Abstract
A recently described Edwardsiella ictaluri type III secretion system (T3SS) with functional similarity to the Salmonella pathogenicity island 2 T3SS is required for replication in channel catfish head-kidney-derived macrophages (HKDM) and virulence in channel catfish. Quantitative PCR and Western blotting identified low pH and phosphate limitation as conducive to expression of the E. ictaluri T3SS, growth conditions that mimic the phagosomal environment. Mutagenesis studies demonstrated that expression is under the control of the EsrAB two-component regulatory system. EsrB also induces upregulation of the AraC-type regulatory protein EsrC, which enhances expression of the EscB/EseG chaperone/effector operon in concert with EsrB and induces expression of the pEI1-encoded effector, EseH. EsrC also induces expression of a putative type VI secretion system translocon protein, EvpC, which is secreted under the same low-pH conditions as the T3SS translocon proteins. The pEI2-encoded effector, EseI, was upregulated under low-pH and low-phosphate conditions but not in an EsrB- or EsrC-dependent manner. Mutations of EsrA and EsrB both resulted in loss of the ability to replicate in HKDM and full attenuation in the channel catfish host. Mutation of EsrC did not affect intracellular replication but did result in attenuation in catfish. Although EsrB is the primary transcriptional regulator for E. ictaluri genes within the T3SS pathogenicity island, EsrC regulates expression of the plasmid-carried effector eseH and appears to mediate coordinated expression of the T6SS with the T3SS.
INTRODUCTION
Edwardsiella ictaluri, the etiological agent of enteric septicemia of catfish (ESC), was isolated, named, and characterized by Hawke (22) and Hawke et al. (23). The disease is found throughout channel catfish (Ictalurus punctatus) growing regions and is the leading cause of disease-related mortality in channel catfish production facilities (2, 3). Recent reports have expanded the host and geographic ranges to the culture of striped catfish (Pangasianodon hypophthalmus) in Vietnam (14, 51) and yellow catfish (Pelteobagrus fulvidraco) in China (29).
Using signature-tagged mutagenesis, Thune et al. (52) identified mutations in 50 E. ictaluri genes that led to an attenuated phenotype in channel catfish, including a type III secretion system (T3SS) apparatus gene. Further sequencing and genome analysis led to the identification of an E. ictaluri pathogenicity island (PAI) encoding a complete T3SS, including Edwardsiella secretion apparatus (esa), chaperone (esc), effector (ese), and regulatory (esr) genes. Mutation of the apparatus gene resulted in an inability to replicate in catfish cells and loss of virulence, indicating that the T3SS is essential to the pathogenesis of E. ictaluri. These systems are widespread in Gram-negative bacteria and function to translocate effector molecules directly from the bacterial cytosol to the host cell cytoplasm.
The Edwardsiella tarda T3SS, which is involved in replication within fish macrophages (38, 49, 50, 57), is homologous to the E. ictaluri T3SS at both the nucleotide (89% identical) and amino acid (33 open reading frames [ORFs] with a range of 70 to 99% similarity) levels. At the amino acid level, individual proteins of the E. ictaluri T3SS are 42 to 77% similar to the same proteins of the Salmonella pathogenicity island 2 (SPI-2) T3SS. Although there is no similarity to Salmonella at the nucleotide level, many of the genes are arranged in similar operons (52). Following phagocytosis, the SPI-2 system translocates effector proteins that are involved in intracellular replication across the phagosomal membrane (11, 24). In addition to the T3SS PAI, the E. ictaluri plasmids, pEI1 and pEI2, encode proteins with similarity to T3SS effector proteins of other pathogens (20, 52). These two plasmids are found in all channel catfish isolates of E. ictaluri (31, 37, 42, 47), suggesting that they are required for virulence.
Encoded within the E. ictaluri T3SS PAI are the putative two-component regulatory proteins EsrA and EsrB, which are a sensor kinase and a response regulator, respectively. These proteins are homologous to EsrAB and SsrAB, which are two-component regulatory systems responsible for T3SS regulation in E. tarda (49, 50) and Salmonella (11), respectively. In addition, an AraC-type regulatory protein, EsrC, is encoded within the E. ictaluri and E. tarda T3SSs (50, 52) but not in Salmonella. The E. tarda EsrC is involved in the regulation of both the T3SS and the type VI secretion system (T6SS) (56, 57). Whereas the SPI-2 T3SS is regulated by environmental conditions such as low pH (5, 15) and phosphate limitation (15), the E. tarda T3SS is active at neutral pH (48, 49, 57) and is repressed by acidic pH (39). Phosphate limitation is not reported to be involved in expression of the E. tarda T3SS.
This study was conducted to evaluate the role of pH and phosphate concentration in the expression of the E. ictaluri T3SS and to determine if the encoded proteins, EsrA, EsrB, and EsrC, are involved in regulation. The results indicate that both the EsrAB two-component regulatory system and EsrC are important for T3SS gene expression and virulence in channel catfish in response to low pH and low phosphate. Furthermore, EsrC regulates expression of a T6SS protein, EvpC, suggesting coordinated expression of two virulence-related secretion systems by the T3SS-encoded regulatory proteins.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. Edwardsiella ictaluri strains were grown at 28°C with brain heart infusion (BHI) broth or agar, minimal medium (MM19) (12), or Luria-Bertani (LB) broth or agar supplemented with 0.35% mannitol (LB-Man). For isolation from channel catfish cells or tissue, E. ictaluri was grown on Trypticase soy agar supplemented with 5% sheep blood (BA; Remel Products, Lenexa, KS). Escherichia coli strains were cultured using LB broth or agar at 37°C. All broth cultures were grown with aeration on a Cel-Gro tissue culture rotator (Lab-Line, Inc., Melrose Park, IL). Antibiotics were added where appropriate in the following concentrations: ampicillin (Ap), 200 μg/ml; colistin (Col), 10 μg/ml; and kanamycin (Km), 50 μg/ml.
