Abstract
Background
Increased cardiac lipid content has been associated with diabetic cardiomyopathy. We recently showed that cardiac lipid content is reduced after 12 weeks of physical activity training in healthy overweight subjects. The beneficial effect of exercise training on cardiovascular risk is well established and the decrease in cardiac lipid content with exercise training in healthy overweight subjects was accompanied by improved ejection fraction. It is yet unclear whether diabetic patients respond similarly to physical activity training and whether a lowered lipid content in the heart is necessary for improvements in cardiac function. Here, we investigated whether exercise training is able to lower cardiac lipid content and improve cardiac function in type 2 diabetic patients.
Methods
Eleven overweight-to-obese male patients with type 2 diabetes mellitus (age: 58.4 ± 0.9 years, BMI: 29.9 ± 0.01 kg/m2) followed a 12-week training program (combination endurance/strength training, three sessions/week). Before and after training, maximal whole body oxygen uptake (VO2max) and insulin sensitivity (by hyperinsulinemic, euglycemic clamp) was determined. Systolic function was determined under resting conditions by CINE-MRI and cardiac lipid content in the septum of the heart by Proton Magnetic Resonance Spectroscopy.
Results
VO2max increased (from 27.1 ± 1.5 to 30.1 ± 1.6 ml/min/kg, p = 0.001) and insulin sensitivity improved upon training (insulin stimulated glucose disposal (delta Rd of glucose) improved from 5.8 ± 1.9 to 10.3 ± 2.0 μmol/kg/min, p = 0.02. Left-ventricular ejection fraction improved after training (from 50.5 ± 2.0 to 55.6 ± 1.5%, p = 0.01) as well as cardiac index and cardiac output. Unexpectedly, cardiac lipid content in the septum remained unchanged (from 0.80 ± 0.22% to 0.95 ± 0.21%, p = 0.15).
Conclusions
Twelve weeks of progressive endurance/strength training was effective in improving VO2max, insulin sensitivity and cardiac function in patients with type 2 diabetes mellitus. However, cardiac lipid content remained unchanged. These data suggest that a decrease in cardiac lipid content in type 2 diabetic patients is not a prerequisite for improvements in cardiac function.
Trial registration
ISRCTN: ISRCTN43780395
Keywords: magnetic resonance spectroscopy, magnetic resonance imaging, ectopic fat, type 2 diabetes mellitus, exercise, cardiomyopathy, lipotoxicity
Background
The prevalence of heart failure continues to increase. The life time risk to develop heart failure is presently 20% [1] and type 2 diabetes mellitus independently increases the risk for developing heart failure [2]. In addition to the well-known diabetes-associated risk of coronary atherosclerosis, also metabolic changes and increased triglyceride deposition in the diabetic myocardium are likely to contribute to the development of compromised cardiac function in patients with type 2 diabetes.
While most fatty acids (FA) in the body are stored in adipose tissue, small but physiologically relevant amounts are stored in non-adipose tissues such as skeletal muscle, liver, pancreas and the heart. In the heart, this (ectopic) fat storage has been suggested to interfere with insulin signaling and to induce apoptosis, which may contribute to impaired cardiac function, a process coined cardiac "lipotoxicity". Thus, lean rodent models with targeted overexpression of genes involved in lipid delivery and synthesis in the myocardium show lipid accumulation-induced dilated cardiomyopathy and heart failure in the absence of obesity-related cardiovascular risk factors (such as hypertension or dyslipidemia) [3-5], indicating that lipid accumulation in the myocardium can cause cardiomyopathy directly. Moreover, treatments ameliorating cardiac lipid accumulation rescued the heart from dilated cardiomyopathy in rodents [6,7].
