Abstract
The serine/threonine kinase Akt has been implicated in the control of cell survival and metabolism. Here we report the disruption of the most ubiquitously expressed member of the akt family of genes, akt1, in the mouse. Akt1−/− mice are viable but smaller when compared to wild-type littermates. In addition, the life span of Akt1−/− mice, upon exposure to genotoxic stress, is shorter. However, Akt1−/− mice do not display a diabetic phenotype. Increased spontaneous apoptosis in testes, and attenuation of spermatogenesis is observed in Akt1−/− male mice. Increased spontaneous apoptosis is also observed in the thymi of Akt1−/− mice, and Akt1−/− thymocytes are more sensitive to apoptosis induced by γ-irradiation and dexamethasone. Finally, Akt1−/− mouse embryo fibroblasts (MEFs) are more susceptible to apoptosis induced by TNF, anti-Fas, UV irradiation, and serum withdrawal.
Keywords: Gamma irradiation, glucose tolerance, seminiferous tubular atrophy, thymocytes, mouse embryo fibroblasts
The serine/threonine kinase Akt or protein kinase B (PKB) is a downstream effector of phosphatidylinositol 3 (PI 3)-kinase. It was shown to be the mediator of growth factor–dependent cell survival in a variety of cell types (for reviews, see Datta et al. 1999; Kandel and Hay 1999). It also has been implicated in executing many of the metabolic functions of insulin and growth factors such as protein and lipid synthesis, carbohydrate metabolism, and transcription (for review, see Kandel and Hay 1999). The kinase activity of Akt is constitutively activated in human cancer as a result of mutation/deletion of the tumor suppressor PTEN, a phospholipid phosphatase that negatively regulates Akt activation (for review, see Simpson and Parsons 2001), amplification of the catalytic subunit of PI 3-kinase (Shayesteh et al. 1999), amplification of the akt genes (Staal 1987; Cheng et al. 1996; Miwa et al. 1996), through activation of growth factor receptors (Porter and Vaillancourt 1998; Liu et al. 1999), and activation of Ras (Downward 1998b).
Three major isoforms of Akt/PKB, termed Akt1 or PKBα, Akt2/PKBβ, and Akt3/PKBγ, encoded by three separate genes with >85% sequence identity, have been found in mammalian cells. All Akt/PKB isoforms are assumed to have identical or similar substrate specificity (for reviews, see Alessi and Cohen 1998; Coffer et al. 1998; Downward 1998a; Kandel and Hay 1999). Among the three Akt isoforms, Akt1 is the predominantly expressed isoform in most tissues.
Despite extensive studies showing association of Akt in mammalian cell survival and metabolism, direct genetic evidence (except the use of dominant-negative forms of Akt) for the requirement of Akt in these processes, and in mammalian organisms in particular, has not been documented.
Results
Targeted disruption of the akt1 gene
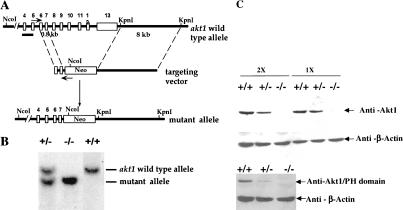
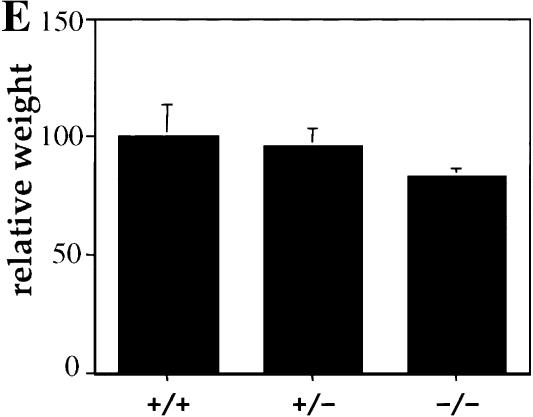
To assess the function of Akt1 in the mouse, Akt1 null mutation was generated through homologous recombination by deleting exon 8–exon 13 of the akt1 gene (Fig. 1A). Southern blot analysis from three genotypes of mice confirmed the correct recombination (Fig. 1B). Western blot analyses using protein lysates derived from mouse embryo fibroblasts (MEFs) of the three genotypes, and two different specific antibodies for the Akt1 protein show no detectable level of Akt1 protein in Akt1−/− MEFs (Fig. 1C). Thus, the expression of Akt1 protein is completely ablated. The Akt1−/− mice (genetic background 50% 129 R1 and 50% C57BL/6) are viable and examination of 20 litters from Akt1 heterozygous (+/−) mice mating showed a Mendelian ratio among wild-type (Akt1+/+), heterozygous (Akt1+/−) and homozygous (Akt1−/−) mice. However, Akt1 homozygous knockout mice are smaller when compared to wild-type and heterozygous littermates (Fig. 1D). The body weight of 1-month-old mice shows that Akt1−/− mice are 15%–20% smaller than wild-type and heterozygous of the same-sex littermates (P < 0.01) (Fig. 1E). The smaller size of Akt1−/− mice also is observed in a different genetic background (50% 129 R1 and 50% CD1; data not shown). This smaller body weight appears to be retained throughout the mouse adulthood.
