Abstract
Malaria is caused by intraerythrocytic protozoan parasites belonging to Plasmodium spp. (phylum Apicomplexa) that produce significant morbidity and mortality, mostly in developing countries. Plasmodium parasites have a complex life cycle that includes multiple stages in anopheline mosquito vectors and vertebrate hosts. During the life cycle, the parasites undergo several cycles of extreme population growth within a brief span, and this is critical for their continued transmission and a contributing factor for their pathogenesis in the host. As with other eukaryotes, successful mitosis is an essential requirement for Plasmodium reproduction; however, some aspects of Plasmodium mitosis are quite distinct and not fully understood. In this review, we will discuss the current understanding of the architecture and key events of mitosis in Plasmodium falciparum and related parasites and compare them with the traditional mitotic events described for other eukaryotes.
SERIAL MITOSIS IS A COMMON THEME IN MALARIA PARASITE REPRODUCTION
There are four critical points in the life cycle of Plasmodium parasites in which a small number of parasites rapidly multiply to generate much larger populations (60). These life cycle stages are male gamete development (72), sporozoite formation (5, 13), liver-stage development (68), and blood-stage asexual reproduction (9, 60). The first two of these processes occur within the mosquito vector, and the second two processes take place in the vertebrate host. During each of these Plasmodium life cycle stages, the parasites increase their numbers by using serial rounds of mitosis to create multinuclear cells and then orchestrating mass cytokinesis events to release their progeny (71). Mitosis is the process by which eukaryotic cells segregate their chromosomes in preparation for cell division (33, 47, 51).
To create male gametes in preparation for sexual reproduction, the parasite begins with a haploid (1n) cell called a microgametocyte which is ingested by the mosquito during a blood meal (34, 72). Within 12 min, this microgametocyte undergoes three rapid rounds of DNA synthesis and mitosis to generate a cell with an 8n genomic complement (35, 36, 73). Over the next 3 min, these genomes separate from one another and eight new haploid (1n) male gametes begin to assemble from the surface of the original cell (4, 69, 71).
Within the mosquito midgut, a small number of the male gametes will fuse with female gametes that have also developed in this compartment, and this fusion will create diploid (2n) zygotes (15). These zygotes develop into motile ookinetes (4n) (36) that ultimately become embedded in the basal lamina beneath the midgut epithelial wall as oocysts (14). Over the course of several days, a single oocyst undergoes 10 to 11 rounds of DNA synthesis and mitosis to create a syncytial cell (sporoblast) with thousands of nuclei (61, 70, 82). In a massive cytokinesis event, thousands of haploid (1n) daughter sporozoites assemble from the surface of the mother cell (61, 67), and these infective sporozoites then migrate to the mosquito salivary glands for transmission to the host.
Of the thousands of sporozoites that are produced from a sporoblast, only a few will be transmitted to the vertebrate host when the mosquito takes another blood meal, and an even smaller number may reach the host liver for further development (60). In liver-stage schizogony (45), a single invading sporozoite grows as a trophozoite (1n to 2n) within a liver cell. The parasite then undergoes 13 to 14 rounds of DNA synthesis, mitosis, and nuclear division to produce a syncytial cell (schizont) with tens of thousands of nuclei. From the surface of this syncytial parasite, tens of thousands (16, 68) of haploid (1n) daughter liver-stage merozoites assemble and are eventually released into the bloodstream in parasite-filled vesicles called merosomes (77).
Once released into the bloodstream, merozoites invade red blood cells and continue to expand their numbers with blood-stage schizogony (10). All of the clinical symptoms of malaria (fever, anemia, and neurological pathologies) are associated with the blood stage of the parasite life cycle (65). In blood-stage schizogony, following invasion, a single invading merozoite begins within the red blood cell as a ring stage and progresses into a trophozoite (1n to 2n) and then undergoes three to four rounds of DNA synthesis, mitosis, and nuclear division to produce a syncytial schizont with 16 to 22 nuclei (6, 10, 44, 53). In a synchronous mass division step, approximately 22 haploid (1n) daughter merozoites (depending on the species) assemble from the surface of the mother schizont, and with the rupture of the red blood cell, the new merozoites are released for more rounds of invasion and expansion (10, 44, 75). In nonimmune hosts, blood-stage parasites may undergo uncontrolled growth unless they are restricted by innate and adaptive immune responses. Splenic clearance is considered a major mechanism of this parasite growth regulation. However, the parasite has developed highly sophisticated mechanisms to evade immune-mediated clearance, such as expressing variant antigens on the surface of infected red cells to sequester them on endothelial cells in different organs. Nonetheless, in both immune and nonimmune hosts, the parasite burden can be maintained by repeated cycles of asexual schizogony from only a few intraerythrocytic parasites.
Thus, the malaria parasite builds large populations from a relatively small number of founding members in every major stage of its development, and each time, the parasite relies on serial mitosis to accomplish this growth. These growth periods enable parasite transmission, and as a by-product, the toxins released by rupturing schizonts help to fuel the pathogenic symptoms of the disease. Similar reproductive strategies have been described for other apicomplexans, such as Sarcocystis (75, 80). This paper highlights some of the distinctive features of mitosis in Plasmodium parasites with a special emphasis on the mitotic spindle and microtubule organizing centers (MTOCs), and we will compare them to some of the visual hallmarks of traditional mitosis that are often associated with higher eukaryotes.
TRADITIONAL VIEWS OF MITOSIS
Historically, mitosis has been divided into stages that visually chronicle chromosome movement and nuclear membrane dynamics (prophase, metaphase, anaphase, telophase); however, it is clear that there is a rich diversity in the methods that eukaryotes use to accomplish mitosis (18, 33, 51). Depending on the particular species and cell type, chromosomes may condense into discrete structures, or they may remain dispersed over large areas. Nuclear membranes may completely disperse, partially disperse, or appear to remain completely intact throughout mitosis (17). Microtubule organizing centers (MTOCs), the complex structures that nucleate and help organize microtubule arrays, may assume a wide variety of intricate conformations that can persist in the cytoplasm or may be found embedded within membranes. These variations can be particularly striking in fungi and in protozoan species such as Plasmodium spp. and other apicomplexan parasites (33).
