Abstract
Polycystic kidney disease (PKD) is a genetic disorder that is characterized by cyst formation in kidney tubules. PKD arises from abnormalities of the primary cilium, a sensory organelle located on the cell surface. Here, we show that the primary cilium of renal epithelial cells contains a protein complex comprising adenylyl cyclase 5/6 (AC5/6), A-kinase anchoring protein 150 (AKAP150), and protein kinase A. Loss of primary cilia caused by deletion of Kif3a results in activation of AC5 and increased cAMP levels. Polycystin-2 (PC2), a ciliary calcium channel that is mutated in human PKD, interacts with AC5/6 through its C terminus. Deletion of PC2 increases cAMP levels, which can be corrected by reexpression of wild-type PC2 but not by a mutant lacking calcium channel activity. Phosphodiesterase 4C (PDE4C), which catabolizes cAMP, is also located in renal primary cilia and interacts with the AKAP150 complex. Expression of PDE4C is regulated by the transcription factor hepatocyte nuclear factor-1β (HNF-1β), mutations of which produce kidney cysts. PDE4C is down-regulated and cAMP levels are increased in HNF-1β mutant kidney cells and mice. Collectively, these findings identify PC2 and PDE4C as unique components of an AKAP complex in primary cilia and reveal a common mechanism for dysregulation of cAMP signaling in cystic kidney diseases arising from different gene mutations.
Keywords: cyclic AMP, PKD2, vHNF1, TCF2, intraflagellar transport
Polycystic kidney disease (PKD) is the most common genetic cause of kidney failure in humans (1). PKD is characterized by kidney enlargement and progressive loss of renal function due to the accumulation of numerous fluid-filled cysts in the renal parenchyma. The cysts arise from renal tubules as a consequence of disturbances in cell proliferation, apoptosis, differentiation, fluid secretion, and planar cell polarity (2). The autosomal dominant form of PKD (ADPKD) is caused by mutations of PKD1 or PKD2, which encode the membrane proteins polycystin-1 (PC1) and polycystin-2 (PC2), respectively (3). PC1 and PC2 are localized in the primary cilium, a whip-like, sensory organelle that projects from the surface of most cells (1, 2). In the kidney, primary cilia are located on the apical surface of renal tubular epithelial cells and project into the tubule lumen. Renal cilia are immotile but bend in response to fluid flow and may have a mechanosensory function (4). In addition to PKD, several other human genetic disorders, collectively called the ciliopathies, are caused by mutations in proteins that are localized in the primary cilium and/or basal body (5).
The synthesis and maintenance of primary cilia requires intraflagellar transport, in which multiprotein complexes are transported along the ciliary axoneme by kinesin-II and dynein motor proteins. We have previously shown that kidney-specific inactivation of the KIF3A subunit of kinesin-II results in the loss of renal cilia and produces kidney cysts in mice (6). Analysis of precystic tubules in Kif3a mutant mice revealed that cells lacking primary cilia have an abnormality in planar cell polarity that may initiate cyst formation (7). Primary cilia have been shown to regulate several intracellular signaling pathways that control planar cell polarity, including Wnt/β-catenin signaling (8, 9); however, the mechanism by which the loss of renal cilia produces kidney cysts remains poorly understood.
The intracellular second messenger cAMP has been implicated in the growth and expansion of kidney cysts (10). Renal cAMP concentrations are elevated in animal models of PKD (8). Treatment of embryonic kidney explants from Pkd1 mutant mice with 8-Br-cAMP results in tubular dilation (11). Moreover, cAMP increases the proliferation of ADPKD cyst epithelial cells by activating the B-Raf/MEK/ERK pathway (12). This effect appears to be Ca2+ dependent because treatment with Ca2+ ionophores inhibits the mitogenic response to cAMP, whereas Ca2+ channel blockers promote proliferation (10). Subcellular compartmentalization of cAMP signaling is mediated by A-kinase anchoring proteins (AKAP), which tether adenylyl cyclases (AC) that synthesize cAMP with downstream effectors such as protein kinase A (PKA), phosphodiesterases (PDE), and exchange factors directly activated by cAMP (Epac) (13). Receptor-mediated agonists of adenylyl cyclase or nonselective phosphodiesterase inhibitors increase cAMP levels in cyst epithelial cells and stimulate fluid secretion and proliferation (14, 15). Conversely, drugs that inhibit cAMP synthesis reduce cyst formation in animal models and are currently being evaluated in clinical trials of human ADPKD (16). However, the mechanism that is responsible for the elevation of cAMP levels in PKD is not known.
Results
Loss of Primary Cilia Activates cAMP Signaling.