Figure 1.
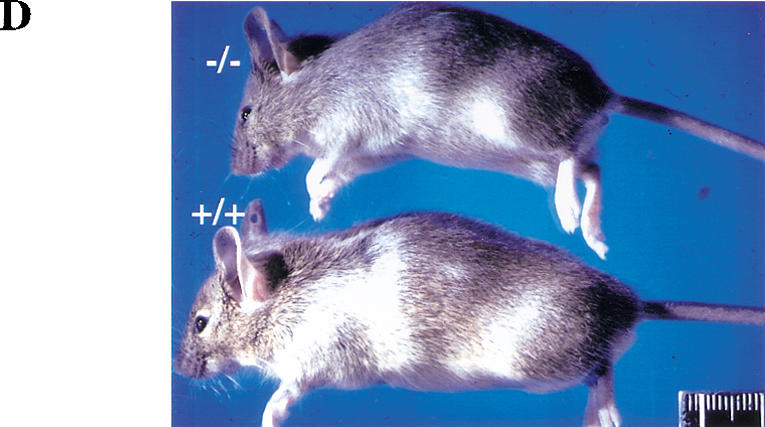
Generation of Akt1−/− mice. (A) Targeted disruption strategy to delete the akt1 gene. Shown from top to bottom, the wild-type akt1 allele with indicated exons, the targeting vector, and the disrupted allele. The locations of the PCR primers used to screen for homologous recombination are depicted by small arrows. A short bar spanning the region between exons 4 and 5 depicts the DNA fragment used as a DNA probe for Southern blots. (B) Mouse genotyping by Southern blot analysis. Genomic DNA extracted from mouse tails was digested by NcoI and hybridized with a DNA fragment spanning the region between exons 4 and 5. The wild-type fragment is ∼10 kb, whereas the deleted akt1 allele is ∼8.5 kb. (C) Western blot analysis of proteins extracted from mouse embryo fibroblasts (MEFs) isolated from wild-type embryos (+/+), heterozygous (+/−), and homozygous mutant embryos (−/−). (Top panel) Western blot analysis of twofold concentrations of extract using Akt1-specific antibodies (UBI). (Bottom panel) Western blot analysis with Akt1 pleckstrin homology (PH) domain-specific antibodies (UBI). Blots were probed with anti-β-actin as a control. (D) Side view of 5-week-old mice from the same littermate. Wild-type Akt1 (+/+) and Akt1 homozygous deletion (−/−) mice are shown. (E) Relative body weight of wild-type (+/+), heterozygous (+/−), and homozygous (−/−) Akt1 mutant mice 30 d after birth. The weight of each mouse is expressed relative to the mean weight of sex-matched, wild-type littermates in each of seven different litters.
Shorter life span of Akt1−/− mice upon exposure to γ-irradiation
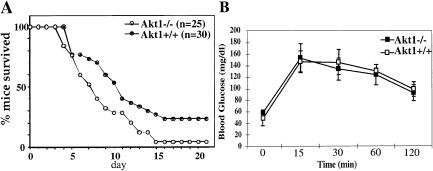
To analyze the response of Akt1−/− mice to genotoxic stress, 15 littermates (including at least two different genotypes) at different ages (from 1–8 mo old) were exposed to γ-irradiation (10 Gy). As shown in Figure 2A, Akt1−/− mice are more sensitive to γ-irradiation when compared to wild-type controls, and the life span of Akt1−/− mice is significantly shorter following γ-irradiation. In the 15 littermates that were tested, 12 Akt1−/− mice were the first to die, and only one out of the 25 Akt1−/− mice survived for 21 d after irradiation. In contrast, 5 of 30 wild-type mice survived for at least 8 wk after γ-irradiation (data not shown).
Figure 2.
(A) Akt1−/− mice are sensitized to genotoxic stress. Survival of Akt1+/+ and Akt1−/− mice was scored for 21 d after exposure to 10 Gy of γ-irradiation. (B) Oral glucose tolerance test (OGTT). Plasma glucose levels of 3-month-old Akt1−/− male mice and their wild-type littermates were measured at 15, 30, 60, and 120 min after oral glucose load. The values are expressed as the mean ±SE (n = 4–6 for each genotype). Similar results were obtained in three independent experiments.
