Abstract
Recent advances in systems biology, omics, and computational studies allow us to carry out data mining for improving biofuel production bioprocesses. Of particular interest are bioprocesses that center on microbial capabilities to biotransform both the hexose and pentose fractions present in crop residues. This called for a systematic exploration of the components of the media to obtain higher-density cultures and more-productive fermentation operations than are currently found. By using a meta-analysis approach of the transcriptional responses to butanol stress, we identified the nutritional requirements of solvent-tolerant strain Clostridium beijerinckii SA-1 (ATCC 35702). The nutritional requirements identified were later validated using the chemostat pulse-and-shift technique. C. beijerinckii SA-1 was cultivated in a two-stage single-feed-stream continuous production system to test the proposed validated medium formulation, and the coutilization of d-glucose and d-xylose was evaluated by taking advantage of the well-known ability of solventogenic clostridia to utilize a large variety of carbon sources such as mono-, oligo-, and polysaccharides containing pentose and hexose sugars. Our results indicated that C. beijerinckii SA-1 was able to coferment hexose/pentose sugar mixtures in the absence of a glucose repression effect. In addition, our analysis suggests that the solvent and acid resistance mechanisms found in this strain are differentially regulated compared to strain NRRL B-527 and are outlined as the basis of the analysis toward optimizing butanol production.
INTRODUCTION
Butanol is an important commodity molecule used in the manufacturing of a number of familiar products such as enamels, lacquers, latex surface coating, and plasticizers and as a solvent in the production of hormones, vitamins, and antibiotics (42). Butanol can also act as a direct replacement for gasoline, since its characteristics as fuel additive make it superior to ethanol with regard to its energy content, lower volatility, less hydroscopic, and less corrosive properties (18). The current market for butanol has been estimated at 7 to 8.4 billion dollars per year (17) with a projected expansion of 3% per year (37), which makes the production of butanol as a biofuel molecule very attractive in today's market. In recent years, high crude oil prices and the adverse effects of greenhouse gas emissions have renewed interest in the production of biobutanol in developed and emerging economies (42). The common goal is to substitute 5 to 10% of fossil fuels with biofuels, including butanol, within the next 5 years. However, food, feedstock, and biofuel productions compete for the same agricultural land, creating a situation that could greatly impact feedstock prices and food availability (20, 63).
As an alternative to sustainable starch and sugar, attractive substrates for microbial biofuel production may well be plant biomass or low-cost non-grain-based feedstocks (i.e., agriculture wastes), which are cheaper and abundant. It is thought that the use of plant biomass could potentially reduce the vast quantity of land area currently dedicated to energy-producing crops. Ongoing research has drawn considerable interest in evaluating the potential of many crop residues, such as straw and wood shavings, for providing the sugars necessary to support biofuel production (24). One of the most abundant sugar components of plant biomass are hemicelluloses, which are heteropolymers of pentose and hexose sugars where d-xylose is the main pentose. However, repression of d-xylose utilization by d-glucose, known as catabolite repression, can cause technological problems in d-xylose utilization when using plant hydrolysates (40, 41). In solventogenic clostridia, catabolite repression of d-xylose utilization by d-glucose (21, 22) and the utilization of arabinose, xylose, and ribose is controlled by AraR, XylR, and RbsR, respectively (60). In Clostridium acetobutylicum, the catabolite repressor protein CcpA appears to regulate transcription of the xylose catabolic genes, and its inactivation allows for cofermentation of glucose and xylose (59). In addition, these regulators may be involved in integrating different metabolic branches to achieve well-balanced growth, as demonstrated by the tight coupling between carbon and nitrogen metabolism in Bacillus subtilis (47). From an economic standpoint, the feasibility of a bioprocess for biofuel production from low-cost non-grain-based feedstocks relies on the producer strain's ability to overcome the glucose repression effect for efficient biotransformation of both the hexose and pentose fractions in the feedstock. Solventogenic clostridia have attracted the attention of the biofuel industry, because they offer a major advantage for producing solvents from a large variety of carbon sources such as mono-, oligo-, and polysaccharides from starches and biomass hydrolysate containing pentose and hexose sugars (15, 55).
Solventogenic clostridia are one of the few organisms able to ferment xylose, arabinose, and ribose to produce acetone, butanol, and ethanol (ABE fermentation) while simultaneously showing a significant resistance to the solvent's toxic effects (43). A vast number of reports describe solvent production by using closed production systems where the final accumulation of solvent is limited by strain sporulation, which is triggered by solvent toxicity. Since solvent stress is closely coupled to sporulation (56), operating continuous butanol production processes can be challenging. Despite these difficulties, the continuous mode operation with high-volume large-scale fermentation process is more advantageous and desirable. Continuous processes allow better control of the cell physiological state (30), attain higher productivity, have lower production costs, and improved process control. Because ABE fermentation is biphasic, where during the acidogenic phase butyric acid is generated and in the solventogenic phase the butyric acid is converted to butanol, a process in a multistage reactor appears to be best suited for allowing us to take further advantage of the self-regulating capacity of the continuous culture system.
Clostridium cell densities during butanol production are typically low, reaching optical density values below 10 or 11 OD600 units (OD600 stands for optical density at 600 nm). Low cell densities have been connected to factors that include but are not limited to low aerotolerance, sporulation, acid intolerance, and strain instability and theoretically connected to the metabolic status of the cells through quorum sensing (55). The sporulation pathway is influenced by a number of factors, including nutritional limitations during growth (1, 33, 45). Standard medium formulations for microbial production of butanol meet the transient needs of many clostridial strains; however, these formulations have not dramatically changed since the time fermentation of clostridia began. It is naïve to assume that a desired optimal production phenotype can be achieved through a “one size fits all” medium. New formulations are essential for cultivation production and can make a considerable impact on selected or genetically engineered strains with a desirable trait requiring key ingredients that are often overlooked. Studies on the behavior and nutritional needs of cells when confronted with stressors that may interfere with a desirable trait should be completed before a multistage continuous process can be implemented. Examining conditions and nutrients for strains or strain derivatives that lead to higher-density cultures require systematic assessments of medium components that can support more-productive fermentation operations (55). However, optimization of medium formulations for clostridial fermentation have been hindered by the inherent instability of butanol-producing strains (7, 31, 35). For this reason, stable butanol-producing strains such as SA-1 (43) need to be reevaluated as part of a course for medium optimization that can lead to improvements in the low-yield traditional transient production processes.
Approaches used to obtain solventogenic Clostridium spp. capable of increased butanol production include strain isolation (10, 62), strain selection (43, 58, 59, 64), gene overexpression (16, 57, 66), and site-directed and random mutagenesis (5, 11, 29). Clostridium beijerinckii mutant SA-1 (ATCC 35702), selected by Lin and Blaschek (43), can withstand butanol concentrations toxic to the parental wild-type strain by delaying the onset of sporulation at high butanol concentrations. We have taken a meta-analysis approach to defining medium requirements for sustaining the growth of SA-1 (ATCC 35702) with the goal of maintaining the culture in a predominantly vegetative state, thereby increasing the number of cells able to efficiently biotransform mixed carbon sources while generating end products, including butanol.
We examined transcriptional data published by Alsaker et al. (2) to identify nutrient requirements of C. acetobutylicum growing under butanol stress. Our analysis was then contrasted with previously described medium compositions (43, 54, 69, 70), the components consolidated and later validated in a formulation focused on improving the production of butanol while using sugar mixtures rich in six- and five-carbon sources (d-glucose and d-xylose). In this study, we report a two-step continuous process/production strategy after identifying distinctive nutritional and environmental conditions that are better suited for butanol production when cultivating C. beijerinckii SA-1.
MATERIALS AND METHODS
Strains, media, and cultivation methods.
The Clostridium beijerinckii SA-1 strain (ATCC 35702) (referred to as SA-1/ATCC 35702) was deposited by Lin and Blaschek (43) as an offspring strain derived from Clostridium acetobutylicum ATCC 824. Later, Johnson et al. (32) identified SA-1as belonging to the C. beijerinckii species by DNA relatedness. After this finding, Blaschek recatalogued their ATCC 824 strain as C. beijerinckii NCIMB 8052 (23). For this work, Clostridium beijerinckii NRRL B-527 was obtained from the Agricultural Research Service (ARS) (Northern Regional Research Laboratory [NRRL]) Culture Collection, and C. beijerinckii SA-1 (ATCC 35702) was obtained from the American Type Culture Collection. Species identity was verified by PCR amplifying the 16S rRNA gene using prokaryotic 16S rRNA gene universal primers 515F (F stands for forward) (5′-GCGGATCCTCTAGACTGCAGTGCCA-3′) and 1492R (R stands for reverse) (5′-GGTTACCTTGTTACGACTT-3′). The organisms were routinely maintained at −80°C on reinforced clostridial medium (RCM) (Oxoid, England) containing glycerol (10%), and this medium was chosen for batch experiments. The compositions of the newly consolidated and validated media are shown in Tables 3 and 4. The base medium components were always sterilized without d-glucose, d-xylose, and trace elements. These components were always sterilized by filtration and added aseptically to the medium reservoir.
Table 3.
