Abstract
Streptococcus salivarius exhibited an anti-inflammatory effect on intestinal epithelial cells (IECs) and monocytes. Strains were screened using a reporter clone, HT-29/kB-luc-E, induced by tumor necrosis factor alpha (TNF-α). Supernatant from each strain downregulated NF-κB activation. The two most efficient strains produced an active metabolite (<3 kDa) which was able to downregulate the secretion of the proinflammatory chemokine interleukin-8 (IL-8).
TEXT
The intestinal microbiota consists of more than 1014 bacteria living in a symbiotic relationship with their host. Commensal microorganisms contribute to host health by supplying nutriment, preventing pathogen colonization, and maintaining intestinal homeostasis. The microbial inhabitants contribute to the maturation of the gastrointestinal tract and its immune system by shaping and maintaining normal mucosal immunity (20). These properties underline the existence of an extensive cross talk between the commensal bacteria and the gut mucosae to maintain beneficial relationships and tolerogenic host response (21). The intestinal epithelial cells (IECs) represent the first point of contact for bacteria within the gut, preventing microbial penetration and eliciting first communication for immune recognition of commensal bacteria. Keeping a balance between tolerant response and aberrant inflammation is the main goal of the cross talk between commensal bacteria and IECs in the digestive tract (1, 20).
In an inflammatory context, several commensal and probiotic bacteria have been shown to modulate mucosal innate immune response and reduce the inflammatory signaling cascade (7). Lactobacillus and Bifidobacterium species have been shown to reduce inflammatory responses, including NF-κB activation and interleukin-8 (IL-8) production, in various models of intestinal epithelial cells (2, 16, 28). Interestingly, the immunomodulation capacities of commensal species from the intestinal microbiota established on IECs are correlated with anti-inflammatory effects in vivo (6, 9, 12, 13, 25, 29). Although close contact between live commensal bacteria and eukaryotic cells leads to many biological activities, some secreted bacterial factors have been characterized as responsible for anti-inflammatory effect (8, 17). These bacterial products may be proposed as tools for prevention and/or treatment of human inflammatory bowel diseases.
Several mechanisms underlying the beneficial effects of commensal and probiotic bacteria identified in vitro involve a downregulation of the NF-κB-dependent transcriptional activity. NF-κB is a dimeric transcription factor whose activation is connected by signaling cascade to several receptors, including Toll-like receptors (TLRs). The different steps of the NF-κB signaling pathway represent potential targets for anti-inflammatory probiotic and commensal bacteria to weaken NF-κB activation and thereby prevent its transcriptional activity. An inhibition of the NF-κB pathway is observed with targeting genes involved in ubiquitination and proteasome processes by Lactobacillus casei anti-inflammatory effect (28), through interference with IκBα degradation of nonpathogenic Salmonella strain effect (14, 18), in the cellular phosphorylation step of the NF-κB pathway by interference with soluble factor of Bifidobacterium breve (9), and finally, in the nuclear export of NF-κB subunit RelA by Bacteroides thetaiotamicron (12).
The commensal bacterium Streptococcus salivarius is one of the early colonizers of oral mucosal surfaces a few hours after birth. This species remains prevalent in the oral cavity and subprevalent in the digestive tract throughout the life span and plays an important role in oral ecology. S. salivarius displays protective effects against pathogens involved in development of tooth decay and periodontitis (26, 27). It influences the inflammatory responses triggered by periodontopathogens and enteric pathogens (8, 24). Recently, the commensal strain Streptococcus salivarius K12 was shown to attenuate NF-κB activation, suggesting a role of this bacterium in inflammation (3). We investigated the regulatory effects of S. salivarius strains on the NF-κB pathway in human IECs. We used NF-κB reporter systems stably expressed in HT-29 (ATCC HTB-38) to analyze the effects of different strains of S. salivarius on NF-κB activation. Five repeats of the NF-κB binding site were cloned in the luciferase reporter plasmid pGL3 enhancer vector (Promega). The obtained plasmid was cotransfected with pTK-Hyg plasmid, a hygromycin selection vector (Clontech), in HT-29 cells using TFX-50 (Promega) according to the manufacturer's instructions. After 3 weeks under hygromycin (200 μg/ml), the HT-29/kB-luc-E clone was selected for its response to tumor necrosis factor alpha (TNF-α; 10 ng/ml; Peprotech). For each experiment, HT-29/kB-luc-E reporter cells were seeded at 50,000 cells per well into 96-well plates and incubated for 48 h in RPMI (Sigma) supplemented with 2 mM l-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 10% heat-inactivated fetal calf serum (FCS; Lonza) in a humidified 5% CO2 atmosphere at 37°C, before stimulation (6 h) with TNF-α (10 ng/ml). The supernatants (Sn) of 9 strains of Streptococcus vestibularis and 32 strains of S. salivarius were tested to determine whether the property to modulate inflammatory response was widely distributed in these species. The strains used in this work were previously described (5) and were grown on M17 (at 37°C) supplemented with glucose (0.5 g/liter). S. salivarius and S. vestibularis bacterial supernatants were collected by centrifugation of cultures and filtered through 0.22-μm filters. Stimulated cells were tested with each supernatant (10%, vol/vol) or with M17 medium adjusted to pH ∼5.5 (pH at the end of the bacterial culture) as a control. Luciferase activity was measured using the luciferase assay system (Promega) and a microplate reader (Infinite 200; Tecan). Relative luminescence units (RLU) are expressed as relative percentage of NF-κB activation compared to positive control, i.e., cells stimulated with growth medium plus the NF-κB activator.
