps of each run were discarded as burn-in.
Neutralization assay.
To examine the antigenic properties of Shandong E6 isolates, a microneutralization assay was carried out in 96-well tissue culture plates using polyclonal antiserum for E6 (Denka, Japan). Briefly, 25 μl of the E6 antiserum, with a 4-fold serial dilution from 1:4 to 1:1,024, was added into the 96-well plates. Virus with 100 50% tissue culture infective doses (TCID50)/25 μl was added to them and incubated for 1 to 2 h at 36°C. Subsequently, RD cells were added to the plate, which was subsequently examined daily within 7 days postinfection. The number of wells with CPE was counted, and the neutralization antibody titers were calculated. All assays were performed in triplicate, and back titration controls were performed for each assay.
Nucleotide sequence accession numbers.
The VP1 nucleotide sequences of E6 isolates described in this study were deposited in the GenBank database under accession numbers GU272016, GQ329778 to GQ329785, HQ399470 to HQ399495, and HQ829944 to HQ829961.
RESULTS
Virus isolation.
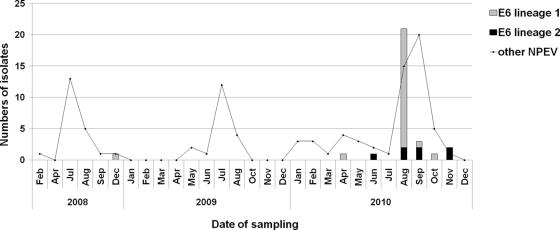
During the environmental surveillance from February 2008 to December 2010, a total of 29 sewage samples were collected, and 128 NPEVs and 18 polioviruses (3, 9, and 6 strains for poliovirus types 1, 2, and 3, respectively) were isolated. As shown in Fig. 1, the numbers of enterovirus isolates reached a peak from July to September. Thirty E6 isolates (23.4% of total NPEVs) were detected from seven sewage samples, with 1 isolate in December 2008, none in 2009, and 29 isolates from six samples in 2010. Most environmental E6 strains (21 isolates) were isolated in August 2010. In other seasons, E6 was detected at a low frequency.
Fig. 1.
Numbers of E6 isolates and other NPEVs from sewage collected in different months from 2008 to 2010 in Jinan city, China.
The RD and HEp-2 cell lines were evaluated in isolating environmental E6, and 63.3% (19/30) of E6 viruses were isolated on the RD cell line, reflecting relatively higher sensitivity to E6 than HEp-2 cell line.
Genetic relationship of environmental E6.
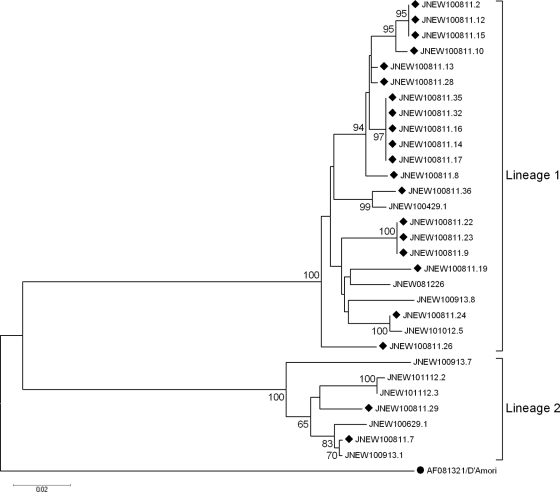
To investigate the genetic relationship among all environmental E6 isolates, especially from the samples collected in August 2010, the 470-nucleotide (nt) partial VP1 coding regions (position 2447 to 2916 on prototype strain D'Amori) were sequenced and analyzed. Phylogenetic analysis revealed two lineages of environmental E6 isolates (Fig. 2), indicating two transmission links of E6 in Jinan. For the 21 isolates from August 2010, 19 isolates segregated into a major lineage (lineage 1), while the other 2 isolates belonged to a minor lineage (lineage 2). For the nine environmental E6 viruses isolated from the other six sewage samples, four were grouped into lineage 1, and the remaining five were assigned to lineage 2. Homologous analysis revealed 78.0 to 80.0% nucleotide identities between the two lineages, 94.8 to 100.0% identity within lineage 1, and 92.7 to 98.5% identity within lineage 2.
