Abstract
We previously described a marine, tellurite-resistant strain of the yeast Rhodotorula mucilaginosa that both precipitates intracellular Te(0) and volatilizes methylated Te compounds when grown in the presence of the oxyanion tellurite. The uses of microbes as a “green” route for the production of Te(0)-containing nanostructures and for the remediation of Te-oxyanion wastes have great potential, and so a more thorough understanding of this process is required. Here, Te precipitation and volatilization catalyzed by R. mucilaginosa were examined in continuously aerated and sealed (low oxygen concentration) batch cultures. Continuous aeration was found to strongly promote Te volatilization while inhibiting Te(0) precipitation. This differs from the results in sealed batch cultures, for which tellurite reduction to Te(0) was found to be very efficient. We show also that volatile Te species may be degraded rapidly in medium and converted to the particulate form by biological activity. Further experiments revealed that Te(0) precipitates produced by R. mucilaginosa can be further transformed to volatile and dissolved Te species. However, it was not clearly determined whether Te(0) is a required intermediate for Te volatilization. Based on these results, we conclude that low oxygen concentrations will be the most efficient for production of Te(0) nanoparticles while limiting the production of toxic volatile Te species, although the production of these compounds may never be completely eliminated.
INTRODUCTION
Tellurium (Te) is a group VIa/16 element that is extensively used in various industries, including metallurgy, electronics, and applied chemical industries. In nature Te exists in four oxidation states: +VI, +IV, 0, and −II. Microbial interactions with Te have been increasingly well documented in recent years, as recently reviewed by Zannoni et al. (41) and Chasteen et al. (9), and there has been interest in the microbial production of Te-containing nanoparticles (3) for applications such as composite or compound nanomaterials in solar cells and as an alternative for bench-scale syntheses. We recently described (26) a series of obligately aerobic, tellurite-resistant microbes isolated from salt marsh sediments that efficiently produce intracellular Te-containing nanostructures in a complex growth medium without any specialized growth requirements. The same strains also volatilized a small proportion of the supplied Te as methylated species. Here we further describe the interactions of one of these organisms, the yeast Rhodotorula mucilaginosa, with Te and how these interactions are modulated by aeration.
In aerobic soils and aquatic environments, tellurium occurs primarily as the tellurite [TeO32− or Te(IV)] and tellurate [TeO42− or Te(VI)] oxyanions. While tellurite is the most water soluble, both are freely available to living organisms. Tellurite has been employed as an antimicrobial therapeutic agent (31, 34), and it is about 10 times more toxic to both Gram-positive and Gram-negative microorganisms than tellurate (37, 39). Tellurite toxicity may be related to its activity as a strong oxidizing agent toward biological materials (17, 25, 34), although some disagree with this model (40).
Microbial tellurite resistance is a widespread phenomenon. In most environments sampled to date, tellurite-resistant organisms comprise ∼10% of the total culturable microbial population (26, 28, 32, 34). Resistance to tellurite has been reported in a few Gram-positive bacteria (2, 26, 33) and in a number of Gram-negative Proteobacteria (1, 7, 10, 13, 22, 27, 38). To date, all tellurite-resistant microbes reduce tellurite to Te(0) (26, 34), and some have been documented to produce volatile, methylated Te species (5, 33). Reduction and methylation is thought to be a protective mechanism used by microorganisms to detoxify Te oxyanions. The previously described salt marsh isolates (26), including strains of R. mucilaginosa, behave similarly and precipitate greater quantities of Te than do representative Gram-negative bacteria.
Here we report the effect of aeration on the precipitation and volatilization of Te by R. mucilaginosa and our efforts to determine the fate of precipitated Te(0) in order to elucidate Te transformation pathways. Tellurite reduction to Te(0) was more efficient in sealed batch cultures than in continuously aerated batch cultures. Conversely, the continuously aerated cultures were found to volatilize much greater amounts of Te than those in sealed vessels. We further demonstrated that cell-associated Te-containing precipitates can be further metabolized to volatile Te species. These results have implications for the application of R. mucilaginosa or other Te(0)-precipitating microbes for industrial and remediation applications.
MATERIALS AND METHODS
Media and reagents.
Chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO), Fisher Scientific (Pittsburgh, PA), or VWR Scientific (West Chester, PA) and were of the highest grade available. High-purity HNO3 was prepared from trace metal-grade HNO3 in a quartz subboiler (Quartz & Silice, Courbevoie, France). The growth medium for all experiments was LB-marine prepared as previously described (26). The medium was solidified with 1.5% (wt/vol) biotechnology-grade agar (Fisher Scientific) for colony isolation. For nonaerated liquid cultures, 2 ml of medium was placed in sealed 18-ml headspace vials (Supelco, Bellefonte, PA) with the indicated concentrations of tellurite and an initial headspace of room air at ambient pressure. Individual cultures were sacrificed at the indicated time points to quantify both precipitated and soluble Te species, with the volatile fraction determined by the difference. Special attention was given to short incubation times (between 24 and 96 h of incubation).
Growth with continuous aeration.