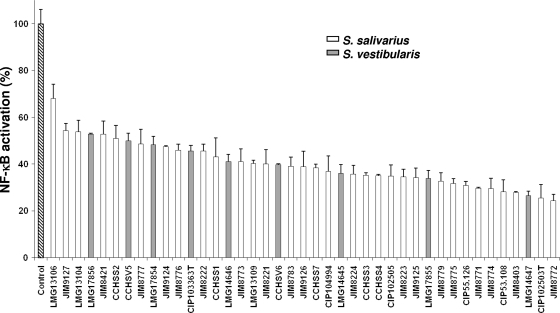
Supernatants of S. salivarius and S. vestibularis strains markedly inhibited TNF-α-induced NF-κB activation (Fig. 1). The inhibition rate, ranging from 30 to 70%, suggested that an active metabolite modulating the inflammatory response was produced and released in the culture medium. The supernatants of JIM8772 and CIP102503T strains, presenting higher activity, were selected to characterize the bacterial molecular factor involved in this modulation. We demonstrated by dilution of these two supernatants that the effect is dose dependent. Indeed, compared to the 70% inhibition obtained with the CIP102503T supernatant, inhibitions of 60%, 30%, and 20% of NF-κB were observed when the supernatant was diluted to 3/4, 1/2, and 1/4, respectively. Similar results were obtained using dilutions of JIM8772 supernatant (data not shown).
Fig. 1.
Effect of the supernatants (Sn) of different strains of S. salivarius and S. vestibularis on TNF-α-induced NF-κB activation in HT-29/kB-luc-E. Luciferase activity was measured after addition of Sn to HT-29/luc-E reporter cells in the presence of TNF-α (10 ng/ml). The control assay corresponds to luciferase activity obtained with HT-29/kB-luc-E reporter cells in the presence of M17 bacterial growth medium with adjusted pH and TNF-α (10 ng/ml). Results are expressed as relative percentages of NF-κB activation compared to the control assay (100%). Results are represented by means ± standard deviations of triplicate measurements from one representative experiment out of a minimum of three independent experiments performed. Data were analyzed by Student's t test (P < 0.05 compared to control for all tested supernatants). Results from S. salivarius (white bars) and S. vestibularis (gray bars) are classified by increasing inhibition strength.
Several experiments were then performed to characterize the nature of the active compound(s) released by S. salivarius. First, we ruled out that the inhibitory effect was due to bacterial metabolites such as butyric and lactic acid that have previously been shown to regulate the NF-κB pathway in several cell lines, including human IECs (10, 11, 22). High-pressure liquid chromatography (HPLC) measurement of organic acids revealed 80 mM lactic acid and the absence of butyrate in S. salivarius supernatant (data not shown). Consequently, we tested the effect of a wide range of concentrations of lactic acid (20 to 120 mM). At these concentrations, lactic acid did not affect NF-κB activation (data not shown). Lastly, S. salivarius supernatants had no effect on the baseline NF-κB activity and did not affect cell viability as controlled using the MTS assay (Promega) (data not shown).