Table 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Description | Reference or source |
|---|---|---|
| Edwardsiella ictaluri | ||
| 93-146 | Wild-type E. ictaluri isolated from a moribund channel catfish from a natural outbreak at a commercial facility in 1993 | LSU aquatic animal diagnostic laboratory |
| 93-146 ΔesrA::km | 93-146 with nucleotides 112-2610 of esrA deleted in frame and a Tn5 kanamycin resistance cassette inserted; Kmr | This work |
| 93-146 ΔesrB::km | 93-146 with nucleotides 58-564 of esrB deleted in frame and a Tn5 kanamycin resistance cassette inserted; Kmr | This work |
| 93-146 ΔesrC | 93-146 with nucleotides 301-633 of esrC deleted in frame | This work |
| 93-146 ΔesrA::km/pesrA | 93-146 ΔesrA::km carrying pesrA; Kmr Apr | This work |
| 93-146 ΔesrB::km/pesrB | 93-146 ΔesrB::km carrying pesrB; Kmr Apr | This work |
| 93-146 ΔesrC/pesrC | 93-146 ΔesrC carrying pesrC; Apr | This work |
| WT/pesrA | 93-146 carrying pesrA; Apr | This work |
| WT/pesrB | 93-146 carrying pesrB; Apr | This work |
| WT/pesrC | 93-146 carrying pesrC; Apr | This work |
| EseB-Flag | 93-146 carrying pBBR1-EseB::Flag; Apr | This work |
| EseG-Flag | 93-146 carrying pBBR1-EseG::Flag; Apr | This work |
| EseH-Flag | 93-146 carrying pBBR1-EseH::Flag; Apr | This work |
| EseI-Flag | 93-146 carrying pBBR1-EseI::Flag; Apr | This work |
| EsrC-Flag | 93-146 carrying pBBR1-EsrC::Flag; Apr | This work |
| Escherichia coli | ||
| XL1 Blue MRF′ | (mcrA)183 (mcrCB-hsdSMR-mrr)173endA1 supE44 thi-1 recA1 gyrA96 relA1 lac [F′ proAB lacIqZM15 Tn5(Km)] | Stratagene, La Jolla, CA |
| CC118λ pir | Δ(ara-leu) araD ΔlacX74 galE galK phoA20 thi-1 rpsE rpoB argE(Am) recA1 λpir lysogen | 25 |
| SM10λ pir | thi1 thr1 leuB supE44 tonA21 lacY1 recA-::RP4-2-Tc::Mu Kmr λ::pir | 46 |
| Plasmids | ||
| pBluescript SK− | Cloning vector; Apr | Stratagene, La Jolla, CA |
| pGP704 | R6K ori mob; Apr; suicide vector used for allelic exchange | 35 |
| pRE107 | R6K ori mobsacB1; Apr; suicide vector used for allelic exchange | 16 |
| pGP::ΔesrA::km | pGP704 carrying ΔesrA::km | This work |
| pGP::ΔesrB::km | pGP704 carrying ΔesrB::km | This work |
| pRE::ΔesrC | pRE107 carrying ΔesrC | This work |
| pBBR1-MCS4 | Broad-host-range expression vector; Apr | 27 |
| pesrA | pBBR1-MCS4 carrying esrA | This work |
| pesrB | pBBR1-MCS4 carrying esrB | This work |
| pesrC | pBBR1-MCS4 carrying esrC | This work |
| pMRFlag | pBluescript SK-km::Flag with an SphI site engineered between km and the Flag epitope | This work |
| pBBR1-EseB::Flag | pBBR1-MCS4 carrying the escC/eseB ORFs fused to the Flag epitope | This work |
| pBBR1-EseG::Flag | pBBR1-MCS4 carrying the escB/eseG operon fused to the Flag epitope | This work |
| pBBR1-EseH::Flag | pBBR1-MCS4 carrying the eseH gene fused to the Flag epitope | This work |
| pBBR1-EseI::Flag | pBBR1-MCS4 carrying the escD/eseI operon fused to the Flag epitope | This work |
| pBBR1-EsrC::Flag | pBBR1-MCS4 carrying the esrC gene fused to the Flag epitope | This work |
Low-phosphate MM19 (MMP) was formulated similarly to MM19, except salts containing phosphate were omitted. For physiological requirements, phosphate was maintained at 0.125 mM, compared to approximately 110 mM phosphate in MM19. To replace the buffering capacity of the phosphate, 80 mM MES (morpholineethanesulfonic acid) and MOPS (morpholinepropanesulfonic acid) were used to buffer pH 5.5 and 7.0 MMP, respectively. The osmolality of the medium was maintained by the addition of 43 mM sodium chloride and 54 mM potassium chloride.
Specific-pathogen-free channel catfish.
Channel catfish egg masses obtained from production facilities with no history of E. ictaluri outbreaks were disinfected with 100 ppm free iodine and hatched in a closed recirculating aquaculture system in the specific-pathogen-free aquatic laboratory at the Louisiana State University (LSU) School of Veterinary Medicine. Catfish used for immersion infections were between 10 and 20 g during exposure to E. ictaluri, while those used for harvesting macrophages were between 500 and 750 g. All guidelines concerning the care and use of laboratory animals as governed by the LSU School of Veterinary Medicine IACUC were followed.
WCL and ECP preparation.
Bacterial whole-cell lysates (WCL) were prepared as described by Moore and Thune (36). Briefly, cultures were grown to late log phase, lysed by sonication using a Fisher 500 sonic dismembranator (Fisher Scientific, Pittsburgh, PA), and centrifuged at 12,000 × g at 4°C for 30 min. The supernatant was collected, and protein concentrations were estimated using the Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA). Supernatants from the same cultures were filter sterilized using a 0.22-μm cellulose acetate low-protein-binding filter (Corning Inc., Corning, NY), and extracellular proteins (ECP) were precipitated by adding trichloroacetic acid (Ricca Chemical Co., Arlington, TX) to a final concentration of 10%. Precipitates were pelleted by centrifugation at 24,000 × g for 30 min at 4°C and resuspended in 1 ml sterile water.
2D-PAGE.
The WCL and ECP were purified by using the ReadyPrep 2-D Cleanup kit (Bio-Rad Laboratories) and separated by two-dimensional polyacrylamide gel electrophoresis (2D-PAGE). Immobilized pH gradient (IPG) strips (11 cm, pH 4 to 7; Bio-Rad Laboratories) were rehydrated with 185 μl rehydration buffer containing 100 μg of WCL or the concentrated ECP from 20 ml of culture. Isoelectric focusing (IEF) was done by using a Protean IEF cell (Bio-Rad Laboratories). Focused IPG strips were equilibrated using ReadyPrep 2-D starter kit equilibration buffers (Bio-Rad Laboratories), and proteins were separated on a Criterion precast 12.5% gel (Bio-Rad Laboratories). Three protein preparations were independently isolated for both the WCL and ECP and were analyzed by 2D-PAGE to confirm reproducibility.
Protein spot identification.
Gels containing separated MM19 pH 5.5 WCL and ECP were sent for mass spectrometric analysis (Nevada Proteomics Center, University of Nevada, Reno, NV). Protein spots of interest were selected, trypsin digested, and analyzed by matrix-assisted laser desorption ionization-time-of-flight tandem mass spectrometry (MALDI-TOF/TOF MS). Proteins were identified by comparison to peptide mass databases and to in silico trypsin digests of proteins encoded in the E. ictaluri genome (GenBank accession no. NC_012779).
Nucleic acid protocols.
Bacterial genomic DNA was isolated by a procedure similar to that described by Ausubel et al. (4). Plasmid DNA was isolated using the Qiagen Miniprep kit (Qiagen, Valencia, CA). Restriction digests were conducted using enzymes purchased from New England BioLabs (Ipswich, MA), and DNA was purified from restriction digests and PCRs using the QIAquick kit or Minelute kit (Qiagen). Total RNA was isolated from cultures grown to late log phase using the Bacteria RNAprotect RNeasy Minikit (Qiagen) and subjected to DNase treatment using Baseline Zero DNase (Epicentre Biotechnologies, Madison, WI) per the manufacturer's instructions. The high-capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA) was used to generate cDNA, which was analyzed by quantitative real-time PCR (qPCR) using the Power SYBR green PCR Master Mix (Applied Biosystems) and gene-specific primers. Target amplicons, their operons, and their gene-specific primers are listed in Table 2. Putative operons were identified by using FGENESB (SoftBerry, Inc., Mount Kisco, NY), and transcriptional linkages were subsequently confirmed by reverse transcription-PCR (RT-PCR) using operon-specific primers.
Table 2.
Primers used for quantitative PCR in this study
| Target | Primer | Primer sequence | Putative T3SS operon gene(s)a | Putative protein function(s) | GenBank accession no. |
|---|---|---|---|---|---|
| escB | 5′ | 5′-CTTTACCTTGCGATTTGCCTGCGT-3′ | escB, eseG | Chaperone and secreted effector | YP_002932393 and YP_002932394 |
| 3′ | 5′-AACAGGCACTCCGCCATATGAAAC-3′ | ||||
| escC | 5′ | 5′-AATGCAAGACCTACAGCAGCGTCA-3′ | escC, escA, eseBCDE | Chaperone and translocon | YP_002932396 to YP_002932401 |
| 3′ | 5′-GCGTTGCGATCTCTTGCTGTAACG-3′ | ||||
| eseH | 5′ | 5′-AAGAGGCTGGATGTCTCTGGTACT-3′ | eseH | Secreted effector | AF244083 |
| 3′ | 5′-GGTAGCCTTGGCATAGGAGTGTTA-3′ | ||||
| eseI | 5′ | 5′-GCAACCCTGCTTAGCAAGTGGAAA-3′ | escD, eseI | Chaperone and secreted effector | AAF85960 |
| 3′ | 5′-TCAAAGCCTTCTGGGCCTAATGGA-3′ | ||||
| esrA | 5′ | 5′-AGAGCGAGCATCTGAACAGCATCA-3′ | esrA | Two-component sensor kinase | YP_002932413 |
| 3′ | 5′-AGTAAGTCATGCTGCTCCTGCGTA-3′ | ||||
| esrB | 5′ | 5′-CAATGCAGCATGCATCACTGGGAA-3′ | esrB | Two-component response regulator | YP_002932414 |
| 3′ | 5′-TCAGCGATATCCCGGTTGCGATTA-3′ | ||||
| esrC | 5′ | 5′-AAAGTTTGGGATGGCGCCGAA-3′ | esaGHIKWKL | AraC-type regulator; apparatus | YP_002932388 |
| 3′ | 5′-GAGAAATGGGCGGCGTTACAGAAT-3′ | ||||
| 16S | 5′ | 5′-AACGCGAAGAACCTTACCTGGTCT-3′ | |||
| 3′ | 5′-GCTCGTTGCGGGAATTAAACCCAA-3′ |
Transcriptional linkages of underlined genes were confirmed by RT-PCR.