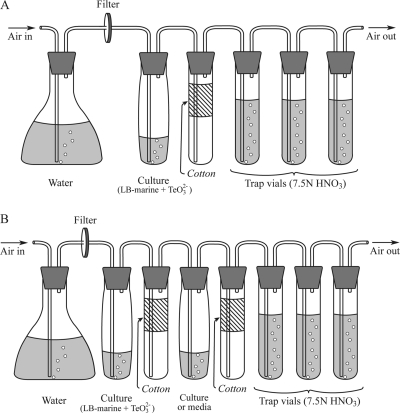
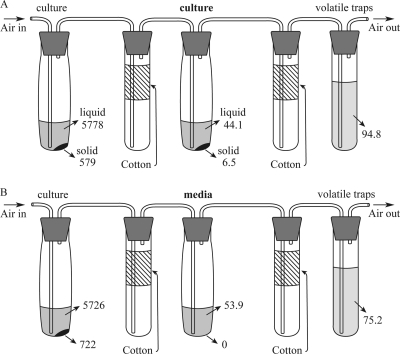
A batch culture system employing continuous aeration along with trapping of volatile compounds was developed based on a system previously described by Momoshima et al. (24) for trapping volatile polonium species. Cultures (10 ml) were immersed in a 30°C water bath and connected to a downstream series of tubes by Teflon tubing passed through silicone rubber stoppers (Fig. 1 A). During culturing, room air filtered through a 0.20-μm membrane filter (Millipore, Billerica, MA) was bubbled into the culture solution to move headspace gas through the system. The volumetric airflow rate was 1.4 ± 0.2 ml min−1, which produced a residence time of 18 ± 2 min for the headspace above the culture tubes. The air stream was then passed through a cotton layer (ca. 7 cm in length) in a test tube to remove aerosol droplets and then introduced to three successive 20-ml glass vials containing 10 ml of 7.5 N HNO3 to trap volatile Te compounds (Fig. 1A). The highest quantity of Te was always observed in the first trap; less than 2% of the Te measured in the first trap was found in the second trap, and no Te was found in the final trap. No Te was recovered from any of the traps when sterile medium was used instead of culture in the first tube of the system.
Fig. 1.
Culture apparatus for growth of R. mucilaginosa with continuous aeration while trapping volatile Te compounds. Cultures were maintained at 30°C in a water bath, and all other components were at room temperature.
Quantification of solid and dissolved tellurium species.
Tellurium content in liquid and solid phases of samples was determined by graphite furnace atomic absorption spectroscopy as described by Ollivier et al. (26). Samples from the dissolved or pellet (solid) fractions were diluted by factors ranging from 5- to 12,000-fold in order to obtain tellurium concentrations between 0.2 and 0.8 μM. Samples containing no added tellurium were analyzed with each batch of samples to ensure that minimal contamination was introduced by laboratory operations. In addition, sterile controls in medium amended with tellurite were incubated to determinate Te precipitation due to abiotic reactions. No Te precipitates were found, suggesting that abiotic reactions are not involved in production of these compounds.
Quantification and speciation of volatile tellurium species.
Tellurium content in trap samples of cultures grown with continuous aeration was determined by graphite furnace atomic absorption spectrometry as above. Trap samples were evaporated to dryness at 90 to 95°C. The residue was dissolved with subboiled HNO3 (a high-purity grade of trace metal-free HNO3), dried at 90 to 95°C, and redissolved in 5% (vol/vol) HNO3. Tellurium was never detected in traps connected to sterile tellurite-amended medium, indicating that Te volatilzation was strictly dependent on the presence of cultures. For cultures grown in sealed vials, the headspace above cultures was sampled with a manual solid-phase microextraction (SPME) holder using a 75-μm carboxen-polydimethylsiloxane fiber (Supelco, Bellefonte, PA) and analyzed by gas chromatography-mass spectrometry as described by Ollivier et al. (26). This extraction method (SPME) is semiquantitative due to the gas transfer from media during sampling and to the equilibration time between the sample and the fiber coating, which may be excessively long. The peaks of dimethyltelluride (DMTe) and dimethylditelluride (DMDTe) displayed retention times of approximately 2.5 to 3.7 and 16.6 to 19.3 min, respectively, and their identities were confirmed by the natural isotopic abundance imparted to mass spectra of these compounds by Te, in comparison with an ∼98% pure standard (see Fig. 3 in Ollivier et al. [26]). Gas chromatography-mass spectrometry (GC-MS) analysis of the headspace above sterile controls did not detect any volatile tellurium species.
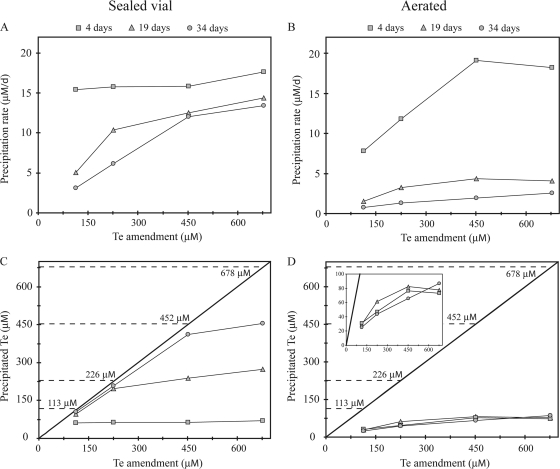
Fig. 3.
Tellurium precipitation dynamics in sealed (A and C) and aerated (B and D) cultures of R. mucilaginosa. (A and B) The relationship between precipitation rate, duration of culture, and the initial concentration of tellurite amended. (C and D) Comparison of tellurium precipitated versus tellurium amended over time clearly indicates that in sealed cultures (C), the extent of tellurium precipitation is governed by the total available tellurite, while aerated cultures (D) do not become tellurite limited, as delineated in the insert, which shows data for 0 to 100 μM precipitated Te.
Production of volatile Te from particulate Te.