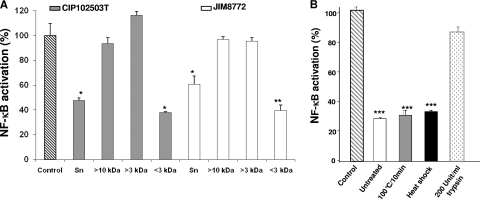
To determine the size and the nature of the active component(s) present in S. salivarius supernatant, we tested the inhibitory activity after passage through 10-kDa- and 3-kDa-cutoff columns. To facilitate the purification process, strains were grown on chemically defined medium (CDM) (23) supplemented with 0.5 g/liter ascorbic acid, 0.1 mM MgCl2, β-glycerophosphate (disodium salt, 6 g/liter), and glucose (0.5 g/liter). Bacterial supernatants were submitted to ultrafiltration through Centriplus YM-3 or YM-10 membranes that retain molecules larger than 3 or 10 kDa, respectively. For both strains, the <3-kDa fraction inhibited NF-κB activity by 60% while retained fractions (>10 kDa and >3 kDa) displayed no effect (Fig. 2 A). Furthermore, a trypsin treatment (200 U/ml; Mag-Trypsin; Ozyme) of the <3-kDa fraction resulted in a drastic loss of the inhibitory effect, with only ∼15% remaining inhibition (Fig. 2B). These results suggest that the partially purified active compound of a molecular mass lower than 3 kDa contained peptidic bonds. Exposure to high temperatures (100°C for 10 min) or to heat shock (100°C for 10 min before freezing in liquid nitrogen) did not affect the inhibitory potential. The compound's resistance to heat treatments confirmed that it is a small molecule that may not display a complex folding. Taken together, these results suggest that S. salivarius strains mediated their anti-inflammatory effects through the release of a low-molecular-weight component of peptidic nature.
Fig. 2.
Determination of molecular mass and nature of the active component secreted by S. salivarius. (A) Fractions were obtained after filtrations of S. salivarius CIP102503T Sn (gray bars) and JIM8772 Sn (white bars) after CDM growth through selective membranes. Retained fractions containing metabolites of >10 kDa and >3 kDa and filtered fraction containing metabolites of <3 kDa were tested for inhibition of NF-κB transcription activity in HT-29/kB-luc-E cells. (B) S. salivarius JIM8772 <3-kDa Sn was treated by exposure to high temperature (100°C for 10 min), to heat shock (100°C for 10 min before freezing in liquid nitrogen), or to trypsin hydrolysis (200 U/ml). Results are from one representative out of at least three independent experiments. P values were <0.05 (*), <0.025 (**), or <0.01 (***) compared to control for the two tested strains. Bars represent standard deviations of the means.
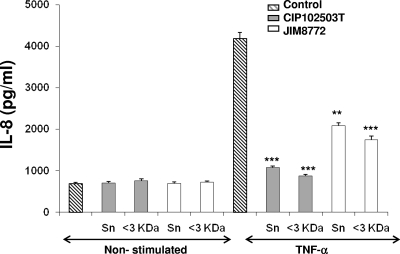
The inhibitory effects of the S. salivarius supernatant on the NF-κB pathway were confirmed on the production of interleukin-8 (IL-8) as assayed by enzyme-linked immunosorbent assay (ELISA; Eli-Pair; Diaclone). Both JIM8772 and CIP102503T supernatants and their corresponding <3-kDa fractions provoked a drastic decrease of IL-8 secretion induced by TNF-α (Fig. 3). The inhibitory effects were similar between raw and fractionated supernatants, with inhibition rates of 75% and 55% for CIP102503T and JIM8772, respectively.
Fig. 3.
Effect of S. salivarius supernatant (Sn) on IL-8 secretion by HT-29/kB-luc-E cells. HT-29 reporter cells were incubated with S. salivarius CIP102503T and JIM8772 Sn and the corresponding <3-kDa Sn of both strains after CDM growth, in the presence or absence of TNF-α (10 ng/ml). Results are from one representative out of three independent experiments. P values were <0.05 (*), <0.025 (**), or <0.01 (***) compared to control for the two tested strains. Bars represent standard deviations of the means.
