Abstract
Microorganisms in diverse terrestrial surface and subsurface environments can anaerobically catalyze the oxidative dissolution of uraninite. While a limited quantity (∼5 to 12 μmol liter−1) of uranium is oxidatively dissolved in pure culture studies, the metabolism is coupled to electron transport, providing the potential of uraninite to support indigenous microbial populations and to solubilize uranium.
TEXT
While uranium-bearing minerals and deposits are naturally abundant, mining and disposal practices of materials associated with nuclear fuels have resulted in natural systems with concentrations of dissolved uranium (U) exceeding the U.S. Environmental Protection Agency's maximum contamination limit (MCL) (30 μg liter−1) (13). In an oxidizing system with a pH of >5, uranium exists predominantly in various soluble U(VI) complexes (3, 13, 19). A uranium bioremediative strategy is based on the biomineralization of uranium, reducing aqueous U(VI) to an insoluble end product(s) such as uraninite, UO2(S) (14, 15). This strategy relies on maintenance of a low reduction potential in order to preserve the U(IV) precipitate in situ. Reoxidation and remobilization of uranium have been observed in laboratory and field experiments via both biotic and abiotic mechanisms (1, 6, 9–11, 17, 24). Anaerobic microbial metabolism catalyzes U(IV) oxidation, with nitrate serving as a potential electron acceptor (1, 9, 24). Due to the use of nitric acid in the processing of nuclear fuels, nitrate is often a cocontaminant in these environments. Thus, this microbially mediated process warrants attention.
The oxidation of U(IV) coupled to the reduction of nitrate at circumneutral pH is thermodynamically favorable, theoretically yielding enough energy to support microbial growth or generation of chemical energy in the form of ATP (ΔG°′ = −352.9 kJ/mol) (see Table SI1 in the supplemental material) according to the following equation: 2.5UO2(am) + NO3− + 5HCO3− + H+ → 2.5UO2(CO3)22− + 0.5N2 + 3H2O [where (am) means amorphous].
To date, a microorganism capable of growth has not been identified and only two have been described to catalyze anaerobic U(IV) oxidation, Geobacter metallireducens and Thiobacillus denitrificans (1, 9). In order to understand the applicability and long-term stability of a reductive uranium immobilization strategy, we investigated the ubiquity and diversity of microorganisms capable of the oxidative dissolution of uraninite. Here we describe terrestrial surface and subsurface microorganisms capable of catalyzing the anaerobic oxidative dissolution of uraninite by utilizing the solid-phase mineral as an electron donor. The quantity (∼5 to 12 μmol liter−1) of uranium oxidatively dissolved is ca. 40- to 100-fold higher than the MCL. Furthermore, the metabolism is coupled to electron transport, indicating the potential of uraninite to support indigenous microbial populations and contribute to uranium mobilization.
Various pure cultures of nitrate-reducing bacteria were screened for the ability to oxidize U(IV) in the presence of nitrate; these included Geothrix fermentans H5 (ATCC 700665) from the Acidobacteria (7), bacteria from diverse subclasses of the Proteobacteria, including Pseudogulbenkiania sp. strain 2002 (ATCC BAA-1479) (21, 22), Acidovorax ebreus TPSY (4), and Azospira suillum PS (ATCC BAA-33) (5) from the Betaproteobacteria, Pseudomonas sp. strain PK from the Gammaproteobacteria, and Magnetospirillum sp. strain VDY (ATCC BAA-1730) from the Alphaproteobacteria (18). Cultures were obtained from glycerol stocks in the University of California—Berkeley laboratory culture collection. Two of the tested strains, Pseudogulbenkiania sp. 2002 and A. ebreus TPSY, were isolated from environments (uncontaminated [22] and uranium/nitrate contaminated [4], respectively) assayed in the enumeration study discussed below. Cells were grown anaerobically on acetate (6.25 mM) and nitrate (10 mM) in anoxic (N2-CO2, 80:20 atmosphere), basal bicarbonate buffered (30 mM, pH 6.8) medium (20, 21), harvested in late log phase by centrifugation (6,000 × g, 10 min), washed twice with anoxic (N2-CO2; 80:20 atmosphere) bicarbonate buffer (30 mM, pH 6.8), and resuspended in anoxic bicarbonate buffer to serve as an inoculum.