Composition of media used to cultivate Clostridium spp.
| Component | Component concn (g/liter) in mediuma: |
|||||
|---|---|---|---|---|---|---|
| Consolidated medium of this study | Wiesenborn et al. (69) | Ounine et al. (54) | Lin and Blaschek (43) | Woolley and Morris (70) | Long et al. (45) | |
| KH2PO4 | 3 | 0.75 | 0.5 | 0.75 | 3 | |
| K2HPO4·3H2O | 0.5 | |||||
| K2HPO4 | 0.75 | 0.75 | ||||
| MgSO4·7H2O | 0.03 | 0.2 | 0.03 | 0.2 | ||
| MgSO4 | 0.4 | 0.02 | ||||
| MnSO4 | 0.01 | 0.01 | 0.01 | |||
| MnCl2·4H2O | 0.01 | 0.01 | 0.01 | |||
| FeSO4·7H2O | 0.01 | 0.01 | 0.01 | |||
| FeSO4·2H2O | 0.01 | 0.01 | 0.01 | |||
| NaCl | 0.01 | 1.0 | 0.01 | 1 | ||
| CH3COONH4 | 2.2 | |||||
| (NH4)2HPO4 | 6 | |||||
| NH4Cl | 3 | 3 | ||||
| (NH4)2SO4 | 0.5 | 2.0 | 2 | 0.5 | ||
| CaCl2 | 0.011 | 0.011 | ||||
| CaCO3 | 20 | |||||
| CuSO4·5H2O | 0.032 | 0.032 | ||||
| CoCl2·6H2O | 0.032 | 0.032 | ||||
| ZnSO4 | 0.044 | 0.044 | 0.05 | |||
| (NH4)6Mo7O24·4H2O | 0.022 | 0.022 | ||||
| Biotin | 0.001 | 1.10−5 | 0.001 | tr | ||
| p-Aminobenzoic acid | 0.002 | 0.001 | 0.002 | 0.001 | ||
| Thiamine HCl | 0.002 | 0.002 | 0.001 | |||
| Cysteine | 0.05 | 0.05 | 0.5 | |||
| Asparagine | 0.05 | 2.0 | 2 | |||
| Yeast extract | 1 | 5.0 | 10 | |||
| EDTA | 0.1 mM | |||||
| Tryptophan | 0.05 | |||||
| Histidine | 0.05 | |||||
| Glutamine | 0.05 | |||||
| Riboflavin | 0.001 | |||||
| Vitamin B12 | 0.001 | |||||
| Carbon source | d-Glucose/d-xylose | d-Glucose | Several | Corn starch | d-Glucose | d-Glucose |
The first or leftmost column shows the composition of the consolidated medium utilized for the cultivation of Clostridium spp. used in this study. The medium was formulated after evaluation of meta-analysis data from cultures of C. acetobutylicum ATCC 824 exposed to butanol (Tables 1 and 2) and through comparisons to media described in the references shown. Concentrations are expressed in grams/liter except for EDTA, which is expressed in millimolar.
Table 4.
Composition of the validated medium formulated in this study to cultivate C. beijerinkii SA-1a
| Component of validated medium | Final concn |
|---|---|
| Medium components | |
| KH2PO4 | 3 g/liter |
| NH4Cl | 3 g/liter |
| (NH4)SO4 | 0.5 g/liter |
| NaCl | 0.01 g/liter |
| EDTA | 0.1 mM |
| Yeast extract | 2 g/liter |
| Tween 80 | 1 ml/liter |
| Trace componentsb | |
| MgSO4·7H2O | 0.1 g/liter |
| CaCl2 | 22 mg/liter |
| MnCl2·4H2O | 20 mg/liter |
| FeSO4·2H2O | 20 mg/liter |
| CuSO4·5H2O | 6.4 mg/liter |
| CoCl2·6H2O | 6.4 mg/liter |
| ZnSO4 | 8.8 mg/liter |
| (NH4)6Mo7O24·4H2O | 4.4 mg/liter |
| Biotin | 1 mg/liter |
| p-Aminobenzoic acid | 2 mg/liter |
| Thiamine HCl | 2 mg/liter |
| Cysteine | 0.1 g/liter |
| Asparagine | 0.1 g/liter |
| Tryptophan | 0.1 g/liter |
| Histidine | 0.1 g/liter |
| Glutamine | 0.1 g/liter |
| Riboflavin | 0.001 g/liter |
| Vitamin B12 | 0.001 g/liter |
Validated medium composition was obtained by applying the pulse-and-shift optimization technique (Fig. 3). The carbon source(s) is not included in the formula.
Trace components were prepared by filtration and aseptically added to the medium.
Growth experiments were performed in 2-liter Biostat B plus fermentors equipped with controllers for pH, temperature, and agitation (Sartorius BBI Systems, Melsungen, Germany). Single-stage experiments were carried out in chemostat mode of operation with a working volume of 900 ml and agitation speed of 200 rpm. Later, experiments employing two reactors operating in cascade were carried out. The first reactor was operated with a working volume of 700 ml, while the second reactor's working volume was varied to obtain the desired retention time. The first reactor was fed with fresh validated medium (see Table 4) by a type 520Du peristaltic pump (Watson-Marlow, Falmouth, United Kingdom). The overflow of the first stage was pumped into the second stage and then out into a collection reservoir.
In order to maintain anaerobic conditions after inoculation, a continuous stream of sterile nitrogen gas (0.1 VVM [volume of air/volume of reactor × minutes]) was passed through the reactors. The gassing was stopped upon cell production of sufficient gases (positive headspace pressure), usually between 24 and 48 h. Sterile 5 M potassium hydroxide (KOH) was used to maintain the desired pH level in the reactors. Antifoam A (Sigma-Aldrich Inc., St. Louis, MO) was incorporated into the media at 0.10 g/liter. Exact conditions for the fermentations are detailed below when the different assays are presented. The purity of the culture was checked daily by direct microscopic examination and by plating dilutions on RCM agar plates.
Chemostat pulse-and-shift experiments were performed in a Biostat B plus chemostat (Sartorius) with a working volume of 600 ml and used throughout these experiments. The temperature was set at 37°C, the agitation speed was set at 200 rpm, and the pH was kept at 6.5 by the automated addition of 1.5 N KOH. The dilution rate was fixed at 0.135 h−1, which corresponded to half of the growth rate (μ/2) and was determined in previous experiments using batch cultures with controlled pH. The residence time (Tr = 1/D where Tr is the residence time and D is the dilution rate) was 7.4 h. Steady states, as assessed by biomass, glucose, xylose, and product concentrations, were reached following at least 3 or 4 volumes of medium replacement. Turbidometric values and d-glucose and d-xylose concentrations at different steady states during the experiment are shown below in Fig. 3. The various compounds that were determined to be necessary using the chemostat pulse-and-shift technique were sterilized by filtration and added to the vessel aseptically.
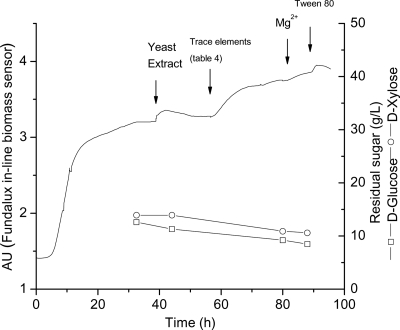
Fig. 3.
Pulse-and-shift technique applied to C. beijerinckii SA-1/ATCC 35702 culture in a chemostat (dilution rate [D] = 0.135 h−1) at 37°C. Time (in hours) versus d-glucose or d-xylose consumed (g/liter) and biomass (in arbitrary units [AU]) are depicted on the graph. The pulse location for each component that was added is indicated on the graph by arrows (↓). Each compound was tested individually by adding to the consolidated medium reservoir (pulse) (Table 3). Once an ingredient limitation was satisfied, an increase in sugar utilization followed by a transient increase in biomass (responses) was observed, and the compounds showing a positive response were added to the medium reservoir (shift) before the next component was tested. A steady state was reestablished before each new element was added and evaluated. Each compound was added to the reactor at the following final concentrations; yeast extract (2 g/liter), trace elements (double the concentration added to the original medium [see Table 4]), MgSO4 (0.1 g/liter), and Tween 80 (1 ml/liter).
Butanol and butyric acid growth tolerance assays.
Comparative growth curves were performed to evaluate the reduction of specific growth rates as a response to the strain's resistance to butanol and butyric acid. Inoculums were obtained from exponential-growth-phase cultures harvested by centrifugation and resuspended in sterile RCM medium to an initial optical density at 600 nm (OD600) of ca. 0.1. The RCM medium was prepared from the same batch, and the desired pH values were adjusted by adding the adequate amount of base to the medium. The doubling time of each culture was calculated from the slope after fitting the exponential-growth-phase data to a linear regression with correlation coefficients (r2) of 0.99. Each point represents the mean of at least two independent assays.
The strain's butanol tolerance was evaluated by inoculating and growing in medium containing increasing concentrations of butanol (0, 40, 45, 50, 55, and 60 mM) (0, 2.96, 3.34, 3.70,4.07, and 4.44 g/liter). To test for butyric acid resistance, the strains were inoculated and grown in medium containing increasing concentrations of butyric acid (0, 1, 2, 3, 4, 5, and 6 g/liter). Since nondisassociated forms of organic acids can diffuse through the plasmatic membrane, the initial pH of the medium was adjusted using the Henderson-Hasselbalch equation (27, 28) to maintain the same initial concentration of the nondisassociated butyrate forms at 0.1 g/liter at the time of inoculation. The pH was also monitored during the experiment in order to calculate the time course development of nondisassociated acid concentration.
Meta-analysis of the transcriptional response of C. acetobutylicum ATCC 824 to butanol stress.
The National Institutes of Health (NIH) defines meta-analyses as systematic methods that use statistical techniques for combining results from different studies to obtain a quantitative estimate of the overall effect of a particular intervention or variable on a defined outcome (http://www.nlm.nih.gov/nichsr/hta101/ta101014.html). Microarray raw intensity values from strain ATCC 824 exposed to butanol or butyric or acetic acid were downloaded from the GEO database (2). The data were log2 transformed and imported into JMP Genomics (SAS, Cary, NC). Afterwards, loess normalization was applied to preprocessed data. Following normalization, gene-specific effects were modeled in terms of the residuals. Two independent model-based approaches were applied to the normalized data: a mixed-model analysis (14) and a step-down quadratic regression model for pattern recognition (44). The outcome of responsive genes from our analysis was then grouped into functional categories (Clusters of Orthologous Groups [COG]) (http://www.ncbi.nlm.nih.gov/COG/grace/uni.html). For statistical significance, P values of ≤0.05 were considered significant.
Analytical methods.
