Abstract
Parasitic protozoa, such as malaria parasites, trypanosomes, and Leishmania, acquire a plethora of nutrients from their hosts, employing transport proteins located in the plasma membrane of the parasite. Application of molecular genetic approaches and the completion of genome projects have allowed the identification and functional characterization of a cohort of transporters and their genes in these parasites. This review focuses on a subset of these permeases that have been studied in some detail, that import critical nutrients, and that provide examples of approaches being undertaken broadly with these and other parasite transporters. Permeases reviewed include those for hexoses, purines, iron, polyamines, carboxylates, and amino acids. Topics of special emphasis include structure-function approaches, critical roles for transporters in parasite viability and physiology, regulation of transporter expression, and subcellular targeting. Investigations of parasite transporters impact a broad spectrum of basic biological problems in these protozoa.
Parasitic protozoa are responsible for a host of devastating diseases worldwide, including malaria, African trypanosomiasis, Chagas' disease, leishmaniases, toxoplasmosis, and many others. While all organisms must acquire nutrients from their environment, the parasitic mode of life has a number of consequences regarding nutrient uptake. First, the parasite must compete with its insect and vertebrate hosts for acquisition of many essential compounds and thus must evolve efficient uptake mechanisms. Second, the typical parasitic life cycle entails at least two hosts, one of which is often an invertebrate vector and the other a vertebrate host. Thus, the microbe cycles between multiple physiologically distinct milieus that may present pronounced differences in available nutrients, pH, temperature, ionic composition, etc. The parasite must express nutrient uptake systems that accommodate these profound alterations in environment and may employ regulatory mechanisms to alter the level of uptake according to nutrient availability and/or life cycle stage. The capacity of a parasite to import critical nutrients is central to its ability to be transmitted, to infect a host, and to cause disease and hence is an important component of pathogenesis.
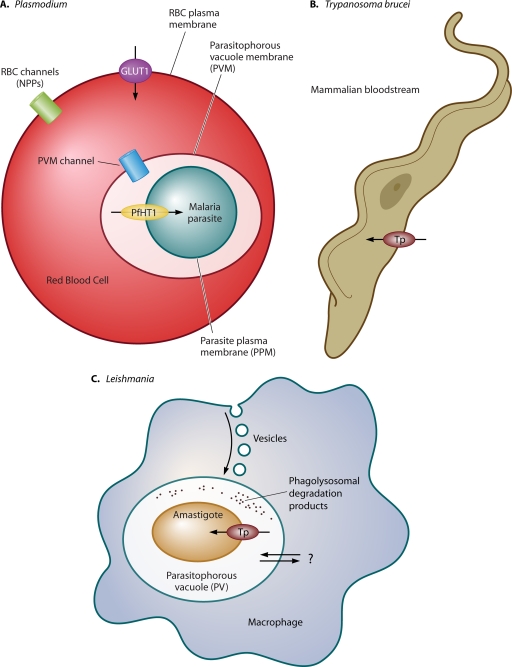
Figure 1 illustrates the interaction with the mammalian host of 3 parasites discussed in this review, Plasmodium, Trypanosoma brucei, and Leishmania, and reveals how the unique niche in the host dictates the mode of nutrient acquisition. African trypanosomes are extracellular parasites that live in the blood and interstitial spaces of the host and thus salvage nutrients directly from these extracellular fluids. In contrast, both Plasmodium and Leishmania live within parasitophorous vacuoles inside the red blood cell and macrophage, respectively. Intracellular parasitism provides constraints and opportunities regarding nutrient acquisition, specifically the need to import nutrients across 3 membrane systems and potentially the opportunity to exploit components of the host cytosol or vacuolar lumen as sources of nutrition.
Fig. 1.
Uptake of nutrients by intraerythrocytic Plasmodium, extracellular T. brucei, and intramacrophage Leishmania parasites. (A) Nutrient uptake by malaria parasites residing within the host red blood cell (RBC) entails flux of hydrophilic compounds across three membranes, the red blood cell plasma membrane, the parasitophorous vacuole membrane (PVM), and the parasite plasma membrane (PPM). The example of glucose uptake illustrates this pathway. Glucose is first imported into infected RBCs by the human glucose transporter GLUT1. It then probably diffuses across the PVM through a low-specificity PVM channel (31) and subsequently enters the parasite via the PPM transporter PfHT1, an ortholog of GLUT1. The new permeation pathway (NPP) channels are thought to be important routes of uptake for a variety of nutrients in the infected RBC (30) but probably do not mediate significant uptake of glucose. This figure was modified from Fig. 13.6 in reference 55 with permission of the publisher. (B) African trypanosomes reside in the mammalian host bloodstream or within interstitial spaces between cells and thus mediate uptake of nutrients from extracellular fluids employing parasite plasma membrane transporters (Tp). Examples of such transporters that have been well studied include those for hexoses, purines, and carboxylates (Table 1). This figure was modified from Fig. 2 in reference 104 with permission of the publisher. (C) Leishmania amastigotes live within parasitophorous vacuoles (PV) of mammalian host macrophages. Amastigotes may obtain nutrients by vesicular transport from the macrophage plasma membrane (88), from degradation products of macromolecules present in the PV lumen (a phagolysosome), or possibly from the host cell cytosol by flux across the PV (double arrows with question mark). Nutrients are imported into the amastigote by transporters located in the parasite plasma membrane. Examples of such transporters include those for hexoses, purines, iron, and polyamines (see Table 1).
To provide focus and to accommodate the space limitations of a minireview, this article will concentrate on a limited number of transporters, those for hexoses, purines, iron, polyamines, amino acids, and carboxylates, that have been studied in sufficient detail in several parasites with well-established experimental systems. However, it has not been possible to cover comprehensively all interesting studies on parasite transporters, and inevitably many transport systems in various parasitic protozoa have not been included. Table 1 summarizes the permeases covered in this review and lists a few relevant references for each transporter that may be consulted as a starting point for further reading. For additional reference, Table 2 lists some other transporters that have been studied intensively but are not covered in this minireview. ABC and drug resistance transporters are not covered in this article.
Table 1.
Principal transporters discussed in this review, tabulated according to substrate specificitya
| Nutrient(s) | Parasite | Transporter(s) | Key reference(s) |
|---|---|---|---|
| Hexoses | P. falciparum | PfHT | 52 |
| T. brucei | THT1, THT2 | 96 | |
| L. mexicana | LmxGT1, LmxGT2, LmxGT3 | 17 | |
| T. gondii | TgGT1 | 13 | |
| Purines | P. falciparum | PfNT1 to PfNT4 | 20, 36, 79 |
| T. brucei | TbAT1, TbNT2 to TbNT12 | 29, 62, 75 | |
| L. donovani, L. major | LdNT1 to LdNT4, LmaNT1 to LmaNT4 | 22, 74, 102 | |
| Iron | T. brucei | ESAG6/ESAG7 heterodimer (transferrin receptor) | 57 |
| L. amazonensis | LIT1 | 47 | |
| Polyamines | L. major | LmaPOT1 | 45 |
| T. cruzi | TcPOT1.1, TcPOT1.2 | 44 | |
| Amino acids | L. donovani | LdAAP3 | 91 |
| Carboxylates | T. brucei | PAD1, PAD2 | 28 |
The list of references is not exhaustive but provides some key articles for further reading.
Table 2.
Some other transporters in P. falciparum, Trypanosoma, and Leishmania species whose genes have been cloned and analyzeda
| Nutrient(s) | Parasite | Transporter(s) | Importance | Key reference(s) |
|---|---|---|---|---|
| Hexoses | T. cruzi | TcHT1 | Sugar uptake | 97 |
| Polyols | L. donovani | LdMIT | myo-Inositol | 35 |
| Folates | Leishmania species | FT1 to FT14 | Essential nutrients | 25, 77 |
| Nucleotide sugars | L. donovani, L. major | LPG2, LPG5 | Golgi apparatus, complex carbohydrate synthesis | 19 |
| Phospholipids | L. donovani | LdMT | Miltefosine drug import | 80 |
| Water, glycerol, osmolytes | L. major | LmAQP1 | Uptake of arsenical drugs, osmolyte sensing | 41 |
| T. brucei | TbAQP | Uptake of water, glycerol, other small molecules | 98 | |
| P. falciparum | PfAQP1 | Water, glycerol | 12 | |
| T. cruzi | TcAQP1 | Acidocalcisomes | 67 | |
| Phosphate | P. falciparum | PfPIT | Phosphate uptake | 89 |
| Phosphorylated C3 compounds | P. falciparum | PfiTPT, PfoTPT | Moving glycolytic derivatives from cytosol to plastid | 68 |
| Cations | T. gondii | Ca2+ ATPase in acidocalcisomes | 60 | |
| L. amazonensis | Lmaa1 | Ca2+ ATPase | 59 | |
| T. brucei | TBA1 | P ATPase | 84 | |
| Leishmania species | P ATPases | 66 | ||
| P. falciparum | Vacuolar H+ ATPase targeted to RBC membrane | 61 | ||
| Fatty acids | T. brucei | GAT1 to -3 | Fatty acid CoA import into glycosome | 49 |
References are not exhaustive but provide some relevant articles that can serve as starting points for more extensive reading. CoA, coenzyme A.
