Abstract
We have examined the distribution of calcium in Neurospora crassa and investigated the role of four predicted calcium transport proteins. The results of cell fractionation experiments showed 4% of cellular calcium in mitochondria, approximately 11% in a dense vacuolar fraction, 40% in an insoluble form that copurifies with microsomes, and 40% in a high-speed supernatant, presumably from large vacuoles that had broken. Strains lacking NCA-1, a SERCA-type Ca2+-ATPase, or NCA-3, a PMC-type Ca2+-ATPase, had no obvious defects in growth or distribution of calcium. A strain lacking NCA-2, which is also a PMC-type Ca2+-ATPase, grew slowly in normal medium and was unable to grow in high concentrations of calcium tolerated by the wild type. Furthermore, when grown in normal concentrations of calcium (0.68 mM), this strain accumulated 4- to 10-fold more calcium than other strains, elevated in all cell fractions. The data suggest that NCA-2 functions in the plasma membrane to pump calcium out of the cell. In this way, it resembles the PMC-type enzymes of animal cells, not the Pmc1p enzyme in Saccharomyces cerevisiae that resides in the vacuole. Strains lacking the cax gene, which encodes a Ca2+/H+ exchange protein in vacuolar membranes, accumulate very little calcium in the dense vacuolar fraction but have normal levels of calcium in other fractions. The cax knockout strain has no other observable phenotypes. These data suggest that “the vacuole” is heterogeneous and that the dense vacuolar fraction contains an organelle that is dependent upon the CAX transporter for accumulation of calcium, while other components of the vacuolar system have multiple calcium transporters.
INTRODUCTION
Calcium is required for growth of eukaryotic organisms yet can be toxic if cytosolic levels rise much above 0.1 μM. Dozens of genes encode proteins involved in calcium signaling and transport (9, 14, 42). In filamentous fungi, calcium has been proposed to play a key role in regulating growth at the hyphal tip (5, 30, 32, 33, 39). However, little is known about how calcium is sequestered in filamentous fungi or about the transporters that maintain calcium homeostasis. Investigators working with Neurospora crassa reported that active transport across the plasma membrane plays a major role in maintaining low levels of cytosolic calcium (28, 34), along with the vacuole, which may buffer intracellular calcium levels (11, 12, 28).
In higher eukaryotes, both plants and animals, three types of Ca2+-ATPases are involved in maintaining the proper level of calcium within cells (8, 36, 38). The PMCA type is localized primarily in the plasma membrane, where it removes excess calcium from the cytosol. The SERCA type, named by its location in the smooth endoplasmic reticulum (ER), has been intensively studied for muscle cells but is also found in the ER of other kinds of cells. The SPCA type (secretory pathway Ca2+-ATPase) functions in the Golgi bodies. In some cells, much of the calcium is sequestered in mitochondria, although no calcium-pumping ATPase has been identified in this organelle (19). Uptake of calcium into mitochondria has been hypothesized to occur via a channel protein, driven by the same electrochemical gradient that drives ATP synthesis (26). Small vesicles and lysosome-like compartments also sequester calcium, presumably transported by Ca2+/H+ exchange proteins (4).
The fungus for which calcium homeostasis has been most thoroughly explored is Saccharomyces cerevisiae. In this organism, more than 95% of cellular calcium is sequestered in the vacuole (20, 22, 24). Two proteins transport it there. Pmc1p is a PMCA-type pump that uses ATP directly to move calcium into the vacuole (15). Vcx1p is a Ca2+/H+ exchange protein that uses the energy in the electrochemical gradient for protons generated by the vacuolar proton-translocating ATPase (V-ATPase) (16, 29). Of these two proteins, Pmc1p appears to play the major role in vivo. Cells lacking a functional Pmc1p protein grow normally in rich medium but accumulate only 20% of the wild-type level of calcium in the vacuole. Deletion of the gene encoding Vcx1p has little effect on vacuolar calcium. Deletion of both PMC1 and VCX1 drops vacuolar calcium to 10% of the level observed in the wild-type strain. Addition of calcium to the medium inhibits the growth of cells lacking a functional Pmc1p protein much more than that of cells lacking Vcx1p. S. cerevisiae also has another P-type Ca2+-ATPase, Pmr1p, that transports calcium, and likely manganese (21), into the Golgi apparatus (1, 31).
Analysis of the genomes of filamentous fungi indicates that these organisms have a larger repertoire of calcium transport proteins than S. cerevisiae. N. crassa has four putative P-type ATPases that function as calcium pumps (3, 42). We have recently investigated the cellular locations of these transport proteins by generating fusion proteins with green fluorescent proteins (GFPs) and/or red fluorescent proteins (RFPs) (6). N. crassa has a SERCA-type Ca2+-ATPase, NCA-1, which we found localized to the ER and the nuclear envelope. It also has two closely related PMCA-type Ca2+-ATPases, NCA-2 and NCA-3. In the region of a hypha within 300 μm of the tip, these two transport proteins are in the vacuole, consistent with the location of Pmc1p in S. cerevisiae. However, in regions further back from the tip, both NCA-2 and NCA-3 predominately localize to the plasma membrane. The fourth Ca2+-ATPase, PMR, appears to be an SPCA type, and preliminary evidence suggests it is located in a component of the Golgi apparatus. N. crassa also has a Ca2+/H+ exchange protein, named CAX, that is homologous to Vcx1p from yeast. This protein localizes to the vacuole as predicted.