At the onset of traditional mitosis (prophase), chromosomes begin to condense into discrete structures within the nucleus and the MTOC duplicates within the cytoplasm (Fig. 1 A, Traditional View of Mitosis [Traditional]). The mammalian MTOCs, called centrosomes, are complex structures consisting of a central pair of cylindrical structures (centrioles) that are tethered to one another within a protein matrix in the cytoplasm (8). Since these MTOCs and their microtubules are usually cytoplasmic, the nuclear membrane must disassemble before the microtubules can contact the chromosomes. Depending on the cell type, the nuclear membrane may disperse partially, or it may disperse completely (Fig. 1B, Traditional) to allow access of the cytoplasmic microtubules. At metaphase (Fig. 1B, Traditional), chromosomes are captured by microtubules from both spindle poles and begin to convene at the center of the spindle called the metaphase plate. The main points of contact between the chromosomes and the microtubules are protein structures on the chromosomes called kinetochores. During anaphase (Fig. 1C, Traditional), the sister chromatids separate and begin to move toward opposite poles as the spindle extends. At telophase (Fig. 1D, Traditional), nuclear membranes assemble around the daughter genomes, chromosomes begin to decondense, and the cell begins to assemble the machinery to divide the daughter cells.
Fig. 1.
Comparison of traditional views of mitosis with current knowledge of mitosis in blood-stage Plasmodium parasites. A schematic cartoon of mitosis indicating plasma membranes (black lines), microtubule organizing centers (MTOCs, red circles), microtubules (green lines), kinetochores (tan ovals), nuclear membranes (dark blue lines), condensed chromosomes (light blue), and uncondensed chromosomes (light blue with stipple pattern). (A) Early mitosis. In early traditional mitosis (prophase), chromosomes begin to condense within the nuclear membrane, and cytoplasmic microtubules are nucleated by two cytoplasmic MTOCs. In early Plasmodium mitosis, chromosomes remain uncondensed, the two MTOCs are embedded in the nuclear membrane, and microtubules begin to form inside the nucleus. (B) Metaphase. In traditional mitosis, the nuclear membrane has disassembled, condensed chromosomes are attached to the bipolar spindle microtubules through the kinetochores, and the kinetochores are aligned at the metaphase plate. In Plasmodium mitosis, the nuclear membrane remains intact, the chromosomes remain uncondensed, and the kinetochores are captured by the bipolar mitotic spindle forming inside the nucleus. (C) Anaphase. In traditional mitosis, sister chromatids and their associated kinetochores separate and begin to move to opposite poles of the spindle. In Plasmodium, sister kinetochores separate and migrate to opposite spindle poles while the sister chromatids remain uncondensed. (D) Telophase. In traditional views, nuclear membranes assemble around each daughter genome, chromosomes begin to decondense, and the cell begins to divide. In Plasmodium blood-stage schizogony, the nuclear membrane divides to separate daughter genomes and the cell does not divide until several cycles of mitosis have produced a multinuclear cell.
These ordered events of mitosis are controlled by complex networks of regulatory proteins, structural scaffolds, motor proteins, and proteases (8, 47, 51, 83). Some of these networks, called checkpoints, can delay the progression of the cell cycle until the critical elements in a particular step have been accomplished. Checkpoints help control the timing of the start of mitosis, the assembly of the spindle, and its coordination with DNA replication, and by doing so they serve important quality control functions for the cell (48, 58). Since mitotic events can take place in various forms in different cells, the components of these regulatory networks can also serve as precise benchmarks for cell cycle progression, provided that these components are identified and their functions defined (51).
MITOSIS IN BLOOD-STAGE SCHIZOGONY
P. falciparum mitosis differs from the traditional view of mitosis in several respects. P. falciparum chromosomes do not appear to condense during mitosis (Fig. 1A to D, Plasmodium Mitosis [Plasmodium]), a feature that is also found in other protozoan and fungal species (33). The uncondensed chromosomes of Plasmodium spp. often appear diffuse or indistinct by fluorescent microscopy (53), and they are also difficult to image by transmission electron microscopy (TEM) because of their low electron density (10). The mitotic MTOCs of Plasmodium parasites (also known as kinetic centers, centriolar plaques, or centrosome equivalents) are electron-dense plaques that appear to be embedded in pores of the nuclear membrane with one face exposed to the nuclear interior and the other face exposed to the cytoplasm (11, 66). The spindle microtubules that form inside the parasite nucleus are anchored to the nuclear face of these MTOCs (Fig. 1A to D, Plasmodium). Thus, an early hallmark of mitosis in Plasmodium spp. is the duplication of membrane-bound MTOCs and the assembly of microtubules in the nucleus (Fig. 1A, Plasmodium). P. falciparum chromosomes do contain kinetochores that are captured by microtubules from both spindle poles in a process that is reminiscent of traditional metaphase (52) (Fig. 1B, Plasmodium; see also Fig. 2 and 3). However, it is not clear whether the parasite kinetochores align in a single midpoint plane of the spindle, and so far, it is not clear whether the parasites use a spindle assembly checkpoint to control the timing of mitosis exit similarly to mammalian cells. In P. falciparum mitosis, the nuclear envelope appears to remain intact throughout mitosis (Fig. 1A to D, Plasmodium), similar to some fungi (42, 53, 66). In the related coccidian parasites Toxoplasma and Eimeria, the mitotic spindle forms through a cone-shaped extension of the nuclear membrane called the centrocone (22, 30, 75); however, the nuclear membrane also remains largely intact in these organisms. Thus, at the conclusion of mitosis, organisms that retain their nuclear envelope (closed mitosis), such as Plasmodium spp., must divide this nuclear envelope to complete genome separation, while organisms that disperse the nuclear envelope (open mitosis) must form a new nuclear membrane around each daughter genome (18) (Fig. 1D).
Fig. 2.
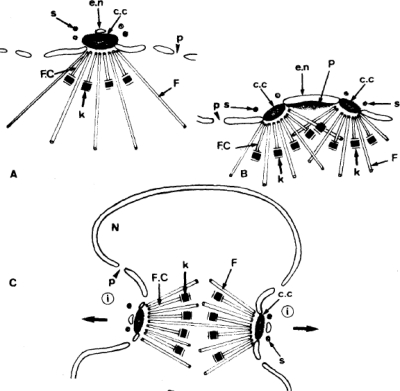
Interpretation of hemispindle and full mitotic spindle development in Plasmodium from TEM. Images of mitosis during sporogony in P. berghei. (A) A single hemispindle. (B) Hemispindle duplication. (C) Formation of a full mitotic spindle. Microtubules (F and F.C.), MTOCs (c.c.), and kinetochores (k) are indicated. p, nuclear pore; e.n., nuclear envelope; s, cystplasmic satellite; P, bridge of dense material; N, nucleus. (Reprinted from reference 66 with permission of the publisher.)
Fig. 3.