Quantitative PCR data were collected using a 7500 Fast Real-Time PCR System and Sequence Detection Software v1.4 (Applied Biosystems). Following real-time amplification, a dissociation curve was established for each sample to ensure amplification of only the target amplicon. cDNA reaction mixtures without reverse transcriptase were used as templates for negative-control reactions. Relative expression was calculated using the ΔΔCT method (30, 44) with 16S rRNA as the endogenous control and MM19 pH 7.0 ΔCT values as calibrators for gene expression in experimental media. Wild-type (WT) MMP pH 5.5 ΔCT values were the calibrators for gene expression in T3SS mutants. All RNA isolations and real-time experiments were repeated three times to confirm reproducibility.
Fusion of the Flag epitope to E. ictaluri T3SS proteins.
The plasmid pMRFlag was constructed for rapid fusion of target proteins to the Flag epitope (DYKDDDDK; Sigma-Aldrich, St. Louis, MO) using primers listed in Table 3. Briefly, the Tn5 kanamycin resistance cassette (km) was amplified with primers encoding the Flag sequence on a 3′ tail. The amplicon was cloned into pBluescript, and an SphI site was introduced between km and flag by inverse PCR, generating pMRFlag. Genes of interest were cloned into pMRFlag using SacI or BglII at the 5′ end and SphI at the 3′ end, resulting in the in-frame fusion of flag to the target gene of interest.
Table 3.
Primers used for fusion of the Flag epitope to T3SS proteins in this study
| Gene | Primer | Sequencea |
|---|---|---|
| km | 5′ | 5′-ATATATGAGCTCGAAGCCCTGCAAAGTAAA-3′ |
| 3′ | 5′-ATATATTCTAGACTATTTATCGTCGTCATCTTTGTAGTCGAAGAACTCGTCAAGAAGG-3′ | |
| kmFLAG | 5′ | 5′-ATATATGCATGCGAAGAACTCGTCAAG AAGGCG-3′ |
| 3′ | 5′-ATATATGCATGCTTCTTCGACTACAAAGATGACGAC-3′ | |
| eseB | 5′ | 5′-ATATATGAGCTCGCCCGCGTAAACAATC GACA-3′ |
| 3′ | 5′-ATATATTCTAGACTATTTATCGTCGTCATCTTTGTAGTCGCGGATATTCTGGGCAATGGA-3′ | |
| eseG | 5′ | 5′-ATATATAGATCTATCGGGCGCTGGATAA GATG-3′ |
| 3′ | 5′-ATATATGCATGCGGCAAAGCTGTGGCGTCG-3′ | |
| eseH | 5′ | 5′-ATATATGAGCTCAGCCATTCACGACACT GCAT-3′ |
| 3′ | 5′-ATATATGCATGCCAAGGAGTGATATACA GGGG-3′ | |
| eseI | 5′ | 5′-ATATATGAGCTCTGGCTCCCTAATCCTG TCTT-3′ |
| 3′ | 5′-ATATATGCATGCGGCTGGGATGAAGA CTCGC-3′ | |
| esrC | 5′ | 5′-ATATATGAGCTCCCAACCCTGACGCATC TTGAA-3′ |
| 3′ | 5′-ATATATGCATGCGCGGTGGTGATGACTGGC-3′ |
Underlined sequences are linkers incorporated into the primers used for cloning. Bold regions are the Flag-encoding DNA sequence. For eseB, eseG, and eseI, the cognate chaperone genes, escC, escB, and escD, respectively, were included in the amplicon.
Selected T3SS translocon, regulatory, and effector genes, including their cognate chaperone genes when present, were amplified for fusion to the flag sequence using primers listed in Table 3. Because the eseB sequence contains an internal SphI site, a flag sequence within the 3′ primer was used, similar to the method used above in the fusion of flag to km. Each specific gene-flag fusion was inserted into the expression vector pBBR1-MCS4 (27), sequenced, and transferred to E. ictaluri by conjugation (33). Transconjugants were selected for Apr and Colr, and the presence of the plasmid was verified by observing a third plasmid in the E. ictaluri plasmid profile on an agarose gel.
Western blot analyses.
Whole-cell lysate proteins were harvested from late-log-phase cultures of E. ictaluri carrying T3SS proteins fused to the Flag epitope, separated by SDS-PAGE, and transferred to polyvinylidene difluoride (PVDF) using the iBlot dry transfer system (Invitrogen). Flag-tagged proteins were labeled with mouse M2 anti-Flag antibody (Sigma). Highly expressed proteins were detected using horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG polyclonal antibody (Thermo Fisher Scientific Inc., Rockford, IL) as the secondary antibody. Poorly expressed proteins were detected using a biotinylated goat anti-mouse IgG (Thermo Fisher Scientific), followed by incubation with streptavidin-poly-HRP (Thermo Fisher Scientific). The SuperSignal West Pico kit (Thermo Fisher Scientific) was used as the substrate for chemiluminescence.
T3SS regulatory gene mutagenesis.
Specific mutations in the T3SS regulatory genes esrA, esrB, and esrC were constructed by PCR using the primers listed in Table 4. Briefly, 5′ and 3′ target sequences were amplified using primers P1 and P2 paired with primers P3 and P4, respectively. The 5′ and 3′ amplicons were digested and ligated to each other via EcoRI sites incorporated into P3 and P4. The ligation product was used as the template for PCR using primers P1 and P2, with the resulting amplicon containing a deletion and an inserted EcoRI site. The deletion construct was cloned into pBluescript, and km was inserted into the EcoRI sites of the ΔesrA and ΔesrB constructs. The ΔesrC construct was left markerless and in frame to preclude polarity effects on the downstream apparatus genes in the operon (Table 2).
Table 4.
Primers used for PCR in the mutagenesis of Edwardsiella ictaluri type III secretion system regulatory genes esrA, esrB, and esrC
| Primer | Sequencea |
|---|---|
| esrA-P1 | 5′-TATATAGGTACCAACCCTACCCATATTGCC-3′ |
| esrA-P2 | 5′-TATATATCTAGAGCTGCCACTGATTCGGAG-3′ |
| esrA-P3 | 5′-TATATAGAATTCGTTCAGCAGCAGCGTCAC-3′ |
| esrA-P4 | 5′-TATATAGAATTCGGCCTGACGTTGGTACAT-3′ |
| esrB-P1 | 5′-ATATATGGTACCCGCGGGACATTATCAGGA-3′ |
| esrB-P2 | 5′-ATATATTCTAGATGCGAGAAAAGCGCGATC-3′ |
| esrB-P3 | 5′-TATATAGAATTCATTACGGATGCCATCGGC-3′ |
| esrB-P4 | 5′-TATATAGAATTCAACCTGATGCGCAAGCTG-3′ |
| esrC-P1 | 5′-TATATAGGTACCCGTCTGCAACGATACGCT-3′ |
| esrC-P2 | 5′-TATATATCTAGACCATTGTTGATGAGGGCC-3′ |
| esrC-P3 | 5′-TATATAGAATTCCAGCCTGAGCATGGTTTC-3′ |
| esrC-P4 | 5′-TATATAGAATTCCACTTCAGTCAGTCGCCA-3′ |
Underlined sequences indicate linkers incorporated into the primers used for cloning.
