Abstract
The balance between excitatory and inhibitory synapses is crucial for normal brain function. Wnt proteins stimulate synapse formation by increasing synaptic assembly. However, it is unclear whether Wnt signaling differentially regulates the formation of excitatory and inhibitory synapses. Here, we demonstrate that Wnt7a preferentially stimulates excitatory synapse formation and function. In hippocampal neurons, Wnt7a increases the number of excitatory synapses, whereas inhibitory synapses are unaffected. Wnt7a or postsynaptic expression of Dishevelled-1 (Dvl1), a core Wnt signaling component, increases the frequency and amplitude of miniature excitatory postsynaptic currents (mEPSCs), but not miniature inhibitory postsynaptic currents (mIPSCs). Wnt7a increases the density and maturity of dendritic spines, whereas Wnt7a-Dvl1–deficient mice exhibit defects in spine morphogenesis and mossy fiber-CA3 synaptic transmission in the hippocampus. Using a postsynaptic reporter for Ca2+/Calmodulin-dependent protein kinase II (CaMKII) activity, we demonstrate that Wnt7a rapidly activates CaMKII in spines. Importantly, CaMKII inhibition abolishes the effects of Wnt7a on spine growth and excitatory synaptic strength. These data indicate that Wnt7a signaling is critical to regulate spine growth and synaptic strength through the local activation of CaMKII at dendritic spines. Therefore, aberrant Wnt7a signaling may contribute to neurological disorders in which excitatory signaling is disrupted.
Keywords: Wnt7a, Dvl1 mutant, plasticity
The formation of functional neuronal circuits requires the assembly of different types of synapses with great specificity. The development of an appropriate balance of glutamatergic excitatory and GABAergic inhibitory synapses (E/I ratio) is essential for proper circuit function (1, 2) because an imbalance in the E/I ratio can result in neurological disorders (3–5). Some synaptogenic factors regulate both excitatory and inhibitory synapses (6, 7), whereas other synaptic organizers are more specific (8–10). However, the precise mechanisms by which synaptic organizing signals regulate excitatory and inhibitory synapses remain poorly understood.
In the central nervous system, most excitatory inputs are located on dendritic spines, postsynaptic protrusions that function as domains where synaptic activity is regulated in a compartmentalized manner (11–13). Several intracellular molecules have been implicated in the formation and maturation of dendritic spines (14–16), but the mechanisms by which extracellular factors signal through intracellular regulators to promote spine development and maturation remain poorly characterized.
Wnt secreted proteins are synaptic organizers that stimulate the formation of central and peripheral synapses (17–19) by promoting presynaptic assembly (20) and the clustering of postsynaptic components (19, 21–24). In cultured neurons, Wnt5a regulates postsynaptic development of both GABAergic and glutamatergic synapses (24, 25). However, it is unclear whether other Wnts play a more selective role in promoting the formation of specific synapses and, therefore, regulating the balance between excitatory and inhibitory inputs.
Here, we demonstrate that Wnt7a signaling specifically regulates the formation and function of excitatory synapses without affecting inhibitory synapses in the hippocampus. Wnt7a signals directly to the postsynaptic dendrite to stimulate spine growth and synaptic strength. Dishevelled-1 (Dvl1), a cytoplasmic core Wnt signaling component that promotes the activation of the Wnt cascade at specific cellular locale (26), is present at the postsynapse and is required for Wnt7a function. Analyses of Wnt signaling-deficient mutant mice demonstrate that Wnt7a-Dvl1 signaling is required for spine morphogenesis and glutamatergic transmission in the hippocampus. Expression of PSD-95-Vim-CFP, a synaptic read-out of Ca2+/Calmodulin-dependent protein kinase II (CaMKII) activity, demonstrates that Wnt7a activates CaMKII at dendritic spines. Importantly, CaMKII is required for Wnt-mediated spine growth and increased synaptic strength. These findings reveal a unique role for Wnt7a signaling in promoting the formation and function of excitatory synapses through CaMKII, a key molecule that regulates the functional and morphological plasticity of synapses.
Results
Wnt7a Specifically Increases the Number and Function of Excitatory Synapses.