Fig. 2.
Phylogenetic tree based on 470-nt 5′ partial VP1 sequences of all environmental E6 isolates in Shandong Province. A circle indicates the prototype strain D'Amori, and diamonds indicate the E6 isolates from the sewage collected on 11 August 2010. The remaining nine E6 isolates were collected at other times. The isolates are identified by a code that consists of JNEW, followed by the sample date (presented as YYMMDD [i.e., year, month, day]), and the tube number.
Origin, evolutionary rate, and molecular clock phylogeny.
The entire VP1 coding regions of representative E6 isolates from environmental surveillance (n = 16), AFP cases (n = 17), and AM patients (n = 2) in Shandong Province (Table 1) and from global sequences (43 out of 113) were selected for divergence time and substitution rate estimation with the Bayesian MCMC method. Different models were used for data analysis, and it was found that UCLD with EG fit our data best, while the HKY and GTR nucleotide substitution models had no significant impact on the analysis (Table 2). The coefficient of variation of the evolutionary rates among branches was 0.335 (95% HPD, 0.209 to 0.465) estimated by the HKY model, indicating the rate heterogeneity that existed among different branches.
Table 1.
Information of E6 isolates from AFP and AM cases
| Isolate | Prefecture | Year | Patient data |
||
|---|---|---|---|---|---|
| Age | Sex | Clinical symptom | |||
| 98186 | Linyi | 1998 | 1 | F | AFP |
| 98215 | Jinan | 1998 | 1 | M | GBSa |
| 00059 | Dezhou | 2000 | 10 | M | AFP |
| 01229 | Linyi | 2001 | 1 | F | Myelitis |
| 01444 | Weifang | 2001 | 8 | F | Myelitis |
| 02203 | Linyi | 2002 | 3 | M | GBS |
| 02295 | Jinan | 2002 | 1 | M | AFP |
| 05397 | Heze | 2005 | 6 | F | AFP |
| 06415 | Dezhou | 2006 | 1 | M | AFP |
| 08346 | Binzhou | 2008 | 3 | M | AFP |
| 08351 | Zibo | 2008 | 1 | F | AFP |
| 09086 | Weifang | 2009 | 3 | M | AFP |
| 10154 | Liaocheng | 2010 | 1 | F | AFP |
| 10165 | Heze | 2010 | 3 | F | AFP |
| 10180 | Taian | 2010 | 6 | M | AFP |
| 10193 | Jining | 2010 | 0.5 | M | AFP |
| 10208 | Linyi | 2010 | 0.5 | M | AFP |
| 2010LY059 | Linyi | 2010 | 1 | M | AM |
| 2010D0010005 | Heze | 2010 | 1 | M | AM |
Guillain-Barré syndrome.
Table 2.
Origin and evolution rate inferred with Bayesian MCMC method on VP1 coding region
| Parameter | Mean value of the parameter (95% HPD) as determined by: |
|
|---|---|---|
| HKY+UCLD+EG | GTR+UCLD+EG | |
| tMRCA cluster A | 2,003.3 (2,001.2–2,005.2) | 2,003.3 (2,001.3–2,005.3) |
| tMRCA cluster B | 1,996.9 (1,994.0–1,999.6) | 1,996.8 (1,992.6–1,999.2) |
| tMRCA cluster C | 1,992.1 (1,989.1–1,994.7) | 1,992.2 (1,989.4–1,994.9) |
| Root ht (tMRCA global E6) | 1,901.7 (1,873.6–1,928.1) | 1,901.2 (1,873.5–1,928.1) |
| Mean evolutionary rate (10−3 substitutions/site/yr)a | 7.047 (5.507–8.803) | 7.351 (5.677–9.110) |
| Coefficient of variation | 0.335 (0.209–0.465) | 0.323 (0.192–0.455) |
Rate of molecular evolution given as numbers of nucleotide substitutions per site per year.