To investigate the role of the primary cilium in the regulation of cAMP signaling, we generated renal epithelial cell lines lacking primary cilia. Kif3aF/– mice carrying one null allele and one floxed allele of the ciliogenic gene Kif3a were crossed with mice expressing temperature-sensitive mutant SV40 large T antigen, and conditionally immortalized renal epithelial cell lines were established. To delete Kif3a, Kif3aF/– cells were infected with a retrovirus encoding self-excising Cre recombinase. The resulting Kif3a−/− cells lacked KIF3A protein and primary cilia, whereas cilia were present on the parental Kif3aF/– cells (Fig. 1A and Fig. S1 A–C). Compared with Kif3aF/– cells, Kif3a−/− cells contained higher levels of cAMP under basal conditions and following treatment with forskolin, a diterpene that maximally activates adenylyl cyclases (Fig. 1B). The increase in cAMP levels was sufficient to activate cAMP-dependent signaling, as indicated by cAMP response element binding protein (CREB) reporter assays (Fig. 1C). Kif3a−/− cells also exhibited enhanced phosphorylation of the PKA substrate Kemptide (Fig. 1 D and E).
Fig. 1.
Activation of cAMP signaling in Kif3a mutant cells and kidneys. (A) Immunostaining of KIF3A (red) and acetylated tubulin (green) shows that KIF3A and primary cilia (arrows) are present in Kif3aF/– cells (a and b) but absent in confluent Kif3a−/− cells (c and d). Nuclei are counterstained with DAPI (blue). (Scale bars: 20 μm.) (B) Basal (open bars, Left axis) and forskolin-stimulated (closed bars, Right axis) cAMP levels are 11-fold and 1.5-fold higher in Kif3a−/− cells than in Kif3aF/– cells. (C) Basal (open bars, Left axis) and forskolin-stimulated (closed bars, Right axis) CREB reporter activity is twofold higher in Kif3a−/− cells than in Kif3aF/– cells. (D) Agarose gel electrophoresis of phosphorylated and nonphosphorylated Kemptide shows that PKA activity is higher in Kif3a−/− cells than in Kif3aF/– cells. Each lane represents a different cell clone. NC, negative control. (E) Quantification of phosphorylated Kemptide shows that basal PKA activity is increased 1.7-fold in Kif3a−/− cells. (F) Immunostaining of phosphorylated PKA substrates (red) shows increased nuclear staining (arrows) in kidney cysts from kidney-specific Kif3a knockout mice (d) compared with wild-type kidneys (a) at age P19. Collecting ducts are stained with dolichos biflorus agglutinin (DBA) (green, b and e). C, cysts. (Scale bars: 10 μm.) (G) Immunostaining of phospho-CREB (red) shows increased nuclear staining (arrows) in kidney cysts from kidney-specific Kif3a knockout mice (d) compared with wild-type kidneys (a). Proximal tubules are stained with lotus tetragonolobus agglutinin (LTA) (green, b and e). (Scale bars: 10 μm.)
In addition to primary cilia, KIF3A is also located in the cytoplasm where it may have other functions (Fig. S1C). To verify that the effects on cAMP were due to the loss of primary cilia, ciliated Kif3aF/– cells were deciliated with dibucaine (17). Treatment with dibucaine resulted in the removal of cilia from ∼95% of cells (Fig. S1 E and F) and increased cAMP levels (Fig. S1G). As a negative control, dibucaine had no effect on the already elevated cAMP levels in nonciliated Kif3a−/− cells. Because the formation of primary cilia depends on cell confluence (18), we also compared cAMP levels in confluent and nonconfluent cells. cAMP levels were higher in nonconfluent (nonciliated) mIMCD3 cells compared with confluent (ciliated) cells (Fig. S1H). Treatment with dibucaine increased cAMP levels in confluent cells to approximately the same level as nonconfluent cells. Although these treatments may have additional effects, the most parsimonious explanation for these findings is that the loss of primary cilia stimulates cAMP-dependent signaling.
To determine whether the loss of cilia activates cAMP signaling in vivo, we analyzed cystic kidneys from kidney-specific Kif3a mutant mice (6). Staining with an antibody that recognizes phosphorylated PKA substrates (RRXXS/T) revealed increased staining in the nuclei of cyst epithelial cells compared with the predominantly cytoplasmic staining in wild-type renal tubules (Fig. 1F). As a positive control, staining was also increased in cultured Kif3a−/− cells compared with Kif3aF/– cells (Fig. S1D). Similarly, staining with an antibody against phospho-CREB, a specific PKA substrate, showed increased nuclear staining in kidney cysts (Fig. 1G). Costaining with fluorescent lectins showed that cAMP signaling was activated in cysts derived from both proximal tubules and collecting ducts (Fig. 1 F and G).