HEXOSE TRANSPORTERS
Malaria and toxoplasma.
Glucose and related hexoses, such as fructose, are nutrients of central importance to many cells. The intraerythrocytic stages of malaria parasites, the life cycle forms responsible for most of the disease pathology, live in the vertebrate bloodstream, which provides a rich source of glucose (∼5 mM). While recent studies indicate that intraerythrocytic parasites in natural infections experience a variety of nutritional states (26), glycolysis is the major route for providing energy to these intracellular microbes and results in high utilization of glucose and robust export of the end product, lactate. To serve as a useful source of metabolic energy for the parasite, glucose must pass across the red blood cell plasma membrane (PM), through the membrane of the parasitophorous vacuole (PV), and across the malaria plasma membrane (Fig. 1A). The red blood cell glucose transporter GLUT1 appears to be the major route across the first barrier (54), and a low specificity channel in the PV membrane (31) allows flux of the sugar into this organelle. The parasite PM possesses its own facilitative (i.e., nonconcentrative) hexose transporter, which is critical for delivery of glucose to the parasite cytosol. The gene encoding the Plasmodium falciparum hexose transporter (PfHT) was identified (106) as a homolog (29.8% amino acid identity) of GLUT1 that appeared in the emerging genome sequence for this parasite. Expression of this transporter in Xenopus oocytes established that PfHT transported glucose (Km, 0.48 mM), fructose, mannose, and galactose, and a transporter-specific antibody was employed to show that the protein is expressed in the PM of the intraerythrocytic parasite. To test a critical role for this permease in parasite viability, Joet and colleagues (52) screened a variety of glucose analogs and determined that 3-O-((undec-10-en)-1-yl)-d-glucose, CM3361, was a high-affinity inhibitor of PfHT (Ki, ∼50 μM) but a very low-affinity inhibitor of GLUT1 (Ki, ∼3 mM). CM3361 inhibited growth of P. falciparum intraerythrocytic parasites in culture with a 50% inhibitory concentration (IC50) of 16 μM, suggesting that uptake of hexoses through PfHT is indeed essential for viability of blood-stage parasites. In addition, treatment of mice infected with Plasmodium berghei with CM3361 caused pronounced suppression of parasitemia, further indicating that PfHT was critical for infectivity and was a valid target for drug development.
More recent work by two groups has confirmed the essential nature of PfHT using genetic approaches and has implied that this permease may play central roles in the nutrition of both mammalian and insect stages of the life cycle. The Krishna group (92) was unable to knock out the PfHT gene in intraerythrocytic parasites unless the transfections were performed in the presence of a complementing expression vector containing the PfHT open reading frame (ORF). In the latter case, the integration vector replaced the endogenous PfHT gene, and the complementing episomal vector rescued the knockout by expressing either wild-type or green fluorescent protein (GFP)-tagged PfHT. Furthermore, transgenic parasites expressing PfHT-GFP exhibited membrane-localized fluorescence throughout the insect-infective stages, including female gametes, zygotes, ookinetes, midgut and sporulating oocysts, and sporozoites within the mosquito salivary glands. The Gupta group (13a) performed similar knockout experiments on the murine parasite P. berghei and demonstrated that a knockout of the gene encoding the P. berghei hexose transporter (PbHT) was possible only if the integrating deletion construct was accompanied by a complementing expression construct encoding either PbHT or PfHT. Insect-stage and liver-stage parasites expressed tagged PbHT in the plasma membrane, demonstrating that this hexose transporter is expressed throughout the parasite life cycle. Furthermore, both mosquito- and liver-stage parasites were attenuated by treatment with CM3361, implying that this transporter is essential throughout the life cycle and that inhibitors of PfHT might be effective against bloodstream-, liver-, and insect-stage parasites. Together these studies underscore the central requirement for glucose uptake and metabolism throughout the malaria life cycle and enhance interest in PfHT as a potential drug target.
In contrast to findings for P. falciparum, the GT1 glucose transporter of the related apicomplexan parasite Toxoplasma gondii (TgGT1) is not essential for parasite viability (13).
African trypanosomes.
The bloodstream forms (BFs) of Trypanosoma brucei live extracellularly in a glucose-rich environment (Fig. 1B). As a result, BF trypanosomes metabolize high levels of glucose by glycolysis and do not possess a complete Krebs cycle or oxidative phosphorylation (95). The parasites are completely dependent upon glucose to maintain viability, and hexose transporters have been proposed as targets for development of antitrypanosomal therapies (7, 8, 11, 95). The genes encoding two hexose transporters, T. brucei HT1 (TbHT1) and TbHT2, were cloned (14) and shown to encode proteins with low but significant sequence identity to GLUT1. Functional expression (10) established that TbHT1 represents a low-affinity, high-capacity hexose transporter expressed almost exclusively in BF trypanosomes, whereas TbHT2 is a high-affinity, low-capacity permease expressed primarily in the procyclic-form (PF) insect-stage parasites.
Two studies have examined small-molecule inhibitors of TbHT1 to determine whether this permease could be targeted for drug development. Triazine compounds, such as Cibacron blue, inhibited TbHT1 with moderate affinity (11). Placing a bromoacetamide group onto the 2-O position of d-glucose or the 6-O position of d-fructose resulted in high-affinity competitive inhibitors of glucose uptake by BF trypanosomes (7). These compounds had 50% lethal dose (LD50) values for BF trypanosomes in culture that were in the low micromolar range, indicating that inhibition of the parasite hexose transporter was lethal to mammalian-stage parasites and confirming the potential for TbHT1 as a possible drug target. To date, no localization experiments have been performed for either TbHT1 or TbHT2, nor have genetic approaches been undertaken to confirm the essential nature of TbHT1 in BF trypanosomes.
Leishmania.
Leishmania parasites are kinetoplastid protozoa, related to the African trypanosomes, that live as flagellated promastigotes inside the sand fly vector and as nonmotile amastigotes within the acidified phagolysosomal vesicles of vertebrate host macrophages (Fig. 1C). Promastigotes in culture can employ either glucose or proline as principal sources of metabolic energy (94), and both are likely to be important nutrients in the sand fly. Hexoses, such as glucose and fructose, are generated in the sand fly gut by digestion of sucrose present in high concentrations in plant nectar, which is a food source for the insect (90), and these sugars probably fluctuate between high and low concentrations depending upon the frequency with which nectar is obtained by the fly. When the promastigote enters a macrophage and is internalized into the phagolysosome, it is thought to enter an environment that is relatively low in carbohydrates but which provides fatty acids and amino acids as energy sources (15, 65, 69).
The Leishmania mexicana genome encodes a cluster of 3 hexose transporter genes, designated LmxGT1, LmxGT2, and LmxGT3 (16). GT1 and GT3 mRNAs are expressed at similar levels in both culture-form promastigotes and intracellular amastigotes, but GT2 mRNA is downregulated ∼15-fold in amastigotes compared to levels in promastigotes. All 3 permeases transport glucose, fructose, mannose, and galactose (86), and GT2 and GT3 transport ribose (71). The GT2 and GT3 proteins are present in the pellicular plasma membrane (PPM) that surrounds the cell body, but they are not present at detectable levels in the flagellar membrane. In contrast, GT1 is localized primarily to the flagellar membrane (81). The function of the flagellar localization of GT1 is still not clear, but two sequences within the unique amino-terminal hydrophilic region of this permease have been implicated in flagellar targeting (70).
A null mutant in the LmxGT gene cluster, in which all 3 GT genes were knocked out, was generated by targeted gene replacement (17). This null mutant could not take up detectable levels of the 4 hexose substrates (86), and knockout promastigotes grew more slowly and to a lower density than wild-type promastigotes. Remarkably, infection of murine macrophages with the Δlmxgt null mutant resulted in dramatically reduced viability of intracellular amastigotes, although the developmental transformation from promastigotes to amastigotes was accomplished following invasion of host cells (37). The reduced intracellular viability of the null mutants may result from pleiotropic effects, including a pronounced decrease in the level of the storage carbohydrate β-mannan, increased sensitivity to oxidative stress, nutrient restriction, and elevated temperature encountered in macrophages (87).