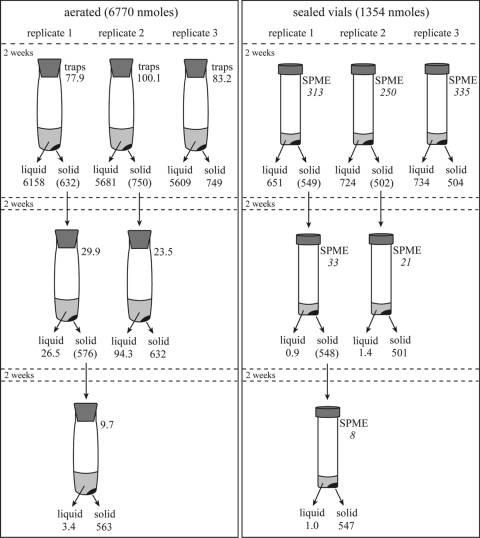
Three replicate 2-ml cultures (in sealed vials) and 10-ml cultures (in the aeration train) (Fig. 1A) of strain 13B were amended with 0.68 mM tellurite and incubated. After a 2-week period of Te(0) accumulation, the volatile traps were analyzed for Te and replaced with fresh traps for the aerated cultures, while the sealed culture headspace was analyzed by SPME-GC-MS. All cultures were then centrifuged (9,000 × g, 25 min), and the supernatant transferred to a Teflon beaker to analyze the soluble fraction of Te. The cell pellet was washed by resuspension with Te-free medium, and cells were collected again by centrifugation. One of the cultures from each condition was sacrificed to determine the amount of Te(0) produced under each condition. An appropriate volume of Te-free medium (2 ml and 10 ml for sealed and aerated samples, respectively) was then added to the remaining cell pellets, and cultures were incubated under the same conditions for another 2 weeks, at which point one culture from each condition was sacrificed to determine the Te in each fraction. The final culture was sacrificed after 2 weeks of further incubation, a total of 6 weeks, 4 of which were in Te-free medium.
To test if Te(0) produced in sealed vials could be further metabolized under aerated conditions, 2-ml cultures of strain 13B were grown in sealed vials. After a 2-week period of Te(0) accumulation, cultures were centrifuged (9,000 × g, 25 min) and the supernatant transferred to a Teflon beaker to analyze the soluble fraction of Te. The cell pellet was washed by resuspension with sterile Te-free medium. Cells were then collected again by centrifugation, followed by the addition of 10 ml of sterile Te-free medium. These cells were incubated in the aerated system for another 2 weeks, at which point the amounts of Te in the precipitate, dissolved, and volatile fractions were assayed as described above.
Production of dissolved and particulate Te species from volatile Te.
A modified aerated culture apparatus (Fig. 1B) was employed to determine whether or not cultures could consume volatile Te species and the products of consumption. An additional tube was inserted in the vapor train immediately downstream of the first cotton aerosol trap tube. The additional tube contained either sterile Te-free LB-marine medium or cultures of R. mucilaginosa in Te-free LB-marine medium. After incubation, the contents of this trap tube were analyzed for dissolved and particulate Te species as described above, as were the source cultures and vapor traps.
RESULTS
Fate of Te in aerated versus sealed cultures of R. mucilaginosa.
Previously, we reported cultures of aerobic, tellurite-resistant marine microbes precipitated most of the supplied tellurite as intracellular Te-containing nanostructures (26). However, these experiments were carried out in sealed headspace vials that likely resulted in a steady decrease in oxygen concentration over time, which may have strongly affected the physiology of the obligately aerobic microbial strains used (26). Therefore, we employed a system for culturing the strains with continuous aeration that would allow quantification of dissolved, particulate, and volatile species of Te (Fig. 1A). Strain 13B, an isolate of the yeast R. mucilaginosa, appeared to be the most proficient for Te precipitation and volatilization in the sealed vial system (26) and was utilized for all experiments reported here.
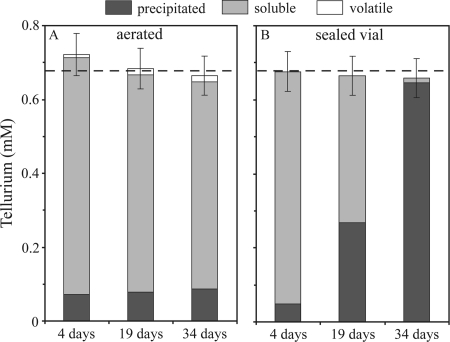
Volatile, dissolved, and precipitated Te species were quantified in aerated cultures of R. mucilaginosa at time points ranging from 4 days to 5 weeks (Fig. 2 A). Volatilization of tellurium appeared to progressively increase with time (up to 2 weeks of growth), resulting in the conversion of 2.8% of the supplied tellurite to volatile species, while precipitated Te species remained constant at ∼12% of the supplied tellurite over 5 weeks of culture (Fig. 2A). This pattern is markedly different from how R. mucilaginosa behaved in sealed vial cultures, in which precipitated Te progressively increased with time until 98% of the supplied tellurite was precipitated after 5 weeks (Fig. 2B) (26). It is noteworthy that, in the aerated system, Te precipitation was rapid but not instantaneous. Indeed, after 1 day of incubation, 25% (18.8 nM) of the maximum Te that could be converted to the particulate form (75.2 nM) was already in the solid phase. No volatile Te species were detected when sterile medium was used instead of cultures in the aerated system (data not shown).
Fig. 2.
Soluble, particulate, and volatile Te for Rhodotorula mucilaginosa strain 13B cultures growing with continuous aeration (A) or in sealed vials (B). Volatile Te compounds above cultures growing in sealed vials do not appear here because they were not quantified (see text for details). A total of 0.68 mM tellurite was added to cultures (denoted by the dashed lines). Errors bars indicate the standard deviations from three independent cultures for each condition. The data in panel B originally appeared in Ollivier et al. (26).