In humans, cardiac lipid accretion has been reported in obesity and even more so in type 2 diabetes [8-10] although human data is still limited due to the fact that cardiac tissue samples are not readily available. Only recently, non-invasive lipid quantification in the septum of the heart using magnetic resonance spectroscopy has been established [11,12]. With this methodology, several studies showed inverse relations between cardiac lipid content and systolic and diastolic function [9-11], suggesting that excessive lipid accumulation in the heart may indeed impair normal cardiac function and result in cardiomyopathy and contribute to the increased risk of heart failure in type 2 diabetic patients [13]. However, whether a decrease in cardiac lipid content is necessary for improvement of cardiac function is presently unknown.
In that respect, we recently showed that endurance training reduced cardiac lipid content in parallel with an improvement in left ventricular ejection fraction in healthy, obese subjects [14]. It is well known that physical exercise training improves insulin sensitivity and reduces cardiac risk factors also in diabetic subjects. Hence, both the American Diabetes Association and American Heart Association promote regular physical exercise to reduce morbidity in type 2 diabetic patients [15]. Whether exercise training is also able to reduce cardiac lipid content in type 2 diabetic patients is presently unknown, and is the aim of the present study.
Methods
Subjects
Eleven male type 2 diabetic patients were included in this study. Diabetes had to be diagnosed for at least one year before the start of the study (on average: 3.5 ± 1.1 years) and was confirmed during screening (based on fasting plasma glucose concentrations of 7.0 mmol/l or higher, in accordance with the definition of the American Diabetes Association, ADA); diabetes was well-controlled in all subjects (HbA1c = 7.1 ± 0.2%). Exclusion criteria were: systolic blood pressure > 160 mm Hg or diastolic blood pressure > 100 mm Hg and known cardiac disease. Subjects' characteristics are listed in table 1. None of the patients was using insulin therapy; only oral anti-diabetic agents were used (metformin only (n = 6), metformin in combination with SU derivatives (n = 2) or SU derivatives only (n = 2)). Lipid-lowering medication was used by seven patients (six patients used statins, one patient used fibrates), some patients used blood pressure lowering medication (n = 5, of which 2 beta blokkers) or blood diluents (n = 4). Patients continued their normal medication throughout the study, except for antidiabetic medication which was stopped one week before the determination of insulin sensitivity. The study was approved by the institutional medical ethical committee and written informed consent was obtained from all participants.
Table 1.
Subjects' characteristics before and after the training intervention period of 12 weeks.
| before training | after training | p-value | |
|---|---|---|---|
| age (y) | 59.5 ± 0.9 | - | - |
| BMI (kg/m2) | 30.5 ± 1.4 | 30.4 ± 1.4 | 0.8 |
| Fat percentage (%) | 30.3 ± 2.1 | 29.1 ± 2.1 | 0.11 |
| VO2max (ml/kg/min) | 27.1 ± 1.5 | 30.1 ± 1.6 | 0.001* |
| delta Rd (μmol/kg/min) | 5.8 ± 1.9 | 10.3 ± 2.0 | 0.02 * |
| glucose (mMol) | 9.1 ± 0.6 | 9.3 ± 0.6 | 0.6 |
| HbA1c (%) | 7.1 ± 0.2 | 7.2 ± 0.3 | 0.5 |
| FA (μmol/l) | 478.4 ± 54.2 | 526.3 ± 71.3 | 0.2 |
| Total cholesterol (mmol/l) | 4.9 ± 0.2 | 4.6 ± 0.3 | 0.3 |
| HDL cholesterol (mmol/l) | 1.2 ± 0.1 | 1.2 ± 0.1 | 0.6 |
| LDL cholesterol (mmol/l) | 2.7 ± 0.2 | 2.6 ± 0.2 | 0.4 |
| Triacylglycerols (mmol/l) | 2.1 ± 0.2 | 1.9 ± 0.2 | 0.4 |
Stars indicate statistically significant changes.
Study protocol
Body composition, maximal oxygen uptake, insulin sensitivity, cardiac function and fat content, and fasting plasma concentration of fatty acids (FA), triacylglycerols, glucose, HbA1c, and cholesterol were measured before and after a 12-week well-controlled exercise program, consisting of a combination of aerobic and resistance exercise with three training sessions per week, carried out in small groups of 4 subjects, supervised by a coach, see [14].