AC5/6 and AKAP150 Are Localized in Renal Primary Cilia.
cAMP is synthesized by adenylyl cyclase, which comprises a family of nine membrane-associated isoenzymes with distinct tissue distributions and subcellular localizations. Adenylyl cyclase 6 (AC6) has previously been localized in primary cilia in renal tubular cells and cholangiocytes (19, 20). In Kif3aF/– renal epithelial cells, adenylyl cyclases 5 and 6 (AC5/6) colocalized with acetylated tubulin, a marker of the primary cilium (Fig. 2A). In Kif3a−/− cells, cilia were absent, and AC5/6 remained associated with the plasma membrane. Because the antibody to AC5 also recognizes AC6, we confirmed the localization of AC5 using epitope-tagged proteins. Consistent with the endogenous staining results, Flag-tagged AC5 and AC6 were localized in the plasma membrane and primary cilia of transfected renal epithelial cells (Fig. 2B). To determine whether AC5 mediates the increase in cAMP levels in Kif3a mutant cells, we treated the cells with NKY80, a selective AC5 inhibitor. Treatment with NKY80 reduced the magnitude of the increase in CREB reporter activity in Kif3a−/− cells. In contrast, the elevated CREB reporter activity was not affected by NKY80 in Kif3aF/– cells (Fig. 2C). Similarly, knockdown of AC5 with siRNA normalized CREB reporter activity in Kif3a−/− cells, whereas knockdown of AC6 had no effect (Fig. 2D).
Fig. 2.
Localization of AC5/6 and AKAP150 in renal primary cilia. (A) Kif3aF/− cells were stained with antibodies that recognize both AC5 and AC6 (red, b) or AC6 only (red, e) and costained with antiacetylated tubulin antibody (green, a and d). The merged image shows colocalization in primary cilia (c and f). (Scale bars: 5 μm.) (B) IMCD3 cells were stably transfected with Flag-tagged AC5 or AC6 and stained with anti-Flag antibody (green). Flag-tagged AC5 (a and b) and AC6 (c and d) are localized in plasma membrane (a and c) and primary cilia (arrows in b and d). (Scale bars: a and c, 10 μm; b and d, 20 μm.) (C) Treatment with NKY80, a specific inhibitor of AC5, produces a dose-dependent decrease in CREB reporter activity in Kif3a−/− cells but has no effect in Kif3aF/− cells. Error bars indicate SD (n = 3). (D) Transfection of Kif3a−/− cells with siRNA to AC5 reduces CREB reporter activity, whereas siRNA to AC6 or scrambled siRNA (control) have no effect. Expression of siRNAs does not affect CREB reporter activity in Kif3aF/– cells (n = 3). (E) mIMCD3 cells were costained with anti-AKAP150 (red in b, e, and h) and antibodies (green) against acetylated tubulin (a), γ-tubulin (d), or α-tubulin (g). Merged images show localization of AKAP150 in primary cilia (c), centrosomes (f), and mitotic spindles (i). (Scale bars: 5 μm.)
The A-kinase anchoring protein AKAP150 (also called AKAP79) has previously been localized in cholangiocyte primary cilia and has been shown to interact with AC5/6 (21). We found that AKAP150 was also localized in primary cilia in renal epithelial cells (Fig. 2E). In addition, AKAP150 was found in centrosomes, mitotic spindles, and the plasma membrane (Fig. 2E). In Kif3a−/− cells that lack primary cilia, expression of AKAP150 was maintained in other cellular compartments. Taken together, these findings suggest that an AKAP150 complex containing AC5/6 is located in renal primary cilia.
Polycystin-2 Interacts with AC5/6 and Regulates cAMP Levels.
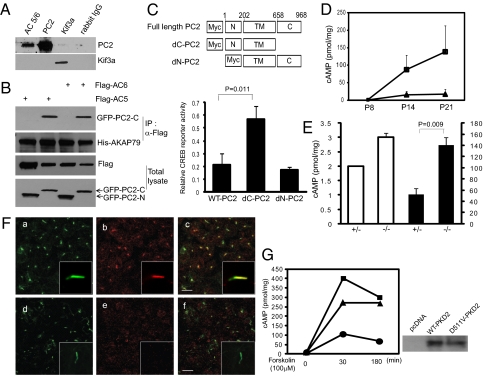
Mutations of PKD2, which encodes the protein PC2, are found in 15–20% of patients with ADPKD (3). PC2 is a Ca2+-permeable channel that is located in the primary cilia of renal epithelial cells where it is required for Ca2+ entry induced by fluid flow (22). Because AC5 and AC6 are Ca2+-sensitive enzymes, we tested the hypothesis that PC2 regulates cAMP through interactions with AC5/6. Immunoprecipitation of endogenous AC5/6 in mIMCD3 renal epithelial cells resulted in coprecipitation of native PC2 (Fig. 3A). To verify these interactions and identify the interacting region of PC2, we performed immunoprecipitation using tagged proteins. Expression of GFP fusion proteins containing the C-terminal and N-terminal domains of PC2, and immunoprecipitation of epitope-tagged AC5/6, revealed that the C terminus of PC2 was required for the interaction with the AKAP150 complex (Fig. 3B). Expression of a PC2 mutant lacking the C-terminal interaction domain stimulated CREB reporter activity, which suggests that the interaction may be important for regulating cAMP signaling (Fig. 3C). Immunofluorescence confocal microscopy verified that the mutant protein localized to the primary cilium (Fig. S2A). In contrast, an N-terminal PC2 deletion mutant did not localize to the cilium and did not affect CREB activity.