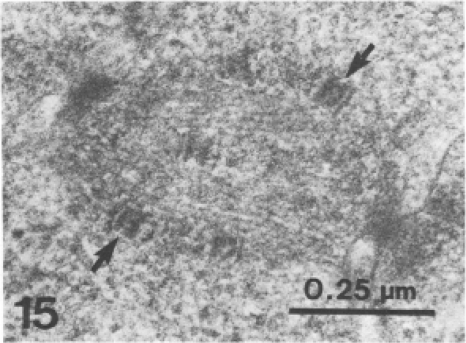
TEM image of a mitotic spindle at the beginning of anaphase in a P. falciparum blood-stage parasite. One thin-section image is shown. MTOCs marking the mitotic spindle poles are visible as dense masses at the northwest and southeast corners of the image. Two kinetochores attached to spindle microtubules are indicated by arrows. The remaining kinetochores are visible in other serial sections from this sample. (Reprinted from reference 52 with permission of the publisher.)
In many eukaryotes, mitosis is followed very closely by the assembly of structures that will divide the cell. In contrast, repeated mitosis without cell division is a means that Plasmodium parasites use to create multinuclear cells as part of their reproductive strategy (Fig. 1D). As the multinuclear cell develops in P. falciparum blood-stage schizogony, most of the nuclei do not divide synchronously (6), and this asynchrony produces numbers of nuclei beyond the expected simple geometric progression (2, 4, 8…) (10, 53). These aspects of Plasmodium biology hamper efforts to determine the timing between DNA synthesis and mitosis (49) and make it difficult to predict the exact number of progeny that any single schizont will produce (6, 40, 49, 53). Even within a clonal P. falciparum line growing in vitro, the number of merozoites produced from a single schizont can vary 2- to 3-fold (21, 54), demonstrating that there is no fixed number of nascent nuclei that are required before cytokinesis can commence.
However, there are a few pieces of evidence that suggest that Plasmodium parasites exert some level of global control over mitosis during schizogony. By visually counting stained nuclei in schizonts with divided merozoites, Reilly et al. observed that the majority contained an even number of final nuclei (54). One possible cause for an even number of progeny could be if the parasite uses one final round of synchronized mitosis at the end of schizogony (31). This explanation would be consistent with the mitotic synchrony that has been noted in the syncytial stages of other apicomplexans (31, 75, 80). Considering that asynchrony is the norm in Plasmodium schizogony, this would indeed be an intriguing finding. A global level of control is also suggested in the apparent coordination of multiple cleavage furrows with a final mitosis in schizogony. Electron microscopy studies have described the alignment of dividing nuclei around the outer edge of the schizont, the assembly of daughter organelles around each spindle pole, and the progression of cleavage furrows around the still-dividing nuclei (10, 11, 44). Coordinated cleavage furrow formation has also been supported by the appearance of simultaneous rings of PfMORN1, a protein that is a component of mitotic spindle and cleavage furrow machinery in Toxoplasma and other apicomplexans (26). Recently, simultaneous furrow ingression at the end of schizogony has also been observed by live, time-lapse microscopy (59). However, in spite of these observations, the molecular mechanisms governing mitotic checkpoints in Plasmodium have not been fully identified. Biochemical, genetic, and genomic studies indicate that apicomplexan (and in particular Plasmodium) signaling networks may not rely on all of the same components that are conserved in other eukaryotes (19, 31, 74).
Despite these variations from traditional modes of mitosis, in the end Plasmodium parasites must achieve the same result from mitosis as all eukaryotes: they must correctly sort and separate their duplicated genomes. In this regard, there are some elements of mitosis that Plasmodium parasites share with higher eukaryotes. Similar to other eukaryotes, Plasmodium parasites use a bipolar, microtubule-based mitotic spindle that is nucleated by two MTOCs and that captures chromosomes for segregation (Fig. 1B). Plasmodium parasites have a clear anaphase, in which the duplicated chromatids separate (observed through kinetochore movement as described below) and the spindle elongates (Fig. 1C), and a clear telophase, in which genome separation completes (Fig. 1D). In addition, some of the components of these structures in parasites are homologous to the structural components found in other eukaryotes.
Recent research has led to the identification of a number of Plasmodium proteins as homologues of known cell cycle regulatory proteins. CDKs (cyclin-dependent kinases), NIMA (never in mitosis gene a) kinases, centrin proteins, and Aurora-related kinases have been identified within this growing list in Plasmodium species (19, 20, 32, 39, 42, 56). In Toxoplasma, an impressive amount of work has recently begun to dissect the specific functions of structural and regulatory proteins in the cell cycle of this organism (29, 31). However, so far, such efforts lag behind in Plasmodium, and only a few of the potential regulatory gene products have been experimentally demonstrated to have defined roles in mitosis (19). Some Plasmodium proteins appear to be essential for blood-stage development since they cannot be knocked out or disrupted in a loss-of-function manner in genetic transfection studies. This inability to recover a genetic-knockout-deficient phenotype also makes it difficult to identify a specific point in the cell cycle in which they are required (for a recent review, see reference 19). In P. falciparum, a high-throughput drug screen has identified several other candidate mitotic regulators that appear to be essential for blood-stage development (28). In P. berghei, a large-scale gene disruption screen of the entire Plasmodium kinome has identified roles for kinases in the development of sexual and asexual blood stages, oocysts, and sporozoites (78). It is likely that some of these gene products do not directly regulate mitosis even though they may be essential for parasite reproduction (23). A conditional knockdown approach (62) will be very useful for future studies that aim to demonstrate specific roles for these proteins in mitosis.
Two Plasmodium genes that do have a defined cell cycle role encode the NIMA-related kinases (Neks) Nek-2 and Nek-4 that have been described in P. falciparum and P. berghei. Both nek-2 and nek-4 have been shown to be essential for the DNA replication that precedes meiosis in the parasite zygote, and disruptions in these genes prevent further development of mosquito-stage parasites. Interestingly, nek-2 and nek-4 are not required for mitosis in blood-stage development (55, 56), while nek-1 and nek-3 could not be disrupted in the blood stage of P. berghei, suggesting that they are essential in this stage (78).