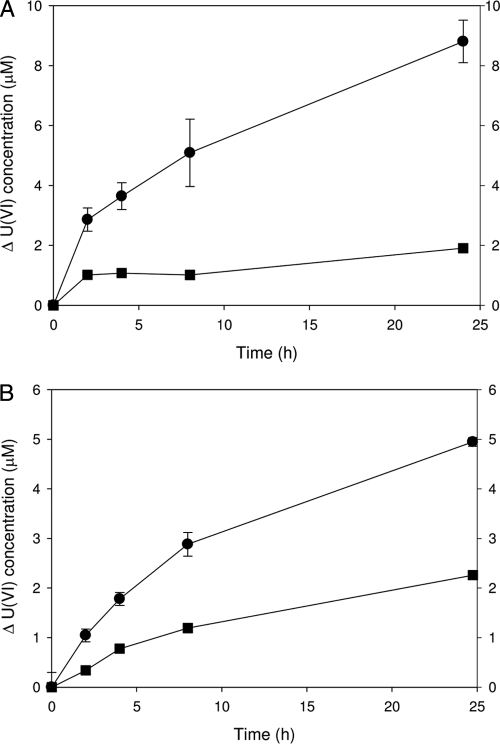
Nongrowth anaerobic cell suspensions of A. ebreus strain TPSY containing chemically precipitated UO2 (final concentration, 100 μmol liter−1, prepared as described in the supplemental material) and nitrate (100 μM) in bicarbonate buffer (30 mM, pH 6.8) oxidized 8 μM U(IV) (as determined by kinetic phosphorescence analysis [KPA] [2]) over 24 h (pseudo-first order rate constant of 0.12 ± 0.02 h−1) (Fig. 1 A). Similarly, nongrowth cell suspensions of strain 2002 amended with UO2 and nitrate also resulted in the oxidation of 5 μM U(IV) over 25 h (pseudo-first order rate constant of 0.12 ± 0.01 h−1). Abiotic oxidation of U(IV) (∼2 μM) was observed in pasteurized control cultures. Comparable experimental results were also obtained with the acidobacterium G. fermentans H5, the gammaproteobacterium Pseudomonas sp. PK, and the alphaproteobacterium Magnetospirillum sp. VDY (see Fig. SI1 in the supplemental material). These results further expand the taxonomic diversity of microorganisms capable of nitrate-dependent U(IV) oxidation beyond the beta- and deltaproteobacteria previously identified (1, 9). Interestingly, many of the bacteria capable of nitrate-dependent U(IV) oxidation are also capable of nitrate-dependent Fe(II) oxidation (1, 9, 18, 21–23). However, nitrate-dependent U(IV) oxidation is not synonymous with the ability to oxidize Fe(II). Repeated experiments with A. suillum strain PS demonstrated that this organism did not anaerobically oxidize U(IV) (see Fig. SI2 in the supplemental material) although it readily mediates nitrate-dependent Fe(II) oxidation.
Fig. 1.
Nongrowth cell suspension experiments were conducted in 30 mM bicarbonate buffer (pH 6.8, N2-CO2 headspace) containing chemically precipitated UO2 and nitrate inoculated with a live, washed cell suspension of A. ebreus strain TPSY (A) and Pseudogulbenkiania sp. strain 2002 (B) grown on acetate and nitrate (6.25 mM and 10 mM, respectively) with results compared to those of pasteurized control cultures. •, live cells; ▪, pasteurized cells. Symbols represent average results for triplicate cultures. Error bars denote standard error of measure.