The ΔesrA and ΔesrB constructs were cloned into pGP704 (35) and introduced into wild-type E. ictaluri as described by Maurer et al. (33). Successful recombinants were verified by PCR and DNA sequencing. The ΔesrC construct was cloned into pRE107, a derivative of pGP704 that carries the sacB1 gene, which is lethal for Gram-negative bacteria in the presence of sucrose (16). The pRE107-ΔesrC construct was transferred to WT E. ictaluri by conjugation, and the recombinant colonies were selected as described by Edwards et al. (16). LB-Man-sucrose agar was used because single-crossover colonies broke through on BHI-sucrose agar. Successful double-crossover recombinants were confirmed by PCR and DNA sequencing. Following construction of the ΔesrC mutant, an esrC esrB double mutant was made using ΔesrC as the parent strain. The ΔesrB::km suicide vector was conjugated to ΔesrC as described above. Successful mutagenesis was verified by PCR and DNA sequencing. Mutation of EsrA removed amino acids 38 to 870 of the 911-amino-acid sequence, eliminating all but one of six transmembrane domains. Mutation of EsrB deleted amino acids 25 to 188 of the 214-amino-acid sequence, removing the helix-turn-helix (HTH) DNA-binding domain. Mutation of EsrC removed amino acids 101 to 211 of the 230 amino acid sequence, removing the two HTH DNA-binding domains. The ΔesrC ΔesrB::km double mutant contains both of the esrB and esrC mutations described above.
Complementation of T3SS regulatory mutants.
The regulatory genes esrA, esrB, and esrC, including upstream DNA containing the respective promoter regions, were amplified using gene-specific primers (Table 5). Amplicons were cloned into pBBR1-MCS4 and sequenced, resulting in the complementation plasmids pesrA, pesrB, and pesrC. The plasmids were transferred to WT E. ictaluri by conjugation (33). Successful transconjugants were verified by observing a third plasmid in the E. ictaluri plasmid profile on an agarose gel.
Table 5.
Primers used for the construction of complementation plasmids for ΔesrA::km, ΔesrB::km, and ΔesrC in this study
| Gene | Primer | Sequencea |
|---|---|---|
| esrA | 5′ | 5′-ATTTAATTTCTAGAATGCAGGTGATGCCG GAAA-3′ |
| 3′ | 5′-TTTAATTCTCGAGGCTGGAGGTTTAATCCGCCT-3′ | |
| esrB | 5′ | 5′-ATTTAATTTCTAGACGATGCATTCCACAA ATCCA-3′ |
| 3′ | 5′-ATTTAATTCTCGAGATACGCTAAAGGGGTT GGCC-3′ | |
| esrC | 5′ | 5′-TTTAATTTCTAGAATCGACTGCCTCAAT GACGC-3′ |
| 3′ | 5′-TTTAATTCTCGAGACCGTGACCATGTTTAGGCG-3′ |
Underlined sequences are linkers incorporated into the primers used for cloning.
Bacterial replication in channel catfish macrophages.
Head-kidney-derived-macrophages (HKDM) were collected from channel catfish and infected with WT and mutant E. ictaluri strains as described by Booth et al. (8), using a gentamicin exclusion assay (17). Macrophage lysates were serially diluted at 0, 5, and 10 h and plated on BA plates, and CFU per well were determined. Fold replication at each time point was calculated by dividing the CFU present at each time by the CFU present at time zero. Gentamicin exclusion experiments were repeated three times to establish reproducibility.
Channel catfish infection challenges.
Twenty-liter tanks were stocked with 25 channel catfish each, and triplicate tanks were challenged by immersion for 1 h with each E. ictaluri strain at a final concentration of 3 × 108 CFU/ml, after which water flow was restored. Dead fish were collected daily, and the presence of E. ictaluri was confirmed by streaking liver tissue from each mortality onto BA plates. Sampling continued until 3 days passed without a death in any treatment.
Statistical analyses.
Statistical differences were determined by analysis of variance (ANOVA) using the mixed procedure (Proc Mixed) of Statistical Analysis Systems v9.2 (SAS Institute, Inc., Cary, NC). Where ANOVA indicated a significant difference, least-square means with post hoc tests were used for pairwise comparisons. For qPCR data, relative expression values were log2 transformed for analysis. Dunnett's test was used to analyze changes in T3SS gene expression in the qPCR studies in response to pH and phosphate levels, as well as to compare the intracellular fold increase in HKDM replication of the T3SS regulatory gene mutants to that of WT E. ictaluri. Tukey's HSD was used to make pairwise comparisons of gene expression in the regulatory mutants and for the percent mortality data. Percent mortality was arcsine transformed prior to statistical analysis, although direct mortality data are reported in the graphs.
RESULTS
Low pH induces secretion of T3SS translocon proteins in vitro.
Three proteins secreted in culture during pH 5.5 medium (Fig. 1, WT) were identified by MALDI-TOF/TOF MS as EseB, EseC, and EseD, which are putative T3SS translocon proteins (52). EseB appears as multiple spots horizontally and vertically. Although the EseD spot is not readily visible because it is coalesced with the larger EseB spot, the protein was identified by MALDI-TOF/TOF MS in that region. A putative T6SS translocon protein, EvpC, was also identified in the ECP from low-pH medium. Similar spots were not visible in the ECP from pH 7.0 cultures, and phosphate limitation at low pH did not result in the appearance of additional protein spots (data not shown). Three proteins that were not present in the ECP were identified by MALDI-TOF/TOF MS in the WCL from pH 5.5 cultures, including the putative T3SS chaperone EscA and the T6SS proteins EvpA and EvpB (data not shown).
Fig. 1.
Two-dimensional polyacrylamide gel electrophoresis analyses of extracellular protein products of wild-type Edwardsiella ictaluri (WT) and T3SS regulatory gene mutants cultured in MM19 at pH 5.5. The T3SS translocon proteins EseB, EseC, and EseD and the T6SS protein EvpC are indicated by arrows. Circles indicate areas on the gel where protein spots occur in the WT ECP but are absent in the mutant ECP. Gel experiments were repeated three times, and representative gels are shown.
Low pH and phosphate limitation induce T3SS gene expression.
In order to further evaluate the effect of low pH and low phosphate concentration on regulatory and effector gene expression, qPCR analyses were conducted. Culture in low-pH medium, with or without phosphate limitation, resulted in a significant increase in expression of escC (Table 6), the first gene of the translocon operon (Table 2). This, along with the 2D-PAGE results, demonstrates that low pH is sufficient for both expression and secretion of the translocon proteins. Culture of E. ictaluri in low-phosphate pH 7.0 medium did not significantly increase expression of any T3SS genes (Table 6), indicating that low phosphate alone is not sufficient to upregulate gene expression. A combination of low pH and low phosphate, however, induced a significant increase in expression of the pEI1-carried effector gene, eseH, as well as the escB operon, which encodes the chaperone, EscB, and its effector, EseG. Although expression of the pEI2-carried effector, eseI, increased 8-fold in low-phosphate medium at pH 5.5, the difference was not statistically significant (P = 0.0646). The AraC-type regulator, esrC, was significantly downregulated by low pH but not by low pH with low phosphate. Expression of the two-component regulators, esrA and esrB, was unaffected by pH or phosphate conditions.
Table 6.
Effects of pH and phosphate on the expression of T3SS genes in Edwardsiella ictaluri
| Medium | Fold change for target transcripta: |
||||||
|---|---|---|---|---|---|---|---|
| escB | escC | eseH | eseI | esrA | esrB | esrC | |
| MM19, pH 5.5 | +3.2 | +6.2** | +1.1 | −1.1 | −2.5 | −2.0 | −5.0* |
| MMP, pH 7.0 | +1.4 | −1.7 | +2.1 | +3.7 | −1.7 | −1.4 | −2.5 |
| MMP, pH 5.5 | +27.5** | +7.3** | +28.6** | +8.1 | +2 | −1.4 | +3.1 |
Values are the fold increase or decrease in transcription of the T3SS genes relative to expression in E. ictaluri cultured in MM19, pH 7.0. Asterisks indicate a significant change in expression relative to MM19 at pH 7: *, P ≤ 0.01; **, P ≤ 0.001.