There is strong evidence in P. falciparum that suggests that the centrin protein PfCEN3 is a component of the mitotic spindle MTOC during blood-stage schizogony (42). Centrin proteins are key components of MTOCs in a wide variety of eukaryotes (8, 63, 64), and centrin-3 proteins are essential for spindle MTOC duplication and stability in human and yeast cells (46, 50, 64). In blood-stage P. falciparum parasites, PfCEN3 localizes to discrete regions of the nuclear membrane that coincide with the mitotic spindle poles (42). Recently, an Aurora-A-related kinase, PfArk1, was also identified in P. falciparum and was shown to localize to mitotic spindle poles during schizogony (57). In higher eukaryotes, Aurora-A is an important mitotic regulator of MTOC maturation and separation, and it has been implicated in the assembly of the mitotic spindle (8, 12). PfArk1 appears to be essential for blood-stage development, as the authors were not able to generate PfArk1-deficient parasites. Interestingly, PfArk1 localizes only to paired MTOCs, and it is only visible in a subset of the schizont nuclei which have short intervening mitotic spindles, suggesting that its function is restricted to a specific phase of mitosis. It will be very interesting to learn whether the appearance of PfArk1 at the spindle poles coincides with a specific stage of spindle progression (spindle assembly, chromosome capture, anaphase), DNA replication state, and/or maturity of MTOC.
We think that further genetic and biochemical studies will be needed to advance our understanding of the molecular basis of the regulation of mitosis in Plasmodium. Nonetheless, our current understanding of mitotic events in these parasites comes from direct observation of mitosis by microscopy. The conserved architectures of the mitotic spindle have allowed the use of microtubules, MTOCs, and DNA as visual landmarks to study the progression of mitosis in Plasmodium parasites. With this in mind, we will examine visual descriptions of these structures in Plasmodium from both electron and light microscopy studies and discuss potential inconsistencies observed between these two microscopy methods.
ELECTRON MICROSCOPY VIEWS OF PLASMODIUM PARASITE MITOSIS
In Plasmodium spp., the best simultaneous views to date of MTOC duplication, mitotic spindle assembly, and chromosome segregation have come from transmission electron microscopy (TEM) studies. TEM has the resolution (∼0.2 nm) to reveal individual microtubules of the mitotic spindle (2, 11, 52) which are normally below the resolution limit of light microscopy (∼0.2 μm). Like other eukaryotes, the Plasmodium mitotic spindle appears to contain at least three types of microtubules: those that extend from both MTOCs to overlap near the midzone or hang freely in the nucleoplasm, those that extend from one MTOC to the other, and those that extend from the MTOCs to contact the chromosomes (66). As mentioned above, since most of the parasite chromatin does not condense during mitosis, TEM does not clearly depict chromosome arms. However, TEM does resolve the kinetochores (52) (Fig. 2 and 3), the protein structures that mediate the attachment of chromosomes to microtubules. Kinetochores can be used to monitor chromosome movements during mitosis by TEM because they are electron dense and they correspond one-to-one with chromosomes. TEM also reveals the spindle MTOCs, which appear as dense clouds of material that originate within nuclear membrane pores and extend into the cytoplasm (11, 52, 66) (Fig. 2 and 3). Mitotic spindles consisting of two MTOCs, a full set of chromosome kinetochores, and the associated microtubules are three-dimensional (3D) structures that are difficult to image in a single TEM section. Thus, the main work that has charted the progression of parasite mitosis at high resolution has relied on serial section three-dimensional TEM.
The earliest sign of Plasmodium parasite mitosis that has been reported by TEM is the appearance of the hemispindle (also called the half-spindle), as seen during sporozoite development in the mosquito (66) (Fig. 2A). The hemispindle is an array of microtubules which radiates into the nucleus from a single MTOC embedded in the nuclear membrane. Hemispindles have been shown in P. falciparum schizogony (74); however, it has not been clearly demonstrated that the first mitotic division begins with a single hemispindle in this parasite stage. It is believed that the hemispindle duplicates (Fig. 2B) and that these two half-spindles join together to form a full, bidirectional mitotic spindle with one MTOC at each pole (66) (Fig. 2C). After the spindle microtubules from both poles capture the chromosomes (approximate metaphase; Fig. 1B), the kinetochores separate to opposite poles (signaling sister chromatid separation) and the mitotic spindle extends in length (anaphase; Fig. 1C) (52). As the spindle elongates, the MTOCs located at the poles move apart from one another and appear to migrate to opposite sides of the nucleus in preparation for nuclear division (telophase; Fig. 1D).
An important and often overlooked piece of information from TEM studies on Plasmodium parasite mitosis is the relative size of these structures. It is essential to keep scale in mind, especially when attempting to correlate TEM-reported structures with the morphology that is visible by light microscopy. TEM studies indicate that the early mitotic spindle structures in P. falciparum are quite small and that even fully assembled bidirectional spindles begin with lengths between 0.5 and 0.7 μm and widths between 0.3 and 0.4 μm (52, 66). Kinetochores for all 14 chromosomes can be found on spindle microtubules (approximate metaphase) when the spindles are roughly only 1 μm long from pole to pole (52) (Fig. 3). Kinetochore separation (anaphase A) is also visible in spindles that are roughly 1 μm long (3, 52, 74). These small spindles are still positioned close to one inner face of the nuclear membrane, as they do not extend far from the paired MTOCs that are embedded in pores of the nuclear membrane (11). This suggests that the events of chromosome capture and separation are likely already completed before the nucleus displays overt signs that would be visible by conventional light microscopy staining techniques. It is also possible that the chromosomes may remain attached to the hemispindle microtubules throughout the cell cycle (75); however, further work will be required to demonstrate this definitively in Plasmodium.
LIGHT MICROSCOPY VIEWS OF P. FALCIPARUM BLOOD-STAGE MITOSIS
Fluorescent light microscopy remains a valuable tool in the study of P. falciparum mitosis and can be used to complement the information from TEM (7, 24, 42, 44, 53, 74). Samples for light microscopy are usually prepared under less-disruptive fixation conditions than TEM samples and thus are less susceptible to certain (but not all) types of artifacts. Importantly, fluorescence microscopy also offers the opportunity to observe organelles in living cells (79) and the possibility of using several markers simultaneously to correlate organelle development with the schizont nuclear cycle (1, 44, 57, 76, 81).
With the use of markers for chromosomes, microtubules, and MTOCs in combination with optical sectioning techniques, it is possible to observe the full three-dimensional structure of the P. falciparum mitotic spindle by light microscopy (Fig. 4). Although individual parasite chromosomes are difficult to image by light microscopy, the staining intensity of DNA in the nucleus can yield useful clues about chromosome disposition (44, 53, 80). Recently, a significant amount of information about the internal organization of the parasite nucleus has become available with the use of markers for telomeres, nucleoli, centromeres, and gene-specific loci (7, 27, 41, 43). This has the advantage of examining the chromosome structures that are currently not visible by TEM, leading to further insights into the fine details of the nuclear cycle in schizonts.