Maximal oxygen uptake (whole body oxidative capacity)
A routine incremental cycling test was used to determine the maximal aerobic capacity as described earlier (Oxycon Beta, Mijnhardt, The Netherlands) [14].
Insulin sensitivity
Insulin sensitivity was measured with a hyperinsulinemic (40 mU/m2/min) euglycemic clamp, see [14]. Patients were asked to keep a constant eating pattern and to refrain from intense physical exercise during the last three days before the clamp.
Hydrostatic weighing
Hydrostatic weighing with simultaneous measurement of lung volume was used to determine whole body fat percentage in the morning in the fasted state. The equation of Siri [16] was used to calculate fat percentage, fat mass and fat-free mass.
Blood sample analysis
Blood samples were collected in EDTA-containing tubes and immediately centrifuged at high speed. Plasma FA, glucose and glycosylated haemoglobin were measured as described previously [14]. Total cholesterol, HDL-cholesterol and triacylglycerol were analyzed in serum enzymatically as described previously [14].
MRI
An electrocardiographically triggered balanced steady state free precession (bSSFP) cine sequence was used to acquire images in two- and four chamber views and to image the whole heart in the short-axis orientation during breath holds (Intera, 1.5T, Philips Healthcare, Best, The Netherlands). Slice thickness was 6 mm with a 4 mm gap (flip angle = 50°; shortest possible TR (3.3 ms) and TE (1.67 ms); field of view = 350 × 350 mm, reconstructed matrix = 256 × 256, number of heart phases = 24). Cine images were analyzed using dedicated software (CAAS, Pie Medical Imaging, Maastricht, The Netherlands) to determine left ventricular end-systolic and end-diastolic volume. From this, parameters of systolic function were calculated (ejection volume, cardiac output, cardiac index). For one patient, the MRI procedure had to be accelerated due to anxiety and another patient had problems holding his breath. From these two subjects, it was impossible to acquire enough slices to cover the whole left ventricle and systolic function could not be determined and is therefore reported for nine subjects.
MRS
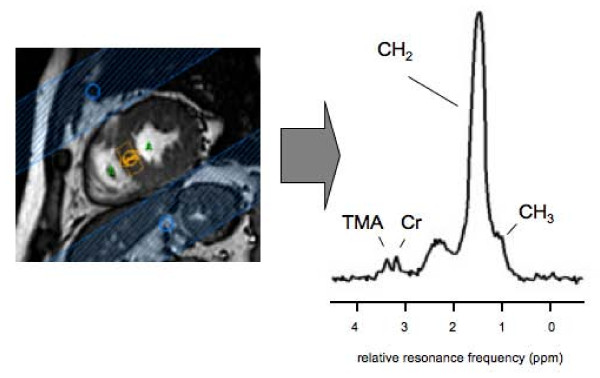
Cardiac lipid content was determined in vivo by Magnetic Resonance Spectroscopy (MRS) as described previously [14]. Signal acquisition was restricted to a volume of interest of 6 cm3 (10 mm × 20 mm × 30 mm) in the septum of the heart (PRESS sequence; spectral bandwidth = 1000 Hz; points acquired = 512; TE = 26 ms; TR = 4 s; n = 128 for lipid spectrum, n = 16 for reference water spectrum), signal acquisition was ECG-triggered to end-systole. Respiratory gating and tracking was performed with a pencil beam navigator placed on the diaphragm. A spectrum of the lipid metabolites was acquired with water suppression (CHESS). As internal reference, water signal intensity was determined in a spectrum with the water suppression pulses off-resonance. Overall acquisition time (imaging and spectroscopy) was 90 minutes. The coefficient of variation in repeated measurements was 11% on average. See figure 1 for a typical spectrum.
Figure 1.
A typical short axis view and a proton magnetic resonance spectrum, acquired from a volume of 10 × 20 × 30 mm3 positioned in the septum of the heart. The peaks of the CH2 and CH3 groups of the fatty acids can be discriminated and the peaks of Creatine (Cr) and trimethylammonium (TMA) are depicted in the figure.