Fig. 3.
Polycystin-2 interacts with AC5/6 and regulates cAMP levels. (A) Immunoprecipitation of mIMCD3 cell lysates with anti-AC5/6, anti-PC2, or anti-Kif3a followed by immunoblot analysis with anti-PC2 and anti-Kif3a shows coimmunoprecipitation of endogenous PC2 and AC5/6. (B) HEK293 cells were cotransfected with plasmids encoding GFP fusion proteins containing the C-terminal or N-terminal domains of PC2, Flag-tagged AC5 or AC6, and His-tagged AKAP79/150. Cell lysates were immunoprecipitated with anti-Flag antibody and subjected to immunoblot analysis. (Upper two panels) AC5 and AC6 interact with AKAP79/150 and the C terminus of PC2, but not the N terminus. (Lower two panels) Total amount of Flag-tagged and GFP-tagged proteins. (C) Schematic diagram of PC2 mutants lacking the C terminus (dC-PC2) or the N terminus (dN-PC2) (Upper). Expression of dC-PC2 in mIMCD3 cells increases CREB reporter activity threefold, whereas expression of wild-type PC2 or dN-PC2 has no effect (Lower). Error bars indicate SD (n = 3). (D) cAMP levels in kidneys from kidney-specific Pkd2 knockout mice (■) are increased eightfold at postnatal day (P) 14 and P21 compared with kidneys from wild-type mice (▲). Error bars indicate SD (n = 3). (E) Forskolin-stimulated cAMP levels (closed bars, Right axis) are threefold higher in Pkd2+/− renal epithelial cells than in Pkd2−/− cells. Basal cAMP levels (open bars, Left axis) are only slightly increased (n = 3). (F) Pkd2+/− cells (a–c) and Pkd2−/− cells (d–f) are costained with antiacetylated tubulin (green) and anti-AC5/6 (red). Higher-magnification images (Insets) show that the ciliary localization of AC5/6 is decreased in Pkd2−/− cells (e vs. b). (Scale bars: 5 μm.) (G) cAMP levels were measured in Pkd2−/− cells that stably express wild-type PC2 (●), D511V mutant PC2 (▲), or a control plasmid (■). Expression of wild-type PC2 reduces cAMP levels, whereas cAMP remains elevated in cells expressing the PC2-D511V mutant (Left). Immunoblot analysis confirms that wild-type PC2 and the PC2-D511V mutant are expressed at similar levels (Right).
To verify that PC2 regulates cAMP signaling in vivo, cAMP levels were measured in the kidneys of mice following kidney-specific deletion of Pkd2 (23). Kidneys from 14- and 21-d-old mice were cystic and contained elevated levels of cAMP compared with wild-type littermates (Fig. 3D). The cysts arose from collecting ducts, and the cells lining the cysts showed increased nuclear expression of phosphorylated PKA substrates, including phospho-CREB (Fig. S2 B and C). cAMP levels were also elevated in cultured Pkd2−/− renal epithelial cells that were produced using a similar approach as Kif3a−/− cells (Fig. 3E). Antibody staining showed that Pkd2+/− and Pkd2−/− cells contained primary cilia (Fig. 3F, a and d). AC5/6 was present in primary cilia in Pkd2+/− cells but was not detected in the cilia of Pkd2−/− cells (Fig. 3F, b and e). Quantitative real-time RT-PCR showed that the expression of AC5 mRNA transcripts was down-regulated by 60% and AC6 mRNA was up-regulated by 40% in Pkd2−/− cells (Fig. S2D). However, Western blot analysis showed that the total amount of AC5/6 protein was unchanged in Pkd2−/− cells (Fig. S2E). These results suggest that PC2 may be required for the ciliary localization of AC5/6.
Next, we examined whether the Ca2+ channel activity of PC2 plays a role in regulating cAMP signaling. Pkd2−/− cells were stably transfected with wild-type PC2 or a PC2 mutant, D511V, which lacks Ca2+ channel activity (24). Expression of wild-type PC2 in Pkd2−/− cells reduced forskolin-stimulated cAMP levels, whereas cAMP levels remained elevated following expression of the PC2-D511V mutant, despite similar levels of protein expression (Fig. 3G). Antibody staining confirmed that the PC2-D511V mutant, similar to wild-type PC2, is localized in the primary cilium (Fig. S2F). These results indicate that the Ca2+ channel activity of PC2 is important for regulating cAMP signaling.