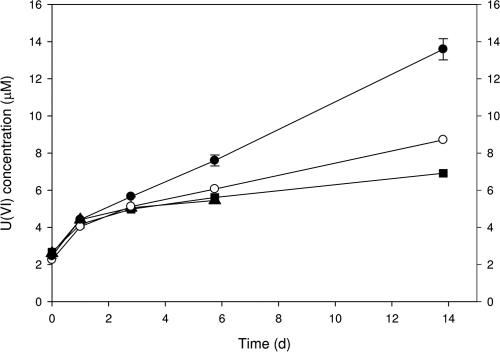
Pseudogulbenkiania sp. 2002 was previously demonstrated to grow autotrophically with nitrate-dependent Fe(II) oxidation (22). Uranium(IV) bio-oxidation by this organism was evaluated under similar growth conditions in autotrophic basal medium (22) amended with nitrate (25 μM) and UO2 (100 μmol liter−1) in place of the Fe(II). After 14 days of incubation, strain 2002 oxidized ∼12 μM U(IV). Approximately 5 μM was oxidized in controls where nitrate was omitted from basal medium, potentially a result of the remaining electron-accepting capacity in the inoculum. However, approximately 5 μM was also oxidized in a pasteurized control, suggesting that some oxidation may alternatively be abiotic. Similar to the pasteurized control, cultures amended with sodium azide (final concentration, 1 mM), an electron transport inhibitor, demonstrated limited oxidation (∼7 μM) (Fig. 2), suggesting that U(IV) oxidation was coupled to electron transport in the live cultures. In addition, U(IV) oxidation was completely inhibited in washed cell suspensions of strain 2002 amended with antimycin A (electron transport complex III inhibitor) (data not shown). These results suggest the direct involvement of the electron transport chain with U(IV) serving as the sole electron donor.
Fig. 2.
Anaerobic, nitrate-dependent U(IV) oxidation by Pseudogulbenkiania sp. strain 2002 under growth conditions in basal bicarbonate buffer (pH 6.8, N2-CO2 headspace) containing chemically precipitated UO2 and nitrate. •, live cells, ▪, pasteurized cells; ○, live cells amended with sodium azide (electron transport inhibitor); ▴, live cells with U(IV) only (no nitrate). Symbols represent average results for triplicate cultures. Error bars denote standard error of measure.
While anaerobic U(IV) oxidation may be coupled to electron transport, the small quantity of U(IV) oxidized precludes microbial growth given the net energy yield. The 11 μmol liter−1 of U(IV) oxidized by strain 2002 under growth conditions would theoretically yield 1.9 J of energy according to the equation above (see Table SI1 in the supplemental material). Based on data previously published on nitrate-dependent Fe(II) oxidation by Pseudogulbenkiania sp. 2002 under growth conditions (22), estimates were calculated to determine the required quantity of U(IV) oxidized to provide enough energy for growth. Assuming 100% efficiency for conversion of energy into biomass, as observed for autotrophic nitrate-dependent Fe(II) oxidation (22), 96 J of energy is required for one cell doubling (growth), significantly more than the 1.9 J netted from the limited U(IV) oxidation in these studies. Thus, U(IV) oxidation would not yield enough energy for cells of strain 2002 to grow under the conditions tested. However, chemical energy in the form of ATP could be generated (ATP = 5.3 × 10−20 J), theoretically yielding 59 μmol of ATP. The consequence of this metabolism is that it can result in the mobilization of uranium via oxidative dissolution in excess of the MCL (30 μg liter−1, 0.126 μM) without cell growth.