Akt1−/− mice do not display a diabetic phenotype
Akt is a downstream effector of many metabolic functions of insulin. It mediates protein synthesis and glycolysis induced by insulin (for review, see Kandel and Hay 1999). It has been shown to induce expression of the glucose transporters GLUT-1 and GLUT-3 (Hajduch et al. 1998; Barthel et al. 1999) and to induce translocation of GLUT-4 to the plasma membrane (Kohn et al. 1996; Cong et al. 1997; Tanti et al. 1997). Akt also can regulate glycogen synthesis through phosphorylation and inactivation of glycogen synthase kinase 3 (GSK-3) (Cross et al. 1995). When Akt1 null mice and their wild-type littermates were examined for blood-glucose and insulin levels, it appeared that both levels were similar in wild-type and Akt1 null mice (data not shown). Glucose-tolerance tests of 3-month-old mice did not show any significant difference between wild-type and Akt1−/− mice (Fig. 2B). In addition, we have not been able to find a significant difference between wild-type and Akt1−/− mice in insulin-tolerance tests (data not shown). It is, therefore, possible that Akt1−/− mice do not display a diabetic phenotype either because of the redundant function of the three Akt isoforms or because of a specific function of Akt2, which is expressed at relatively high levels in insulin-responsive tissues (Altomare et al. 1995, 1998). Indeed, it has been shown recently that Akt2−/− mice display insulin resistance (data not shown; Cho et al. 2001) and exhibit a diabetic phenotype (Cho et al. 2001).
Attenuation of spermatogenesis and spontaneous apoptosis in testes of Akt1−/− mice
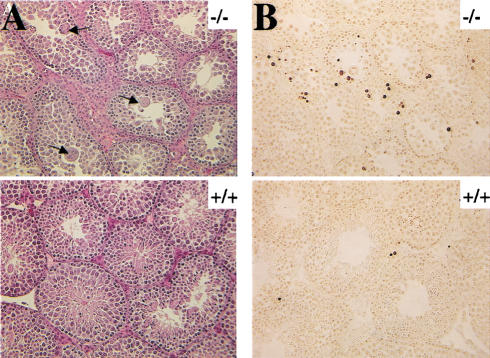
Histopathological examination shows no significant difference in spleen, bone marrow, salivary gland, pancreas, heart, lung, kidney, adrenal, bladder, liver, tongue, lymph nodes, stomach, small intestine, cecum, colon, brain, decal-head femur, skin, breast, ovary, and uterus between wild-type, Akt1+/−, and Akt1−/− mice. However, qualitative histopathological evaluation showed abnormalities in testes in the Akt1−/− male mice. Tubular atrophy in the testes and decreased diameter of seminiferous tubules were observed as early as 4 wk of age in Akt1−/− males. The arrangement of normal layer of differentiating germ cells is disturbed and the thickness of germ cells in the tubules is uneven in the 5-week-old males (Fig. 3A). Spermatogonia A and B, preleptotene spermatocytes, pachytene spermatocytes, and spermatids can be recognized in the tubules of Akt1−/− testes. However, only few matured sperms could be found in the Akt1−/− tubules when compared to wild-type and heterozygous tubules. Starting at 5 wk of age, many enlarged spermatids are found in the luminal compartment of semiferous tubules and several multinucleated giant cells are present in the lumen (Fig. 3A, upper panels). These giant cells were observed rarely in the wild-type and Akt1+/− males before reaching 6 mo of age (Takano and Abe 1987; data not shown).
Figure 3.
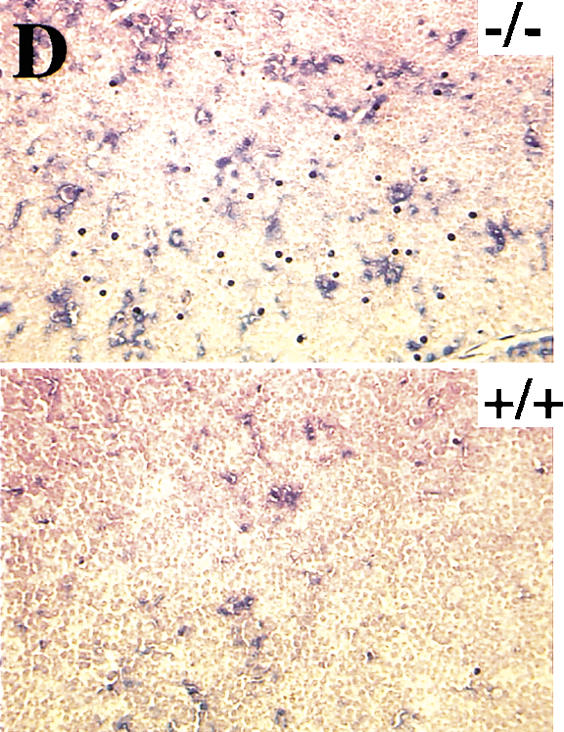
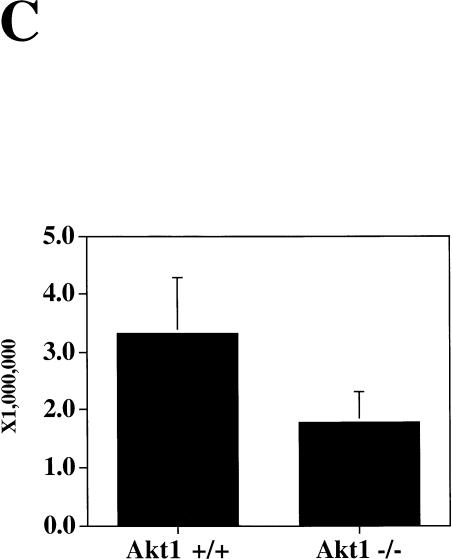
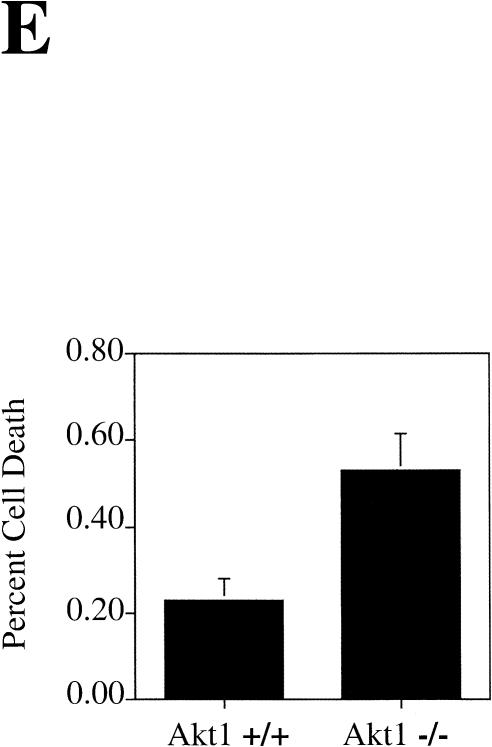