HNF-1β–Regulated PDE4C Interacts with the AKAP150 Complex in Primary Cilia.
In addition to stimulation of cAMP synthesis, the increased steady-state levels of cAMP in Kif3aF/– cells could also arise from decreased cAMP degradation. Hydrolysis of cyclic nucleotides is mediated by PDEs, a multigene family of enzymes with distinct subcellular localizations and substrate specificities. Antibody staining revealed that a cAMP-specific member of the PDE family, PDE4C, was localized in the primary cilia of renal epithelial cells (Fig. 4A). To determine whether PDE4C interacts with other components of the ciliary AKAP150 complex, coimmunoprecipitation was performed. Expression of either Flag-tagged PDE4C or Flag-tagged AKAP150 followed by immunoprecipitation with anti-Flag antibody resulted in coprecipitation of endogenous PDE4C, AKAP150, a regulatory subunit of protein kinase A (PKA-RIIα), and AC5/6, which indicated that PDE4C was a component of the AKAP150 complex (Fig. 4B). Because cilia constitute only a very minor portion of the cell, the strength of the coimmunoprecipitation signal suggested that the protein interactions may also occur outside the primary cilium. To examine this possibility, we performed coimmunoprecipitation on nonconfluent mIMCD3 cells that lack primary cilia. The PC2-AC5/6-AKAP150-PDE4C complex was detected in both confluent mIMCD3 cells and nonconfluent cells. However, the abundance of the complex was higher in confluent cells that formed primary cilia (Fig. S1I).
Fig. 4.
PDE4C is localized in primary cilia and is regulated by the transcription factor HNF-1β. (A) Immunostaining of acetylated tubulin (green) and PDE4C (red) shows that PDE4C is localized in primary cilia in uninduced 53A cells (a–c) but is not detectable in cilia following treatment with mifepristone to induce expression of dominant-negative mutant HNF-1β (d–f). (B) mIMCD3 cells were transfected with either Flag-tagged PDE4C (Left) or Flag-tagged AKAP150 (Right), and cell lysates were immunoprecipitated with anti-Flag antibody. Immunoblot analysis shows coimmunoprecipitation of PDE4C, AKAP150, PKA regulatory subunit, and AC5/6. (C) ChIP of the promoter region of PDE4C using an antibody to HNF-1β (Right lane) and primers P1 and P2 (Upper and Middle panels). No product is seen using control IgG (Center lane) or primers that amplify an irrelevant region of the promoter (Lower). (D) PDE4C mRNA transcripts are reduced by 80% in 53A cells that are treated with mifepristone (Mif) to induce expression of dominant-negative mutant HNF-1β. Error bars indicate SD (n = 3). (E) cAMP levels are two- to 4.2-fold higher in mifepristone-treated 53A cells (▲) compared with uninduced cells (■) (n = 3). (F) Basal (open bars, Left axis) and forskolin-stimulated (closed bars, Right axis) CREB reporter activity is twofold and threefold higher in mifepristone-treated 53A cells (n = 3). (G) cAMP levels are 5.6-fold higher in kidneys from kidney-specific HNF-1β knockout mice compared with wild-type littermates (age P7, n = 2). (H) mIMCD3 cells were transfected with four different PDE4C siRNAs or a scrambled siRNA (control), and CREB reporter activity was measured. siRNAs 1, 2, and 4 reduced PDE4C mRNA levels (Right) and increased CREB reporter activity 1.3- to 5.4-fold (Left). (I) Immunostaining of acetylated tubulin (green) and PDE4C (red) in Pkd2+/− cells (a–c) and Pkd2−/− cells (d–f) shows that the ciliary localization of PDE4C is decreased in Pkd2−/− cells (e and f). (Scale bars: 30 μm.)
We previously identified PDE4C in a genome-wide screen for genes that were regulated by the transcription factor HNF-1β in the kidney (25). This result was of interest because mutations of HNF-1β produce kidney cysts in humans and mice (26, 27). Chromatin immunoprecipitation and DNA microarray analysis (ChIP-on-chip) identified PDE4C as a potential HNF-1β target gene (Fig. S3). ChIP assays showed that HNF-1β binds to the PDE4C promoter in chromatin from mIMCD3 cells and mouse kidney (Fig. 4C). Binding to the PDE4C promoter was specific, because no binding was detected on the promoter of another PDE4 family member, PDE4A. We identified two consensus HNF-1β binding sites in the PDE4C promoter located 582 and 860 bp upstream from the translation start site (Fig. S4 A and B). The sequence located 582 bp upstream was evolutionarily conserved between mouse and human. Luciferase reporter assays showed that HNF-1β activated the PDE4C promoter (Fig. S4C), and mutations of either binding site or expression of dominant-negative mutant HNF-1β inhibited promoter activity (Fig. S4 D and E). These findings demonstrate that HNF-1β directly regulates PDE4C gene transcription.
cAMP Levels Are Elevated in HNF-1β Mutant Cells and Kidneys.