JIM8772 and CIP102503T <3-kDa supernatants were also tested on NF-κB activation in the monocyte-like cell clone THP-1 blue (Invivogen) and the colonic epithelial cell clone Caco-2/kB-seap-7 (15), both bearing an NF-κB reporter system with secreted alkaline phosphatase (SEAP) as reporter gene. SEAP was revealed using Quanti-Blue reagent (Invivogen). JIM8772 and CIP102503T <3-kDa fraction supernatant led to 40% and 20% inhibition of NF-κB activity on THP-1 reporter cells after activation with lipopolysaccharide (LPS) or TNF-α, respectively (see Fig. S1A in the supplemental material). Similarly, the two fractions induced a 60% inhibition of NF-κB activity in Caco-2/kB-seap-7 cells stimulated with IL-1β (see Fig. 1B in the supplemental material). Thus, the effect of S. salivarius supernatant is not restricted to epithelial cells but is also observed on monocytic cells. We confirmed the recent studies that have highlighted anti-inflammatory properties of live S. salivarius strains in vitro. An S. salivarius strain was shown to weaken IL-8 production induced by the periodontopathogen Aggregatibacter actinomycetemcomitans on human oral epithelial cells (24). Furthermore, S. salivarius reduced NF-κB activation and IL-8 production triggered by Yersinia enterocolitica in HT-29 cells (8) and by Pseudomonas aeruginosa or flagellin in a human bronchial epithelial cell line as well as primary cultures of keratinocytes (3). Beneficial effects on inflammatory response and maintenance of intestinal homeostasis were reported for Streptococcus thermophilus, a dairy probiotic bacterium and the third known species belonging to the salivarius group (17, 19). Therefore, these results together with our study suggest that species belonging to the streptococcal salivarius group are presenting beneficial effects on host inflammatory processes. Their phylogenetic nearness suggests that they may share a related metabolite production pathway inherited from their common ancestor (4).
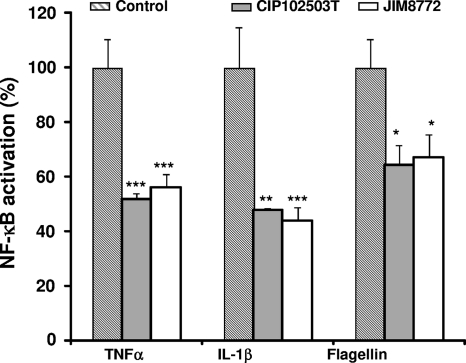
Finally, we showed that the supernatant of S. salivarius also inhibited NF-κB activity induced in HT-29 cells with another proinflammatory cytokine, IL-1β, and the TLR5 ligand, flagellin (Fig. 4). Thus, the target of the inhibitory compound seemed localized downstream of the receptors and at a step common to all tested TNF, IL-1, and TLR5 receptors.
Fig. 4.
Effect of S. salivarius Sn on NF-κB activation in HT-29/kB-luc-E induced by different inducers. Sn fractions (<3 kDa) from S. salivarius JIM8772 (white bars) and CIP102503T (gray bars) after CDM growth were added to the HT-29/kB-luc-E cells induced by TNF-α (10 ng/ml), IL-1β (10 ng/ml), or flagellin (10 μg/ml). Results are from one representative out of three independent experiments. P values were <0.05 (*), <0.025 (**), or <0.01 (***) compared to control for the two tested strains. Bars represent standard deviations of the means.
In summary, we have shown that all strains from a representative collection of S. salivarius and S. vestibularis strains inhibited the activation of NF-κB. We partially purified a low-molecular-weight metabolite from S. salivarius supernatant that harbored anti-inflammatory properties in vitro on IECs as well as immune cells. Thus, S. salivarius strains are involved in the molecular cross talk with beneficial potential for the host mucosal immune system. The precise characterization of the active molecule and its mechanism of action will facilitate the development of promising therapeutic strategies for inflammatory disorders, such as the safe use of this metabolite as a drug or the use of S. salivarius as a probiotic in oral and intestinal pathologies.
Supplementary Material
Acknowledgments
This work was supported by the Institut National de la Recherche Agronomique and by the EU MetaHit (Metagenomics of the Human Intestinal Tract) project HEALTH-F4-2007-201052.
We thank Pascal Courtin for the HPLC analysis of organic acids.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 20 May 2011.
