Abstract
The RET receptor tyrosine kinase has a critical role in kidney organogenesis and the development of the enteric nervous system. Two major isoforms, RET9 and RET51, differ in the amino acid sequence of the C-terminal tail as a result of alternative splicing. To determine the roles of these isoforms in vivo, we used targeted mutagenesis to generate mice that express either RET9 or RET51. Monoisoformic RET9 mice, which lack RET51, are viable and appear normal. In contrast, monoisoformic RET51 animals, which lack RET9, have kidney hypodysplasia and lack enteric ganglia from the colon. To study the differential activities of the two RET isoforms further, we generated transgenic mice expressing ligand-dependent and constitutively active forms of RET9 or RET51 under the control of the Hoxb7 regulatory sequences. Such RET9 transgenes are capable of rescuing the kidney agenesis in RET-deficient mice or causing kidney hypodysplasia in wild-type animals. In contrast, similar RET51 transgenes fail to rescue the kidney agenesis or cause hypodysplasia. Our findings show that RET9 and RET51 have different signaling properties in vivo and define specific temporal and spatial requirements of c-Ret function during renal development and histogenesis of the enteric nervous system.
Keywords: c-Ret, signaling, Hirschsprung's disease, nephrogenesis
The c-Ret proto-oncogene encodes a receptor tyrosine kinase (RTK) that is expressed widely in mammalian embryos and has diverse roles in development and disease (Takahashi et al. 1985, 1988; Taraviras and Pachnis 1999; Baloh et al. 2000; Jhiang 2000; Parisi and Kapur, 2000; Schedl and Hastie 2000). During embryogenesis, the main sites of c-Ret expression are the excretory and the nervous systems (Pachnis et al. 1993; Tsuzuki et al. 1995). In the nervous system, c-Ret is expressed in the progenitors of the enteric nervous system (ENS), in the enteric, autonomic, and sensory neurons of the peripheral nervous system (PNS), and in the motor and catecholaminergic neurons of the central nervous system (CNS).
Mice homozygous for a targeted mutation of c-Ret (Ret.k−) have severe hypodysplasia or aplasia of the kidneys (Schuchardt et al. 1994, 1996; Durbec et al. 1996; Srinivas et al. 1999). The mammalian kidney is generated by reciprocal inductive interactions between the ureteric bud (UB) and the undifferentiated metanephric mesenchyme. Whereas the mesenchyme is important for the growth and branching of the UB, which gives rise to the renal collecting system, the tips of the UB branches induce the surrounding mesenchymal cells to condense into epithelial vesicles, which differentiate into the various segments of the nephrons (Saxen 1987). c-Ret is expressed in the UB and its function is necessary for the evagination, growth, and branching of this structure (Pachnis et al. 1993; Schuchardt et al. 1994, 1996; Sainio et al. 1997; Ehrenfels et al. 1999; Schedl and Hastie 2000).
c-Ret also has a critical role in the development of the ENS. The majority of enteric neurons and glia are derived from a subset of neural crest (NC) cells that emigrate from the neural tube at the level of somites 1–7. On invading the foregut mesenchyme, enteric NC (ENC) cells migrate in a rostrocaudal direction and colonize the wall of the gastrointestinal tract (Yntema and Hammond 1954; Le Douarin and Teillet 1973; Kapur et al. 1992; Durbec et al. 1996). Mutations of c-RET in humans lead to absence of enteric ganglia from the distal colon and congenital megacolon (Hirschsprung's disease, HSCR; Parisi and Kapur 2000), whereas Ret.k−/Ret.k− mice lack all enteric ganglia posterior to the stomach (intestinal aganglionosis; Schuchardt et al. 1994; Durbec et al. 1996). Although some of the cellular processes controlled by RET (such as survival, migration, and differentiation of ENC) have been identified, the mechanisms that lead to the localized absence of enteric ganglia in HSCR patients remain unclear.
Gain-of-function mutations of c-Ret have also been described in humans and reproduced in mice by targeted mutagenesis. These mutations result in the constitutive activation of RET and are associated with multiple endocrine neoplasia (MEN) types 2A and 2B and familial medullary thyroid carcinoma (FMTC), inherited cancer syndromes characterized by tumors of neuroendocrine origin (Jhiang 2000; Smith-Hicks et al. 2000).
RET is the signaling component of multisubunit receptor complexes for glial cell line-derived neurotrophic factor (GDNF) family ligands (GFLs), which belong to the TGF-β superfamily (Baloh et al. 2000; Saarma 2000). The interaction between the GFLs and RET is mediated by glycosylphosphatidylinositol (GPI)-linked cell-surface glycoproteins, called GFRα1–4 (Airaksinen et al. 1999). Among the signaling pathways activated by RET are the MAP kinase (MAPK) and the PI3-K pathway (van Weering and Bos 1998). As is the case for other RTKs, phosphorylated tyrosine (Tyr) residues in RET are critical for the formation of docking sites for intracellular adaptor and effector signaling molecules, such as phospholipase C-γ, Shc, Enigma, SNT/FRS2, Gab1, Nck, Crk, Grb2, and p62DOC (van Weering and Bos 1998; Airaksinen et al. 1999; Hansford and Mulligan 2000). Among these Tyr residues, Tyr 1062 has a critical role in signal initiation during embryogenesis and tumorigenesis, serving as a docking site for protein complexes that can activate both the MAPK and the PI3-K pathways (Besset et al. 2000; Hayashi et al. 2000).
In mammals, c-Ret encodes two major isoforms, RET9 and RET51, which are generated by alternative splicing and differ only at their C-terminal tails; RET9 has a nine-amino-acid tail, which in RET51, is replaced by 51 unrelated amino acids (Tahira et al. 1990). Although the two isoforms behave similarly in a number of in vitro assays, several observations have suggested that they have different and possibly tissue-specific effects on embryogenesis and tumorigenesis. To address this issue, we used targeted mutagenesis in embryonic stem (ES) cells and pronuclear DNA microinjection to generate mice that express single RET isoforms. Our experiments reveal that RET9 is sufficient to support normal embryogenesis and postnatal life. Mice expressing only RET51, however, have severe defects in the innervation of the gut and renal development. Furthermore, activated forms of RET9 and RET51 differ in their ability to induce dominant renal malformations in transgenic mice. We discuss the implications of these findings in ENS histogenesis and nephrogenesis.
Results
Generation of mice expressing single RET isoforms
To study the relative roles of the RET isoforms in vivo, we first targeted the mouse c-Ret locus and inserted a cDNA fragment encoding the intracellular portion of the human RET9 or RET51 isoforms. Our goal was to disrupt the wild-type gene and replace it with alleles encoding single chimeric receptors composed of the extracellular domain of mouse RET fused in-frame to the intracellular segment of human RET9 or RET51 (Fig. 1A,B). We named these alleles monoisoformic Ret9 (miRet9) and miRet51. ES cell clones carrying the miRet9 or miRet51 alleles (Fig. 1C) were used to generate animals with various combinations of wild-type and miRet alleles.
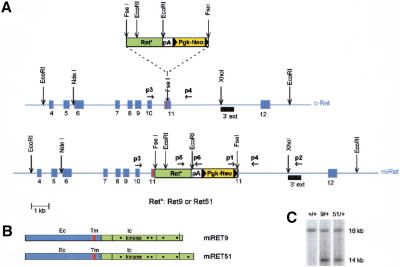
Figure 1.
Targeting of the c-Ret locus. (A) Schematic representation of the targeting strategy. A cassette (top) consisting of cDNA fragments encoding the intracellular part of human RET9 or RET51 (Ret*), the β-globin polyadenylation signal (pA), and the NeoR gene (Pgk-Neo) was inserted in frame into exon 11 of the mouse locus, immediately after the segment encoding the transmembrane domain (red stripe, middle). The targeted locus is shown at the bottom. Solid arrowheads indicate loxP sites. (B) The targeted loci encode single chimeric RET receptors with identical extracellular (Ec) and transmembrane (Tm) domains and the intracellular (Ic) segment of human RET9 or RET51. The positions of Tyr-residues thought to be important for RET signaling are indicated by dots. (C) Southern blot analysis of DNA from ES cell clones using the probe shown in A (3′ ext). On digestion with EcoRI, the wild-type and targeted loci generate 18-kb and 14-kb fragments, respectively.
Heterozygous (+/miRet9 or +/miRet51), heteroallelic (miRet9/miRet51), and homozygous miRet9 mice were viable and displayed no abnormalities, indicating that expression of the miRet alleles was not by itself detrimental. In contrast, the majority of miRet51/miRet51 mice died as neonates and <5% survived to 2–3 mo of age, exhibiting severe growth retardation.
