Abstract
22q11 Deletion Syndrome (22q11DS) is a common microdeletion syndrome with multisystem expression. Phenotypic features vary with age, ascertainment, and assessment. We systematically assessed 78 adults (36 M, 42 F; mean age 31.5, SD 10.5 years) with a 22q11.2 deletion ascertained through an adult congenital cardiac clinic (n = 35), psychiatric-related sources (n = 39), or as affected parents of subjects (n = 4). We recorded the lifetime prevalence of features requiring attention, with 95% confidence intervals (CI) not overlapping zero. Subtle learning difficulties, hypernasality and facial gestalt were not included. We investigated ascertainment effects using non-overlapping subgroups ascertained with tetralogy of Fallot (n = 31) or schizophrenia (n = 31). Forty-three features met inclusion criteria and were present in 5% or more patients, including several of later onset (e.g., hypothyroidism, cholelithiasis). Number of features per patient (median 9, range 3–22) correlated with hospitalizations (P=0.0002) and, when congenital features were excluded, with age (P=0.02). Adjusting for ascertainment, 25.8% (95% CI, 9.5–42.1%) of patients had cardiac anomalies and 22.6% (95% CI, 7.0–38.2%) had schizophrenia. Ascertainment subgroups were otherwise similar in median number and prevalence of features. Non-characteristic features are common in 22q11DS. Adjusting for ascertainment effects is important. Many treatable conditions may be anticipated and features may accumulate over time. The results have implications for clinical assessment and management, genetic counseling and research into pathophysiological mechanisms.
Keywords: 22q11 deletion syndrome, adult phenotype, ascertainment, natural history, morbidity
INTRODUCTION
22q11 deletion syndrome (22q11DS) (OMIM #188400/#192430) is a multisystem disorder associated with chromosome 22q11.2 interstitial deletions. At an estimated prevalence of 1/4,000, 22q11DS is the most common microdeletion syndrome in humans [Goodship et al., 1998; Scambler, 2000]. Standard fluorescence in-situ hybridization (FISH) techniques detect the common 3.0 Mb, and nested 1.5 Mb, hemizygous deletions [Driscoll et al., 1993]. Most deletions occur de novo, with autosomal dominant inheritance observed in 15% or fewer cases [Swillen et al., 2000]. The phenotype is highly variable in severity but expression appears to be unrelated to the length of the deletion [Lindsay, 2001]. Accurate estimates of the prevalence of associated features are important for recognizing the syndrome, genetic counseling, and planning long-term health supervision of patients diagnosed with 22q11DS [McDonald-McGinn et al., 2001], and have implications for understanding pathophysiology. However, the true frequency of features is uncertain as rates vary with developmental stage [Vantrappen et al., 1999; Greenhalgh et al., 2003; Oskarsdottir et al., 2005], ascertainment, and completeness of assessment [Cohen et al., 1999].
Some characteristic features such as cardiac and other birth defects (e.g., congenital thymic hypoplasia), and neonatal hypocalcemia are recognizable in infancy. Others are usually identified later, including learning difficulties and the hypernasal speech often associated with velopharyngeal insufficiency and/or submucous cleft palate [Shprintzen et al., 1978]. Ascertainment of 22q11DS commonly involves major clinical syndromes which share overlapping groups of these characteristics and the 22q11.2 deletion. DiGeorge (DGS), velocardiofacial (VCFS), and conotruncal anomaly face syndromes all feature cardiovascular malformations (CVM), including tetralogy of Fallot [Matsuoka et al., 1998]. Rates of major CVM are routinely reported as >70% in 22q11DS [Ryan et al., 1997; Matsuoka et al., 1998; McDonald-McGinn et al., 1999], with somewhat lower rates observed in older children [Vantrappen et al., 1999; Greenhalgh et al., 2003; Oskarsdottir et al., 2005].
Studying adults could provide more information on the prevalence of lifetime features of 22q11DS. For example, a review of adult 22q11DS cases reported 36 (30%) of 120 patients had CVM [Cohen et al., 1999]. However, apart from a high prevalence of psychiatric illness, especially schizophrenia [Murphy et al., 1999], there is limited information on the adult phenotype [Cohen et al., 1999]. Also, there is an ascertainment bias to transmitting parents, who often have a mild phenotype [Ryan et al., 1997; Cohen et al., 1999; McDonald-McGinn et al., 2001]. Few studies document other ascertainment biases or involve systematic assessment of features.
We comprehensively investigated the lifetime prevalence of clinical features in 78 adults with 22q11DS. We hypothesized that features other than those characteristic of 22q11DS would be common, and that the rates of individual features would differ between two non-overlapping subgroups of patients ascertained with tetralogy of Fallot or schizophrenia.
METHODS
We investigated adults (≥17 years) diagnosed with 22q11DS and confirmed to have a chromosome 22q11.2 deletion by standard methods using metaphase chromosomes from peripheral blood lymphocytes and FISH techniques using a probe, most commonly TUPLE1 (Vysis) or N25 (ONCOR), from the commonly deleted 22q11.2 region [Driscoll et al., 1993]. Informed consent was obtained in writing, and the study approved by the Research Ethics Boards of the University of Toronto, Centre for Addiction and Mental Health, and University Health Network.
