Abstract
Conformational changes in sigma 54 (ς54) and ς54-holoenzyme depend on nucleotide hydrolysis by an activator. We now show that ς54 and its holoenzyme bind to the central ATP-hydrolyzing domains of the transcriptional activators PspF and NifA in the presence of ADP–aluminum fluoride, an analog of ATP in the transition state for hydrolysis. Direct binding of ς54 Region I to activator in the presence of ADP–aluminum fluoride was shown and inferred from in vivo suppression genetics. Energy transduction appears to occur through activator contacts to ς54 Region I. ADP–aluminum fluoride-dependent interactions and consideration of other AAA+ proteins provide insight into activator mechanochemical action.
Keywords: Sigma 54, activators, transcription, ADP · AlFx, AAA+ proteins
Transcription by RNA polymerase (RNAP) is often regulated by interactions with control proteins to link specific gene expression to environmental signals and temporal cues. Often activators help recruit RNAP to promoters to increase initiation rates (Busby and Ebright 1999). In contrast, activity of the bacterial ς54 containing RNAP holoenzyme is regulated at the DNA melting step (for review, see Buck et al. 2000). Hydrolysis of an NTP by an activator drives a change in configuration of the ς54-holoenzyme, converting the initial closed complex to an open complex to allow interaction with the template DNA for mRNA synthesis (Wedel and Kustu 1995). Preopening of DNA templates does not overcome the requirement for NTP hydrolysis by an activator to promote engagement of the holoenzyme with the melted DNA (Wedel and Kustu 1995; Cannon et al. 1999).
The activators of ς54-holoenzyme are members of the large AAA+ protein family, which use ATP binding and hydrolysis to remodel their substrates (Neuwald et al. 1999; Cannon et al. 2000, 2001). The greater part of the central domain of ς54 activators corresponds to the AAA core structure, and includes ATP-binding and hydrolyzing determinants. The ς54 protein is known to be the primary target for the NTPase of activators, but how activators use NTP binding and hydrolysis is not well understood (Cannon et al. 2000). Similarly, the nature of the interaction between ς54 and the activator is not well described, but an interaction with ς54 can be detected in the case of the DctD activator by protein cross-linking (Lee and Hoover 1995). Here we show that the use of ADP–aluminum fluoride, an analog of ATP that mimics ATP in the transition state for hydrolysis, allows formation of a stable complex among the activator PspF, the PspF and NifA central activating domains, and ς54. The binding assay was used to help define determinants in ς54 and the activator needed for their interaction, and to show that binding can lead to an altered ς54–DNA footprint. The need for a transition-state analog of ATP for protein–protein binding is discussed in relation to the required ATPase activity of activators of ς54-dependent transcription. In particular, it seems that altered functional states of activators exist as ATP is hydrolyzed. This suggests a parallel to some switch and motor proteins that use nucleotide binding and hydrolysis to establish alternate functional states (Hirose and Amos 1999).
Results
Assay system
Enhancer-binding activators of the ς54-holoenzyme are typically composed of three domains (Drummond et al. 1986; Morett and Segovia 1993). These include a C-terminal enhancer DNA-binding domain and an N-terminal domain. The latter functions in regulation, often by acting on the central domain (Lee et al. 2000). Interactions with the ς54-holoenzyme and ATP-binding and hydrolyzing activities directly involve the activator central domain. The PspFΔHTH protein we have employed here represents mainly the central domain of the ς54 activators (Fig. 1a). The PspF activator of Escherichia coli lacks a regulatory N-terminal domain, being subject instead to control by PspA (Jovanovic et al. 1999; Dworkin et al. 2000). Interactions of ς54 and the activator PspFΔHTH were explored in the presence of MgADP and compounds that are known to mimic the transfer of the γ-phosphate at hydrolysis of ATP (transition state analogs) with several proteins, as defined by X-ray crystallography of the nucleotide-containing complexes (Fersht 1998). In particular, we were interested in exploring the possibility of isolating ς54-activator complexes that depend on nucleotide interactions with the activator. The basic assay consisted of incubating the activator with the transition state analog ADP–aluminum fluoride (ADP · AlFx) together with ς54 (or holoenzyme), or a DNA complex thereof, and resolving the mixture on a native polyacrylamide gel. Typically either one of the protein components or DNA was 32P-end labeled, and in some experiments complexes were visualized by Coomassie staining.
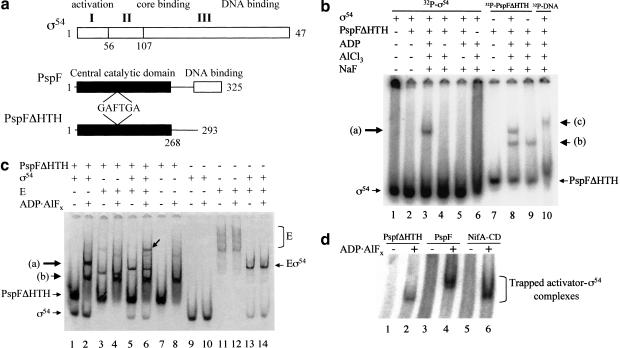
Figure 1.
(a) Schematics of ς54 and the activator PspF. The three regions of Klebsiella pneumoniae ς54 and their associated functions are indicated (Buck et al. 2000). The functional domains of the Escherichia coli ς54 activator PspF and its derivative PspFΔHTH deleted for its DNA-binding domain are shown (Jovanovic et al. 1999). The approximate position of the highly conserved GAFTGA motif implicated as part of the Switch 1 region in ς54 activators (Rombel et al. 1998; Yan and Kustu 1999) is indicated. (b) Gel mobility-shift assay for ADP–aluminum fluoride-dependent complex formation between PspFΔHTH and ς54 using 32P-HMK-tagged protein. Reactions were with 32P-HMK-tagged ς54 or 32P-HMK-tagged PspFΔHTH (100 nM), unlabeled PspFΔHTH (10 μM), and unlabeled ς54 (1 μM). Lane 10 contains ς54 (1 μM), PspFΔHTH (10 μM) with 32P-end-labeled Sinorhizobium meliloti nifH promoter 88-nt DNA (16 nM). Arrow (a) indicates position of complexes formed between ς54 and PspFΔHTH in the presence of ADP · AlFx; arrow (b) indicates the position of PspFΔHTH complex formed in the presence of ADP · AlFx, and arrow (c) indicates the position of DNA–ς54–PspFΔHTH complex in the presence of ADP · AlFx. (c) Gel mobility-shift assay for ADP · AlFx-dependent complex formation between PspFΔHTH and ς54 and ς54-holoenzyme detected by Coomassie staining. Reaction conditions were as in a except for PspFΔHTH (20 μM), ς54 (4 μM or 600 nM when present with core RNAP [E]), and core RNAP (300nM). The arrow on the gel indicates the holoenzyme trapped activator complex in lane 6. Arrows (a) and (b) point to complexes as indicated in a. (d) Wild-type PspF and the NifA central domain form an ADP · AlFx-dependent complex with ς54. Reaction conditions were as in b with 32P-HMK ς54 (50 nM), PspFΔHTH (10 μM), wild-type PspF (3 μM), and NifA central domain (3 μM). PspFΔHTH (∼36 kD), wild-type PspF (∼37.5 kD), and NifA-CD (∼32 kD).
ADP–aluminum fluoride induces formation of a stable complex between the activator and ς54
Initially either ς54 or PspFΔHTH was 32P-end-labeled through an engineered heart muscle kinase (HMK) tag (Casaz and Buck 1997). Under conditions where ADP · AlFx (where x must be 3 or 4) can form, but not otherwise, the end-labeled protein (either ς54 or PspFΔHTH) was found in a new, slow-running complex when the nonlabeled protein (activator or ς54, respectively) was added (Fig. 1b, lanes 3,8). Similar results were also achieved using proteins lacking the heart muscle kinase tag and in the absence of α-lactoalbumin (a nonspecific carrier protein, see Materials and Methods), with complexes being detected by Coomassie staining (Fig. 1c). PspFΔHTH in the presence of ADP · AlFx also bound ς54-holoenzyme (Eς54; Fig. 1c, lane 6). Results show that ς54 and its holoenzyme can detectably associate with PspFΔHTH to form a stable complex in the presence of ADP · AlFx. Hereafter we use the term “trapped” to refer to the form of activator bound to ς54 or ς54-holoenzyme in the presence of ADP · AlFx.
Controls using core RNAP (E) alone did not result in the increased formation of a complex between core RNAP and PspFΔHTH–ADP · AlFx, suggesting that ς54 is the main target of the activator within the holoenzyme (Fig. 1c, cf. lanes 3 and 8 with lane 4). Interestingly, PspFΔHTH can interact with core RNAP in the absence of ADP · AlFx (Fig. 1c, cf. lane 3 with lanes 1, 7, and 11).
