Abstract
Selenium-reducing microorganisms produce elemental selenium nanoparticles with particular physicochemical properties due to an associated organic fraction. This study identified high-affinity proteins associated with such bionanominerals and with nonbiogenic elemental selenium. Proteins with an anticipated functional role in selenium reduction, such as a metalloid reductase, were found to be associated with nanoparticles formed by one selenium respirer, Sulfurospirillum barnesii.
TEXT
Certain anaerobic microorganisms utilize selenite and selenate as terminal electron acceptors for respiration and growth (dissimilatory reduction), producing elemental selenium, either as internal accumulations that can be released to the medium or extracellularly (5, 11, 12). Despite these differences in mechanism of formation, biogenic elemental selenium generally does not form large crystals but rather spherical nanoparticles (7, 8, 12). It has been hypothesized that biogenic selenium nanoparticles can be stabilized against crystallization due to the presence of proteins (5), yet hitherto, no proteins associated with nanoparticles of respiratory selenium reducers have been identified. This study investigated such proteins using two dissimilatory selenium reducers, Bacillus selenatarsenatis (DSMZ 18680) and Sulfurospirillum barnesii (DSMZ 10660). For comparison, Rhodospirillum rubrum (DSMZ 467), which can induce selenium precipitation, although not in a dissimilatory manner, was investigated.
Microorganisms were grown anaerobically in media according to the culture collection (DSMZ) with the addition of 20 mM selenate (B. selenatarsenatis, S. barnesii) or 1 mM selenite (R. rubrum). B. selenatarsenatis was first pregrown aerobically without the addition of selenium and transferred to anaerobic medium during the exponential growth phase. S. barnesii was grown using a N2-CO2 (80:20 [vol/vol]) headspace instead of N2. R. rubrum was grown under light. Selenium nanoparticles were harvested in all batches in the late stationary phase, since formation in R. rubrum occurs during that phase (5). As a control, lysed cells and culture medium were incubated with conventionally synthesized elemental selenium (hereinafter referred to as “nonbiogenic”) (Sigma Aldrich, Buchs, Switzerland). For this, pure cultures were grown in the absence of selenium oxyanions and subsequently lysed by means of an ultrasonic probe (Labsonic M; Sartorius, Tagelswangen, Switzerland).
High-affinity proteins associated with elemental selenium (biogenic, nonbiogenic) were isolated from low-affinity/nonassociated proteins and residual biomass using density-based centrifugation in sodium polytungstate solution (SPTS) modified according to references 10 and 13. Batch medium (50 ml) was centrifuged (30 min; 5,000 × g), and pellets were washed twice with Tris buffer (50 mM, pH 7.2) and transferred to the top of SPTS (density, 3 g/ml) (TC Tungsten Compounds, Grub, Germany). Samples were then subjected to low-speed centrifugation (30 min, 2,000 × g), the supernatants containing low-affinity/nonassociated proteins and biomass were discarded, and the pellets were transferred into tubes containing fresh SPTS. Controls using biomass grown without selenium received the same treatment. Since no visible pellet was formed in the latter, 200 μl of SPTS was transferred instead. Centrifugation (five repetitions) was followed by washing with Tris-buffer (seven repetitions) to exhaustively remove remaining SPTS.
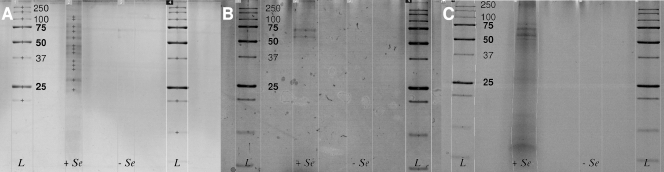
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was used to separate proteins following standard protocols (6). Sypro orange (limit of detectcion [LOD], 1 to 2 ng/band) (Fig. 1) and colloidal blue staining (LOD, <10 ng/band) revealed a number of protein bands for all microbial species used. Protein bands were found to be most abundant at apparent molecular masses of between 23 and 60 kDa (B. selenatarsenatis) (Fig. 1A), 61 and 69 kDa (R. rubrum) (Fig. 1B) and 52 and 80 kDa (S. barnesii) (Fig. 1B).
Fig. 1.
SDS-PAGE gel images (Sypro orange stain) of proteins associated with biogenic selenium nanoparticles (+ Se) and controls (− Se) produced by Bacillus selenatarsenatis (A), Rhodospirillum rubrum (B), and Sulfurospirillum barnesii (C) after density-based centrifugation. Molecular size standards (L) are in kilodaltons.