Sample
There were 78 (36 male, 42 female) patients: 72 (92.3%) were caucasian, three (3.9%) black, one (1.28%) Asian, and two (2.56%) other ethnicity (native-caucasian). We ascertained subjects through four methods: 35 patients attending the Toronto Congenital Cardiac Centre for Adults (TCCCA, a tertiary centre following adults with congenital cardiac defects, where we screen patients, primarily those with tetralogy of Fallot, for 22q11DS features); 39 patients through psychiatric-related sources: 31 diagnosed with schizophrenia, and 8 referred for psychiatric assessment of other disorders; and 4 parents of four other adults in the sample. All patients met clinical screening criteria for 22q11DS in adults: 2 or more of 7 broad categories of features (dysmorphic facies, hypernasal speech, history of learning difficulties, cardiac defect, thymic hypoplasia, other birth defect, or hypocalcemia) [Bassett and Chow, 1999]. 54 (69.2%) received a clinical diagnosis of 22q11DS after age 17 years. FISH was performed at a median age of 24 (range 7–67) years.
Evaluations
We used a combination of semi-structured direct interviews with patients and family members and comprehensive review of lifetime medical records, including pediatric records, to systematically evaluate medical, surgical, and psychiatric history. Only features assessed by a specialist and/or requiring significant investigation and/or management were included. Hospitalizations included documented emergency room visits and day surgery. We systematically compiled and coded diagnoses and results of investigations, including ionized calcium and thyroid stimulating hormone testing, electrocardiograms, echocardiograms, and abdominal ultrasounds for all patients. As previously described [Scutt et al., 2001; Bassett et al., 2003], we confirmed DSM-IV lifetime psychiatric diagnoses using standard methods, determined DSM-IV diagnosis of mental retardation from functioning and IQ testing results, assessed hypernasality of speech and directly measured height, weight, and maximum occiptofrontal head circumference. We determined body mass index (BMI) using the formula: weight (kg)/height (m)2; BMI ≥ 30 was considered obesity. Percentiles for height were assessed using norms for Caucasian North Americans (http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/datafiles.htm). Features directly assessed are noted in Table I.
TABLE I.
Lifetime Features in 78 Adults (36 M, 42 F, Mean Age 31.5, SD 10.5) With 22q11 Deletion Syndrome
| Featurea | Number | Sample | % | 95% CI
|
|
|---|---|---|---|---|---|
| Lower | Upperb | ||||
| General features | |||||
| Intellectual disability* (any degree) | 72 | 78 | 92.3 | 86.3 | 98.4 |
| Borderline intellect | 40 | 78 | 51.3 | 39.9 | 62.6 |
| Mild mental retardation | 26 | 78 | 33.3 | 22.6 | 44.0 |
| Moderate mental retardation | 6 | 78 | 7.7 | 1.6 | 13.7 |
| Microcephaly* (<3rd percentile) | 5 | 78 | 6.4 | 0.9 | 12.0 |
| Short stature* (<3rd percentile) | 16 | 78 | 20.5 | 11.3 | 29.7 |
| Obesity (BMI ≥ 30)* | 27 | 78 | 34.6 | 23.8 | 45.4 |
| Psychiatric | |||||
| Psychiatric disorder(s)*c,d | 18 | 31 | 58.1 | 39.7 | 76.5 |
| Schizophreniac | 7 | 31 | 22.6 | 7.0 | 38.2 |
| Neurologic | |||||
| Recurrent seizures | 31 | 78 | 39.7 | 28.6 | 50.8 |
| Epilepsy | 4 | 78 | 5.1 | 0.1 | 12.5 |
| Hemiparesis | 5 | 78 | 6.4 | 0.9 | 12.0 |
| Endocrinologic | |||||
| Hypocalcemia* | 50 | 78 | 64.1 | 53.2 | 75.0 |
| Neonatal hypocalcemia | 11 | 78 | 14.1 | 6.2 | 22.0 |
| Hypothyroidism* | 16 | 78 | 20.5 | 11.3 | 29.7 |
| Hyperthyroidism* | 4 | 78 | 5.1 | 0.1 | 12.5 |
| Palatal | |||||
| Submucous cleft palate and/or velopharyngeal insufficiency | 33 | 78 | 42.3 | 31.1 | 53.5 |
| Surgical repair(s) | 24 | 78 | 30.8 | 20.3 | 41.2 |
| Cardiovascular | |||||
| Cardiovascular malformations*e,f | 8 | 31 | 25.8 | 9.5 | 42.1 |
| Surgical repair(s)e | 6 | 31 | 19.4 | 4.6 | 34.1 |
| Varicose veins | 7 | 78 | 9.0 | 2.5 | 15.5 |
| Respiratory | |||||
| Recurrent pneumonia | 30 | 78 | 38.5 | 27.4 | 49.5 |
| Atelectasis | 16 | 78 | 20.5 | 11.3 | 29.7 |
| Asthma | 10 | 78 | 12.8 | 5.2 | 20.4 |
| Chronic obstructive pulmonary disease | 6 | 78 | 7.7 | 1.6 | 13.7 |
| Hematologic | |||||
| Thymic hypoplasia | 8 | 78 | 10.3 | 3.4 | 17.1 |
| Splenomegaly | 8 | 78 | 10.3 | 3.4 | 17.1 |
| Thrombocytopenia | 22 | 78 | 28.2 | 18.0 | 38.4 |
| Idiopathic thrombocytopenia | 5 | 78 | 6.4 | 0.9 | 12.0 |
| Anemia | 9 | 78 | 11.8 | 4.3 | 18.8 |
| Otorhinolaryngologic | |||||
| Recurrent or chronic otitis media | 27 | 78 | 34.6 | 23.8 | 45.4 |
| Surgical treatment(s) | 23 | 78 | 29.5 | 19.1 | 39.8 |
| Hearing deficit (any) | 22 | 78 | 28.2 | 18.0 | 38.4 |