In this report, we used a cell fractionation procedure to examine where calcium is sequestered in hyphae of N. crassa. We also investigated the roles of NCA-1, NCA-2, NCA-3, and CAX in maintaining calcium homeostasis. We analyzed the phenotypes of strains in which the protein-coding region for each of these transporters was deleted from the genome (10). For each mutant strain, we asked if the strain formed hyphae with normal morphology, measured the amount and distribution of calcium in the hyphae, and tested the sensitivity of growth to changes in the concentration of calcium in the medium. The presence of a SERCA-type Ca2+-ATPase and the plasma membrane locations of two PMCA-type ATPases suggested that calcium homeostasis in N. crassa and other filamentous fungi may be maintained in ways significantly different than reported for S. cerevisiae.
MATERIALS AND METHODS
Media, strains, and growth procedures.
N. crassa was grown either in Vogel's medium N with 2% sucrose (17) or in Vogeloid medium, which was developed by Robert Metzenberg at the University of Wisconsin (unpublished). Vogeloid has no phosphate or sulfate and can be made with high concentrations of calcium without precipitation. A 10× stock of Vogeloid medium contained 100 mM NH4Cl, 20 mM MgCl2·6H2O, 100 mM KCl, 20 mM methionine, 100 μg biotin per ml, the same trace elements as those in Vogel's medium, and either 200 mM morpholineethanesulfonic acid (MES) buffer adjusted to pH 5.8 with NaOH or 200 mM HEPES buffer adjusted to pH 7.5 with NaOH. The 10× stock solution was diluted to 1×, supplemented with 2% sucrose, and sterilized in an autoclave. Phosphorylethanolamine was added from a 100 mM stock (sterilized by filtration) to give a final concentration of 1 mM, and CaCl2 was added as desired from a 2 M sterile solution.
The N. crassa deletion mutant strains in this study were generated by the Neurospora Genome Project (10) and were obtained from the Fungal Genetics Stock Center at the University of Missouri, Kansas City, MO (27). We used the following strains: wild-type strains 74A (FGSC987) and 74a (FGSC988) and the Δcax::hph+ mat a (FGSC11248), Δcax::hph+ mat A (FGSC11249), Δnca-1::hph+ mat a (FGSC13287), Δnca-2::hph+ mat A (FGSC13071), Δnca-3::hph+ mat a (FGSC13036), and Δnca-3::hph+ mat A (FGSC13037) mutant strains. We backcrossed each of the mutant strains to the wild type and identified progeny containing the desired gene deletion by PCR analysis. We generated strains with multiple mutations by genetic crosses, using standard procedures for N. crassa (17), and again verified the gene deletions by PCR analysis.
The growth yield of wild-type and mutant strains in Vogeloid medium, pH 7.5, with various concentrations of calcium was determined by inoculating 6 ml of medium with 6 × 105 conidia (counted with a hemocytometer) and incubating them at 30°C for 2 days. Mycelia were collected by filtration, dried, and weighed. At least two independent experiments were done for all strains, with 2 to 4 replicates for each data point in each experiment. Data were plotted, with error bars showing standard errors.
Measurement of calcium uptake by cells.
We measured calcium uptake using 45Ca with a specific activity of >10 Ci/g (Perkin Elmer Life Sciences). Vials with 6 ml of normal Vogel's medium (0.68 mM CaCl2) or Vogeloid medium containing the indicated amounts of radioactive and nonradioactive calcium were inoculated with 6 × 106 conidia and incubated at 30°C for 25 h. Immediately before the conidia were harvested, 6 ml of growth medium containing 20 mM nonradioactive CaCl2 was added. The cultures were collected by filtration, using 25 mm acetatePlus filters (5.0 μm; Micron Separations, Inc., Westport, MA). We rinsed the filters containing mycelium 3 times with 6 ml of growth medium containing 20 mM nonradioactive CaCl2, removed the mycelial pad from the filter, and measured the amount of radioactivity with a liquid scintillation counter. All uptake experiments were done at least twice, with three replicates per data point in each experiment.
The amount of calcium in conidia was measured by growing wild-type strain 74A in 2 ml of Vogel's medium with the normal concentration of calcium (0.68 mM) and with 1.6 μCi of 45Ca. We incubated the cultures at 30°C for 3 days, placed them in the light at room temperature for an additional 6 days, and collected a scoopful of conidia from the hyphae growing above the liquid. Next, we suspended the conidia in water, counted them, measured the amount of radioactivity, and calculated the content of calcium/conidium.
Measurement of calcium in cell fractions.
To collect cell fractions, we used a well-characterized procedure developed to isolate mitochondria, vacuoles, and other organelles from N. crassa (7, 13, 40). Strains were grown in 1 or 2 liters of Vogel's medium containing approximately 50 μCi of 45Ca. The cultures were inoculated with 106 conidia per ml and grown for approximately 15 h at 25°C with vigorous aeration. Mycelia were collected by filtration through cheesecloth, placed in a solution of cold 1 M sorbitol and 10 mM Tris buffer, pH 7.5, and mixed vigorously with glass beads in a BeadBeater (BioSpec Products, Inc., Bartlesville, OK). The resulting homogenate was centrifuged at 1,000 × g for 10 min to remove cell walls and unbroken cells. The supernatant was centrifuged for 30 min at 15,000 × g, yielding a pellet of dense organelles. This pellet was resuspended in 1 M sorbitol, 10 mM Tris, pH 7.5, and layered on top of a step gradient made of 7 ml 50% sucrose, 2 ml 40% sucrose, and 2 ml 30% sucrose, all in 10 mM Tris, pH 7.5. After centrifugation for 1 h at 20,000 × g, the mitochondria formed a thick layer on top of the 40% sucrose, while the dense vacuoles went to the pellet. An aliquot of the supernatant from the 30-min centrifugation at 15,000 × g was centrifuged for 30 min at 250,000 × g, yielding a microsomal fraction. We suspended all fractions in 10 mM Tris, pH 7.5, and measured radioactivity with a liquid scintillation counter. Protein was assayed with the Bio-Rad protein assay dye reagent (Bio-Rad Laboratories, Hercules, CA). Arginine and polyphosphate were assayed as described previously (12). The fractionation experiment was done at least twice with each strain.