We also used ADP · AlFx with the full-length PspF activator (i.e., with its DNA-binding domain) and the central domain of the nitrogen fixation A protein, NifA-CD (Money et al. 2001), another activator of the ς54-holoenzyme, so as to trap stable complexes with ς54 (Fig. 1d). As predicted from the presence of the activator PspFΔHTH within the trapped complex that formed with ς54, and the different molecular weights of the PspFΔHTH, PspF, and NifA-CD, these three ς54 trapped complexes each had a different native gel mobility (Fig. 1d).
Order of addition experiments in which either 32P-ς54 (50 nM) and PspFΔHTH (10 μM) were preincubated prior to formation of ADP · AlFx (as in the standard reaction; see Materials and Methods) or PspFΔHTH was exposed to ADP · AlFx before addition of ς54, resulted in 24% and 1% of the 32P-ς54 bound in the trapped complex, respectively. Formation of the ς54-holoenzyme trapped complex was subject to the same order of addition effects (data not shown). This strongly suggests that the transition-state analog ADP · AlFx acts to stabilize a preexisting unstable complex between ς54 and PspFΔHTH.
Addition of 20 mM phosphate or 10 mM ATP after trapped complexes had been allowed to form did not diminish the amount of trapped ς54–PspFΔHTH complex, indicating that the ADP · AlFx is stably bound in the complex (data not shown). ADP without aluminum fluoride, use of the alternative transition-state analog ADP · Vi (ADP in the presence of vanadate ion), or nonhydrolyzable analogs AMPPNP or ATPγS did not result in formation of a stable ς54–PspFΔHTH complex (data not shown; Cannon et al. 2000, 2001). Other sigma factors (E. coli ς70 or ς38) did not associate with PspFΔHTH–ADP · AlFx to give the slow-migrating trapped complex (data not shown). Therefore, We conclude that ADP · AlFx acts specifically to increase the binding of activator to ς54 and its holoenzyme.
Using Coomassie staining we estimated the amount of ς54 and PspFΔHTH in trapped complexes isolated from a native gel (data not shown). Repeated experiments indicated that not less than five PspFΔHTH monomers are present per ς54 monomer. This implies that an oligomeric form of activator binds to ς54. Simple steric effects may also limit the number of ς54 molecules bound per activator oligomer.
ADP · AlFx changes self-association and ATPase activity of PspFΔHTH
The native gel mobility of the PspFΔHTH activator is changed when ADP · AlFx is allowed to form (Fig. 1b, cf. lanes 7 and 9; Fig. 1c, cf. lanes 7 and 8). This could be caused by differences in oligomerization state and/or conformation. Activators, in particular NtrC, of the ς54-holoenzyme are known to form higher-order oligomers (Wyman et al. 1997), and this is also true for PspF and PspFΔHTH (see below). Activators of ς54 belong to the AAA+ protein family (Neuwald et al. 1999; Vale 2000), crystal structures of which show nucleotide interactions in one protomer and contact to the γ-phosphate from an adjacent protomer within a hexameric assembly (Neuwald et al. 1999). Preliminary gel filtration experiments and analytical ultracentrifigation analyses have shown that ADP · AlFx does increase the association state of the PspFΔHTH protein (data not shown). Because the PspF protein is known to interact with ATP (Jovanovic et al. 1999), we infer that the self-associated activator is in an ADP · AlFx-bound form. The ATPase activity of PspFΔHTH and PspF were inhibited by ADP · AlFx. With PspFΔHTH (3.0 μM) or PspF (1.0 μM) and ATP (0.4 mM), the presence of ADP · AlFx reduced ATPase activities by 40% and 95%, respectively. ς54 is not known to interact directly with nucleotides, suggesting that binding of ς54 to PspFΔHTH is stabilized through interactions made between ADP · AlFx and activator.
Role of activator self-association in binding ς54
Trapping experiments were performed using wild-type PspF at concentrations above that at which it fully self-associates and forms a higher-order oligomer (data not shown). Addition of ADP · AlFx did not alter the native gel mobility of the wild-type PspF but did allow it to bind ς54 (data not shown; Fig. 1d, lane 4). Addition of ATP, ADP, or ATPγS did not allow wild-type PspF to bind stably to ς54 (data not shown). Formation of a higher-order oligomer per se does not therefore allow PspF to stably bind ς54. Rather, a distinct form of PspF associated with the presence of the transition-state analog ADP · AlFx is required for a stable interaction between activator and ς54. The ADP · AlFx-dependent self-association of PspFΔHTH may reflect loss of a contribution by the HTH to self-association that allows the effects of binding the ATP analog to be visualized in terms of oligomerization changes. Binding of ADP · AlFx between protomers can help account for the self-association of PspFΔHTH.
ς54 Region I is essential for binding activator
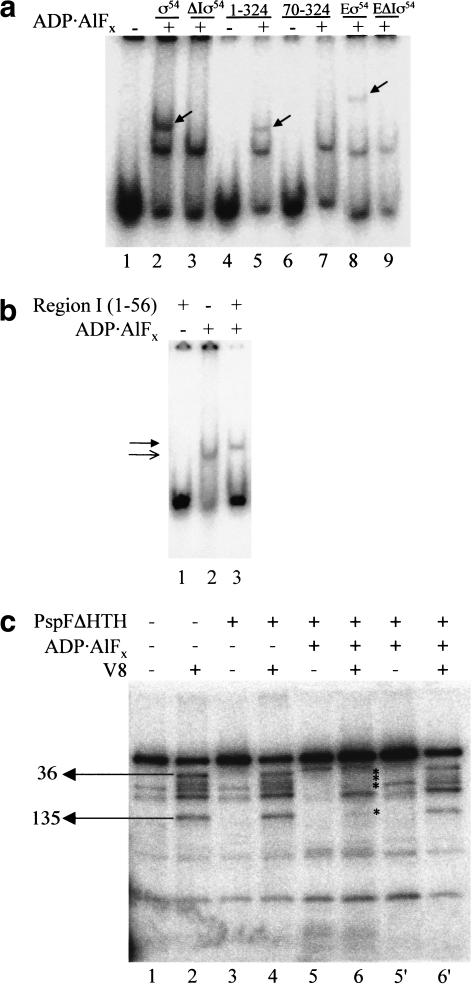
ς54 fragments 57–477 (ς54 deleted for Region I, ΔIς54), 1–324, 70–324, and a series of ς54 Region I three alanine substitution mutants from residue 6 to 50 (Casaz et al. 1999), both alone or as part of the holoenzyme, were screened for trapped complex formation with end-labeled PspFΔHTH activator (Fig. 2a; data not shown). It is clear that the ς54 N-terminal Region I sequences (residues 1–56) are important for the binding reaction with PspFΔHTH–ADP · AlFx. No single three alanine substitution mutant in ς54 Region I diminished formation of the trapped complex with ς54 or ς54-holoenzyme as greatly as did removal of Region I (Fig. 2a; data not shown). However, clear patterns of reduced binding were apparent, suggesting that several sequences in Region I contribute to binding of the activator (Casaz et al. 1999). With 32P-HMK-tagged PspFΔHTH at 100 nM and ς54-holoenzyme at 300 nM, Region I residues 6–11, 33–38, and 45–47 stood out as important patches for binding PspFΔHTH–ADP · AlFx to holoenzyme. Triple alanine substitutions across these positions bound 20%, 30%, and 50% of the PspFΔHTH–ADP · AlFx compared to wild-type holoenzyme, respectively (data not shown). For residues 33–38 and 45–47, binding activity correlates with the critical role of these patches in activated transcription (Syed and Gralla 1998; Casaz et al. 1999; Gallegos and Buck 2000). Importantly, two mutants in ς54 (deletion 310–328 and R336A) that share with certain ς54 Region I mutants and the Region I deletion form of ς54 the property of activator-independent transcription in vitro (Chaney and Buck 1999; Chaney et al. 2000), efficiently formed trapped complexes with PspFΔHTH (data not shown). This further supports the argument that ς54 Region I may directly interact with PspFΔHTH. Experiments using ς54 fragments 70–324 and 1–324 together with ΔIς54 (residues 57–477) and wild-type ς54 showed that the NifA-CD had the same specificity for Region I as did PspFΔHTH in the trapping reaction (Fig. 2a; data not shown).
Figure 2.
(a) Gel mobility-shift assay for ADP · AlFx-dependent complex formation between PspFΔHTH and ς54, ς54 peptides, and ς54-holoenzyme with and without Region I. Reactions contained 32P-HMK-tagged PspFΔHTH (100 nM), ς54, and ΔIς54 (1 μM). Peptides 1–324 and 70–324 (50 μM). Eς54 and EΔIς54 were formed with ς54 (600 nM) and E (300 nM). Trapped activator–ς54 fragment complexes are marked with an arrow. (b) Gel mobility shift assay for ADP · AlFx-dependent complex formation between PspFΔHTH and ς54 Region I. Reactions contained 32P-HMK-tagged PspFΔHTH (100 nM) and ς54 Region I (50 μM). The lower, unfilled arrowhead indicates the PspFΔHTH–ADP · AlFx complex and the upper, filled arrowhead the trapped PspFΔHTH–Region I complex. (c) V8 footprinting of the trapped PspFΔHTH–ς54 complex. Reactions contained 32P-HMK-tagged ς54 (200nM) and PspFΔHTH (20 μM). V8-treated reactions are marked with + (lanes 2,4,6,6‘) and untreated reactions are marked with − (lanes 1,3,5,5′). Lanes 5′ and 6‘ contain the free ς54 isolated from reactions in lanes 5 and 6, respectively. V8 cleavage sites are as marked.