Culturing of the Δlmxgt null mutant in glucose-replete medium for several months resulted in the outgrowth of suppressor strains that were able to import hexoses, grow more rapidly than the null mutant in glucose-containing medium, and survive within macrophages (37). This suppressor had amplified as an extrachromosomal circle an ∼10-kb region of genomic DNA that encoded a homolog of the LmxGT genes, designated LmxGT4. The latter permease is a very low-affinity hexose transporter and may have an unknown compound as its natural substrate. However, at high levels of amplification (∼38-fold), GT4 is overexpressed to a sufficient degree to mediate substantial uptake of hexoses, such as glucose and fructose.
PURINE TRANSPORTERS
Leishmania.
All parasitic protozoa examined to date are unable to synthesize purines de novo and must salvage these essential nutrients from their hosts. Salvage entails transport of preformed purine nucleosides or nucleobases, followed by interconversion and metabolism to phosphorylated nucleotides (43). The first parasite purine transporters whose genes were identified were the Leishmania donovani nucleoside transporters (NTs) L. donovani NT1 (LdNT1) (substrates adenosine and the pyrimidine nucleosides [102]) and LdNT2 (substrates inosine, guanosine, xanthosine [22]). These proteins were related in sequence to the human equilibrative nucleoside transporter ENT1 (hENT1), which had been identified shortly before (42), and were members of a new cohort of transporters ultimately designated the SLC29 family (http://www.bioparadigms.org/slc/menu.asp). Subsequently, two additional members of this gene family were identified in the then-emerging genome sequence of Leishmania major. These genes were designated NT3 (74) and NT4 (73) and were shown by functional expression to encode purine nucleobase transporters (the substrates hypoxanthine, xanthine, adenine, and guanine). While hENT1 is a nonconcentrative permease, all 4 of the Leishmania purine transporters are active proton-coupled symporters that harness energy from the electrochemical gradient across the plasma membrane to concentrate their substrates within the parasite (73, 93). Active (i.e., concentrative, energy-requiring) transport, plus the high affinity of the microbial transporters, allows the parasite to compete efficiently with host tissues for import of nutrients that are essential for the parasite.
Mutagenesis of LdNT1 (99–101, 103) and LdNT2 (3–5) has identified a variety of amino acids that play critical roles in transport or substrate specificity. In addition, two computational models, one employing “threading” of the NT2 structure onto the crystal structure of the Escherichia coli glycerol 3-phosphate transporter (3) and the other employing ab initio prediction of the NT1 structure (101), gave similar structures and predicted that NT1 and NT2 assume 3-dimensional configurations that are related to major facilitator superfamily transporters, such as the E. coli lactose permease. These models predict that most of the mutants of NT1 that greatly impair transport activity or alter substrate specificity line the central permeation pore, as might be expected for mutations that induce strong transport phenotypes. Furthermore, the ab initio model (101) predicted that two aromatic residues in transmembrane helices 1 and 2 of NT1 interact to bring these helices in close apposition and close off the central pore when the permease is in the outside-closed/inside-open conformation. Hence, these residues are predicted to constitute part of the extracellular “gate.” Results of site-directed mutagenesis also supported a gating function for these residues. Homology models of purine transporters from P. falciparum (9) and T. brucei (78) also predict similar 3-dimensional folds for those carriers.
Gene knockout studies have revealed that none of these 4 purine permeases is essential for viability of either promastigotes (insect stage) or amastigotes (intramacrophage vertebrate stage) (58, 74). However, while a double null mutant of the nucleoside transporters (Δnt1 Δnt2) was viable (58), it was impossible to generate a dual null mutant (Δnt3 Δnt4) of the nucleobase transporters (74). This result implies that purine nucleobases play a particularly important role in purine salvage. One significant distinction between NT3 and NT4 is that the former operates optimally at neutral pH, whereas the latter has a transport optimum for all 4 purine nucleobases in the acidic pH range (73). This result suggests that NT4 is designed to function optimally in the acidic environment of the phagolysosomal vesicle in which the mammalian infectious amastigote-form resides (2). Indeed, recent experiments (Diana Ortiz, Marco Sanchez, and Scott Landfear, unpublished results) indicate that a Δnt4 null mutant of L. major is strongly impaired in its ability to form lesions in BALB/c mice, whereas null mutants in the other 3 NT genes are minimally impaired in virulence.
Classic studies by Gottlieb on the kinetoplastid parasite Crithidia fasciculata (40) demonstrated that starvation for purines elicited an ∼1,000-fold upregulation of the purine salvage enzyme 3′-nucleotidase and suggested that other determinants of purine salvage might respond acutely to purine limitation. Two recent studies have examined expression of purine transporters in L. donovani and L. major parasites that have been restricted for uptake of essential purines, either by eliminating purines from the growth medium (24) or by restricting purine uptake in single or double null mutants for purine transporters (76). In each study, purine restriction resulted in a pronounced upregulation (45- to 85-fold) of purine transport activities that resulted from an increase in the rate of transporter mRNA translation but did not significantly alter the abundance of the mRNA itself. An increase in the level of several purine salvage enzymes was also detected in parasites starved for purines (24). These results imply that Leishmania parasites sense purines and respond to decreasing levels of these essential nutrients by upregulating translation of mRNAs encoding purine transporters and salvage enzymes. Elucidating both the purine sensory apparatus and the mechanism for translational control of protein expression constitutes exciting avenues for future investigation.
A different regulatory mechanism that affects expression of NTs in Leishmania parasites was discovered by selecting for mutants that could grow in the presence of several cytotoxic purine analogs (53) that are imported by these permeases. These mutant lines had a greatly reduced capacity for transport of nucleosides and nucleobases and had also amplified an ∼55-kb extrachromosomal element that conferred the resistance phenotype. Subsequently, a 2.3-kb ORF (toxic nucleoside resistance gene, or TOR) from this amplicon was cloned and shown to be responsible for the reduced transport of purines and toxic purine analogs (32). Further studies (33) revealed that the TOR protein interacts with the large intracellular loop of the NT1 permease, resulting in internalization of the transporter to a tubular lysosomal compartment and degradation by a ubiquitin-dependent mechanism. Additional studies suggest that TOR is a regulator of multiple transporters that operates at the level of protein degradation. The natural biological role of regulation by TOR has not been established.
African trypanosomes.
The observation that the adenosine/adenine transporter of T. brucei, now designated TbAT1, mediates the uptake of the arsenical drug melarsoprol (23) and a second antitrypanosomal drug, pentamidine (21), focused early interest on this purine permease. TbAT1 was the first purine transporter gene identified in T. brucei (62), and polymorphisms in this transporter have been shown to modulate drug sensitivity in both laboratory and field strains of the parasites (63). However, other routes for drug uptake exist, since a Δtbat1 null mutant still retains significant sensitivity to melarsoprol (64). Trypanosomes encode a complex family of nucleoside and nucleobase permeases (Table 1), consisting of at least 12 members with various substrate specificities and expression patterns during the trypanosome life cycle (29, 56, 75). The reason for the expansion of this gene family in trypanosomes, compared to other kinetoplastid parasites, such as Leishmania, is not clear.
Malaria.
Malaria parasites, like other parasitic protozoa, are unable to synthesize purines and must salvage them from their hosts. Purines are transported across the plasma membrane of the parasitized red blood cell primarily by host purine transporters, such as hENT1 and the human facilitative nucleobase transporter hFNT1 (82). The parasite-encoded channel-like new permeation pathway (NPP), located in the membrane of the infected red blood cell (Fig. 1A), does not appear to play a major role in purine uptake. However, purines must permeate the parasite plasma membrane using innate parasite-specific uptake systems. Two articles (20, 79) identified an ORF, designated PfNT1 (P. falciparum NT1), in the malaria parasite genome sequence that was homologous to hENT1 and other members of the SLC29 family. Expression of PfNT1 in Xenopus oocytes confirmed that this permease was a broad-specificity nucleoside transporter, and one of these studies (79) also identified purine nucleobases as substrates. An antibody raised against the amino-terminal domain of PfNT1 demonstrated that the protein is localized to the parasite plasma membrane and that it is expressed throughout the intraerythrocytic cycle but reaches its highest levels of expression in late trophozoites and schizonts (83).