| Recurrent/chronic sinusitis | 9 | 78 | 11.5 | 4.3 | 18.8 |
| Sialorrhea | 5 | 78 | 6.4 | 0.9 | 12.0 |
| General surgery | |||||
| Hernia repair(s): inguinal, umbilical, and/or femoral | 17 | 78 | 21.8 | 12.4 | 31.2 |
| Pilonidal sinus, cyst or abcess surgery | 5 | 78 | 6.4 | 0.9 | 12.0 |
| Gastrointestinal | |||||
| Cholelithiasis, cholecystitis | 15 | 78 | 19.2 | 10.3 | 28.2 |
| Cholecystectomy | 10 | 78 | 12.8 | 5.2 | 20.4 |
| Gastroesophageal reflux | 6 | 78 | 7.7 | 1.6 | 13.7 |
| Anal fissure(s) | 6 | 78 | 7.7 | 1.6 | 13.7 |
| Fatty liver | 5 | 78 | 6.4 | 0.9 | 12.0 |
| Urogenital | |||||
| Renal agenesis* | 5 | 78 | 6.4 | 0.9 | 12.0 |
| Hydronephrosis/hydroureter | 6 | 78 | 7.7 | 1.6 | 13.7 |
| Renal failure | 8 | 78 | 10.3 | 3.4 | 17.1 |
| Hydroceleg | 5 | 36 | 13.9 | 2.0 | 25.8 |
| Ovarian cyst(s)h | 7 | 42 | 16.7 | 4.9 | 28.4 |
| Musculoskeletal | |||||
| Scoliosis | 37 | 78 | 47.4 | 36.1 | 58.8 |
| Surgically treated | 5 | 78 | 6.4 | 0.9 | 12.0 |
| Patellar dislocation | 9 | 78 | 11.5 | 4.3 | 18.8 |
| Degenerative disc disease | 6 | 78 | 7.7 | 1.6 | 13.7 |
| Ophthalmologic | |||||
| Strabismus, including duane syndrome | 12 | 78 | 15.4 | 7.2 | 23.6 |
| Nasolacrimal duct occlusion requiring surgical probing | 4 | 78 | 5.1 | 0.1 | 12.5 |
| Dermatologic | |||||
| Severe acne | 18 | 78 | 23.1 | 13.5 | 32.6 |
| Seborrhea/dermatitis | 27 | 78 | 34.6 | 23.8 | 45.4 |
Features considered characteristic of 22q11DS commonly used to make a syndrome diagnosis are highlighted in italics; these do not include common but highly variable features: typical facies, hypernasal speech, or learning difficulties. Indented features are subsets of the feature listed immediately above. An asterisk (*) indicates features directly assessed.
Upper limit of CI adjusted for frequency <5; see Methods.
Tetralogy of Fallot ascertainment subgroup only.
Schizophrenia, major depression, anxiety disorders, attention deficit hyperactivity disorder, impulse control disorders, substance use disorders.
Schizophrenia ascertainment subgroup only.
Tetralogy of Fallot, ventricular septal defect (VSD) with atrial septal defect, VSD.
Males only.
Females only.
Statistical Analysis
Features recorded in four or more (≥5%) patients were included in this study; lower frequencies were considered insufficient to provide reliable estimates. Broad 22q11DS clinical screening criteria (dysmorphic facies, hypernasal speech, non-specific learning difficulties) were excluded, as were common but non-specific features like headache or constipation, and post-operative complications. In all, over 200 features, mainly due to low frequency, were excluded. Of the 43 features included (see Table I), all were considered to be observable and/or potentially requiring monitoring or other medical attention in adulthood. To assess the effects of major ascertainment biases, we used two non-overlapping subgroups: 31 TCCCA patients with tetralogy of Fallot (16 male, 15 female, mean age 28.1 SD 7.9 years) and 31 patients ascertained with schizophrenia (16 male, 15 female, mean age 34.8 SD 8.6 years); small sample size precluded using other subgroups.
Ninety-five percent confidence intervals (CI) for frequency of features, with adjustments for frequencies <5, were calculated [Richardson et al., 2000]. Chi-square or two-tailed Fisher’s exact tests were used to compare these frequencies and categorical variables between subgroups of patients. Wilcoxon rank sum tests and Spearman rank correlations were used to compare ordinal data, and two-tailed Student’s t tests for continuous variables, using SAS 8.2 (SAS Institute, Cary, NC); P values <0.05 were considered significant.
RESULTS
Lifetime Features of 22q11DS
Forty-three lifetime features, and 12 subcategories of these features, met our inclusion criteria. Their respective prevalences with 95% CI are presented in Table I, grouped into 15 areas representing most medical and surgical specialties. The features included 38 that were not part of typical clinical criteria for 22q11DS. There were no significant differences in frequency of features between males and females (results not shown), except for hernias (14 males, 3 females; P = 0.0009). Excluding neonatal hypocalcemia which may resolve in infancy [Weinzimer, 2001], non-neonatal hypocalcemia was detected in 50% (95% CI, 38.7–61.3%) of patients in this sample. Median ages of onset (and ranges) in years were consistently available for this and three other later onset conditions: schizophrenia 21 (14–36), hypothyroidism 24 (8–56), non-neonatal hypocalcemia 25 (5–48), and cholelithiasis requiring cholecystectomy 27.5 (15–56).