To examine the products of the miRet alleles, RNA from embryos or newborn brain was analyzed by RT–PCR using allele-specific primers. As expected, homozygous miRet9 or miRet51 mice expressed miRet9 or miRet51 mRNA, respectively, and lacked wild-type c-Ret transcripts, whereas +/miRet9 and +/miRet51 animals expressed both the wild-type and the appropriate chimeric isoforms (Fig. 2A). Semiquantitative RT–PCR showed that the levels of transcripts encoding RET9 and RET51 in monoisoformic embryos and brain were comparable with those of isoform specific transcripts in wild-type tissues (Fig. 2B; data not shown).
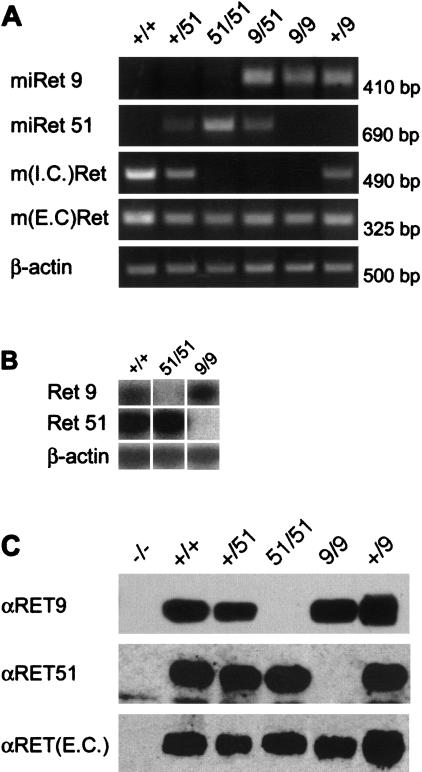
Figure 2.
Expression of the miRet alleles. (A) RT–PCR analysis of total RNA from neonatal brain. Products specific for miRet51 or miRet9 mRNAs are generated only in samples derived from animals carrying at least one copy of miRet51 or miRet9, respectively. Although no signal specific for sequences encoding the intracellular domain of mouse RET [m(I.C.)Ret] was detected in either homozygous or heteroallelic monoisoformic mice, sequences corresponding to the extracellular domain of mouse RET [m(E.C.)Ret] were amplified irrespective of the genotype. Amplification of β-actin mRNA verified the integrity of the mRNA. Shown at top are the genotypes of the animals used (51 = miRet51 and 9 = miRet9). For all genotypes n = 9. (B) Semi-quantitive RT–PCR on neonatal brain. Isoform-specific primers that correspond to identical human and murine sequences generate products of similar intensity in samples of neonatal brain RNA derived from +/+ and homozygous miRet51 and miRet9 animals. For all genotypes n = 4. (C) Expression of RET isoforms in miRet mice. Protein extracts from neonatal brain were immunoprecipitated with antibodies against the extracellular (EC) domain of RET and subjected to Western blot analysis using isoform-specific antibodies. Homozygous miRet51 mice do not express RET9 whereas homozygous miRet9 mice do not express RET51. An antibody directed against the EC domain of mouse RET [αRET(E.C.)] detects comparable levels of the receptor in all samples. For all genotypes n = 6. (D) Cell-specific expression of miRet alleles. In situ hybridization on sections of E13.5 +/+ (a,d,g), homozygous miRet9 (b,e,h) and homozygous miRet51 (c,f,i) embryos using a riboprobe that corresponds to the EC domain of mouse RET. Specific signal was present in motoneurons (mn), sensory neurons of dorsal root ganglia (drg), the ganglia of the myenteric plexus (mp), and the tips of the ureteric bud branches (ub). e, Endoderm of the gut. For all genotypes n = 12.
To further analyze the products of the miRet alleles, protein extracts from embryos and newborn brain were immunoprecipitated with antibodies specific for the extracellular domain of RET and the immunoprecipitates were characterized by Western blotting using isoform specific antibodies. Consistent with the RT–PCR analysis, animals carrying at least one wild-type c-Ret allele produced both RET9 and RET51 isoforms (Fig. 2C). In contrast, homozygous miRet9 or miRet51 animals generated RET9 or RET51 exclusively, respectively. Furthermore, the levels of RET9 and RET51 expressed in monoisoformic mice were comparable with those in wild-type animals (Fig. 2C). In summary, the novel miRet alleles we have generated encode single RET isoforms at levels comparable to those detected in wild-type animals.
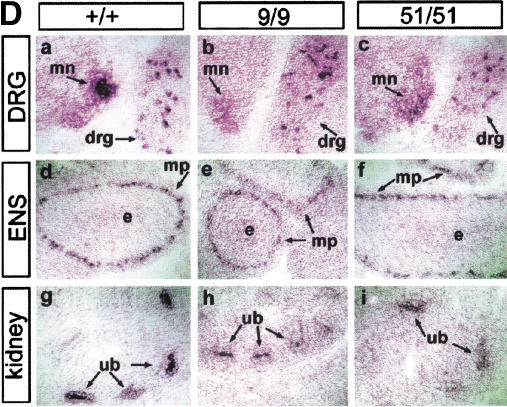
To examine the tissue specificity of miRet9 or miRet51 expression during embryogenesis, we analyzed sections of monoisoformic mouse embryos by in situ hybridization using riboprobes corresponding to the extracellular domain of RET. As is the case for c-Ret, both miRet9 and miRet51 were expressed in the motoneurons of the spinal cord and in subsets of peripheral sensory neurons of the dorsal root ganglia (Fig. 2D, panels a–c), as well as in enteric (Fig. 2D, panels d–f), and autonomic ganglia (data not shown). Outside the nervous system, miRet9 and miRet51 mRNA were detected in the Wolffian duct (data not shown) and the branches of the UB throughout nephrogenesis (Fig. 2D, panels g–i). We conclude that miRet9 and miRet51 are expressed with a spatial and temporal pattern indistinguishable from that of wild-type c-Ret.
The role of RET9 and RET51 in the development of the excretory system
Given the critical role of RET signaling in nephrogenesis, we examined the excretory system of newborn miRet animals. Consistent with the normal appearance and healthy status of miRet9 heterozygotes and homozygotes, their kidneys were morphologically and histologically normal (Fig. 3A,B, panels a and b). In contrast, the kidneys of miRet51 homozygotes were always smaller (one-fourth to one-third the volume of +/+ controls), cystic and had fewer nephrons (Fig. 3A, panel c and B, panel d). In addition, the peripheral nephrogenic zone, in which UB branching and induction of new nephrons take place, was mostly absent. These abnormalities were similar to, although less severe than, those observed in newborn Ret.k− homozygotes (Schuchardt et al. 1994, 1996). No abnormalities were observed in the kidneys of +/miRet51 or miRet9/miRet51 animals (Fig. 3A, panels d,e and B, panel c).
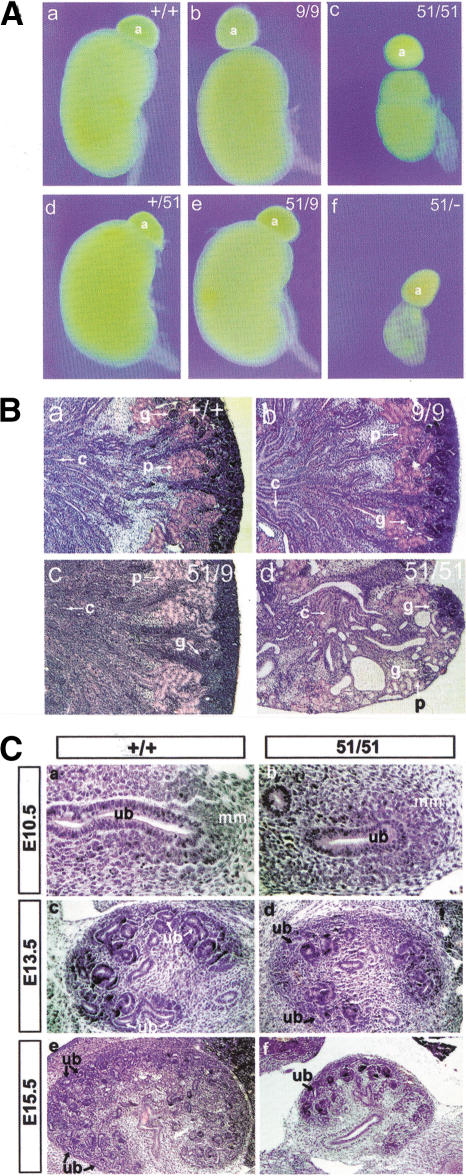
Figure 3.