Peaks were fitted with Gaussian curves, imposing relative ratios of the amplitudes and line widths, using the AMARES algorithm [17] in jMRUI [18]. The results are displayed as ratios of the amplitudes of the CH2 peak relative to the water peak (uncorrected for T1 and T2 relaxation).
Statistics
All data are presented as mean ± SEM. Statistical analyses were performed using SPSS 16.0 for Mac (SPSS Inc., Chicago, IL). The effect of the intervention was determined by a two-sided, paired student t-test (considered significant if p < 0.05). Sample size (n = 11) was determined with β = 0.8 and α = 0.05, assuming a similar effect size as we found earlier
Results
Maximal oxygen uptake
The training program resulted in an improved maximal performance (from 200 ± 11 to 235 ± 13 Watt (p < 0.001). Accordingly, maximal whole body oxygen uptake improved with training (from 27.1 ± 1.5 to 30.1 ± 1.6 ml/min/kg (p = 0.001)).
Body weight and whole body fat percentage
Body weight did not change with training (96.1 ± 4.0 and 95.6 ± 4.4 kg, pre- and post training, respectively, p = 0.8). Similarly, fat percentage, total body fat and fat-free mass remained unchanged (see table 1 for fat percentage).
Plasma parameters
The training program did not affect fasting plasma glucose, HbA1c, FA and triacylglycerol plasma concentration. Total cholesterol concentrations, HDL and LDL cholesterol concentrations were unchanged after training (see table 1).
Insulin sensitivity
Insulin sensitivity measured as insulin-stimulated glucose disposal (delta Rd) during a hyperinsulinemic, euglycemic clamp improved significantly (from 5.8 ± 1.9 at baseline to 10.3 ± 2.0 mmol/kg/min after training, p = 0.02).
Cardiac function and lipid content
Left ventricular end-diastolic volume was not affected by training (180.3 ± 8.7 to 179.0 ± 6.5 ml, p = 0.8) while end-systolic volume was reduced (from 89.6 ± 6.5 to 79.6 ± 4.5 ml, p = 0.004). The decreased end-systolic volume after training translates into improvements of similar magnitude (about 10%) of ejection fraction (from 50.5 ± 2.0 to 55.6 ± 1.5%, p = 0.01, see figure 2), cardiac index (from 2.87 ± 1.61 to 3.14 ± 1.08 l*min-1*m-2, p = 0.026) and cardiac output (from 5.8 ± 0.3 to 6.4 ± 0.2 ml/min, p = 0.04) after the training intervention. Strikingly, these adaptive changes occurred in the absence of changes in cardiac lipid content (0.80 ± 0.22% to 0.95 ± 0.21%, p = 0.15, as shown in figure 3.
Figure 2.
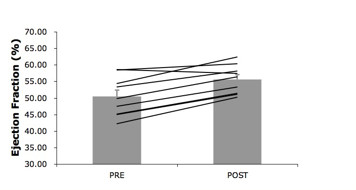
Left ventricular ejection fraction improved with the training intervention (p = 0.01).
Figure 3.
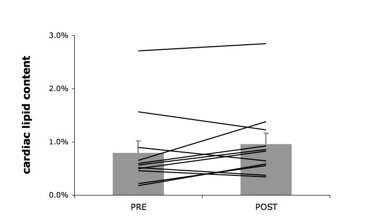
Cardiac lipid content. Cardiac lipid content before and after the training intervention. Cardiac lipid content is expressed as the relative intensity of the CH2 peak, compared to the unsuppressed water resonance. Cardiac lipid content is unchanged after the training intervention (p = 0.8).
Ejection fraction, cardiac index and cardiac output did not correlate with cardiac lipid content. Similarly, changes in cardiac function were not related to changes in cardiac lipid content.