Interactions of ς54 Region I with activator
To explore the possibility that Region I (residues 1–56 of ς54) sequences might directly bind activator, we added purified Region I to PspFΔHTH (Fig. 2b). A distinct, buffer-independent, small reduction in gel mobility was seen when PspFΔHTH–ADP · AlFx was formed in the presence of Region I, compared to controls without Region I (Fig. 2b, cf. lanes 2 and 3). No stable interaction was seen between PspFΔHTH and Region I in the absence of ADP · AlFx (Fig. 2b, lane 1; data not shown). This result provides direct evidence for a PspFΔHTH–ADP · AlFx–Region I interaction. The absence of other regions of the ς54 protein may allow Region I to interact with the PspFΔHTH monomer so as to inhibit activator self-association in the presence of ADP · AlFx. This could explain why lane 3 does not also contain a band with the mobility corresponding to self-associated activator as seen in lane 2.
Experiments using heterobifunctional cross-linking reagents revealed that determinants for nucleotide-independent binding of ς54 to the activator DctD were outside of ς54 Region I (Lee and Hoover 1995; Kelly et al. 2000). Therefore, we performed a competition assay wherein an increasing concentration of Region I or ΔIς54 was added to a fixed amount of ς54 and PspFΔHTH. At a ratio of 4:1 (Region I or ΔIς54:32P-ς54) added prior to trapping, Region I reduced the amount of ς54 in the trapped complex by 68% and ΔIς54 reduced the amount by 20% (data not shown). Together these results suggest that Region I is the region of primary contact between PspFΔHTH and ς54 before and after trapping but that additional determinants for an activator–ς54 interaction prior to trapping do exist outside of Region I. Consistent with the Region I–trapped activator interaction assays, protein footprints of the stable PspFΔHTH–ς54 complex formed with ADP · AlFx showed that much of the Region I sequence was protected from protease attack (Fig. 2c, lane 6). In contrast, unbound ς54 from the same trapping and footprinting reaction was not protected across Region I (Fig. 2c, cf. lanes 6 and 6‘). Protection in trapped complexes extended as far as amino acid 135, within the acidic Region II of ς54. Overall we conclude that PspFΔHTH–ADP · AlFx and ς54 form a complex that involves direct protein–protein contacts between Region I of ς54 and the activator.
ς54 mutants implicate interactions between the GAFTGA motif and Region I in vivo
A signature of activators of the ς54-holoenzyme is the six-amino-acid GAFTGA motif within the C3 region, which is involved in transcriptional activation and implicated in energy coupling (Morett and Segovia 1993; Wang et al. 1997; Gonzalez et al. 1998; Rombel et al. 1998). In an attempt to identify the determinants of the ς54-holoenzyme involved in the interaction with activator proteins, we searched for mutants of ς54 able to recover activator function of activation-defective mutants in the GAFTGA motif and in an adjacent residue in the C3 region of Bradyrhizobium japonicum nifA (Gonzalez et al. 1998). This strategy is based on the premise that in a macromolecular assembly the activity of a mutation that affects one of the members can be suppressed through a compensatory mutation in an interacting member.
Randomly generated mutants across Regions I and II of ς54 were screened for suppression of the NifA E298D (outside of GAFTGA) and NifA T308S (within GAFTGA) mutants, which give a low (<1% compared to wild-type NifA) transcription activity in vivo (Gonzalez et al. 1998). From an initial pool of ∼50,000 mutants, two rounds of screening resulted in selection of a mutant that consistently maintained the suppression phenotype. The nucleotide sequence of this clone revealed six nucleotide changes, resulting in four amino acid replacements and two silent changes (Q20L, CAG to CTG; H53N, CAC to AAC; D89D, GAT to GAC; D159G, GAA to GGA; R196R, CGT to CGC; and D231V, GAC to GTC). To determine which amino acid replacements were responsible for the phenotype, the four amino acid changes were segregated. The suppression phenotype was only maintained when Q20L and H53N mutations were present simultaneously (clone Q20L/H53N in Table 1a). The data presented in Table 1a suggest that mutant ς54 Q20L/H53N suppressed mutant NifA T308S in an allele-specific manner, because its activity was increased 23-fold but the activity of NifA E298D was increased only fivefold. Expression in combination with the wild-type NifA does not seem to be affected.
Table 1.
β-galactosidase activity from K. pneumoniae nifH and S. meliloti nifH promoters in an rpoN-background
| (a) The mean β-galactosidase activity of ς54 wild type and the ς54 Q20L/H53N double mutant at the K. pneumoniae nifH promoter in the presence of NifA wild type and NifA T308S and E298D mutants. |
| NifA
|
ς54
|
ς54 Q20L/H53N
|
|---|---|---|
| wild type | 35055 ± 2212 | 39640 ± 640 |
| T308S | 102 ± 10 | 2385 ± 408 |
| E298D | 50 ± 0.57 | 279 ± 112 |
| − | 55 ± 10 | nd |
| (b) The mean β-galactosidase activity of ς54 wild type and the ς54 Q20L/H53N double mutant at the S. meliloti nifH promoter in the presence of PspFΔHTH and PspFΔHTH carrying mutations T86S or T86V. |
| PspFΔHTH
|
ς54
|
ς54 Q20L/H53N
|
|---|---|---|
| PspFΔHTH | 9131 ± 701 | 17155 ± 774 |
| T86S | 2183 ± 107 | 10106 ± 845 |
| T86V | 2019 ± 99 | 3540 ± 475 |
| − | 2057 ± 211 | 4085 ± 89 |
| (c) The β-galactosidase activity from the K. pneumoniae nifH promoter of a series of double mutations at position Q20 and H53 in the presence of NifA wild type and NifA T308S. |
| ς5420/53
|
NifA
|
NifA T308Sa
|
|---|---|---|
| leu/leu | 32044 | 1426 ± 3 |
| leu/phe | 34314 | 3998 ± 885 |
| leu/asn | 32860 | 1634 ± 333 |
| leu/asn | 28627 | 1699 ± 235 |
| val/ala | 29499 | 1076 ± 200 |
| leu/ala | 35444 | 1374 ± 243 |
| leu/thr | 46478 | 1734 ± 410 |
| leu/glu | 31568 | 1327 ± 109 |
| leu/ser | 32401 | 1537 ± 108 |
| leu/asp | 29293 | 1248 ± 144 |
The β-galactosidase activity of ς54 wild type with NifA T308S was as in a.
The suppression potential of ς54 Q20L/H53N was also examined with region C3 GAFTGA mutants in PspF. To do so, the corresponding amino acid substitutions T308S and T308V of B. japonicum NifA were made in PspFΔHTH by site-directed mutagenesis, resulting in PspFΔHTH T86S and PspFΔHTH T86V. A Sinorhizobium meliloti nifH–lacZ fusion was used to determine activity because this promoter can be activated in trans by PspFΔHTH. The data in Table 1b show that the changes generated in the GAFTGA motif of PspFΔHTH also strongly affected the activation function, as in the case of NifA, and that the ς54 Q20L/H53N suppressed the PspFΔHTH T86S mutant. As previously observed, in vivo expression of the S. meliloti nifH promoter in the absence of a plasmid-borne activator is greater than the Klebsiella pneumoniae nifH promoter. This is likely caused by cross-activation at this strong promoter by other ς54 activators present in the cell. Interestingly, expression from ς54 Q20L/H53N in the absence of plasmids carrying PspFΔHTH was about twice that seen with wild-type ς54, suggesting that cross-activation is more efficient with ς54 Q20L/H53N.
To identify other possible combinations of amino acids that could result in the same or a clearer suppression phenotype, codons 20 and 53 of ς54 were mutagenized to saturation using a pair of oligonucleotides with NNG/C at these positions. The resulting mutants were screened for suppression of the NifA T308S mutation. Ten colonies were detected that displayed a suppressor phenotype with the NifA T308S mutant. The plasmids of selected colonies were segregated and retransformed and their β-galactosidase activity was determined (Table 1c).
Nine out of the 10 ς54 suppressor mutants of NifA T308S had leucine at position 20, as did the original suppressor ς54 Q20L/H53N; the remaining mutant had Q20V. This indicates a clear requirement for a hydrophobic amino acid, leucine, at this position to suppress the NifA T308S phenotype. Moreover, the combination leucine/asparagine was selected several times with different codons for leucine, which discounts duplication of siblings. A range of functional substitutions at position 53, in addition to asparagine, suppressed the low transcription activity phenotype of NifA T308S (Table 1c). Position 53 seems to be more accessible to substitutions that lead to suppression than position 20. It is noteworthy that Q20L plus H53F had activity double that of the parental mutant (Table 1c). Although the double mutant had considerable activity with NifA T308A, no mutants were isolated that could recover the transcription activity of NifA E298D (data not shown).