To determine if inducing conditions led to T3SS protein synthesis, Flag-tagged proteins were analyzed by using Western blotting. The EseH::Flag fusion was not detected under any conditions (data not shown). In agreement with the qPCR data, expression of EseB::Flag (escC operon) was upregulated in acidic medium independent of phosphate concentration, while EseG::Flag (escB operon) was upregulated only in acidic, low-phosphate medium (Fig. 2). Detection of EseI::Flag under low-pH, low-phosphate conditions indicates that the statistically insignificant increase in eseI expression in qPCR under low-pH, low-phosphate conditions is biologically significant (Table 6). Although expression of esrC was significantly reduced at low pH but not at low pH with low phosphate (Table 6), EsrC::Flag was detected equally under either condition (Fig. 2). This indicates that the small, but statistically significant, change in expression detected by qPCR is not biologically significant and that esrC is expressed at low pH regardless of phosphate concentration.
Fig. 2.
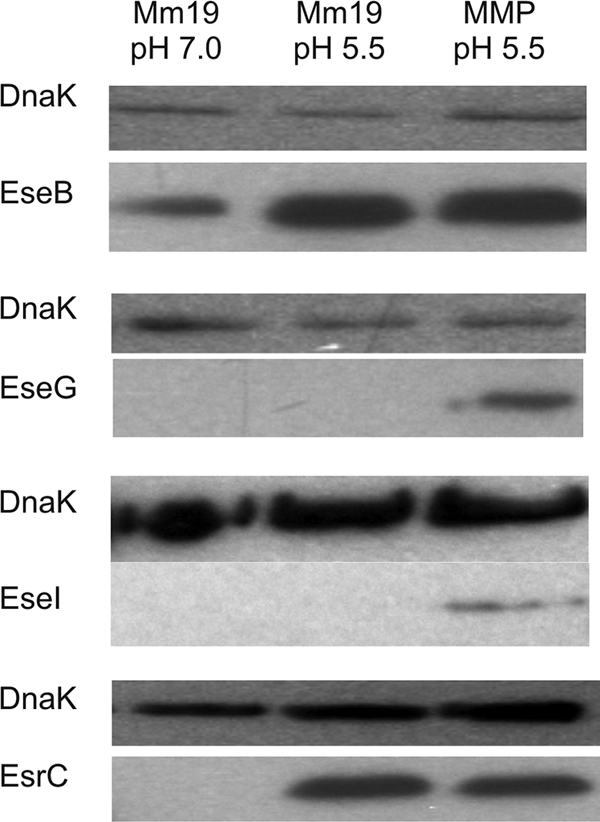
Western blot detection of T3SS proteins fused to the Flag epitope in Edwardsiella ictaluri whole-cell lysates. Strains carrying the fusions were cultured in MM19 at pH 7.0, MM19 at pH 5.5, and MMP at pH 5.5. Proteins carrying the Flag epitope were labeled with anti-Flag antibody followed by HRP-conjugated goat anti-mouse antibody to detect EsrC, EseB, and EseG. Detection of EseI required mouse anti-Flag antibody followed by biotinylated goat anti-mouse antibody and HRP-conjugated streptavidin. Loading of equivalent protein amounts across lanes was verified using mouse anti-DnaK antibody, biotinylated goat anti-mouse antibody, and HRP-conjugated streptavidin.
Replication within HKDM is dependent on EsrA and EsrB but not EsrC.
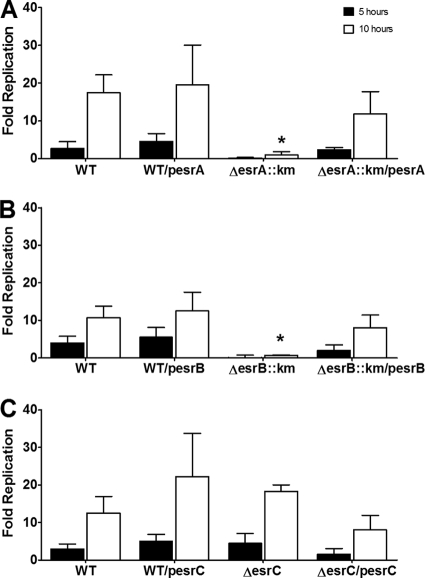
The ΔesrA::km and ΔesrB::km T3SS regulatory mutants exhibited severe deficiencies in replication within channel catfish HKDM (Fig. 3 A and B). The replication deficiency of both ΔesrA::km and ΔesrB::km was reversed by providing the WT genes in trans. In contrast, ΔesrC replicated as well as the 93-146 parent strain (Fig. 3C). The esrC esrB double mutant had a replication deficiency similar to that of the ΔesrB::km strain (data not shown). The results demonstrate that the regulatory activities of EsrA and EsrB, but not EsrC, are required for replication of E. ictaluri in HKDM.
Fig. 3.
Replication of Edwardsiella ictaluri strains carrying mutations in T3SS regulatory gene esrA (A), esrB (B), or esrC (C) in HKDM. Strains carrying complementation plasmids are labeled with pesrA, pesrB, or pesrC. Bars indicate the mean fold replication (± standard error of the mean [SEM]) at 5 and 10 h postinfection for triplicate assays. Asterisks indicate a significant difference from the WT fold increase at the same time point (P ≤ 0.05).
Mutation of T3SS regulatory genes attenuates E. ictaluri in channel catfish immersion challenges.
As predicted by the inability to replicate in HKDM, the ΔesrA::km and ΔesrB::km strains did not cause mortality in channel catfish (Fig. 4 A and B). Again, virulence was restored by expressing WT esrA and esrB in trans. Although the ΔesrC mutant maintained the ability to replicate within channel catfish HKDM, it was avirulent in channel catfish (Fig. 4C), indicating an important function for EsrC in virulence. Unlike for the ΔesrA::km and ΔesrB::km mutants, however, ΔesrC virulence was not fully rescued by the esrC complementation plasmid. Furthermore, WT E. ictaluri carrying the esrC complementation plasmid exhibited decreased virulence in channel catfish, suggesting that multiple-copy expression of the regulatory gene from the plasmid resulted in reduced virulence.
Fig. 4.
Cumulative mortality of channel catfish following infection by Edwardsiella ictaluri strains carrying mutations in T3SS regulatory gene esrA (A), esrB (B), or esrC (C). Strains carrying complementation plasmids are labeled with pesrA, pesrB, or pesrC. Bars indicate the mean daily cumulative percent mortality (± SEM) in triplicate challenge tanks. Mortality curves with the same letter show no significant difference in cumulative percent mortality (P > 0.05), based on analysis of arcsine-transformed data.
Effects of regulatory gene mutations on T3SS gene expression.
Mutations of esrA and esrB both resulted in a significant decrease in expression of the escB/eseG chaperone/effector genes, the pEI1 effector gene eseH, and the escC translocon operon (Table 7). Mutations of esrA and esrB also resulted in a significant reduction of esrC expression, indicating that expression of the AraC-type transcriptional activator is dependent on EsrAB. In each instance when expression of the target gene was reduced, the reduction was significantly greater in the esrB mutant than in the esrA mutant.
Table 7.
Effects of T3SS regulatory gene mutations on expression of T3SS genes in Edwardsiella ictaluri cultured in MMP at pH 5.5
| Mutant genotype | Fold change for target transcripta: |
||||||
|---|---|---|---|---|---|---|---|
| escB | escC | eseH | eseI | esrA | esrB | esrC | |
| ΔesrA::km | −6.3 A** | −4.8 A** | −3.9 A** | −1.6 A | +1.6 A | −6.0 A** | |
| ΔesrB::km | −155.5 B** | −49.2 B** | −22.0 B** | −2.2 A | −2.3 A | −85.9 B** | |
| ΔesrC | −11.9 A** | −2.1 A | −18.6 B** | −1.6 A | +2.3 B | +2.3 A | |
| ΔesrC ΔesrB::km | −156.7 B** | −52.3 B** | −28.7 B** | −2.4 A | −2.5 A | ||
Values are the fold increase or decrease in transcription of the T3SS genes relative to expression in WT E. ictaluri. Values within columns with the same letters are not significantly different (P > 0.01). Asterisks indicate a significant difference from WT: *, P ≤ 0.01; **, P ≤ 0.001.