Biomass proliferation in the fermentation tank was monitored using an in-line biomass sensor (Fundalux; Sartorius BBI Systems, Melsungen, Germany) and also by measuring the OD600 on a digital spectrophotometer (SmartSpec Plus; Bio-Rad). Direct cell counts were made by phase-contrast microscopy using an improved Neubauer hemocytometer slide chamber. Substrate (d-glucose and d-xylose) consumption and accumulation of end products were performed by high-performance liquid chromatography (HPLC) and gas chromatography (GC-8A) fitted with a flame ionization detector (Shimadzu Corporation, Kyoto, Japan). d-Glucose and d-xylose were quantified with an HPLC under isocratic conditions at 65°C and a mobile phase of 5 mM sulfuric acid (H2SO4) at a 0.4-ml/min flow rate using an Alltech IOA-1000 organic acids column (300 mm by 7.8 mm) (Alltech, IL) and coupled to a refractive-index detector. The products of the fermentations (butanol, butyric acid, acetone, acetic acid, and ethanol) were separated in a GC SS Porapak Q 80/100 column (OV, Marietta, OH) using 200 kPa of nitrogen as the mobile phase with an injection temperature of 220°C and a column temperature of 140°C.
RESULTS
Comparative analysis of the specific growth rates of Clostridium beijerinckii strains growing in the presence of increasing butanol concentrations.
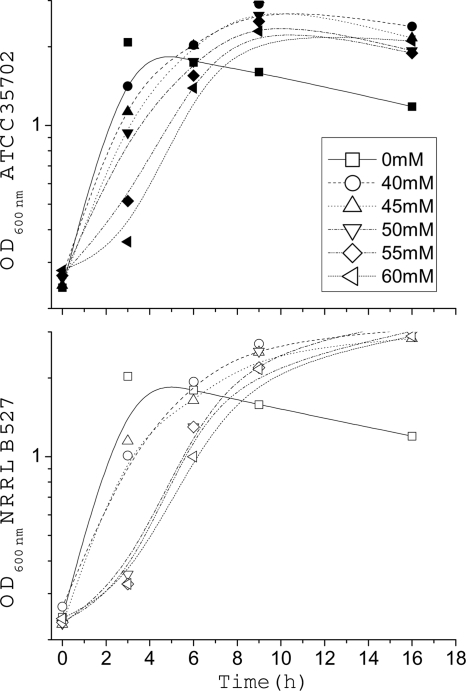
The literature reports that the C. beijerinckii selected strain (SA-1/ATCC 35702) is able to tolerate higher concentrations of butanol when grown in broth. To confirm the phenotype, we measured the specific growth rates (μ·h−1) on the C. beijerinckii strains (NRRL B-527 and SA-1/ATCC 35702) grown in the presence and absence of butanol. Each strain was grown separately in serial individual flasks each containing increasing concentrations of butanol. Figure 1 shows the growth curves of the NRRL B-527 and the SA-1/ATCC 35702 strains challenged to butanol at different concentrations (0, 40, 45, 50, 55, and 60 mM). When comparing the growth rate of the NRRL B-527 and SA-1/ATCC 35702 strains in the presence of increasing butanol concentrations, the derivative strain SA-1/ATCC 35702 maintained higher specific growth rates, evident by the steeper slopes, in medium containing butanol concentrations greater than 45 mM (Fig. 1). Therefore, we confirm here that strain SA-1/ATCC 35702 maintains an increased resistance to butanol and a retarded sporulation cycle compared to NRRL B-527 strain. Higher resistance to butanol toxicity should theoretically allow the strain to produce greater quantities of butanol.
Fig. 1.
Batch growth kinetics of C. beijerinckii NRRL B-527 and SA-1/ATCC 35702 strains grown at 37°C in reinforced clostridial medium (RCM) containing increasing concentrations of butanol (0, 40, 45, 50, 55, and 60 mM). C. beijerinckii (SA-1/ATCC 35702) is represented by solid symbols connected with solid lines, while the C. beijerinckii (NRRL B-527) is represented by open symbols connected with solid lines.
Transcriptional meta-analysis of C. acetobutylicum ATCC 824 response to butanol stress.
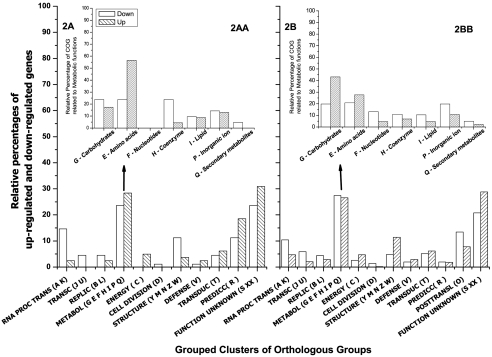
In order to identify the nutrients required for optimal growth and butanol production, we analyzed publically available microarray data obtained from stress experiments of samples exposed to 50 mM butanol (3). Figure 2 shows the results of the analysis and grouping of the responsive genes when cells from C. acetobutylicum ATCC 824 were exposed to 50 mM butanol compared to nonexposed controls. The analysis includes short-time-course microarray experiments of culture samples exposed to butanol or grown in the absence of butanol for 10, 30, 45, 60, 120, 360, 720, and 1,440 min. Figure 2A shows frequencies of genes demonstrating a kinetic linear pattern of up- or downregulation compared to genes showing a flat pattern or no regulation. Figure 2B shows the frequencies of significantly (P = 0.05) up- and downregulated genes exposed to 50 mM butanol compared to nonexposed controls [e.g., differential of time × treatment = (360 min butanol) − (360 min control)].
Fig. 2.
Transcriptional response of C. acetobutylicum ATCC 824 after the cells were challenged with 50 mM butanol. The bar graphs denote significantly upregulated and downregulated genes as percentages of the total number of responsive genes. Significant responsive genes were grouped into functional categories (Clusters of Orthologous Groups [COGs] [www.ncbi.nlm.nih.gov/COG/grace/uni.html]) after the normalized series were analyzed by short-time-course microarray (44) and mixed-model analysis (14). (A) Frequencies of genes showing a significant kinetic linear pattern (time course microarray series 10, 30, 45, 60, 120, 360, 720, and 1,440 min) compared to genes that show a flat pattern (no regulation); (AA) percentages of genes that belong only to the metabolic grouped category (COG groups G, E, F, H, I, P, and Q) found in panel A; (B) frequencies of genes showing a significant responsive after mixed-model analysis [time × treatment = (360 min butanol) − (360 min control)]; (BB) percentages of genes that belong only to the metabolic grouped category (COG groups G, E, F, H, I, P, and Q) found in panel B.
Independent of the type of analysis performed to the series (3), a high percentage of the responsive genes can be grouped into either the metabolism category or into the “gene unknown function” category (Fig. 2). Furthermore, by disaggregating the genes within Clusters of Orthologous Group (COG) functions grouped into each metabolic category, we found genes altered by this change now fitting in larger proportions into the COGs of transport and metabolism of carbohydrates and amino acids, as well as coenzymes and inorganic salt transport (Fig. 2AA and BB). Tables 1 and 2 show selected C. acetobutylicum ATCC 824 genes with statistically significant responses following exposure to 50 mM butanol compared to nonexposed controls after applying Stromberg analysis (44) or mixed-model analysis (14).
Table 1.
Selected genes showing a significant response identified after meta-analysis of microarray data of C. acetobutylicum ATCC 824 exposed to 50 mM butanol compared to nonexposed controls applying the step-down quadratic regression methoda
| Product type and substrateb | Butanol-downregulated or -upregulated product namec | COG EHIPd | Locus tag | Overall P value |
|---|---|---|---|---|
| Butanol-downregulated products | ||||
| VitB12 | Cobyrinic acid a,c-diamide synthase CobB/CbiA (CBIB protein) | H | CAC0582 | 0.001301656 |
| Phosphatidylserine synthase | I | CAC0676 | 0.00234798 | |
| Predicted permease | E | CAC0875 | 0.00077822 | |
| Co | Cobalt transport (ATPase component) | P | CAC1368 | 0.002664104 |
| VitB12 | Precorrin 8X methylmutase | H | CAC1376 | 0.000268975 |
| VitB12 | Precorrin 6B methylase CbiT | H | CAC1378 | 3.97206E−07 |
| VitB12 | Precorrin 4 methylase CbiF | H | CAC1380 | 0.000110082 |
| THF | Enzyme of dihydrofolate reductase family, ortholog YWFD of B. subtilis | H | CAC1495 | 0.00520426 |
| Lipid | 3-Ketoacyl-(acyl carrier protein) reductase | I | CAC2626 | 0.00025712 |
| Proline/glycine betaine ABC-type transport system, permease component fused to periplasmic component | E | CAC2849 | 0.000922292 | |
| Proline/glycine betaine ABC-type transport system, ATPase component | E | CAC2850 | 1.31008E−05 | |
| Oligopeptide ABC transporter, ATPase component | P | CAC3182 | 0.0047743 | |
| Oligopeptide transport permease protein | E | CAP0177 | 0.000635715 | |
| Butanol-upregulated products | ||||
| Fe | Nitrogenase iron protein (nitrogenase component II) gene nifH | P | CAC0253 | 0.000816333 |
| Peptidase T | E | CAC0476 | 0.001467584 | |
| His | ATP phosphoribosyltransferase catalytic subunit | E | CAC0936 | 2.40248E−05 |
| His | Phosphoribosylformimino-5-aminoimidazole carboxamide ribonucleotide (ProFAR) isomerase | E | CAC0940 | 7.91786E−05 |
| Lipoprotein, attached to the cytoplasmic membrane, NLPA family | P | CAC0986 | 0.003698933 | |
| Lipid | Phosphatidylglycerophosphate synthase related protein (fragment) | I | CAC2024 | 0.009199846 |
| Urea | N-Acetylornithine aminotransferase | E | CAC2388 | 2.72258E−05 |
| Urea | Acetylglutamate kinase | E | CAC2389 | 0.000250975 |
| Urea | N-Acetyl-gamma-glutamyl-phosphate reductase | E | CAC2390 | 0.003281643 |
| Dipeptidase PepV | E | CAC2723 | 0.002038606 | |
| Se | Possible selenocysteine lyase (aminotransferase of NifS family) | E | CAC2805 | 0.000640894 |
| Lys | Lysine-specific permease | E | CAC3164 | 0.003458938 |
| Met | Homocysteine methyltransferase | E | CAC3348 | 0.000180346 |
| ABC-type polar amino acid transport system, ATPase component | E | CAC3618 | 0.000132942 | |
| Amino acid ABC transporter, permease component | E | CAC3619 | 0.006894683 |
The meta-analysis includes time course microarray experiments at 0, 10, 30, 45, 60, 120, 360, 720, and 1,440 min). Figure 2A shows the frequencies of genes displaying a kinetic linear pattern of up- and downregulation compared to genes showing a flat pattern (or no regulation) after applying Stromberg analysis (44). The step-down quadratic regression method was applied to normalized data for pattern recognition (44). Data were obtained from cells cultivated in clostridial growth medium (CGM [69]).