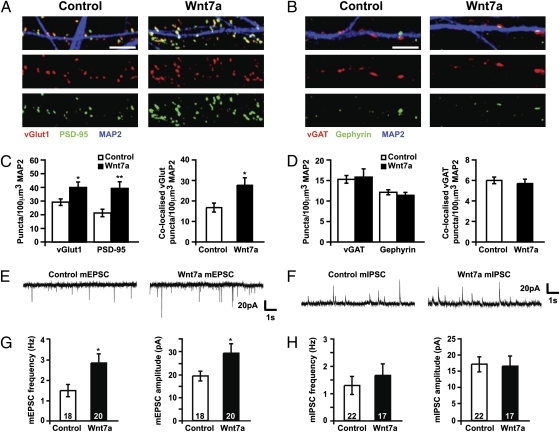
To address the role of Wnts in the formation of specific synapse types, we focused on the hippocampus, because Wnt7a/b protein is present in neurons of the dentate gyrus, CA1, and CA3 at the peak of synaptogenesis (Fig. S1). We therefore examined whether Wnt7a stimulates excitatory and inhibitory synapse formation in cultured hippocampal neurons at this developmental stage. Wnt7a increases the density of vGlut and PSD-95 puncta (excitatory pre- and postsynaptic markers, respectively) within 1 h, with further increases after 3 and 16 h treatment (Fig. 1 A and C and Fig. S2A). In contrast, the density of vGAT and Gephyrin puncta (inhibitory pre- and postsynaptic markers, respectively) are unchanged at all time-points studied (Fig. 1 B and D and Fig. S2B). Crucially, the density of vGlut puncta apposed to PSD-95 (putative excitatory synapses) displays a robust time-dependent increase after Wnt7a treatment, whereas the density of putative inhibitory synapses (vGAT puncta apposed to Gephyrin) is unchanged (Fig. 1 C and D and Fig. S2A and Fig. S2B). Consistently, whole-cell patch-clamp recordings show that 3-h Wnt7a treatment increases the frequency and amplitude of mEPSCs (Fig. 1 E and G), whereas mIPSCs are unaffected (Fig. 1 F and H). Together our results show that Wnt7a specifically increases the number and strength of excitatory synapses, while leaving inhibitory synapses unaffected.
Fig. 1.
Wnt7a specifically stimulates the formation and function of excitatory synapses. (A and B) Fourteen days in vitro (DIV) hippocampal culture were treated for 3 h with Wnt7a. Excitatory presynaptic (vGlut1) and postsynaptic (PSD-95) sites (A) and inhibitory presynaptic (vGAT) and postsynaptic (Gephyrin) sites along processes (MAP2) of neurons (B). (Scale bars: 5 μm.) (C and D) Quantification of excitatory and inhibitory puncta density normalized to neurite volume, as assessed by MAP2 staining. *P < 0.05, **P < 0.01 by Student's t test. (E and F) Representative 10-s traces of mEPSCs (E) and mIPSCs (F) from 14 DIV hippocampal cells treated with control or Wnt7a. (G and H) Quantification of mEPSC (G) and mIPSC (H) frequency and amplitude. *P < 0.05 by Mann–Whitney test for frequency and Student's t test for amplitude. All experiments were performed by using at least three independent cultures.
Wnt Signaling Through Dvl1 Regulates Dendritic Spine Morphogenesis.
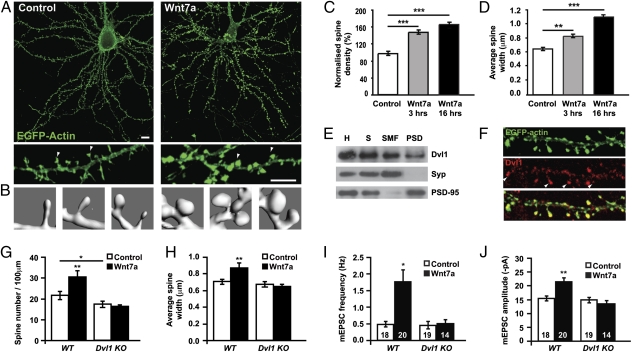
Given the specific effect of Wnt7a on excitatory synapses, we examined the potential role of Wnt7a signaling on the formation of dendritic spines, which receive the majority of central excitatory inputs (11). Sixteen-hour Wnt7a treatment increases spine number and spine head size by 65 ± 5.7% and 66 ± 3%, respectively (Fig. 2 A–D, Movie S1, and Movie S2). Three-hour Wnt7a exposure also increases spine number and spine head size by 48 ± 6.1% and 27 ± 3.3%, respectively (Fig. 2 C and D). Increased spine head size is an indication of spine maturity (11, 27), indicating that Wnt7a increases the formation and maturation of dendritic spines.
Fig. 2.
Wnt7a acts through Dvl1 to regulate spine morphogenesis. (A) Representative EGFP-actin–transfected hippocampal neurons exposed to control or Wnt7a for 16 h. Arrow heads indicate spines. (B) 3D reconstructions of typical examples of dendritic spines from control or Wnt7a-treated neurons. (C and D) Quantification of spine number (C) and size (D) in control and Wnt7a-treated neurons. (E) Synaptosome preparation from brain lysates: S, synaptosomal fraction; SMF, synaptosomal membrane fraction; PSD, postsynaptic density fraction; H, brain homogenate; Syp, synaptophysin. (F) Endogenous Dvl1 localization in dendritic spines visualized by EGFP-actin. (G and H) Quantification of spine number (G) and size (H) in Dvl1 mutant neurons exposed to Wnt7a. (I and J) Quantification of mEPSC (I) frequency and amplitude (J) in Dvl1 mutant neurons exposed to Wnt7a. The numbers at the base of bars reflect the number of cells, recorded from at least three independent cultures. *P < 0.05, **P < 0.01, ***P < 0.001 by Student's t test or ANOVA for spine number and mPSC amplitude, and Kruskal-Wallis test or Mann–Whitney test for spine size and mPSC frequency.