Upon completion of the malaria genome project (39), 3 more NT genes (PfNT2 to PfNT4) were discovered, but the transport properties of the corresponding permeases are largely unknown, with the possible exception of the endoplasmic reticulum-resident PfNT2, which may transport uridine (34). To determine the role of PfNT1 in intraerythrocytic parasites, Ben El Bissati and coworkers (36) disrupted the PfNT1 gene using a double-crossover strategy. The PfNT1 null mutants were viable if purines were provided in the medium at a concentration of 50 μM or higher, but they did not survive at physiological (low micromolar) concentrations of purines that support growth of wild-type P. falciparum. Transport measurements performed on parasites released from red blood cells revealed a severe reduction in hypoxanthine import but only a modest reduction in uptake of purine nucleosides. The authors concluded that purine nucleosides that enter the infected red blood cell are converted to hypoxanthine by host adenosine deaminase and purine nucleoside phosphorylases and that hypoxanthine, transported by PfNT1, is the principal purine source for the intracellular parasites. The observation that a functional PfNT1 gene is required for viability of the parasites under physiological conditions implies that the transporter is essential and might be targeted for novel antimalarial drug therapy.
IRON TRANSPORTER IN LEISHMANIA
Iron, usually in the form of Fe2+ or ferrous iron, is a cofactor that is required for the function of many proteins. However, Fe2+ must be maintained at low concentrations in biological systems due to the generation of toxic hydroxyl radicals by the Fenton reaction. Ferric iron (Fe3+) is insoluble and is maintained almost exclusively as high-affinity complexes with iron binding proteins, such as transferrin, ferritin, or lactoferrin. Furthermore, parasites such as Leishmania that reside inside phagolysosomes of their host cells are in an especially iron-restricted environment due to the lysosomal Fe2+ pump Nramp1 (38), which depletes phagosomes of ferrous iron.
Studies of Leishmania chagasi (105) demonstrated that the parasites take up iron primarily in the form of Fe2+ and also express a membrane-bound NADPH-dependent iron reductase that converts Fe3+ to Fe2+ at the extracellular surface of the parasite. Thus, in the macrophage phagolysosome, the parasites presumably convert Fe+3 bound to proteins such as transferrin into Fe2+ that can be imported by a permease in the parasite plasma membrane. In a search for such an iron import protein, Andrews and colleagues identified two identical ORFs within the L. major genome sequence that had 30% amino acid identity to the Arabidopsis thaliana Fe2+ transporter IRT1, a member of the ZIP family of divalent metal cation transporters (47, 48). These ORFs were designated LIT1-1 and LIT1-2, for Leishmania iron transporter 1. Functional expression of LIT1 in a Saccharomyces cerevisiae strain deficient in iron uptake allowed growth of the transformants in iron-depleted medium, strongly suggesting that it was a bona fide iron transporter. Subsequently, overexpression of the LIT1 protein in transgenic axenic amastigotes of Leishmania amazonensis induced the ability to transport 55Fe2+, confirming the iron transport function of this protein. LIT1 mRNA was present in both promastigotes and amastigotes of wild-type L. amazonensis. However, immunofluorescence using an anti-LIT1 antibody demonstrated that the LIT1 protein was not expressed in promastigotes or axenic amastigotes, both grown in iron-replete medium. Furthermore, the LIT1 protein was not detected in intracellular amastigotes early after infection of bone marrow-derived murine macrophages, but fluorescence was detectable at the plasma membrane of the intracellular parasites by 24 h after invasion. Hence, while mRNA is present in promastigotes, the LIT1 protein is not expressed until the parasites differentiate into amastigotes and are exposed to the iron-poor environment of the phagolysosome. Furthermore, when a GFP-LIT1 fusion protein was overexpressed in promastigotes, it accumulated internally in vesicles that may be parasite lysosomes, suggesting that LIT1 does not accumulate in promastigotes because it is degraded intracellularly.
A Δlit1 null mutant of L. amazonensis was prepared by targeted gene replacement. These mutants could invade macrophages and transform into amastigotes, but they could not replicate within bone marrow-derived macrophages. Complementation of the Δlit1 null mutant with the LIT1 gene on an episomal expression vector restored the ability to replicate within macrophages. Inoculation of BALB/c mice with Δlit1 mutants resulted in no lesion formation, but complementation with LIT1 either on an episome or integrated into the chromosome partially restored virulence. Of interest, reduced but significant numbers of amastigotes persisted in murine tissues inoculated with the Δlit1 null mutants despite the absence of overt pathology.
In summary, LIT1 is a Fe2+ transporter that is required for replication of amastigotes within macrophages. LIT1 mRNA is present throughout the life cycle, but the protein is detected only following transformation into amastigotes in the iron-poor environment of the phagolysosome (but not in the iron-rich medium used to generate axenic amastigotes). Hence, LIT1 expression appears to be regulated at the posttranslational level by iron availability and is thus induced only after parasites invade macrophages. Other uptake processes must provide promastigotes or axenic amastigotes with iron when they are cultured in relatively iron-rich environments, since the Δlit1 null mutant is not impaired in growth under these conditions.
A recent article (51) has examined the function of LIT1 using site-directed mutagenesis. These studies revealed both conserved and nonconserved domains of LIT1 that are critical for its function of importing Fe2+ when the parasite is exposed to an iron-limiting environment within the mammalian host.
In contrast to the intracellular Leishmania parasites (Fig. 1C), extracellular African trypanosomes (Fig. 1B) import iron using a unique heterodimeric transferrin receptor in which one subunit is tethered to the extracellular surface of the flagellar pocket membrane by a glycosyl phosphatidylinositol anchor (57). This unusual receptor delivers iron-laden host transferrin to intracellular vesicles of bloodstream trypanosomes, from which iron is mobilized to the parasite cytosol.
POLYAMINE TRANSPORTERS
Cationic polyamines, such as putrescine, cadaverine, and spermidine, are required for multiple biological processes, such as cell growth, development, protein synthesis, and progression through the cell cycle. Many organisms, including Leishmania species, can both synthesize polyamines de novo and salvage them by transport across their plasma membranes. Some microorganisms that live in polyamine-poor environments, such as T. brucei, are completely dependent upon polyamine biosynthesis. d,l-α-Difluoromethylornithine (DFMO) is a suicide inhibitor of ornithine decarboxylase, the rate-limiting enzyme in polyamine biosynthesis, and is an effective drug against African sleeping sickness caused by Trypanosoma brucei gambiense (18). In contrast to those of Leishmania and T. brucei, the T. cruzi (causative agent of Chagas disease) genome does not encode a gene for ornithine decarboxylase, and this parasite cannot synthesize putrescine and must import polyamines from the cytosol of mammalian host cells (46). Hence, polyamine transporters and polyamine biosynthesis play distinct functions in providing these essential nutrients to different parasites.
Leishmania.
The first gene encoding a eukaryotic plasma membrane polyamine transporter was cloned several years ago from L. major (45), and the corresponding protein was named L. major POT1 (LmPOT1). This protein is a member of the amino acid/polyamine/organocation (APC) superfamily and has highest sequence identity to members of the L-type amino acid transporter family. Functional expression of LmPOT1 cRNA in Xenopus oocytes established that it is a putrescine/spermidine transporter with Km values in the low micromolar range, and heterologous expression of the gene in T. brucei, a parasite with a low uptake capacity for polyamines, confirmed the functional characterization from oocytes. Other related polycationic compounds, such as spermine, arginine, agmatine, and pentamidine, were not transported by LmPOT1. An antibody was raised against LmPOT1 and used to monitor expression and subcellular localization of the protein. An ∼90-kDa protein was present in immunoblots of promastigote lysates but not in lysates from intracellular amastigotes, indicating that LmPOT1 is a promastigote-specific permease. Similarly, immunofluorescence revealed a strong signal on the plasma membrane of promastigotes, but no signal was present in amastigotes. Since this protein is not expressed in amastigotes and Leishmania parasites have an intact polyamine biosynthetic pathway (46), LmPOT1 is not likely to be important for viability of the disease-causing stage of the life cycle.
T. cruzi.
A query of the T. cruzi genome database identified two ORFs, designated TcPOT1.1 and TcPOT1.2, whose products have 41% amino acid identity to LmPOT1 (44). These two closely related (95% identical) ORFs are heterozygous alleles derived from the two haplotypes present in the sequenced CL Brenner strain. Overexpression of each allele in T. cruzi culture form epimastigotes exhibited pronounced increases in high-affinity uptake capacity for putrescine and cadaverine (Km values of 158 nM and 18 μM, respectively, for TcPOT1.1). Findings of earlier studies on uptake in whole T. cruzi parasites suggest that they also scavenge spermine and spermidine. Searches of the T. cruzi genome uncovered two additional homologs of TcPOT1.1 and TcPOT1.2, but these permeases remain uncharacterized.