Discussion
The most important finding of the present study is that in this group of type 2 diabetic patients, a twelve-week training program, which increased aerobic capacity and insulin sensitivity resulted in an improvement of systolic function, while cardiac lipid content remained unchanged. These data suggest that a decrease in cardiac lipid content in type 2 diabetic patients is not a prerequisite for improvements in cardiac function. A limitation of the current study is the relatively small number of patients included.
Exercise training is well established as a powerful tool to reduce the risk for cardiovascular disease in type 2 diabetic patients [19-23]; however it is less clear whether training can actually improve cardiac function in this population. Data on the effect of training on systolic function in type 2 diabetic subjects is limited, with only one study reporting a positive effect [24] while others do not [25,26]. In diabetic animal models, however, the improvement in ejection fraction and fractional shortening with endurance training is well documented [27,28]. We previously reported improved ejection fraction in obese but otherwise healthy human subjects in response to exercise training [14]. Here, we show that a modest but significant improvement in ejection fraction can also occur in type 2 diabetic patients, in response to a relatively short period of training.
Rodent studies strongly support the concept that accumulation of triglycerides in cardiomyocytes causes cardiac lipotoxicity, impairing cardiac function. In addition, several human studies report negative correlations of triglyceride content with cardiac function as well [9,11]. Some [29], but not all [30] human studies report lowered cardiac lipid content upon thiazolidinediones. Recently, we reported that in healthy overweight-to-obese subjects, the training-induced improvement in systolic function is accompanied by a decrease in cardiac lipid, in line with the cardiac lipid hypothesis [14]. Intriguingly, though, exercise-induced improvement in cardiac contractile function was not accompanied by a decrease in lipid content in the patients with type 2 diabetes, rather cardiac lipid content tends to increase in this population, although not significantly. Why type 2 diabetic patients are unresponsive towards training-induced decrease in cardiac lipid content cannot be deduced from the present study. It is important to note that the cardiac lipid content in the present group of diabetic subjects is similar as reported in healthy overweight subjects of similar age and BMI [14]. However, when compared to healthy lean young men, cardiac lipid content is clearly elevated by ~300% in the present group of patients [31]. In other words, cardiac lipid content is elevated as expected based on BMI and age, however, it is not further elevated due to type 2 diabetes. This may be due to the relatively healthy patients investigated in the current study. Although diabetic, patients did not have any severe diabetes-related complications. Additionally, plasma concentrations of FFA and TG were also normal. However, although not elevated more severely than expected based on BMI and age at baseline, the cardiac lipid content was unresponsive to an exercise intervention in the diabetic subjects and interestingly, this differs from the response in healthy overweight subjects. It is yet unclear what is at the basis of this differential response, but it is well known that cardiac metabolism is altered in diabetes. For example, cardiac insulin resistance, decreased metabolic flexibility, mitochondrial dysfunction and excessive cardiac fat uptake and oxidation all have been associated with the diabetic state. Furthermore, a study in a diabetic animal model demonstrated decreased responsiveness to beta adrenergic stimulation in diabetic animals upon exercise training while beta adrenergic stimulation increased in control animals [32]. Future studies are needed to investigate whether these factors are contributing to the differential response in diabetic patients to exercise training resulting in the unchanged cardiac lipid content.
Furthermore, the present findings demonstrate that the training-induced improvement of systolic function can occur independently of changes in cardiac lipid content. Generally, it is believed that the beneficial effect of training on systolic- and diastolic function is based on alterations of the Ca2+ regulatory systems involved in the excitation-contraction coupling and relaxation processes [33]. According to the cardiac lipotoxicity theory, there are potential mechanisms by which lipid accumulation may interact directly with excitation-contraction coupling. We here demonstrate that this is not pivotal: the improved function with exercise training can occur independently of changes in lipid content. Apparently, the improvement of cardiac function does not necessarily require decrease in cardiac lipids in type 2 diabetic subjects. Similarly, it has been shown that pioglitazone improves diastolic function in type 2 diabetic subjects without changes in cardiac lipid content [30].