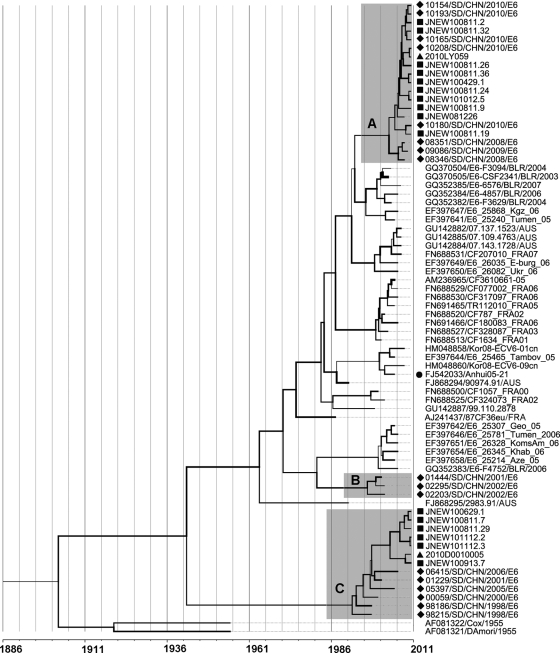
In the molecular clock phylogenetic tree (Fig. 3), Shandong clinical E6 isolates segregated into three clusters, designated A, B, and C, composed of isolates from 2008 to 2010, 2001 to 2002, and 1998 to 2010, respectively. Lineage 1 and 2 environmental E6 isolates belonged to clusters A and C, respectively. The Anhui isolates from an aseptic meningitis outbreak in Anhui Province, China, in 2005 did not fall into any of the three clusters. High intracluster similarities were observed among VP1 sequences of environmental and clinical isolates. In cluster A, environmental E6 had 95.3 to 97.2% identity with the isolate 2010LY059 from an AM patient and 91.8 to 98.5% identities with AFP isolates. Similarly, isolate JNEW100913.7 in cluster C had only 2.1% nucleotide divergence with the isolate 2010D0010005 from an AM case.
Fig. 3.
MCMC tree of the VP1 sequences of E6 isolates throughout the world visualized in FigTree. The width of a branch reflects the evolution rate of individual sequences and their reconstructed ancestors. E6 strains from Shandong segregated into three clusters (A, B, and C). A circle indicates an isolate from aseptic meningitis outbreak in the Anhui Province of China, squares indicate Shandong environmental isolates, diamonds indicate isolates from AFP surveillance in Shandong Province, and triangles indicate isolates from aseptic meningitis cases. Names of environmental isolates (JNEW prefix) are shown as described in the legend of Fig. 2. AUS, Australia; AZE, Azerbaijan; BLR, Belarus; CHN, China; FRA, France; GEO, Georgia; KEO, South Korea; KGZ, Kyrgyzstan; RUS, Russia; UKR, Ukraine; USA, the United States of America.
Different evolution rates for different branches were observed, and the mean evolution rate was 7.047 × 10−3 substitutions per site per year (HKY). The most recent common ancestor of global E6 can be traced back to 1901 (range, 1873 to 1928). The tMRCA estimates for Shandong clusters were dated to 2003 (A), 1996 (B), and 1992 (C), and the tMRCA estimates for environmental lineages 1 and 2 were dated to 2005.2 and 2003.7, respectively.
Antigenic property.
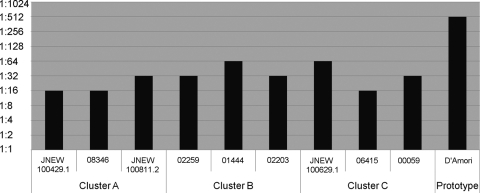
We tested the antigenicity of nine Shandong E6 isolates from clusters A, B, and C (three for each cluster) with E6-specific antiserum. The neutralization to prototype D'Amori served as a control. It was found that all isolates were neutralized with E6-specific antiserum, but the degree of neutralization of these viruses was dramatically less than the result of the prototype strain and varied slightly between isolates (Fig. 4). The variation in the degree of neutralization with E6 antiserum did not depend on the cluster.
Fig. 4.
Results of the neutralization assay of random selected Shandong E6 isolates (three isolates for each cluster) with E6-specific antisera. The E6 neutralization antibody titers in the presence of 100 TCID50/25 μl of the viruses are shown.