Overall, the in vivo data provide strong indirect evidence for a functional interaction between the GAFTGA motif and Region I of ς54. Q20 and H53 lie within ς54 sequences that are protected from protease attack by PspFΔHTH–ADP · AlFx (Fig. 2c) and that directly bind to PspFΔHTH–ADP · AlFx (Fig. 2b). Taken together, these data suggest that the Q20L and H53F suppression phenotype may be caused by an enhanced affinity for activator.
The PspF GAFTGA motif is a determinant of ς54 activator binding
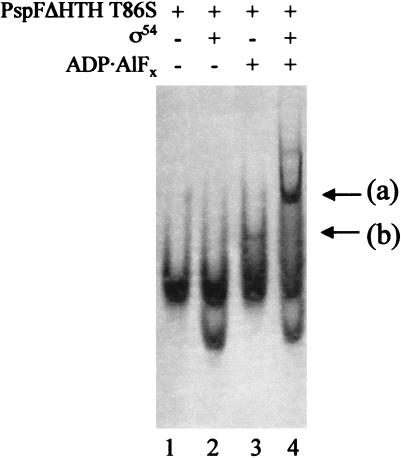
Using PspFΔHTH GAFTGA mutants with T → S, T → A, or T → V mutations (amino acid 86), we examined whether the integrity of this motif was required for binding to the ς54 protein and its holoenzyme under trapping conditions (see below). In an in vitro transcription assay that allowed only one round of transcription from the S. meliloti nifH promoter (10 nM) with ς54-holoenzyme (100 nM) and PspFΔHTH (4.0 μM), the T86S mutant gave 52% whereas the T86A and T86V mutants each gave less than 1% of the activity of the wild-type PspFΔHTH. The higher than expected activity of the T86S mutant compared to the in vivo data (Table 1b) may be explained in part by the effect of transcription reinitiation kinetics that are not measured in the single-round transcription assay. ATPase assays showed that at 2.0 μM protein monomer, the T86S, T86A, and T86V mutants had ATPase activities equivalent to wild type (data not shown). These data discount a simple defect in nucleotide binding. Trapping assays suggest that sequences within the GAFTGA motif function in binding ς54 Region I. The T86A and T86V mutants failed to give the characteristic self-associated complex seen with PspFΔHTH–ADP · AlFx in the absence of ς54 and did not detectably form ADP · AlFx-dependent complexes with ς54 or the holoenzyme (data not shown). The T → S mutant was defective for forming the ADP · AlFx-dependent self-associated complex seen with PspFΔHTH in the absence of ς54 (cf. Fig. 3, lane 3 with Fig. 1c, lane 8) but did form trapped complexes with ς54 and holoenzyme (cf. Fig. 3, lane 4 with Fig. 1c, lane 2; data not shown). Binding of ς54 to the T86S mutant may stabilize its oligomeric state in the presence of ADP · AlFx. Results with the T86 mutants correlate to the known defects in the GAFTGA motif for transcription activation and ς54 isomerization (Gonzalez et al. 1998; Cannon et al. 2000), and suggest they are closely linked to changes in binding one functional state of the activator that is established upon interaction with ADP · AlFx. Clearly, although binding of ς54 need not be directly or exclusively to the GAFTGA motif, it critically involves it.
Figure 3.
Gel mobility-shift assay for ADP · AlFx-dependent complex formation between the PspFΔHTH T86S GAFTGA mutant and ς54 detected by Coomassie staining. Reactions contained PspFΔHTH T86S (20 μM) and ς54 (2 μM). Arrow (a), trapped ς54–PspFΔHTH T86S complex; (b), PspFΔHTH T86S–ADP · AlFx complex.
ς54–DNA interactions in trapped complexes
To determine the DNA-binding properties of the trapped activator–ς54 complexes and compare these to ς54, we conducted band shift and footprint assays. We wished to learn what the functional consequences of binding trapped activator were with respect to the DNA interacting properties of ς54 that are central to maintaining the closed promoter complex and to establishing the open promoter complex.
The trapped activator–ς54 complex binds promoter DNA
PspFΔHTH lacks a DNA-binding domain and its use simplifies band-shift assays that employ DNA probes. We showed that ς54 bound to DNA probes derived from the S. meliloti nifH promoter also formed trapped complexes with PspFΔHTH (Fig. 4a). The new activator and ADP · AlFx-dependent slow-running DNA complex has a slightly lower mobility than the DNA-free trapped PspFΔHTH–ς54 complex (Fig. 1b, cf. lane 10 with lanes 3 and 8). Controls showed that PspFΔHTH activator alone could not band shift the DNA irrespective of the presence of the trapping conditions (data not shown) and that the mobility of the ς54–DNA complexes remained unchanged under trapping conditions when activator protein was omitted (data not shown). When ΔIς54 (ς54 lacking Region I) was bound to DNA it did not form a new complex in the presence of PspFΔHTH–ADP · AlFx, as was the case for trapping reactions without DNA (Fig. 2a). Trapped complexes containing promoter DNA were able to form when ς54 was bound to DNA before trapping and when the ς54 activator-trapped complex was allowed to form prior to addition of DNA. Compared to homoduplex DNA, a −12 to −11 heteroduplex (early melted DNA), representing DNA melted early in transcription initiation, is bound six- to eightfold more strongly by ς54 (Cannon et al. 2000). The trapped complex did not show a loss of preference for binding the early melted DNA compared to the homoduplex probe, indicating that trapping does not greatly change ς54–early melted DNA interactions (Fig. 4a, cf. lanes 3 and 6). Because Region I sequences of ς54 direct its tight binding to early melted DNA (Cannon et al. 1999, 2000; Gallegos and Buck 1999; Guo et al. 1999), this activity appears unaltered when ς54 is bound by PspFΔHTH–ADP · AlFx, even though the activator is interacting with Region I.
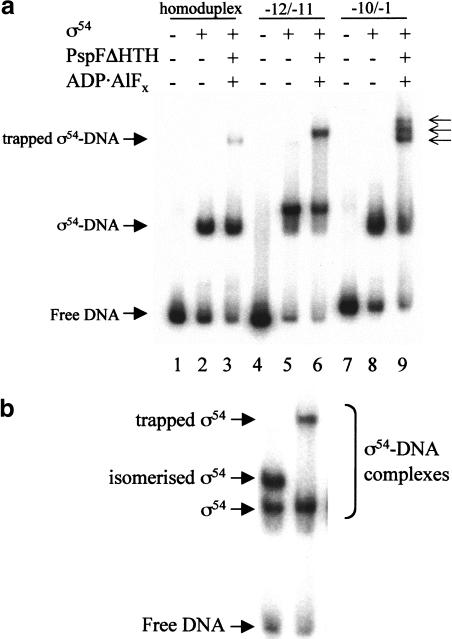
Figure 4.
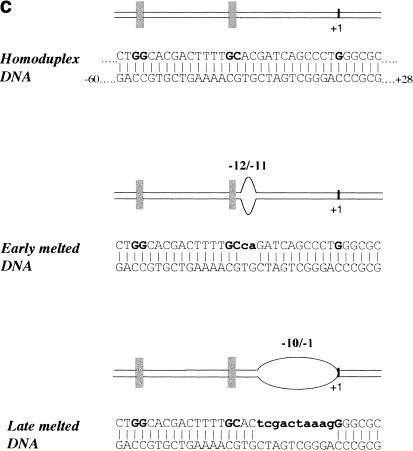
Gel mobility shift assay for ς54 bound to DNA in the presence of PspFΔHTH ADP · AlFx. (a) Reactions contained 32P-end-labeled Sinorhizobium meliloti nifH promoter 88-nt DNA (16 nM), ς54 (1 μM), and PspFΔHTH (10 μM). The three arrows indicate the multiple bands obtained with the −10/−1 (late melted) heteroduplex DNA. (b) Mobility of the isomerized ς54–DNA supershifted complex (lane 1; Cannon et al. 2000) compared with the trapped PspFΔHTH–ς54–DNA complex (lane 2). ATP was used for isomerization. (c) DNA molecules used in this experiment. The consensus GG and GC of the ς54 binding sites are indicated by the vertical bars. Mismatched regions that create the early and late melted DNA templates are indicated.
A third DNA template used in the trapping experiments was an S. meliloti nifH heteroduplex from −10 to −1 (late melted DNA), representing DNA melted later in the transcription initiation process (Cannon et al. 1999, 2000). The late melted heteroduplex gave a close set of slow-running bands, suggesting that an interaction between ς54 and the region of melted DNA could be changed by binding of PspFΔHTH–ADP · AlFx to ς54 Region 1 (Fig. 4a, lane 9).