REFERENCES
- 1. Artis D. 2008. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 8:411–420 [DOI] [PubMed] [Google Scholar]
- 2. Candela M., et al. 2008. Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int. J. Food Microbiol. 125:286–292 [DOI] [PubMed] [Google Scholar]
- 3. Cosseau C., et al. 2008. The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect. Immun. 76:4163–4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Delorme C., Bartholini C., Bolotine A., Ehrlich S. D., Renault P. 2010. Emergence of a cell wall protease in the Streptococcus thermophilus population. Appl. Environ. Microbiol. 76:451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Delorme C., Poyart C., Ehrlich S. D., Renault P. 2007. Extent of horizontal gene transfer in evolution of streptococci of the salivarius group. J. Bacteriol. 189:1330–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ewaschuk J. B., et al. 2008. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 295:G1025–G1034 [DOI] [PubMed] [Google Scholar]
- 7. Ewaschuk J. B., Dieleman L. A. 2006. Probiotics and prebiotics in chronic inflammatory bowel diseases. World J. Gastroenterol. 12:5941–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frick J. S., et al. 2007. Identification of commensal bacterial strains that modulate Yersinia enterocolitica and dextran sodium sulfate-induced inflammatory responses: implications for the development of probiotics. Infect. Immun. 75:3490–3497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heuvelin E., et al. 2009. Mechanisms involved in alleviation of intestinal inflammation by Bifidobacterium breve soluble factors. PLoS One 4:e5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Inan M. S., et al. 2000. The luminal short-chain fatty acid butyrate modulates NF-kappaB activity in a human colonic epithelial cell line. Gastroenterology 118:724–734 [DOI] [PubMed] [Google Scholar]
- 11. Kellum J. A., Song M., Li J. 2004. Lactic and hydrochloric acids induce different patterns of inflammatory response in LPS-stimulated RAW 264.7 cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286:R686–R692 [DOI] [PubMed] [Google Scholar]
- 12. Kelly D., et al. 2004. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat. Immunol. 5:104–112 [DOI] [PubMed] [Google Scholar]
- 13. Kim S. W., et al. 2010. Bifidobacterium lactis inhibits NF-kappaB in intestinal epithelial cells and prevents acute colitis and colitis-associated colon cancer in mice. Inflamm. Bowel Dis. 16:1514–1525 [DOI] [PubMed] [Google Scholar]
- 14. Kumar A., et al. 2007. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. EMBO J. 26:4457–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lakhdari O., et al. 2010. Functional metagenomics: a high throughput screening method to decipher microbiota-driven NF-kappaB modulation in the human gut. PLoS One 5:e13092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin P. W., et al. 2009. Lactobacillus rhamnosus blocks inflammatory signaling in vivo via reactive oxygen species generation. Free Radic. Biol. Med. 47:1205–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Menard S., et al. 2004. Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut 53:821–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neish A. S., et al. 2000. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science 289:1560–1563 [DOI] [PubMed] [Google Scholar]
- 19. Resta-Lenert S., Barrett K. E. 2003. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 52:988–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Round J. L., Mazmanian S. K. 2009. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9:313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Round J. L., O'Connell R. M., Mazmanian S. K. 2010. Coordination of tolerogenic immune responses by the commensal microbiota. J. Autoimmun. 34:J220–J225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Segain J. P., et al. 2000. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn's disease. Gut 47:397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sissler M., et al. 1999. An aminoacyl-tRNA synthetase paralog with a catalytic role in histidine biosynthesis. Proc. Natl. Acad. Sci. U. S. A. 96:8985–8990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sliepen I., et al. 2009. Microbial interactions influence inflammatory host cell responses. J. Dent. Res. 88:1026–1030 [DOI] [PubMed] [Google Scholar]
- 25. Sokol H., et al. 2008. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. U. S. A. 105:16731–16736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tanzer J. M., Kurasz A. B., Clive J. 1985. Competitive displacement of mutans streptococci and inhibition of tooth decay by Streptococcus salivarius TOVE-R. Infect. Immun. 48:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Teughels W., et al. 2007. Bacteria interfere with A. actinomycetemcomitans colonization. J. Dent. Res. 86:611–617 [DOI] [PubMed] [Google Scholar]
- 28. Tien M. T., et al. 2006. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J. Immunol. 176:1228–1237 [DOI] [PubMed] [Google Scholar]
- 29. Zoumpopoulou G., et al. 2008. Lactobacillus fermentum ACA-DC 179 displays probiotic potential in vitro and protects against trinitrobenzene sulfonic acid (TNBS)-induced colitis and Salmonella infection in murine models. Int. J. Food Microbiol. 121:18–26 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.