The esrC mutant had a significant reduction in expression of the escB/eseG operon and eseH but not the escC translocon operon. The reduction in expression of the escB/eseG chaperone/effector operon was significantly greater in the esrB mutant than in the esrC mutant. Expression of eseH, however, decreased to similar levels in the esrB and esrC mutants. None of the mutations had a significant impact on expression of the pEI2-carried eseI effector gene. The esrC esrB double mutant was phenotypically identical to the esrB mutant.
T3SS regulatory gene mutants exhibit various deficiencies in protein secretion.
Although mutation of esrA resulted in significantly reduced transcription of the escC translocon operon (Table 7), the translocon proteins were readily detectable in both the ΔesrA::km and ΔesrC ECP (Fig. 1). Mutation of esrB, however, completely abrogated translocon protein secretion. Furthermore, the T6SS translocon protein, EvpC, was secreted by ΔesrA::km but not by ΔesrB::km or ΔesrC (Fig. 1), suggesting that the T6SS can be regulated by EsrB and EsrC through a pathway not requiring EsrA. The 2D-PAGE analysis of WCL from EsrB and EsrC mutants revealed that EvpC was not detected, indicating that the effect on EvpC was not due to an effect on secretion but was due to the loss of EvpC expression in both mutants (data not shown).
DISCUSSION
Thune et al. (52) reported that the E. ictaluri T3SS is required for replication in channel catfish HKDM and virulence. The results presented here demonstrate that expression of the E. ictaluri T3SS is upregulated under low-pH and phosphate-limited conditions, which mimic the intracellular milieu of a phagosome (18, 32, 41). The upregulation of this system, including the AraC-type regulator EsrC, is under the control of the EsrAB two-component regulatory system. EsrC is involved in the regulation of the T6SS protein, EvpC, suggesting coregulation of the two systems. EsrAB mutants are unable to replicate in HKDM and are avirulent. An EsrC mutant retains its ability to replicate in HKDM but is attenuated in vivo.
Model for regulation of the E. ictaluri T3SS.
Final analysis of the data presented here enabled the development of a model for regulation of the E. ictaluri T3SS (Fig. 5). Transcriptional analysis shows that the regulatory genes esrA and esrB are expressed but are unaffected by pH or phosphate (Table 6). In response to low pH, EsrB is presumably phosphorylated and upregulates expression of the translocon genes and the transcriptional regulatory gene, esrC. Mutation of esrA, however, does not reduce T3SS gene expression as much as mutation of esrB, indicating that EsrB can be phosphorylated in the absence of EsrA. Although low pH alone increases expression of esrC and the translocon proteins, a combination of low phosphate and low pH is required for upregulation of the putative E. ictaluri T3SS effectors, eseG and eseH, both of which require EsrC for optimal expression. Expression of eseI was upregulated by low pH/low phosphate, but was not affected by mutation of either esrB or esrC. This indicates that factors other than EsrBC are involved in the E. ictaluri T3SS signaling cascade or that EseI is a secreted effector for a system other than the T3SS.
Fig. 5.
Proposed model for pH and phosphate regulation of the E. ictaluri T3SS.
Despite significant transcriptional upregulation in low-pH/low-phosphate medium (Table 6), EseH::Flag is not observed under any condition, even using biotin/streptavidin to enhance detection. The lack of EseH::Flag may be due either to a low level of translation or to mRNA secondary structure. Translation of some Yersinia T3SS effector proteins is regulated by the secondary structure of the message near the ribosome-binding site, which prevents ribosome binding (1). Upon further signaling, the mRNA contacts the T3SS machinery, resolving the secondary structure and allowing simultaneous translation and translocation of the protein.
Expression of the apparatus proteins was not evaluated in this study. Expression of EsrC at low pH (Fig. 2), however, suggests that the seven apparatus genes included in the esrC operon (Table 2) are also expressed. Because translocon proteins were secreted and secretion depends on the secretion apparatus, it is evident that the remaining apparatus genes of the T3SS PAI were also expressed and a functional needle complex was constructed.
Secretion of E. ictaluri T6SS proteins is influenced by EsrB and EsrC.
The putative T6SS translocon protein EvpC is secreted by E. ictaluri in low-pH medium (Fig. 1). Mutation of either esrB or esrC abrogates EvpC expression, indicating a central role for EsrB and EsrC. Because EsrC expression is lost in an EsrB mutant (Table 7), it is likely that EsrB has an indirect effect on EvpC expression through its regulation of EsrC. EsrB and EsrC of E. tarda positively affect T6SS expression and secretion (49, 54, 57), similar to the results observed here (Fig. 1). In E. tarda, EvpC is a putative translocon protein that forms a tube-like structure (26) and is required for secretion of the E. tarda T6SS proteins EvpI and EvpP (56). Zheng et al. (56) reported that individual mutations in 14 T6SS genes, including evpC, evpI, and evpP, attenuate virulence of E. tarda in fish, but the effects on intracellular replication were not reported.
Bioinformatic analysis of the region of the E. ictaluri genome surrounding EvpC identified a 16-gene PAI encoding a T6SS with 80 to 99% amino acid identity to that of E. tarda. EvpO is a putative T6SS apparatus protein in the bacterial membrane (9, 45) that has 43% homology to 350 amino acids at the amino terminus of the Salmonella T6SS protein SciS. The EsrB homologue in Salmonella, SsrB, represses expression of sciS, delaying it until 24 to 27 h postinfection of macrophages. Mutation of SciS results in intracellular hyperreplication and hypervirulence, suggesting that the Salmonella T6SS acts to control intracellular bacterial levels at later stages of infection and attenuates virulence (40). The E. ictaluri esrC mutant fails to produce EvpC but retains its ability to replicate in HKDM. This suggests that the E. ictaluri T6SS is not required for intracellular replication and the possibility that the E. ictaluri T6SS has a similar function in limiting intracellular replication.
The T3SS regulatory proteins are required for E. ictaluri pathogenesis.
The current study demonstrates that mutation of either esrA or esrB results in a significant reduction in T3SS gene expression, resulting in severe attenuation of intracellular replication and virulence. While an esrC mutant maintains the ability to replicate in HKDM, it is avirulent in channel catfish, demonstrating that the EsrC regulon is essential for E. ictaluri pathogenesis. The ability of the ΔesrC mutant to replicate in HKDM suggests that the EsrC-dependent T3SS proteins, EseH and EseG, as well as the T6SS protein EvpC are not required for intracellular replication. EseH has homology to both SspH1 and SspH2 of Salmonella (20, 52), which do not affect intracellular replication when mutated (34). EseG is similar to SseG of Salmonella (52), which is required for intracellular replication (43), but an eseG mutation in E. tarda does not attenuate virulence (55). As discussed above, the E. tarda EvpC is required for virulence, but there are no reports concerning its role in intracellular replication.
Comparison of the E. ictaluri, E. tarda, and Salmonella T3SSs.
Although the E. tarda T3SS is very similar to that of E. ictaluri (52), there are significant differences in expression and secretion of key translocon proteins. First, Zheng et al. (57) determined that EsrC is required for expression of translocon and effector genes of E. tarda. The data reported here demonstrate that EsrC of E. ictaluri has little or no involvement in translocon expression but has an important role in the expression of the putative effector, eseH (Table 7). Second, the E. tarda T3SS response to low pH is the opposite of that described here for E. ictaluri. Expression and secretion of the E. tarda translocon proteins are inhibited by low pH (39), while neutral or alkaline conditions promote expression and secretion (39, 48, 49, 57). Furthermore, the pH of phagosomes that contain WT E. tarda is neutral, but it is acidic when phagosomes contain a T3SS-deficient strain (39). The authors suggest that the E. tarda T3SS secretes proteins that prevent acidification of the phagosome and promote E. tarda survival and growth. In contrast, E. ictaluri encodes an acid-activated urease thought to modulate phagosomal pH (7), suggesting that the E. ictaluri T3SS is not involved in phagosomal pH modulation.