Conclusion
In conclusion, we report here that a twelve-week training program, which increased maximal whole body oxygen uptake and insulin sensitivity, resulted in an improvement in ejection fraction, while cardiac lipid content was unchanged. These results demonstrate that the training-induced improvement in systolic function does not necessarily require lowering of cardiac lipid availability. More studies are needed to get insight in the clinical relevance of cardiac lipid content and its relation with cardiac metabolism and diabetic cardiomyopathy.
Competing interests
MS is employed by Philips Health Care, which does not pose a conflict of interest.
Authors' contributions
VBSH: performing measurements, writing manuscript. RCM, TW: performing measurements, discussion of manuscript. TL: Discussion of data analysis and manuscript. MS, EK, HL: Helping to set up methodology, discussion of data and manuscript. JEW, JFCG: discussion of data and manuscript. PS, MKCH: experimental set-up, discussion of data and manuscript.
Contributor Information
Vera B Schrauwen-Hinderling, Email: v.schrauwen@maastrichtuniversity.nl.
Ruth CR Meex, Email: ruth.meex@monash.edu.
Matthijs KC Hesselink, Email: matthijs.hesselink@maastrichtuniversity.nl.
Tineke van de Weijer, Email: t.vandeweijer@maastrichtuniversity.nl.
Tim Leiner, Email: timleiner@gmail.com.
Michael Schär, Email: michael.schar@philips.com.
Hildo J Lamb, Email: h.j.lamb@lumc.nl.
Joachim E Wildberger, Email: j.wildberger@mumc.nl.
Jan FC Glatz, Email: glatz@maastrichtuniversity.nl.
Patrick Schrauwen, Email: p.schrauwen@maastrichtuniversity.nl.
M Eline Kooi, Email: eline.kooi@mumc.nl.
Acknowledgements
This study has been supported by the European Foundation for the Study of Diabetes (EFSD), Düsseldorf, Germany. Dr. P.Schrauwen is supported by a 'VICI' Research Grant for innovative research from the Netherlands Organization for Scientific Research, The Hague, The Netherlands (Grant 918.96.618). Dr. M. Hesselink and Ruth Meex are supported by a 'VIDI' Research Grant for innovative research from the Netherlands Organization for Scientific Research, The Hague, The Netherlands (Grant 917.66.359). T. van de Weijer was supported by the Center for Translational Molecular Medicine (CTMM), project PREDICCt (grant 01C-104).
References
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C. et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Bibbins-Domingo K, Lin F, Vittinghoff E, Barrett-Connor E, Hulley SB, Grady D, Shlipak MG. Predictors of heart failure among women with coronary disease. Circulation. 2004;110:1424–1430. doi: 10.1161/01.CIR.0000141726.01302.83. [DOI] [PubMed] [Google Scholar]
- Yagyu H, Chen G, Yokoyama M, Hirata K, Augustus A, Kako Y, Seo T, Hu Y, Lutz EP, Merkel M. et al. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest. 2003;111:419–426. doi: 10.1172/JCI16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, Saffitz JE, Schaffer JE. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–822. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Ding G, Qin Q, Huang Y, Lewis W, He N, Evans RM, Schneider MD, Brako FA, Xiao Y. et al. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med. 2004;10:1245–1250. doi: 10.1038/nm1116. [DOI] [PubMed] [Google Scholar]
- Nielsen LB, Bartels ED, Bollano E. Overexpression of apolipoprotein B in the heart impedes cardiac triglyceride accumulation and development of cardiac dysfunction in diabetic mice. J Biol Chem. 2002;277:27014–27020. doi: 10.1074/jbc.M203458200. [DOI] [PubMed] [Google Scholar]