VitB12, vitamin B12; THF, tetrahydrofolate.
CBIB, cobyrinic acid a,c-diamide synthase; YWFD, synonym for bacilysin biosynthesis oxidoreductase BacC; NLPA, family of lipoproteins.
COGs, clusters of orthologous groups (http://www.ncbi.nlm.nih.gov/COG/grace/uni.html); EHIP, COG groups E, H, I, and P.
Table 2.
Selected C. acetobutylicum ATCC 824 genes showing a significant response after exposure to 50 mM butanol compared to nonexposed controls by using a mixed-model analysisa
| Substrate | Product nameb | COG EHIPc | Locus tag | Differenced | −Log10P value |
|---|---|---|---|---|---|
| S | O-Acetylhomoserine sulfhydrylase | E | CAC0102 | 2.31573 | 13.35289 |
| S | Adenylylsulfate kinase | P | CAC0103 | 2.31299 | 10.6627 |
| S | ABC-type probable sulfate transporter, periplasmic binding protein | P | CAC0106 | 2.19078 | 9.92702 |
| S | ABC-type sulfate transporter, ATPase component | P | CAC0107 | 2.22341 | 4.68265 |
| S | ABC-type probable sulfate transporter, permease protein | P | CAC0108 | 1.63887 | 6.53352 |
| S | Sulfate adenylyltransferase subunit 2 | E | CAC0109 | 2.44843 | 10.70661 |
| S | GTPase, sulfate adenylate transferase subunit 1 | P | CAC0110 | 2.13862 | 7.30271 |
| Asp | Aspartate semialdehyde dehydrogenase | E | CAC0568 | 0.90651 | 5.24713 |
| Lys | Diaminopimelate decarboxylase LisA | E | CAC0608 | 0.94998 | 2.35148 |
| Fe | Ferrichrome transport permease | P | CAC0788 | 0.68284 | 4.07679 |
| Cys | γ-Cystathionine synthase | E | CAC0930 | 1.52732 | 7.36916 |
| His | ATP phosphoribosyltransferase catalytic subunit | E | CAC0936 | 1.28815 | 3.9961 |
| His | Histidinol dehydrogenase | E | CAC0937 | 1.63942 | 10.93148 |
| His | Imidazoleglycerol-phosphate dehydratase | E | CAC0938 | 1.30102 | 7.31492 |
| His | Phosphoribosylformimino-5-aminoimidazole carboxamide ribonucleotide (ProFAR) isomerase | E | CAC0940 | 0.87342 | 6.57009 |
| His | Phosphoribosyl-AMP cyclohydrolase | E | CAC0942 | 0.57879 | 3.28315 |
| Arg | Argininosuccinate lyase | E | CAC0974 | 1.65485 | 10.71147 |
| 5-Formyltetrahydrofolate cycloligase | H | CAC1090 | 0.70186 | 2.19531 | |
| Asp | Aspartate kinase I | E | CAC1810 | 0.707 | 2.50719 |
| Mo | Molybdopterin biosynthesis enzyme, MoeA, fused to the molybdopterin-binding domain | P | CAC2020 | 0.92291 | 3.22569 |
| Cys | Cysteine synthase/cystathionine beta-synthase, CysK | E | CAC2235 | 0.40506 | 2.13166 |
| Arg/Lys | N-Acetylornithine aminotransferase | E | CAC2388 | 1.51803 | 7.14475 |
| Urea | Acetylglutamate kinase | E | CAC2389 | 1.07553 | 4.10307 |
| Urea | N-Acetyl-gamma-glutamyl-phosphate reductase | E | CAC2390 | 1.7949 | 7.75867 |
| Fe | Ferric uptake regulator (FUR family), YGAG B. subtilis ortholog | P | CAC2634 | 2.70695 | 4.15283 |
| 3-Hydroxybutyryl-CoA dehydrogenase | I | CAC2708 | 0.54094 | 2.51473 | |
| Lipid | Butyryl-CoA dehydrogenase | I | CAC2711 | 0.61838 | 3.75509 |
| 3-Hydroxybutyryl-CoA dehydratase | I | CAC2712 | 0.56573 | 3.00566 | |
| Lys | Lysine-specific permease | E | CAC3164 | 0.92611 | 5.19834 |
| Dihydroxy-acid dehydratase | E | CAC3170 | 0.55559 | 2.16606 | |
| Met | Homocysteine methyltransferase | E | CAC3348 | 0.4169 | 2.22381 |
| Lipid | 3-Oxoacyl-acyl carrier protein reductase | I | CAC3462 | 0.78625 | 5.23967 |
| Lipid | Phosphatidylglycerophosphate synthase | I | CAC3596 | 1.88139 | 5.43757 |
| K | K+-transporting ATPase, c chain | P | CAC3680 | 0.65812 | 3.02242 |
| K | Potassium-transporting ATPase subunit B | P | CAC3681 | 0.74665 | 3.01109 |
The mixed-model analysis (JMP Genomics [SAS, Cary, NC]) was performed on data obtained from microarray results (3) comparing the cells exposed to butanol for 360 min [e.g., differential of time × treatment = (360 min butanol) − (360 min control)]. Figure 2B shows the frequencies of genes displaying up- or downregulation after mixed-model analysis (14). Data were obtained from cells cultivated in clostridial growth medium (CGM [69]).
YGAG, synonym for the PerR protein, which encodes a FUR homolog in B. subtilis; CoA, coenzyme A.
COGs (clusters of orthologous groups) (www.ncbi.nlm.nih.gov/COG/grace/uni.html).
Difference in P values are shown.
Formulation of a consolidated medium for cultivation of solventogenic Clostridium spp.
The compositions of the commonly used media (43, 45, 54, 69, 70) for clostridial cultivation are presented in Table 3. Typical characteristics of the media utilized for Clostridium cultivation are the inclusion of nitrogen-rich components and phosphate limitation. Nitrogen-rich components increase the overall process cost and may complicate product recovery. On the other hand, phosphate limitation is a condition that appears to contribute to the initiation of solventogenesis and to the stability of C. acetobutylicum growing in continuous cultures. However, phosphates are present in many complex substrates used for solvent production, which would entail removal via preprocessing, thereby increasing the price of butanol (9). Generally, the utilization of media simpler in composition is limited in carbon or ammonia and often creates problems with culture stability by triggering early sporulation and low solventogenesis performance (46). Thus, we did not consider phosphate limitation during our effort to obtain a higher concentration of biomass with our validated medium while cofermenting both d-glucose and d-xylose to produce initial threshold concentrations of butyrate or acetate.
Therefore, to formulate a new medium capable of sustaining growth and solventogenesis, we surveyed microarray data to identify metabolic pathways differentially expressed in C. acetobutylicum ATCC 824 during butanol stress. As a result, we incorporated the following components into the growth medium: vitamin B12, riboflavin, tryptophan, glutamine, asparagine, cysteine, and histidine (Table 3). Histidine as a limiting factor was interesting, since examination of the literature illustrated that induction of genes for histidine biosynthesis has recently been shown to contribute to the acid tolerance response in Lactobacillus casei (12). The decision for adding the above compounds was made by comparisons with various clostridial growth media and by the findings of our meta-analysis (Table 1 and 2). We then compared and contrasted these findings with medium formulations traditionally used for growth of Clostridium spp. (43, 45, 54, 69, 70) and developed a new consolidated medium formulation. The new consolidated formulation was subsequently adjusted and later validated by applying the chemostat pulse-and-shift technique (6, 38, 49), using a nonlimiting carbon source (comprised of a mixture of d-glucose and d-xylose) and specified ammonium concentrations (Table 3 and 4).
Validation of the consolidated medium by the chemostat pulse-and-shift technique.
To ensure the proliferation of C. beijerinckii and avoid nutrient limitation as a consequence of butanol and butyric acid toxicity (4, 12, 19, 43, 50, 54), nutrients were independently added to the reactor (pulse) and when the observed response was an increase in biomass after pulsing a particular compound into the reactor, that compound (the limiting factor) was subsequently incorporated into the culture medium reservoir, which is referred to as the “shift.” Compounds causing decrease or no change in biomass were not included in the culture medium. Final concentrations of each component incorporated in the working volume are shown in Table 4. Pulse-and-shift experiments were conducted in the presence of excess sugars, d-glucose (30 g/liter), and d-xylose (15 g/liter). In our experiments, both sugars were simultaneously consumed, and catabolite repression was not observed during the fermentation. Iron limitation during ABE fermentations has been previously studied (34) and was also one of the components showing an early positive effect during the pulse-and-shift experiment. The inclusion of this component confirmed the results seen in our meta-analysis (Tables 1 and 2). Also, in preliminary chemostat pulse-and-shift experiments, we determined the nutritional requirement of the cells for bioavailable iron and lipids (data not shown). Therefore, the chemical siderophore EDTA (0.1 mM) was added to the medium to guarantee iron bioavailability and also to protect the cells from oxidative stress (13). The final pulse-shift experiment demonstrated that growth of strain SA-1/ATCC 35702 was limited in yeast extract, trace elements, MgSO4, and Tween 80 (Fig. 3). Consequently, concentrated solutions of yeast extract (2 g/liter), trace elements (double that of the original medium; see Table 3), MgSO4 (0.1 g/liter), and Tween 80 (1 ml/liter) were added to the fermentor. A reduced amount of yeast extract (0.2%) was used in the medium composition to overcome the nitrogen limitation, while keeping carbon in excess to achieve high levels of solvent production (48, 61). The integration of these medium components was developed into a validated medium formulation (Table 4).
Analysis of growth and butanol production in continuous culture.