Expression of esrB in E. tarda is upregulated by PhoPQ at 23 to 35°C or when Mg2+ concentrations are reduced to 1 mM (10). Edwardsiella ictaluri infections occur primarily within a temperature range of 22 to 28°C (21), indicating that water temperature is important in pathogenesis. Expression of esrB in E. ictaluri is stable at both pH levels and phosphate concentrations studied here, but all cultures were grown at 28°C in MM19, which has an Mg2+ concentration of 1 mM (12). Thus, conditions were always conducive to the expression of esrB according to the results for E. tarda. PhoPQ are present in E. ictaluri, but further research is required to determine if temperature and Mg2+ concentration contribute to expression of esrB.
The reason for the differences in pathogenesis between E. tarda and E. ictaluri is unclear. Except for the PAI-encoded effector EseG, which acts to destabilize microtubules in E. tarda-infected host cells (55), T3SS effectors remain unidentified for E. tarda. Although the two secretory machines are highly homologous, analysis of the E. tarda genome failed to detect sequences with homology to the putative E. ictaluri effector EseH or EseI. The differences in pathogenesis may be related to differences in the effector proteins, but further identification of translocated effectors and their functions is required to clarify the situation.
Similar to the case for E. tarda, regulation of ssrAB in SPI-2 is through the global regulator PhoPQ, but low phosphate is the primary cue, rather than temperature (15). Although low pH induces expression (13) and secretion (5) of the SPI-2 T3SS translocon proteins, similar to the case for E. ictaluri, pH does not have an effect on ssrAB expression (15). A second two-component system, OmpR/EnvZ, also has regulatory control over ssrAB (19), possibly functioning shortly after internalization in the macrophage, while PhoPQ has activity later (6). The transcriptional regulator SlyA also acts through SsrAB, exhibiting redundancy with OmpR/EnvZ (28). Although expression of esrA or esrB in E. ictaluri was not affected by low-pH or low-phosphate conditions (Table 6), PhoP and/or OmpR may exhibit regulatory control in response to changes in other parameters, like temperature or low Mg+2 in E. tarda. Like in Salmonella (53), however, it is apparent that EsrB can be phosphorylated in the absence of EsrA, possibly by PhoP or OmpR. Analysis of the E. ictaluri genome (GenBank accession no. NC_012779) indicates that E. ictaluri encodes proteins similar to PhoPQ, OmpR/EnvZ, and SlyA, but a role in E. ictaluri virulence is not documented.
In conclusion, we have demonstrated that the EsrAB two-component regulatory system regulates the expression of the E. ictaluri T3SS and is essential for virulence. We propose that exposure of E. ictaluri to the initial acidic pH and phosphate limitation within the phagosome induces EsrB-dependent expression of T3SS apparatus and translocon proteins, as well as the transcriptional activator EsrC. Regulatory activity of EsrB and EsrC results in expression of T3SS effector proteins and EvpC, a T6SS protein. Further investigation of the E. ictaluri T3SS is required to determine what specific effects it has on host cell function and what effect the interaction with the T6SS has on pathogenesis.
ACKNOWLEDGMENTS
This research was supported by the National Research Initiative of the U.S. Department of Agriculture Cooperative State Research, Education, and Extension Service with grant number 2009-35204-05312 to Ronald L. Thune. The project described was supported by NIH grant number P20 RR-016464 from the INBRE Program of the National Center for Research Resources.
Footnotes
Published ahead of print on 6 May 2011.
REFERENCES
- 1. Anderson D. M., Schneewind O. 1997. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science 278:1140–1143 [DOI] [PubMed] [Google Scholar]
- 2. Anonymous 2003. Catfish 2003. I. Reference of fingerling catfish health and production practices in the United States. N406.1103. USDA, APHIS, VS, CEAH, National Animal Health Monitoring System, Fort Collins, CO: http://www.aphis.usda.gov/animal_health/nahms/aquaculture/downloads /catfish03/Cat03_dr_PartI.pdf [Google Scholar]
- 3. Anonymous 2003. Catfish 2003. II. Reference of foodsize catfish health and production practices in the United States. N406.1103. USDA, APHIS, VS, CEAH, National Animal Health Monitoring System, Fort Collins, CO: http://www.aphis.usda.gov/animal_health/nahms/aquaculture/downloads /catfish03/Cat03_dr_PartII.pdf [Google Scholar]
- 4. Ausubel F. M., et al. 1994. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, NY [Google Scholar]
- 5. Beuzon C. R., Banks G., Deiwick J., Hensel M., Holden D. W. 1999. pH-dependent secretion of SseB, a product of the SPI-2 type III secretion system of Salmonella typhimurium. Mol. Microbiol. 33:806–816 [DOI] [PubMed] [Google Scholar]
- 6. Bijlsma J. J., Groisman E. A. 2005. The PhoP/PhoQ system controls the intramacrophage type three secretion system of Salmonella enterica. Mol. Microbiol. 57:85–96 [DOI] [PubMed] [Google Scholar]
- 7. Booth N. J., Beekman J. B., Thune R. L. 2009. Edwardsiella ictaluri encodes an acid-activated urease that is required for intracellular replication in channel catfish (Ictalurus punctatus) macrophages. Appl. Environ. Microbiol. 75:6712–6720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Booth N. J., ElKamel A., Thune R. L. 2006. Intracellular replication of Edwardsiella ictaluri in channel catfish macrophages. J. Aquat. Anim. Health 18:101–108 [Google Scholar]
- 9. Boyer F., Fichant G., Berthod J., Vandenbrouck Y., Attree I. 2009. Dissecting the bacterial type VI secretion system by a genome wide in silico analysis: what can be learned from available microbial genomic resources? BMC Genomics 10:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chakraborty S., et al. 2010. Temperature and Mg2+ sensing by a novel PhoP-PhoQ two-component system for regulation of virulence in Edwardsiella tarda. J. Biol. Chem. 285:38876–38888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cirillo D. M., Valdivia R. H., Monack D. M., Falkow S. 1998. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol. Microbiol. 30:175–188 [DOI] [PubMed] [Google Scholar]
- 12. Collins L. A., Thune R. L. 1996. Development of a defined minimal medium for the growth of Edwardsiella ictaluri. Appl. Environ. Microbiol. 62:848–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coombes B. K., Brown N. F., Valdez Y., Brumell J. H., Finlay B. B. 2004. Expression and secretion of Salmonella pathogenicity island-2 virulence genes in response to acidification exhibit differential requirements of a functional type III secretion apparatus and SsaL. J. Biol. Chem. 279:49804–49815 [DOI] [PubMed] [Google Scholar]
- 14. Crumlish M., Thanh P. C., Koesling J., Tung V. T., Gravningen K. 2010. Experimental challenge studies in Vietnamese catfish, Pangasianodon hypophthalmus (Sauvage), exposed to Edwardsiella ictaluri and Aeromonas hydrophila. J. Fish Dis. 33:717–722 [DOI] [PubMed] [Google Scholar]
- 15. Deiwick J., Nikolaus T., Erdogan S., Hensel M. 1999. Environmental regulation of Salmonella pathogenicity island 2 gene expression. Mol. Microbiol. 31:1759–1773 [DOI] [PubMed] [Google Scholar]
- 16. Edwards R. A., Keller L. H., Schifferli D. M. 1998. Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene 207:149–157 [DOI] [PubMed] [Google Scholar]
- 17. Elsinghorst E. A. 1994. Measurement of invasion by gentamicin resistance. Methods Enzymol. 236:405–420 [DOI] [PubMed] [Google Scholar]
- 18. Eriksson S., Lucchini S., Thompson A., Rhen M., Hinton J. C. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47:103–118 [DOI] [PubMed] [Google Scholar]