Next, we examined whether the HNF-1β–dependent transcription of PDE4C plays a role in the regulation of cAMP signaling. Expression of dominant-negative mutant HNF-1β in renal epithelial cells decreased the levels of PDE4C mRNA transcripts and inhibited the expression of PDE4C protein in the primary cilia (Fig. 4 A and D). Consistent with down-regulation of PDE4C, cAMP levels and CREB reporter activity were increased in cells expressing dominant-negative mutant HNF-1β (Fig. 4 E and F). The increase in cAMP signaling was not due to changes in the expression or ciliary localization of AC5/6 and AKAP150 (Fig. S4 F–H). To confirm these findings in vivo, cAMP levels were measured in mice following kidney-specific deletion of HNF-1β (27). Deletion of HNF-1β produced cystic kidneys that contained higher levels of cAMP compared with wild-type kidneys (Fig. 4G). To verify that down-regulation of PDE4C was sufficient to increase cAMP levels, we reduced its expression in wild-type renal epithelial cells using siRNAs. Knockdown of PDE4C with four different siRNAs increased CREB reporter activity, and the magnitude of the increase correlated with the degree of PDE4C knockdown (Fig. 4H). Next, we examined PDE4C expression in Pkd2−/− mutant kidney cells. Similar to previous findings in Pkd2WS25/– mutant mice (28), we found that Pkd2−/− and Pkd2+/− renal epithelial cells had comparable levels of PDE4C mRNA transcripts (Fig. S2D). However, ciliary staining of PDE4C was not detected in Pkd2−/− cells (Fig. 4I), indicating that the ciliary localization of the AKAP150 complex containing PDE4C is dependent on PC2.
Discussion
cAMP promotes cyst expansion by stimulating fluid secretion and increasing cell proliferation (10). Drugs that reduce cAMP levels inhibit cyst growth in experimental animals and retard kidney enlargement in humans with ADPKD (2, 8, 29). However, the molecular mechanism that is responsible for the accumulation of cAMP in PKD is not known. Here, we show that deletion of the ciliogenic gene Kif3a results in the loss of the primary cilium and produces increased cAMP levels. Because KIF3A may have functions outside the primary cilium, we used two additional approaches, pharmacological deciliation and growth under nonconfluent conditions, to confirm that primary cilia are required for the proper regulation of cAMP signaling. These findings support the concept that the primary cilium is a subcellular cAMP signaling compartment.
Previous studies have shown that primary cilia on cholangiocytes contain components of the cAMP signaling machinery, including AKAP150; AC4, AC6, and AC8; protein kinase A; and Epac2 (21). Here, we show that a protein complex comprising AKAP150, AC5/6, and PKA exists in primary cilia in renal epithelial cells (Fig. S5). Moreover, we identify PC2 and PDE4C as unique components of the ciliary AKAP complex. Under normal conditions, the ciliary AKAP complex constrains cAMP signaling: PDE4C promotes the hydrolysis of cAMP, and PC2, functioning as a Ca2+ entry channel, may mediate local accumulation of Ca2+ that inhibits the activity of Ca2+-sensitive AC5 and AC6. PC2 also appears to be required for the ciliary localization of the AKAP150 complex. Consistent with the latter finding, the N terminus of PC2 contains a 15-amino acid sequence that mediates trafficking to the cilium (30). In addition to the primary cilium, the AKAP150 complex is also located in the cytoplasm where it is also likely to be important for proper regulation of cAMP signaling.
Primary cilia have been shown to contain G protein-coupled receptors (GPCR) that signal through cAMP, including receptors for somatostatin and odorants (31). Recently, the type 2 vasopressin receptor (V2R) has been identified in primary cilia on renal epithelial cells where it colocalizes with AC5/6 (31). Treatment of isolated cilia with vasopressin in the presence of the phosphodiesterase inhibitor IBMX results in increased cAMP production, which highlights the potential importance of ciliary phosphodiesterases. The AKAP150 complex identified in the present study contains PDE4C, which may function to negatively regulate cAMP signaling that is coupled to GPCRs such as V2R.
The identification of an AKAP complex containing PC2 and PDE4C in the primary cilium reveals a common mechanism for dysregulation of cAMP signaling in cystic kidney diseases arising from different gene mutations. Mutations of Kif3a lead to loss of cilia, disinhibition of AC5, and increased cAMP levels, indicating that the ciliary localization of the complex is necessary for the proper regulation of cAMP signaling. Missense mutations of Pkd2 that inhibit the channel activity of PC2 (e.g., D511V) may reduce the local Ca2+ within the cilium and activate Ca2+-inhibitable adenylyl cyclases, such as AC5/6. Previous observations that cAMP concentrations are elevated in Pkd2+/− vascular smooth muscle cells and in wild-type cells in which [Ca2+]i is lowered with verapamil or BAPTA-AM, further support the tight link between Ca2+ and cAMP signaling (32).