The next series of experiments were performed in order to evaluate the suitability of strain SA-1/ATCC 35702 for butanol production in a continuous, high-density bioprocessing system. First, we analyzed the environmental conditions necessary to obtain higher concentration of products. We sought to produce butanol and control the strain's capacity to maintain its physiological vegetative state at different dilution rates in a single-step fermentation process using a mixture of d-glucose and d-xylose. Taking into account the fact that the biphasic nature of ABE fermentation is best accommodated by a multistage continuous mode of operation, we later evaluated a production process in two stages to accommodate the generation of butyric or acetic acid in the first phase and the generation of butanol or acetone in the second phase.
Physiological behavior of C. beijerinckii SA-1/ATCC 35702 in a single-stage chemostat culture.
C. beijerinckii SA-1/ATCC 35702 was cultivated in a single-stage chemostat culture containing d-glucose (30 g/liter) and d-xylose (40 g/liter) in the medium (Table 4). The starting concentrations of fermentable sugars were adjusted to a traditionally dilute sugar solution, because these biotransformations are influenced by the toxicity of butanol accumulation at low concentrations (1.5% to 2.0%). During the fermentation, the pH was held at 6 and temperature was maintained at 37°C. The dilution rate was varied from 0.040 to 0.135 h−1 for each steady state, and three samples were collected and tested with three retention time intervals between each sample taken (Table 5). As expected, butyric acid appeared as the major fermentation end product for all dilution rates tested, which is in agreement with the expected behavior of the strain during the acidogenic phase (8, 9). Interestingly, we observed that strain SA-1/ATCC 35702 initiated the solventogenic phase at higher retention times when a small fraction of butyric acid was converted to butanol. Furthermore, catabolite repression was not observed, since cofermentation of d-glucose and d-xylose was noticeable in the different steady states and the largest amount of sugars was consumed at a dilution rate (D) of 0.04 h−1 (40.7 g/liter). For all dilution rates tested, the amount of d-glucose consumed was double the amount of d-xylose consumed. The lowest product yield (0.30 g/g of carbohydrates) was observed at D = 0.040 h−1, while product yield increased (0.39 g/g of carbohydrates) at higher dilution rates (D = 0.135 h−1). Since the butyric acid productivity increased with the dilution rate, we choose this maximum value (D = 0.135 h−1) (Table 5) and subsequently focused on testing alternative environmental conditions (pH and temperature), using a single-step chemostat culture.
Table 5.
Results of cultivating C. beijerinckii SA-1/ATCC 35702 in a single-stage chemostat containing d-glucose and d-xylose in the validated mediuma
| D (h−1)b | Mean concn (g/liter) ± SD of the following fermentation product: |
Final concn (g/liter) of the following consumed sugar: |
Productivity of butyrate (g/liter h) | |||||
|---|---|---|---|---|---|---|---|---|
| Acetate | Butyrate | Ethanol | Acetone | Butanol | d-Glucose | d-Xylose | ||
| 0.04 | 4.20 ± 0.69 | 7.53 ± 1.43 | 0.14c | 0.06c | 0.55 ± 0.06 | 25.93 ± 0.37 | 14.95 ± 0.68 | 0.30 |
| 0.08 | 4.08 ± 0.75 | 5.44 ± 0.13 | 0.11 ± 0.01 | 0.08 ± 0.04 | 0.30 ± 0.13 | 18.71 ± 1.50 | 10.53 ± 3.00 | 0.43 |
| 0.102 | 4.31 ± 0.08 | 5.61 ± 0.18 | 0.11 ± 0.01 | 0.04 ± 0.01 | 0.15 ± 0.07 | 20.04 ± 0.65 | 11.25 ± 0.88 | 0.57 |
| 0.135 | 4.35 ± 0.20 | 4.56 ± 0.23 | 0.14 ± 0.09 | 0.09 ± 0.01 | 0.36 ± 0.09 | 16.23 ± 0.36 | 8.13 ± 0.96 | 0.62 |
C. beijerinckii SA-1/ATCC 35702 was grown in a single-stage chemostat containing d-glucose (30 g/liter) and d-xylose (40 g/liter) in the validated medium described in Table 4 at pH 6.0 and 37°C.
D, dilution rate.
Determination of the optimum operational parameters of the reactor with a fixed dilution rate.
The same validated medium (Table 4) obtained previously containing glucose (30 g/liter) and d-xylose (15 g/liter) was employed. Note the change in the d-glucose/d-xylose ratio based on the results obtained in the former assay. The culture pH was varied by 0.5 unit from pH 6.0 to 7.0, and three culture temperatures were tested, 30, 37 and 40°C. The results for the different parameters tested in the chemostat are shown in Table 6. For all physiological states tested, the cells were able to simultaneously consume both d-glucose and d-xylose. We observed the highest butyrate productivity and improved strain stability at D = 0.135 h−1. An additional advantage pertaining to operational convenience was the short time needed (retention time of 7.4 h) to establish steady state.
Table 6.
Results for parameters for C. beijerinckii SA-1/ATCC 35702 grown in a single-stage chemostat containing d-glucose and d-xylose in the validated mediuma
| Temp (°C) | pH | Mean concn (g/liter) ± SD of the following fermentation product: |
Mean final concn (g/liter) ± SD of the following consumed sugar: |
|||||
|---|---|---|---|---|---|---|---|---|
| Acetate | Butyrate | Ethanol | Acetone | Butanol | d-Glucose | d-Xylose | ||
| 30 | 6.0 | 1.56 ± 0.20 | 0.99 ± 0.13 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.21 ± 0.04 | 8.53 ± 0.65 | 0.70 ± 0.40 |
| 37 | 6.0 | 2.02 ± 0.37 | 1.16 ± 0.04 | 0.15 ± 0.00 | 1.31 ± 0.05 | 2.42 ± 0.04 | 19.40 ± 0.57 | 5.07 ± 0.27 |
| 40 | 6.0 | 2.44 ± 0.22 | 2.38 ± 0.10 | 0.16 ± 0.02 | 0.58 ± 0.17 | 1.18 ± 0.31 | 14.65 ± 0.49 | 4.86 ± 0.19 |
| 37 | 6.5 | 4.35 ± 0.20 | 4.56 ± 0.23 | 0.14 ± 0.09 | 0.09 ± 0.01 | 0.36 ± 0.09 | 16.23 ± 0.38 | 8.13 ± 0.96 |
| 40 | 6.5 | 3.05 ± 0.11 | 2.97 ± 0.13 | 0.25 ± 0.01 | 0.22 ± 0.08 | 0.62 ± 0.15 | 17.35 ± 0.07 | 6.55 ± 0.41 |
| 40 | 7.0 | 2.18 | 1.19 | 0.14 | 0.14 | 0.20 | 8.50 | 2.06 |
C. beijerinckii SA-1/ATCC 35702 was grown in a single-stage chemostat containing d-glucose (30 g/liter) and d-xylose (15 g/liter) in the validated medium described in Table 4 at D = 0.135 h−1. The culture pH and three culture temperatures (30°C, 37°C, and 40°C) were evaluated.
The standard deviations were not determined for the final condition (40°C and pH 7.0).
The data in Table 6 illustrate the advantage of our cultivation techniques by exposing the C. beijerinckii SA-1/ATCC 35702 cells to less acid stress (butyric and acetic acid). Clearly, while it is important to attain our objective of maximized efficiency of the acidogenic phase, the cells were less stressed by maintaining the dissociation of butyric acid to the less-toxic butyrate anion. Within the working conditions tested, the highest butyric acid concentration (4.56 g/liter) was obtained at 37°C and pH 6.5. By using these conditions, we also observed a reduced final concentration of acetone (0.09 g/liter). The combined total amount of butyric acid plus butanol remained constant (approximately 3.6 g/liter) and was independent of the pH and temperature variables tested, including 37°C and pH 6.0, 40°C and pH 6.0, and 30°C and pH 6.5. Conversely, suboptimal operational conditions, such as 30°C and pH 6.0 and 40°C and pH 7, lead to reduced product accumulation of butyric acid and butanol. Interestingly, while steady-state culture conditions at 40°C were achievable, slight deviations in dilution rate, pH, and temperature during routine operation of the continuous fermentation processes easily triggered cell sporulation. Therefore, we established that an operating temperature of 37°C was necessary for a stable continuous fermentation process using C. beijerinckii SA-1/ATCC 35702.
In traditional processes, butanol production is favored at an acidic pH (8, 25, 61, 65); however, interestingly, strain SA-1/ATCC 35702 produced significant amounts of butanol at pH 6.0. This demonstrates the ability of SA-1/ATCC 35702 to initiate solventogenesis in parallel with the acidogenic phase at a growth rate that significantly improves butanol volumetric productivity.
Growth kinetics of C. beijerinckii in the presence of increasing butyric concentrations.
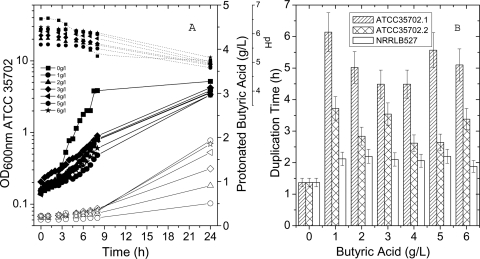
Butyric acid is known to directly influence the pH of the media; therefore, we investigated the effect of butyric acid on the growth of the strains of C. beijerinckii (C. beijerinckii SA-1/ATCC 35702 and NRRL B-527). Cell growth was challenged by the presence or absence of butyric acid; each strain was grown separately in individual flasks containing increasing concentration of butyric acid (0, 1, 2, 3, 4, 5, and 6 g/liter). All through the butyric acid exposure studies, the initial concentration of undissociated butyrate in each flask was made constant by adjusting the initial pH of the RCM in each flask (Fig. 4A), thus allowing only a maximum of 0.1 g/liter of the butyric acid to readily enter the cells. During cell growth, the pH decreased, and the amount of protonated acid in each culture increased (Fig. 4A). This effect was more pronounced with greater concentrations of butyric acid. When comparing strains NRRL B-527 and SA-1/ATCC 35702 in the presence of increased concentrations of butyric acid, SA-1/ATCC 35702 demonstrated longer duplication times than NRRL B-527 (Fig. 4A and B). In particular, concentrations of butyric acid greater than 1 g/liter reduced specific growth rates of SA-1/ATCC 35702 compared to NRRL B-527. Therefore, our results show that SA-1/ATCC 35702 has an increased sensitivity to butyric acid compared to NRRL B-527. Butyric acid sensitivity was an unexpected phenotype, considering the fact that the final solvent accumulation in the biphasic process is normally correlated with higher accumulation of organic acids in the fermentation broth. Since butyric acid stress can be a limiting factor of cell growth (Fig. 4), the amount of butyric acid in the culture can be reduced by growing C. beijerinckii in a more neutral pH in continuous fermentation system, thus allowing the cells to propagate and continue producing butanol. Accordingly, in our experiments, SA-1/ATCC 35702 was able to transform sugars more efficiently and accumulate more butanol when the organic acid toxicity was reduced under steady-state conditions (Table 4).