Other Clinical Findings
All patients had facial features of 22q11DS, ranging from subtle to characteristic, as previously reported [Bassett et al., 1998; Scutt et al., 2001]. Most (89.7%) had some hypernasality of speech. Estimated IQ ranged from 45 to 95 (mean 71.8; SD 11.3), although all patients had a history of some learning difficulties in school. Patients had a median of 6 (range 0–19) documented non-psychiatric hospitalizations, of which a median of 1 (range 0–10) occurred at age 17 years or older. There were three (3.8%) deaths: two female patients at 18 and 68 years, neither of whom had a major congenital cardiac defect, and a 23-year-old man with tetralogy of Fallot. Causes of death were: shock secondary to acute pancreatitis after elective endoscopic retrograde cholangiopancreatography, stroke, and arrhythmia, respectively.
Ascertainment Effects
As expected, after adjusting for ascertainment source, rates of patients with major CVM (25.8%; 95% CI 9.5, 42.1) and schizophrenia (22.6%; 95% CI 7.0, 38.2) were lower than unadjusted frequencies in the total sample: 41/78 (52.6%) and 39/78 (50.0%), respectively. However, there were no significant differences in frequencies of other individual features between the two ascertainment subgroups (results not shown), despite older age of the schizophrenia group (t = −3.20, P =0.002). There were also no significant differences between the tetralogy of Fallot and schizophrenia ascertainment subgroups, respectively, in mean percentile for height (19.2 SD 19.4, 18.0 SD 19.9; t = 0.24, P = 0.81), BMI (27.1 SD 4.5, 27.3 SD 4.5; t = −0.09, P = 0.93), or estimated IQ (71.8 SD 11.0, 67.8 SD 9.4; t = −1.56, P = 0.12).
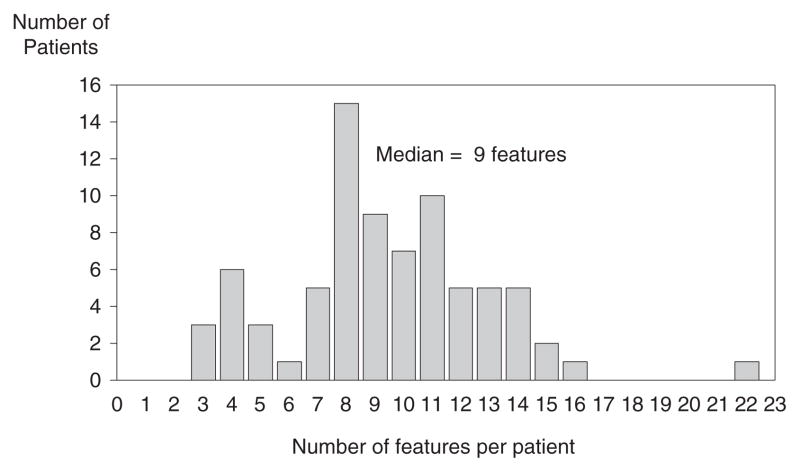
Number of Lifetime Features Per Patient
Figure 1 shows the distribution of features per patient. Patients had a median of 9 (range 3–22) of the 43 clinical features studied. The patient with the most features (22) died at age 18 years. There were no significant differences in median number of features per patient between males (n = 10) and females (n = 9), or between the two ascertainment subgroups (tetralogy of Fallot n = 10; schizophrenia n = 10). The number of features per patient was significantly correlated with the number of lifetime hospitalizations (Spearman rho = 0.41; P = 0.0002). After excluding constitutional features (short stature, microcephaly) and major congenital anomalies (CVM, thymic hypoplasia, renal agenesis), the number of features per patient also showed a modest correlation with age at assessment (Spearman rho = 0.27; P = 0.02).
Fig. 1.
Frequency of number of lifetime features per patient with 22q11DS.
DISCUSSION
This is the largest sample of adults with 22q11DS investigated to date and the first to systematically assess multiple features, present CI for frequencies meeting standard criteria for significance (95% CI not overlapping zero), and assess potential effects of ascertainment. The methods used increase the probability that the features included are directly or indirectly related to syndrome expression rather than co-occurring by chance alone. The results thus provide an overview of 43 conditions commonly encountered in 22q11DS that would pertain to individuals who meet conventional clinical criteria for the syndrome and live to adulthood. The lifetime burden of illness in 22q11DS appears substantial, and includes congenital and later onset conditions, many chronic disorders and risk factors for other conditions. However, many of the associated conditions are treatable once detected, highlighting the importance of appropriate follow-up.