Examination of TcPOT1 localization and expression revealed an intriguing regulatory mechanism. When parasites expressing TcPOT1.1-GFP were cultured in polyamine-deficient medium, the apparent Vmax increased ∼4- to 6-fold compared to results for cells cultured in polyamine-replete medium, but the Km value was unaffected. In contrast, for parasites expressing TcPOT1.2-GFP, no increase in Vmax was detected in polyamine-deficient medium, but the Km decreased ∼4-fold. When TcPOT1.1-GFP (or another construct containing a hemagglutinin tag instead of GFP) was localized by fluorescence microscopy, it was restricted to a ring near the base of the flagellum (44) in parasites cultured in polyamine-replete medium. In striking contrast, when these parasites were shifted to polyamine-deficient medium, TcPOT1.1-GFP was also localized extensively on the cell surface (44). Hence, this permease is released to the cell surface when polyamine levels are restricted, a retargeting process that is likely responsible for the increased polyamine uptake capacity of polyamine-starved cells. Remarkably, TcPOT1.2-GFP was not released to the cell surface in polyamine-deficient medium, consistent with the absence of increased polyamine transport capacity in parasites expressing this fusion protein. The mechanism whereby trafficking of TcPOT1.1 is affected by polyamine levels and the differences in response to polyamine starvation between TcPOT1.1 and TcPOT1.2 represent intriguing areas for future research.
AMINO ACID TRANSPORTERS
Leishmania arginine permease.
Amino acids serve multiple biological functions in eukaryotes, including functioning as osmolytes and as precursors for protein biosynthesis and other biosynthetic pathways, such as those for polyamines and pyrimidines. Individual eukaryotes synthesize a subset of amino acids, but others are not made de novo and must be imported. Even for amino acids that are synthesized by a cell, there may exist permeases that supplement the supply provided by biosynthesis. Amino acid transporters represent one of the largest families of permeases encoded within the genomes of many eukaryotes, and these proteins fall into many classes and several superfamilies of solute carriers (http://www.bioparadigms.org/slc/menu.asp). Indeed, among the kinetoplastid parasites whose genomes have been sequenced (6), putative amino acid permeases are the largest group of transporters (∼30 to 40 genes [each] in L. major, T. brucei, and T. cruzi), with the possible exception of ABC transporters. Various amino acid carriers have been studied extensively in microorganisms such as yeast and in mammalian systems, but there is a paucity of knowledge about this family of permeases among the parasitic protozoa.
The first amino acid transporter gene in Leishmania to be cloned and functionally characterized encodes the AAP3 arginine carrier from L. donovani (LdAAP3) (91). The protein belongs to the amino acid auxin permease (AAP) superfamily (1); however, the Leishmania members of this family are quite divergent and represent a separate clade. The LdAAP3 protein was expressed in a yeast strain deficient in multiple amino acid transport activities and was shown to transport arginine, with a Km of 1.9 μM, but no other amino acids. Arginine is an essential amino acid in Leishmania parasites and must be salvaged by import from the hosts (72). This amino acid is used biosynthetically only for protein synthesis and as the precursor for synthesis of polyamines (85). To determine whether import of arginine might be regulated by internal arginine levels, parasites were starved for arginine and found to reduce the internal arginine pools from ∼6 mM to ∼3 mM. Starvation resulted in an ∼5-fold increase in arginine uptake after 4 h of amino acid depletion, and the levels of both mRNA and the LdAAP3 protein were similarly increased (27). The increase in expression of the LdAAP3 protein was abrogated by supplementing amino acid-starved parasites with arginine but not with other individual amino acids. Furthermore, mutants that were deficient in polyamine biosynthesis (null mutants for the genes encoding ornithine decarboxylase or spermidine synthase) and which thus did not use arginine for that biosynthetic process had reduced levels of arginine uptake. These observations suggest that the parasites sense the intracellular level of arginine and either increase or decrease transport to maintain homeostasis. However, the mechanism whereby these parasites sense arginine and modulate the levels of LdAAP3 mRNAs remains to be deciphered. Recently, a lysine transporter gene from L. donovani (LdAAP7) and its ortholog from T. cruzi (TcAAP7) were cloned and functionally expressed (50). The inability to knock out the LdAAP7 gene suggests that this is the sole lysine transporter in L. donovani and that this permease is essential to promote the uptake of this required amino acid.
CARBOXYLATE TRANSPORTERS
A particularly intriguing example of transporter involvement in life cycle differentiation emerged recently from studies on African trypanosomes (28). Long slender (LS) bloodstream trypanosomes are parasites that replicate within the bloodstream, whereas short stumpy (SS) forms differentiate from LS forms but do not divide. Rather, SS parasites differentiate into insect procyclic forms (PFs) upon uptake into the gut of the tsetse fly vector. Differentiation of SS to PF parasites can be induced in vitro by incubation in the presence of citrate and cis-aconitate at 20°C. Microarray studies of an SS-to-PF-differentiation-deficient mutant revealed differential expression, compared to wild-type parasites, of two transcripts encoded by adjacent genes on chromosome 7. These genes predicted ORFs with 14 putative transmembrane domains and with sequence similarity to nodulin-like transporters from plants and major facilitative transporter superfamily members but with closest similarity to carboxylate transporters. Because of their suspected role in SS-to-PF differentiation, they were designated “proteins associated with differentiation,” or PAD1 and PAD2. PAD1 and PAD2 induced uptake of [14C]citrate when expressed in Xenopus oocytes, suggesting that they function as bona fide carboxylate transporters. PAD1 mRNA was upregulated in SS compared to LS or PF trypanosomes, whereas PAD2 mRNA was most strongly expressed in PF parasites. Immunoblots with specific peptide antibodies revealed that the PAD1 protein is detectable only in SS parasites, and PAD2 is present in both SS and PF parasites but is expressed most abundantly in the later life cycle stage. PAD2 protein expression was induced ∼4-fold when parasites were incubated at 20°C compared to results at 37°C (reflecting the temperature shift that occurs when SS parasites enter the tsetse fly), and the protein was located primarily at the cell surface at the lower temperature but was restricted to the flagellar pocket at 37°C. Reduction of expression of PAD proteins by RNA interference (RNAi) directed against their transcripts impaired differentiation of SS parasites to PF parasites upon incubation with cis-aconitate at 20°C. Collectively, these data implicate the PAD proteins as carboxylate transporters present in SS forms that mediate differentiation of SS parasites to PF parasites. Differentiation is apparently induced by PAD-mediated uptake of citrate and cis-aconitate at 20°C.
PERSPECTIVES AND CHALLENGES FOR THE FUTURE
A substantial body of work, including both studies referenced here and others that could not be included in this brief review, has greatly advanced our understanding of nutrient uptake in parasitic protozoa at the molecular level. Functional cloning employing various strategies has provided us with genes encoding a variety of permeases with known transport properties. The completion of parasite genome projects has also allowed identification of likely permeases by sequence similarity to transporters with known functions in other organisms. Nucleic acid and antibody probes have allowed the discovery and initial dissection of intriguing regulatory processes that control expression and function of parasite transporters under a variety of physiological conditions. Site-directed mutagenesis and computational modeling have provided insights into how the structure of individual permeases determines their function as solute carriers. Examination of subcellular localization has identified transporters that traffic to specific membrane systems or organelles within parasites, sometimes with strikingly distinct distributions. Finally, targeted gene replacement approaches have helped define which transporters are essential for viability of various parasites in different stages of their life cycles. Thus, the field has advanced a long way since the days when investigators could only study uptake of compounds in intact parasites but had little understanding of the molecular processes involved.
There are a number of frontiers that present both challenges and opportunities for the near future. First, none of the transporters discussed in this article has a close homolog whose 3-dimensional structure has been determined by X-ray crystallography or electron diffraction. In the absence of experimental structures, computational modeling has provided some discerning insights and promises to deliver more. Nonetheless, obtaining experimentally defined structures of these or closely related permeases, ideally in various biochemically relevant conformations, represents a critical unachieved milestone. Transporters whose expression or function is acutely regulated by substrate availability or other physiological conditions provide fascinating albeit challenging opportunities to investigate mechanisms for regulating expression of genes and/or proteins. Gene regulation remains a central but poorly understood aspect of the biology of these microorganisms, and permeases may provide valuable experimental models to dissect regulation. Defining at the molecular level how transporters traffic selectively to discrete subcellular locations should offer intriguing insights into the cell biology of these protozoa. At a more applied level, could some parasite transporters that serve essential roles in delivering nutrients to these pathogens be targeted for development of desperately needed antiparasitic drugs? Continuing studies of nutrient transporters promise to provide insights into many aspects of basic parasite biology and potentially to deliver novel therapies for treatment of the widespread diseases caused by these protozoa.
