Abstract
Serum levels of apolipoprotein CIII (apoCIII) are increased in type 1 diabetic patients, and when β cells are exposed to these diabetic sera, apoptosis occurs, an effect abolished by an antibody against apoCIII. We have investigated the BB rat, an animal model that develops a human-like type 1 diabetes, and found that apoCIII was also increased in sera from prediabetic rats. This increase in apoCIII promoted β-cell death. The endogenous levels of apoCIII were reduced by treating prediabetic animals with an antisense against this apolipoprotein, resulting in a significantly delayed onset of diabetes. ApoCIII thus serves as a diabetogenic factor, and intervention with this apolipoprotein in the prediabetic state can arrest disease progression. These findings suggest apoCIII as a target for the treatment of type 1 diabetes.
Keywords: BB rat model, cytoplasmic free Ca2+ concentration, insulin release, β-cell destruction, diabetes onset
We have previously shown that there is a group of patients with type 1 diabetes (T1D) whose sera induce an increased activity of voltage-gated Ca2+ channels in pancreatic β cells, resulting in increased cytoplasmic free Ca2+ concentration ([Ca2+]i) and apoptosis (1). T1D serum was found to contain increased concentrations of apolipoprotein CIII (apoCIII), and we could later demonstrate that this serum factor was responsible for the [Ca2+]i increase and β-cell death in vitro (2). Consequently, the effects of both T1D serum and apoCIII on [Ca2+]i and β-cell death are abolished when β cells are coincubated with an antibody against apoCIII (2).
To clarify the extent to which apoCIII is also involved in β-cell death in vivo, we have used the animal model diabetes-prone BB rat (DPBB), which spontaneously, at around the age of 60 d, develops T1D that resembles the human form of the disease (3, 4). Diabetes-resistant BB rats (DRBB) were developed from selective breeding from diabetes-prone (DP) forebears and were used as controls (5).
This model gives an opportunity to study the impact of prediabetic interventions to prevent or delay onset of the disease. In this study, three prediabetic age groups (40, 50, and 60 d) were investigated both in vitro and in vivo, and we could demonstrate that lowering the endogenous levels of apoCIII by antisense treatment markedly delayed onset of diabetes.
Results
Morphological and Immunohistochemical Characterization.
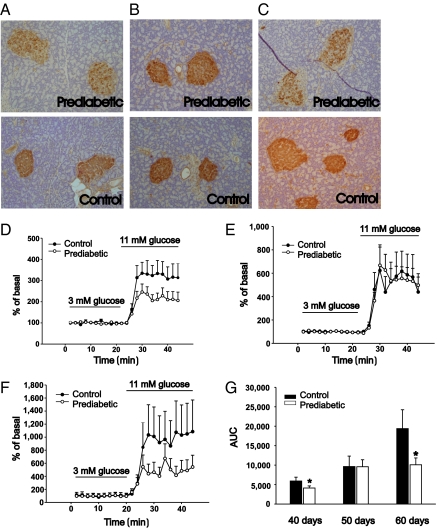
Qualitative morphological analysis showed that pancreas taken from prediabetic rats at the age of 40 d had a lower number of insulin-containing cells than the age-matched controls. The shape of the islets was round to oval with an intact thin layer of collagen around the islets. No inflammation was seen (Fig. 1A). At the age of 50 d, the number of insulin-containing cells did not differ compared with islets from control rats. Islet shape and border did not differ from control rats. No inflammation was seen (Fig. 1B). At 60 d of age, there was a clear decrease in the number of insulin-positive cells. The shape of the islet was irregular, and in some islets, exocrine cells were found. There was no distinct border between the islets and the surrounding exocrine tissue, and signs of inflammation were frequently found (Fig. 1C).
Fig. 1.
Morphology, immunohistochemistry, and release of insulin from pancreatic islets from prediabetic and control BB rats. (A) Prediabetic rats, age 40 d, have a lower number of insulin-containing cells than control rats (n = 3). (B) In rats age 50 d, there is no difference in the number of insulin-positive cells (n = 3). (C) In the pancreas from 60-d-old prediabetic rats, there is a low number of insulin-containing cells and the islets of Langerhans have lost their surrounding collagen capsule. Also, exocrine cells are in close contact with the endocrine cells (n = 3). Glucose-stimulated insulin release measured from islets taken at the ages of (D) 40 d (n = 11), (E) 50 d (n = 10), and (F) 60 d (n = 7). (G) The amount of released insulin expressed as area under the curve (AUC). Statistical significance was evaluated by Student's t test, and P values < 0.05 were considered significant. Data are presented as means ± SEM. *P < 0.05.
Insulin Secretion, apoCIII Levels, and Changes in [Ca2+]i.
Glucose-stimulated insulin release was impaired in islets from 40- and 60-d-old rats, whereas there was no difference in islets from 50-d-old rats, all compared with age-matched controls (Fig. 1 D–G).