To estimate the concentration of calcium in cells, we measured the correspondence between wet weight, dry weight, and protein. Strain 74A was grown in 1 liter of Vogel's medium as described for the cell fractionation experiments. After growth for 15 h, three 50-ml aliquots were collected by vacuum filtration; the damp pad of mycelium was weighed, then dried, and weighed again. After measuring the volume of the rest of the culture, we disrupted the cells in the BeadBeater as described above and measured the protein content. We calculated that an aliquot of cells at 1 mg (dry weight) was 6.7 mg (wet weight) and contained 0.21 mg of protein. Therefore, we assumed that 1 mg (dry weight) of mycelium corresponded to 5.7 μl of cell water and that 1 mg of protein from a cell extract corresponded to 27 μl of cell water.
RESULTS
Calcium uptake by cells versus concentration of calcium in the medium.
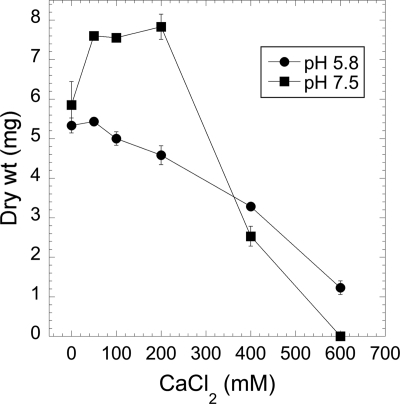
At high concentrations, calcium readily precipitates in the most widely used growth medium for N. crassa, Vogel's medium N (17). In the experiments whose results are shown in Fig. 1 and 2, we used an alternative medium, Vogeloid (described in Materials and Methods), which does not contain citrate, phosphate, or sulfate. This medium can be made with high concentrations of Ca2+ while avoiding precipitation of calcium phosphate and calcium sulfate. As other investigators have shown previously (32), N. crassa grows well even if no calcium is added to the medium, apparently using the trace amounts of calcium present in other components of the medium (Fig. 1). CaCl2 at 400 mM or higher significantly inhibited growth. The effects of calcium were different in acidic and alkaline media. In pH 5.8 medium (the pH of Vogel's medium N), there was a roughly linear decline in the amount of growth as the concentration of calcium increased. In pH 7.5 medium, growth was stimulated approximately 35% by the addition of 10 to 200 mM CaCl2 and then strongly inhibited at higher concentrations.
Fig. 1.
The growth of N. crassa is inhibited by high concentrations of calcium. Cells were grown for 3 days at 25°C in Vogeloid medium (pH 5.8 or 7.5) as described in Materials and Methods.
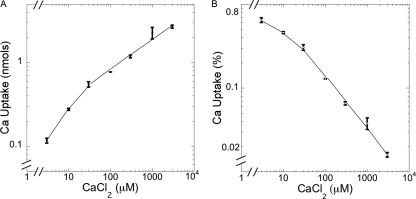
Fig. 2.
The accumulation of calcium in mycelia is dependent on the concentration of calcium in the medium. Wild-type strain 74A was grown for 25 h at 30°C in Vogel's medium N as described in Materials and Methods. (A) Uptake is expressed as nmol per 6 ml culture. (B) Uptake is expressed as percentage of total calcium in the medium.
The accumulation of calcium in cells is highly dependent on the concentration of calcium in the medium. We measured uptake of 45Ca into cells after 25 h of growth in pH 5.8 medium with various concentrations of nonradioactive calcium (Fig. 2). The accumulation of calcium in cells was not directly proportional to the concentration of calcium in the medium. A 1,000-fold increase in calcium (3 to 3,000 μM) caused only a 23-fold increase in cellular calcium levels (Fig. 2A). Figure 2B shows the same data, expressed as percentages of calcium in the medium taken up by cells. In 3 μM CaCl2, cells took up 0.65% of the total calcium, but at 3,000 μM CaCl2, the cells took up only 0.015%. By excluding calcium from cells as the external concentration rose, the cells apparently avoided accumulating toxic levels.
Calcium in conidia.
Because we used a conidial inoculum for growth experiments, we wanted to know how much calcium was in the conidia, i.e., if there was enough for a significant amount of calcium to be added to the medium. As described in Materials and Methods, we measured the amount of calcium in conidia from cultures grown in a standard growth medium, Vogel's medium N, containing 0.68 mM CaCl2. On average, each conidium contained 0.095 fmol of calcium. Based on the measurement of 50 conidia, we assumed that the calcium was inside a sphere with a diameter of 6.2 μm. The internal concentration of calcium was 0.76 mM, approximately 3- to 8-fold more concentrated than in mycelium (see below). In the growth experiments, we inoculated medium with 105 conidia per ml. If all calcium in the conidia were released into the medium, the concentration would be only 9.4 nM.
Calcium in cell fractions.
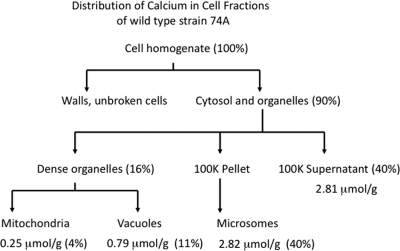
To determine where calcium is located within hyphae, we used a fractionation procedure developed to isolate mitochondria, vacuoles, and lighter membranes (Fig. 3) (7, 13, 40). We grew wild-type mycelium in bubbling cultures with vigorous aeration in Vogel's medium N containing 45Ca (0.68 mM CaCl2), broke the hyphae with glass beads, separated membrane fractions by differential centrifugation, and estimated the amount of calcium in each fraction by counting 45Ca. Approximately 10% of the calcium was in the pellet with cell walls and unbroken cells (Fig. 3). The rest (90%) was distributed in three fractions, a dense organelle fraction composed mainly of mitochondria and vacuoles, a heterogeneous “microsome” fraction, and a high-speed supernatant fraction. Mitochondria, although very abundant, contained only 4% of the calcium. This is likely an overestimate caused by contamination of mitochondria with broken vacuoles (13). (As shown below, mitochondria isolated from the Δcax mutant strain, which has essentially no calcium in the dense vacuolar fraction, contained only 1% of the calcium.) Eleven percent of the calcium was found in the dense vacuolar fraction of wild-type cells.