Spontaneous apoptosis in the testes and thymus of Akt1−/− mice. (A) Morphology and histology of testes from Akt1+/+ and Akt1−/−. Testes were fixed with 4% paraformaldehyde, processed with paraffin, sectioned, and stained with hematoxylin/eosin. (Top panel) Seminiferous tubules of Akt null (−/−); (bottom panel) seminiferous tubules of wild-type (+/+) littermate (200× magnification). Arrows indicate multinucleated giant cells. (B) Spontaneous apoptosis in tubules from Akt1−/− mice. Comparison of TUNEL-positive cells in tubules from Akt1−/− mice (top panel) and from wild-type littermate (bottom panel). Cells with dark stained nuclei are apoptotic cells (200× magnification). (C) Sperm count of Akt1+/+ male mice (n = 8, ±SE) and Akt1−/− male mice (n = 10, ±SE; P < 0.001). (D) Spontaneous apoptosis is observed throughout the thymi of Akt1−/− mice. TUNEL assays were performed on sections from thymi of Akt1−/− (top panel) and wild-type (bottom panel) mice. Cells with dark stained nuclei are apoptotic cells. Magnification, 400×. (E) Percentage of apoptotic cells as measured by TUNEL-positive cells in sections from thymi of age-matched (1–2 mo old), wild-type, and Akt1−/− mice as measured by counting 10 fields of each section (P < 0.001).
To determine if apoptosis contributes to the abnormality in testes, TUNEL assays were performed on testis sections. The apoptotic cells defined by TUNEL-positive nuclei were found in the peripheral region near the basement of semiferous tubules (Fig. 3B). These apoptotic cells are confined to spermatogonium. The frequency of TUNEL-positive cells is variable among the tubules, but overall there are significantly more apoptotic cells in the semiferous tubules in Akt1−/− males compared to wild-type and Akt1+/− littermates. Quantitation of apoptosis is summarized in Table 1 and is representative of testes sections analyses from at least three mice of each genotype. Multinucleated giant cells, however, are not TUNEL-positive, indicating that these cells are not undergoing apoptosis. As stated above, the giant cells do not appear in wild-type and Akt1+/− mice before 6 mo of age, indicating that in addition to increased apoptosis, the testes of Akt1−/− males show signs of premature aging. Alternatively, meiotic division is impaired in the testes of Akt1−/− mice as was observed in mice with reduced level of p53 (Rotter et al. 1993).
Table 1.
Quantitation of TUNEL-positive cells from testes section
| Genotype
|
Positive tubes
|
Positive cells
|
Negative tubes
|
Positive cells/tube
|
|---|---|---|---|---|
| Aktl+/+ | 16 | 36 | 135 | 0.24 |
| Aktl−/− | 23 | 65 | 52 | 0.86 |
The phenotype showing that apoptosis is confined mostly to germ cells is similar, albeit less severe, to the phenotype observed in “knockin” mice of Stem Cell Factor/Kit receptor mutated in the docking site for the regulatory subunit of PI 3-kinase (Blume-Jensen et al. 2000). It was suggested that the inability of the mutated Kit receptor to activate Akt and promote cell survival leads to the death of germ cells (Blume-Jensen et al. 2000). The results presented here provide a direct evidence for this assumption. Unlike the Kit receptor mutant phenotype, Akt1−/− mice are fertile although the number of mature sperm cells is two to three times less in Akt1−/− mice when compared to wild-type littermates (Fig. 3C). The phenotype of Akt1 null mice is less severe than the Kit receptor mutant phenotype likely because of the expression of other Akt isoforms in the testes.