Renal hypodysplasia in miRet51animals. (A) Kidneys from miRet9/miRet9 (b), +/miRet51 (d) and miRet9/miRet51 (e) animals appeared identical to the wild type (a). The kidneys of miRet51/miRet51 (c) or miRet51/Ret.k− (f) animals, however, were hypodysplastic. +/+, n = 134; 9/9, n = 43; 51/51, n = 103; +/51, n = 197; 9/51, n = 35; 51/−, n = 17. a, Adrenal gland. (B) Histological analysis of kidneys of miRet neonates. The histoarchitecture of miRet9/miRet9 (b) and miRet9/miRet51 (c) kidneys was indistinguishable from the wild type (a). However, the kidneys of miRet51/miRet51 neonates (d) show many cysts and a reduced number of nephrons. g, Glomerulus; p, proximal convoluting tubule; c, collecting tubule. For all genotypes n = 7. (C) Reduced branching of the UB in miRet51 homozygote embryos. Sections through the metanephros of E10.5 +/+ (a) and miRet51 (b) embryos reveal no difference in the evagination of the UB (ub) and the invasion of the metanephric mesenchyme (mm). Note the normal condensation of the mesenchyme in the miRet51embryo. At E13.5, a small reduction in the number of UB branches is detected in the miRet51 embryos (d) in comparison with +/+ (c). This difference becomes clearer at subsequent developmental stages (E15.5; cf. panels e and f). For all genotypes and stages n = 5.
To analyze the developmental basis of the renal abnormalities of miRet51 homozygotes, we compared histological sections of +/+ and miRet51/miRet51 embryos at various stages. Although the first stages of metanephric kidney development (i.e., evagination of the UB and its initial branching between E10.5 and E12.5), occurred normally in miRet51 embryos (Fig. 3C, panels a,b), a reduction in the number of UB branches was first observed at E13.5 and more pronounced at later stages (Fig. 3C, panels c–f). This suggested that although signaling by RET51 is capable of supporting the early stages of UB outgrowth and branching, it is unable to sustain normal nephrogenesis at later stages of embryogenesis.
To address the possibility that the kidney defects observed in miRet51 homozygotes were attributable to excessive RET signaling, we generated mice heteroallelic for miRet51 and the null Ret.k− allele. The kidneys of these animals were even more severely affected than those of miRet51 homozygotes (Fig. 3A, cf. panels c and f), suggesting that overactivation of the RET signaling pathway is not the cause of the renal abnormalities in miRet51 animals.
Molecular analysis of kidney development in monoisoformic RET mice
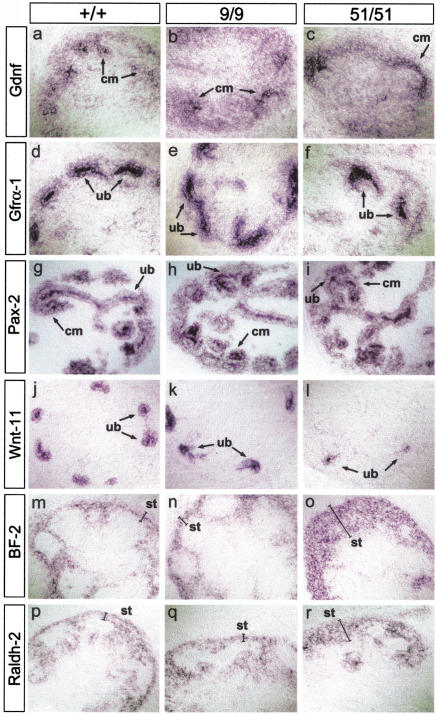
We first analyzed the expression of Gdnf, Gfrα1, and Pax2 (Schedl and Hastie 2000) but we observed no differences in the expression of these genes between the metanephroi of wild-type and miRet51/miRet51 embryos (Fig. 4, panels a–i).
Figure 4.
Molecular analysis of metanephric development in miRet mice. In situ hybridization with riboprobes for Gdnf (a–c); Gfrα1 (d–,f); Pax2 (g–i); Wnt11 (j–l); BF-2 (m–o); and Raldh2 (p–r) on serial sections of E13.5 kidneys from +/+ (a,d,g,j,m,p), miRet9 (b,e,h,k,n,q), and miRet51 (c,f,i,l,o,r) embryos. cm, Condensing mesenchyme; ub, ureteric bud tips; st, stroma. For each genotype and probe n = 4.
The Wnt family of signaling molecules has an important role in renal development (Vainio et al. 1999; Schedl and Hastie 2000), and one of them, Wnt11, is normally coexpressed with c-Ret at the tips of the growing UB. We found that the levels of Wnt11 mRNA were reduced in the metanephroi of miRet51 homozygotes relative to miRet9/miRet9 or +/+ embryos (Fig. 4j–l), indicating that the expression of Wnt11 is differentially regulated by RET51 and RET9.
In addition to the UB and the nephrogenic mesenchyme, stromal cells also have an important role in kidney development (Bard 1996; Hatini et al. 1996; Batourina et al. 2001). Genes that are expressed specifically in stromal cells and are important for normal renal development include BF-2, which encodes a winged-helix transcription factor (Bard 1996; Hatini et al. 1996) and Raldh2, which is essential for retinoic acid (RA) synthesis (Niederreither et al. 1999). In E13.5 +/+ and miRet9/miRet9 embryos, these two genes were co-expressed in a thin layer of peripheral stromal cells and in narrow strips of cells intercalated between the mesenchymal condensates and the UB tips (Fig. 4, panels m,n,p,q). In contrast, in E13.5 miRet51 homozygotes, the layer of peripheral stromal cells expressing BF-2 and Raldh2 was significantly wider whereas the intercalated bands of positive stromal cells were reduced (Fig. 4o,r). Interestingly, this defect appears similar to that in kidneys from mice doubly mutant for RA receptors (RAR) α and β2. The renal defects in RARαβ2 mutant mice were attributed to an indirect effect of the mutation on the UB, which resulted in progressively reduced expression of RET (all isoforms), and could be rescued with a Hoxb7–Ret9 transgene (Batourina et al. 2001).
A Hoxb7–Ret51 transgene, unlike Hoxb7–Ret9, does not rescue kidney development in Ret.k− mice
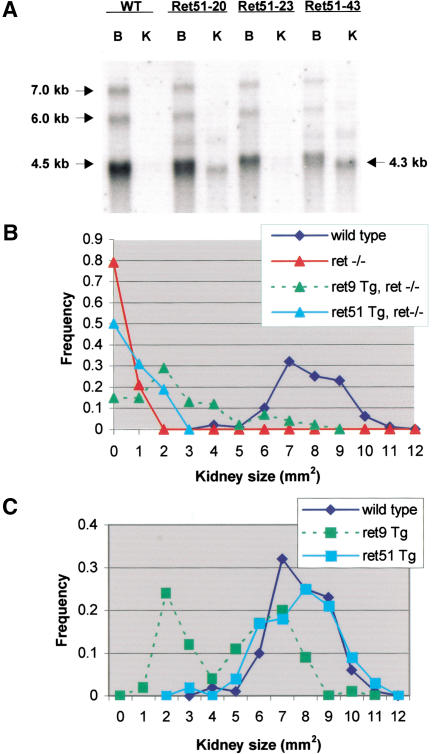
We have described previously a series of transgenic (Tg) mice that expressed a Ret9 cDNA under control of the Hoxb7 promoter, which is active throughout the UB and its derivatives (the epithelium of the collecting system in the mature kidney; Srinivas et al. 1999). Consistent with the normal kidney development observed in miRet9 mice, the Hoxb7–Ret9 transgene was capable of fully rescuing kidney development in Ret.k− homozygotes (Srinivas et al. 1999). To independently assess the role of RET51 in kidney development, a similar series of Hoxb7–Ret51 Tg mice was generated. Three Hoxb7–Ret51 Tg lines were found to express the transgene in adult kidneys by Northern and Western blotting, at a range of levels comparable with the Hoxb7–Ret9 transgene (Fig. 5A; data not shown). When the Hoxb7–Ret51 transgene was crossed into a Ret.k−/Ret.k− background, it had only a minimally beneficial effect on kidney development (Fig. 5B). As kidney size correlated inversely with the degree of dysplasia, the size distribution provides a rough quantitative measure of normal kidney development (Srinivas et al. 1999). Hoxb7–Ret51 reduced the frequency of renal agenesis, and slightly increased the sizes of the kidneys formed, but never provided a full rescue. These experiments support the conclusion that RET51 cannot support normal kidney development in the absence of RET9.
Figure 5.