Although homoduplex and heteroduplex DNAs each gave slow-running complexes under trapping conditions, only the early melted DNA gives the higher mobility nucleotide hydrolysis-dependent isomerized complex in which activator is not stably bound (Fig. 4b, lane 1; Cannon et al. 2000).
The trapped complex produces an extended DNA footprint
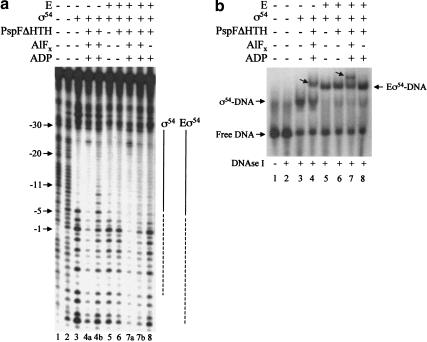
To explore further whether the trapped activator–ς54 complex interacts differently with promoter DNA, we conducted DNA footprinting of the trapped ς54–promoter complexes. Using DNase I we found that the footprint of the trapped complex on homoduplex DNA was extended compared to ς54, and was clearly increased toward the start of transcription (Fig. 5, lane 4a). Untrapped ς54–DNA complexes from the same trapping and footprinting reaction did not show this footprint extension (Fig. 5, lane 4b). Unlike the ATP hydrolysis-dependent extended footprint of ς54, which requires the use of the early melted heteroduplex (Cannon et al. 2000, 2001), the extended trapped complex footprint was not DNA template-dependent. The trapped complex gave a similar extended DNase I footprint on both the early melted and homoduplex DNA (data not shown). Similar extended footprints on both DNA templates were also observed with trapped ς54-holoenzyme complexes (Fig. 5, lane 7a; data not shown). We conclude that the extended footprint occurs as a consequence of activator interacting with ς54 Region I at the point of ATP hydrolysis. The extended footprint could be caused by changed ς54–DNA interactions and/or protection by the presence of PspFΔHTH at the downstream side of the promoter.
Figure 5.
DNase I footprints of trapped complexes bound to promoter DNA. (a) Complexes were formed with Escherichia coli glnHp2 homoduplex 88-nt DNA (100 nM, bottom strand end-labeled), ς54 (1 μM) or its holoenzyme (Eς54, 100 nM), and PspFΔHTH (20 μM) in the absence or presence of ADP · AlFx. Samples were treated with DNase I (1.75 × 10−3 units; Amersham Life Sciences) for 1 min and the reaction was stopped by the addition of EDTA (10 mM). Bound and unbound complexes were separated and excised from a native gel (b) and the DNA was eluted into H2O overnight at 37°C. Equal amounts of DNA were denatured and then electrophoresed through a 10% denaturing gel. Additions to each binding reaction are indicated above each lane. (Lane 1) Untreated DNA; (lane 2) DNA alone treated with DNase I. Lanes 4a and 7a show extended DNase I footprints (dashed lines) from the trapped ς54–DNA and ς54-holoenzyme–DNA complexes shown in b (marked with an arrow in lanes 4 and 7, respectively). Because of the fragment sizes migrating close to the gel front it was not possible to precisely define the downstream end of the extended footprint in lane 7a. (Lanes 4b,7b) Footprints of untrapped ς54–DNA and ς54-holoenzyme–DNA complexes shown in b (lanes 4 and 7, respectively). (b) Native gel showing DNase I-treated complexes described in a. Additions to each binding reaction are indicated above each lane.
Use of a hydrolyzable nucleotide and activator results in some ς54-dependent DNA melting with early melted heteroduplex DNA, suggestive of a single strand DNA-binding activity being revealed within ς54 (Cannon et al. 2000, 2001). We attempted to measure DNA opening within DNA complexes formed between ς54 and PspFΔHTH in the presence of ADP · AlFx. However, complexes were rapidly destroyed by KMnO4 or diethylpyrocarbonate, and we were unable to obtain data showing any DNA melting.
Trapped activator does not induce efficient single-stranded DNA binding or open complex formation
We investigated whether trapped activator can induce single strand DNA binding on a preopened DNA template by examining trapped holoenzyme interactions with the late melted heteroduplex DNA. Holoenzyme formed with wild-type ς54 is able to form a heparin stable complex on late melted S. meliloti nifH promoter DNA if activator and a hydrolyzable nucleoside triphosphate are present (Wedel and Kustu 1995; Cannon et al. 1999). Trapping of activator did not allow the ς54-holoenzyme to form a heparin stable complex on the late melted DNA, even when initiating nucleotide was present (data not shown). This suggests that the interaction of trapped activator with the ς54-holoenzyme does not induce all the conformational changes in the holoenzyme that allow stable interactions with melted DNA. Consistent with this, the ς54-holoenzyme bound to promoter DNA with heteroduplex from −7 to −3, from −5 to −1, or from −3 to −1 did not acquire heparin stability in the presence of activator and ADP · AlFx (data not shown). It seems that although trapping can alter ς54–DNA interactions (Fig. 4a), these changes are not sufficient to lead to properties seen in activated complexes of the ς54-holoenzyme.
To test further if the trapped activator can induce formation of an open complex, we attempted to make specific transcripts from the trapped ς54-holoenzyme using supercoiled S. meliloti nifH promoter DNA templates. This failed, but can be rationalized through the demonstrated failure of trapped holoenzyme to efficiently engage melted DNA (see above) and the known requirement of ς54 Region I sequences for stabilizing the open complex (Cannon et al. 1999; Gallegos et al. 1999). The latter requirement may not be efficiently met when Region I remains bound by the trapped activator, possibly because of restricting movements in Region I that seem to occur during stable open-complex formation (Casaz and Buck 1997; Wigneshweraraj et al. 2001). Results suggest that although trapped activator binds Region I of ς54, the binding does not lead to engagement of the holoenzyme with melted DNA. Further conformational changes in ς54 likely to be associated with completion of the ATP hydrolysis cycle appear necessary.
Discussion
Activator–RNAP contacts are known to be critical for regulated transcription initiation, and activators of the ς54-holoenzyme catalyze formation of open promoter complexes through hydrolysis of a nucleoside triphosphate. We have shown, using the central ATP-hydrolyzing domains of the activators PspF and NifA together with wild-type PspF, that in the presence of ADP–aluminum fluoride a stable complex between the activator and ς54 or its holoenzyme is formed. It seems that we may have isolated a new functional state of the activator. The combination of ADP and ions (Al+3 and F−) is likely to form a planar complex that mimics the atomic arrangement of the ATP γ-phosphate in the transition state (Wittinghofer 1997). We propose that this transition-state analog of ATP (ADP · AlFx) is interacting with activator to allow adoption of a functional state that has increased affinity for ς54, compared to the nucleotide-free form of the activator, or when nonhydrolyzable analogs such as GTPγS and ATPγS are used. ADP did not substitute for its aluminum fluoride form, suggesting a critical role for the ATP γ-phosphate in establishing the conformation of activator that binds ς54. We infer that one role of ATP hydrolysis in activation of the ς54-holoenzyme is to promote formation of a functional state of the activator that tightly binds ς54. Hydrolysis of ATP would indicate this functional state is not long lived, and that Pi and ADP release return the activator to an alternate state that has decreased binding for ς54 (Fig. 4b).
Characteristics of the ς54-activator binding interaction
Because activators exist in different functional states depending on the progress of nucleotide hydrolysis, different sets of binding interactions between the activator and ς54 seem likely, and may relate to different steps in the process of formation of the open complex. ς54 Region I sequences function before open-complex formation to maintain the closed complex, and after to stabilize the open complex, suggesting that a series of linked interactions between the activator and ς54 Region I may occur and need not all be direct (Cannon et al. 1999; Gallegos et al. 1999; Gallegos and Buck 2000; Guo et al. 2000). The trapped ς54 and ς54-holoenzyme–PspFΔHTH complexes were only able to form efficiently when the proteins were allowed to associate prior to formation of ADP · AlFx. This suggests a pathway in which ς54 and activator associate weakly prior to interaction with nucleotide. We also showed that ς54 deleted for Region I (ΔIς54) was able to compete weakly with full-length ς54 for PspFΔHTH prior to trapping, implying that at least some of the interactions between activator and ς54 prior to trapping lie outside of Region I. Additional evidence for a range of interactions is provided by results of cross-linking assays between ς54 and the activator DctD (Lee and Hoover 1995; Wang et al. 1997; Kelly et al. 2000). In these experiments the determinants for binding ς54 to DctD were outside of ς54 Region I, and in the N-terminal half of the activator C3 region.
In our experiments the addition of ADP · AlFx results in a new stable complex between ς54 and activator that directly requires the regulatory Region I of ς54 for its formation. This suggests that ATP hydrolysis is used to promote a new binding interaction between ς54 Region I and activator. The dependence on ADP · AlFx for detecting the complex indicates that the stable complex with ς54 is normally transient and is not seen when using ATP because of the short life of the transition state of ATP at hydrolysis. One inference is that coupling ς54 and activator interactions to ATP hydrolysis can lead to high rates of transcription through rapidly directing ς54 to a new functional state suitable for open-complex formation.