ACKNOWLEDGMENTS
Funding for work described in this article was provided by grants AI02590 and AI44138 from the National Institutes of Health.
I thank Nicola Carter and Marie-Pierre Hasne for critical comments on the manuscript.
Footnotes
Published ahead of print on 7 January 2011.
REFERENCES
- 1. Akerman M., Shaked-Mishan P., Mazareb S., Volpin H., Zilberstein D. 2004. Novel motifs in amino acid permease genes from Leishmania. Biochem. Biophys. Res. Commun. 325:353–366 [DOI] [PubMed] [Google Scholar]
- 2. Antoine J. C., Prina E., Jouanne C., Bongrand P. 1990. Parasitophorous vacuoles of Leishmania amazonensis-infected macrophages maintain an acidic pH. Infect. Immun. 58:779–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arastu-Kapur S., Arendt C. S., Purnat T., Carter N. S., Ullman B. 2005. Second-site suppression of a nonfunctional mutation within the Leishmania donovani inosine-guanosine transporter. J. Biol. Chem. 280:2213–2219 [DOI] [PubMed] [Google Scholar]
- 4. Arastu-Kapur S., Ford E., Ullman B., Carter N. S. 2003. Functional analysis of an inosine-guanosine transporter from Leishmania donovani: the role of conserved residues, aspartate 389 and arginine 393. J. Biol. Chem. 278:33327–33333 [DOI] [PubMed] [Google Scholar]
- 5. Arendt C. S., Ullman B. 2010. Role of transmembrane domain 4 in ligand permeation by Crithidia fasciculata equilibrative nucleoside transporter 2 (CfNT2). J. Biol. Chem. 285:6024–6035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aslett M., Aurrecoechea C., Berriman M., Brestelli J., Brunk B. P., Carrington M., Depledge D. P., Fischer S., Gajria B., Gao X., Gardner M. J., Gingle A., Grant G., Harb O. S., Heiges M., Hertz-Fowler C., Houston R., Innamorato F., Iodice J., Kissinger J. C., Kraemer E., Li W., Logan F. J., Miller J. A., Mitra S., Myler P. J., Nayak V., Pennington C., Phan I., Pinney D. F., Ramasamy G., Rogers M. B., Roos D. S., Ross C., Sivam D., Smith D. F., Srinivasamoorthy G., Stoeckert C. J., Jr., Subramanian S., Thibodeau R., Tivey A., Treatman C., Velarde G., Wang H. 2010. TriTrypDB: a functional genomic resource for the Trypanosomatidae. Nucleic Acids Res. 38:D457–D462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Azema L., et al. 2004. Interaction of substituted hexose analogues with the Trypanosoma brucei hexose transporter. Biochem. Pharmacol. 67:459–467 [DOI] [PubMed] [Google Scholar]
- 8. Bakker B. M., Westerhoff H. V., Opperdoes F. R., Michels P. A. 2000. Metabolic control analysis of glycolysis in trypanosomes as an approach to improve selectivity and effectiveness of drugs. Mol. Biochem. Parasitol. 106:1–10 [DOI] [PubMed] [Google Scholar]
- 9. Baldwin S. A., McConkey G. A., Cass C. E., Young J. D. 2007. Nucleoside transport as a potential target for chemotherapy in malaria. Curr. Pharm. Des. 13:569–580 [DOI] [PubMed] [Google Scholar]
- 10. Barrett M. P., Tetaud E., Seyfang A., Brignaud F., Baltz T. 1998. Trypanosome glucose transporters. Mol. Biochem. Parasitol. 91:195–205 [DOI] [PubMed] [Google Scholar]
- 11. Bayele H. K. 2001. Triazinyl derivatives that are potent inhibitors of glucose transport in Trypanosoma brucei. Parasitol. Res. 87:911–914 [DOI] [PubMed] [Google Scholar]
- 12. Beitz E., Pavlovic-Djuranovic S., Yasui M., Agre P., Schultz J. E. 2004. Molecular dissection of water and glycerol permeability of the aquaglyceroporin from Plasmodium falciparum by mutational analysis. Proc. Natl. Acad. Sci. U. S. A. 101:1153–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blume M., et al. 2009. Host-derived glucose and its transporter in the obligate intracellular pathogen Toxoplasma gondii are dispensable by glutaminolysis. Proc. Natl. Acad. Sci. U. S. A. 106:12998–13003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a. Blume M., et al. 17 December 2010, posting date. A constitutive pan-hexose permease for the Plasmodium life cycle and transgenic models for screening of antimalarial sugar analogs. FASEB J. doi:10.1096/fj.10-173278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bringaud F., Baltz T. 1993. Differential regulation of two distinct families of glucose transporter genes in Trypanosoma brucei. Mol. Cell. Biol. 13:1146–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burchmore R. J., Barrett M. P. 2001. Life in vacuoles—nutrient acquisition by Leishmania amastigotes. Int. J. Parasitol. 31:1311–1320 [DOI] [PubMed] [Google Scholar]
- 16. Burchmore R. J. S., Landfear S. M. 1998. Differential regulation of multiple glucose transporter genes in the parasitic protozoan Leishmania mexicana. J. Biol. Chem. 273:29118–29126 [DOI] [PubMed] [Google Scholar]
- 17. Burchmore R. J. S., et al. 2003. Genetic characterization of glucose transporter function in Leishmania mexicana. Proc. Natl. Acad. Sci. U. S. A. 100:3901–3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burri C., Brun R. 2003. Eflornithine for the treatment of human African trypanosomiasis. Parasitol. Res. 90(Supp. 1):S49–S52 [DOI] [PubMed] [Google Scholar]
- 19. Capul A. A., Hickerson S., Barron T., Turco S. J., Beverley S. M. 2007. Comparisons of mutants lacking the Golgi UDP-galactose or GDP-mannose transporters establish that phosphoglycans are important for promastigote but not amastigote virulence in Leishmania major. Infect. Immun. 75:4629–4637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carter N. S., et al. 2000. Isolation and functional characterization of the PfNT1 nucleoside transporter gene from Plasmodium falciparum. J. Biol. Chem. 275:10683–10691 [DOI] [PubMed] [Google Scholar]
- 21. Carter N. S., Berger B. J., Fairlamb A. H. 1995. Uptake of diamidine drugs by the P2 transporter in melarsen-sensitive and -resistant Trypanosoma brucei brucei. J. Biol. Chem. 270:28153–28157 [DOI] [PubMed] [Google Scholar]
- 22. Carter N. S., et al. 2000. Cloning of a novel inosine-guanosine transporter gene from Leishmania donovani by functional rescue of a transport-deficient mutant. J. Biol. Chem. 275:20935–20941 [DOI] [PubMed] [Google Scholar]
- 23. Carter N. S., Fairlamb A. H. 1993. Arsenical-resistant trypanosomes lack an unusual adenine/adenosine transporter. Nature 361:173–175 [DOI] [PubMed] [Google Scholar]
- 24. Carter N. S., et al. 2010. Adaptive responses to purine starvation in Leishmania donovani. Mol. Microbiol. 78:92–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cunningham M. L., Beverley S. M. 2001. Pteridine salvage throughout the Leishmania infectious cycle: implications for antifolate chemotherapy. Mol. Biochem. Parasitol. 113:199–213 [DOI] [PubMed] [Google Scholar]
- 26. Daily J. P., et al. 2005. In vivo transcriptome of Plasmodium falciparum reveals overexpression of transcripts that encode surface proteins. J. Infect. Dis. 191:1196–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Darlyuk I., et al. 2009. Arginine homeostasis and transport in the human pathogen Leishmania donovani. J. Biol. Chem. 284:19800–19807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dean S., Marchetti R., Kirk K., Matthews K. R. 2009. A surface transporter family conveys the trypanosome differentiation signal. Nature 459:213–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Koning H. P., Bridges D. J., Burchmore R. J. 2005. Purine and pyrimidine transport in pathogenic protozoa: from biology to therapy. FEMS Microbiol. Rev. 29:987–1020 [DOI] [PubMed] [Google Scholar]
- 30. Desai S. A., Bezrukov S. M., Zimmerberg J. 2000. A voltage-dependent channel involved in nutrient uptake by red blood cells infected with the malaria parasite. Nature 406:1001–1005 [DOI] [PubMed] [Google Scholar]
- 31. Desai S. A., Krogstad D. J., McCleskey E. W. 1993. A nutrient-permeable channel on the intraerythrocytic malaria parasite. Nature 362:643–646 [DOI] [PubMed] [Google Scholar]