- Lee Y, Naseem RH, Duplomb L, Park BH, Garry DJ, Richardson JA, Schaffer JE, Unger RH. Hyperleptinemia prevents lipotoxic cardiomyopathy in acyl CoA synthase transgenic mice. Proc Natl Acad Sci USA. 2004;101:13624–13629. doi: 10.1073/pnas.0405499101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavock JM, Lingvay I, Zib I, Tillery T, Salas N, Unger R, Levine BD, Raskin P, Victor RG, Szczepaniak LS. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 2007;116:1170–1175. doi: 10.1161/CIRCULATIONAHA.106.645614. [DOI] [PubMed] [Google Scholar]
- Rijzewijk LJ, van der Meer RW, Smit JW, Diamant M, Bax JJ, Hammer S, Romijn JA, de Roos A, Lamb HJ. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol. 2008;52:1793–1799. doi: 10.1016/j.jacc.2008.07.062. [DOI] [PubMed] [Google Scholar]
- Kankaanpaa M, Lehto HR, Parkka JP, Komu M, Viljanen A, Ferrannini E, Knuuti J, Nuutila P, Parkkola R, Iozzo P. Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metab. 2006;91:4689–4695. doi: 10.1210/jc.2006-0584. [DOI] [PubMed] [Google Scholar]
- Szczepaniak LS, Dobbins RL, Metzger GJ, Sartoni-D'Ambrosia G, Arbique D, Vongpatanasin W, Unger R, Victor RG. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med. 2003;49:417–423. doi: 10.1002/mrm.10372. [DOI] [PubMed] [Google Scholar]
- van der Meer RW, Doornbos J, Kozerke S, Schar M, Bax JJ, Hammer S, Smit JW, Romijn JA, Diamant M, Rijzewijk LJ. et al. Metabolic imaging of myocardial triglyceride content: reproducibility of 1H MR spectroscopy with respiratory navigator gating in volunteers. Radiology. 2007;245:251–257. doi: 10.1148/radiol.2451061904. [DOI] [PubMed] [Google Scholar]
- Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971-1993. Diabetes Care. 1998;21:1138–1145. doi: 10.2337/diacare.21.7.1138. [DOI] [PubMed] [Google Scholar]
- Schrauwen-Hinderling VB, Hesselink MK, Meex R, van der Made S, Schar M, Lamb H, Wildberger JE, Glatz J, Snoep G, Kooi ME, Schrauwen P. Improved ejection fraction after exercise training in obesity is accompanied by reduced cardiac lipid content. J Clin Endocrinol Metab. 2010;95:1932–1938. doi: 10.1210/jc.2009-2076. [DOI] [PubMed] [Google Scholar]
- Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, Fonseca V, Gerstein HC, Grundy S, Nesto RW. et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2007;30:162–172. doi: 10.2337/dc07-9917. [DOI] [PubMed] [Google Scholar]
- Siri W. The gross composition of the body. Adv Biol Med Physiol. 1956;4:239–280. doi: 10.1016/b978-1-4832-3110-5.50011-x. [DOI] [PubMed] [Google Scholar]
- Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129:35–43. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, Graveron-Demilly D. Java-based graphical user interface for the MRUI quantitation package. Magma. 2001;12:141–152. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care. 2010;33:2692–2696. doi: 10.2337/dc10-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balducci S, Zanuso S, Nicolucci A, De Feo P, Cavallo S, Cardelli P, Fallucca S, Alessi E, Fallucca F, Pugliese G. Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: a randomized controlled trial: the Italian Diabetes and Exercise Study (IDES) Arch Intern Med. 2010;170:1794–1803. doi: 10.1001/archinternmed.2010.380. [DOI] [PubMed] [Google Scholar]
- Monteiro LZ, Fiani CR, Freitas MC, Zanetti ML, Foss MC. Decrease in blood pressure, body mass index and glycemia after aerobic training in elderly women with type 2 diabetes. Arq Bras Cardiol. 2010;95:563–570. doi: 10.1590/S0066-782X2010005000135. [DOI] [PubMed] [Google Scholar]