The Hoxb7–Ret51 transgene fails to rescue kidney development in Ret.k−/Ret.k− mice, or to induce dominant hypodysplasia in wild-type mice, unlike the Hoxb7–Ret9 transgene (Srinivas et al. 1999). (A) Northern blot of 5 μg of poly(A)+ RNA from adult brain and kidney of wild-type and Hoxb7–Ret51 Tg mice, hybridized with a c-Ret probe. The major endogenous c-Ret transcripts are indicated at left. The transgene encodes a major 4.3-kb mRNA, and several larger bands of unknown origin. (WT) Wild-type control. RET51-20, RET51-23, and RET51-43 are three different Tg lines. Brain RNA (B) expresses endogenous c-Ret mRNA in wild-type and Tg mice, whereas kidney (K) does not express detectable c-Ret mRNA in wild-type adult, but expresses the Tg mRNA. (B,C) Kidney sizes in newborn mice (a value of zero indicates no kidney). (B) Although the Hoxb7–Ret9 transgene increased kidney size in Ret.k−/Ret.k− mice, occasionally into the normal range, the Hoxb7–Ret51 transgene had only a small beneficial effect. (C) On a wild-type background, although the Hoxb7–Ret9 transgene caused renal hypodysplasia in ∼50% of Tg mice, Hoxb7–Ret51 had no significant effect. Data for Ret.k− homozygotes (ret−/−) and Hoxb7–Ret9 Tg mice are from Srinivas et al. (1999). ret−/−, n = 38; +/+, n = 138; Ret9 Tg, ret−/−, n = 92; Ret51 Tg, ret−/−, n = 32; Ret9 Tg, n = 76; Ret51 Tg, n = 128. Data were pooled for three Hoxb7–Ret51 and three Hoxb7–Ret9 Tg lines.
On a wild-type background, the Hoxb7–Ret9 transgene caused an incompletely penetrant, dominant renal hypodysplasia through an unknown mechanism thought to result from ectopic expression of RET9 throughout the UB (Srinivas et al. 1999). In contrast, no such dominant defect was seen in the Hoxb7–Ret51 Tg mice, based on their normal distribution of kidney sizes (Fig. 5C) and histology (data not shown).
Constitutively active forms of RET9, but not RET51, induce abnormal growth of the UB in transgenic mice
We reported previously that an oncogenic form of RET9 (Ret9–PTC2), when expressed under the Hoxb7 promoter, induced the formation of abnormal renal nodules, apparently formed by deregulated growth and branching of the UB (Srinivas et al. 1999). To compare the effects of activated forms of RET9 and RET51, we constructed four more Hoxb7–Ret transgenes that expressed two additional oncogenic forms of each of the two RET isoforms. One pair of transgenes contained the C634K mutation found in the MEN2A syndrome (Donis-Keller et al. 1993; Mulligan et al. 1993). The second pair contained the M918T substitution associated with the related syndrome MEN2B (Carlson et al. 1994; Hofstra et al. 1994).
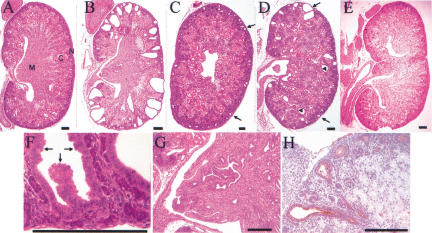
Because oncogenic Hoxb7–Ret transgenes may cause perinatal lethality (Srinivas et al. 1999), we sacrificed and examined the founder Tg mice at E18.5. Three out of five E18.5 Hoxb7–Ret9–MEN2A Tg founders had hyperplastic renal cysts, primarily in the nephrogenic zone (and extending into the cortex), and abnormal branching of collecting ducts in the medulla (Fig. 6B,C,F–H). Similarly, two out of three E18.5 Hoxb7–Ret9–MEN2B Tg founders displayed cystic dilation of UB tips in the nephrogenic zone as well as cysts derived from more proximal maturing tubules in the cortex (Fig. 6D). Although the renal defects induced by Ret9–MEN2A and Ret9–MEN2B differed somewhat from those induced by Ret9–PTC2 (Srinivas et al. 1999), they also appeared to result from the abnormal growth, branching, and proliferation of the UB and collecting ducts.
Figure 6.
Constitutively active forms of RET9 but not RET51 induce cystic kidneys. Histological sections of kidneys from a +/+ mouse (A), two Hoxb7–Ret9–MEN2A Tg mice (B,C), a Hoxb7–Ret9–MEN2B mouse (D) and a Hoxb7–Ret51–MEN2A mouse, all at E18.5. The medulla (M), cortex (C), and nephrogenic zone (N) are labeled in A. (B) A severe case of cystic dysplasia caused by Hoxb7–Ret9–MEN2A. The cysts, which appear to derive from UB tips, replace much of the nephrogenic zone, and display hyperplastic features, such as stratification of the epithelium, as shown at higher magnification in F (arrows). In addition, the medulla displays abnormal branching of the collecting ducts, as shown at higher magnification in G. (C) A less severe case of cystic UB tips (arrows) in a different Hoxb7–Ret9–MEN2A founder. (H) A section for the same kidney as in C, stained with the UB-specific lectin dolichos biflorus (brown stain) (D'Agati and Trudel 1992), confirming the origin of the epithelial cysts. (D) a kidney from a Hoxb7–Ret9–MEN2B founder displays cystic UB tips in the peripheral nephrogenic zone (arrows) as well as cysts deeper in the cortex (arrowheads). (E) A bifid kidney from a Hoxb7–Ret51–MEN2A Tg mouse, with duplication of the ureter, pelvis, and renal pyramid.
In contrast, no abnormalities of this type were observed in the kidneys of Tg mice expressing either mutant form of RET51. None of five Hoxb7–Ret51–MEN2B or 11 Hoxb7–Ret51–MEN2A Tg founders showed any cortical cysts or abnormally branched medullary collecting ducts. Interestingly, two of the Hoxb7–Ret51–MEN2A mice had a unilateral bifid kidney, which contained a duplicated ureter, renal pelvis, and pyramid (Fig. 6E), but were otherwise normal in size and histology. Bifid kidneys, which sometimes occur in humans, are believed to result from the evagination of two UBs from the Wolffian duct (N'Guessan and Stephens 1983). This suggests that an activated form of RET51, although not perturbing the growth and branching of the UB within the developing kidney as do similar forms of RET9, can cause ectopic outgrowth of a secondary UB.
The role of RET9 and RET51 in the development of the ENS of the mouse
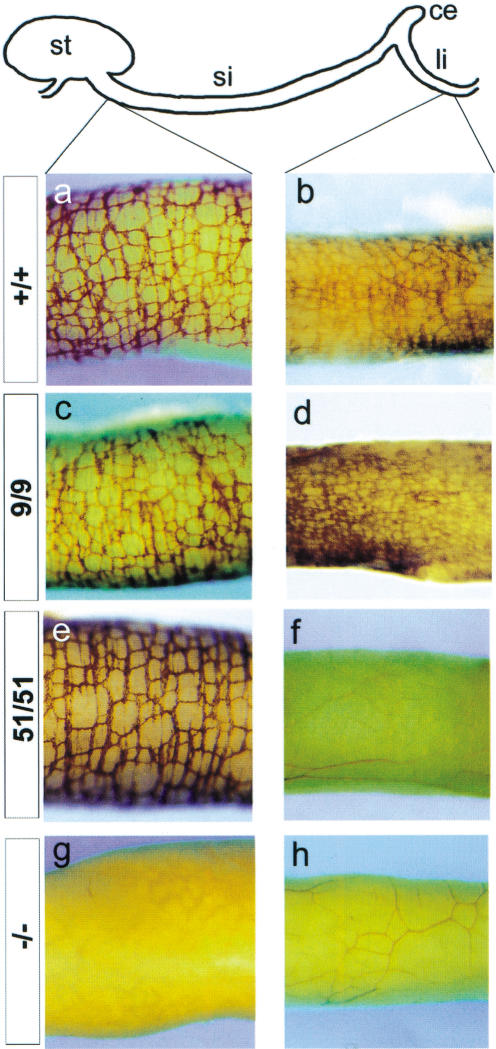
Genetic studies in humans and mice have shown that signaling by RET has a pivotal role in the development of the ENS (Taraviras and Pachnis 1999). To examine the individual roles of RET9 and RET51 during ENS histogenesis, we first analyzed the myenteric plexus of +/+, miRet9/miRet9, and miRet51/miRet51 newborn mice by whole-mount acetylcholinesterase (AchE) histochemistry (Enomoto et al. 1998). We observed that the number of enteric ganglia and their distribution along the length of the gut were indistinguishable between +/+ and miRet9/miRet9animals (Fig. 7a–d). Similarly, an apparently normal myenteric plexus was present in the stomach and small intestine of miRet51 homozygotes (Fig. 7e). A severe reduction or complete absence of enteric ganglia, however, was reproducibly observed in the terminal three-fourths of the colon of miRet51 mice at birth (Fig. 7f). Careful examination of gut preparations from these animals using molecular markers of enteric neurons, such as PGP9.5, indicated that a reduced number of neurons was also detectable in the proximal quarter of the colon (data not shown).