Although Wnt proteins can signal through multiple intracellular cascades, recipient cells require the cytoplasmic protein Dishevelled (Dvl), a core Wnt pathway component that functions as a hub to promote Wnt signaling within specific cellular compartments (26). Three mouse Dvl genes (Dvl1–Dvl3) are expressed broadly in the postnatal and adult brain (Allen Brain Atlas). However, Dvl1 has been the most studied because Dvl1 mutant mice exhibit defects in presynaptic assembly (20) and in social behavior (28). Importantly, Wnt7a requires Dvl1 to regulate axonal remodelling and presynaptic differentiation (20). To determine the downstream requirements for Wnt7a signaling in spines, the localization of Dvl1 was examined. Endogenous Dvl1 is present in the postsynaptic density (PSD) fraction (Fig. 2E) and at dendritic spines (Fig. 2F), suggesting that Wnt7a could regulate spine development by signaling directly via Dvl1 within dendritic spines.
We next investigated whether Dvl1 is required for Wnt7a function. Cultured hippocampal neurons from Dvl1 mutant mice exhibit a mild defect in spine morphogenesis compared with wild-type neurons (Fig. 2 G and H). Importantly, Dvl1 mutant neurons do not respond to Wnt7a, because the number and size of spines and the frequency and amplitude of mEPSCs remain unchanged after Wnt7a exposure (Fig. 2 G–J and Fig. S3). Thus, Dvl1 is required downstream of Wnt7a to regulate spine morphogenesis and excitatory synaptic function.
Wnt Signaling Is Required in Vivo for Dendritic Spine Morphogenesis and Excitatory Synaptic Function.
We next examined possible defects in spine morphogenesis in Wnt signaling-deficient mice. We analyzed the double Wnt7a; Dvl1 mutant mouse because previous studies have shown that this mutant exhibits stronger synaptic defects than single mutants in the cerebellum (20). Analyses of organotypic brain slices transfected with EGFP-actin reveal a significant decrease in spine density (30%) and spine head width (15%) in the double mutant mice compared with wild-type animals (Fig. S4).
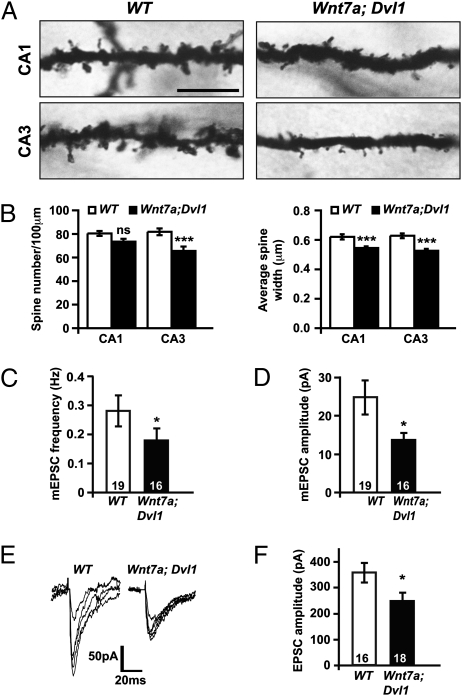
The in vivo role of Wnt7a signaling was investigated by Golgi staining. Double Wnt7a; Dvl1 mutant mice exhibit a 20% decrease in the number of spines on CA3 cells, although no significant differences were detected in CA1 cells (Fig. 3 A and B), consistent with the stronger Wnt7a/b expression observed in the CA3 of wild-type mice (Fig. S1). In contrast, spine size is significantly reduced in both CA1 and CA3 (Fig. 3 A and B). Wnt7a single knockout mice display a milder phenotype to double knockout mice, because spine number is not significantly affected in the CA3 (Fig. S5A), suggesting other Wnts may partially compensate for the loss of Wnt7a in vivo. Collectively, these results demonstrate that Wnt7a-Dvl1 signaling is required in vivo for the proper formation and growth of dendritic spines in the hippocampus.
Fig. 3.
Wnt mutant mice exhibit defects in dendritic spine morphogenesis and excitatory synaptic function in the hippocampus. (A) Representative sections of Golgi-stained dendrites of CA1 and CA3 cells from hippocampi of postnatal day (P)21 WT or Wnt7a; Dvl1 mice. (Scale bar: 10 μm.) (B) Quantification of spine number and size in CA3 and CA1 cells from WT and Wnt7a; Dvl1 mice. (C and D) Quantification of mEPSC frequency (C) and amplitude (D) in CA3 cells. (E) Overlays of five consecutive EPSCs evoked at MF-CA3 synapses in acute hippocampal slices from P14 WT or Wnt7a; Dvl1 mice. (F) Quantification of evoked EPSCs in Wnt7a; Dvl1 slices. Numbers at the base of bars reflect the number of cells, recorded from three mice per genotype. *P < 0.05, **P < 0.01, ***P < 0.001 by Student's t test for spine number and EPSC amplitude and Mann–Whitney test for spine size and mEPSC frequency. ns, not significant.