- Okada S, Hiuge A, Makino H, Nagumo A, Takaki H, Konishi H, Goto Y, Yoshimasa Y, Miyamoto Y. Effect of exercise intervention on endothelial function and incidence of cardiovascular disease in patients with type 2 diabetes. J Atheroscler Thromb. 2010;17:828–833. doi: 10.5551/jat.3798. [DOI] [PubMed] [Google Scholar]
- Leehey DJ, Moinuddin I, Bast JP, Qureshi S, Jelinek CS, Cooper C, Edwards LC, Smith BM, Collins EG. Aerobic exercise in obese diabetic patients with chronic kidney disease: a randomized and controlled pilot study. Cardiovasc Diabetol. 2009;8:62. doi: 10.1186/1475-2840-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miche E, Herrmann G, Nowak M, Wirtz U, Tietz M, Hurst M, Zoller B, Radzewitz A. Effect of an exercise training program on endothelial dysfunction in diabetic and non-diabetic patients with severe chronic heart failure. Clin Res Cardiol. 2006;95(Suppl 1):i117–124. doi: 10.1007/s00392-006-1106-z. [DOI] [PubMed] [Google Scholar]
- Loimaala A, Groundstroem K, Rinne M, Nenonen A, Huhtala H, Vuori I. Exercise training does not improve myocardial diastolic tissue velocities in Type 2 diabetes. Cardiovasc Ultrasound. 2007;5:32. doi: 10.1186/1476-7120-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordern MD, Coombes JS, Cooney LM, Jeffriess L, Prins JB, Marwick TH. Effects of exercise intervention on myocardial function in type 2 diabetes. Heart. 2009;95:1343–1349. doi: 10.1136/hrt.2009.165571. [DOI] [PubMed] [Google Scholar]
- Loganathan R, Bilgen M, Al-Hafez B, Zhero SV, Alenezy MD, Smirnova IV. Exercise training improves cardiac performance in diabetes: in vivo demonstration with quantitative cine-MRI analyses. J Appl Physiol. 2007;102:665–672. doi: 10.1152/japplphysiol.00521.2006. [DOI] [PubMed] [Google Scholar]
- Korte FS, Mokelke EA, Sturek M, McDonald KS. Exercise improves impaired ventricular function and alterations of cardiac myofibrillar proteins in diabetic dyslipidemic pigs. J Appl Physiol. 2005;98:461–467. doi: 10.1152/japplphysiol.00551.2004. [DOI] [PubMed] [Google Scholar]
- Zib I, Jacob AN, Lingvay I, Salinas K, McGavock JM, Raskin P, Szczepaniak LS. Effect of pioglitazone therapy on myocardial and hepatic steatosis in insulin-treated patients with type 2 diabetes. J Investig Med. 2007;55:230–236. doi: 10.2310/6650.2007.00003. [DOI] [PubMed] [Google Scholar]
- van der Meer RW, Rijzewijk LJ, de Jong HW, Lamb HJ, Lubberink M, Romijn JA, Bax JJ, de Roos A, Kamp O, Paulus WJ. et al. Pioglitazone improves cardiac function and alters myocardial substrate metabolism without affecting cardiac triglyceride accumulation and high-energy phosphate metabolism in patients with well-controlled type 2 diabetes mellitus. Circulation. 2009;119:2069–2077. doi: 10.1161/CIRCULATIONAHA.108.803916. [DOI] [PubMed] [Google Scholar]
- Bilet L, van de Weijer T, Hesselink MK, Glatz JF, Lamb HJ, Wildberger J, Kooi ME, Schrauwen P, Schrauwen-Hinderling VB. Exercise-induced modulation of cardiac lipid content in healthy lean young men. Basic Res Cardiol. pp. 307–315. [DOI] [PMC free article] [PubMed]
- Lahaye Sle D, Gratas-Delamarche A, Malarde L, Vincent S, Zguira MS, Morel SL, Delamarche P, Zouhal H, Carre F, Bekono FR. Intense exercise training induces adaptation in expression and responsiveness of cardiac beta-adrenoceptors in diabetic rats. Cardiovasc Diabetol. 2010;9:72. doi: 10.1186/1475-2840-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemi OJ, Ellingsen O, Smith GL, Wisloff U. Exercise-induced changes in calcium handling in left ventricular cardiomyocytes. Front Biosci. 2008;13:356–368. doi: 10.2741/2685. [DOI] [PubMed] [Google Scholar]