Our studies also reveal a unique role of the transcription factor HNF-1β in the regulation of cAMP signaling. HNF-1β plays a central role in the transcriptional regulation of cystic disease genes such as Pkd2 and Pkhd1 (27, 33). Here, we show that wild-type HNF-1β activates transcription of PDE4C, whereas mutations of HNF-1β inhibit the expression of PDE4C and increase cAMP levels. Because HNF-1β also regulates the transcription of Pkd2 (27), down-regulation of PC2 and impaired ciliary trafficking of the AKAP complex may also contribute to the elevation in cAMP. Drugs that reduce cAMP levels are currently in clinical trials and may be effective in inhibiting cyst growth in humans with mutations of HNF-1β.
Methods
Animals.
Mice with kidney-specific inactivation of Kif3a, Pkd2, and HNF-1β (Tcf2) were generated by Cre/loxP recombination as described previously (6, 23, 27). H-2Kb-tsA58 mice expressing temperature-sensitive mutant SV40 large T antigen were obtained from Charles River. All experiments involving animals were performed under the auspices of the University of Texas Southwestern Institutional Animal Care and Use Committee.
Antibody Staining.
Cells were grown on coverslips and fixed with 4% paraformaldehyde in PBS or ice-cold methanol, then permeabilized in PBS containing 0.2% Triton X-100. Kidneys were fixed with 4% paraformaldehyde and then cryosectioned. Antibody staining and immunofluorescence microscopy were performed as described previously (6).
Reporter Gene Assays.
Cells were transfected with pCRE-Luc or PDE4C promoter-reporter plasmids using Effectene (Qiagen), Lipofectamine 2000 (Invitrogen), or FuGene (Roche) according to the manufacturer's directions. Cotransfection with pRL-TK (Promega) was used to control for transfection efficiency. After 48 h, the cells were lysed in 500 μL of passive lysis buffer (Promega), freeze-thawed once, and centrifuged. Supernatants (20 μL) were added to 96-well plates, and Photinus and Renilla luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer's directions.
cAMP Assays.
cAMP levels were measured using an enzyme immunoassay (Assay Designs) according to the manufacturer's directions. Protein concentration was determined with the Coomassie Plus Bradford Assay (Pierce).
Statistical Analysis.
Statistical analysis was performed using two-tailed unpaired Student t test. For multiple comparisons, ANOVA and Dunnett's post hoc test of significance were performed using GraphPad Prism software. Statistical significance was defined as P < 0.05. Additional methods can be found in SI Methods.
Supplementary Material
Acknowledgments
We thank Patricia Cobo-Stark, Rajiv Parmar, and Yimei Gong for expert technical assistance and Fangming Lin for assistance with the generation of cell lines. We thank Leo Tsiokas, John Scott, Daniel Silver, and Ron Taussig for providing reagents. We acknowledge support from National Institutes of Health Grants R01DK042921 (to P.I.), R01DK067565 (to P.I.), University of Texas Southwestern O'Brien Kidney Research Core Center Grant P30DK079328, the PKD Foundation (A.S. and Z.M.), and the National Kidney Foundation (A.S.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016214108/-/DCSupplemental.