Fig. 3.
Distribution of calcium in cell fractions from wild-type strain 74A. The cell fractionation procedure is described in Materials and Methods. Note that because of small measurement errors in some steps of the procedure, the numbers do not total exactly 100%. The microsomal fraction contains membranes from broken vacuoles, the ER, and the Golgi apparatus. The 100K supernatant contains soluble material from broken organelles and the cytosol.
Much of the calcium, 40%, was in the microsomal fraction. This fraction contains the less dense particulate matter and the less dense organelles and membranes, including the ER, the Golgi apparatus, nuclei, plasma membranes, and various transport vesicles. Surprisingly, the vacuolar and microsomal calcium fractions were in different physical states. We suspended each fraction in 1 mM Tris buffer, pH 7.5, a procedure that osmotically lyses organelles, and centrifuged them for 30 min at 250,000 × g. All of the calcium in the vacuolar fraction was solubilized, while 90% of the microsomal calcium remained in the pellet. If the microsomal calcium was in a crystalline mineral form, such as hydroxylapatite, it should be soluble in strongly acidic solutions. Suspension of the microsomal fraction in 0.1 M HCl completely solubilized the calcium.
The rest of the calcium, 40%, was in the high-speed supernatant. Although the mycelium was rinsed several times in water after being harvested, some external calcium may have remained in the cell homogenate. However, most of the calcium in the high-speed supernatant likely came from broken vacuoles. Previous analyses of the compartmentation of arginine have shown that more than 95% of soluble cellular arginine and other basic amino acids are sequestered in vacuoles (40, 41). By measuring soluble arginine, Cramer et al. (13) estimated that 8 to 15% of the arginine and therefore 8 to 15% of the vacuoles were in the fraction with the dense organelles (13, 40). We measured soluble arginine in our cell fractions and found 17% of the total cell arginine in the vacuolar fraction (data not shown), consistent with the published results.
The mycelium used for the fractionation experiments accumulated approximately 7 nmol of calcium per mg of protein in the cell extract, yielding an average concentration in cell water of 0.26 mM. As other investigators have suggested, the calcium is likely bound to a polyanion (12, 22). Both the vacuolar and the microsomal fractions contained large concentrations of polyphosphate. After acid hydrolysis of the polyphosphate, we found that the ratios of phosphate to calcium were 12 ± 1.2 to 1 in the vacuolar fraction and 14 ± 1.6 to 1 in the microsomal fraction.
Calcium accumulation in mutant strains lacking calcium transporters.
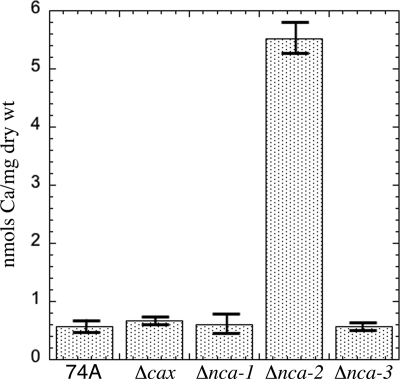
We examined calcium uptake in strains of N. crassa lacking either a calcium-transporting ATPase (the Δnca-1, Δnca-2, and Δnca-3 strains) or a Ca2+/H+ exchange protein (the Δcax strain). The amounts of calcium accumulated in the cells were similar for wild-type strain 74A and the Δcax, Δnca-1, and Δnca-3 mutant strains (Fig. 4). We calculated that the average calcium concentration in these mycelia was 0.1 mM. The Δnca-2 strain produced strikingly different results, accumulating 10-fold more calcium than the others. The Δnca-2 gene is a homolog of the PMC1 gene in S. cerevisiae. Yeast cells lacking PMC1 take up 80% less calcium than wild-type strains (15). Our contrasting results with N. crassa are consistent with the previous observation that NCA-2 fused with GFP is located in the plasma membrane as well as in vacuolar membranes in this organism (6). If NCA-2 functions to pump calcium out of the cell, elevated levels of intracellular calcium may be expected to occur in strains lacking NCA-2.
Fig. 4.
Accumulation of calcium in wild-type strain 74A and in strains lacking calcium transporters. Mycelia were grown in Vogel's medium N for 24 h at 30°C as described in Materials and Methods.
Calcium in cell fractions obtained from mutant strains.
We determined the distribution of calcium in the mutant strains as done with the wild-type strain (Fig. 3 and Table 1). For the Δnca-1 and Δnca-3 strains, the amount of calcium in each fraction was not significantly different from that observed in the wild-type strain. In agreement with the data in Fig. 4, the Δnca-2 strain had larger amounts of calcium in all fractions. The observation that disruption of this single gene caused an elevation in the calcium in the high-speed supernatant supports the conclusion that most of this calcium comes from within the cell, not from binding to the outside. In the cell fractionation experiments (Table 1), the calcium levels were elevated approximately 4-fold in Δnca-2, compared to 10-fold in the whole-cell uptake experiment (Fig. 4). This difference may be due to how the cells were grown, 24 h in standing cultures (Fig. 4) versus 15 h with vigorous aeration (Table 1). Both types of experiments, performed multiple times, gave consistent results.