Figure 7.
Aganglionosis of the colon of miRet51/miRet51 animals. Whole-mount acetylcholinesterase histochemistry of neonatal duodenum (a,c,e,g) and colon (b,d,f,h) of +/+ (a,b), miRet9/miRet9 (c,d), miRet51/miRet51 (e,f) and Ret.k−/Ret.k− (−/−; g,h) neonates. In miRet51 homozygotes, although an apparently normal myenteric plexus forms in the small intestine, no enteric ganglia were present in the colon. RET-deficient animals fail to develop the myenteric plexus throughout the length of the intestine. (Top) Diagrammatic representation of the various gut segments. st, Stomach; si, small intestine; ce, cecum; li, large intestine. +/+, n = 37; 9/9, n = 18; 51/51, n = 23; −/−, n = 3.
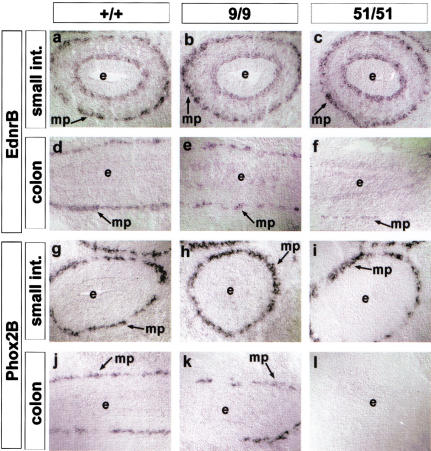
Aganglionosis in miRet51 mice could result from failure of the ENS progenitors to enter the colon during embryogenesis or from their inability to survive within its microenvironment. As a first step toward distinguishing between these possibilities, we analyzed the distribution of ENC cells and their derivatives in the gut at E11.5–E13.5, a period during which ENS progenitors are colonizing the gut (Kapur et al. 1992; Durbec et al. 1996). In situ hybridization for markers of ENS progenitors, such as Ednrb, Phox2b, and Gfra1 revealed no obvious differences in the number or distribution of ENC-derived cells throughout the gastrointestinal (GI) tract of +/+ and miRet9 homozygotes (Fig. 8a,b,d,e,g,h,j,k). Similarly, normal numbers of Ednrb+, Phox2B+, and Gfra1+ cells were present in the stomach and the small intestine of miRet51 embryos (Fig. 8c,i). However, no ENC-derived cells were present in the distal three-fourths of the colon at any stage analyzed (Fig. 8f,l). These findings suggest that the absence of enteric ganglia from miRet51/miRet51 neonates results from failure of ENC cells to enter the colon during embryogenesis.
Figure 8.
The progenitors of the ENS fail to colonize the colon of miRet51/miRet51 embryos. In situ hybridization with riboprobes specific for EdnrB (a–f) and Phox2B (g–l) mRNAs on serial sections of small intestine (a–c,g–i) and colon (d–f,j–l) of +/+ (a,d,g,j), miRet9/miRet9 (b,e,h,k) and miRet51/miRet51 (c,f,j,l) E13.5 embryos. mp, Myenteric plexus. For all genotypes n = 3.
The colonic aganglionosis of miRet51 mice is reproduced in vitro
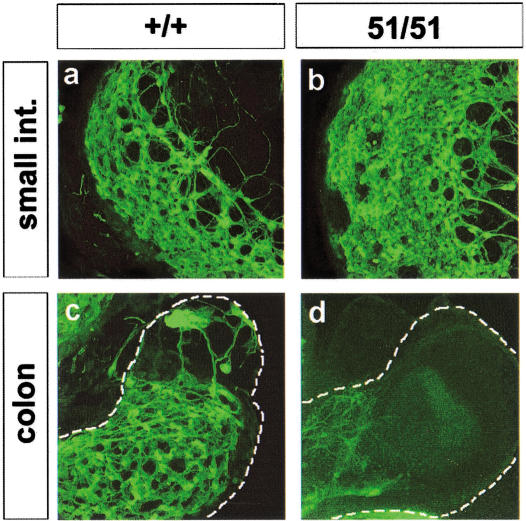
We previously developed a fetal gut culture system in which key steps of ENS histogenesis, such as ENC cell migration and differentiation, can be reproduced in vitro (Natarajan et al. 1999). For this system, gut is isolated from E11.0–E11.5 mouse embryos (a stage at which NC cells have migrated up to the cecum) and cultured in defined medium. After 1–3 wk, ENC cells have colonized the entire gut and a large fraction have differentiated into neuronal and glial cells. Although the identity of the morphologically distinct segments of the gut (esophagus, stomach, small intestine, cecum, and large intestine) is maintained, the overall growth and elongation of the organ in culture is limited in comparison with its in vivo counterpart (Natarajan et al. 1999). To determine whether the colonic aganglionosis in miRet51 homozygotes can be reproduced under conditions in which the gut undergoes limited growth, gut from +/+ and miRet51/miRet51 E11.0 embryos was cultured for 4 d. The extent of colonization by ENS progenitors and their differentiation was assayed by immunostaining with the neuronal markers PGP9.5 and TuJ1. A large number of PGP9.5 positive cells were detected throughout the length of the gut derived from +/+ and miRet9/miRet9 embryos (Fig. 9a,c). Similarly, an apparently normal complement of neurons was present in the stomach and small intestine of parallel cultures derived from miRet51/miRet51 embryos (Fig. 9b). However, the colon of these explants was almost completely devoid of neurons and contained only PGP9.5-positive axonal projections that originated in the more proximal small intestine (Fig. 9d). These data indicate that the aganglionic phenotype of miRet51 animals is reproduced in the fetal gut culture system.
Figure 9.
The colonic aganglionosis phenotype of miRet51/miRet51 is reproduced in organ culture. Guts were removed from +/+ and miRet51/miRet51 (51/51) E11.5 embryos, cultured for 4 d and processed for immunostaining with PGP9.5. (a,b) Fluorescent images of segments of the small intestine of +/+ and miRet51/miRet51 gut. (c,d) The corresponding colonic segments. Note the absence of a ganglionic plexus from the colon of explants derived from miRet51/miRet51 embryos. +/+, n = 5; 51/51, n = 4.
Discussion
To gain insight into the differential roles of the RET isoforms, we generated monoisoformic mouse strains that express only RET9 or RET51 in place of the normal complement of c-Ret gene products. Our genetic and embryological studies demonstrated that signaling by RET9 is sufficient for normal embryonic development and postnatal life. In contrast, signaling by RET51, in the absence of RET9, resulted in characteristic defects in the development of the excretory and enteric nervous systems. Further evidence for differential signaling between the RET isoforms was provided by transgenic experiments in which RET9, but not RET51, when expressed in the UB and its branches was capable of rescuing the renal defects of Ret.k− mice (this study; Srinivas et al. 1999). These findings indicate that the RET isoforms have distinct signaling properties and suggest that RET51 is dispensable during embryogenesis, whereas RET9 is necessary and sufficient for normal development of the ENS and the excretory system. We cannot exclude the possibility that the miRet9 animals have subtle developmental abnormalities, however, an issue that is currently being addressed by further experimentation.
Several lines of evidence suggest that the phenotypic differences between the monoisoformic RET strains reflect different signaling properties of the two isoforms, rather than differences in the expression levels of the miRet9 and miRet51 alleles. First, we introduced equivalent cDNA fragments encoding intracellular portions of RET9 and RET51 into an identical position of the c-Ret locus, thereby generating alleles that were very similar in sequence and organization. Second, similar levels of expression of the isoform-specific transcripts were detected in RET9 and RET51 homozygous animals. Third, using the in vitro survival of DRG neurons (in the presence of GDNF or neurturin) as a measure of RET activation, we have observed that RET9 and RET51 can activate the anti-apoptotic machinery of peripheral sensory neurons to the same extent (E. de Graaf, S. Taraviras, and V. Pachnis, unpubl.). Finally, strong support for the differential signaling of RET9 and RET51 is derived from transgenic experiments showing that both ligand-dependent and -independent forms of the two RET isoforms have distinct biological effects on the developing kidney.