Spontaneous apoptosis in thymi of Akt1−/− mice
TUNEL assays also were performed on other tissue sections including spleen, pancreas, thymus, heart, and brain to determine spontaneous apoptosis. A significant increase in spontaneous apoptosis was found in the thymi of 4- to 8-week-old Akt1−/− mice. Spontaneous apoptosis was observed throughout the thymus of Akt1−/− mice when compared to the thymus of wild-type mice littermates (Fig. 3D). Quantitation of apoptosis showed about two- to threefold more apoptotic cells in the thymus of Akt1−/− mice (Fig. 3E). In contrast, when TUNEL assays were performed in spleen sections of littermates, no detectable differences were observed between Akt1−/− and wild-type spleens (data not shown). No significant increase in apoptosis was observed in all other examined tissues.
Akt1−/− thymocytes and MEFs are more susceptible to apoptosis
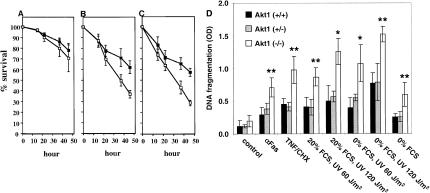
Thymocytes derived from Akt1−/− mice were examined further for the response to γ-irradiation and dexamethasone. Upon exposure to γ-irradiation and dexamethasone, wild-type thymocytes undergo apoptosis. These two stimuli of apoptosis operate through two distinct mechanisms as p53 deletion protects from γ-irradiation and not from dexamethasone-induced apoptosis (Clarke et al. 1993; Lowe et al. 1993). When thymocytes from both wild-type and Akt1−/− mice were cultured, there was no significant difference in their survival (Fig. 4A). However, thymocytes derived from Akt1−/− mice are significantly more sensitive to both γ-irradiation and dexamethasone treatment when compared to wild-type thymocytes (Fig. 4B,C). This is consistent with a recent observation showing that activated Akt targeted to thymocytes in transgenic mice provides a survival advantage upon exposure to γ-irradiation and dexamethasone (Jones et al. 2000).
Figure 4.
Akt1−/− thymocytes and MEFs are more susceptible to apoptosis. (A) Survival of thymocytes in the absence of treatment. (B) Survival of thymocytes following exposure to γ-irradiation (500 rad). (C) Survival of thymocytes upon treatment with 1 μM of dexamethasone. Values represent the average of four independent experiments of thymocytes derived from three mice of each genotype (±SE). (█) Percentage survival of wild-type thymocytes; (□) percentage survival of Akt1−/− thymocytes as measured by trypan blue exclusion. (D) Akt1−/− MEFs are sensitized to apoptosis by various apoptotic stimuli. MEFs derived from Akt1+/+, Akt1+/−, and Akt1−/− embryos were treated with 1 μg/mL anti-Fas antibody (Pharmingen), 10 ng/mL TNFα (Life Technologies), 5 μg/mL cycloheximide CHX (Sigma), UV irradiation (60 or 120 J/m2) in the presence (20% FCS) or absence (0% FCS) of fetal calf serum in the medium and apoptosis was quantitated 24 h following treatment. Apoptosis induced just by serum deprivation (0% FCS) was quantitated 48 h following serum deprivation. Apoptosis was measured by quantitation of DNA fragmentation using the cell death detection ELISA method (Boehringer Mannheim). The average of 3 independent experiments (±SE) is shown (*, P < 0.01; **, P < 0.05).
Because Akt1 is the predominantly expressed Akt isoform in MEFs (data not shown), MEFs derived from Akt1−/− mice were subjected to various apoptotic stimuli. As shown in Figure 4D, Akt1−/− MEFs are significantly more susceptible to apoptosis induced by anti-Fas, tumor necrosis factor (TNF), ultraviolet (UV) irradiation, and serum withdrawal. Depending on the apoptotic stimulus, Akt1−/− MEFs show a two- to threefold increase in DNA fragmentation compared to wild-type MEFs, indicating that the knock-out of Akt1 sensitizes cells to apoptosis. Thus, Akt provides protection from both death-receptor and non-death receptor-mediated apoptosis. This is consistent with the observation that PTEN−/− MEFs are more resistant to both death receptor and non-death receptor-mediated apoptosis (Stambolic et al. 1998).