Fig. 4.
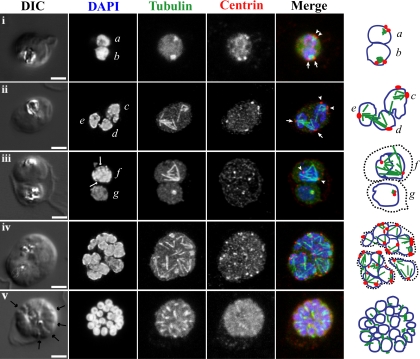
Fluorescence light microscopy images of mitotic spindle during blood-stage schizogony in P. falciparum parasites. 3D confocal microscopy views of blood-stage P. falciparum parasite cell morphology (DIC microscopy), nuclei (DAPI, blue in merged images), mitotic spindle (anti-alpha-tubulin antibody, green in merged images), and mitotic spindle MTOCs (anti-PfCEN3 antibody in rows i, ii, iv, and v or anti-Cr Centrin1 antibody 20H5 in row iii, red in merged images) as previously described (42). Schematic cartoons are drawn for each example, indicating spindle MTOCs (red circles), microtubules (green lines), and outlines of stained DNA (blue lines). Dotted black lines indicate separate parasites in multiply infected host cells. Row i, a and b, short mitotic spindles bounded by MTOCs. The spindle in nucleus b which resembles an oblong tubulin spot has a pole-to-pole distance that is approximately 1 μm long, similar to the lengths of anaphase spindles as described by TEM (52) (also see Fig. 3). Rows ii and iii, c to f, larger spindle structures. In d and e, spindle extends across separate nuclear bodies. In f, a dark line is visible down the center of the DAPI-stained DNA near the spindle midzone (DAPI, arrows). Row iv, asynchronous schizont nuclei have spindles with different geometries in multiple stages of extension. Row v, segmented parasites after the final mitosis of schizogony. Furrows are visible between daughters (arrows), nuclei are condensed, and microtubules appear in the daughter cytoplasms. Row i is a single confocal slice from a 3D series. Rows ii to v are maximum-projection images of full 3D series. Rows ii to iv were processed with deconvolution software. Scale bar, 2 μm.
Tubulin proteins, the building blocks of microtubules, are highly conserved among eukaryotes, and there are several antibodies and other reagents for tubulins that have been used to stain P. falciparum microtubules for fluorescence microscopy (24, 42, 53, 74). Studies using fluorescent probes against tubulins have highlighted the variety of P. falciparum microtubule structures (53) that are comprised of differentially expressed tubulin isotypes and posttranslational modifications (24, 38, 53). In addition, tubulin fluorescence has been used to correlate spindle development with the nuclear cycle (7, 42, 53) and to characterize the disruption of mitotic structures in response to antimalarial drug treatments (25, 74). These studies have indicated that during the later stages of schizogony when the parasite becomes crowded with nuclei, the maximum lengths of the mitotic spindles appear to become progressively smaller in the limited space (42, 53). After the final mitosis of schizogony, anti-tubulin reagents label the cytoplasmic microtubules of the nascent daughter parasites (53, 74).
However, there are some aspects of fluorescent tubulin structures that appear to be inconsistent with TEM. In some studies using fluorescent reagents against tubulins, relatively long (2 to 4 μm) and sometimes astral microtubules have been described inside nuclei in blood-stage P. falciparum (24, 25, 53), and it has been postulated that these structures are hemispindles similar to those described by TEM studies of mitosis (Fig. 2). However, the early mitotic hemispindles described by TEM are small (∼0.5 to 0.7 μm long) (66, 74), as are the early stages of the fully assembled spindles (∼1 μm long) (3, 52) (Fig. 3). From these data, it is difficult to determine when these longer microtubule structures arise during the process of spindle formation and dissolution, but it seems likely that they are products of the later stages of mitosis (Fig. 1D and Fig. 4, row iii, f). In another example of apparent scale inconsistency, fluorescence studies have also identified short, discrete plaques in P. falciparum nuclei that stain intensely with anti-microtubule reagents (7, 24, 25, 53), and these structures have been interpreted as the MTOCs that are visible by TEM. These tubulin-rich plaques have been reported in the literature with a variety of sizes. Many of these tubulin-rich plaques are larger than the membrane-embedded MTOCs reported by TEM (0.2 to 0.3 μm) (52, 66) and instead appear to be closer in size to the early mitotic spindles (0.4 to 0.5 μm wide and 0.5 to 1.0 μm long) (3, 52) (Fig. 3). Also, without the use of an additional reference marker for the nuclear membrane and three-dimensional sectioning, it is difficult to distinguish potential tubulin-rich MTOCs in nuclear membrane pores from the spindle microtubules inside the nuclear lumen, particularly when they are labeled with the same tubulin marker. We suggest the possibility that some of the tubulin-rich plaques in these size ranges that are detected by fluorescence microscopy may actually represent small mitotic spindles in different stages of extension (Fig. 4, row i). We think it is important to reconcile inconsistencies such as these between TEM and fluorescence microscopy because the orders of events in MTOC duplication, movement, and microtubule extension that we infer from these observations are critical pieces of data for our understanding of P. falciparum spindle formation (33).
As discussed above, PfCEN3 and PfArk1 are two markers that may be used to track P. falciparum mitotic MTOCs independently of microtubules (42, 57). It will be useful to validate both markers by immuno-EM to confirm their accuracy and to colocalize them with other MTOC components. Regardless of the specific reagents that are used, simultaneously viewing spindle microtubules and MTOCs with separate markers provides the advantages of confirming which microtubules are components of the spindle and determining the orientation of the small spindles by identifying their poles and by helping to estimate spindle length as a measure of mitotic progression. Adding a third marker for individual chromosomes (such as a fluorescent kinetochore marker) would significantly help to correlate the timing of events between TEM and fluorescence microscopy. More confirmed markers for each of these structures are needed in Plasmodium.