For mass spectrometry (MS) analysis, colloidal blue-stained bands were excised, reduced with dithiothreitol (10 mM, 2 h, 37°C), alkylated with iodoacetamide (50 mM, 15 min, at room temperature and in darkness), trypsin digested (125 ng trypsin, sequencing grade, 18 h, 37°C), and concentrated in a SpeedVac concentrator. Capillary liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) was conducted with an Orbitrap FT hybrid instrument (Thermo Finnigan, San Jose, CA) (17). The MS/MS spectra were searched against the NCBI data bank (using the Matrix Science portal) by applying the following constraints: carbamidomethyl cysteine as fixed and N-acetylation and oxidized methionine as variable modifications; tryptic specificity allowing two missed cleavages; peptide tolerance, 10 ppm; MS/MS tolerance, 0.6 Da; taxonomy setting, bacteria. The significance threshold was set to 0.05. The search output was filtered in a stringent manner, omitting all proteins with less than four peptides matched and proteins with molecular weights outside 100% ± 20% of the apparent molecular weight determined by SDS.
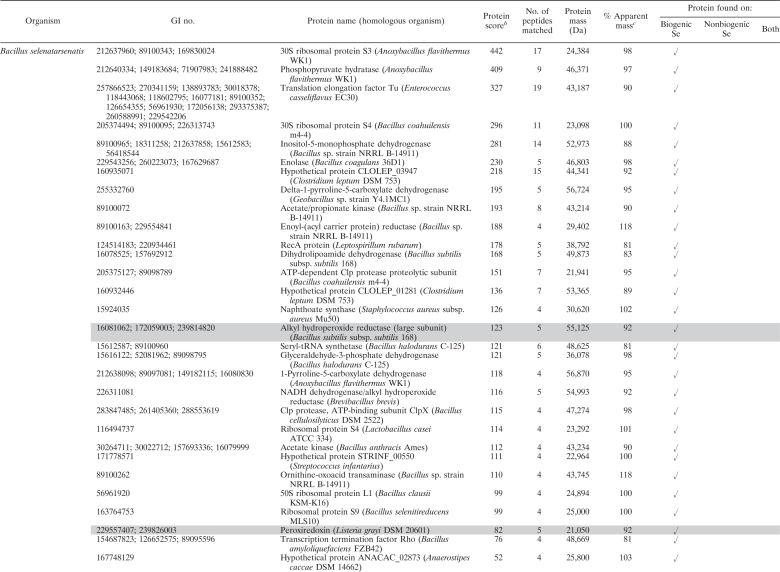
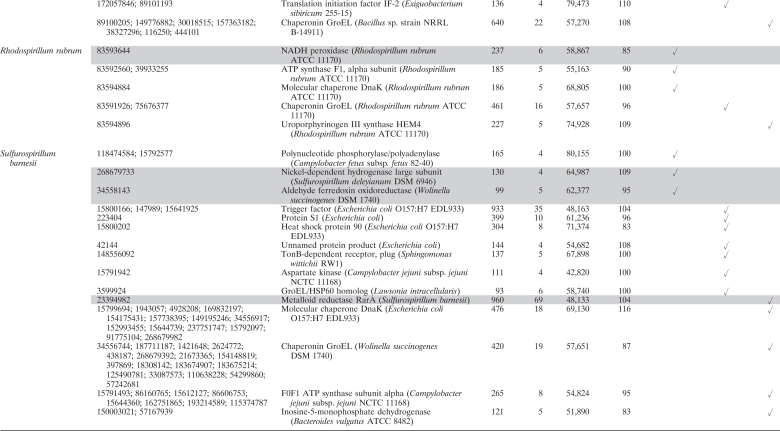
Our results allowed for the first time a qualitative characterization of the high-affinity protein fraction associated with selenium bionanominerals of different microbial origins. For all microorganisms used here, a plenitude of proteins with diverse cellular functions were identified (Table 1) . Furthermore, we could demonstrate that nonbiogenic selenium, as well, associates with a number of proteins produced by dissimilatory selenium reducers. Taking into account the total number of proteins expressed in the studied organisms, it is striking that several proteins were found on both biogenic and nonbiogenic particles. For silver nanoparticles in contact with Escherichia coli cell extracts, it has been shown that proteins associated did not simply reflect the most abundant proteins but instead are due to higher affinities of some proteins to the particles (18). In this regard, it is most striking that the protein with the highest number of peptides matched in this study was the so-called “metalloid reductase RarA” found on both biogenic (S. barnesii) and nonbiogenic selenium nanoparticles (Table 1). Numerous peptide motifs, so-called aptamers, have to date been described to specifically bind inorganic nanomaterials, among others, chalcogen-based (CdS, PbS, ZnS) and metallic (Au, Ag, Si, etc.) nanomaterials, yet not elemental selenium (see reference 16 and references therein). It may thus well be that the metalloid reductase RarA found in this study contains a natural peptide motif conferring its high affinity to elemental selenium. Such peptide motifs are of high interest, since they can be used to bind elemental selenium surfaces in particular applications, e.g., in bioremediation. Here, a current technically unresolved challenge is to remove the nanosized biogenic selenium from the aqueous phase (7). Although its name suggests a selenate-reducing enzymatic activity, unfortunately no further information is available at present regarding the enzymatic characterization of this protein. A role as outer membrane porin can be anticipated from sequence similarities with porins from closely related Sulfurospirillum deleyianum (59% identity) and Geobacter lovleyi (49% identity, NCBI BLAST). It is thus possible that the metalloid reductase found here is involved in the initial uptake of selenate to the periplasm, where other selenate reductases have been found (15).