Discussion
The relatively subtle phenotype of Akt1−/− mice suggests that Akt2 and Akt3 may substitute to some extent for Akt1 as was shown for the Akt1 and Akt2 in Caenorhabditis elegans (Paradis and Ruvkun 1998). As Akt2 and Akt3 are expressed in both testes and thymus, it is not clear why only these particular organs are affected by the ablation of Akt1. One possibility is that germ cells and thymus cells are exclusively dependent on Akt for their survival and therefore even a reduced threshold level of Akt activity is sufficient to affect their survival. Alternatively, despite a similar level of expression of the other Akt isoforms in these organs, Akt1 is more profoundly activated in the cells of these organs and/or may have exclusive protein substrates in these cells. Further studies including deletions of akt2 and akt3 genes are required to verify these possibilities.
In Drosophila, the PTEN/PI 3-kinase/Akt signaling pathway is associated with cell survival, organismal size, metabolism, and disruption of the Akt gene in Drosophila impaired normal cell survival during embryogenesis, and results in a decreased cell size (Goberdhan et al. 1999; Huang et al. 1999; Verdu et al. 1999; Gao et al. 2000; Scanga et al. 2000). The disruption of the akt-1 gene in the mouse, although by itself does not impair embryogenesis, has been shown here to affect cell survival and organismal size and growth retardation in adult mice. It has to be seen if the combined disruption of the three akt genes in the mouse would result in embryonic lethality as a result of impaired cell survival during embryogenesis.
Surprisingly, despite multiple downstream effectors of Akt and the ubiquitous expression of Akt1, ablation of Akt1 by itself does not have a gross phenotypic impact. This observation implies that reduced threshold level of Akt activity can be tolerated and therefore suggests that small molecules aimed at reducing Akt activity could be excellent therapeutic regimens for the treatment of cancers in which the PI 3-kinase/Akt pathway is constitutively activated.
Materials and methods
Gene targeting and generation of homozygous mutant mice
The targeting vector contains a neo gene cassette from pPNT (Tybulewicz et al. 1991) as a positive selective marker, and the diphtheria toxin gene cassette was used as a negative selective marker into the Not1 site of the vector. A fragment of the 3′ untranslated region in exon 13 of the mouse akt1 gene was used to screen a 129 genomic library from Stratagene. An isolated 8-kb KpnI fragment from the 3′-end, noncoding region of the akt1 gene then was used as the long arm and was inserted into the KpnI site of the targeting vector. The oligonucleotides 5′-GTAGAGGTTGCCCACACGCTTAC-3′ and 5′-CTCGCCCCCGTTGGCATACTCC-3′ were used to isolate a 1.0-kb fragment containing exons 6 and 7 of akt1 as the short arm. This fragment was inserted into the HindIII/NotI sites of the targeting vector. The targeting vector was linearized by digestion with ClaI, and electroporated into R1 embryonic stem (ES) cells. The G418-resistant clones were screened initially by PCR. Two positive clones were expanded for Southern blot analysis following digestion of genomic DNA by NcoI. An external probe (a DNA fragment between exons 4 and 5 isolated using the oligo primers 5′-CCACCGCCATTCAGACTGTG-3′, and 5′-CAGGTACTCAAACTCGTTCATGG-3′) and an internal probe were used to verify correct targeting. All PCR fragments were confirmed by sequencing. The two positive ES-cell clones were injected into C57BL/6J blastocysts. Chimeras from these clones were transmitted through germ line. The heterozygous mice were intercrossed to obtain homozygous mice. The genotype of the mice was confirmed by Southern blot analysis.
γ-Irradiation and survival of mice
Fifteen littermates including 30 Akt1+/+ mice and 25 Akt1−/− mice from 1–8 mo old were irradiated with 10 Gy in a 137Cs irradiator. Mice were scored for survival for 8 wk.
Isolation of MEFs, thymocytes, and splenic T cells
MEFs were isolated from 14.5-day-old embryos from heterozygous Akt1 mice mating, and expanded for another two passages before the assay. Thymocytes and splenic T cells were prepared by gently pressing the thymus or the spleen between two sterile slides, then were washed with PBS following cold Hank's balanced salt solution (HBSS), and were resuspended in Iscove's modified Dulbecco's medium (IMDM) with 15% fetal calf serum (FCS) and 50 μM β-mercaptoethanol at 106 cells/mL.
Protein analysis
Western blot analysis was performed as described previously (Kennedy et al. 1997). Protein extracts from MEFs derived from wild type, Akt1+/−, and Akt1−/− were used for the Western blot analysis using anti-Akt1 antibodies (catalog no. 06-558) and Anti-Akt1 PH domain antibodies (catalog no. 06-608) from Upstate Biotechnology.