CONCLUSION
Several decades of studies in electron microscopy and light microscopy have identified some of the architectural components of mitosis and shed light on the major sequence of events in Plasmodium nuclear development. Nevertheless, we are still missing many of the intervening details in these processes, and so mitosis in Plasmodium remains poorly understood. Specifically, mitosis in Plasmodium still lacks a fully reconciled picture of the earliest steps of MTOC duplication, mitotic spindle formation, and chromosome capture. Due to the complexities of this parasite's biology that include asynchronous multinuclear cells, many of these questions are best answered by the direct observation of individual parasites, preferably in three dimensions. Ideally, Plasmodium microtubule and kinetochore dynamics should be followed at a level of detail similar to that used to investigate the overlap between S and M phases in Saccharomyces cerevisiae (37). We are also missing a complete picture of the molecular signaling networks that regulate mitosis in Plasmodium. Further biochemical studies as well as genetic knockout and knockdown experiments will be essential for identifying additional components of this regulatory machinery and for dissecting the precise functions of such networks in mitosis. The unique aspects of mitosis in malaria parasites may yet include targets to address the pathogenesis and transmission of the disease. As molecular and instrument technologies advance and improve both our spatial and temporal resolution of these processes in Plasmodium, it is hoped that we will gain a better understanding of the reproduction of this important parasite and come to appreciate the lessons it can teach us about eukaryotic mitosis.
ACKNOWLEDGMENTS
We thank Thomas McCutchan, Denis Larochelle, Jill Blankenship, Tetsuya Furuya, and Jody Franke for critical comments on the manuscript. We thank the NIAID Microscopy Facility for help with confocal microscopy.
This work was supported by intramural funding from the Food and Drug Administration.
Footnotes
Published ahead of print on 11 February 2011.
REFERENCES
- 1. Adisa A., et al. 2007. Re-assessing the locations of components of the classical vesicle-mediated trafficking machinery in transfected Plasmodium falciparum. Int. J. Parasitol. 37:1127–1141 [DOI] [PubMed] [Google Scholar]
- 2. Aikawa M. 1971. Parasitological review. Plasmodium: the fine structure of malarial parasites. Exp. Parasitol. 30:284–320 [DOI] [PubMed] [Google Scholar]
- 3. Aikawa M., Beaudoin R. L. 1968. Studies on nuclear division of a malarial parasite under pyrimethamine treatment. J. Cell Biol. 39:749–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alano P., Billker O. 2005. Gametocytes and gametes, p. 191–219 In Sherman I. W. (ed.), Molecular approaches to malaria. ASM Press, Washington, DC [Google Scholar]
- 5. Aly A. S. I., Vaughan A. M., Kappe S. H. I. 2009. Malaria parasite development in the mosquito and infection of the mammalian host. Annu. Rev. Microbiol. 63:195–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arnot D. E., Gull K. 1998. The Plasmodium cell-cycle: facts and questions. Ann. Trop. Med. Parasitol. 92:361–365 [DOI] [PubMed] [Google Scholar]
- 7. Arnot D. E., Ronander E., Bengtsson D. C. 2011. The progression of the intra-erythrocytic cell cycle of Plasmodium falciparum and the role of the centriolar plaques in asynchronous mitotic division during schizogony. Int. J. Parasitol. 41:71–80 [DOI] [PubMed] [Google Scholar]
- 8. Azimzadeh J., Bornens M. 2007. Structure and duplication of the centrosome. J. Cell Sci. 120:2139–2142 [DOI] [PubMed] [Google Scholar]
- 9. Bannister L., Mitchell G. 2003. The ins, outs and roundabouts of malaria. Trends Parasitol. 19:209–213 [DOI] [PubMed] [Google Scholar]
- 10. Bannister L. H., Hopkins J. M., Fowler R. E., Krishna S., Mitchell G. H. 2000. A brief illustrated guide to the ultrastructure of Plasmodium falciparum asexual blood stages. Parasitol. Today 16:427–433 [DOI] [PubMed] [Google Scholar]
- 11. Bannister L. H., Hopkins J. M., Fowler R. E., Krishna S., Mitchell G. H. 2000. Ultrastructure of rhoptry development in Plasmodium falciparum erythrocytic schizonts. Parasitology 121(pt. 3):273–287 [DOI] [PubMed] [Google Scholar]
- 12. Barr A. R., Gergely F. 2007. Aurora-A: the maker and breaker of spindle poles. J. Cell Sci. 120:2987–2996 [DOI] [PubMed] [Google Scholar]
- 13. Baton L. A., Ranford-Cartwright L. C. 2005. Spreading the seeds of million-murdering death: metamorphoses of malaria in the mosquito. Trends Parasitol. 21:573–580 [DOI] [PubMed] [Google Scholar]
- 14. Beier J., Vanderberg J. 1998. Sporogonic development in the mosquito, p. 49–61 In Sherman I. W. (ed.), Malaria: parasite biology, pathogenesis, and protection. ASM Press, Washington, DC [Google Scholar]
- 15. Beier J. C. 1998. Malaria parasite development in mosquitoes. Annu. Rev. Entomol. 43:519–543 [DOI] [PubMed] [Google Scholar]
- 16. Bray R. S., Garnham P. C. 1982. The life-cycle of primate malaria parasites. Br. Med. Bull. 38:117–122 [DOI] [PubMed] [Google Scholar]
- 17. De Souza C. P., Osmani S. A. 2007. Mitosis, not just open or closed. Eukaryot. Cell 6:1521–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Souza C. P., Osmani S. A. 2009. Double duty for nuclear proteins—the price of more open forms of mitosis. Trends Genet. 25:545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Doerig C., et al. 2009. Signalling in malaria parasites. The MALSIG consortium. Parasite 16:169–182 [DOI] [PubMed] [Google Scholar]
- 20. Doerig C., Endicott J., Chakrabarti D. 2002. Cyclin-dependent kinase homologues of Plasmodium falciparum. Int. J. Parasitol. 32:1575–1585 [DOI] [PubMed] [Google Scholar]
- 21. Dorin-Semblat D., Sicard A., Doerig C., Ranford-Cartwright L. 2008. Disruption of the PfPK7 gene impairs schizogony and sporogony in the human malaria parasite Plasmodium falciparum. Eukaryot. Cell 7:279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dubremetz J. F. 1973. [Ultrastructural study of schizogonic mitosis in the coccidian, Eimeria necatrix (Johnson 1930)]. J. Ultrastruct. Res. 42:354–376 (In French.) [PubMed] [Google Scholar]