Table 1.
Calcium in cell fractionsa
| Strain or genotype | Ca concn (μmol/g protein) in: |
||||
|---|---|---|---|---|---|
| Cell extract (2.5K supernatant) | Mitochondria | Vacuoles | Microsomes | High-speed supernatant | |
| 74A | 6.99 ± 2.47 | 0.25 ± 0.05 | 0.79 ± 0.16 | 2.82 ± 0.47 | 2.81 ± 0.96 |
| Δcax | 6.52 ± 0.22 | 0.09 ± 0.04 | 0.05 ± 0.04 | 2.58 ± 0.00 | 3.15 ± 0.39 |
| Δnca-1 | 7.94 ± 0.34 | 0.33 ± 0.10 | 0.64 ± 0.36 | 2.50 ± 0.17 | 3.27 ± 0.07 |
| Δnca-2 | 29.45 ± 6.25 | 0.89 ± 0.47 | 2.12 ± 1.40 | 10.1 ± 4.24 | 13.22 ± 4.47 |
| Δnca-3 | 6.86 ± 0.96 | 0.22 ± 0.07 | 0.60 ± 0.30 | 3.13 ± 0.49 | 2.33 ± 1.31 |
Cell fractions were obtained as described in Materials and Methods.
Another interesting result was the small amount of calcium detected in the vacuolar fraction from the Δcax mutant strain. Molecules previously shown to be in this vacuolar fraction, arginine and polyphosphate, were present at nearly normal levels (data not shown). Consistent with the results for uptake into whole cells (Fig. 4), the amount of calcium in the whole-cell extract was not significantly different from that observed in the wild type. The amount of calcium in the mitochondrial fraction was smaller, but this was expected because small amounts of molecules stored in the dense vacuoles, such as arginine, have been shown to appear in the mitochondrial fraction (13). Thus, the Δcax mutant strain presents a puzzle: if most of the calcium in the high-speed supernatant is from broken vacuoles, why does this fraction have a normal level of calcium while the level in the dense vacuolar fraction is greatly diminished in Δcax mutants? We address this question in Discussion.
Phenotypes of mutant strains.
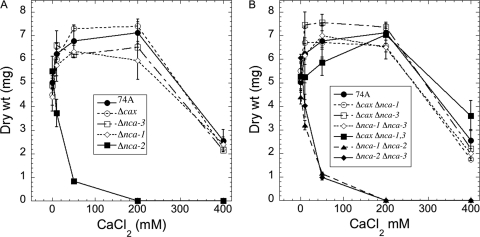
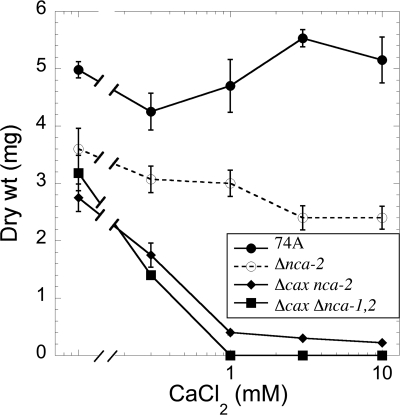
To test the sensitivity of the mutant strains to calcium, we grew them in liquid medium, pH 7.5, with increasing concentrations of CaCl2 (Fig. 5 and 6). The growth of the Δcax, Δnca-1, and Δnca-3 strains was very similar to that of wild-type strain 74A. The Δnca-2 strain was clearly different, showing significant inhibition of growth at 50 mM CaCl2 (Fig. 5A). Strains with both Δcax and Δnca-2 mutations (Δcax Δnca-2 and Δcax Δnca-1 Δnca-2) were particularly sensitive to calcium. CaCl2 concentrations of 1 mM or greater almost completely inhibited the growth of these strains (Fig. 6). The growth of the Δnca-1 Δnca-2 and Δnca-2 Δnca-3 strains was indistinguishable from that of the Δnca-2 strain (Fig. 5A and B). All combinations of mutations that did not include Δnca-2 had patterns of growth similar to that of the wild-type strain (Fig. 5B).
Fig. 5.
Effect of calcium on the growth of strains lacking calcium transporters. Growth was measured after 2 days at 30°C in liquid Vogel's medium N, pH 7.5, as described in Materials and Methods. Panel A shows results for wild-type 74A and single mutant strains; panel B shows results for strains with multiple mutations.
Fig. 6.
Effect of calcium on the growth of strains lacking both cax and nca-2 genes. Growth was measured after 2 days in liquid Vogel's medium N, pH 7.5, as described in Materials and Methods. Note that the x axis has a logarithmic scale.
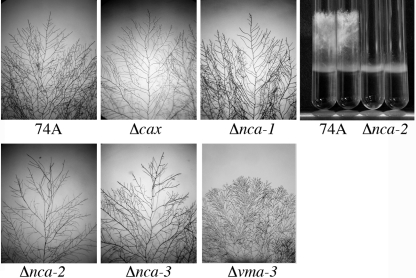
Because calcium has been hypothesized to play an important role in polar growth in filamentous fungi (5, 30, 33, 39), we looked for impairment of growth and fertility in the mutant strains. When grown on agar medium, the hyphal branching pattern of the Δcax, Δnca-1, Δnca-2, and Δnca-3 strains did not appear to be significantly different from that of the wild type (Fig. 7). For comparison, we show in Fig. 7 the hyperbranching phenotype of the Δvma-3 strain, a strain lacking the V-ATPase. We found no fertility defects in the Δcax, Δnca-1, or Δnca-3 strain. The Δnca-2 strain, however, produced fewer conidia and was infertile as a female parent. In addition, the Δnca-2 progeny from a cross with wild-type strain 74A grew more slowly than wild-type progeny (Fig. 7, right panel). Eventually, the Δnca-2 strain produced aerial hyphae that grew to half the height observed for the wild type. The defects of Δnca-2 were the same in medium with no added calcium (data not shown).