The phenotype of miRet51 mice is similar, albeit milder, to that of RET-deficient animals. Therefore, whereas Ret.k− animals have either renal agenesis or very small and highly dysplastic kidney rudiments, all miRet51 mice develop somewhat larger kidney rudiments. The reproducible formation of a kidney rudiment is consistent with the observation that the first stages of metanephric development (i.e., evagination of the UB and its initial branching at E10.5–E12.5) are apparently normal in these animals, and that defects become evident only during later stages of nephrogenesis. Analysis of Hoxb7–Ret51 Tg mice also showed that although this transgene failed to support normal kidney development in Ret.k− homozygotes, it reduced the frequency of renal agenesis and slightly increased the kidney size, consistent with a limited role for RET51 in early UB growth. A milder phenotype was also observed in the ENS. Whereas Ret.k− animals have total intestinal aganglionosis, miRet51 mice have an apparently normal complement of enteric ganglia up to the cecum, but fail to develop an ENS plexus in the post-cecal colon. The apparently normal status of heteroallelic (miRet9/miRet51) animals strongly suggests that the intracellular part of the chimeric receptors encoded by the miRet alleles are capable of normal signaling in mouse cells. Also, the failure to detect novel phenotypes in miRet animals (in comparison with Ret.k− homozygotes) indicates that these alleles do not interfere with other unrelated signaling pathways. Finally, the normal status of +/miRet9 and +/miRet51 mice indicates that neither miRet9 nor miRet51 have dominant-negative effects. Based on these findings, we suggest that miRet51 represents a hypomorphic allele of c-Ret.
The failure of RET51 to support normal embryogenesis is surprising. In addition to all the Tyr-based signaling motifs it shares with RET9, RET51 contains two additional Tyr residues (corresponding to Tyr 1090 and Tyr 1096 of human RET). At least one of these (Tyr 1096) forms a docking site for the adaptor protein Grb2 (Liu et al. 1996; Besset et al. 2000), and biochemical studies have shown that it contributes to the activation of the PI3-K and the MAP-kinase signaling pathways (Besset et al. 2000). Nevertheless, our in vivo analysis implies that Tyr 1096, and the entire RET51-specific C-terminal tail, is not necessary for normal embryogenesis and that RET9 is sufficient for activation of all intracellular signaling pathways required for normal ENS histogenesis and renal development. In light of this, it is interesting to note that the RET9-specific C-terminal tail is identical in all vertebrates examined so far, whereas the RET51-specific sequence is less well conserved (Tahira et al. 1990; GenBank accession nos.: mouse, AF209436; rat, AJ299016 and AJ299017; chicken, Z49898; zebrafish, AF007949; pufferfish, U58673; and Xenopus, AF286643).
One hypothesis to explain the differential activities of RET9 and RET51 is that their specific carboxy-terminal tails may modulate the efficiency with which active signaling complexes are assembled. In this regard, it is interesting that the two isoforms diverge in sequence one amino acid after Tyr 1062, a residue essential for binding of the adaptor protein Shc and the assembly of signaling complexes (Besset et al. 2000; De Vita et al. 2000; Hayashi et al. 2000). Because amino acids flanking Tyr-residues can determine the efficiency of active complex formation and intracellular signaling, it is possible that in the context of RET51, Tyr 1062 forms a less efficient docking site. Consistent with this hypothesis, the C-terminal tail of RET isoforms can influence the strength of binding to the SH2 and PTB domains of Shc (Lorenzo et al. 1997; Ohiwa et al. 1997; Ishiguro et al. 1999). Furthermore, it is possible that other more distantly located amino acids of the RET51-specific tail have an inhibitory effect on active complex formation on the intracellular part of the receptor. Alternatively, the RET9-specific sequences that are absent from RET51 may have previously unrecognized signaling properties. Although at present we cannot distinguish between these possibilities, the strategy we have employed here allows us to test efficiently in vivo recombinant cDNAs encoding mutant forms of the intracellular segments of RET9 or RET51 isoforms.
The differential signaling by the RET isoforms could also result from the segregation of RET9 and RET51 in distinct cell membrane subdomains. This could influence their interactions with downstream signaling components, coreceptors or ligands. The availability of mouse strains that express single RET isoforms could facilitate studies on the differential localization of these molecules in polarized cells (such as the epithelial cells of the UB) or nonpolarized cells (such as the progenitors of the ENS).
The role of RET isoforms in nephrogenesis
In contrast to the frequent failure of UB outgrowth observed in Ret.k− mouse embryos (Schuchardt et al. 1996), the early stages of metanephric development, including the formation of the UB, invasion of the metanephric mesenchyme and the first branching events, occurred normally in miRet51 homozygous embryos. As development progressed, however, the ability of the tips of the UB to branch further was reduced, and the kidney failed to achieve a normal size and histoarchitecture. Therefore, either RET51 or RET9 can transduce the signals for the outgrowth of the UB from the mesonephric duct and its initial branching, whereas only RET9 is able to support the further development of the metanephros. Although some studies have suggested that GDNF/RET signaling is required primarily for UB initiation (Sainio et al. 1997), our results indicate that RET signaling (specifically RET9) is also required for later stages of UB growth and branching. Our studies also provide the first suggestion that the intracellular signaling events downstream of RET during the early stages of UB outgrowth and branching may be distinct from those required at later stages of metanephric development.
These conclusions were supported further by the analysis of Hoxb7–Ret51 Tg embryos on a Ret.k−/Ret.k− background, where the transgene partially rescued the renal agenesis phenotype (reflecting a partial rescue of UB outgrowth) but failed to support normal kidney development. This contrasts with the complete rescue of kidney development achieved by a Hoxb7–Ret9 transgene (Srinivas et al. 1999). We also found that mutant, ligand-independent forms of the two RET isoforms had distinct dominant effects on the development of UB derivatives in the kidney. Hoxb7–Ret9 (but not Hoxb7–Ret51) containing the activating mutations associated with human MEN2A or MEN2B frequently caused cystic dilation, hyperplasia, or abnormal branching of UB-derived collecting duct epithelia. Therefore, constitutive RET9 but not RET51 signaling seems able to perturb the normal growth and development of the collecting ducts. In contrast, Hoxb7–Ret51–MEN2A mice sometimes displayed a duplicated ureter, which most likely results from evagination of duplicate UBs. This finding appears consistent with the specific ability of RET51 to support the early stages of UB growth.
The role of RET isoforms in the development of ENS
Although the ENS of miRet9 mice appeared to develop normally, the gut of miRet51 animals was only partially colonized by the progenitors of the ENS; enteric ganglia were present in the esophagus, stomach, and small intestine but were absent from the colon. The aganglionic phenotype of miRet51 mice reproduces that of human congenital megacolon (HSCR), which is usually characterized by partial aganglionosis of the colon. In this respect, the miRet51 strain provides an excellent model for studies on the pathogenetic mechanisms underlying this neural deficiency. For example, an unresolved issue in the field of HSCR is the state of the ENS in the apparently normoganglionic proximal segments of the gastrointestinal tract. This is of utmost medical importance, given that a large percentage of HSCR patients continue to have abnormal gut motility, even following a successful surgical resection of the aganglionic segment and anastomosis. Experiments are currently in progress to determine whether subtle histological defects of the ENS are present in the stomach and small intestine of miRet51 animals.
With regard to the mechanisms of aganglionosis in miRet51 homozygous mice, our studies suggest that it results from failure of ENS progenitors to colonize efficiently the post-cecal part of the gastrointestinal tract. Therefore, whereas a normal number of ENS progenitors is present in the stomach and small intestine at E13.5, a drastically reduced number of ENC cells is found in the post-cecal colon. But, why can loss-of-function Ret alleles (such as miRet51 or other alleles described in patients with HSCR) support normal colonization of the gut by ENS progenitors up to the cecum, but not in the post-cecal gut? One possible explanation is based on the view that the developing gut is a relatively homogeneous structure whose colonization is determined mainly by the number of ENC cells, their migratory efficiency, and the rate of gut elongation. Taking into account the role of c-Ret in ENC cell proliferation and migration (Chalazonitis et al. 1998; Hearn et al. 1998; Heuckeroth et al. 1998; Taraviras et al. 1999; Young et al. 2001), one hypothesis is that, under normal circumstances, a constantly expanding population of migrating ENS progenitors is able to keep up with a rapidly growing organ and fully colonize it. Therefore, any mutations that affect RET signaling would impair the proliferation and migration of ENC cells, resulting in incomplete colonization of the gut. In this case, the colon would be aganglionic only because it is the most distal, and therefore hardest to reach, segment of the gut. An alternative hypothesis is that the ENS progenitors, as they migrate along the gut, are exposed to region-specific microenvironmental signals that modify the ability of RET to control cell proliferation and migration. Therefore, in miRet51 embryos, signaling by RET51 would be sufficient for normal development of the ENS up to the cecum, but not in the colon. According to this view, it is the unique properties of the cecum and the colon that are critical and not their distance from the entry point of the ENC cells in the foregut. The reproduction of the aganglionic phenotype of miRet51 animals in an organ culture system, which allows the proliferation, migration, and differentiation of ENC cells but fails to support extensive growth of the gut, supports the second model. We suggest that local signals produced by the microenvironment of the colon further reduce the ability of the already weakened RET51 receptor to signal efficiently, resulting in failure of its colonization by ENC cells. The identification of such signals is a subject of our current experimentation.