Screening of samples collected from diverse environments indicated that this metabolism is ubiquitous. Groundwater, soil, and sediment samples were collected from various sites that were anthropogenically contaminated with U and nitrate (U.S. Department of Energy, Office of Science, Environmental Remediation Sciences, Oak Ridge Field Research Center [ORFRC]) as well as various uncontaminated sites. The ORFRC sites selected were the background site (pH 7 to 8), area 1 (pH 3.25 to 6.5; nitrate, 48 to 10,400 mg/liter; uranium, <7.5 mg/liter), and area 2 (pH 6 to 7; nitrate, <100 mg/liter; uranium, <12 mg/liter) (3). Freshwater lake sediment was collected from Campus Lake, Southern Illinois University, Carbondale, IL (22), and surface soil was collected from an agricultural field in Nebraska (41°22′19"N, 97°01′23"W). From the environmental samples collected, most probable number enumeration (MPN) series were initiated by adding 1 g of soil/sediment or 1 ml groundwater to 9 ml anoxic (N2-CO2; 90:10 headspace), low-nutrient medium (see the supplemental material) buffered with 20 mM PIPPS [piperazine-N,N-bis(3-propanesulfonic acid), pH <6] or 20 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid), pH >6], adjusted to the environmental pH, and amended with 1.0 mmol liter−1 UO2 and 0.5 mM nitrate. Acetate (0.1 mM) was added as an additional carbon source. After 8 weeks of static incubation in the dark at room temperature, ca. 20°C, tubes positive for U(IV) oxidation were identified by quantitative analysis of aqueous and solid-associated U(VI) (8) via a high-performance liquid chromatography (HPLC) colorimetric post column dye assay (12) with comparison to killed controls. Enumeration of U(IV)-oxidizing microorganisms in aquatic and sediment samples collected from freshwater lake, surface soil, subsurface sediment, and groundwater revealed anaerobic U(IV)-oxidizing microorganisms ranging in abundance from 9.30 × 101 to 2.40 × 103 cells ml−1 groundwater or g−1 soil/sediment (Table 1). Uranium- and nitrate-contaminated groundwater and sediment samples collected from ORFRC were among sites positive for U(IV)-oxidizing microorganisms. Microbial U(IV)-oxidizing activity was also identified in uncontaminated subsurface sediments (2.40 × 103 cells g−1 sediment). Additionally, freshwater lake sediments and surface soil also harbored nitrate-dependent U(IV)-oxidizing microorganisms, suggesting that exposure to high uranium concentrations was not necessary for U(IV) oxidation. No visible growth was observed in the dilution series, and 5 to 225 μM U(IV) was oxidized in MPN enrichments (Table 1). Given the reactivity of nitrate reduction intermediates (NO2−, NO, or N2O) with U(IV) (17), the possibility of a partial role of abiotic interactions in U(IV) oxidation cannot be excluded.
Table 1.
MPN enumeration of microorganisms capable of nitrate-dependent U(IV) biooxidation from samples collected from ORFRC sites and other sites in the continental United States
| Site and/or sample | Cells ml−1 or cells g−1 soil/sediment | Cornish-Fisher confidence limitsa | Concn range of biologically oxidized U(IV) (μM)b |
|---|---|---|---|
| Background sediment FB618 | 2,398 | 475.6–9,652 | 9.15–225 |
| Groundwater FW300 | NDc | ||
| Area 1 sediment FWB063-04-23 | 239.8 | 47.56–965.2 | 57.0–94.7 |
| Sediment FNB063-01-44 | 93.3 | 20.66–270.9 | 28.3–30.6 |
| Area 1 groundwater FW021 | 93.1 | 20.67–269.8 | 1.68–13.1 |
| Area 1 sediment FB062 | 239.8 | 47.56–965.2 | 99.5–109 |
| Area 1 sediment FB063-04-23 | 239.8 | 47.6–965.2 | 57.0–94.7 |
| Area 2 groundwater TBP16 | 427 | 103.4–1,385 | 2.03–85.0 |
| Freshwater lake sediment, Crab Orchard Lake, Carbondale, IL | 93 | 20.66–270.9 | 52.4–67.6 |
| Subsurface sediment, Longhorn, TX | 2,398 | 475.6–9,652 | 1.03–47.1 |
| Surface soil, agricultural field, Linwood, NE | 932.8 | 206.6–2,709 | 0.616–42.2 |
Cells ml−1 or cells g−1 soil/sediment.
Values are reported as concentration of biological U(VI) determined by subtracting the average U(VI) concentrations in triplicate killed controls from the U(VI) concentrations in each tube of the MPN series. Values represent the calculated minimum and maximum U(VI) concentrations. Tubes in which U(VI) concentrations did not exceed the killed-control value were recorded as zero. Non-zero values are reported.
ND, not detected.