TUNEL and apoptotic assays
Tissues were dissected and fixed in 4% paraformaldehyde overnight, dehydrated, and embedded in paraffin, then subjected to TUNEL assay. TUNEL assay was performed according to Boehringer Mannheim's in situ cell death detection instructions as described previously (Chen et al. 1994). For quantitation of apoptosis in testes, sections of testes derived from mice with different genotypes and from the same litter were subjected to TUNEL assay. The number of both TUNEL-positive and TUNEL-negative tubules was determined, and TUNEL-positive cells per each tubule were counted. For quantitation of apoptosis in thymus, sections of thymus derived from mice both with different genotypes and from the same littermate. The percentage of TUNEL-positive cells was determined by counting both the positive and negative cells from 10 random fields in each section. The average of the 10 fields was used and standard deviation (SD) was also from those 10 fields. For determining survival of cultured thymocytes, thymocytes were γ-irradiated with 500 rad or treated with 1 μM of dexamethasone and then cultured at 106 cell/mL on a 96-well plate in IMDM with 15% FCS and 50 μM β-mercaptoethanol. The cell viability was determined by trypan-blue exclusion at different time points. MEFs were treated with different apoptotic stimuli (1 mg/mL anti-Fas antibody, 10 ng/mL TNFα, 5 μg/mL cycloheximide, 60 or 120 J/m2 UV irradiation, and 0% FCS in DME). About 100,000 cells per 3-cm plate were allowed to attach for 36 h. After induction of apoptosis, floating and attached cells were harvested 24 h after the treatment, except for the serum-deprived cells that were harvested 48 h after serum deprivation. Cells were lysed at room temperature for 30 min and dilutions of these extracts were used for the cell death ELISA that was performed according to the manufacturer's protocol (Boehringer Mannheim). P values were determined using paired t-test.
Sperm count
Testes were dissected from the animals and were kept in PBS. Small dissecting scissors were used to open and release the sperm from the testis. The sperm was counted under the microscope (only stages 14–16 were included).
Oral glucose tolerance test
Oral glucose tolerance tests were carried out as described previously (Tamemoto et al. 1994).
Acknowledgments
This work was supported by a grant from the U.S. Army Medical Research and Materiel Command DAMD17-96-1-6051 to W.C. and by NIH grants AG 16927 and CA 90764 to N.H.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL nhay@uic.edu; FAX (312) 355-2032.
E-MAIL billyschen@hotmail.com.
Article and publication are at www.genesdev.org/cgi/doi/10.1101/gad.913901.
References
- Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- Altomare DA, Guo K, Cheng JQ, Sonoda G, Walsh K, Testa JR. Cloning, chromosomal localization and expression analysis of the mouse Akt2 oncogene. Oncogene. 1995;11:1055–1060. [PubMed] [Google Scholar]
- Altomare DA, Lyons GE, Mitsuuchi Y, Cheng JQ, Testa JR. Akt2 mRNA is highly expressed in embryonic brown fat and the AKT2 kinase is activated by insulin. Oncogene. 1998;116:2407–2411. doi: 10.1038/sj.onc.1201750. [DOI] [PubMed] [Google Scholar]
- Barthel A, Okino ST, Liao J, Nakatani K, Li J, Whitlock J, Jr, Roth RA. Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. J Biol Chem. 1999;274:20281–20286. doi: 10.1074/jbc.274.29.20281. [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P, Jiang G, Hyman R, Lee KF, O'Gorman S, Hunter T. Kit/stem cell factor receptor-induced activation of phosphatidylinositol 3′-kinase is essential for male fertility. Nat Genet. 2000;24:157–162. doi: 10.1038/72814. [DOI] [PubMed] [Google Scholar]
- Chen WS, Manova K, Weinstein DC, Duncan SA, Plump AS, Prezioso VR, Bachvarova RF, Darnell JE., Jr Disruption of the HNF-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Genes & Dev. 1994;8:2466–2477. doi: 10.1101/gad.8.20.2466. [DOI] [PubMed] [Google Scholar]
- Cheng JQ, Ruggeri B, Klein WM, Sonoda G, Altomare DA, Watson DK, Testa JR. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci. 1996;93:3636–3641. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, III, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB β) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Purdie CA, Harrison DJ, Morris RG, Bird CC, Hooper ML, Wyllie AH. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993;362:849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Coffer PJ, Jin J, Woodgett JR. Protein kinase B (c-Akt): A multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong LN, Chen H, Li Y, Zhou L, McGibbon MA, Taylor SI, Quon MJ. Physiological role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. Mol Endocrinol. 1997;11:1881–1890. doi: 10.1210/mend.11.13.0027. [DOI] [PubMed] [Google Scholar]