Concentration-rate dependence and order of Te species appearance with R. mucilaginosa.
We examined the influence of tellurite concentration on rates of Te conversion and fate of Te in cultures of R. mucilaginosa grown with continuous aeration or in sealed culture vials over a 5-week time course. In sealed vials, the initial integrated rate of Te precipitation measured at day 4 was 16.2 μM day−1, independent of the tellurite concentration (Fig. 3 A). Beyond 19 days of growth, the Te precipitation rate decreased dramatically in cultures with lower tellurite concentrations, which was clearly due to exhaustion of the supplied tellurite (Fig. 3C): 96% and 97% of 113 μM and 226 μM tellurite had been converted to the particulate form at this time, while only 58% and 47% of 452 μM and 678 μM tellurite was precipitated (Fig. 3C). Thus, in sealed vials, it appeared that the Te precipitation rate was constant as long as sufficient tellurite was available. Based on the rate of 16.2 μM Te day−1, we calculated that the total amended Te would be completely precipitated after 7 days, 14 days, 28 days, and 42 days in cultures amended with 113 μM, 226 μM, 452 μM, and 678 μM tellurite, which was in excellent agreement with our observations. In contrast, the initial Te precipitation rate in continuously aerated cultures was strongly dependent on the tellurite concentration (Fig. 3B). Note that at high tellurite concentrations (452 μM and 678 μM), the initial Te precipitation rate in aerated cultures, 17.9 μM day−1, was similar to that in the sealed vial cultures. However, the precipitation rate decreased in aerated cultures to essentially zero after 4 days of growth at all tellurite concentrations, resulting in the precipitation of only 10% to 28% of the supplied tellurite.
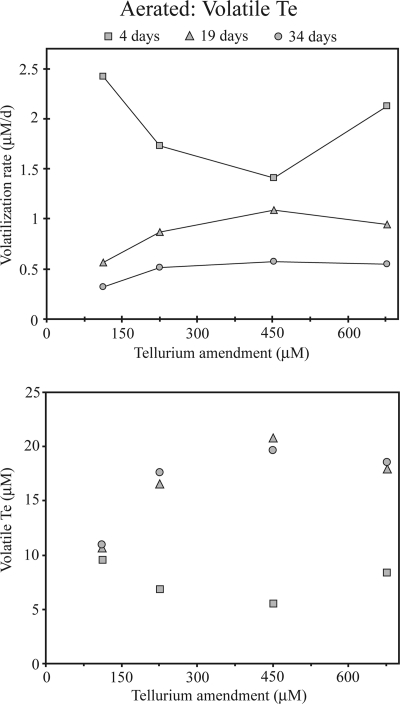
With respect to volatile Te species in aerated cultures, their production rate through day 4 was low and not dependent on tellurite concentration (Fig. 4). Between 4 days and 19 days of growth, the amount of volatile Te in headspace samples above cultures increased by 2- to 4-fold, depending on the concentration of supplied tellurite, except for cultures amended with 113 μM tellurite, which showed little increase. Little production of volatile species was observed after day 19.
Fig. 4.
Effect of initial tellurite concentration on the production of volatile Te above cultures inoculated with R. mucilaginosa 13B after 4 days, 19 days, and 34 days of growth in the aerated system.
Conversion of Te precipitates to volatile Te species by R. mucilaginosa.
The observation that aerated cultures of strain 13B still displayed a significant rate of Te volatilization after 4 days of incubation while the rate of Te precipitation decreased dramatically may indicate that precipitated Te is a substrate in the pathway of volatile Te production. This hypothesis was tested in both aerated and sealed cultures by allowing R. mucilaginosa to precipitate Te followed by transfer of the Te(0)-containing cells to fresh Te-free medium and following Te species during continued incubation (Fig. 5).
Fig. 5.
The production of volatile and dissolved Te species from intracellular Te(0) by R. mucilaginosa strain 13B. The amounts of Te (in nmol) found in the precipitated (solid), dissolved (liquid), and volatile (traps) fractions after the indicated incubation times are noted. Values in italics are the relative amounts of DMTe (as the integrated DMTe count [×106]) in the headspace sample, determined by SPME-GC-MS analysis. Values in parentheses are the amounts of precipitated Te, calculated by adding all measurements for each replicate coming from this precipitate in the following experiments. The mass balance observed was 98% + 3%, indicating that all pools of Te were recovered. In sealed vials, the volatile Te could not determined at the nanomolar level, and these vials were thus not included in the calculation. The difference in the total amended tellurite is due to the difference in volume between the aerated (10 ml) and sealed (2 ml) culture volumes.
During the 2-week Te precipitation phase, all samples produced volatile Te (at a rate in the aerated samples of 2.4 μM Te day−1) and precipitated Te(0) at similar rates to those above (aerated, 0.54 μM day−1; sealed, 18.0 μM Te day−1), although most of the Te was recovered from the soluble fraction (Fig. 5, first row). The sealed vial cultures produced primarily DMTe followed by dimethylditelluride DMDTe and methylated sulfur compounds, primarily dimethyldisulfide (DMDS), but also dimethylsulfide (DMS) and dimethyltrisulfide (DMTS) and the mixed species dimethyltellurenyl sulfide (DMTeS). In the 2 weeks following the transfer to Te-free medium (Fig. 5, second row), the rate of Te volatilization decreased by a factor of 10 (aerated, 0.2 μM Te day−1) and decreased again by ∼3-fold following a second transfer to fresh Te-free medium (Fig. 5, third row; rate for aerated culture, 0.07 μM Te day−1). Even though the SPME-GC-MS data from the sealed vials cannot be directly quantified to derive a specific volatilization rate, the intensity of the DMTe peak decayed by 10-fold and 4-fold (Fig. 5, values in italics) during the same transfer regimen as used for the aerated cultures, suggesting that the volatilization rate in sealed cultures similarly decreased during the incubations. The volatile species produced in the sealed incubations were DMTe and the same volatile sulfur compounds as above, while DMDTe and DMTeS were not detected.