Table 1.
Proteins identified by LC-ESI-MS/MS associated with biogenic and nonbiogenic elemental seleniuma
For matches relating to homologous proteins in different organisms, all accession numbers (GI numbers) are given, yet only the organism with the highest ion score is mentioned. Proteins with an anticipated role in selenium reduction are highlighted in gray.
A protein score greater than 42 is considered significant (P < 0.05).
% Apparent mass, percentage of the apparent mass as determined by SDS.
Furthermore, we were able to identify proteins with additional anticipated functional roles in selenium reduction in spatial association with the formed bionanominerals. Such proteins include, next to the metalloid reductase RarA, proteins involved in electron transport during microbial respiration, peptides with reactive thiol functional groups, and enzymes involved in reactive oxygen species degradation (Table 1).
For S. barnesii, we first observed respiratory electron transport chain proteins associated with the bionanominerals, i.e., a nickel-dependent hydrogenase and an aldehyde ferredoxin oxidoreductase. It can thus be interpreted that electrons necessary for reduction of selenate can be supplied by the hydrogenase, and this mechanism has been described for microbial reduction of other toxic oxyanions (1). Electrons can then be transferred via ferredoxin, as has been shown for selenate reduction in Synechocystis (9) and selenite reduction in Clostridium (19).
Selenite can be reduced to elemental selenium by reaction with reactive thiol groups of proteins/peptides in the so called “Painter-type” reaction, which has been suggested as a general microbial detoxification reaction to oxyanions (3). The latter idea might be further supported by the present study, since we were able to identify peroxiredoxins in B. selenatarsenatis, which can contain such catalytic cysteine-thiols (2, 14). In addition to reacting via their thiol, functional groups, the peroxiredoxins found here (peroxiredoxin, alkyl hydroperoxide reductase) can have a further general role in reaction to toxic selenium oxyanions, since they are involved in degradation of reactive oxygen species. These, among other products, are generated during the latter Painter-type reactions (3, 4). The same general detoxification role applies to NADH peroxidase found in the nonrespiratory selenium reducer R. rubrum.
This study shows that selenium nanoparticles can be associated with a plenitude of high-affinity proteins, despite their microbial origin and/or biogenic character. It is important to note that identification of proteins associated with selenium nanoparticles critically depends on the successful isolation of proteins associated intrinsically from adventitiously associated proteins that are potentially copurified in the density-based centrifugation in SPTS. In this regard, the modification of the centrifugation procedure as applied in this study allowed us first to definitively distinguish between these protein fractions, since selenium-free controls did not show any protein bands in the SDS gels (Fig. 1). Indisputably, the protein modification on the selenium particles will change the physicochemical properties of the selenium solid to some extent and, in consequence, influence the environmental fate of selenium, as has been demonstrated for other bionanominerals (10). Consequently, this study represents a very first step toward understanding the mechanisms underlying the formation of such associations. The high-affinity proteins identified here might be used to design specific probes for, e.g., immunofluorescence or immunoelectron microscopy to further study and visualize the mechanisms of nanoparticle formation. On one hand, owing to the high importance of selenium in animal and human health, there is a clear need to further study the association of the protein fraction to nanoparticles, e.g., by proteomic analysis focusing on expression of proteins/enzymes found here. On the other hand, future studies should also consider the dissociation or biodegradation of the proteic fraction on selenium (or other bionanominerals), hence opening a fascinating yet challenging future field of interdisciplinary biogeochemical research.
Acknowledgments
The work was supported by the Swiss National Science Foundation (SNF 200021_126899).