Materials and methods
Generation of targeted or transgenic mice expressing either RET9 or RET51
A 10-kb NdeI–XhoI genomic fragment encompassing exons 6–11 of c-Ret was used as the backbone for the targeting construct (Fig. 1A). The genomic region encoding the extracellular domain of murine RET was fused in-frame to cDNA fragments encoding the intracellular segments of human RET9 or RET51 (which are 95% identical to the corresponding segments of the mouse isoforms), a human β-globin polyadenylation signal and a floxed PGK–NeoR fragment (Fig. 1A). Human cDNA sequences were used to allow us to distinguish the expression of the monoisoformic from the endogenous alleles. Targeted clones of E14.2 ES cells were identified by PCR using primers p1, 5′-gccttctatcgccttcttgacgag-3′ and p2, 5′-ctagcaggctcttgagtgatcttgg-3′ (Fig. 1A) and confirmed by Southern blot analysis (Fig. 1C; data not shown). Construction of the Hoxb7–Ret9 transgenes has been described previously (Srinivas et al. 1999). To generate the other transgenes, the RET9 cDNA was replaced by equivalent cDNAs encoding RET51, or human RET9 and RET51 bearing the MEN2A (Cys634Lys) and MEN2B (Met918Thr) mutations.
Expression analysis
Expression of endogenous c-Ret was assayed by RT–PCR using mouse-specific primers from exons 11 (5′-ggagaaccaggttcctgtggactct-3′) and 14 (5′-ttctgccaagtactgcatacccctc-3′). For detection of both wild-type and targeted c-Ret, cDNA was amplified with two primers (forward, 5′-ctgtgtgatgcgctgtgccgca-3′; reverse, 5′-gtcacaggaacctggttctccg-3′) in the part of exon 11 that was not affected by the targeting strategy. Specific expression of the targeted allele was determined using the p5 and p6 primers (Fig. 1A). Primers for actin (Stratagene) were used as an internal control.
For semiquantitative RT–PCR, we used primers specific for RET9− or RET51 that are identical between human and mouse. The forward primer for both isoforms was in exon 19 (5′-gatggagaggccagacaactgca-3′); the Ret9 mRNA-specific primer was in exon 19 (5′-ctagaatctagtaaatgcatg-3′), whereas the Ret51 mRNA-specific primer was in exon 20 (5′-aggactctctccaggccagttcg-3′).
For Western blots, neonatal brains and E13.5 embryos were homogenized in 2% NP40, 40 mM Tris-HCl (pH 7.5), 20 mM EDTA supplemented with protease inhibitors (Complete, Roche), incubated on ice for 30 min and centrifuged at 6500 rpm at 4°C. Immunoprecipitation and Western blotting was performed according to standard procedures using protein A sepharose beads. For immunoprecipitation, we used a cocktail of monoclonal antibodies directed against the extracellular domain of RET (Lo and Anderson 1995). Immunoblotting was done using polyclonal antibodies specific for RET9 (Immuno-Biological Laboratories), RET51 (Santa Cruz Biotechnologies) or the common extracellular domain (R&D Systems) at a 1/250 dilution.
Histological analysis and in situ hybridization was performed according to standard protocols. Oganotypic culture of fetal gut was performed as described previously (Natarajan et al. 1999). Details of the knock-in constructs and strategy and our protocols are available on request.
Acknowledgments
The authors are grateful to Amanda Hewett and Mary Dawson for animal husbandry. We thank David Anderson for the RET monoclonal antibodies and Jacqueline Deschamps for the Hoxb7 promoter. We are indebted to Richard Maas, Andy McMahon, Cathy Mendelsohn, Pierre Chambon, Michael Gershon, and Christo Goridis for probes. We also thank members of the Pachnis and Costantini laboratories and Iris Salecker, Bruce Ponder, and Aaron Cranston for valuable suggestions and discussions. EdG is a recipient of an EMBO fellowship (ALTF 260-1996). This work was supported by the Medical Research Council (UK), the National Institutes of Health, and the Cancer Research Campaign (UK).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL vpachni@nimr.mrc.ac.uk; FAX 44-20-89138536.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.205001.
References
- Airaksinen MS, Titievsky A, Saarma M. GDNF family neurotrophic factor signaling: Four masters, one servant? Mol Cell Neurosci. 1999;13:313–325. doi: 10.1006/mcne.1999.0754. [DOI] [PubMed] [Google Scholar]
- Baloh RH, Enomoto H, Johnson EM, Jr, Milbrandt J. The GDNF family ligands and receptors— implications for neural development. Curr Opin Neurobiol. 2000;10:103–110. doi: 10.1016/s0959-4388(99)00048-3. [DOI] [PubMed] [Google Scholar]
- Bard J. A new role for the stromal cells in kidney development. Bioessays. 1996;18:705–707. doi: 10.1002/bies.950180905. [DOI] [PubMed] [Google Scholar]
- Batourina E, Gim S, Bello N, Shy M, Clagett-Dame M, Srinivas S, Costantini F, Mendelsohn C. Vitamin A controls epithelial/mesenchymal interactions through ret expression. Nat Genet. 2001;27:74–78. doi: 10.1038/83792. [DOI] [PubMed] [Google Scholar]
- Besset V, Scott RP, Ibanez CF. Signaling complexes and protein–protein interactions involved in the activation of the ras and phosphatidylinositol 3-kinase pathways by the c-Ret receptor tyrosine kinase. J Biol Chem. 2000;275:39159–39166. doi: 10.1074/jbc.M006908200. [DOI] [PubMed] [Google Scholar]
- Carlson KM, Dou S, Chi D, Scavarda N, Toshima K, Jackson CE, Wells SA, Jr, Goodfellow PJ, Donis-Keller H. Single missense mutation in the tyrosine kinase catalytic domain of the RET protooncogene is associated with multiple endocrine neoplasia type 2B. Proc Natl Acad Sci. 1994;91:1579–1583. doi: 10.1073/pnas.91.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalazonitis A, Rothman TP, Chen J, Gershon MD. Age-dependent differences in the effects of GDNF and NT-3 on the development of neurons and glia from neural crest-derived precursors immunoselected from the fetal rat gut: Expression of GFRα-1 in vitro and in vivo. Dev Biol. 1998;204:385–406. doi: 10.1006/dbio.1998.9090. [DOI] [PubMed] [Google Scholar]
- D'Agati V, Trudel M. Lectin characterization of cystogenesis in the SBM transgenic model of polycystic kidney disease. J Am Soc Nephrol. 1992;3:975–983. doi: 10.1681/ASN.V34975. [DOI] [PubMed] [Google Scholar]
- De Vita G, Melillo RM, Carlomagno F, Visconti R, Castellone MD, Bellacosa A, Billaud M, Fusco A, Tsichlis PN, Santoro M. Tyrosine 1062 of RET-MEN2A mediates activation of Akt (protein kinase B) and mitogen-activated protein kinase pathways leading to PC12 cell survival. Cancer Res. 2000;60:3727–3731. [PubMed] [Google Scholar]
- Donis-Keller H, Dou S, Chi D, Carlson KM, Toshima K, Lairmore TC, Howe JR, Moley JF, Goodfellow P, Wells SA., Jr Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum Mol Genet. 1993;2:851–856. doi: 10.1093/hmg/2.7.851. [DOI] [PubMed] [Google Scholar]
- Durbec PL, Larsson-Blomberg LB, Schuchardt A, Costantini F, Pachnis V. Common origin and developmental dependence on c-ret of subsets of enteric and sympathetic neuroblasts. Development. 1996;122:349–358. doi: 10.1242/dev.122.1.349. [DOI] [PubMed] [Google Scholar]
- Ehrenfels C W, Carmillo P J, Orozco O, Cate R L, Sanicola M. Perturbation of RET signaling in the embryonic kidney. Dev Genet. 1999;24:263–272. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<263::AID-DVG9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Araki T, Jackman A, Heuckeroth RO, Snider WD, Johnson EM, Jr, Milbrandt J. GFR α1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron. 1998;21:317–324. doi: 10.1016/s0896-6273(00)80541-3. [DOI] [PubMed] [Google Scholar]