Dissolved Te species were also produced in both the aerated and sealed incubations (Fig. 5, second and third rows). The rates in the first Te-free transfer (Fig. 5, second row) were 0.43 μM Te day−1 in aerated and 0.04 μM Te day−1 in sealed incubations. The rate in aerated cultures dramatically decreased in the second Te-free incubation (Fig. 5, third row) to 0.02 μM Te day−1, while the Te(0) dissolution rate in the sealed incubation was constant at 0.04 μM Te day−1.
A final experiment examined whether Te precipitated in sealed cultures of R. mucliaginosa was converted to volatile or dissolved species following transfer to aerated conditions. R. mucilaginosa was cultured in a 2-ml sealed vial with 0.67 mM tellurite (i.e., 1,354 μmol total Te) for 2 weeks, at which point the cells were harvested and transferred to 10 ml Te-free medium and incubated with aeration for 2 weeks. Following 2 weeks of incubation with aeration, 391 μmol Te (80%) was found in the precipitate fraction and 82.2 μmol Te (17%) was found in the dissolved fraction, with 12.6 μmol Te found in the volatile fraction (3%). This corresponds to a volatilization rate of 0.09 μM Te day−1, or half the initial Te volatilization rate observed in cells allowed to accumulate Te(0) under aerated conditions and then transferred to Te-free medium (aerated rate, = 0.2 μM Te day−1) (Fig. 5, second row).
Conversion of volatile Te species by R. mucilaginosa.
The prior experiments suggested that there may be a route for the conversion of either precipitated or volatile Te species to a dissolved species that is favored in aerated cultures. To test for the conversion of volatile species, a modified aerated culture apparatus was constructed so that strains could be exposed to volatile Te species produced by the same culture in otherwise-Te-free medium (Fig. 1B and 6). The ability of cultures to produce dissolved and precipitated Te species over 14 days was compared to sterile LB-marine medium. The strains behaved as expected in the tellurite-amended medium in the first tube of the culturing apparatus that was used to produce volatile Te species. Strain 13B produced 129 to 145 nmol of volatile Te, of which 42% was captured in cell-free medium as dissolved species, with no precipitated Te species observed. In contrast, when cells of strain 13B were present, only 30% of the total volatile Te was converted to dissolved species, along with another 6% recovered as precipitated Te species. The fact that the dissolved species of Te were consistently lower when cells of either strain were present strongly argues that the conversion of volatile Te to dissolved species is an abiotic reaction. However, the precipitation of Te from either volatile or dissolved species, which this experiment cannot distinguish, requires the presence of viable cells.
Fig. 6.
The production of dissolved Te and precipitated Te from volatile Te in tubes containing either cultures of R. mucilaginosa in Te-free LB-marine medium (A) or sterile Te-free LB-marine medium (B). The amounts of Te (in nmol) found in the precipitated (solid), dissolved (liquid), and volatile (traps) fractions are noted. See the text for details.
DISCUSSION
Here we have further documented the processes of tellurium precipitation and volatilization by the tellurite-resistant marine yeast R. mucilaginosa. Strain 13B precipitates Te(0) as intracellular bundles of needles when grown in the presence of tellurite (26). Tellurium-containing nanoparticles have attracted considerable interest because of their semiconductor [i.e., Te(0)] or fluorescence properties (i.e., CdTe or CdSe quantum dots) (4, 6, 8, 16, 19). “Green” chemical methods are being sought for the synthetic manufacture of these materials (23).
R. mucilaginosa precipitated greater quantities of tellurium nanoparticles in sealed vials than when cultured in a continuously aerated system. In sealed vials, elemental Te precipitates were a major end product of tellurite metabolism (98% of tellurium added after 5 weeks growth) for R. mucilaginosa, while they represented only 12% of added tellurite after 5 weeks of growth in cultures growing in a continuously aerated system (Fig. 2). Little to no work on tellurite precipitation kinetics has been performed, but the observations detailed above broadly parallel those of induced anaerobic selenite reduction in the bacterium Shewanella oneidensis (22) and Rhodospirillum rubrum (21) and the strong inhibition of selenite precipitation by exposure of Stenotrophomonas maltophilia to aerobic conditions (14).
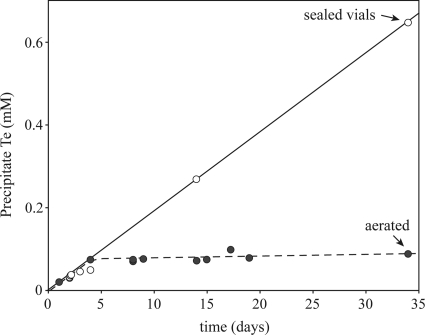
The precipitation of Te(0) was sustained over long periods in the batch cultures of the R. mucilaginosa cultures amended with 0.68 mM tellurite in sealed vials, but not in the aerated system (Fig. 7). The initial rate of Te(0) precipitation was similar in aerated and nonaerated cultures during the first 4 days of incubation. After this time, Te(0) precipitation appeared to stop in the aerated system while continuing at the same rate in sealed vials. This long-term persistence of the tellurite precipitation rate is unique relative to selenite precipitation with S. oneidensis, where Se(0) precipitation roughly parallels cell growth (22). Similarly, in R. rubrum, Se(0) precipitation was maximal during the transition between the logarithmic and stationary growth phases (21).