Fig. 7.
Morphology of hyphal growth of wild-type strain 74A and of strains lacking transporters. Strains were grown on plates with Vogel's medium N and 2% agar for 24 h (or 48 h for the Δvma-3 strain) at 30°C. The morphologies of hyphal growth were similar in all strains except for the Δvma-3 strain, which had a highly compact and highly branched pattern of growth. In the panel on the right, wild-type 74A and the Δnca-2 strain were grown in tubes with 2 ml of Vogel's medium N at 30°C for 3 days.
DISCUSSION
N. crassa requires very little calcium for growth. It grows well with the trace amounts of calcium found in other components of growth media. However, it tolerates extremely high levels of calcium, growing well in standard medium (pH 5.8) supplemented with 200 mM calcium. In N. crassa, as in other organisms, the cytosolic calcium concentration is approximately 0.1 μM (28, 39). Growth media typically contain much higher concentrations, e.g., 0.68 mM in Vogel's medium N. The low levels in the cytosol can be maintained by using active transport mechanisms to pump calcium out of the cell and/or to sequester calcium in organelles. We have found that N. crassa, grown in Vogel's medium N, accumulates significant amounts of calcium. If the calcium-sequestering organelles make up 10% of the cell volume, the concentration of calcium in these organelles will be roughly 2.5 mM, 25,000-fold higher than the concentration in the cytosol.
Cells can accumulate large amounts of calcium if much of it is bound to macromolecules. The cell fractionation experiments in this paper show that approximately 40% of the calcium is in an insoluble form. A significant amount of insoluble calcium was also reported to occur in S. cerevisiae (22). Because the insoluble calcium is pelleted only at high centrifugal forces, it may be in the form of tiny particles, probably bound to polyphosphate. Several previous reports have concluded that almost all calcium in fungal cells is in the vacuole, which contains polyphosphate as the predominant polyanion (12). Polyphosphate granules within vacuoles have long been reported (35, 40), although some investigators have argued that the particulate nature of the polyphosphate in electron micrographs is an artifact of the staining procedure (2). In living cells, we frequently observed tiny particles in large vacuoles, moving rapidly by Brownian motion.
In this study, most of the calcium was found in a soluble form in the dense vacuolar pellet (10 to 15%) and the high-speed supernatant (∼40%). In the cell fractionation procedure, 8 to 15% of the vacuoles behave as small dense organelles and are “pelletable”; however, most vacuoles break and release their contents (13). The yield of vacuoles has been estimated by measuring total cell soluble arginine, almost all of which is in the vacuole (40, 41). The proportion of total calcium in the vacuolar pellet was approximately the same as the proportion of total soluble arginine. Therefore, the calcium in the high-speed supernatant most likely came from broken vacuoles. Overall, our data are consistent with the hypothesis that over 90% of cell calcium is sequestered in vacuoles, with a significant proportion in an insoluble form. However, we cannot rule out the possibility that some calcium is in other organelles in the microsomal fraction or that some calcium in the high-speed supernatant was released from other organelles.
One of the most interesting observations in this study was that the Δcax strain accumulated very little calcium in the vacuolar pellet, yet the total amount of calcium in the cell was similar to levels in the wild-type strain (Fig. 4 and Table 1). The heterogeneity in size and shape of vacuoles could explain this finding. Previously, we used microscopy to visualize the vacuolar markers CAX, NCA-2, NCA-3, VMA-1 (a subunit of the V-ATPase), and VAM-3 (a vacuolar SNARE) fused to green or red fluorescent proteins. In living cells, all of these proteins appear both on spherical vacuoles with a wide range of sizes and in a network of interconnected tubules near the hyphal tip (6). The cell fractionation procedure yields small (1 μm or less), dense vesicles in the “vacuolar pellet” (13). This fraction appears to be highly enriched in a vacuolar compartment that is dependent on the CAX protein for sequestration of calcium (Table 1). The microscopy experiments showed small vesicles near the hyphal tip that were highly enriched in CAX and the V-ATPase but did not have VAM3, NCA-2, or NCA-3 (6). These organelles are candidates for the CAX-dependent, calcium-sequestering compartment in the vacuolar pellet. Organelles of similar size and composition, named “acidocalcisomes,” have been described, initially in trapanosomatids but more recently also in bacteria and algae (18). These organelles have proton and calcium pumps and contain high concentrations of polyphosphate, calcium, and magnesium. More data are needed to definitively link acidocalcisomes and the vesicles that we observed in N. crassa.
Larger vacuoles, which also contain calcium, are more likely to break during cell fractionation. We hypothesize that in the CAX strain calcium is transported into the larger vacuoles by other transporters. An analysis of the N. crassa genome revealed at least seven other genes encoding proteins with a high degree of sequence similarity to CAX (42). Several of these proteins, tagged with GFP or RFP, localize to all the components of the vacuolar system, except for the small vesicles near the hyphal tip where only CAX and the vacuolar ATPase appear (B. Bowman, unpublished experiments).