Fig. 4.
(A) Growth kinetics of C. beijerinckii SA-1/ATCC 35702 exposed to increasing concentrations of butyric acid. Each flask contained identical initial concentrations of undissociated butyric acid. Symbols connected with dotted lines show pH evolution, symbols connected with solid lines show growth of the SA-1/ATCC 35702 cells, and open symbols with solid lines show the amount of undissociated butyric acid in each flask. The growth curves for strain SA-1/ATCC 35702 are biphasic. (B) Comparison of duplication times of C. beijerinckii (NRRL B-527) and C. beijerinckii (SA-1/ATCC 35702) challenged with increasing butyric acid concentrations. The duplication times of both slopes for SA-1/ATCC 35702 are labeled ATCC 35702.1 and ATCC 35702.2.
Evaluation of cell performance during the solventogenic phase at different physiological states employing a two-stage continuous process.
Finally, a two-stage process was tested in order to evaluate the acidogenic and solventogenic phases. Dissociating the overall process into two independent steps increased the process stability by favoring cell proliferation and production, thus keeping the toxic effects of acetic acid or butyrate under control during the first stage of fermentation and allowing solventogenesis to continue in the second stage of the fermentation. At a pH of 6.5, the concentration of the undissociated acid species in the medium is minimized, thereby reducing cell stress; therefore, the pH in both reactors was maintained at 6.5. As a result, the number of physiologically active vegetative cells that continuously reseed the second fermentor should be maximized, as the second fermentor is the location where the main biotransformation of acid to butanol should be achieved.
Two fermentors were connected in a series, and the harvest of the first fermentor became the feed stream to the second fermentor. Both fermentors were inoculated simultaneously and cultivated in batch conditions at 37°C with a pH of 6.5. The carbon sources were increased to 40 g/liter for glucose and 20 g/liter for d-xylose. When the cells in the first fermentor reached the log phase of growth, the medium feed was initiated. The volume in the first fermentor was maintained at 700 ml, while the volume of the second fermentor was modified according to the desired dilution rate. Three samples were taken once the steady state of the reactor was established with at least three retention times between each of the samples. Concurrently, cell stability based on the percentage of sporulation in the culture for both fermentors was monitored daily to confirm stationary consistency at the respective steady states.
Throughout the assay, the environmental parameters (37°C and pH 6.5) and the dilution rate of D = 0.102 h−1 were maintained in the first fermentor in order to continuously produce physiologically active vegetative cells. The temperature of the second fermentor was also maintained at 37°C, while the overall dilution rate was varied from 0.04 to 0.05 and finally to 0.06 h−1. In the second fermentor, the pH was initially fixed at 6.5 for all dilution rates. In order to evaluate acidic conditions, we also assessed the conditions in the second stage, where the pH was no longer controlled, thus allowing the pH to drop and stabilize at a value of pH 5 for all dilution rates tested. The results of this assay are shown in Table 7.
Table 7.
Results for C. beijerinckii SA-1/ATCC 35702 cultivated in a two-stage, one-feed-stream chemostat culture containing d-glucose and d-xylose in the validated mediuma
| Reactor | Overall D (h−1) | pH | Mean concn (g/liter) ± SD of the following fermentation product: |
Proportion (%) of sugar that was consumed |
Butanol yield (mol/mol of substrate) | No. of vegetative cells | No. of spores | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetate | Butyrate | Acetone | Butanol | d-Glucose | d-Xylose | ||||||
| First reactor | 0.102 | 6.5 | 3.3 ± 0.6 | 6.3 ± 0.5 | 0.2 ± 0.1 | 0.5 ± 0.3 | 69.2 | 31.0 | |||
| Second reactor | 0.04 | 6.5 | 1.6 ± 0.8 | 6.4 ± 3.0 | 0.2 ± 0.1 | 0.4 ± 0.2 | 93.3 | 59.1 | 0.018 | 7.3 E7 | 2.3 E8 |
| 0.05 | 6.5 | 1.2 ± 1.0 | 4.0 ± 0.8 | 0.5 ± 0.2 | 1.8 ± 0.8 | 96.9 | 67.6 | 0.078 | 1.4 E8 | 1.5 E8 | |
| 0.06 | 6.5 | 2.0 ± 1.0 | 1.6 ± 1.0 | 5.3 ± 0.6 | 7.2 ± 0.2 | 83.2 | 52.9 | 0.380 | 1.8 E8 | 1.3 E8 | |
| 0.04 | Free | 2.7 ± 0.2 | 3.2 ± 1.2 | 1.1 ± 0.1 | 3.7 ± 0.4 | 95.7 | 47.5 | 0.183 | |||
| 0.05 | Free | 2.0 ± 0.4 | 3.5 ± 0.2 | 0.5 ± 0.1 | 2.0 ± 0.1 | 96.6 | 32.7 | 0.105 | |||
| 0.06 | Free | 2.0 ± 0.1 | 3.2 ± 0.9 | 0.4 ± 0.1 | 2.1 ± 0.5 | 93.5 | 57.6 | 0.100 | |||
C. beijerinckii SA-1/ATCC 35702 was cultivated in a two-stage, one-feed-stream chemostat culture containing d-glucose (40 g/liter) and d-xylose (20 g/liter) in the validated medium described in Table 4 at 37°C and pH 6.5. The dilution rate (D) and pH were varied in the second reactor.
Overall, the highest concentration of butanol (7.2 g/liter) and the majority of end products were obtained at a dilution rate of 0.06 h−1 and a pH of 6.5. By using a pH of 6.5, we found butanol accumulation improved as dilution rates were elevated until a value of 43.2% (wt/wt), indicating that acid stress toxicity is reduced at this pH. In all steady-state conditions evaluated, butyric acid and lactate were also produced as by-products. Although butanol was detected in the culture medium of the first fermentor, microscopic examination revealed the absence of spores in the medium, therefore implying that C. beijerinckii SA-1/ATCC 35702 is capable of producing butanol in the vegetative stage. The number of vegetative cells and spores counted in the second fermentor at different dilution rates are shown in Table 7.
The presence of more vegetative cells in the culture and higher butanol concentrations (Table 7) were observed when the second fermentor operated at an overall D of 0.06 h−1 and pH 6.5. A greater number of spores was detected when the continuous culture of the second fermentor was operated at an overall D of 0.04 h−1, which also coincided with lower butanol concentrations. Since the literature extensively supports the concept that acidic conditions favor solventogenesis (8, 25, 33, 65), we also evaluated this environmental condition (i.e., acidity). However, as expected, due to the acid sensitivity of strain SA-1/ATCC 35702, our data showed improvement in butanol production at the lower overall retention time tested at pH 6.5. Furthermore, steady-state concentrations of butyric acid remained constant under all conditions tested when the pH of the culture was allowed to fluctuate (Table 7).
Degeneration of the strain in terms of acid drift or morphological changes did not occur during the 40-day experimentation period. The strain maintained its capability to produce butanol; therefore, a stable continuous culture of C. beijerinckii SA-1/ATCC 35702 on d-glucose/d-xylose mixtures for extended periods of time was achieved.
DISCUSSION
Clostridium spp. must constantly deal with factors that can affect their growth and viability with two of the major threats being acid and solvent stress. Acid stress occurs from the passive movement of nondissociated organic acids across the cell wall and the resulting accumulation of acid in the cytoplasm. Solvent stress happens when there is an accumulation of butanol, acetone, and ethanol in the growth medium. One appealing strain to study butanol production and tolerance is C. beijerinckii ATCC 35702 (SA-1 butanol-resistant strain), an offspring of the Clostridium reference strain NCIMB 8052 (23). Lin and Blaschek (43) characterized SA-1/ATCC 35702 as a strain that maintains a higher resistance to butanol toxicity than NCIMB 8052 and exhibits characteristics of delayed lysis during sporulation similar to a C. acetobutylicum lyt-l mutant (67). Our results confirmed the ability of C. beijerinckii SA-1/ATCC 35702 to tolerate higher concentrations of solvents (Fig. 1), which should allow for the production of greater amounts of butanol (43). In addition, C. beijerinckii SA-1/ATCC 35702 exhibits unique hydrolytic and xylanolytic activities (40, 41), making it possible to obtain reliable butanol production in a continuous production process using a mixture of d-glucose and d-xylose.
It has traditionally been assumed that when cells are exposed to stress, the presence of complete biochemical pathways for the synthesis of metabolic intermediaries meet the conditions that are sufficient for the cells to survive and proliferate. However, the transcriptomic response to cell stress clearly shows a pronounced demand for some intermediaries when cells are exposed to increasing concentrations of stressors. Advances in genomic and proteomic technology allow us to analyze previously generated microarray data, so that valuable information can be obtained that assists us in understanding complex cellular processes or to use this information in drug discovery (39). These technologies have the potential to increase our understanding of cellular behavior and to integrate this knowledge into the design of future bioprocesses. In particular, gene expression profiling of industrial microorganisms generate valuable information that can be used to enhance the yield and productivity of processes (26). We performed meta-analysis on the extensive transcriptomic data of C. acetobutylicum ATCC 824 exposed to the fermentation acids acetate and butyrate and the solvent product butanol. These transcriptome data were generated by the Papoutsakis group, publicly available at the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) (2). The information obtained from our meta-analysis of the butanol stress response of C. acetobutylicum ATCC 824 was utilized to identify limiting growth factors for clostridial cells when confronted with butanol stress. After intensive statistical analysis and screening of the data, we were able to identify metabolic responsive genes that correlate with requirements for compounds that appeared to be limiting during butanol stress (Table 2). The responsive genes were classified by their membership into the following metabolic categories (Clusters of Orthologous Groups [COG] groups G, E, F, H, I, P, and Q). The growth medium was subsequently adjusted by adding the components associated with the gene function that showed a significant upregulated response. Thus, the nutritional demands of the strain under stress were met by including optimal concentrations of metals and/or vitamins in the medium.