- 23. Dvorin J. D., et al. 2010. A plant-like kinase in Plasmodium falciparum regulates parasite egress from erythrocytes. Science 328:910–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fennell B. J., Al-shatr Z. A., Bell A. 2008. Isotype expression, post-translational modification and stage-dependent production of tubulins in erythrocytic Plasmodium falciparum. Int. J. Parasitol. 38:527–539 [DOI] [PubMed] [Google Scholar]
- 25. Fennell B. J., Naughton J. A., Dempsey E., Bell A. 2006. Cellular and molecular actions of dinitroaniline and phosphorothioamidate herbicides on Plasmodium falciparum: tubulin as a specific antimalarial target. Mol. Biochem. Parasitol. 145:226–238 [DOI] [PubMed] [Google Scholar]
- 26. Ferguson D. J., et al. 2008. MORN1 has a conserved role in asexual and sexual development across the apicomplexa. Eukaryot. Cell 7:698–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Figueiredo L. M., et al. 2005. The unusually large Plasmodium telomerase reverse-transcriptase localizes in a discrete compartment associated with the nucleolus. Nucleic Acids Res. 33:1111–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gamo F. J., et al. 2010. Thousands of chemical starting points for antimalarial lead identification. Nature 465:305–310 [DOI] [PubMed] [Google Scholar]
- 29. Gubbels M. J., et al. 2008. Forward genetic analysis of the apicomplexan cell division cycle in Toxoplasma gondii. PLoS Pathog. 4:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gubbels M. J., Vaishnava S., Boot N., Dubremetz J. F., Striepen B. 2006. A MORN-repeat protein is a dynamic component of the Toxoplasma gondii cell division apparatus. J. Cell Sci. 119:2236–2245 [DOI] [PubMed] [Google Scholar]
- 31. Gubbels M. J., White M., Szatanek T. 2008. The cell cycle and Toxoplasma gondii cell division: tightly knit or loosely stitched? Int. J. Parasitol. 38:1343–1358 [DOI] [PubMed] [Google Scholar]
- 32. Halbert J., et al. 2010. A Plasmodium falciparum transcriptional cyclin-dependent kinase-related kinase with a crucial role in parasite proliferation associates with histone deacetylase activity. Eukaryot. Cell 9:952–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heath I. B. 1980. Variant mitoses in lower eukaryotes: indicators of the evolution of mitosis. Int. Rev. Cytol. 64:1–80 [DOI] [PubMed] [Google Scholar]
- 34. Janse C. J., et al. 1988. DNA synthesis in gametocytes of Plasmodium falciparum. Parasitology 96(pt. 1):1–7 [DOI] [PubMed] [Google Scholar]
- 35. Janse C. J., van der Klooster P. F., van der Kaay H. J., van der Ploeg M., Overdulve J. P. 1986. DNA synthesis in Plasmodium berghei during asexual and sexual development. Mol. Biochem. Parasitol. 20:173–182 [DOI] [PubMed] [Google Scholar]
- 36. Janse C. J., Van der Klooster P. F., Van der Kaay H. J., Van der Ploeg M., Overdulve J. P. 1986. Rapid repeated DNA replication during microgametogenesis and DNA synthesis in young zygotes of Plasmodium berghei. Trans. R. Soc. Trop. Med. Hyg. 80:154–157 [DOI] [PubMed] [Google Scholar]
- 37. Kitamura E., Tanaka K., Kitamura Y., Tanaka T. U. 2007. Kinetochore microtubule interaction during S phase in Saccharomyces cerevisiae. Genes Dev. 21:3319–3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kooij T. W., et al. 2005. Plasmodium berghei alpha-tubulin II: a role in both male gamete formation and asexual blood stages. Mol. Biochem. Parasitol. 144:16–26 [DOI] [PubMed] [Google Scholar]
- 39. Kozlov S., Waters N. C., Chavchich M. 2010. Leveraging cell cycle analysis in anticancer drug discovery to identify novel plasmodial drug targets. Infect. Disord. Drug Targets 10:165–190 [DOI] [PubMed] [Google Scholar]
- 40. Leete T. H., Rubin H. 1996. Malaria and the cell cycle. Parasitol. Today 12:442–444 [DOI] [PubMed] [Google Scholar]
- 41. Li F., Sonbuchner L., Kyes S. A., Epp C., Deitsch K. W. 2008. Nuclear non-coding RNAs are transcribed from the centromeres of Plasmodium falciparum and are associated with centromeric chromatin. J. Biol. Chem. 283:5692–5698 [DOI] [PubMed] [Google Scholar]
- 42. Mahajan B., et al. 2008. Centrins, cell cycle regulation proteins in human malaria parasite Plasmodium falciparum. J. Biol. Chem. 283:31871–31873 [DOI] [PubMed] [Google Scholar]
- 43. Mancio-Silva L., Rojas-Meza A. P., Vargas M., Scherf A., Hernandez-Rivas R. 2008. Differential association of Orc1 and Sir2 proteins to telomeric domains in Plasmodium falciparum. J. Cell Sci. 121:2046–2053 [DOI] [PubMed] [Google Scholar]
- 44. Margos G., et al. 2004. Correlation of structural development and differential expression of invasion-related molecules in schizonts of Plasmodium falciparum. Parasitology 129:273–287 [DOI] [PubMed] [Google Scholar]
- 45. Meis J. F., et al. 1990. Plasmodium falciparum: studies on mature exoerythrocytic forms in the liver of the chimpanzee, Pan troglodytes. Exp. Parasitol. 70:1–11 [DOI] [PubMed] [Google Scholar]
- 46. Middendorp S., et al. 2000. A role for centrin 3 in centrosome reproduction. J. Cell Biol. 148:405–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mitchison T. J., Salmon E. D. 2001. Mitosis: a history of division. Nat. Cell Biol. 3:E17–E21 [DOI] [PubMed] [Google Scholar]
- 48. Musacchio A., Salmon E. D. 2007. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 8:379–393 [DOI] [PubMed] [Google Scholar]
- 49. Naughton J. A., Bell A. 2007. Studies on cell-cycle synchronization in the asexual erythrocytic stages of Plasmodium falciparum. Parasitology 134:331–337 [DOI] [PubMed] [Google Scholar]
- 50. Paoletti A., et al. 2003. Fission yeast cdc31p is a component of the half-bridge and controls SPB duplication. Mol. Biol. Cell 14:2793–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pines J., Rieder C. L. 2001. Re-staging mitosis: a contemporary view of mitotic progression. Nat. Cell Biol. 3:E3–E6 [DOI] [PubMed] [Google Scholar]
- 52. Prensier G., Slomianny C. 1986. The karyotype of Plasmodium falciparum determined by ultrastructural serial sectioning and 3D reconstruction. J. Parasitol. 72:731–736 [PubMed] [Google Scholar]