Activator–nucleotide interactions
The changed native gel mobility of PspFΔHTH resulting from incubation with ADP · AlFx supports the idea that nucleotide binding can change the quaternary structure of this mutant activator. Tight binding of the transition state of ATP by PspFΔHTH may stabilize formation of an oligomer through one protomer binding the nucleotide base while an adjacent protomer contributes a contact to the γ-phosphate. Implicit in this view is the idea that the activator senses the γ-phosphate of the hydrolyzable nucleoside triphosphate, and that hydrolysis and changing interactions with the γ-phosphate allow the activator to interconvert between different functional states. The ATP-bound form of the activator NtrC is reported to interact with ς54 in a manner dependent on the presence of the γ-phosphate (Guo et al. 2000). These interconversions may also involve the amino acids located in regions of the ATP-binding fold, and may include residues equivalent to those that are known to comprise the Switch 1 and Switch 2 sequences of GTP-hydrolyzing signaling proteins (Gamblin and Smerdon 1998; Rombel et al. 1998). There appears to be a strong similarity between activators of the ς54-holoenzyme and motor proteins and signaling switch proteins that use nucleotide binding and hydrolysis to interconvert between functional states with different affinities for their targets. In the case of activators of the ς54-holoenzyme, activator–ς54 interactions that depend on ATP hydrolysis lead to an increased DNA interaction by ς54 to enable open complex formation (Cannon et al. 2000, 2001).
A notable property of the mutants in the GAFTGA motif of PSPFΔHTH was their failure to efficiently self-associate in the presence of ADP · AlFx (Fig. 3; data not shown). The normal levels of ATPase activity and its unchanged dependence on activator concentration for these mutants (J. Schumacher, unpubl.) support the argument that the self-association and interactions of the mutant proteins with ATP for hydrolytic cleavage are largely intact. Differences seen with ADP · AlFx could be related to alterations in interactions with ADP or a subtle defect in self-association at some step after the hydrolytic cleavage of ATP. Although the PSPFΔHTH T86S mutant gave little self-associated product in the absence of ς54 (Fig. 3), the level of trapped ς54–PSPFΔHTH T86S complex was normal, suggesting that ς54 stabilizes an interaction with ADP · AlFx that occurs through the GAFTGA motif.
The tight binding of trapped activator with ς54 and holoenzyme seen in the absence of promoter DNA raises the possibility that the enhancer-bound activator might recruit the holoenzyme to promoters that are weak binding sites for the holoenzyme. However, careful kinetic analysis to include consideration of the short lifetime of the transition state of ATP hydrolysis and the dissociation rate of the closed complex would be required to show such an effect was meaningful when ATP was being hydrolyzed. Nevertheless, it is clear that one particular binding interaction between ς54 and activator only occurs efficiently when the activator is in a particular “on” state that is transiently created when ATP is being turned over.
The role of ς54 Region I
The regulatory Region I of ς54 was clearly a determinant in the formation of the stable trapped complex with activator. This is consistent with the central role Region I has in activated transcription and in controlling the DNA-binding properties of ς54 and its holoenzyme that partly distinguish the closed and open promoter complexes (Wang et al. 1995; Casaz and Buck 1999; Guo et al. 1999; Gallegos and Buck 2000; Pitt et al. 2000). Binding assays with ς54 Region I alone strongly suggest that activator makes a direct contact to it. Analysis of 15 triple alanine substitution mutants spanning Region I (amino acids 6–50) showed none had a defect as great as deletion of the entire Region I, implying multiple determinants. Region I sequences localize in the core RNAP near the active site for RNA synthesis (Wigneshweraraj et al. 2000) and over the promoter region that is near the start of DNA melting. We have termed this protein–promoter DNA focus the regulatory center of the holoenzyme closed complex (Wigneshweraraj et al. 2001). Mutations in Region I lead to changes in the holoenzyme and ς54–DNA interactions that can lead to activator-independent transcription in vitro (Wang et al. 1995; Syed and Gralla 1998; Casaz et al. 1999). The trapped state of the activator may therefore make a contact to ς54 Region I within the regulatory center to start to change the conformation of the protein components of the closed complex.
DNA interactions of the trapped complex
In the light of the distinctive changes in ς54 binding to late melted DNA, it appears that binding of ς54 by the trapped activator may begin to change the DNA-binding properties of ς54. Experiments using DNA heteroduplex from −10 to −1 showed multiple banding with the trapped ς54–PspFΔHTH complex. This suggests that an altered interaction with start site proximal single strand DNA is possible when ς54 Region I is stably engaged with trapped activator. When ATP or GTP is used, the activator drives ς54 to melt DNA from −9 to −6 (Cannon et al. 2001). We attempted to see whether the ς54 trapped complex had resulted in any DNA strand denaturation, using KMnO4 or diethylpyrocarbonate as a probe for DNA base unstacking. Unfortunately, the sensitivity of the protein complex to both KMnO4 and diethylpyrocarbonate precluded any meaningful interpretation of the DNA footprints.
The extended promoter DNase I footprint we see with ς54 and holoenzyme trapped activator complexes could be directly caused by any single component in the complex being proximal to the DNA downstream of the −12 promoter DNA. Because Region I of ς54, the binding site for activator, is located over the −12 promoter element (Wigneshweraraj et al. 2001), activator bound to Region I could be responsible, at least in part, for directly blocking DNase I access. Two observations are consistent with this idea. First, the isomerized complexes that form with the ς54, activator, and hydrolyzable NTP and give extended DNase I footprints require the use of early melted DNA (Cannon et al. 2000), whereas the extended footprint in trapped complexes was evident with homoduplex and early melted DNA. Therefore, the extended footprint seen with the trapped complex may not be owing to ς54 isomerization. Rather, the footprint may reflect an intermediate state of ς54, not the fully isomerized form, bound to activator. Second, the isomerized ς54–DNA complexes bind core RNAP poorly, and holoenzyme does not efficiently form the isomerized complex (Cannon et al. 2001). This contrasts with the clear extended footprint seen with the trapped holoenzyme–activator complex on homoduplex and early melted DNA, again supporting the argument that the extended footprint may not be caused by ς54 isomerization but, rather, to the presence of activator downstream of the −12 promoter DNA.
The transition state–dependent interaction between activator and ς54 occurs prior to open complex formation
The full ATP-hydrolysis cycle by activator results in a remodeling of the ς54-holoenzyme–DNA complex, with conformational changes being evident in ς54 and DNA (Cannon et al. 2000, 2001). Characterization of the trapped PspFΔHTH ς54-holoenzyme complex interaction with DNA (see above) and the failure to transcribe from supercoiled DNA suggest that these changes have not fully occurred when ADP · AlFx replaces ATP. It seems that an enzymatic transition in the activator needed for the major conformational change in ς54 and its holoenzyme occurs after the transition state of ATP hydrolysis has been established. We envisage that on formation of the transition state between activator and ATP the switch in ς54-holoenzyme, probably Region I of ς54, tightly interacts with activator through direct protein–protein contact. It seems that ADP · AlFx locks the ς54–activator complex in a tense state. Subsequently, upon phosphate and/or ADP release, enzymatic transitions in the activator operate the switch (Region I) in ς54 to allow holoenzyme to form an open complex and so complete the mechanochemical cycle that couples ATP hydrolysis to a change in configuration of the ς54 protein and its holoenzyme. Region I of ς54 interacts with other parts of ς54 and core RNAP and is involved in establishing the closed complex and in maintaining open complexes (Cannon et al. 1999; Gallegos et al. 1999; Gallegos and Buck 2000). Together these activities point to a need for Region I to be repositioned in order for transcription initiation to occur.
For AAA+ family proteins, nucleotide is located close to the interface between protomers, with the active site being contributed to by residues from the adjacent protomer (Vale 2000). Our results with ADP · AlFx strongly suggest that for PspF and NifA, and likely all other activators of the ς54-holoenzyme, sensing the state of the γ-phosphate of ATP may serve to critically link ATP hydrolysis to changes in protomer structure to enable engagement with the ς54 subunit. Hence the basis of the mechanochemical functioning of activator may be in propagating interactions with the γ-phosphate to changes in activator conformation to allow cycles of binding and rebinding of activator and ς54 that rely on an ATP hydrolysis cycle. Intriguingly, structural alignment of PspF with the AAA+ protein p97 shows that the position of the GAFTGA motif of PspF, important for ADP · AlFx-dependent binding to ς54, is predicted to be dependent on the nucleotide-bound state of the activator (X. Zhang, pers. comm.). We suggest that other AAA+ proteins may also use ATP hydrolysis to manipulate the structure of their targets through the formation of a new functional state at the transition state of ATP hydrolysis.