Effects of Ascertainment and Age
As expected, we detected marked ascertainment bias effects with respect to the features directly related to the sampling strategies used. Adjusted rates of both CVM and schizophrenia were about half the unadjusted rates. The adjusted rate of CVM observed is consistent with studies involving adults or patients with velopharyngeal insufficiency [Cohen et al., 1999; Boorman et al., 2001; Shooner et al., 2005], while the unadjusted rate is more comparable to reported rates using samples that may have predominantly originated through congenital cardiac services [Ryan et al., 1997; Matsuoka et al., 1998; McDonald-McGinn et al., 1999]. The adjusted rate of schizophrenia observed is consistent with smaller samples of individuals over age 17 years using systematic [Momma et al., 2001] or diverse ascertainment [Murphy et al., 1999]. The results confirm schizophrenia as a common characteristic of 22q11DS.
None of the 41 other features showed significantly different rates between the two ascertainment subgroups although sample sizes would have limited power to detect differences for features with lower frequencies. There was also no difference in quantitative measures assessed or median number of features between the two subgroups. Patients who were older at assessment had significantly more non-congenital features, indicating that, even within an adult sample, age appears to be a factor in expression of 22q11DS.
Specific Features
The significant morbidity of the associated features is indicated by the number of features per patient, the lifelong clinical implications of many of these, and the high correlation with number of hospitalizations, predominantly in the pediatric age group. As expected, given the clinical diagnostic strategy, five of the 43 features studied (intellectual disabilities, hypocalcemia, palatal anomalies, CVM, and thymic hypoplasia) are characteristic features of 22q11DS. The high lifetime rate of hypocalcemia is consistent with previous reports, including late-occurring onset or recurrence [Ryan et al., 1997; Brauner et al., 2003; Greenhalgh et al., 2003]. Routine assessment through our clinic contributed to the high prevalence and late median age at onset/detection. Hypocalcemia in 22q11DS, which is usually due to functional hypoparathyroidism, likely contributed to the high rate of recurrent seizures documented [Ryan et al., 1997]. A generally lowered seizure threshold may also be part of the central nervous system involvement in the 22q11DS phenotype. Psychiatric disorders are common in 22q11DS, especially schizophrenia but including an array of other disorders such as attention deficit hyperactivity disorder, mood and anxiety disorders [Bassett et al., 1998; Cohen et al., 1999; Murphy et al., 1999]. Psychiatric illness and learning difficulties represent important contributors to functional disability in 22q11DS [Swillen et al., 2000; Momma et al., 2001], and may interfere with obtaining appropriate medical care and maintaining compliance with recommended management.
Many of the other features observed are commonly reported ancillary findings in 22q11DS, including infections (pneumonia and otitis media), scoliosis, thrombocytopenia, hernias, and short stature [Ryan et al., 1997; Matsuoka et al., 1998; McDonald-McGinn et al., 1999; Swillen et al., 2000]. Other features, such as obesity, hypothyroidism, hearing deficits, and dermatologic conditions have been reported less frequently [Wilson et al., 1993; Lindsay et al., 1995; Bassett et al., 1998; Digilio et al., 2001], perhaps due to later onset, lesser severity, and/or lack of recording.
Limitations
We recognize the limitations of this cross-sectional study. Larger, prospective studies would provide more details about onset of conditions, changes with development, relationships between features, effects of medications, lifestyle and other factors, and death as an outcome in 22q11DS. Each feature warrants individual study. Detailed molecular studies would be needed to determine if there were length of deletion or parent of origin effects, although most studies have not found these [Scambler, 2000; Lindsay, 2001]. Incorporation bias effects [Richardson et al., 2000] would predict that rates for features commonly used in case finding for 22q11DS may be overestimates for the total population of adults with 22q11DS. On the other hand, frequencies reported in Table I may represent underestimates for features associated with early mortality or those not systematically assessed using specific investigations, e.g., nasendoscopy for velopharyngeal insufficiency. Infrequent features, such as overt cleft palate (n = 2/78), were not reported. We are confident however that features directly assessed and those contributing to hospitalization and/or surgery were recorded, given access to universal health care in Canada and diligence in obtaining lifetime medical records. There is a wide variability of severity of features reported. This is consistent with previous studies of 22q11DS which show variable expression in both severity of individual features and the number of features observed, even within families or between identical twins [Vincent et al., 1999]. Some features (e.g., obesity, hypothyroidism) are common in aneuploidies [Conway, 2004], and many are common in the general population. Further studies would be needed to determine sensitivity and specificity of individual features to the diagnosis of 22q11DS. For example, schizophrenia is rarely associated with other genetic syndromes but is 20–25 times more common in 22q11DS than in the general population [Bassett et al., 2000]. Demographically comparable general population frequencies would be needed to derive relative risks for 22q11DS. The prevalences of most of the features reported would, however, appear to exceed general population expectations for young to middle-aged adults. The long term outcome of older individuals remains unknown.
Implications
This study provides an overview of features likely to be found in young to middle-aged adults with 22q11DS over their lifetime. The phenotype is broader than the characteristic features usually associated with the syndrome, and several features would not be apparent at young ages and/or with limited assessments. The results provide a sense of the frequency of commonly associated conditions that case reports, questionnaire surveys and studies of infants cannot. The variable expression and incomplete penetrance suggest it may be difficult to denote a particular phenotype as “atypical.” The clinical heterogeneity of 22q11DS and limited data on later onset manifestations have contributed to its under-recognition. The results could thus be useful for suggesting new patient populations in which to consider 22q11DS, if suggestive facial features (see Fig. 2), hypernasal voice, and learning difficulties are present.
Fig. 2.

Adult patient with 22q11DS.