Footnotes
Published ahead of print on 20 May 2011.
REFERENCES
- 1. De Luca G., de Philip P., Dermoun Z., Rousset M., Vermeglio A. 2001. Reduction of technetium(VII) by Desulfovibrio fructosovorans is mediated by the nickel-iron hydrogenase. Appl. Environ. Microbiol. 67:4583–4587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Georgiou G., Masip L. 2003. An overoxidation journey with a return ticket. Science 300:592–594 [DOI] [PubMed] [Google Scholar]
- 3. Harrison J. J., Ceri H., Turner R. J. 2007. Multimetal resistance and tolerance in microbial biofilms. Nat. Rev. Microbiol. 5:928–938 [DOI] [PubMed] [Google Scholar]
- 4. Kessi J., Hanselmann K. W. 2004. Similarities between the abiotic reduction of selenite with glutathione and the dissimilatory reaction mediated by Rhodospirillum rubrum and Escherichia coli. J. Biol. Chem. 279:50662–50669 [DOI] [PubMed] [Google Scholar]
- 5. Kessi J., Ramuz M., Wehrli E., Spycher M., Bachofen R. 1999. Reduction of selenite and detoxification of elemental selenium by the phototrophic bacterium Rhodospirillum rubrum. Appl. Environ. Microbiol. 65:4734–4740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laemmli U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 7. Lenz M., Smit M., Binder P., Van Aelst A. C., Lens P. N. L. 2008. Biological alkylation and colloid formation of selenium in methanogenic UASB reactors. J. Environ. Qual. 37:1691–1700 [DOI] [PubMed] [Google Scholar]
- 8. Lenz M., Van Aelst A. C., Smit M., Corvini P. F. X., Lens P. N. L. 2009. Biological production of selenium nanoparticles from waste waters. Adv. Mater. Res. 71–73:721–724 [Google Scholar]
- 9. Marteyn B., Domain F., Legrain P., Chauvat F., Cassier-Chauvat C. 2009. The thioredoxin reductase-glutaredoxins-ferredoxin crossroad pathway for selenate tolerance in Synechocystis PCC6803. Mol. Microbiol. 71:520–532 [DOI] [PubMed] [Google Scholar]
- 10. Moreau J. W., et al. 2007. Extracellular proteins limit the dispersal of biogenic nanoparticles. Science 316:1600–1603 [DOI] [PubMed] [Google Scholar]
- 11. Narasingarao P., Haggblom M. M. 2007. Identification of anaerobic selenate-respiring bacteria from aquatic sediments. Appl. Environ. Microbiol. 73:3519–3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oremland R. S., et al. 2004. Structural and spectral features of selenium nanospheres produced by Se respiring bacteria. Appl. Environ. Microbiol. 70:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pearce C. I., et al. 2009. Investigating different mechanisms for biogenic selenite transformations: Geobacter sulfurreducens, Shewanella oneidensis and Veillonella atypica. Environ. Technol. 30:1313–1326 [DOI] [PubMed] [Google Scholar]
- 14. Poole L. B. 2005. Bacterial defenses against oxidants: mechanistic features of cysteine-based peroxidases and their flavoprotein reductases. Arch. Biochem. Biophys. 433:240–254 [DOI] [PubMed] [Google Scholar]
- 15. Schröder I., Rech S., Krafft T., Macy J. M. 1997. Purification and characterization of the selenate reductase from Thauera selenatis. J. Biol. Chem. 272:23765–23768 [DOI] [PubMed] [Google Scholar]
- 16. Shiba K. 2010. Exploitation of peptide motif sequences and their use in nanobiotechnology. Curr. Opin. Biotechnol. 21:412–425 [DOI] [PubMed] [Google Scholar]
- 17. Vaupotic T., Veranic P., Jenoe P., Plemenitas A. 2008. Mitochondrial mediation of environmental osmolytes discrimination during osmoadaptation in the extremely halotolerant black yeast Hortaea werneckii. Fungal Genet. Biol. 45:994–1107 [DOI] [PubMed] [Google Scholar]
- 18. Wigginton N. S., et al. 2010. Binding of silver nanoparticles to bacterial proteins depends on surface modifications and inhibits enzymatic activity. Environ. Sci. Technol. 44:2163–2168 [DOI] [PubMed] [Google Scholar]
- 19. Yanke L. J., Bryant R. D., Laishley E. J. 1995. Hydrogenase I of Clostridium pasteurianum functions as a novel selenite reductase. Anaerobe 1:61–67 [DOI] [PubMed] [Google Scholar]