Anaerobic U(IV)-oxidizing bacteria are capable of directly mediating the oxidation of U(IV). Acidovorax ebreus TPSY was isolated from an MPN dilution series initiated from the same groundwater sample collected from area 2 of the ORFRC (4) in which U(IV)-oxidizing microorganisms were identified. Additionally, Pseudogulbenkiania sp. 2002 was isolated from a non-U-impacted site that also exhibited U(IV) oxidation in the MPN series. The identification of uranium(IV)-oxidizing microorganisms in these various environments suggest that they are ubiquitous in environmental systems and are not necessarily dependent on contaminant U exposure. Thus, the ubiquity of U(IV)-oxidizing microorganisms presents significant challenges to minimizing uranium mobility in anoxic, aqueous systems, with U(IV) in soils/sediments.
To date, various studies have documented the subsequent reoxidation of U(IV) in situ (9, 16, 17). A recent study conducted by Wu and colleagues demonstrated in situ reoxidation of U(IV) following the addition of nitrate (25). The authors attributed U(IV) oxidation to abiotic reduction by biogenic Fe(III). However, given the ubiquity of microorganisms oxidizing U(IV), direct oxidation is a plausible explanation. Specifically, Geothrix spp. were suggested to play a role in this environment (25), presumably oxidizing Fe(II) coupled to nitrate reduction. Here we demonstrated the ability of G. fermentans to directly oxidize U(IV) in the absence of Fe(II). Thus, the direct oxidation of U(IV) by the indigenous microbial community could also be operative in bioreduced regions, leading to the subsequent mobilization of uranium.
Supplementary Material
Acknowledgments
We acknowledge the aid of Joesfa de la Cruz, Philip M. Jardine, and David Watson in the successful completion of this work.
The research presented in the study was supported by grant number DE-FG02-07ER64390 from the Department of Energy Environmental Remediation Sciences Program, awarded to J.D.C., L.A.A., and K.A.W. Manuscript preparation was supported by NSF grant 0811250 (ADVANCE-Nebraska) to K.A.W.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 13 May 2011.
REFERENCES
- 1. Beller H. R. 2005. Anaerobic, nitrate-dependent oxidation of U(IV)oxide minerals by the chemolithoautotrophic bacterium Thiobacillus denitrificans. Appl. Environ. Microbiol. 71:2170–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brina R., Miller A. G. 1992. Direct detection of trace levels of uranium by laser-induced kinetic phosphorimetry. Anal. Chem. 64:1413–1418 [Google Scholar]
- 3. Brooks S. C. 2001. Waste characteristics of the former S-3 ponds and outline of uranium chemistry relevant to NABIR Field Research Center studies. Oak Ridge National Laboratory technical report ORNL/TM-2001/27. Oak Ridge National Laboratory, Oak Ridge, TN: http://www.osti.gov/bridge [Google Scholar]
- 4. Byrne-Bailey K. G., et al. 2010. Completed genome sequence of the anaerobic iron-oxidizing bacterium Acidovorax ebreus strain TPSY. J. Bacteriol. 192:1475–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chaudhuri S. K., Lack J. G., Coates J. D. 2001. Biogenic magnetite formation through anaerobic biooxidation of Fe(II). Appl. Environ. Microbiol. 67:2844–2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chinni S., Anderson C. R., Ulrich K. U., Giammar D. E., Tebo B. M. 2008. Indirect UO2 oxidation by Mn(II)-oxidizing spores of Bacillus sp strain SG-1 and the effect of U and Mn concentrations. Environ. Sci. Technol. 42:8709–8714 [DOI] [PubMed] [Google Scholar]
- 7. Coates J. D., Ellis D. J., Gaw C. V., Lovley D. R. 1999. Geothrix fermentans gen. nov. sp. nov., a novel Fe(III)-reducing bacterium from a hydrocarbon-contaminated aquifer. Int. J. Syst. Bacteriol. 49:1615–1622 [DOI] [PubMed] [Google Scholar]