The results reinforce the need for a thorough assessment at diagnosis, genetic counseling, and long term follow-up with active monitoring for frequently associated conditions, most of which are readily treatable once identified. We have suggested some guidelines for the assessment and follow-up of adults with 22q11DS in Table II. As for other syndromes [Conway, 2004], specialty clinics for patients with 22q11DS and a multidisciplinary approach could help individual physicians coordinate care and provide necessary long term follow-up, systematic monitoring, and interventions appropriate to the patient’s developmental stage and manifestations [Swillen et al., 2000; Greenhalgh et al., 2003; Oskarsdottir et al., 2005].
TABLE II.
Proposed Initial Assessments and Health Monitoring for Adults With 22q11DS
| Baseline work-up at adult diagnosis of 22q11DS | Initial adult assessment at transition (childhood diagnosis of 22q11DS) | Annual or biennial follow-up as an adult | |
|---|---|---|---|
| Recommended clinical assessments | Comprehensive medical history; psychiatric assessment; cognitive assessment; family history; physical examination; BMI | Comprehensive medical history; psychiatric assessment; update family history; physical examination; BMI | Systems review; interim medical, surgical, psychiatric, family history; targeted physical examination; BMI |
| Recommended genetic assessments and management | Consultation with medical geneticist and/or clinic experienced in 22q11DS; FISH for 22q11.2 deletion and karyotype; genetic counseling for proband; genetic counseling and testing for parents (siblings if parents unavailable) and offspring | Genetic counseling update; family planning and prenatal counseling, as needed | Genetic counseling update; family planning and prenatal counseling, as needed |
| Recommended investigations | Complete blood count; TSH; pH-corrected ionized calcium, PTH; creatinine; abdominal ultrasound; echocardiogram | Complete blood count; TSH; pH-corrected ionized calcium, PTH; creatinine | Complete blood count; TSH; pH-corrected ionized calcium, PTH; creatinine; others as needed based on history and physical examination; Seizure work-up to include pH-corrected ionized calcium, magnesium |
| Consider | ECG; audiological assessment; EEG; brain MRI; cervical spine x-rays | Cognitive reassessment | Fasting glucose and lipid profile; ECG; pulmonary function studies |
| As needed | Endocrinological consultation; psychiatric consultation; other consultations | Relevant/targeted investigations; audiological assessment; ECG; EEG; abdominal ultrasound; antibiotic prophylaxis (if CVM); diet/exercise counseling; endocrinological consultation; psychiatric consultation; other consultations; vocational counseling; life skills assessment; disability pension |
The results of this study also have implications for genetic counseling in 22q11DS. In particular, counseling about common later onset conditions, including psychiatric disorders, and the importance of early detection and treatment will represent an important addition to standard practice [Hodgkinson et al., 2001]. The results from this study and others indicate that the majority of patients with 22q11DS do not have major cardiac anomalies [Cohen et al., 1999; Boorman et al., 2001] and most survive childhood [Ryan et al., 1997; McDonald-McGinn et al., 1999]. It therefore appears likely that general population prevalence rates reported for 22q11DS [Goodship et al., 1998], which are largely based on major cardiac and other anomalies leading to molecular testing, are underestimates [Liling et al., 1999].
The frequency and medical importance of associated features invite further study to determine their origins. Most of the dozens of genes in the 22q11.2 region are well characterized [Scambler, 2000; Lindsay, 2001; Maynard et al., 2003], and recent studies are advancing our understanding of the pathogenesis of specific congenital features of 22q11DS [Yagi et al., 2003; Liao et al., 2004]. However, much of the mechanism of expression of 22q11DS remains unknown. Some of the lifetime features of 22q11DS may be long term consequences of developmental abnormalities, including those of the neural crest [Budarf and Emanuel, 1997]. Endocrinological disorders may involve functional disruption of the pharyngeal apparatus [Lindsay, 2001], and/or autoimmune mechanisms related to abnormal thymic development [Weinzimer, 2001]. Schizophrenia and other neuropsychiatric aspects may involve disrupted neurodevelopment and neuronal function [Maynard et al., 2003]. However, developmental and functional anomalies of multiple other systems may underlie other features. Models of 22q11DS will need to take into account the broader phenotype to improve representativeness [Lindsay, 2001; Maynard et al., 2003].
In summary, an increased index of suspicion for 22q11DS, informed management of those diagnosed, and further longitudinal studies could benefit not only patients and their families but could also help further our understanding of the complex pathogenesis of this common condition.
Acknowledgments
The authors thank the patients and their families for their participation, colleagues for referring patients, Dr. Nighat Parveen and Dr. Tahir Tayyeb, and Mirona Gheorghiu, Heather Dorman, Linda Chiu, Sheri O’Neill, Jillian Murphy, Mark Watson, and Laura Scutt, who assisted in the collection of data for the study, Vivian Wong for statistical assistance, and Dr. Patricia Heard and Dr. Gary Costain for reviewing versions of the manuscript. This research was supported by grants from the W. Garfield Weston Foundation, Ontario Mental Health Foundation, Medical Research Council of Canada (MOP-38099), and by a Distinguished Investigator Award from the National Alliance for Research on Schizophrenia and Depression (ASB) and Canada Research Chair in Schizophrenia Genetics (ASB).