- 32. Detke S. 1997. Identification of a transcription factor like protein at the TOR locus in Leishmania mexicana amazonensis. Mol. Biochem. Parasitol. 90:505–511 [DOI] [PubMed] [Google Scholar]
- 33. Detke S. 2007. TOR-induced resistance to toxic adenosine analogs in Leishmania brought about by the internalization and degradation of the adenosine permease. Exp. Cell Res. 313:1963–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Downie M. J., et al. 2010. PfNT2, a permease of the equilibrative nucleoside transporter family in the endoplasmic reticulum of Plasmodium falciparum. J. Biol. Chem. 285:20827–20833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Drew M. E., et al. 1995. Functional expression of a myo-inositol/H+ symporter from Leishmania donovani. Mol. Cell. Biol. 15:5508–5515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. El Bissati K., et al. 2006. The plasma membrane permease PfNT1 is essential for purine salvage in the human malaria parasite Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 103:9286–9291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Feng X., et al. 2009. Amplification of an alternate transporter gene suppresses the avirulent phenotype of glucose transporter null mutants in Leishmania mexicana. Mol. Microbiol. 71:369–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fortier A., Min-Oo G., Forbes J., Lam-Yuk-Tseung S., Gros P. 2005. Single gene effects in mouse models of host:pathogen interactions. J. Leukoc. Biol. 77:868–877 [DOI] [PubMed] [Google Scholar]
- 39. Gardner M. J., et al. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gottlieb M. 1985. Enzyme regulation in a trypanosomatid: effect of purine starvation on levels of 3′-nucleotidase activity. Science 227:72–74 [DOI] [PubMed] [Google Scholar]
- 41. Gourbal B., et al. 2004. Drug uptake and modulation of drug resistance in Leishmania by an aquaglyceroporin. J. Biol. Chem. 279:31010–31017 [DOI] [PubMed] [Google Scholar]
- 42. Griffiths M., et al. 1997. Cloning of a human nucleoside transporter implicated in the cellular uptake of adenosine and chemotherapeutic drugs. Nat. Med. 3:89–93 [DOI] [PubMed] [Google Scholar]
- 43. Hammond D. J., Gutteridge W. E. 1984. Purine and pyrimidine metabolism in the Trypanosomatidae. Mol. Biochem. Parasitol. 13:243–261 [DOI] [PubMed] [Google Scholar]
- 44. Hasne M. P., Coppens I., Soysa R., Ullman B. 2010. A high-affinity putrescine-cadaverine transporter from Trypanosoma cruzi. Mol. Microbiol. 76:78–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hasne M. P., Ullman B. 2005. Identification and characterization of a polyamine permease from the protozoan parasite Leishmania major. J. Biol. Chem. 280:15188–15194 [DOI] [PubMed] [Google Scholar]
- 46. Heby O., Roberts S. C., Ullman B. 2003. Polyamine biosynthetic enzymes as drug targets in parasitic protozoa. Biochem. Soc. Trans. 31:415–419 [DOI] [PubMed] [Google Scholar]
- 47. Huynh C., Andrews N. W. 2008. Iron acquisition within host cells and the pathogenicity of Leishmania. Cell Microbiol. 10:293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huynh C., Sacks D. L., Andrews N. W. 2006. A Leishmania amazonensis ZIP family iron transporter is essential for parasite replication within macrophage phagolysosomes. J. Exp. Med. 203:2363–2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Igoillo-Esteve M., Mazet M., Deumer G., Wallemacq P., Michels P. A. 2011. Glycosomal ABC transporters of Trypanosoma brucei: characterization of their expression, topology and substrate specificity. Int. J. Parasitol. 41:429–438 [DOI] [PubMed] [Google Scholar]
- 50. Inbar E., et al. Lysine transporters in human trypanosomatid pathogens. Amino Acids, in press [DOI] [PubMed] [Google Scholar]
- 51. Jacques I., Andrews N. W., Huynh C. 2010. Functional characterization of LIT1, the Leishmania amazonensis ferrous iron transporter. Mol. Biochem. Parasitol. 170:28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Joet T., Eckstein-Ludwig U., Morin C., Krishna S. 2003. Validation of the hexose transporter of Plasmodium falciparum as a novel drug target. Proc. Natl. Acad. Sci. U. S. A. 100:7476–7479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kerby D. R., Detke S. 1993. Reduced purine accumulation is encoded on an amplified DNA in Leishmania mexicana amazonensis resistant to toxic nucleosides. Mol. Biochem. Parasitol. 60:171–185 [DOI] [PubMed] [Google Scholar]
- 54. Kirk K., Saliba K. J. 2007. Targeting nutrient uptake mechanisms in Plasmodium. Curr. Drug Targets 8:75–88 [DOI] [PubMed] [Google Scholar]
- 55. Landfear S. M. 2010. Glucose transporters in parasitic protozoa. Methods Mol. Biol. 637:245–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Landfear S. M., Ullman B., Carter N., Sanchez M. 2004. Nucleoside and nucleobase transporters in parasitic protozoa. Eukaryot. Cell 3:245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ligtenberg M. J. L., et al. 1994. Reconstitution of a surface transferrin binding complex in insect form Trypanosoma brucei. EMBO J. 13:2565–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu W., et al. 2006. Functional characterization of nucleoside transporter gene replacements in Leishmania donovani. Mol. Biochem. Parasitol. 150:300–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lu H.-G., Zhong L., Chang K.-P., Decampo R. 1997. Intracellular Ca+2 pool content and signaling and expression of a calcium pump are linked to virulence in Leishmania mexicana amazonensis amastigotes. J. Biol. Chem. 272:9464–9473 [DOI] [PubMed] [Google Scholar]
- 60. Luo S., Vieira M., Graves J., Zhong L., Moreno S. N. 2001. A plasma membrane-type Ca(2+)-ATPase co-localizes with a vacuolar H(+)-pyrophosphatase to acidocalcisomes of Toxoplasma gondii. EMBO J. 20:55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Marchesini N., Vieira M., Luo S., Moreno S. N., Docampo R. 2005. A malaria parasite-encoded vacuolar H(+)-ATPase is targeted to the host erythrocyte. J. Biol. Chem. 280:36841–36847 [DOI] [PubMed] [Google Scholar]
- 62. Mäser P., Sütterlin C., Kralli A., Kaminsky R. 1999. A nucleoside transporter from Trypanosoma brucei involved in drug resistance. Science 285:242–244 [DOI] [PubMed] [Google Scholar]
- 63. Matovu E., et al. 2001. Genetic variants of the TbAT1 adenosine transporter from African trypanosomes in relapse infections following melarsoprol therapy. Mol. Biochem. Parasitol. 117:73–81 [DOI] [PubMed] [Google Scholar]
- 64. Matovu E., et al. 2003. Mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot. Cell 2:1003–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McConville M. J., de Souza D., Saunders E., Likic V. A., Naderer T. 2007. Living in a phagolysosome; metabolism of Leishmania amastigotes. Trends Parasitol. 23:368–375 [DOI] [PubMed] [Google Scholar]
- 66. Meade J. C., Coombs G. H., Mottram J. C., Steele P. E., Stringer J. R. 1991. Conservation of cation-transporting ATPase genes in Leishmania. Mol. Biochem. Parasitol. 45:29–38 [DOI] [PubMed] [Google Scholar]
- 67. Montalvetti A., Rohloff P., Docampo R. 2004. A functional aquaporin co-localizes with the vacuolar proton pyrophosphatase to acidocalcisomes and the contractile vacuole complex of Trypanosoma cruzi. J. Biol. Chem. 279:38673–38682 [DOI] [PubMed] [Google Scholar]
- 68. Mullin K. A., et al. 2006. Membrane transporters in the relict plastid of malaria parasites. Proc. Natl. Acad. Sci. U. S. A. 103:9572–9577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Naderer T., McConville M. J. 2008. The Leishmania-macrophage interaction: a metabolic perspective. Cell Microbiol. 10:301–308 [DOI] [PubMed] [Google Scholar]
- 70. Nasser M. I. A., Landfear S. M. 2004. Sequences required for the flagellar targeting of an integral membrane protein. Mol. Biochem. Parasitol. 135:89–100 [DOI] [PubMed] [Google Scholar]