- 19. Feng X., Oropeza R., Kenney L. J. 2003. Dual regulation by phospho-OmpR of ssrA/B gene expression in Salmonella pathogenicity island 2. Mol. Microbiol. 48:1131–1143 [DOI] [PubMed] [Google Scholar]
- 20. Fernandez D. H., Pittman-Cooley L., Thune R. L. 2001. Sequencing and analysis of the Edwardsiella ictaluri plasmids. Plasmid 45:52–56 [DOI] [PubMed] [Google Scholar]
- 21. Francis-Floyd R., Beleau M. H., Waterstrat P. R., Bowser P. R. 1987. Effect of water temperature on the clinical outcome of infection with Edwardsiella ictaluri in channel catfish. J. Am. Vet. Med. Assoc. 191:1413–1416 [PubMed] [Google Scholar]
- 22. Hawke J. P. 1979. A bacterium associated with disease of pond-cultured channel catfish, Ictalurus punctatus. J. Fish. Res. Board Can. 36:1508–1512 [Google Scholar]
- 23. Hawke J. P., McWhorter A. C., Steigerwalt A. G., Brenner D. J. 1981. Edwardsiella ictaluri sp. nov., the causative agent of enteric septicemia of catfish. Int. J. Syst. Bacteriol. 31:396–400 [Google Scholar]
- 24. Hensel M., et al. 1998. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol. Microbiol. 30:163–174 [DOI] [PubMed] [Google Scholar]
- 25. Herrero M., de Lorenzo V., Timmis K. N. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 172:6557–6567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jobichen C., et al. 2010. Structural basis for the secretion of EvpC: a key type VI secretion system protein from Edwardsiella tarda. PLoS One 5:e12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kovach M. E., et al. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176 [DOI] [PubMed] [Google Scholar]
- 28. Linehan S. A., Rytkonen A., Yu X. J., Liu M., Holden D. W. 2005. SlyA regulates function of Salmonella pathogenicity island 2 (SPI-2) and expression of SPI-2-associated genes. Infect. Immun. 73:4354–4362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu J. Y., Li A. H., Zhou D. R., Wen Z. R., Ye X. P. 2010. Isolation and characterization of Edwardsiella ictaluri strains as pathogens from diseased yellow catfish Pelteobagrus fulvidraco (Richardson) cultured in China. Aquacult. Res. 41:1835–1844 [Google Scholar]
- 30. Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 31. Lobb C. J., Ghaffari S. H., Hayman J. R., Thompson D. T. 1993. Plasmid and serological differences between Edwardsiella ictaluri strains. Appl. Environ. Microbiol. 59:2830–2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lober S., Jackel D., Kaiser N., Hensel M. 2006. Regulation of Salmonella pathogenicity island 2 genes by independent environmental signals. Int. J. Med. Microbiol. 296:435–447 [DOI] [PubMed] [Google Scholar]
- 33. Maurer K. J., Lawrence M. L., Fernandez D. H., Thune R. L. 2001. Evaluation and optimization of a DNA transfer system for Edwardsiella ictaluri. J. Aquat. Anim. Health 13:163–167 [Google Scholar]
- 34. Miao E. A., et al. 1999. Salmonella typhimurium leucine-rich repeat proteins are targeted to the SPI1 and SPI2 type III secretion systems. Mol. Microbiol. 34:850–864 [DOI] [PubMed] [Google Scholar]
- 35. Miller V. L., Mekalanos J. J. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moore M. M., Thune R. L. 1999. Evaluation of antigenic and nonantigenic proteins of Edwardsiella ictaluri at two culture temperatures and in enriched and minimal media using two-dimensional polyacrylamide gel electrophoresis. J. Aquat. Anim. Health 11:262–274 [Google Scholar]
- 37. Newton J. C., Bird R. C., Blevins W. T., Wilt G. R., Wolfe L. G. 1988. Isolation, characterization and molecular cloning of cryptic plasmids isolated from Edwardsiella ictaluri. Am. J. Vet. Res. 49:1856–1860 [PubMed] [Google Scholar]
- 38. Okuda J., et al. 2006. Intracellular replication of Edwardsiella tarda in murine macrophage is dependent on the type III secretion system and induces an up-regulation of anti-apoptotic NF-κB target genes protecting the macrophage from staurosporine-induced apoptosis. Microb. Pathog. 41:226–240 [DOI] [PubMed] [Google Scholar]
- 39. Okuda J., et al. 2009. Characterization of proteins secreted from a type III secretion system of Edwardsiella tarda and their roles in macrophage infection. Dis. Aquat. Org. 84:115–121 [DOI] [PubMed] [Google Scholar]
- 40. Parsons D. A., Heffron F. 2005. sciS, an icmF homolog in Salmonella enterica serovar Typhimurium, limits intracellular replication and decreases virulence. Infect. Immun. 73:4338–4345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rathman M., Sjaastad M. D., Falkow S. 1996. Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect. Immun. 64:2765–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reid W. S., Boyle J. A. 1989. Plasmid homologies in Edwardsiella ictaluri. Appl. Environ. Microbiol. 55:3253–3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Salcedo S. P., Holden D. W. 2003. SseG, a virulence protein that targets Salmonella to the Golgi network. EMBO J. 22:5003–5014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schmittgen T. D., Livak K. J. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3:1101–1108 [DOI] [PubMed] [Google Scholar]
- 45. Shrivastava S., Mande S. S. 2008. Identification and functional characterization of gene components of type VI secretion system in bacterial genomes. PLoS One 3:e2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Simon R., Priefer U., Puhler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Nat. Biotechnol. 1:784–791 [Google Scholar]
- 47. Speyerer D., Boyle J. A. 1987. The plasmid profile of Edwardsiella ictaluri. J. Fish Dis. 10:461–469 [Google Scholar]
- 48. Srinivasa Rao P. S., Lim T. M., Leung K. Y. 2003. Functional genomics approach to the identification of virulence genes involved in Edwardsiella tarda pathogenesis. Infect. Immun. 71:1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Srinivasa Rao P. S., Yamada Y., Tan Y. P., Leung K. Y. 2004. Use of proteomics to identify novel virulence determinants that are required for Edwardsiella tarda pathogenesis. Mol. Microbiol. 53:573–586 [DOI] [PubMed] [Google Scholar]
- 50. Tan Y. P., Zheng J., Tung S. L., Rosenshine I., Leung K. Y. 2005. Role of type III secretion in Edwardsiella tarda virulence. Microbiology 151:2301–2313 [DOI] [PubMed] [Google Scholar]
- 51. Thinh N. H., et al. 2009. Combined immersion and oral vaccination of Vietnamese catfish (Pangasianodon hypophthalmus) confers protection against mortality caused by Edwardsiella ictaluri. Fish Shellfish Immunol. 27:773–776 [DOI] [PubMed] [Google Scholar]
- 52. Thune R. L., et al. 2007. Signature-tagged mutagenesis of Edwardsiella ictaluri identifies virulence-related genes, including a Salmonella pathogenicity island 2 class of type III secretion systems. Appl. Environ. Microbiol. 73:7934–7946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Walthers D., et al. 2007. The response regulator SsrB activates expression of diverse Salmonella pathogenicity island 2 promoters and counters silencing by the nucleoid-associated protein H-NS. Mol. Microbiol. 65:477–493 [DOI] [PubMed] [Google Scholar]
- 54. Wang X., et al. 2009. Edwardsiella tarda T6SS component evpP is regulated by esrB and iron, and plays essential roles in the invasion of fish. Fish Shellfish Immunol. 27:469–477 [DOI] [PubMed] [Google Scholar]
- 55. Xie H. X., et al. 2010. EseG, an effector of the type III secretion system of Edwardsiella tarda, triggers microtubule destabilization. Infect. Immun. 78:5011–5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zheng J., Leung K. A. 2007. Dissection of a type VI secretion system in Edwardsiella tarda. Mol. Microbiol. 66:1192–1206 [DOI] [PubMed] [Google Scholar]
- 57. Zheng J., Tung S. L., Leung K. Y. 2005. Regulation of a type III and a putative secretion system in Edwardsiella tarda by EsrC is under the control of a two-component system, EsrA-EsrB. Infect. Immun. 73:4127–4137 [DOI] [PMC free article] [PubMed] [Google Scholar]