Fig. 7.
Time course of tellurium precipitation by Rhodotorula mucilaginosa 13B in the presence of 0.68 mM tellurite with continuous aeration or in sealed vials.
The production of toxic volatile Te species is of concern if R. mucilaginosa or other strains are to be employed for the production of nanocrystalline Te or the remediation of tellurite-containing wastes. Aerated cultures of R. mucilaginosa 13B volatilized up to 10% of added Te (as found for cultures amended with 113 μM tellurite) after only 4 days of growth (Fig. 4). Volatile tellurium increased between 4 days and 2 weeks of growth in the aerated system (Fig. 2 and 4), as previously described for cultures in sealed vials (26). However, after 2 weeks of growth, the amount of volatile Te remained constant above cultures growing in the aerated system, and it decreased by a factor of 4 above cultures growing in sealed vials. These observations suggest that cells can both produce and consume volatile Te species under nonaerated conditions. However, we cannot say if volatile Te species are directly or indirectly consumed, because some alkyl species may be degraded and dissolved in the medium. Indeed, it has been noted that the partitioning of DMSe between water and gaseous headspace in equilibrium (Henry's law constant) always favors the aqueous phase, with 10 to 20 times as much dissolved in solution as is found in the headspace at normal temperatures (18, 20, 35). The volatile Te produced under aerobic conditions would be less affected, because it is continually removed from cultures by airflow. The degradation of volatile Te in culture medium may also explain the decrease with time in the amount of volatile Te measured in the headspace in the sealed cultures. Previous work showed a degradation of DMDSe in aqueous solution before solution/gas phase equilibration could be reached (20). As illustrated in Fig. 6, we have shown here that 42% of total volatile Te compounds (129.1 nmol and 74.3 nmol) issued from cultures of Bacillus sp. strain 6A and R. mucilaginosa 13B amended with 0.68 mM tellurite is trapped into Te-free and cell-free medium. The chemical composition of soluble Te compounds was not determined, but because of the aerobic condition of the experiment, some of these compounds could be oxidation and/or hydrolysis products of volatile Te [DMTeO and DMTe(OH)2].
The production rate of volatile Te from cultures of strain 13B amended with 0.68 mM tellurite in the aerated system decreased with time, changing from 2.1 μM Te day−1 during the first 4 days of incubation to 0.6 μM Te day−1 between 4 days and 19 days of incubation, finally reaching <0.05 μM Te day−1 between 19 and 34 days of incubation (Fig. 4). R. mucilaginosa was thus more efficient at Te volatilization during the early phase of long-term incubations. Previous studies indicated that the rate of DMTe production by Pseudomonas fluorescens K27 was maximal as the culture reached stationary phase (5), and more recent work showed that volatile Te above cultures of Bacillus sp. STG-83 was produced during log phase (30). Our results are consistent with these reports, but a more thorough investigation of volatilization rates in short-term incubations is required to determine how Te volatilization is linked to cell growth in R. mucilaginosa.
Our experiments were equivocal about the pathway of volatile Te production, specifically whether or not Te(0) is a required intermediate. Transfer of cells containing Te(0) to fresh Te-free medium showed conclusively that volatile Te can be produced from Te(0), whether the Te(0) accumulated in aerated or sealed cultures. In phototrophic bacteria, Te(0) addition resulted in the production of methylated volatile Te above cultures of Rhodocyclus tenuis, Rhodospirillum rubrum S1, and Rhodospirillum rubrum G9 (36). Similarly, soil and sewage samples also convert Se(0) to volatile selenium compounds (12, 29), and the formation of DMSe by a soil Corynebacterium sp. was preceded by formation of Se(0) (11). Taken together with the observation that Te volatilization is prolonged relative to Te precipitation in aerated cultures, this suggests a model where the elemental species is an intermediate on the pathway of metalloid volatilization in widely divergent microbes.
However, other experiments are consistent with the direct volatilization of soluble Te species. Aeration promoted the formation of volatile species by R. mucilaginosa, as between 1.5% and 4% of precipitated Te(0) could be converted to volatile Te with the concurrent production of dissolved Te species. An explanation may be that the oxidation of Te(0) in an aerated system leads to increased availability of soluble species for volatilization. Kessi et al. (21) reported a slight increase in selenite concentration during the stationary phase for cultures of R. rubrum under aerobic conditions, which was interpreted to be due to the slow reoxidation of Se(0). Similarly, we observed that the conversion of intracellular particulate Te(0) to soluble Te in R. mucilaginosa was greater in aerated cultures [up to 12.6% of starting Te(0)] relative to nonaerated cultures [<0.3% of Te(0)]. The fact that the dissolution and volatilization of Te(0) declined with subsequent transfers suggests that two pools of Te(0) exist in these organisms: a small reactive pool and a larger stable pool that is a terminal product. Finally, only DMTe was observed as the product of volatile Te production from Te(0), whereas DMTe, DMTeS, and DMDTe were produced by R. mucilaginosa cultures containing both tellurite and Te(0). This difference in product spectrum under the two conditions may indicate that multiple pathways of Te volatilization operate in cells, depending on the available form of Te.