References
- 1.Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2002;13:2384–2398. doi: 10.1097/01.asn.0000028643.17901.42. [DOI] [PubMed] [Google Scholar]
- 2.Patel V, Chowdhury R, Igarashi P. Advances in the pathogenesis and treatment of polycystic kidney disease. Curr Opin Nephrol Hypertens. 2009;18:99–106. doi: 10.1097/MNH.0b013e3283262ab0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallagher AR, Germino GG, Somlo S. Molecular advances in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17:118–130. doi: 10.1053/j.ackd.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Praetorius HA, Spring KR. A physiological view of the primary cilium. Annu Rev Physiol. 2005;67:515–529. doi: 10.1146/annurev.physiol.67.040403.101353. [DOI] [PubMed] [Google Scholar]
- 5.Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: An emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 6.Lin F, et al. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA. 2003;100:5286–5291. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel V, et al. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet. 2008;17:1578–1590. doi: 10.1093/hmg/ddn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres VE, Harris PC. Mechanisms of disease: Autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol. 2006;2:40–55. doi: 10.1038/ncpneph0070. quiz 55. [DOI] [PubMed] [Google Scholar]
- 9.Berbari NF, O'Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol. 2009;19:R526–R535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calvet JP. Strategies to inhibit cyst formation in ADPKD. Clin J Am Soc Nephrol. 2008;3:1205–1211. doi: 10.2215/CJN.05651207. [DOI] [PubMed] [Google Scholar]
- 11.Magenheimer BS, et al. Early embryonic renal tubules of wild-type and polycystic kidney disease kidneys respond to cAMP stimulation with cystic fibrosis transmembrane conductance regulator/Na(+),K(+),2Cl(−) co-transporter-dependent cystic dilation. J Am Soc Nephrol. 2006;17:3424–3437. doi: 10.1681/ASN.2006030295. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi T, et al. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int. 2003;63:1983–1994. doi: 10.1046/j.1523-1755.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 13.Zaccolo M, Magalhães P, Pozzan T. Compartmentalisation of cAMP and Ca(2+) signals. Curr Opin Cell Biol. 2002;14:160–166. doi: 10.1016/s0955-0674(02)00316-2. [DOI] [PubMed] [Google Scholar]
- 14.Mangoo-Karim R, et al. Renal epithelial fluid secretion and cyst growth: The role of cyclic AMP. FASEB J. 1989;3:2629–2632. doi: 10.1096/fasebj.3.14.2480260. [DOI] [PubMed] [Google Scholar]
- 15.Belibi FA, et al. The effect of caffeine on renal epithelial cells from patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2002;13:2723–2729. doi: 10.1097/01.asn.0000025282.48298.7b. [DOI] [PubMed] [Google Scholar]
- 16.Gattone VH., 2nd Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med. 9:1323–1326. doi: 10.1038/nm935. [DOI] [PubMed] [Google Scholar]
- 17.Overgaard CE, et al. Deciliation is associated with dramatic remodeling of epithelial cell junctions and surface domains. Mol Biol Cell. 2009;20:102–113. doi: 10.1091/mbc.E08-07-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joly D, et al. The polycystin 1-C-terminal fragment stimulates ERK-dependent spreading of renal epithelial cells. J Biol Chem. 2006;281:26329–26339. doi: 10.1074/jbc.M601373200. [DOI] [PubMed] [Google Scholar]
- 19.Masyuk AI, et al. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology. 2006;131:911–920. doi: 10.1053/j.gastro.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chien CL, et al. Impaired water reabsorption in mice deficient in the type VI adenylyl cyclase (AC6) FEBS Lett. 2010;584:2883–2890. doi: 10.1016/j.febslet.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Masyuk AI, et al. Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. Am J Physiol Gastrointest Liver Physiol. 2008;295:G725–G734. doi: 10.1152/ajpgi.90265.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nauli SM, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 23.Nishio S, et al. Loss of oriented cell division does not initiate cyst formation. J Am Soc Nephrol. 2010;21:295–302. doi: 10.1681/ASN.2009060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma R, et al. PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol Cell Biol. 2005;25:8285–8298. doi: 10.1128/MCB.25.18.8285-8298.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Z, et al. Mutations of HNF-1β inhibit epithelial morphogenesis through dysregulation of SOCS-3. Proc Natl Acad Sci USA. 2007;104:20386–20391. doi: 10.1073/pnas.0705957104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Igarashi P, Shao X, McNally BT, Hiesberger T. Roles of HNF-1β in kidney development and congenital cystic diseases. Kidney Int. 2005;68:1944–1947. doi: 10.1111/j.1523-1755.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- 27.Gresh L, et al. A transcriptional network in polycystic kidney disease. EMBO J. 2004;23:1657–1668. doi: 10.1038/sj.emboj.7600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Ward CJ, Harris PC, Torres VE. Cyclic nucleotide signaling in polycystic kidney disease. Kidney Int. 2010;77:129–140. doi: 10.1038/ki.2009.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hogan MC, et al. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010;21:1052–1061. doi: 10.1681/ASN.2009121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geng L, et al. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci. 2006;119:1383–1395. doi: 10.1242/jcs.02818. [DOI] [PubMed] [Google Scholar]
- 31.Raychowdhury MK, et al. Vasopressin receptor-mediated functional signaling pathway in primary cilia of renal epithelial cells. Am J Physiol Renal Physiol. 2009;296:F87–F97. doi: 10.1152/ajprenal.90509.2008. [DOI] [PubMed] [Google Scholar]
- 32.Kip SN, et al. [Ca2+]i reduction increases cellular proliferation and apoptosis in vascular smooth muscle cells: Relevance to the ADPKD phenotype. Circ Res. 2005;96:873–880. doi: 10.1161/01.RES.0000163278.68142.8a. [DOI] [PubMed] [Google Scholar]
- 33.Hiesberger T, et al. Mutation of hepatocyte nuclear factor-1β inhibits Pkhd1 gene expression and produces renal cysts in mice. J Clin Invest. 2004;113:814–825. doi: 10.1172/JCI20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.