DISCUSSION
As a supplemental method to AFP surveillance for global poliomyelitis eradication, environmental surveillance is of great importance in investigating the circulation of wild-type poliovirus or vaccine-derived polioviruses (VDPV) (4, 10, 27, 32). In addition, environmental surveillance constitutes a sensitive method for monitoring NPEV and estimating the extent and duration of enterovirus circulation in a population (23, 24, 26).
Enterovirus environmental surveillance in China was first conducted in the Shandong Provincial Poliovirus Laboratory in February 2008. The sewage examined in this study was obtained monthly from treatment plant 2, which services the west half of Jinan City. By the end of 2010, E6 was isolated in sewage collected in 2008 and 2010. No E6 was present in sewage in 2009. There was just one isolate detected in each sample until an abrupt increase in the E6 isolates occurred for the sewage collected in August 2010. Taking into account the similar sewage concentrations and inoculation methods in these years, it is reasonable to assume that the increase in E6 isolates is a reflection of high local E6 activity at that time.
Moreover, the VP1 sequences and phylogenetic analysis provided more information on environmental E6 circulating in Jinan City. Normally, the divergence among enteroviruses isolated in an outbreak of EV-associated diseases, such as AM, was no more than 2% (5, 16). The two lineages in environmental E6 and the relatively high intralineage genetic divergence (up to 7%) revealed by phylogenetic analysis indicated that the E6 isolates from the sewage samples in August 2010 were not simply the result of current transmission activity. By Bayesian MCMC evolutionary analysis, the most recent common ancestors for environmental lineages 1 and 2 were dated to 2005.2 and 2003.7, respectively, indicating that the two lineages have circulated for 5 to ∼7 years.
Enteroviruses were known to be active in summer and early autumn (24). So, the E6 circulation in recent years might be enlarged in the summer of 2010, resulting in the peak detection via environmental surveillance in Jinan in August. The two lineages of environmental E6 probably represented the local prevalent and minor circulating E6 strains at that time. Furthermore, the cocirculation of two E6 transmission lineages in the same region and period might lead to the occurrence of intratypic recombination, which might affect E6 evolution or even result in the antigenicity and virulence alterations.
E6 can cause serious illnesses such as aseptic meningitis. However, no specialized enterovirus surveillance system for associated diseases has been established in China. Therefore, we cannot obtain sufficient clinical isolates for comparison. Considering the high rate of inapparent infection for enteroviruses, including E6, the prevalent and minor circulating E6 strains detected via environmental surveillance in this study offered unique information of E6 strains currently circulating in Jinan. Two E6 isolates from AM patients in Shandong Province, initially reported as Japanese encephalitis (JE) clinical cases, were grouped into the two clusters of environmental viruses, with high intracluster identities (Fig. 3), indicating that the environmental viruses might also be causative for AM. So, it is reasonable to conclude an E6-associated AM epidemic might occur in Jinan City in the summer of 2010.
The EV genome evolved at a rate of 1% to ∼2% mutation per year (8), and the previously estimated substitution rate of the E6 VP1 gene was 8.597 × 10−3 substitutions per site per year (2), slightly different from the results of this study (7.047 × 10−3 substitution/site/year). Moreover, global E6 strains investigated in this study shared a recent common ancestor that originated in 1901, 16 years earlier than the tMRCA estimated previously (2). The differentiation in estimated origin year and evolution rate was thought to come from the joining of Shandong E6 sequences, and analysis of more sequences with time information will yield more precise data to determine the origin and evolution of E6 strains. Shandong E6 isolates were grouped into three clusters. Isolates from cluster B were from 2001 to 2002, and 8 years has passed since the last isolation, indicating that cluster B viruses may have been eliminated from Shandong Province. Cluster A consisted of isolates from 2008 to 2010, suggesting that it may have been introduced into the Shandong area recently. The isolation years of cluster C spanned from 1998 to 2010, revealing the long-time circulation of this cluster in Shandong Province.
Another Chinese isolate (Anhui05-21) from Anhui Province was not grouped into any of the Shandong clusters, demonstrating that the E6 circulating in China consisted of different transmission lineages with high genetic divergence among them. However, the information on Chinese E6 isolates was limited, and more sequences are needed to determine domestic E6 transmission and evolution. The results of phylogenetic analysis also suggested that E6 isolates from the same cluster might come from different regions. As for the three clusters of E6 in Shandong Province, no geographic accumulation of isolates was found, and they were all distributed throughout the province.