R. mucilaginosa volatilized approximately 2-fold less Te at a tellurite concentration of 113 μM than at 226 μM, 452 μM, or 678 μM after 5 weeks of growth in the aerated system. Cultures amended with 226 μM, 452 μM, and 678 μM tellurite displayed very similar amounts of volatile Te species, suggesting a maximum rate of volatilization of Te that could be directly related to the maximum capacity of strains to methylate Te (Fig. 4). This parallels work with Se in which a Corynebacterium sp. volatilized approximately 10-fold more Se at a selenite concentration of 0.63 mM than at 0.06 mM (12) and in which a euryhaline alga (Chlorella sp.) volatilized 6- to 10-fold more Se when the selenite concentration in cultures was increased from 10 μM to 1,300 μM (15).
While our experiments have not yet clearly delineated pathways of volatile Te production in R. mucilaginosa, they provide important insights regarding the role of aeration in controlling the balance between Te volatilization and precipitation. Given the observation that the precipitation rate for Te appears constant over at least 5 weeks in sealed batch cultures, we suggest that R. mucilaginosa may be an efficient and stable catalyst to precipitate Te nanoparticles in fed batch or batch processes without a requirement for precise oxygen tension control, perhaps by using a pulsed dose scheme as proposed by Baesman et al. (3). While these data clearly indicate that Te(0) can be converted to volatile Te, more detailed experiments will be required to determine if Te(0) is a required precursor for volatile Te formation and to compare tellurite metabolism in R. mucilaginosa to currently better-studied Gram negative bacteria systems.
Acknowledgements
This work was supported in part by a grant from the National Science Foundation (OCE-0425199 to T.M.C. and T.E.H.) and utilized common equipment provided in part by grant P20-RR116472-04 from the National Institutes of Health.
Footnotes
Published ahead of print on 20 May 2011.
REFERENCES
- 1. Alvazeri C., et al. 1997. Tellurite reductase activity of nitrate reductase is responsible for the basal resistance of Escherichia coli to tellurite. Microbiology 143: 1181–1189 [DOI] [PubMed] [Google Scholar]
- 2. Amoozegar M. A., et al. 2008. Isolation and initial characterization of the tellurite reducing moderately halophilic bacterium, Salinicoccus sp. strain QW6. Microbiol. Res. 163: 456–465 [DOI] [PubMed] [Google Scholar]
- 3. Baesman S. M., et al. 2007. Formation of tellurium nanocrystals during anaerobic growth of bacteria that use Te oxyanions as respiratory electron acceptors. Appl. Environ. Microbiol. 73: 2135–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bailey R. E., Strausburg J. B., Nie S. 2004. A new class of far-red and near-infrared biological labels based on alloyed semiconductor quantum dots. J. Nanosci. Nanotechnol. 4: 569–574 [DOI] [PubMed] [Google Scholar]
- 5. Basnayake R. S. T., Bius J. H., Akpolat O. M., Chasteen T. G. 2001. Production of dimethyl telluride and elemental tellurium by bacteria amended with tellurite or tellurate. Appl. Organomet. Chem. 15: 499–510 [Google Scholar]
- 6. Batabyal S. K., Basu C., Das A. R., Sanyal G. S. 2006. Nanorods of tellurium: synthesis and self-assembly. J. Nanosci. Nanotechnol. 6: 719–725 [DOI] [PubMed] [Google Scholar]
- 7. Borsetti F., et al. 2003. Reduction of potassium tellurite to elemental tellurium and its effect on the plasma membrane redox components of the facultative phototroph Rhodobacter capsulatus. Protoplasma 221: 153–161 [DOI] [PubMed] [Google Scholar]
- 8. Chalmers N. I., et al. 2007. Use of quantum dot luminescent probes to achieve single-cell resolution of human oral bacteria in biofilms. Appl. Environ. Microbiol. 73: 630–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chasteen T. G., Fuentes D. E., Tantalean J. C., Vasquez C. C. 2009. Tellurite: history, oxidative stress, and molecular mechanisms of resistance. FEMS Microbiol. Rev. 33: 820–832 [DOI] [PubMed] [Google Scholar]
- 10. Di Tomaso G., et al. 2002. The membrane-bound respiratory chain of Pseudomonas pseudoalcaligenes KF707 cells grown in the presence or absence of potassium tellurite. Microbiology 148: 1699–1708 [DOI] [PubMed] [Google Scholar]
- 11. Doran J. W. 1982. Microorganisms and the biological cycling of selenium. Adv. Microb. Ecol. 6: 1–32 [Google Scholar]
- 12. Doran J. W., Alexander M. 1977. Microbial transformations of selenium. Appl. Environ. Microbiol. 33: 31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dungan R. S., Frankenberger W. T. 2000. Factors affecting the volatilization of dimethylselenide by Enterobacter cloacae SLD1a-1. Soil Biol. Biochem. 32: 1353–1358 [Google Scholar]
- 14. Dungan R. S., Yates S. R., Frankenberger W. T. 2003. Transformations of selenate and selenite by Stenotrophomonas maltophilia isolated from a seleniferous agricultural drainage pond sediment. Environ. Microbiol. 5: 287–295 [DOI] [PubMed] [Google Scholar]
- 15. Fan T. W. M., Lane A. N., Higashi R. M. 1997. Selenium biotransformations by a euryhaline microalga isolated from a saline evaporation pond. Environ. Sci. Technol. 31: 569–576 [Google Scholar]