Our experiments also point to the important role of NCA-2 in controlling calcium levels. N. crassa has two closely related proteins, NCA-2 and NCA-3, in the PMCA family of Ca2+-ATPases. In animal cells, PMCA enzymes reside in the plasma membrane and pump calcium out of the cell, while in S. cerevisiae Pmc1p is in the vacuolar membrane. In N. crassa, NCA-2 and NCA-3 proteins tagged with GFP and RFP are found in both locations, in the vacuolar compartments near the hyphal tip but predominately in the plasma membrane in other regions of the hyphae (6). The phenotypes of the Δnca-2 and Δnca-3 mutant strains strongly suggest that NCA-2 plays the major role in pumping calcium out of the cell. The Δnca-2 strain will not grow in high concentrations of calcium, and it accumulates calcium to excessively high levels, a phenotype opposite to that of the Δpmc1 strain of S. cerevisiae, in which calcium levels are 80% lower than in the wild type (15). The Δnca-3 strain, unlike the Δnca-2 strain, behaves like the wild type in growth and calcium accumulation. Our experiments failed to provide clues about the function of NCA-3. The gene is expressed in wild-type cells, as shown by an early report and recent microarray analyses (3, 25, 37). We found no defects in the Δnca-3 mutant strain, nor did it confer any additional defects when present in strains with mutations in other Ca2+-transporting genes.
In contrast to N. crassa, which has two genes in the PMCA family of Ca2+-ATPases, Aspergillus nidulans has five genes in the PMCA family. In a recent investigation, A. nidulans strains lacking either pmcA or both pmcA and pmcB were generated (23). Tests of the ability of these two strains to grow on solid medium containing 700 mM CaCl2 showed that the pmcA strain was slightly sensitive to calcium, and the double mutant strain was more strongly inhibited. It will be challenging but important to determine why filamentous fungi have multiple PMCA-type calcium pumps.
The Δnca-1 mutant strain of N. crassa was indistinguishable from the wild type in growth, tolerance of high calcium, and calcium accumulation in Vogel's medium. NCA-1 was predicted to be in the ER, and observation of NCA-1–GFP confirmed that prediction (6). However, the results presented here suggest that the ER contains little calcium. Different levels of calcium in Δnca-1 compared to the level in the wild type may be too small to be detected in our experiments.
In summary, our results indicate that N. crassa, a representative filamentous fungus, maintains calcium homeostasis in the cytosol by pumping calcium out of the cell and by sequestering calcium in vacuolar compartments. The “vacuole” is heterogeneous in size and structure. An intriguing question is whether the vacuole is heterogeneous in function, with different regions specialized for different tasks. Other organelles, such as the mitochondria and the ER (which includes the nuclear envelope), likely contain only small amounts of calcium. However, because the concentration of calcium in the cytosol is so low, 0.1 μM, release of calcium from an organelle containing only 1% of total cell calcium could raise the cytosolic concentration significantly, at least 10-fold. For this reason, the results in this report neither negate nor support the hypothesis that a tip-high concentration gradient of calcium regulates polar growth in fungal hyphae.
ACKNOWLEDGMENTS
This study was supported by Public Health Service grant GM058903 from the Institute of General Medicine to B. Bowman.
We thank Teddy Solbes and Christopher Chavez for contributions to early work relating to this project.
Footnotes
Published ahead of print on 18 February 2011.
REFERENCES
- 1. Antebi A., Fink G. R. 1992. The yeast Ca2+-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol. Biol. Cell 3:633–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ashford A. E., Vesk P. A., Orlovich D. A., Markovina A. L., Allaway W. G. 1999. Dispersed polyphosphate in fungal vacuoles in Eucalyptus pilularis/Pisolithus tinctorius ectomycorrhizas. Fungal Genet. Biol. 28:21–33 [DOI] [PubMed] [Google Scholar]
- 3. Benito B., Garciadeblas B., Rodriguez-Navarro A. 2000. Molecular cloning of the calcium and sodium ATPases in Neurospora crassa. Mol. Microbiol. 35:1079–1088 [DOI] [PubMed] [Google Scholar]
- 4. Berridge M. J., Bootman M. D., Roderick H. L. 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4:517–529 [DOI] [PubMed] [Google Scholar]
- 5. Bok J. W., et al. 2001. Structure and function analysis of the calcium-related gene spray in Neurospora crassa. Fungal Genet. Biol. 32:145–158 [DOI] [PubMed] [Google Scholar]
- 6. Bowman B. J., Draskovic M., Freitag M., Bowman E. J. 2009. Structure and distribution of organelles and cellular location of calcium transporters in Neurospora crassa. Eukaryot. Cell 8:1845–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bowman E. J., Bowman B. J. 1988. Purification of vacuolar membranes, mitochondria, and plasma membranes from Neurospora crassa and modes of discriminating among the different H+-ATPases. Methods Enzymol. 157:562–573 [DOI] [PubMed] [Google Scholar]
- 8. Carafoli E. 2002. Calcium signaling: a tale for all seasons. Proc. Natl. Acad. Sci. U. S. A. 99:1115–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clapham D. E. 2007. Calcium signaling. Cell 131:1047–1058 [DOI] [PubMed] [Google Scholar]
- 10. Colot H. V., et al. 2006. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. U. S. A. 103:10352–10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cornelius G., Nakashima H. 1987. Vacuoles play a decisive role in calcium homeostasis in Neurospora crassa. J. Gen. Microbiol. 133:2341–2347 [Google Scholar]
- 12. Cramer C. L., Davis R. H. 1984. Polyphosphate-cation interaction in the amino acid-containing vacuole of Neurospora crassa. J. Biol. Chem. 259:5152–5157 [PubMed] [Google Scholar]