Materials and methods
DNA manipulations
Plasmid pSRW–HMKPSPFΔHTH encodes E. coli PspFΔHTH protein with a 6-His tag followed by a heart muscle kinase (HMK) site at its N-terminal end. Plasmid pSRW–HMKPSPFΔHTH was constructed from pMJ15 (Jovanovic et al. 1999). The NlaIII site, containing the pspF ATG codon, was replaced with an NdeI site by PCR mutagenesis. The PCR product was digested with NdeI and HindIII, and the resulting mutagenized fragment was cloned into plasmid HMK–pET28b+, in which the sequence SSGLV in pET28b+ (Novagen) was changed to RRASV to create an HMK site. Construction of mutant plasmids encoding PspFΔHTH T86S, T86A, or T86V was by oligonucleotide site-directed mutagenesis using pMJ15 (Jovanovic et al. 1999). For protein expression the mutated PspFΔHTH DNA was inserted into pQE32 (QIAGEN). The full-length pspF gene was amplified by PCR from the chromosome of E. coli (MRE600) and inserted into the expression vector pET28b+. Random PCR mutagenisis (6mM MgCl2) of Regions I and II of ς54 was performed using plasmid pMM70 as template (Merrick and Gibbins 1985). The resulting mutagenic fragment was inserted into plasmid pVB009, a derivative of pTZ19R (Pharmacia) that contains sequences encoding Region III of ς54 from pMM70. Codons 20 and 53 of rpoN were mutagenized to saturation using a pair of oligonucleotides with NNG/C at these positions (Miyazaki and Arnold 1999).
Protein purification
The ς54 and PspFΔHTH proteins were overproduced and purified as described previously (Cannon et al. 1995, 1999; Gallegos and Buck 1999; Jovanovic et al. 1999). The ΔIς54 (residues 57–477) was without His tag. ς54 (residues 1–477) was used with and without an N-terminal His tag. PspFΔHTH and all other ς54 fragments had an N-terminal His tag. For 32P end-labeling, a heart muscle kinase site was added to the C-terminal end of ς54 (Casaz and Buck 1997) or at the N-terminal end of PspFΔHTH (Cannon et al. 2000). Expression of the full-length PspF protein was carried out in E. coli strain C41(DE3) (Miroux and Walker 1996). Essentially, growth of cells was in LB to an A600 of 0.6 at 37°C. Induction was carried out with 0.5 mM IPTG, and cultures were transferred to 16°C (with shaking) for a further 12 h. Cells were disrupted, and subsequent purification was by Ni-chelate affinity chromatography using a Hi-Trap column (Pharmacia) and FPLC (Pharmacia). Purified protein was eluted in TGED (10 mM Tris at pH 8.0, 5% glycerol, 0.1 mM EDTA, 1 mM DTT) containing approximately 300 mM NaCl, and protein was dialyzed and stored in TGED (as before with 50% glycerol) containing 500 mM NaCl. Purified protein was stored at −80°C.
Promoter DNA
Purified synthetic DNA fragments based on the −60- to −28-bp sequence of the S. meliloti nifH promoter were used to construct the DNA molecules used in this study (Cannon et al. 2000). DNA strands were annealed to create a duplex, with one strand 32P-kinased and the other unlabeled strand at a twofold molar excess.
Trapping
ADP–aluminum fluoride trapping was essentially performed as described previously for myosin subfragment 1 (Moshe et al. 1992). The desired amount of activator was incubated at 30°C for 5 min in STA buffer (25 mM Tris-actetate at pH 8.0, 8 mM Mg-actetate, 10 mM KCl, 1 mM DTT, 3.5% w/v PEG 6000) with ADP (0.2 mM), NaF (5.0 mM), and other proteins as required. After addition of AlCl3 (0.2 mM) the reactions were incubated for a further 10 min and directly loaded onto a native gel. Reactions (10 μL) containing 32P-end-labeled proteins also contained α-lactoalbumin (50 ng).
Gel-shift assays
Gel-shift assays were conducted using either end-labeled protein or linear end-labeled S. meliloti nifH promoter fragments as described previously (Cannon et al. 2000). Either 88-nt homoduplex or heteroduplex molecules from −60 to +28 bp, the latter with non-template-strand sequences mismatched, were used. Binding reactions were in STA buffer at 30°C, and bound and unbound protein or DNA was resolved on 4.5% native polyacrylamide Bio Rad mini protean II gels run at room temperature at 120 V in 25 mM Tris, 200 mM glycine buffer (pH 8.6). Where added, ATP was at 4 mM.
V8 protease footprints
Reactions (20 μL) contained the trapped activator–32P end-labeled ς54 complex (see above). After incubation for 10 min at 37°C, 150 ng of V8 protease (Sigma) was added for 2 min followed by the addition of 500 μM dichloroisocoumarin (Sigma) to stop digestion. Samples were run on a 4.5% native gel. Complexes were isolated from gel either as free protein or as part of a trapped activator complex, eluted in 1× Lamelli dye (Sigma), and run on a 12.5% SDS gel.
In vivo activation assays
Plasmids carrying the mutated rpoN gene were transformed into the E. coli ΔrpoN TH1 strain harboring a K. pneumoniae nifH–lacZ fusion in plasmid pRT22 (Cmr) and plasmid pRJ7511 (Tcr) carrying the wild-type or mutant derivatives of the B. japonicum nifA gene. Plasmid pWKS130 (Kmr) carried wild-type or mutant derivatives of the E. coli pspFΔHTH gene. The screen for suppressor mutants was made on minimal medium plates containing X-gal to select those colonies that displayed an enhanced blue color (Grande et al. 1999). The β-galactosidase assays were performed as described previously (Grande et al. 1999).
Estimation of stoichiometry
Trapped complexes containing PspFΔHTH and ς54 were resolved on a native gel. The center of the trapped-complex band was isolated. The proteins contained in the gel slice were then separated on denaturing 12.5% SDS PAGE and stained with Coomassie blue, and the respective bands were analyzed by densitometry. The ratio of PspFΔHTH to ς54 in the trapped complex was calculated by comparing optical density (OD) readings with standard curves (mass/OD) prepared at the same time for PspFΔHTH and ς54.
Acknowledgments
This work was supported by funding from the BBSRC and Wellcome trust to M.B. We thank Ray Dixon and Jason Barrett for the gift of the Azotobacter vinelandii NifA central domain; Leticia Olvera, Maricela Olvera, and Rene Hernandez for their help with the mutagenesis studies; Deep Holmlund for help with protein purification; and X. Zhang for valuable discussions on AAA proteins.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL m.buck@ic.ac.uk; FAX 0207-594-5419.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.205501.