Once the medium components for culturing C. beijerinckii SA-1/ATCC 35702 were defined and validated, we characterized the responses of Clostridium spp. to environmental fluctuations in pH, temperature, growth, and production rates. We performed one- and two-stage chemostat experiments to demonstrate the reliability and productivity of our enhanced bioprocess compared to the performance of previous bioprocesses employing C. acetobutylicum (36, 52, 53). For example, our formulation allowed for the stable propagation of the butanol-resistant C. beijerinckii SA-1/ATCC 35702 strain in chemostat culture using a mixture of sugars for over 2 months.
The results obtained after examining the batch growth kinetics of the two strains in the presence of butanol or butyric acid emphasizes the complexity of solvent and acid toxicity in clostridia, reinforcing the need for a flexible genomic approach for discovering genes involved in solvent tolerance. For example, surprisingly, we identified an increased sensitivity to butyric acid by strain SA-1/ATCC 35702 (Fig. 4) (43). This discovery was unexpected, since cells require acid accumulation in the fermentation broth as a prerequisite for triggering butanol formation and sporulation.
Due to the biphasic nature of butanol production by clostridia, we designed our process in two stages, a technique that has been successfully employed for C. acetobutylicum DSM 1731 (9). The goal in the first stage was to determine the conditions that would support the highest possible butyric acid concentration, which would subsequently trigger solventogenesis. In the second stage, the goal was to obtain the highest butanol concentration possible. C. beijerinckii SA-1/ATCC 35702 tends to sporulate under standard growth conditions; therefore, our new medium formulation was used to evaluate the process parameters and variables that addressed product formation, cell density, and culture stability.
Our experiments confirm that continuous production of vegetative cells in the first fermentor allows for continuous feeding into a second fermentor, therefore avoiding process shutdown due to toxic acid accumulation or sporulation. The two-stage process makes it possible to continually generate butanol at a concentration of 7.2 g/liter by cofermenting a mixture of d-glucose and d-xylose. The generated butanol is similar to concentrations that have been previously reported using a continuous culture with different carbon sources (4, 51, 52). The butanol yield obtained for C. beijerinckii SA-1/ATCC 35702 varied from 0.018 to 0.38 mol (butanol produced per mole of substrate consumed) and was dependent on the working conditions of the second reactor. The highest butanol yield was obtained when the dilution rate of the second reactor was 0.06 h−1 and had a pH of 6.5. Yields from the second reactor are in the range of those previously reported for batch and continuous culture data using different carbon sources (68). Not surprisingly, the optimal pH results confirmed the sensitivity of C. beijerinckii SA-1/ATCC 35702 to butyric acid (Fig. 4). This phenotype explains the optimum environmental conditions of reactor operation and improved our understanding of organic acid metabolism for the initiation of solvent production by C. beijerinckii SA-1/ATCC 35702.
Improving the growth of low-density solventogenic clostridial cultures has been hindered by the complexities involved in formulating an optimal medium for a more productive fermentation process (55). Our results show that by using meta-analyses and a functional approach such as the pulse-and-shift technique, we were able to formulate and validate a medium to support the growth and butanol production by cells displaying a phenotype with enhanced butanol tolerance, C. beijerinckii SA-1/ATCC 35702. Furthermore, we were able to optimize the environmental conditions that influence the continuous growth and production of butanol by C. beijerinckii SA-1/ATCC 35702 and improve the reproducibility and consistency of the final process. We also show the ability of C. beijerinckii SA-1/ATCC 35702 to grow to cell densities between 10 and 11 OD600 units by cofermenting d-glucose and d-xylose in the absence of glucose repression and initiating solventogenesis at neutral pH. Summarizing, C. beijerinckii SA-1/ATCC 35702 was cultured in a continuous fermentation process, we were able to improve the genetic stability of the strain, prolong its vegetative state, and sustain the production of butanol.
ACKNOWLEDGMENTS
This work was supported in part by the College of Life Sciences and the North Carolina Agricultural Research Service.
We thank Satya Makwana for technical assistance.
Footnotes
Published ahead of print on 20 May 2011.
REFERENCES
- 1. Alsaker K. V., Papoutsakis E. T. 2005. Transcriptional program of early sporulation and stationary-phase events in Clostridium acetobutylicum. J. Bacteriol. 187:7103–7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alsaker K. V., Paredes C., Papoutsakis E. T. 2010. Metabolite stress and tolerance in the production of biofuels and chemicals: gene expression based systems analysis of butanol, butyrate, and acetate stresses in the anaerobe Clostridium acetobutylicum. Biotechnol. Bioeng. 105:1131–1147 [DOI] [PubMed] [Google Scholar]
- 3. Alsaker K. V., Paredes C. J., Papoutsakis E. T. 2007. Metabolite stress in Clostridium acetobutylicum ATCC 824. Microarray Data SuperSeries GSE5020. http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE5020 National Center for Biotechnology Information, Bethesda, MD.
- 4. Andrade J. C., Vasconcelos I. 2003. Continuous cultures of Clostridium acetobutylicum: culture stability and low-grade glycerol utilisation. Biotechnol. Lett. 25:121–125 [DOI] [PubMed] [Google Scholar]
- 5. Annous B. A., Blaschek H. P. 1991. Isolation and characterization of Clostridium acetobutylicum mutants with enhanced amylolytic activity. Appl. Environ. Microbiol. 57:2544–2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Antier P., Moulin G., Galzy P. 1990. Influence of composition of the culture medium on the behaviour of Kluyveromyces fragilis in chemostat culture. Process Biochem. 25:9–13 [Google Scholar]
- 7. Antoni D., Zverlov V. V., Schwarz W. H. 2007. Biofuels from microbes. Appl. Microbiol. Biotechnol. 77:23–35 [DOI] [PubMed] [Google Scholar]
- 8. Bahl H., Andersch W., Braun K., Gottschalk G. 1982. Effect of pH and butyrate concentration on the production of acetone and butanol by Clostridium acetobutylicum grown in continuous culture. Eur. J. Appl. Microbiol. Biotechnol. 14:17–20 [Google Scholar]
- 9. Bahl H., Andersch W., Gottschalk G. 1982. Continuous production of acetone and butanol by Clostridium acetobutylicum in a two stage phosphate limited chemostat. Eur. J. Appl. Microbiol. Biotechnol. 15:201–205 [Google Scholar]
- 10. Bieszkiewicz E., Szymanska D. 1987. Studies on the resistance of activated sludge bacteria to high concentrations of methanol, butanol, glycol, cyclohexanone and cyclohexylamine. Acta Microbiol. Pol. 36:259–265 [PubMed] [Google Scholar]
- 11. Blaschek H. P., Annous B. A., Formanek J., Chen C. K. March 2002. Method of producing butanol using a mutant strain of Clostridium beijerinckii U.S. patent 6,358,717.
- 12. Broadbent J. R., Larsen R. L., Deibel V., Steele J. L. 2010. Physiological and transcriptional response of Lactobacillus casei ATCC 334 to acid stress. J. Bacteriol. 192:2445–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bruno-Barcena J. M., Azcarate-Peril M. A., Hassan H. M. 2010. Role of antioxidant enzymes in bacterial resistance to organic acids. Appl. Environ. Microbiol. 76:2747–2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cui X., Churchill G. A. 2003. Statistical tests for differential expression in cDNA microarray experiments. Genome Biol. 4:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dellomonaco C., Fava F., Gonzalez R. 2010. The path to next generation biofuels: successes and challenges in the era of synthetic biology. Microb. Cell Fact. 9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Desmond C., Fitzgerald G. F., Stanton C., Ross R. P. 2004. Improved stress tolerance of GroESL-overproducing Lactococcus lactis and probiotic Lactobacillus paracasei NFBC 338. Appl. Environ. Microbiol. 70:5929–5936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Donaldson G. K., et al. 2007. Fermentative production of four carbon alcohols. International patent WO2007/041269. [Google Scholar]
- 18. Durre P. 2007. Biobutanol: an attractive biofuel. Biotechnol. J. 2:1525–1534 [DOI] [PubMed] [Google Scholar]
- 19. El Kanouni A., et al. 1998. The improvement of glucose/xylose fermentation by Clostridium acetobutylicum using calcium carbonate. World J. Microbiol. Biotechnol. 14:431–435 [Google Scholar]
- 20. EU Commission 2008. Directive 2008/30/EC of the European Parliament and of the Council of 11 March 1008 amending Directive 2006/43/EC on statutory audits of annual accounts and consolidated accounts, as regard the implementing powers conferred on the Commission. Off. J. Eur. Union 51:L 81/53–L 81/56 [Google Scholar]
- 21. Fond O., Engasser J. M., Mattaelamouri G., Petitdemange H. 1986. The acetone butanol fermentation on glucose and xylose. I. Regulation and kinetics in batch cultures. Biotechnol. Bioeng. 28:160–166 [DOI] [PubMed] [Google Scholar]
- 22. Fond O., Engasser J. M., Mattaelamouri G., Petitdemange H. 1986. The acetone butanol fermentation on glucose and xylose. II. Regulation and kinetics in fed-batch cultures. Biotechnol. Bioeng. 28:167–175 [DOI] [PubMed] [Google Scholar]
- 23. Formanek J., Mackie R., Blaschek H. P. 1997. Enhanced butanol production by Clostridium beijerinckii BA101 grown in semidefined P2 medium containing 6 percent maltodextrin or glucose. Appl. Environ. Microbiol. 63:2306–2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gomez L. D., Steele-King C. G., McQueen-Mason S. J. 2008. Sustainable liquid biofuels from biomass: the writing's on the walls. New Phytol. 178:473–485 [DOI] [PubMed] [Google Scholar]
- 25. Grupe H., Gottschalk G. 1992. Physiological events in Clostridium acetobutylicum during the shift from acidogenesis to solventogenesis in continuous culture and presentation of a model for shift induction. Appl. Environ. Microbiol. 58:3896–3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gupta P., Lee K. H. 2007. Genomics and proteomics in process development: opportunities and challenges. Trends Biotechnol. 25:324–330 [DOI] [PubMed] [Google Scholar]
- 27. Hasselbalch K. A. 1917. Die Berechnung der Wasserstoffzahl des Blutes ausder freien und gebundenen kohlensaure desselben, und die Sauerstoffbindung des Blutes als function der Wasserstoffzahl. Biochem. Z. 78:112–144 [Google Scholar]
- 28. Henderson L. J. 1908. Concerning the relationship between the strength of acids and their capacity to preserve neutrality. Am. J. Physiol. 21:173–179 [Google Scholar]
- 29. Jain M. K., Beacom D., Datta R. 1993. Mutant strain of C. acetobutylicum and process for making butanol. U.S. patent 5,192,673.