- 13. Cramer C. L., Ristow J. L., Paulus T. J., Davis R. H. 1983. Methods for mycelial breakage and isolation of mitochondria and vacuoles of Neurospora. Anal. Biochem. 128:384–392 [DOI] [PubMed] [Google Scholar]
- 14. Cui J., Kaandorp J. A., Sloot P. M., Lloyd C. M., Filatov M. V. 2009. Calcium homeostasis and signaling in yeast cells and cardiac myocytes. FEMS Yeast Res. 9:1137–1147 [DOI] [PubMed] [Google Scholar]
- 15. Cunningham K. W., Fink G. R. 1994. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J. Cell Biol. 124:351–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cunningham K. W., Fink G. R. 1996. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:2226–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davis R. H. 2000. Neurospora: contributions of a model organism. Oxford University Press, Oxford, United Kingdom [Google Scholar]
- 18. Docampo R., de Souza W., Miranda K., Rohloff P., Moreno S. N. 2005. Acidocalcisomes—conserved from bacteria to man. Nat. Rev. Microbiol. 3:251–261 [DOI] [PubMed] [Google Scholar]
- 19. Duchen M. R., Verkhratsky A., Muallem S. 2008. Mitochondria and calcium in health and disease. Cell Calcium 44:1–5 [DOI] [PubMed] [Google Scholar]
- 20. Dunn T., Gable K., Beeler T. 1994. Regulation of cellular Ca2+ by yeast vacuoles. J. Biol. Chem. 269:7273–7278 [PubMed] [Google Scholar]
- 21. Durr G., et al. 1998. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol. Biol. Cell 9:1149–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eilam Y., Lavi H., Grossowicz N. 1985. Cytoplasmic Ca2+ homeostasis maintained by a vacuolar Ca2+ transport-system in the yeast Saccharomyces cerevisiae. J. Gen. Microbiol. 131:623–629 [DOI] [PubMed] [Google Scholar]
- 23. Findon H., et al. 2010. Analysis of a novel calcium auxotrophy in Aspergillus nidulans. Fungal Genet. Biol. 47:647–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Halachmi D., Eilam Y. 1989. Cytosolic and vacuolar Ca2+ concentrations in yeast cells measured with the Ca2+-sensitive fluorescence dye indo-1. FEBS Lett. 256:55–61 [DOI] [PubMed] [Google Scholar]
- 25. Kasuga T., et al. 2005. Long-oligomer microarray profiling in Neurospora crassa reveals the transcriptional program underlying biochemical and physiological events of conidial germination. Nucleic Acids Res. 33:6469–6485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kirichok Y., Krapivinsky G., Clapham D. E. 2004. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427:360–364 [DOI] [PubMed] [Google Scholar]
- 27. McCluskey K. 2003. The Fungal Genetics Stock Center: from molds to molecules. Adv. Appl. Microbiol. 52:245–262 [DOI] [PubMed] [Google Scholar]
- 28. Miller A. J., Vogg G., Sanders D. 1990. Cytosolic calcium homeostasis in fungi: roles of plasma membrane transport and intracellular sequestration of calcium. Proc. Natl. Acad. Sci. U. S. A. 87:9348–9352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pozos T. C., Sekler I., Cyert M. S. 1996. The product of HUM1, a novel yeast gene, is required for vacuolar Ca2+/H+ exchange and is related to mammalian Na+/Ca2+ exchangers. Mol. Cell. Biol. 16:3730–3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prokisch H., Yarden O., Dieminger M., Tropschug M., Barthelmess I. B. 1997. Impairment of calcineurin function in Neurospora crassa reveals its essential role in hyphal growth, morphology and maintenance of the apical Ca2+ gradient. Mol. Gen. Genet. 256:104–114 [DOI] [PubMed] [Google Scholar]
- 31. Rudolph H. K., et al. 1989. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell 58:133–145 [DOI] [PubMed] [Google Scholar]
- 32. Schmid J., Harold F. M. 1988. Dual roles for calcium ions in apical growth of Neurospora crassa. J. Gen. Microbiol. 134:2623–2631 [DOI] [PubMed] [Google Scholar]
- 33. Silverman-Gavrila L. B., Lew R. R. 2003. Calcium gradient dependence of Neurospora crassa hyphal growth. Microbiology 149:2475–2485 [DOI] [PubMed] [Google Scholar]
- 34. Stroobant P., Scarborough G. A. 1979. Active transport of calcium in Neurospora plasma membrane vesicles. Proc. Natl. Acad. Sci. U. S. A. 76:3102–3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Strullu D. G., Harley J. L., Gourret J. P., Garrec J. P. 1983. A note on the relative phosphorus and calcium contents of metachromatic granules in Fagus mycorrhiza. New Phytol. 94:89–94 [Google Scholar]
- 36. Sze H., Liang F., Hwang I., Curran A. C., Harper J. F. 2000. Diversity and regulation of plant Ca 2+ pumps: insights from expression in yeast. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51:433–462 [DOI] [PubMed] [Google Scholar]
- 37. Tian C., Kasuga T., Sachs M. S., Glass N. L. 2007. Transcriptional profiling of cross pathway control in Neurospora crassa and comparative analysis of the Gcn4 and CPC1 regulons. Eukaryot. Cell 6:1018–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ton V. K., Rao R. 2004. Functional expression of heterologous proteins in yeast: insights into Ca2+ signaling and Ca2+-transporting ATPases. Am. J. Physiol. Cell Physiol. 287:C580–C589 [DOI] [PubMed] [Google Scholar]
- 39. Torralba S., Heath I. B. 2001. Cytoskeletal and Ca2+ regulation of hyphal tip growth and initiation. Curr. Top. Dev. Biol. 51:135–187 [DOI] [PubMed] [Google Scholar]
- 40. Vaughn L. E., Davis R. H. 1981. Purification of vacuoles from Neurospora crassa. Mol. Cell. Biol. 1:797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weiss R. L. 1976. Compartmentation and control of arginine metabolism in Neurospora. J. Bacteriol. 126:1173–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zelter A., Bencina M., Bowman B. J., Yarden O., Read N. D. 2004. A comparative genomic analysis of the calcium signaling machinery in Neurospora crassa, Magnaporthe grisea, and Saccharomyces cerevisiae. Fungal Genet. Biol. 41:827–841 [DOI] [PubMed] [Google Scholar]