Consistent with the observed spine phenotype, recordings of mEPSCs in CA3 cells of acute hippocampal slices reveal a significant reduction in mEPSC frequency and amplitude in the double mutant compared with wild-type animals (Fig. 3 C and D and Fig. S5B). Evoked recordings at the mossy fiber-CA3 synapse also reveal a decrease in EPSC amplitude (Fig. 3 E and F). These results demonstrate that Wnt7a-Dvl1 signaling is required for normal spine morphogenesis and excitatory synaptic transmission in the hippocampus.
Postsynaptic Activation of Wnt Signaling Is Sufficient to Stimulate Spine Growth.
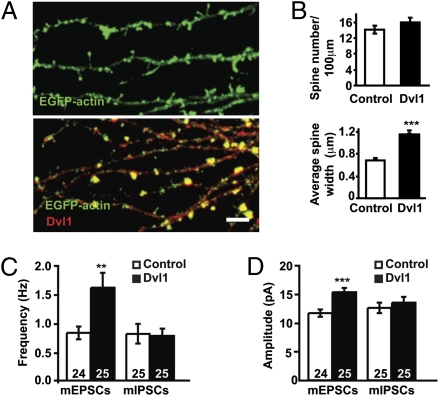
Wnt signaling is required in axons to regulate synaptic differentiation (20). Therefore, changes in spine morphogenesis induced by Wnt7a could be an indirect effect due to increased presynaptic assembly. Alternatively, Wnt7a may signal directly to dendrites as suggested by the presence of endogenous Dvl1 at postsynaptic sites (Fig. 2 E and F). To determine whether postsynaptic Wnt signaling is required for spine regulation, we used sparse Dvl1 transfection to produce Dvl1-expressing neurons that were contacted mainly by nontransfected axons. Postsynaptic expression of Dvl1 increases the width of spine heads by almost twofold compared with control cells (Fig. 4 A and B). Interestingly spine density does not change (Fig. 4 A and B), suggesting that postsynaptic activation of Wnt signaling stimulates spine growth without affecting spine formation.
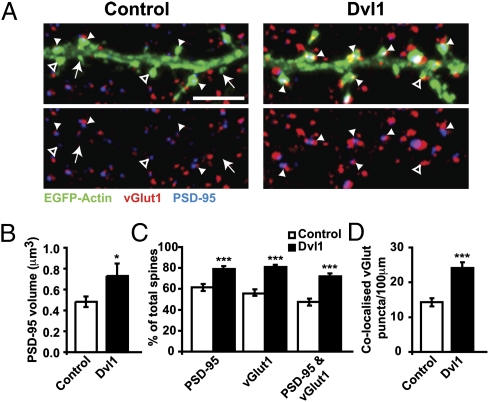
Fig. 4.
Postsynaptic expression of Dvl1 induces spine maturation and enhances excitatory synaptic function. (A) Hippocampal neurons expressing Dvl1-HA exhibit larger EGFP-actin–labeled dendritic spines. (Scale bar: 2 μm.) (B) Quantification of dendritic spine number and size after Dvl-HA expression. (C and D) Quantification of the frequency (C) and the amplitude (D) of mEPSCs and mIPSCs in control and Dvl1-expressing cells. The numbers at the base of bars reflect the number of cells recorded, from at least three independent cultures. **P < 0.01, ***P < 0.001 by Student's t test for spine number and mEPSC amplitude and Mann–Whitney test for spine size and mEPSC frequency.
Postsynaptic Dvl Expression Increases Excitatory Inputs and Synaptic Strength.
To determine the functional effect of postsynaptic Wnt signaling, mEPSCs and mIPSCs were recorded from control or Dvl1-transfected neurons. Dvl1 increases both the frequency and amplitude of mEPSCs, whereas mIPSCs are unaffected (Fig. 4 C and D and Fig. S6A). Thus, gain of function of Dvl1 specifically regulates excitatory connections.
The finding that postsynaptic Dvl1 expression increases mEPSC frequency was intriguing because Dvl1 does not affect dendritic spine number (Fig. 4 A and B). Therefore, we investigated whether postsynaptic activation of Wnt signaling increases presynaptic input onto preexisting spines. Indeed, Dvl increases the percentage of spines receiving presynaptic inputs (vGlut1 puncta; Fig. 5 A and C). In addition, Dvl1 increases the percentage of spines containing PSD-95, and spines contain larger PSD-95 puncta (Fig. 5 A–C). Crucially, the percentage of spines containing PSD-95 in direct apposition with vGlut1, and the density of vGlut1 puncta associated with PSD-95-positive spines, also increases (Fig. 5 A, C, and D). Interestingly, Dvl does not change the density or size of vGat puncta (Fig. S6 B–D). These results demonstrate that postsynaptic Dvl1 expression promotes the assembly of excitatory pre- and postsynaptic structures at preexisting spines, thereby specifically stimulating the formation of excitatory synapses.
Fig. 5.