- 30. Janssen H., et al. 2010. A proteomic and transcriptional view of acidogenic and solventogenic steady-state cells of Clostridium acetobutylicum in a chemostat culture. Appl. Microbiol. Biotechnol. 87:2209–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jobses I. M. L., Roels J. A. 1983. Experience with solvent production by Clostridium beijerinckii in continuous culture. Biotechnol. Bioeng. 25:1187–1194 [DOI] [PubMed] [Google Scholar]
- 32. Johnson J. L., Toth J., Santiwatanakul S., Chen J. S. 1997. Cultures of “Clostridium acetobutylicum” from various collections comprise Clostridium acetobutylicum, Clostridium beijerinckii, and two other distinct types based on DNA-DNA reassociation. Int. J. Syst. Bacteriol. 47:420–424 [DOI] [PubMed] [Google Scholar]
- 33. Jones D. T., Woods D. R. 1986. Acetone-butanol fermentation revisited. Microbiol. Rev. 50:484–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Junelles A. M., Janatiidrissi R., Petitdemange H., Gay R. 1988. Iron effect on acetone butanol fermentation. Curr. Microbiol. 17:299–303 [Google Scholar]
- 35. Kashket E. R., Cao Z. Y. 1995. Clostridial strain degeneration. FEMS Microbiol. Lett. 17:307–315 [Google Scholar]
- 36. Kim B. H., Bellows P., Datta R., Zeikus J. G. 1984. Control of carbon and electron flow in Clostridium acetobutylicum fermentations: utilization of carbon monoxide to inhibit hydrogen production and to enhance butanol yields. Appl. Environ. Microbiol. 48:764–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kirschner M. 2006. n-Butanol. Chem. Market Rep. 269:42 [Google Scholar]
- 38. Kuhn H., Friederich U., Fiechter A. 1979. Defined minimal medium for a thermophilic Bacillus sp. developed by A chemostat pulse and shift technique. Eur. J. Appl. Microbiol. Biotechnol. 6:341–349 [Google Scholar]
- 39. Lee K. H. 2001. Proteomics: a technology-driven and technology-limited discovery science. Trends Biotechnol. 19:217–222 [DOI] [PubMed] [Google Scholar]
- 40. Lee S. F., Forsberg C. W., Gibbins L. N. 1985. Cellulolytic activity of Clostridium acetobutylicum. Appl. Environ. Microbiol. 50:220–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee S. F., Forsberg C. W., Gibbins L. N. 1985. Xylanolytic activity of Clostridium acetobutylicum. Appl. Environ. Microbiol. 50:1068–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee S. Y., et al. 2008. Fermentative butanol production by clostridia. Biotechnol. Bioeng. 101:209–228 [DOI] [PubMed] [Google Scholar]
- 43. Lin Y. L., Blaschek H. P. 1983. Butanol production by a butanol-tolerant strain of Clostridium acetobutylicum in extruded corn broth. Appl. Environ. Microbiol. 45:966–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu H., et al. 2005. Quadratic regression analysis for gene discovery and pattern recognition for non-cyclic short time-course microarray experiments. BMC Bioinformatics 6:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Long S., Jones D. T., Woods D. R. 1983. Sporulation of Clostridium acetobutylicum P262 in a defined medium. Appl. Environ. Microbiol. 45:1389–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Long S., Jones D. T., Woods D. R. 1984. Initiation of solvent production, clostridial stage and endospore formation in Clostridium acetobutylicum P262. Appl. Biochem. Biotechnol. 20:256–261 [Google Scholar]
- 47. Ludwig H., Meinken C., Matin A., Stulke J. 2002. Insufficient expression of the ilv-leu operon encoding enzymes of branched-chain amino acid biosynthesis limits growth of a Bacillus subtilis ccpA mutant. J. Bacteriol. 184:5174–5178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Madihah M. S., Ariff A. B., Sahaid K. M., Suraini A. A., Karim M. I. A. 2001. Direct fermentation of gelatinized sago starch to acetone butanol ethanol by Clostridium acetobutylicum. World J. Microbiol. Biotechnol. 17:567–576 [Google Scholar]
- 49. Mateles R. I., Battat E. 1974. Continuous culture used for media optimization. Appl. Microbiol. 28:901–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Meshartree M., Saddler J. N. 1982. Butanol production of Clostridium acetobutylicum grown on sugars found in hemicellulose hydrolysates. Biotechnol. Lett. 4:247–252 [Google Scholar]
- 51. Monot F., Engasser J. M. 1983. Continuous production of acetone butanol on an optimized synthetic medium. Eur. Appl. Microbiol. Biotechnol. 18:246–248 [Google Scholar]
- 52. Monot F., Martin J. R., Petitdemange H., Gay R. 1982. Acetone and butanol production by Clostridium acetobutylicum in a synthetic medium. Appl. Environ. Microbiol. 44:1318–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Oh S. E., Van Ginkel S., Logan B. E. 2003. The relative effectiveness of pH control and heat treatment for enhancing biohydrogen gas production. Environ. Sci. Technol. 37:5186–5190 [DOI] [PubMed] [Google Scholar]
- 54. Ounine K., Petitdemange H., Raval G., Gay R. 1983. Acetone butanol production from pentoses by Clostridium acetobutylicum. Biotechnol. Lett. 5:605–610 [Google Scholar]
- 55. Papoutsakis E. T. 2008. Engineering solventogenic clostridia. Curr. Opin. Biotechnol. 19:420–429 [DOI] [PubMed] [Google Scholar]
- 56. Paredes C. J., Alsaker K. V., Papoutsakis E. T. 2005. A comparative genomic view of clostridial sporulation and physiology. Nat. Rev. Microbiol. 3:969–978 [DOI] [PubMed] [Google Scholar]
- 57. Peters M. W., Meinhold P., Buelter T., Landwehr M. 2007. Engineered microorganisms for increasing product yield in biotransformations, related methods and systems. Publication no. WO2008/013996. Patent application no. PCT/US2007/017013. Filing date 7/27/2007.
- 58. Quratulain S., Qadeer M. A., Chaudhry M. Y., Kausar A. R. 1995. Development and characterization of butanol resistant strain of Clostridium acetobutylicum in molasses medium. Folia Microbiol. 40:467–471 [Google Scholar]
- 59. Ren C., et al. 2010. Identification and inactivation of pleiotropic regulator CcpA to eliminate glucose repression of xylose utilization in Clostridium acetobutylicum. Metab. Eng. 12:446–454 [DOI] [PubMed] [Google Scholar]
- 60. Rodionov D. A., Mironov A. A., Gelfand M. S. 2001. Transcriptional regulation of pentose utilisation systems in the Bacillus/Clostridium group of bacteria. FEMS Microbiol. Lett. 205:305–314 [DOI] [PubMed] [Google Scholar]
- 61. Roos J. W., McLaughlin J. K., Papoutsakis E. T. 1985. The effect of pH on nitrogen supply, cell lysis, and solvent production in fermentations of Clostridium acetobutylicum. Biotechnol. Bioeng. 27:681–694 [DOI] [PubMed] [Google Scholar]
- 62. Sardessai Y., Bhosle S. 2002. Organic solvent tolerant bacteria in mangrove ecosystem. Curr. Sci. 82:622–623 [Google Scholar]
- 63. Searchinger T., et al. 2008. Use of US croplands for biofuels increases greenhouse gases through emissions from land-use change. Science 319:1238–1240 [DOI] [PubMed] [Google Scholar]
- 64. Soucaille P., Joliff G., Izard A., Goma G. 1987. Butanol tolerance and autobacteriocin production by Clostridium acetobutylicum. Curr. Microbiol. 14:295–299 [Google Scholar]
- 65. Terracciano J. S., Kashket E. R. 1986. Intracellular conditions required for initiation of solvent production by Clostridium acetobutylicum. Appl. Environ. Microbiol. 52:86–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tomas C. A., Welker N. E., Papoutsakis E. T. 2003. Overexpression of groESL in Clostridium acetobutylicum results in increased solvent production and tolerance, prolonged metabolism, and changes in the cell's transcriptional program. Appl. Environ. Microbiol. 69:4951–4965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Van Der Westhuizen A., Jones D. T., Woods D. R. 1982. Autolytic activity and butanol tolerance of Clostridium acetobutylicum. Appl. Environ. Microbiol. 44:1277–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wayman M., Yu S. 1985. Acetone butanol fermentation of xylose and sugar mixtures. Biotechnol. Lett. 7:255–260 [Google Scholar]
- 69. Wiesenborn D. P., Rudolph F. B., Papoutsakis E. T. 1988. Thiolase from Clostridium acetobutylicum ATCC 824 and its role in the synthesis of acids and solvents. Appl. Environ. Microbiol. 54:2717–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Woolley R. C., Morris J. G. 1990. Stability of solvent production by Clostridium acetobutylicum in continuous culture—strain differences. J. Appl. Bacteriol. 69:718–728 [Google Scholar]