- 16. Fountaine T. J., Wincovitch S. M., Geho D. H., Garfield S. H., Pittaluga S. 2006. Multispectral imaging of clinically relevant cellular targets in tonsil and lymphoid tissue using semiconductor quantum dots. Mod. Pathol. 19: 1181–1191 [DOI] [PubMed] [Google Scholar]
- 17. Gharieb M. M., Kierans M., Gadd G. M. 1999. Transformation and tolerance of tellurite by filamentous fungi: accumulation, reduction, and volatilization. Mycol. Res. 103: 299–305 [Google Scholar]
- 18. Guo L., Jury W. A., Frankenberger W. T., Zhang Y. Q. 2000. Characterizing kinetics of sequential selenium transformation in soil. J. Environ. Qual. 29: 1041–1048 [Google Scholar]
- 19. Jayadevan K. P., Tseng T. Y. 2005. One-dimensional semiconductor nanostructures as absorber layers in solar cells. J. Nanosci. Nanotechnol. 5: 1768–1784 [DOI] [PubMed] [Google Scholar]
- 20. Karlson U., Frankenberger W. T., Jr., Spencer W. F. 1994. Physicochemical properties of dimethyl selenide and dimethyl diselenide. J. Chem. Eng. Data 39: 608–610 [Google Scholar]
- 21. Kessi J., Ramuz M., Wehrli E., Spycher M., Bachofen R. 1999. Reduction of selenite and detoxification of elemental selenium by the phototrophic bacterium Rhodospirillum rubrum. Appl. Environ. Microbiol. 65: 4734–4740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klonowska A., Heulin T., Vermeglio A. 2005. Selenite and tellurite reduction by Shewanella oneidensis. Appl. Environ. Microbiol. 71: 5607–5609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lu Q. Y., Gao F., Komarneni S. 2005. A green chemical approach to the synthesis of tellurium nanowires. Langmuir 21: 6002–6005 [DOI] [PubMed] [Google Scholar]
- 24. Momoshima N., Fukuda A., Ishida A., Yoshinaga C. 2007. Impact of microorganism on polonium volatilization. J. Radioanal. Nucl. Chem. 272: 413–417 [Google Scholar]
- 25. Moore M. D., Kaplan S. 1992. Identification of intrinsic high-level resistance to rare-earth oxides and oxyanions in members of the class Proteobacteria: characterization of tellurite, selenite and rhodium sesquioxide reduction in Rhodobacter sphaeroides. J. Bacteriol. 174: 1505–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ollivier P. R. L., et al. 2008. Volatilization and precipitation of tellurium by aerobic, tellurite-resistant marine microbes. Appl. Environ. Microbiol. 74: 7163–7173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rajwade J. M., Paknikar K. M. 2003. Bioreduction of tellurite to elemental tellurium by Pseudomonas mendocina MCM B-180 and its practical application. Hydrometallurgy 71: 243–248 [Google Scholar]
- 28. Rathgeber C., Yurkova N., Stackebrandt E., Beatty J. T., Yurkov V. 2002. Isolation of tellurite- and selenite-resistant bacteria from hydrothermal vents of the Juan de Fuca Ridge in the Pacific Ocean. Appl. Environ. Microbiol. 68: 4613–4622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reamer D. C., Zoller W. H. 1980. Selenium biomethylation products from soil and sewage sludge. Science 208: 500–502 [DOI] [PubMed] [Google Scholar]
- 30. Soudi M. R., Ghazvini P. T. M., Khajeh K., Gharavi S. 2009. Bioprocessing of seleno-oxyanions and tellurite in a novel Bacillus sp. strain STG-83: a solution to removal of toxic oxyanions in presence of nitrate. J. Haz. Mater. 165: 71–77 [DOI] [PubMed] [Google Scholar]
- 31. Summers A. O., Jacoby G. A. 1977. Plasmid-determined resistance to tellurium compounds. J. Bacteriol. 129: 276–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Summers A. O., Silver S. 1978. Microbial transformation of metals. Annu. Rev. Microbiol. 32: 637–672 [DOI] [PubMed] [Google Scholar]
- 33. Swearingen J. W., Jr., et al. 2004. Identification of biogenic organotellurides in Escherichia coli K-12 headspace gases using solid-phase microextraction and gas chromatography. Anal. Biochem. 331: 106–114 [DOI] [PubMed] [Google Scholar]
- 34. Taylor D. E. 1999. Bacterial tellurite resistance. Trends Microbiol. 7: 111–115 [DOI] [PubMed] [Google Scholar]
- 35. Van Fleet-Stalder V., Gurleyuk H., Bachofen R., Chasteen T. G. 1997. Effects of growth conditions on production of methyl selenides in cultures of Rhodobacter sphaeroides. J. Ind. Microbiol. Biotechnol. 19: 98–103 [Google Scholar]
- 36. Van Fleet-Stalder V., Chasteen T. G. 1998. Using fluorine-induced chemiluminescence to detect organo-metalloids in the headspace of phototrophic bacterial cultures amended with selenium and tellurium. J. Photochem. Photobiol. B 43: 193–203 [Google Scholar]
- 37. Viñas P., López-García G. I., Merino-Meroño B., Hernández-Córdoba M. 2005. Ion chromatography-hydride generation-atomic fluorescence spectrometry speciation of tellurium. Appl. Organometal. Chem. 19: 930–934 [Google Scholar]
- 38. Yamada A., Miyagishima N., Matsunaga T. 1997. Tellurite removal by marine photosynthetic bacteria. J. Mar. Biotechnol. 5: 46–49 [Google Scholar]
- 39. Yurkov V. V., Csotonyi J. T. 2003. Aerobic anoxygenic phototrophs and heavy metalloid reducers from extreme environments. Res. Dev. Bacteriol. 1: 247–300 [Google Scholar]
- 40. Zannoni D., Borsetti F., Harrison J. J., Turner R. J. 2008. The bacterial response to the chalcogen metalloids Se and Te. Adv. Microb. Physiol. 53: 1–71 [DOI] [PubMed] [Google Scholar]