- 8. Elias D. A., Senko J. M., Krumholz L. R. 2003. A procedure for quantitation of total oxidized uranium for bioremediation studies. J. Microbiol. Methods 53:343–353 [DOI] [PubMed] [Google Scholar]
- 9. Finneran K. T., Housewright M. E., Lovley D. R. 2002. Multiple influences of nitrate on uranium solubility during bioremediation of uranium-contaminated subsurface sediments. Environ. Microbiol. 4:510–516 [DOI] [PubMed] [Google Scholar]
- 10. Ginder-Vogel M., Criddle C. S., Fendorf S. 2006. Thermodynamic constraints on the oxidation of biogenic UO2 by Fe(III) (hydr) oxides. Environ. Sci. Technol. 40:3544–3550 [DOI] [PubMed] [Google Scholar]
- 11. Komlos J., Peacock A., Kukkadapu R. K., Jaffe P. R. 2008. Long-term dynamics of uranium reduction/reoxidation under low sulfate conditions. Geochim. Cosmochim. Acta 72:3603–3615 [Google Scholar]
- 12. Lack J. G., et al. 2002. Immobilization of radionuclides and heavy metals through anaerobic bio-oxidation of Fe(II). Appl. Environ. Microbiol. 68:2704–2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Langmuir D. 1997. Aqueous environmental geochemistry. Prentice-Hall, Upper Saddle River, NJ [Google Scholar]
- 14. Lovley D. R. 1995. Bioremediation of organic and metal contaminants with dissimilatory metal reduction. J. Ind. Microbiol. 14:85–93 [DOI] [PubMed] [Google Scholar]
- 15. Lovley D. R. 1991. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol. Rev. 55:259–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moon H. S., Komlos J., Jaffe P. 2007. Uranium reoxidation in previously bioreduced sediment by dissolved oxygen and nitrate. Environ. Sci. Technol. 41:4587–4592 [DOI] [PubMed] [Google Scholar]
- 17. Senko J. M., Istok J. D., Suflita J. M., Krumholz L. R. 2002. In-situ evidence for uranium immobilization and remobilization. Environ. Sci. Technol. 36:1491–1496 [DOI] [PubMed] [Google Scholar]
- 18. Thrash J. C., et al. 2007. Electrochemical stimulation of microbial perchlorate reduction. Environ. Sci. Technol. 41:1740–1746 [DOI] [PubMed] [Google Scholar]
- 19. Waite T. D., Davis J. A., Payne T. E., Waychunas G. A., Xu N. 1994. Uranium(VI) adsorption to ferrihydrite: application of a surface complexation model. Geochim. Cosmochim. Acta 58:5464–5478 [Google Scholar]
- 20. Weber K. A., Coates J. D. 2007. Microbially-mediated anaerobic iron(II) oxidation at circumneutral pH, p. 1147–1154 In Hurst C. J., et al. (ed.), Manual of environmental microbiology. ASM Press, Washington, DC [Google Scholar]
- 21. Weber K. A., et al. 2009. Physiological and taxonomic description of the novel autotrophic, metal oxidizing bacterium, Pseudogulbenkiania sp. strain 2002. Appl. Microbiol. Biotechnol. 83:555–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weber K. A., et al. 2006. Anaerobic nitrate-dependent iron(II) bio-oxidation by a novel lithoautotrophic betaproteobacterium, strain 2002. Appl. Environ. Microbiol. 72:686–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weber K. A., Urrutia M. M., Churchill P. F., Kukkadapu R. K., Roden E. E. 2006. Anaerobic redox cycling of iron by freshwater sediment microorganisms. Environ. Microbiol. 8:100–113 [DOI] [PubMed] [Google Scholar]
- 24. Wilkins M. J., Livens F. R., Vaughan D. J., Beadle I., Lloyd J. R. 2007. The influence of microbial redox cycling on radionuclide mobility in the subsurface at a low-level radioactive waste storage site. Geobiology 5:293–301 [Google Scholar]
- 25. Wu W. M., et al. 2010. Effects of nitrate on the stability of uranium in a bioreduced region of the subsurface. Environ. Sci. Technol. 44:5104–5111 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.