Prototype strains of enterovirus serotypes were mostly isolated decades ago, and many have become extinct. The antigenicity of current circulating enteroviruses has changed a lot in comparison with that of prototype strains. This constituted a reason for the frequent failure in serotyping via neutralization testing using combined sera directed at the prototype enteroviruses. Likewise, the present study revealed a considerable differentiation in antigenicity between Shandong E6 isolates and the prototype D'Amori. These isolates could be serotyped by RIVM combined sera in a neutralization test, but a prolonged observation time could result in the failure of serotyping. Hence, a single antiserum directed at currently circulating viruses should be developed as a substitution.
In conclusion, the cocirculation of two E6 transmission lineages in Jinan City, China, was revealed via environmental surveillance and VP1 sequence analysis. The evolutionary genetics of global E6 were investigated, and three clusters of Shandong E6 were revealed. This is the first report of the application of environmental surveillance in monitoring circulating enterovirus in the population in China.
ACKNOWLEDGMENTS
This study was supported by a grant for Research on Emerging and Reemerging Infectious Diseases from the Ministry of Health, Labor and Welfare of Japan and two grants from the Ministry of Science and Technology of China (2008BAI56B02 and 2008ZX10004-008).
We gratefully acknowledge the excellent technical assistance of He Yang and Qingying Fan of Shandong University and express sincere thanks to the colleagues of Division of EPI, Shandong CDC.
Footnotes
Published ahead of print on 8 April 2011.
REFERENCES
- 1. Ashwell M. J., Smith D. W., Phillips P. A., Rouse I. L. 1996. Viral meningitis due to echovirus types 6 and 9: epidemiological data from Western Australia. Epidemiol. Infect. 117:507–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bailly J. L., et al. 6 July 2010, posting date Repeated genomic transfers from echovirus 30 to echovirus 6 lineages indicate co-divergence between co-circulating populations of the two human enterovirus serotypes. Infect. Genet. Evol. doi:10.1016/j.meegid.2010.06.019 [DOI] [PubMed] [Google Scholar]
- 3. Bingjun T., et al. 2008. Molecular typing and epidemiology of non-polio enteroviruses isolated from Yunnan province, the People's Republic of China. J. Med. Virol. 80:670–679 [DOI] [PubMed] [Google Scholar]
- 4. Blomqvist S., et al. 2004. Characterization of a highly evolved vaccine-derived poliovirus type 3 isolated from sewage in Estonia. J. Virol. 78:4876–4883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blomqvist S., et al. 2010. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J. Clin. Virol. 48:49–54 [DOI] [PubMed] [Google Scholar]
- 6. Brown B., Oberste M. S., Maher K., Pallansch M. A. 2003. Complete genomic sequencing shows that polioviruses and members of human enterovirus species C are closely related in the noncapsid coding region. J. Virol. 77:8973–8984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chomel J. J., Antona D., Thouvenot D., Lina B. 2003. Three echovirus serotypes responsible for outbreak of aseptic meningitis in Rhone-Alpes region, France. Eur. J. Clin. Microbiol. Infect. Dis. 22:191–193 [DOI] [PubMed] [Google Scholar]
- 8. Drake J. W. 1993. Rates of spontaneous mutation among RNA viruses. Proc. Natl. Acad. Sci. U. S. A. 90:4171–4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drummond A. J., Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. El Bassioni L., et al. 2003. Prolonged detection of indigenous wild polioviruses in sewage from communities in Egypt. Am. J. Epidemiol. 158:807–815 [DOI] [PubMed] [Google Scholar]
- 11. Hall T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95–98 [Google Scholar]