Postsynaptic expression of Dvl1 increases excitatory input onto spines. (A) Excitatory sites were labeled with vGlut and PSD-95 in control or Dvl-1 transfected neurons coexpressing EGFP-actin. Filled arrowheads, PSD95-positive spines with associated vGlut1; open arrowheads, spines labeled with one marker; arrows, spines lacking PSD95 and vGlut1 markers. (Scale bar: 5 μm.) (B) Dvl1 increases PSD-95 punctum volume. (C) Percentage of spines associated with vGlut and PSD-95. (D) Density of vGlut1 puncta apposed to PSD95-positive spines. *P < 0.05, **P < 0.01, ***P < 0.001 by Mann–Whitney test in B and Student's t test in C and D on pooled data from three independent cultures.
Wnt Signaling Activates CaMKII to Regulate Spine Morphogenesis.
To examine the mechanism by which Wnt signaling regulates spine morphogenesis, we focused our attention on CaMKII. This serine/threonine kinase is enriched at the postsynaptic density and plays a central role in spine morphogenesis and synaptic strength (14, 29). Importantly, Wnt signaling can activate CaMKII during early embryonic patterning (30, 31).
CaMKII is activated by increases in intracellular calcium levels (16, 32). Consistent with this notion, the Ca2+ blockers 2-APB and SKF 96365 and the intracellular Ca2+ chelator BAPTA-AM suppress the effect of Wnt7a on spine growth (Fig. S7A). Importantly, Wnt7a activates endogenous CaMKII in hippocampal neurons as measured by the levels of Thr286 phosphorylation (pCaMKII), a read-out of CaMKII activity (16, 33). When normalized to total CaMKII, Wnt7a induces a rapid increase in pCaMKII of 64% within 2 min, followed by a >100% increase over the next 20 and 60 min (Fig. 6 A and B).
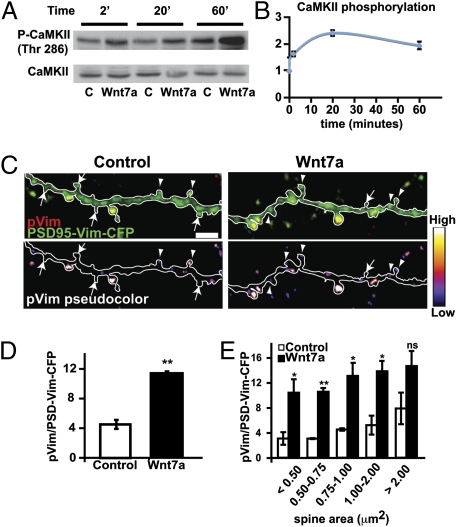
Fig. 6.
Wnt7a induces CaMKII phosphorylation and promotes CaMKII activity at spines. (A) CaMKII phosphorylation of Thr286 by Wnt7a. (B) CaMKII phosphorylation at Threonine 286 over a period of 2–60 min after Wnt7a treatment, normalized to total CaMKII. (C) CaMKII activity at spines assessed by the phosphorylation of Vimentin (pVim) in PSD-95-Vim-CFP (Upper). Lower is a pseudocolor representation indicating the intensity of pVim. Arrowheads, spines containing pVim; arrows, spines without pVim. (Scale bar, 5 μm.) (D) Intensity ratio between pVim and PSD-Vim-CFP. (E) Levels of CaMKII activity in relation to spine size. *P < 0.05, **P < 0.01 by Student's t test. ns, not significant.
To assess whether Wnt7a activates CaMKII specifically at spines, we used the fusion protein PSD-95-Vim-CFP. This construct contains the head of Vimentin, which is phosphorylated by CaMKII. When fused to PSD-95, Vimentin localizes to spines, and the ratio between the fluorescence intensity of phosphorylated Vimentin (pVim) and PSD-95-Vim-CFP provides a local read-out of CaMKII activity within spines (34, 35). Activation of CaMKII with glutamate plus glycine phosphorylates the Vimentin domain in spines, and this phosphorylation is blocked by the myristoylated autocamide-2 related inhibitory peptide (AIP), a specific CaMKII inhibitor (36) (Fig. S7B). Ten-minute exposure to Wnt7a increases pVim levels at spines by 149% (Fig. 6 C and D). Analyses of the distribution of CaMKII activation by spine head size reveals that Wnt7a induces a significant increase in pVim levels in spines <2 μm2, but not in larger spines (Fig. 6E). Together, these results demonstrate that Wnt7a rapidly induces CaMKII activation at smaller spines.
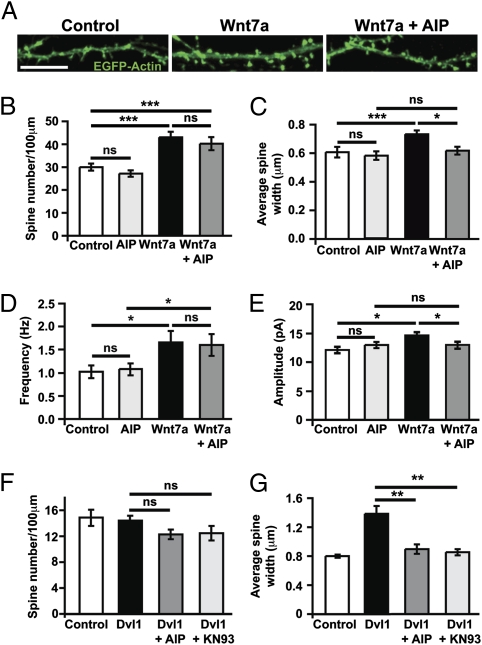
To test the contribution of CaMKII to the effect of Wnt7a on spines, the activity of CaMKII was blocked during Wnt7a exposure. AIP and the widely used CaMK inhibitor KN93 abolish the effect of Wnt7a on spine size without blocking the effect of Wnt7a on spine number (Fig. 7 A–C and Fig. S8 A and B). Therefore, CaMKII activation is required for the Wnt7a-mediated increase in spine size.