References
- Buck M, Gallegos MT, Studholme DJ, Guo Y, Gralla JD. The bacterial enhancer-dependent ς54 (ςN) transcription factor. J Bacteriol. 2000;182:4129–4136. doi: 10.1128/jb.182.15.4129-4136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby S, Ebright RH. Transcription activation by catabolite activator protein (CAP) J Mol Biol. 1999;293:199–213. doi: 10.1006/jmbi.1999.3161. [DOI] [PubMed] [Google Scholar]
- Cannon W, Missailidis S, Smith C, Cottier A, Austin S, Moore M, Buck M. Core RNA polymerase and promoter DNA interactions of purified domains of ςN: Bipartite functions. J Mol Biol. 1995;248:781–803. doi: 10.1006/jmbi.1995.0260. [DOI] [PubMed] [Google Scholar]
- Cannon W, Gallegos MT, Casaz P, Buck M. Amino-terminal sequences of ςN (ς54) inhibit RNA polymerase isomerization. Genes & Dev. 1999;13:357–370. doi: 10.1101/gad.13.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WV, Gallegos MT, Buck M. Isomerization of a binary ς–promoter DNA complex by transcription activators. Nat Struct Biol. 2000;7:594–601. doi: 10.1038/76830. [DOI] [PubMed] [Google Scholar]
- ————— DNA melting within a binary ς54-promoter DNA complex. J Biol Chem. 2001;276:386–394. doi: 10.1074/jbc.M007779200. [DOI] [PubMed] [Google Scholar]
- Casaz P, Buck M. Probing the assembly of transcription initiation complexes through changes in ςN protease sensitivity. Proc Natl Acad Sci. 1997;94:12145–12150. doi: 10.1073/pnas.94.22.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Region I modifies DNA-binding domain conformation of ς54 within the holoenzyme. J Mol Biol. 1999;285:507–514. doi: 10.1006/jmbi.1998.2328. [DOI] [PubMed] [Google Scholar]
- Casaz P, Gallegos MT, Buck M. Systematic analysis of ς54 N-terminal sequences identifies regions involved in positive and negative regulation of transcription. J Mol Biol. 1999;292:229–239. doi: 10.1006/jmbi.1999.3076. [DOI] [PubMed] [Google Scholar]
- Chaney M, Buck M. The ς54 DNA-binding domain includes a determinant of enhancer responsiveness. Mol Microbiol. 1999;33:1200–1209. doi: 10.1046/j.1365-2958.1999.01566.x. [DOI] [PubMed] [Google Scholar]
- Chaney M, Pitt M, Buck M. Sequences within the DNA cross-linking patch of ς54 involved in promoter recognition, sigma isomerization, and open complex formation. J Biol Chem. 2000;275:22104–22113. doi: 10.1074/jbc.M002253200. [DOI] [PubMed] [Google Scholar]
- Drummond M, Whitty P, Wooton J. Sequence and domain relationships of NtrC and NifA from Klebsiella pneumoniae: Homologies to other regulatory proteins. EMBO J. 1986;5:441–447. doi: 10.1002/j.1460-2075.1986.tb04230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin J, Jovanovic G, Model P. The PspA protein of Escherichia coli is a negative regulator of ς54-dependent transcription. J Bacteriol. 2000;182:311–319. doi: 10.1128/jb.182.2.311-319.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fersht A. Structure and mechanism in protein science. New York: Freeman; 1998. [Google Scholar]
- Gallegos MT, Buck M. Sequences in ςN determining holoenzyme formation and properties. J Mol Biol. 1999;288:539–553. doi: 10.1006/jmbi.1999.2704. [DOI] [PubMed] [Google Scholar]
- ————— Sequences in ς54 region I required for binding to early melted DNA and their involvement in ς–DNA isomerisation. J Mol Biol. 2000;297:849–859. doi: 10.1006/jmbi.2000.3608. [DOI] [PubMed] [Google Scholar]
- Gallegos MT, Cannon WV, Buck M. Functions of the ς54 region I in trans and implications for transcription activation. J Biol Chem. 1999;274:25285–25290. doi: 10.1074/jbc.274.36.25285. [DOI] [PubMed] [Google Scholar]
- Gamblin SJ, Smerdon SJ. GTPase-activating proteins and their complexes. Curr Opin Struct Biol. 1998;8:195–201. doi: 10.1016/s0959-440x(98)80038-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez V, Olvera L, Soberon X, Morett E. In vivo studies on the positive control function of NifA: A conserved hydrophobic amino acid patch at the central domain involved in transcriptional activation. Mol Microbiol. 1998;28:55–67. doi: 10.1046/j.1365-2958.1998.00772.x. [DOI] [PubMed] [Google Scholar]
- Grande RA, Valderrama B, Morett E. Suppression analysis of positive control mutants of NifA reveals two overlapping promoters for Klebsiella pneumoniae rpoN. J Mol Biol. 1999;294:291–298. doi: 10.1006/jmbi.1999.3232. [DOI] [PubMed] [Google Scholar]
- Guo Y, Wang L, Gralla JD. A fork junction DNA–protein switch that controls promoter melting by the bacterial enhancer-dependent ς factor. EMBO J. 1999;18:3736–3745. doi: 10.1093/emboj/18.13.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Lew CM, Gralla JD. Promoter opening by ς54 and ς70 RNA polymerases: ς factor-directed alterations in the mechanism and tightness of control. Genes & Dev. 2000;14:2242–2255. doi: 10.1101/gad.794800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Amos LA. Three-dimensional structure of motor molecules. Cell Mol Life Sci. 1999;56:184–199. doi: 10.1007/s000180050421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic G, Rakonjac J, Model P. In vivo and in vitro activities of the Escherichia coli ς54 transcription activator, PspF, and its DNA-binding mutant, PspFDHTH. J Mol Biol. 1999;285:469–483. doi: 10.1006/jmbi.1998.2263. [DOI] [PubMed] [Google Scholar]
- Kelly MT, Ferguson JA, Hoover TR. Transcription initiation-defective forms of ς54 that differ in ability to function with a heteroduplex DNA template. J Bacteriol. 2000;182:6503–6508. doi: 10.1128/jb.182.22.6503-6508.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Hoover TR. Protein crosslinking studies suggest that Rhizobium meliloti C4-dicarboxylic acid transport protein D, a ς54-dependent transcriptional activator, interacts with ς54 and the β subunit of RNA polymerase. Proc Natl Acad Sci. 1995;92:9702–9706. doi: 10.1073/pnas.92.21.9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Owens JT, Hwang I, Meares C, Kustu S. Phosphorylation-induced signal propagation in the response regulator ntrC. J Bacteriol. 2000;182:5188–5195. doi: 10.1128/jb.182.18.5188-5195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick MJ, Gibbins JR. The nucleotide sequence of the nitrogen-regulation gene ntrA of Klebsiella pneumoniae and comparison with conserved features in bacterial RNA polymerase ς factors. Nucleic Acids Res. 1985;13:7607–7620. doi: 10.1093/nar/13.21.7607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miroux B, Walker JE. Over-production of proteins in Escherichia coli: Mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J Mol Biol. 1996;260:289–298. doi: 10.1006/jmbi.1996.0399. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Arnold FH. Exploring nonnatural evolutionary pathways by saturation mutagenesis: Rapid improvement of protein function. J Mol Evol. 1999;49:716–720. doi: 10.1007/pl00006593. [DOI] [PubMed] [Google Scholar]
- Money T, Barrett J, Dixon R, Austin S. Protein–protein interactions in the complex between the enhancer binding protein NIFA and the sensor NIFL from Azotobacter vinelandii. J Bacteriol. 2001;183:1359–1368. doi: 10.1128/JB.183.4.1359-1368.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morett E, Segovia L. The ς54 bacterial enhancer-binding protein family: Mechanism of action and phylogenetic relationship of their functional domains. J Bacteriol. 1993;175:6067–6074. doi: 10.1128/jb.175.19.6067-6074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshe M, Werber Y, Peyser M, Muhlrad Characterization of stable beryllium fluoride, aluminum fluoride, and vanadate containing myosin subfragment 1–nucleotide complexes. Biochemistry. 1992;31:7190–7197. doi: 10.1021/bi00146a023. [DOI] [PubMed] [Google Scholar]
- Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- Pitt M, Gallegos MT, Buck M. Single amino acid substitution mutants of Klebsiella pneumoniae ς54 defective in transcription. Nucleic Acids Res. 2000;28:4419–4427. doi: 10.1093/nar/28.22.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombel I, North A, Hwang I, Wyman C, Kustu S. The bacterial enhancer-binding protein NtrC as a molecular machine. Cold Spring Harb Symp Quant Biol. 1998;63:157–166. doi: 10.1101/sqb.1998.63.157. [DOI] [PubMed] [Google Scholar]
- Syed A, Gralla JD. Identification of an N-terminal region of ς54 required for enhancer responsiveness. J Bacteriol. 1998;180:5619–5625. doi: 10.1128/jb.180.21.5619-5625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale RD. AAA proteins. Lords of the ring. J Cell Biol. 2000;150:F13–F19. doi: 10.1083/jcb.150.1.f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JT, Syed A, Hsieh M, Gralla JD. Converting Escherichia coli RNA polymerase into an enhancer-responsive enzyme: Role of an NH2-terminal leucine patch in ς54. Science. 1995;270:992–994. doi: 10.1126/science.270.5238.992. [DOI] [PubMed] [Google Scholar]
- Wang YK, Lee JH, Brewer JM, Hoover TR. A conserved region in the ς54-dependent activator DctD is involved in both binding to RNA polymerase and coupling ATP hydrolysis to activation. Mol Microbiol. 1997;26:373–386. doi: 10.1046/j.1365-2958.1997.5851955.x. [DOI] [PubMed] [Google Scholar]
- Wedel A, Kustu S. The bacterial enhancer-binding protein NTRC is a molecular machine: ATP hydrolysis is coupled to transcriptional activation. Genes & Dev. 1995;9:2042–2052. doi: 10.1101/gad.9.16.2042. [DOI] [PubMed] [Google Scholar]
- Wigneshweraraj SR, Fujita N, Ishihama A, Buck M. Conservation of ς-core RNA polymerase proximity relationships between the enhancer-independent and enhancer-dependent ς classes. EMBO J. 2000;19:3038–3048. doi: 10.1093/emboj/19.12.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigneshweraraj SR, Chaney MK, Ishihama A, Buck M. Regulatory sequences in ς54 localise near the start of DNA melting. J Mol Biol. 2001;306:681–701. doi: 10.1006/jmbi.2000.4393. [DOI] [PubMed] [Google Scholar]
- Wittinghofer A. Signaling mechanistics: Aluminum fluoride for molecule of the year. Curr Biol. 1997;7:R682–R685. doi: 10.1016/s0960-9822(06)00355-1. [DOI] [PubMed] [Google Scholar]
- Wyman C, Rombel I, North AK, Bustamante C, Kustu S. Unusual oligomerization required for activity of NtrC, a bacterial enhancer-binding protein. Science. 1997;275:1658–1661. doi: 10.1126/science.275.5306.1658. [DOI] [PubMed] [Google Scholar]
- Yan D, Kustu S. “Switch I” mutant forms of the bacterial enhancer-binding protein NtrC that perturb the response to DNA. Proc Natl Acad Sci. 1999;96:13142–13146. doi: 10.1073/pnas.96.23.13142. [DOI] [PMC free article] [PubMed] [Google Scholar]