- 12. Iwai M., et al. 2006. Molecular epidemiology of echoviruses 11 and 13, based on an environmental surveillance conducted in Toyama Prefecture, 2002–2003. Appl. Environ. Microbiol. 72:6381–6387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khetsuriani N., Lamonte-Fowlkes A., Oberste S., Pallansch M. A. 2006. Enterovirus surveillance—United States, 1970–2005. MMWR Surveill. Summ. 55:1–20 [PubMed] [Google Scholar]
- 14. Kimura M. 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111–120 [DOI] [PubMed] [Google Scholar]
- 15. Lee H. Y., et al. 2010. Clinical features of echovirus 6 and 9 infections in children. J. Clin. Virol. 49:175–179 [DOI] [PubMed] [Google Scholar]
- 16. Mao N., et al. 2010. An aseptic meningitis outbreak caused by echovirus 6 in Anhui province, China. J. Med. Virol. 82:441–445 [DOI] [PubMed] [Google Scholar]
- 17. Miwa C., Sawatari S. 1994. Epidemic of aseptic meningitis with echovirus type 6 in Gifu Prefecture in 1992. Kansenshogaku Zasshi 68:1063–1067 [DOI] [PubMed] [Google Scholar]
- 18. Oberste M. S., Maher K., Kilpatrick D. R., Pallansch M. A. 1999. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J. Virol. 73:1941–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oberste M. S., Schnurr D., Maher K., al-Busaidy S., Pallansch M. 2001. Molecular identification of new picornaviruses and characterization of a proposed enterovirus 73 serotype. J. Gen. Virol. 82:409–416 [DOI] [PubMed] [Google Scholar]
- 20. Oberste M. S., et al. 2005. Enteroviruses 76, 89, 90 and 91 represent a novel group within the species human enterovirus A. J. Gen. Virol. 86:445–451 [DOI] [PubMed] [Google Scholar]
- 21. Oberste M. S., et al. 2006. Species-specific RT-PCR amplification of human enteroviruses: a tool for rapid species identification of uncharacterized enteroviruses. J. Gen. Virol. 87:119–128 [DOI] [PubMed] [Google Scholar]
- 22. Oberste M. S., et al. 2007. Molecular identification of 13 new enterovirus types, EV79∼88, EV97, and EV100∼101, members of the species human enterovirus B. Virus Res. 128:34–42 [DOI] [PubMed] [Google Scholar]
- 23. Pallin R., Wyn-Jones A. P., Place B. M., Lightfoot N. F. 1997. The detection of enteroviruses in large volume concentrates of recreational waters by the polymerase chain reaction. J. Virol. Methods 67:57–67 [DOI] [PubMed] [Google Scholar]
- 24. Sedmak G., Bina D., MacDonald J. 2003. Assessment of an enterovirus sewage surveillance system by comparison of clinical isolates with sewage isolates from Milwaukee, Wisconsin, collected august 1994 to December 2002. Appl. Environ. Microbiol. 69:7181–7187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sellwood J., Dadswell J. V., Slade J. S. 1981. Viruses in sewage as an indicator of their presence in the community. J. Hyg. (Lond.) 86:217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shieh Y. S., Baric R. S., Sobsey M. D. 1997. Detection of low levels of enteric viruses in metropolitan and airplane sewage. Appl. Environ. Microbiol. 63:4401–4407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shulman L. M., et al. 2000. Molecular and antigenic characterization of a highly evolved derivative of the type 2 oral poliovaccine strain isolated from sewage in Israel. J. Clin. Microbiol. 38:3729–3734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stanway G., et al. 2005. Family Picornaviridae, p. 757–778 In Fauquet C. M., Mayo M. A., Maniloff J., Desselberger U., Ball L. A. (ed.), Virus taxonomy. Eighth report of the International Committee on Taxonomy of Viruses Academic Press, London, United Kingdom [Google Scholar]
- 29. Tamura K., Dudley J., Nei M., Kumar S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 30. Tao Z., et al. 2010. Isolation of a recombinant type 3/type 2 poliovirus with a chimeric capsid VP1 from sewage in Shandong, China. Virus Res. 150:56–60 [DOI] [PubMed] [Google Scholar]
- 31. World Health Organization 2004. Polio laboratory manual, 4th ed World Health Organization, Geneva, Switzerland [Google Scholar]
- 32. Yoshida H., et al. 2002. Prevalence of vaccine-derived polioviruses in environment. J. Gen. Virol. 83:1107–1111 [DOI] [PubMed] [Google Scholar]