Fig. 7.
Wnt7a regulates spine size through CaMKII. (A) EGFP-transfected hippocampal neurons treated with Wnt7a and the peptide CaMKII inhibitor AIP. (Scale bar: 10 μm.) (B–E) Quantification of spine number (B), size (C), mEPSC frequency (D), and amplitude (E) in neurons treated with the CaMKII inhibitor AIP. (F and G) Quantification of spine number (F) and size (G) in Dvl1-expressing cells treated with the CaMKII inhibitors. *P < 0.05, **P < 0.01, ***P < 0.001 by ANOVA for spine number and mEPSC amplitude, Kruskal-Wallis test for spine size, and mEPSC frequency on pooled data from at least three independent cultures. ns, not significant.
Because synaptic strength is correlated with spine size, we reasoned that CaMKII inhibitors might also block the effect of Wnt7a on synaptic strength. Indeed, the Wnt7a-mediated increase in mEPSC amplitude, but not frequency, is abolished by AIP (Fig. 7 D and E and Fig. S8C). These results suggest that Wnt7a signals through CaMKII to promote spine enlargement and increase excitatory synaptic strength, whereas the changes in spine number and mEPSC frequency are CaMKII independent. Furthermore, in the presence of AIP or KN93, Dvl1 expression is unable to increase spine head width (Fig. 7 F and G and Fig. S8D). Together, our data suggest that Wnt7a signals postsynaptically through Dvl1 and CaMKII to promote spine growth and increase excitatory synaptic strength.
Discussion
Here, we demonstrate a unique role for Wnt7a signaling in specifically promoting the formation and function of excitatory synapses in the hippocampus through the regulation of spine morphogenesis and synaptic strength.
Although a role for Wnts in the formation of both central and peripheral synapses is well established (37, 38), a crucial unanswered question is whether certain Wnt isoforms promote the assembly of specific types of synapses, thereby affecting the ratio of excitatory/inhibitory inputs. In sharp contrast to other Wnts, like Wnt5a (23, 25), Wnt7a specifically promotes the formation and function of excitatory synapses, whereas inhibitory synapses are unaffected. Thus, although certain Wnts may function as broad synaptogenic factors, others, like Wnt7a, may promote the formation of specific types of synapse. Aberrant Wnt signaling could therefore result in the altered balance between excitatory and inhibitory signaling that is characteristic of neurological disorders such as epilepsy.
Wnt7a signaling promotes excitatory synapses through the formation and maturation of dendritic spines, with a concomitant increase in excitatory synaptic transmission. The scaffold protein Dvl1 is required postsynaptically, because Wnt7a is unable to promote spine morphogenesis or changes in mEPSCs in neurons from Dvl1 mutant mice. In vivo, the double mutant Wnt7a; Dvl1 mice exhibit a stronger phenotype than Wnt7a single mutant mice. Interestingly, defects in spine morphogenesis are stronger in the CA3 than in the CA1 region, consistent with the pattern of Wnt7a/b protein observed. Importantly, evoked recordings at the mossy fiber-CA3 synapse reveal defects in amplitude in the Wnt7a; Dvl1 mutant. Together, these findings demonstrate a unique function of Wnt7a signaling in spine maturation and synaptic strength in the hippocampus.
Previous studies have shown that Wnt7a signaling stimulates presynaptic differentiation at early stages of central synaptogenesis (20, 39). Wnt7a increases the number of presynaptic release sites through a mechanism that requires Dvl1 and activation of the canonical/Gsk3β Wnt pathway in axons (20, 39). Electrophysiological recordings suggest that Wnt7a also regulates glutamate release probability (20, 40). Here, we demonstrate that Wnt7a also directly regulates postsynaptic assembly. Together these studies demonstrate that Wnt7a signals bidirectionally to regulate the assembly and function of excitatory synapses.
How does Wnt7a signaling regulate spine morphogenesis? Although Wnt7a increases spine number and size, activation of the Wnt pathway on the postsynaptic side promotes only spine growth. This result suggests that Wnt7a regulates spine number indirectly by increasing the number of presynaptic release sites, which could then stimulate spine formation.