- Hansford JR, Mulligan LM. Multiple endocrine neoplasia type 2 and RET: From neoplasia to neurogenesis. J Med Genet. 2000;37:817–827. doi: 10.1136/jmg.37.11.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes & Dev. 1996;10:1467–1478. doi: 10.1101/gad.10.12.1467. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Ichihara M, Iwashita T, Murakami H, Shimono Y, Kawai K, Kurokawa K, Murakumo Y, Imai T, Funahashi H, et al. Characterization of intracellular signals via tyrosine 1062 in RET activated by glial cell line-derived neurotrophic factor. Oncogene. 2000;19:4469–4475. doi: 10.1038/sj.onc.1203799. [DOI] [PubMed] [Google Scholar]
- Hearn CJ, Murphy M, Newgreen D. GDNF and ET-3 differentially modulate the numbers of avian enteric neural crest cells and enteric neurons in vitro. Dev Biol. 1998;197:93–105. doi: 10.1006/dbio.1998.8876. [DOI] [PubMed] [Google Scholar]
- Heuckeroth RO, Lampe PA, Johnson EM, Milbrandt J. Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitors in vitro. Dev Biol. 1998;200:116–129. doi: 10.1006/dbio.1998.8955. [DOI] [PubMed] [Google Scholar]
- Hofstra RM, Landsvater RM, Ceccherini I, Stulp RP, Stelwagen T, Luo Y, Pasini B, Hoppener JW, van Amstel H K, Romeo G, et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature. 1994;367:375–376. doi: 10.1038/367375a0. [DOI] [PubMed] [Google Scholar]
- Ishiguro Y, Iwashita T, Murakami H, Asai N, Iida K, Goto H, Hayakawa T, Takahashi M. The role of amino acids surrounding tyrosine 1062 in ret in specific binding of the shc phosphotyrosine-binding domain. Endocrinology. 1999;140:3992–3998. doi: 10.1210/endo.140.9.7003. [DOI] [PubMed] [Google Scholar]
- Jhiang SM. The RET proto-oncogene in human cancers. Oncogene. 2000;19:5590–5597. doi: 10.1038/sj.onc.1203857. [DOI] [PubMed] [Google Scholar]
- Kapur RP, Yost C, Palmiter RD. A transgenic model for studying development of the enteric nervous system in normal and aganglionic mice. Development. 1992;116:167–175. doi: 10.1242/dev.116.Supplement.167. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Teillet M A. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973;30:31–48. [PubMed] [Google Scholar]
- Liu X, Vega QC, Decker RA, Pandey A, Worby CA, Dixon JE. Oncogenic RET receptors display different autophosphorylation sites and substrate binding specificities. J Biol Chem. 1996;271:5309–5312. doi: 10.1074/jbc.271.10.5309. [DOI] [PubMed] [Google Scholar]
- Lo L, Anderson DJ. Postmigratory neural crest cells expressing c-RET display restricted developmental and proliferative capacities. Neuron. 1995;15:527–539. doi: 10.1016/0896-6273(95)90142-6. [DOI] [PubMed] [Google Scholar]
- Lorenzo MJ, Gish GD, Houghton C, Stonehouse TJ, Pawson T, Ponder BA, Smith DP. RET alternate splicing influences the interaction of activated RET with the SH2 and PTB domains of Shc, and the SH2 domain of Grb2. Oncogene. 1997;14:763–771. doi: 10.1038/sj.onc.1200894. [DOI] [PubMed] [Google Scholar]
- Mulligan LM, Kwok JB, Healey CS, Elsdon MJ, Eng C, Gardner E, Love DR, Mole SE, Moore JK, Papi L, et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature. 1993;363:458–460. doi: 10.1038/363458a0. [DOI] [PubMed] [Google Scholar]
- Natarajan D, Grigoriou M, Marcos-Gutierrez CV, Atkins C, Pachnis V. Multipotential progenitors of the mammalian enteric nervous system capable of colonising aganglionic bowel in organ culture. Development. 1999;126:157–168. doi: 10.1242/dev.126.1.157. [DOI] [PubMed] [Google Scholar]
- N'Guessan G, Stephens FD. Supernumerary kidney. J Urol. 1983;130:649–653. doi: 10.1016/s0022-5347(17)51385-3. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- Ohiwa M, Murakami H, Iwashita T, Asai N, Iwata Y, Imai T, Funahashi H, Takagi H, Takahashi M. Characterization of Ret–Shc–Grb2 complex induced by GDNF, MEN 2A, and MEN 2B mutations. Biochem Biophys Res Commun. 1997;237:747–751. doi: 10.1006/bbrc.1997.7225. [DOI] [PubMed] [Google Scholar]
- Pachnis V, Mankoo B, Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development. 1993;119:1005–1017. doi: 10.1242/dev.119.4.1005. [DOI] [PubMed] [Google Scholar]
- Parisi MA, Kapur RP. Genetics of Hirschsprung disease. Curr Opin Pediatr. 2000;12:610–617. doi: 10.1097/00008480-200012000-00017. [DOI] [PubMed] [Google Scholar]
- Saarma M. GDNF—A stranger in the TGF-β superfamily? Eur J Biochem. 2000;267:6968–6971. doi: 10.1046/j.1432-1327.2000.01826.x. [DOI] [PubMed] [Google Scholar]
- Sainio K, Suvanto P, Davies J, Wartiovaara J, Wartiovaara K, Saarma M, Arumae U, Meng X, Lindahl M, Pachnis V, et al. Glial-cell-line-derived neurotrophic factor is required for bud initiation from ureteric epithelium. Development. 1997;124:4077–4087. doi: 10.1242/dev.124.20.4077. [DOI] [PubMed] [Google Scholar]
- Saxen L. Organogenesis of the kidney. Cambridge, UK: Cambridge University Press; 1987. [Google Scholar]
- Schedl A, Hastie ND. Cross-talk in kidney development. Curr Opin Genet Dev. 2000;10:543–549. doi: 10.1016/s0959-437x(00)00125-8. [DOI] [PubMed] [Google Scholar]
- Schuchardt A, D'Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Schuchardt A, D'Agati V, Pachnis V, Costantini F. Renal agenesis and hypodysplasia in ret-k-mutant mice result from defects in ureteric bud development. Development. 1996;122:1919–1929. doi: 10.1242/dev.122.6.1919. [DOI] [PubMed] [Google Scholar]
- Smith-Hicks CL, Sizer KC, Powers JF, Tischler AS, Costantini F. C-cell hyperplasia, pheochromocytoma and sympathoadrenal malformation in a mouse model of multiple endocrine neoplasia type 2B. EMBO J. 2000;19:612–622. doi: 10.1093/emboj/19.4.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Wu Z, Chen CM, D'Agati V, Costantini F. Dominant effects of RET receptor misexpression and ligand-independent RET signaling on ureteric bud development. Development. 1999;126:1375–1386. doi: 10.1242/dev.126.7.1375. [DOI] [PubMed] [Google Scholar]
- Tahira T, Ishizaka Y, Itoh F, Sugimura T, Nagao M. Characterization of ret proto-oncogene mRNAs encoding two isoforms of the protein product in a human neuroblastoma cell line. Oncogene. 1990;5:97–102. [PubMed] [Google Scholar]
- Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985;42:581–588. doi: 10.1016/0092-8674(85)90115-1. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Buma Y, Iwamoto T, Inaguma Y, Ikeda H, Hiai H. Cloning and expression of the ret proto-oncogene encoding a tyrosine kinase with two potential transmembrane domains. Oncogene. 1988;3:571–578. [PubMed] [Google Scholar]
- Taraviras S, Pachnis V. Development of the mammalian enteric nervous system. Curr Opin Genet Dev. 1999;9:321–327. doi: 10.1016/s0959-437x(99)80048-3. [DOI] [PubMed] [Google Scholar]
- Taraviras S, Marcos-Gutierrez CV, Durbec P, Jani H, Grigoriou M, Sukumaran M, Wang LC, Hynes M, Raisman G, Pachnis V. Signalling by the RET receptor tyrosine kinase and its role in the development of the mammalian enteric nervous system. Development. 1999;126:2785–2797. doi: 10.1242/dev.126.12.2785. [DOI] [PubMed] [Google Scholar]
- Tsuzuki T, Takahashi M, Asai N, Iwashita T, Matsuyama M, Asai J. Spatial and temporal expression of the ret proto-oncogene product in embryonic, infant and adult rat tissues. Oncogene. 1995;10:191–198. [PubMed] [Google Scholar]
- Vainio SJ, Itaranta PV, Perasaari JP, Uusitalo MS. Wnts as kidney tubule inducing factors. Int. J Dev Biol. 1999;43:419–423. [PubMed] [Google Scholar]
- van Weering DH, Bos JL. Signal transduction by the receptor tyrosine kinase Ret. Recent Results Cancer Res. 1998;154:271–281. doi: 10.1007/978-3-642-46870-4_18. [DOI] [PubMed] [Google Scholar]
- Yntema CL, Hammond WS. The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. J Comp Neurol. 1954;101:515–542. doi: 10.1002/cne.901010212. [DOI] [PubMed] [Google Scholar]
- Young HM, Hearn CJ, Farlie PG, Canty AJ, Thomas PQ, Newgreen DF. GDNF is a chemoattractant for enteric neural cells. Dev Biol. 2001;229:503–516. doi: 10.1006/dbio.2000.0100. .. [DOI] [PubMed] [Google Scholar]