- Cross DA, Aless DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: A play in three Akts. Genes & Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998a;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- ————— Ras signalling and apoptosis. Curr Opin Genet Dev. 1998b;8:49–54. doi: 10.1016/s0959-437x(98)80061-0. [DOI] [PubMed] [Google Scholar]
- Gao X, Neufeld TP, Pan D. Drosophila PTEN regulates cell growth and proliferation through PI3K-dependent and -independent pathways. Dev Biol. 2000;221:404–418. doi: 10.1006/dbio.2000.9680. [DOI] [PubMed] [Google Scholar]
- Goberdhan DC, Paricio N, Goodman EC, Mlodzik M, Wilson C. Drosophila tumor suppressor PTEN controls cell size and number by antagonizing the Chico/PI3-kinase signaling pathway. Genes & Dev. 1999;13:3244–3258. doi: 10.1101/gad.13.24.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajduch E, Alessi DR, Hemmings BA, Hundal HS. Constitutive activation of protein kinase B α by membrane targeting promotes glucose and system A amino acid transport, protein synthesis, and inactivation of glycogen synthase kinase 3 in L6 muscle cells. Diabetes. 1998;47:1006–1013. doi: 10.2337/diabetes.47.7.1006. [DOI] [PubMed] [Google Scholar]
- Huang H, Potter CJ, Tao W, Li DM, Brogiolo W, Hafen E, Sun H, Xu T. PTEN affects cell size, cell proliferation and apoptosis during Drosophila eye development. Development. 1999;126:5365–5372. doi: 10.1242/dev.126.23.5365. [DOI] [PubMed] [Google Scholar]
- Jones RG, Parsons M, Bonnard M, Chan VS, Yeh WC, Woodgett JR, Ohashi PS. Protein kinase B regulates T lymphocyte survival, nuclear factor κB activation, and Bcl-X(L) levels in vivo. J Exp Med. 2000;191:1721–1734. doi: 10.1084/jem.191.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ES, Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp Cell Res. 1999;253:210–229. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes & Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- Liu W, Li J, Roth RA. Heregulin regulation of Akt/protein kinase B in breast cancer cells. Biochem Biophys Res Commun. 1999;261:897–903. doi: 10.1006/bbrc.1999.1144. [DOI] [PubMed] [Google Scholar]
- Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Miwa W, Yasuda J, Murakami Y, Yashima K, Sugano K, Sekine T, Kono A, Egawa S, Yamaguchi K, Hayashizaki Y, et al. Isolation of DNA sequences amplified at chromosome 19q13.1–q13.2 including the AKT2 locus in human pancreatic cancer. Biochem Biophys Res Commun. 1996;225:968–974. doi: 10.1006/bbrc.1996.1280. [DOI] [PubMed] [Google Scholar]
- Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes & Dev. 1998;12:2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter AC, Vaillancourt RR. Tyrosine kinase receptor-activated signal transduction pathways which lead to oncogenesis. Oncogene. 1998;17:1343–1352. doi: 10.1038/sj.onc.1202171. [DOI] [PubMed] [Google Scholar]
- Rotter V, Schwartz D, Almon E, Goldfinger N, Kapon A, Meshorer A, Donehower LA, Levine AJ. Mice with reduced levels of p53 protein exhibit the testicular giant-cell degenerative syndrome. Proc Natl Acad Sci. 1993;90:9075–9079. doi: 10.1073/pnas.90.19.9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanga SE, Ruel L, Binari RC, Snow B, Stambolic V, Bouchard D, Peters M, Calvieri B, Mak TW, Woodgett JR, et al. The conserved PI3′K/PTEN/Akt signaling pathway regulates both cell size and survival in Drosophila. Oncogene. 2000;19:3971–3977. doi: 10.1038/sj.onc.1203739. [DOI] [PubMed] [Google Scholar]
- Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills GB, Gray JW. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- Simpson L, Parsons R. PTEN: Life as a tumor suppressor. Exp Cell Res. 2001;264:29–41. doi: 10.1006/excr.2000.5130. [DOI] [PubMed] [Google Scholar]
- Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: Amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci. 1987;84:5034–5037. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- Takano H, Abe K. Age-related histologic changes in the adult mouse testis. Arch Histol Jpn. 1987;50:533–544. doi: 10.1679/aohc.50.533. [DOI] [PubMed] [Google Scholar]
- Tamemoto H, Kadowaki T, Tobe K, Yagi T, Sakura H, Hayakawa T, Terauchi Y, Ueki K, Kaburagi Y, Satoh S, et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature. 1994;372:182–186. doi: 10.1038/372182a0. [DOI] [PubMed] [Google Scholar]
- Tanti JF, Grillo S, Gremeaux T, Coffer PJ, Van Obberghen E, Le Marchand-Brustel Y. Potential role of protein kinase B in glucose transporter 4 translocation in adipocytes. Endocrinology. 1997;138:2005–2010. doi: 10.1210/endo.138.5.5136. [DOI] [PubMed] [Google Scholar]
- Tybulewicz VL, Crawford CE, Jackson PK, Bronson RT, Mulligan RC. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- Verdu J, Buratovich MA, Wilder EL, Birnbaum MJ. Cell-autonomous regulation of cell and organ growth in Drosophila by Akt/PKB. Nat Cell Biol. 1999;1:500–506. doi: 10.1038/70293. [DOI] [PubMed] [Google Scholar]