Previous studies have reported that Wnt7a does not affect the postsynaptic side (23, 40). This apparent discrepancy with our results could be due to the different sources of Wnt7a used (23, 40). Importantly, here we show that postsynaptic activation of Wnt signaling through Dvl1 expression promotes spine growth and excitatory synaptic transmission. Consistently, Dvl1 mutant dendrites do not respond to exogenous Wnt7a. Because Dvl1 functions as a molecular hub to activate Wnt cascades at specific cellular locales (26), the presence of Dvl1 at spines suggests that Wnt signaling can be activated within this synaptic compartment. Indeed, Wnt7a rapidly induces the local activation of CaMKII at dendritic spines, whereas blockade of CaMKII suppresses the ability of Wnt7a to stimulate spine growth and to increase synaptic strength. Our results therefore reveal a unique mechanism by which secreted Wnt factors can modulate synaptic growth and strength through the activation of CaMKII in dendritic spines.
Wnt signaling has been implicated in synaptic and morphological plasticity (32, 41–44). For example, Wnt7a/b protein levels increase in the CA3 region of the hippocampus after environmental enrichment (41). Neuronal activity also regulates the expression or secretion of Wnts (32, 43) and the synaptic localization of the Wnt receptor Fz5 (45). Importantly, synapse formation induced by high-frequency stimulation is mediated by Wnt-Fz5 signaling (45). Until now, the molecular mechanisms by which Wnt signaling regulates morphological plasticity have remained poorly characterized. CaMKII plays a critical role in postsynaptic morphological and functional plasticity (14, 29, 46, 47). The results reported here provide a possible mechanism by which neuronal activity, through Wnt-CaMKII signaling, could modulate the morphological and functional plasticity of neuronal circuits.
Material and Methods
Neuronal Cultures.
Primary hippocampal cultures were prepared from embryonic day 18 Sprague–Dawley rat embryos. For more details, see SI Materials and Methods.
Electrophysiology.
Cells were patched in whole cell voltage-clamp configuration. EPSCs were recorded in the presence of 10 μM bicuculine. IPSCs were recorded in the presence of 10 μM DNQX and 50 μM AP-5 (100 nM TTX was included when recording mPSCs). For more details, see SI Materials and Methods.
For further details of materials and methods used, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Daniel Sussman, Tony Wynshaw-Boris, and Andy McMahon for mutant mice; Jeremy Nathans, Yukiko Goda, Robert Malenka, and Ann Marie Craig for constructs; Drs. Rob Malenka and Tom Soderling for suggestions on CaMKII experiments; Eleanna Stamatakou for breeding and genotyping of mice; and Dr. Alex Kolodkin and members of our laboratory for useful discussion and comments on the manuscript. The Wellcome Trust, Biotechnology and Biological Sciences Research Council, and Medical Research Council supported this work.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018132108/-/DCSupplemental.
References
- 1.Kenet T, Froemke RC, Schreiner CE, Pessah IN, Merzenich MM. Perinatal exposure to a noncoplanar polychlorinated biphenyl alters tonotopy, receptive fields, and plasticity in rat primary auditory cortex. Proc Natl Acad Sci USA. 2007;104:7646–7651. doi: 10.1073/pnas.0701944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- 3.Rubenstein JL, Merzenich MM. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leite JP, et al. Plasticity, synaptic strength, and epilepsy: What can we learn from ultrastructural data? Epilepsia. 2005;46(Suppl 5):134–141. doi: 10.1111/j.1528-1167.2005.01021.x. [DOI] [PubMed] [Google Scholar]
- 5.Kehrer C, Maziashvili N, Dugladze T, Gloveli T. Altered excitatory-Inhibitory balance in the NMDA-hypofunction model of schizophrenia. Front Mol Neurosci. 2008;1:6. doi: 10.3389/neuro.02.006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vicario-Abejón C, Collin C, McKay RD, Segal M. Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultured hippocampal neurons. J Neurosci. 1998;18:7256–7271. doi: 10.1523/JNEUROSCI.18-18-07256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li AJ, Suzuki S, Suzuki M, Mizukoshi E, Imamura T. Fibroblast growth factor-2 increases functional excitatory synapses on hippocampal neurons. Eur J Neurosci. 2002;16:1313–1324. doi: 10.1046/j.1460-9568.2002.02193.x. [DOI] [PubMed] [Google Scholar]
- 8.Biederer T, et al. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- 9.Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- 10.Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bloodgood BL, Sabatini BL. Ca(2+) signaling in dendritic spines. Curr Opin Neurobiol. 2007;17:345–351. doi: 10.1016/j.conb.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Segal M. Dendritic spines and long-term plasticity. Nat Rev Neurosci. 2005;6:277–284. doi: 10.1038/nrn1649. [DOI] [PubMed] [Google Scholar]
- 14.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saneyoshi T, et al. Activity-dependent synaptogenesis: regulation by a CaM-kinase kinase/CaM-kinase I/betaPIX signaling complex. Neuron. 2008;57:94–107. doi: 10.1016/j.neuron.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 17.Salinas PC, Zou Y. Wnt signaling in neural circuit assembly. Annu Rev Neurosci. 2008;31:339–358. doi: 10.1146/annurev.neuro.31.060407.125649. [DOI] [PubMed] [Google Scholar]