- 53. Read M., Sherwin T., Holloway S. P., Gull K., Hyde J. E. 1993. Microtubular organization visualized by immunofluorescence microscopy during erythrocytic schizogony in Plasmodium falciparum and investigation of post-translational modifications of parasite tubulin. Parasitology 106(pt. 3):223–232 [DOI] [PubMed] [Google Scholar]
- 54. Reilly H. B., Wang H., Steuter J. A., Marx A. M., Ferdig M. T. 2007. Quantitative dissection of clone-specific growth rates in cultured malaria parasites. Int. J. Parasitol. 37:1599–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Reininger L., et al. 2005. A NIMA-related protein kinase is essential for completion of the sexual cycle of malaria parasites. J. Biol. Chem. 280:31957–31964 [DOI] [PubMed] [Google Scholar]
- 56. Reininger L., et al. 2009. An essential role for the Plasmodium Nek-2 Nima-related protein kinase in the sexual development of malaria parasites. J. Biol. Chem. 284:20858–20868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reininger L., Wilkes J. M., Bourgade H., Miranda-Saavedra D., Doerig C. 2011. An essential Aurora-related kinase transiently associates with spindle pole bodies during Plasmodium falciparum erythrocytic schizogony. Mol. Microbiol. 79:205–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rieder C. L. 2010. Mitosis in vertebrates: the G2/M and M/A transitions and their associated checkpoints. Chromosome Res. [Epub ahead of print.] doi:10.1007/s10577-010-9178-z [DOI] [PubMed] [Google Scholar]
- 59. Riglar D. T., et al. 2011. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe 9:9–20 [DOI] [PubMed] [Google Scholar]
- 60. Rosenberg R. 2008. Malaria: some considerations regarding parasite productivity. Trends Parasitol. 24:487–491 [DOI] [PubMed] [Google Scholar]
- 61. Rosenberg R., Rungsiwongse J. 1991. The number of sporozoites produced by individual malaria oocysts. Am. J. Trop. Med. Hyg. 45:574–577 [DOI] [PubMed] [Google Scholar]
- 62. Russo I., Oksman A., Vaupel B., Goldberg D. E. 2009. A calpain unique to alveolates is essential in Plasmodium falciparum and its knockdown reveals an involvement in pre-S-phase development. Proc. Natl. Acad. Sci. U. S. A. 106:1554–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Salisbury J. L. 1995. Centrin, centrosomes, and mitotic spindle poles. Curr. Opin. Cell Biol. 7:39–45 [DOI] [PubMed] [Google Scholar]
- 64. Salisbury J. L. 2007. A mechanistic view on the evolutionary origin for centrin-based control of centriole duplication. J. Cell. Physiol. 213:420–428 [DOI] [PubMed] [Google Scholar]
- 65. Schofield L. 2007. Intravascular infiltrates and organ-specific inflammation in malaria pathogenesis. Immunol. Cell Biol. 85:130–137 [DOI] [PubMed] [Google Scholar]
- 66. Schrevel J., Asfaux-Foucher G., Bafort J. M. 1977. Ultrastructural study of multiple mitoses during sporogony of Plasmodium b. berghei. J. Ultrastruct. Res. 59:332–350(In French.) [DOI] [PubMed] [Google Scholar]
- 67. Schrevel J., et al. 2008. Vesicle trafficking during sporozoite development in Plasmodium berghei: ultrastructural evidence for a novel trafficking mechanism. Parasitology 135:1–12 [DOI] [PubMed] [Google Scholar]
- 68. Shortt H. E., Fairley N. H., Covell G., Shute P. G., Garnham P. C. 1951. The pre-erythrocytic stage of Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 44:405–419 [DOI] [PubMed] [Google Scholar]
- 69. Sinden R. 1998. Gametocytes and sexual development, p. 25–48 In Sherman I. W. (ed.), Malaria: parasite biology, pathogenesis, and protection. ASM Press, Washington, DC [Google Scholar]
- 70. Sinden R., Matuschewski K. 2005. The sporozoite, p. 169–190 In Sherman I. W. (ed.), Molecular approaches to malaria. ASM Press, Washington, DC [Google Scholar]
- 71. Sinden R. E. 1991. Mitosis and meiosis in malarial parasites. Acta Leiden. 60:19–27 [PubMed] [Google Scholar]
- 72. Sinden R. E. 2009. Malaria, sexual development and transmission: retrospect and prospect. Parasitology 136:1427–1434 [DOI] [PubMed] [Google Scholar]
- 73. Sinden R. E., Canning E. U., Bray R. S., Smalley M. E. 1978. Gametocyte and gamete development in Plasmodium falciparum. Proc. R. Soc. Lond. B Biol. Sci. 201:375–399 [DOI] [PubMed] [Google Scholar]
- 74. Sinou V., Boulard Y., Grellier P., Schrevel J. 1998. Host cell and malarial targets for docetaxel (Taxotere) during the erythrocytic development of Plasmodium falciparum. J. Eukaryot. Microbiol. 45:171–183 [DOI] [PubMed] [Google Scholar]
- 75. Striepen B., Jordan C. N., Reiff S., van Dooren G. G. 2007. Building the perfect parasite: cell division in apicomplexa. PLoS Pathog. 3:e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Struck N. S., et al. 2005. Re-defining the Golgi complex in Plasmodium falciparum using the novel Golgi marker PfGRASP. J. Cell Sci. 118:5603–5613 [DOI] [PubMed] [Google Scholar]
- 77. Sturm A., et al. 2006. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science 313:1287–1290 [DOI] [PubMed] [Google Scholar]
- 78. Tewari R., et al. 2010. The systematic functional analysis of Plasmodium protein kinases identifies essential regulators of mosquito transmission. Cell Host Microbe 8:377–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tilley L., McFadden G., Cowman A., Klonis N. 2007. Illuminating Plasmodium falciparum-infected red blood cells. Trends Parasitol. 23:268–277 [DOI] [PubMed] [Google Scholar]
- 80. Vaishnava S., et al. 2005. Plastid segregation and cell division in the apicomplexan parasite Sarcocystis neurona. J. Cell Sci. 118:3397–3407 [DOI] [PubMed] [Google Scholar]
- 81. van Dooren G. G., et al. 2005. Development of the endoplasmic reticulum, mitochondrion and apicoplast during the asexual life cycle of Plasmodium falciparum. Mol. Microbiol. 57:405–419 [DOI] [PubMed] [Google Scholar]
- 82. Vaughan J. A. 2007. Population dynamics of Plasmodium sporogony. Trends Parasitol. 23:63–70 [DOI] [PubMed] [Google Scholar]
- 83. Zheng Y. 2010. A membranous spindle matrix orchestrates cell division. Nat. Rev. Mol. Cell Biol. 11:529–535 [DOI] [PMC free article] [PubMed] [Google Scholar]