- 71. Naula C. M., Logan F. J., Wong P. E., Barrett M. P., Burchmore R. J. 2010. A glucose transporter can mediate ribose uptake: definition of residues that confer substrate specificity in a sugar transporter. J. Biol. Chem. 285:29721–29728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Opperdoes F. R., Coombs G. H. 2007. Metabolism of Leishmania: proven and predicted. Trends Parasitol. 23:149–158 [DOI] [PubMed] [Google Scholar]
- 73. Ortiz D., Sanchez M. A., Koch H. P., Larsson H. P., Landfear S. M. 2009. An acid-activated nucleobase transporter from Leishmania major. J. Biol. Chem. 284:16164–16169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ortiz D., et al. 2007. Molecular genetic analysis of purine nucleobase transport in Leishmania major. Mol. Microbiol. 64:1228–1243 [DOI] [PubMed] [Google Scholar]
- 75. Ortiz D., Sanchez M. A., Quecke P., Landfear S. M. 2009. Two novel nucleobase/pentamidine transporters from Trypanosoma brucei. Mol. Biochem. Parasitol. 163:67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ortiz D., et al. 2010. Purine restriction induces pronounced translational upregulation of the NT1 adenosine/pyrimidine nucleoside transporter in Leishmania major. Mol. Microbiol. 78:108–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ouameur A. A., Girard I., Legare D., Ouellette M. 2008. Functional analysis and complex gene rearrangements of the folate/biopterin transporter (FBT) gene family in the protozoan parasite Leishmania. Mol. Biochem. Parasitol. 162:155–164 [DOI] [PubMed] [Google Scholar]
- 78. Papageorgiou I., De Koning H. P., Soteriadou K., Diallinas G. 2008. Kinetic and mutational analysis of the Trypanosoma brucei NBT1 nucleobase transporter expressed in Saccharomyces cerevisiae reveals structural similarities between ENT and MFS transporters. Int. J. Parasitol. 38:641–653 [DOI] [PubMed] [Google Scholar]
- 79. Parker M. D., et al. 2000. Identification of a nucleoside/nucleobase transporter from Plasmodium falciparum, a novel target for anti-malarial chemotherapy. Biochem. J. 349:67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Perez-Victoria J. M., et al. 2001. Alkyl-lysophospholipid resistance in multidrug-resistant Leishmania tropica and chemosensitization by a novel P-glycoprotein-like transporter modulator. Antimicrob. Agents Chemother. 45:2468–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Piper R. C., Xu X., Russell D. G., Little B. M., Landfear S. M. 1995. Differential targeting of two glucose transporters from Leishmania enriettii is mediated by an NH2-terminal domain. J. Cell Biol. 128:499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Quashie N. B., et al. 2008. A comprehensive model of purine uptake by the malaria parasite Plasmodium falciparum: identification of four purine transport activities in intraerythrocytic parasites. Biochem. J. 411:287–295 [DOI] [PubMed] [Google Scholar]
- 83. Rager N., Ben Mamoun C., Carter N. S., Goldberg D. E., Ullman B. 2001. Localization of the Plasmodium falciparum PfNT1 nucleoside transporter to the parasite plasma membrane. J. Biol. Chem. 276:41095–41099 [DOI] [PubMed] [Google Scholar]
- 84. Revelard P., Pays E. 1991. Structure and transcription of a P-ATPase gene from Trypanosoma brucei. Mol. Biochem. Parasitol. 46:241–252 [DOI] [PubMed] [Google Scholar]
- 85. Roberts S. C., et al. 2004. Arginase plays a pivotal role in polyamine precursor metabolism in Leishmania. Characterization of gene deletion mutants. J. Biol. Chem. 279:23668–23678 [DOI] [PubMed] [Google Scholar]
- 86. Rodriguez-Contreras D., Feng X., Keeney K. M., Bouwer H. G., Landfear S. M. 2007. Phenotypic characterization of a glucose transporter null mutant in Leishmania mexicana. Mol. Biochem. Parasitol. 153:9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rodriguez-Contreras D., Landfear S. M. 2006. Metabolic changes in glucose transporter-deficient Leishmania mexicana and parasite virulence. J. Biol. Chem. 281:20068–20076 [DOI] [PubMed] [Google Scholar]
- 88. Russell D. G., Xu S., Chakraborty P. 1992. Intracellular trafficking and the parasitorphorous vacuole of Leishmania mexicana-infected macrophages. J. Cell Sci. 103:1193–1210 [DOI] [PubMed] [Google Scholar]
- 89. Saliba K. J., et al. 2006. Sodium-dependent uptake of inorganic phosphate by the intracellular malaria parasite. Nature 443:582–585 [DOI] [PubMed] [Google Scholar]
- 90. Schlein Y. 1986. Sandfly diet and Leishmania. Parasitol. Today 2:175–177 [DOI] [PubMed] [Google Scholar]
- 91. Shaked-Mishan P., et al. 2006. A novel high-affinity arginine transporter from the human parasitic protozoan Leishmania donovani. Mol. Microbiol. 60:30–38 [DOI] [PubMed] [Google Scholar]
- 92. Slavic K., et al. 2010. Life cycle studies of the hexose transporter of Plasmodium species and genetic validation of their essentiality. Mol. Microbiol. 75:1402–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Stein A., et al. 2003. Equilibrative nucleoside transporter family members from Leishmania donovani are electrogenic proton symporters. J. Biol. Chem. 278:35127–35134 [DOI] [PubMed] [Google Scholar]
- 94. ter Kuile B. H. 1993. Glucose and proline transport in kinetoplastids. Parasitol. Today 9:206–210 [DOI] [PubMed] [Google Scholar]
- 95. ter Kuile B. H. 1991. Glucose uptake mechanisms as potential targets for drugs against trypanosomatids, p. 635 In Coombs G. H., North M. (ed.), Biochemical protozoology. Taylor and Francis, London, United Kingdom [Google Scholar]
- 96. Tetaud E., Barrett M. P., Bringaud F., Baltz T. 1997. Kinetoplastid glucose transporters. Biochem. J. 325:569–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tetaud E., Bringaud F., Chabas S., Barrett M. P., Baltz T. 1994. Characterization of glucose transport and cloning of a hexose transporter gene in Trypanosoma cruzi. Proc. Natl. Acad. Sci. U. S. A. 91:8278–8282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Uzcategui N. L., et al. 2004. Cloning, heterologous expression, and characterization of three aquaglyceroporins from Trypanosoma brucei. J. Biol. Chem. 279:42669–42676 [DOI] [PubMed] [Google Scholar]
- 99. Valdes R., Liu W., Ullman B., Landfear S. M. 2006. Comprehensive examination of charged intramembrane residues in a nucleoside transporter. J. Biol. Chem. 281:22647–22655 [DOI] [PubMed] [Google Scholar]
- 100. Valdes R., Vasudevan G., Conklin D., Landfear S. M. 2004. Transmembrane domain 5 of the LdNT1.1 nucleoside transporter is an amphipathic helix that forms part of the nucleoside translocation pathway. Biochemistry 43:6793–6802 [DOI] [PubMed] [Google Scholar]
- 101. Valdes R. M., Arastu-Kapur S. A., Landfear S. M., Shinde U. 2009. An ab initio structural model of a nucleoside permease predicts functionally important residues. J. Biol. Chem. 284:19067–19076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Vasudevan G., et al. 1998. Cloning of Leishmania nucleoside transporter genes by rescue of a transport-deficient mutant. Proc. Natl. Acad. Sci. U. S. A. 95:9873–9878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Vasudevan G., Ullman B., Landfear S. M. 2001. Point mutations in a nucleoside transporter gene from Leishmania donovani confer drug resistance and alter substrate selectivity. Proc. Natl. Acad. Sci. U. S. A. 98:6092–6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Vickerman K. 1985. Developmental cycles and biology of pathogenic trypanosomes. Br. Med. Bull. 41:105–114 [DOI] [PubMed] [Google Scholar]
- 105. Wilson M. E., Lewis T. S., Miller M. A., McCormick M. L., Britigan B. E. 2002. Leishmania chagasi: uptake of iron bound to lactoferrin or transferrin requires an iron reductase. Exp. Parasitol. 100:196–207 [DOI] [PubMed] [Google Scholar]
- 106. Woodrow C. J., Penny J. I., Krishna S. 1999. Intraerythrocytic Plasmodium falciparum expresses a high affinity facilitative hexose transporter. J. Biol. Chem. 274:7272–7277 [DOI] [PubMed] [Google Scholar]