- 18.Speese SD, Budnik V. Wnts: up-and-coming at the synapse. Trends Neurosci. 2007;30:268–275. doi: 10.1016/j.tins.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henriquez JP, et al. Wnt signaling promotes AChR aggregation at the neuromuscular synapse in collaboration with agrin. Proc Natl Acad Sci USA. 2008;105:18812–18817. doi: 10.1073/pnas.0806300105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad-Annuar A, et al. Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J Cell Biol. 2006;174:127–139. doi: 10.1083/jcb.200511054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerpa W, Dinamarca MC, Inestrosa NC. Structure-function implications in Alzheimer's disease: Effect of Abeta oligomers at central synapses. Curr Alzheimer Res. 2008;5:233–243. doi: 10.2174/156720508784533321. [DOI] [PubMed] [Google Scholar]
- 22.Packard M, et al. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002;111:319–330. doi: 10.1016/s0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farías GG, et al. Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J Biol Chem. 2009;284:15857–15866. doi: 10.1074/jbc.M808986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuitino L, et al. Wnt-5a modulates recycling of functional GABAA receptors on hippocampal neurons. J Neurosci. 2010;30:8411–8420. doi: 10.1523/JNEUROSCI.5736-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varela-Nallar L, Alfaro IE, Serrano FG, Parodi J, Inestrosa NC. Wingless-type family member 5A (Wnt-5a) stimulates synaptic differentiation and function of glutamatergic synapses. Proc Natl Acad Sci USA. 2010;107:21164–21169. doi: 10.1073/pnas.1010011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao C, Chen YG. Dishevelled: The hub of Wnt signaling. Cell Signal. 2010;22:717–727. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- 28.Lijam N, et al. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- 29.Jourdain P, Fukunaga K, Muller D. Calcium/calmodulin-dependent protein kinase II contributes to activity-dependent filopodia growth and spine formation. J Neurosci. 2003;23:10645–10649. doi: 10.1523/JNEUROSCI.23-33-10645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon MD, Nusse R. Wnt signaling: Multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 31.Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 32.Wayman GA, et al. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron. 2006;50:897–909. doi: 10.1016/j.neuron.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Griffith LC. Calcium/calmodulin-dependent protein kinase II: an unforgettable kinase. J Neurosci. 2004;24:8391–8393. doi: 10.1523/JNEUROSCI.2888-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rose J, Jin SX, Craig AM. Heterosynaptic molecular dynamics: locally induced propagating synaptic accumulation of CaM kinase II. Neuron. 2009;61:351–358. doi: 10.1016/j.neuron.2008.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inagaki N, et al. Activation of Ca2+/calmodulin-dependent protein kinase II within post-synaptic dendritic spines of cultured hippocampal neurons. J Biol Chem. 2000;275:27165–27171. doi: 10.1074/jbc.M003751200. [DOI] [PubMed] [Google Scholar]
- 36.Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H. A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem Biophys Res Commun. 1995;212:806–812. doi: 10.1006/bbrc.1995.2040. [DOI] [PubMed] [Google Scholar]
- 37.Budnik V, Salinas PC. Wnt signaling during synaptic development and plasticity. Curr Opin Neurobiol. 2011;21:151–159. doi: 10.1016/j.conb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci. 2010;11:77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- 39.Hall AC, et al. Valproate regulates GSK-3-mediated axonal remodeling and synapsin I clustering in developing neurons. Mol Cell Neurosci. 2002;20:257–270. doi: 10.1006/mcne.2002.1117. [DOI] [PubMed] [Google Scholar]
- 40.Cerpa W, et al. Wnt-7a modulates the synaptic vesicle cycle and synaptic transmission in hippocampal neurons. J Biol Chem. 2008;283:5918–5927. doi: 10.1074/jbc.M705943200. [DOI] [PubMed] [Google Scholar]
- 41.Gogolla N, Galimberti I, Deguchi Y, Caroni P. Wnt signaling mediates experience-related regulation of synapse numbers and mossy fiber connectivities in the adult hippocampus. Neuron. 2009;62:510–525. doi: 10.1016/j.neuron.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 42.Chiang A, Priya R, Ramaswami M, Vijayraghavan K, Rodrigues V. Neuronal activity and Wnt signaling act through Gsk3-beta to regulate axonal integrity in mature Drosophila olfactory sensory neurons. Development. 2009;136:1273–1282. doi: 10.1242/dev.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ataman B, et al. Rapid activity-dependent modifications in synaptic structure and function require bidirectional Wnt signaling. Neuron. 2008;57:705–718. doi: 10.1016/j.neuron.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J Biol Chem. 2006;281:11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- 45.Sahores M, Gibb A, Salinas PC. Frizzled-5, a receptor for the synaptic organizer Wnt7a, regulates activity-mediated synaptogenesis. Development. 2010;137:2215–2225. doi: 10.1242/dev.046722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asrican B, Lisman J, Otmakhov N. Synaptic strength of individual spines correlates with bound Ca2+-calmodulin-dependent kinase II. J Neurosci. 2007;27:14007–14011. doi: 10.1523/JNEUROSCI.3587-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okamoto K, Narayanan R, Lee SH, Murata K, Hayashi Y. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc Natl Acad Sci USA. 2007;104:6418–6423. doi: 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.