References
- Bassett AS, Chow EWC. 22q11 Deletion syndrome: A genetic subtype of schizophrenia. Biol Psychiatry. 1999;46:882–891. doi: 10.1016/s0006-3223(99)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Hodgkinson K, Chow EWC, Correia S, Scutt LE, Weksberg R. 22q11 deletion syndrome in adults with schizophrenia. Am J Med Genet (Neuropsychiatr Genet) 1998;81:328–337. [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Chow EWC, Weksberg R. Chromosomal abnormalities and schizophrenia. Am J Med Genet (Semin Med Genet) 2000;97:45–51. doi: 10.1002/(sici)1096-8628(200021)97:1<45::aid-ajmg6>3.0.co;2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Chow EWC, AbdelMalik P, Gheorghiu M, Husted J, Weksberg R. The schizophrenia phenotype in 22q11 deletion syndrome. Am J Psychiatry. 2003;160:1580–1586. doi: 10.1176/appi.ajp.160.9.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman JG, Varma S, Ogilvie CM. Velopharyngeal incompetence and chromosome 22q11 deletion. Lancet. 2001;357:774. doi: 10.1016/S0140-6736(00)04183-0. [DOI] [PubMed] [Google Scholar]
- Brauner R, Harivel de Gonneville Al, Kindermans C, Bidois Jl, Prieur M, Lyonnet S, Souberbielle J-C. Parathyroid function and growth in 22q11.2 deletion syndrome. J Pediatr. 2003:504–508. doi: 10.1067/mpd.2003.156. [DOI] [PubMed] [Google Scholar]
- Budarf ML, Emanuel BS. Progress in the autosomal segmental aneusomy syndromes (SASs): Single or multi-locus disorders? Hum Mol Genet. 1997;6:1657–1665. doi: 10.1093/hmg/6.10.1657. [DOI] [PubMed] [Google Scholar]
- Cohen E, Chow EWC, Weksberg R, Bassett AS. Phenotype of adults with the 22q11 deletion syndrome: A review. Am J Med Genet. 1999;86:359–365. doi: 10.1002/(sici)1096-8628(19991008)86:4<359::aid-ajmg10>3.0.co;2-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway GS. Considerations for transition from paediatric to adult endocrinology: Women with Turner’s syndrome. Growth Horm IGF Res. 2004;14:77–84. doi: 10.1016/j.ghir.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Digilio MC, Marino B, Cappa M, Cambiaso P, Giannotti A, Dallapiccola B. Auxological evaluation in patients with DiGeorge/velocardiofacial syndrome (deletion 22q11.2 syndrome) Genet Med. 2001;3:30–33. doi: 10.1097/00125817-200101000-00007. [DOI] [PubMed] [Google Scholar]
- Driscoll DA, Salvin J, Sellinger B, Budarf ML, McDonald-McGinn DM, Zackai EH, Emanuel BS. Prevalence of 22q11 microdeletions in DiGeorge and velocardiofacial syndromes: Implications for genetic counselling and prenatal diagnosis. J Med Genet. 1993;30:813–817. doi: 10.1136/jmg.30.10.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodship J, Cross I, LiLing J, Wren C. A population study of chromosome 22q11 deletions in infancy. Arch Dis Child. 1998;79:348–351. doi: 10.1136/adc.79.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh KL, Aligiania IA, Bromilow G, Cox H, Stait Y, Leech BJ, Lunt PW. 22q11 deletion: A multisystem disorder requiring multi-disciplinary input. Arch Dis Child. 2003;88:523–524. doi: 10.1136/adc.88.6.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson K, Murphy J, O’Neill S, Brzustowicz L, Bassett AS. Genetic counselling for schizophrenia in the era of molecular genetics. Can J Psychiatry. 2001;46:123–130. doi: 10.1177/070674370104600202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Kochilas L, Nowotschin S, Arnold JS, Aggarwal VS, Epstein JA, Brown MC, Adams J, Morrow BE. Full spectrum of malformations in velo-cardio-facial syndrome/DiGeorge syndrome mouse models by altering Tbx1 dosage. Hum Mol Genet. 2004;13:1577–1585. doi: 10.1093/hmg/ddh176. [DOI] [PubMed] [Google Scholar]
- Liling J, Cross I, Burn J, Daniel CP, Tawn EJ, Parker L. Frequency and predictive value of 22q11 deletion. J Med Genet. 1999;36:794–795. doi: 10.1136/jmg.36.10.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay EA. Chromosomal microdeletions: Dissecting Del22q11 syndrome. Nat Rev Genet. 2001;2:858–868. doi: 10.1038/35098574. [DOI] [PubMed] [Google Scholar]
- Lindsay EA, Greenberg F, Shaffer LG, Shapira SK, Scambler PJ, Baldini A. Submicroscopic deletions at 22q11.2: Variability of the clinical picture and delineation of a commonly deleted region. Am J Med Genet. 1995;56:191–197. doi: 10.1002/ajmg.1320560216. [DOI] [PubMed] [Google Scholar]
- Matsuoka R, Kimura M, Scambler PJ, Morrow BE, Imamura S, Minoshima S, Shimizu N. Molecular and clinical study of 183 patients with conotruncal anomaly face syndrome. Hum Genet. 1998;103:70–80. doi: 10.1007/s004390050786. [DOI] [PubMed] [Google Scholar]
- Maynard TM, Haskell GT, Peters AZ, Sikich L, Liberman JA, LaMantia A-S. A comprehensive analysis of 22q11 gene expression in the developing and adult brain. Proc Natl Acad Sci USA. 2003;100:14433–14438. doi: 10.1073/pnas.2235651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Kirschner RE, Goldmuntz E, Sullivan K, Eicher P, Gerdes M, Moss E, Solot C, Wang P, Jacobs I, Handler S, Knightly C, Heher K, Wilson M, Ming JE, Grace K, Driscoll D, Pasquariello P, Randall P, Larossa D, Emanuel BS, Zackai EH. The Philadelphia story: The 22q11.2 deletion: Report on 250 patients. Genet Couns. 1999;10:11–24. [PubMed] [Google Scholar]
- McDonald-McGinn DM, Tonnesen MK, Laufer-Cahana A, Finucane B, Driscoll DA, Emanuel BS, Zachai EH. Phenotype of the 22q11.2 deletion in individuals identified through an affected relative: Cast a wide FISHing net! Genet Med. 2001;3:23–29. doi: 10.1097/00125817-200101000-00006. [DOI] [PubMed] [Google Scholar]
- Momma K, Takao A, Matsuoka R, Imai Y, Muto A, Osawa M, Takayama M. Tetralogy of Fallot associated with chromosome 22q11.2 deletion in adolescents and young adults. Genet Med. 2001;3:56–60. doi: 10.1097/00125817-200101000-00012. [DOI] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56:940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- Oskarsdottir S, Persson C, Eriksson BO, Fasth A. Presenting phenotype in 100 children with the 22q11 deletion syndrome. Eur J Pediatr. 2005;164:146–153. doi: 10.1007/s00431-004-1577-8. [DOI] [PubMed] [Google Scholar]
- Richardson WS, Wilson MC, Williams JWJ, Moyer VA, Naylor CD. Users’ guides to the medical literature: XXIV. How to use an article on the clinical manifestations of disease. J Am Med Assoc. 2000;284:869–875. doi: 10.1001/jama.284.7.869. [DOI] [PubMed] [Google Scholar]
- Ryan AK, Goodship JA, Wilson DI, Philip N, Levy A, Seidel H, Schuffenhauer S, Oechsler H, Belohradsky B, Prieur M, Aurias A, Raymond FL, Clayton-Smith J, Hatchwell E, McKeown C, Brueton L, Brøndum-Nielsen K, Stewart F, Essen TV, Patton M, Paterson J, Scambler PJ. Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: A European collaborative study. J Med Genet. 1997;34:798–804. doi: 10.1136/jmg.34.10.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scambler PJ. The 22q11 deletion syndromes. Hum Mol Genet. 2000;9:2421–2426. doi: 10.1093/hmg/9.16.2421. [DOI] [PubMed] [Google Scholar]
- Scutt L, Chow EWC, Weksberg R, Honer WG, Bassett AS. Patterns of dysmorphic features in schizophrenia. Am J Med Genet (Neuropsychiatr Genet) 2001;105:713–723. doi: 10.1002/ajmg.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shooner KA, Rope AF, Hopkin RJ, Andelfinger GU, Benson DW. Genetic analyses in two extended families with deletion 22q11 syndrome: Importance of extracardiac manifestations. J Pediatr. 2005;146:382–387. doi: 10.1016/j.jpeds.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ, Goldberg RB, Lewin ML, Sidoti EJ, Berkman MD, Argamaso RV, Young D. A new syndrome involving cleft palate, cardiac anomalies, typical facies, and learning disabilities: Velo-cardio-facial syndrome. Cleft Palate J. 1978;15:56–62. [PubMed] [Google Scholar]
- Swillen A, Vogels A, Devriendt K, Fryns JP. Chromosome 22q11 Deletion Syndrome: Update and review of the clinical features, cognitive-behavioral spectrum, and psychiatric complications. Am J Med Genet (Semin Med Genet) 2000;97:128–135. doi: 10.1002/1096-8628(200022)97:2<128::aid-ajmg4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Vantrappen G, Devriendt K, Swillen A, Rommel N, Vogels A, Eyskens B, Gewillig M, Feenstra L, Fryns JP. Presenting symptoms and clinical features in 130 patients with velo-cardio-facial syndrome. The Leuven experience. Genet Couns. 1999;10:3–9. [PubMed] [Google Scholar]
- Vincent M-C, Heitz F, Tricoire J, Bourrouillou G, Kuhlein E, Rolland M, Calvas P. 22q11 deletion in DGS/VCFS monozygotic twins with discordant phenotypes. Genet Couns. 1999;10:43–49. [PubMed] [Google Scholar]
- Weinzimer SA. Endocrine aspects of the 22q11.2 deletion syndrome. Genet Med. 2001;3:19–22. doi: 10.1097/00125817-200101000-00005. [DOI] [PubMed] [Google Scholar]
- Wilson DI, Burn J, Scambler P, Goodship J. DiGeorge syndrome: Part of CATCH 22. J Med Genet. 1993;30:852–856. doi: 10.1136/jmg.30.10.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi H, Furutani Y, Hamada H, Sasaki T, Asakawa S, Minoshima S, Ichida F, Joo K, Kimura M, Imamura S, Kamatani N, Momma K, Takao A, Nakazawa M, Shimizu N, Martsuoka R. Role of TBX1 in human del22q11.2 syndrome. Lancet. 2003;362:1366–1373. doi: 10.1016/s0140-6736(03)14632-6. [DOI] [PubMed] [Google Scholar]