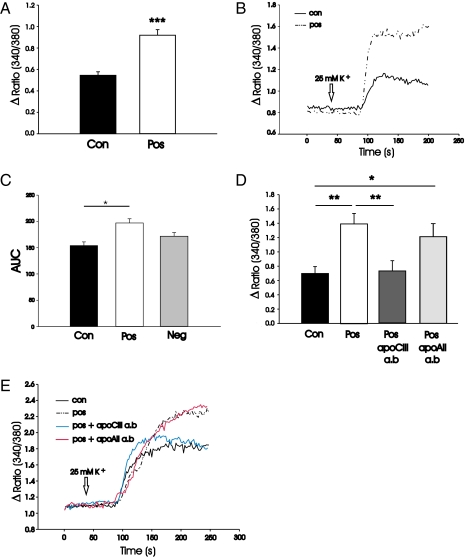
Islet cells from control rats were exposed overnight to sera taken from the three different age groups of prediabetic DPBB rats. When the cells where depolarized with 25 mM KCl, the increase in [Ca2+]i was measured and compared with the response obtained in cells exposed to age-matched control sera. Those sera that induced a higher increase in [Ca2+]i, compared with cells exposed to control sera, are referred to as positive (pos) and those that do not differ are called negative (neg). Two of 11 sera taken from prediabetic rats at the age of 40 d, 1 of 9 taken at the age of 50 d, and 30 of 51 taken at the age of 60 d induced a significantly higher increase in [Ca2+]i (Fig. 2 A and B).
Fig. 2.
Effects on [Ca2+]i and amounts of apoCIII. The increase in [Ca2+]i upon depolarization with 25 mM K+ was investigated in islet cells from control rats incubated overnight with (A) pos sera from 60-d-old rats or age-matched controls (n = 30) and (D) control sera, pos 60-d sera, and pos 60-d sera with an antibody against apoCIII or apoAII (n = 3–6). Examples of typical traces showing the increase in [Ca2+]i upon depolarization with 25 mM K+ in (B) cells exposed to pos or control sera and (E) pos sera, control sera, pos sera, and an antibody against apoCIII or apoAII. (C) Relative levels of apoCIII in three pos, three neg, and three control sera, given as area under the curve (AUC). Statistical significance was evaluated by Student's t test, and P values < 0.05 were considered significant. Data are presented as means ± SEM. ***P < 0.001, **P < 0.01, and *P < 0.05.
The relative amounts of apoCIII in three pos, three neg, and three control sera from 60-d-old rats were evaluated and the content in control sera was significantly lower than in pos sera (Fig. 2C). The effect of pos sera on [Ca2+]i was abolished when coincubating the cells with an antibody against apoCIII (Academy Biomedical), whereas an antibody against apoAII, used as control, was without effect (Fig. 2 D and E).
Effect of Prediabetic Sera on Cell Viability.
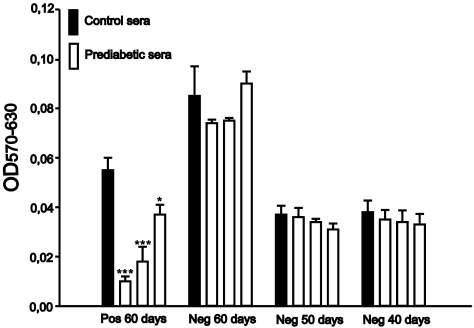
Islet cells from control rats were incubated for 24 h with control sera and with sera from three prediabetic animals from the following groups: pos sera from 60-d-old and neg sera from 40-, 50-, and 60-d-old rats. Viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay and was significantly reduced in cells exposed to the three pos sera from 60-d-old rats, whereas none of the other sera had any effect on cell viability (Fig. 3).
Fig. 3.
Effect of positive sera from prediabetic rats on cell viability. Cell viability was measured with an MTT assay in islet cells taken from control rats incubated for 24 h with pos 60-d sera (n = 7), neg 60-d sera (n = 6), neg 50-d sera (n = 5), and neg 40-d sera (n = 7). Statistical significance was evaluated by Student's t test, and P values < 0.05 were considered significant. Data are presented as means ± SEM. ***P < 0.001, *P < 0.05.
Treatment with Antisense to apoCIII.
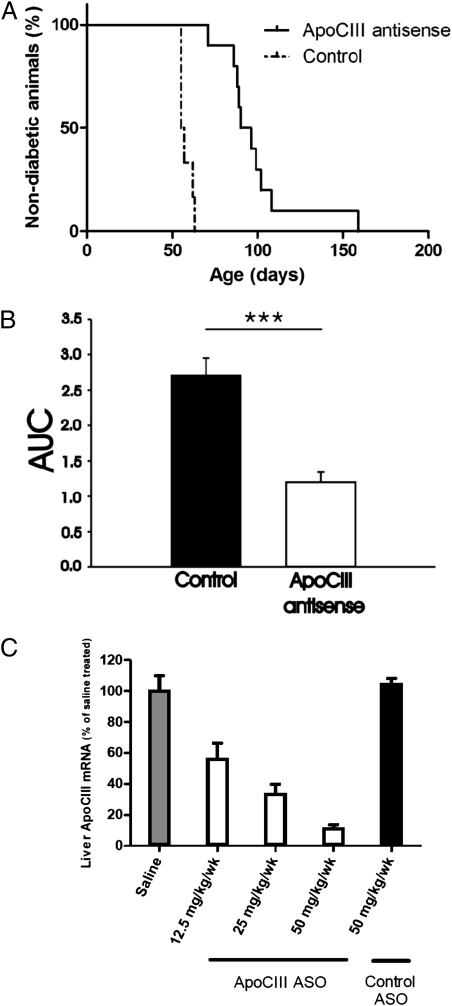
The median time to onset of diabetes was significantly prolonged to 93 d (range 71–158) in DPBB rats treated with antisense against apoCIII compared with the control group that received inactive antisense and had a median debut age of 56 d (range 55–63 d) (Fig. 4A). Blood samples taken at the last day of treatment, when the rats were 40 d old, confirmed that the active treatment had lowered the levels of endogenous apoCIII (Fig. 4B). The efficacy of the antisense in reducing mRNA expression of apoCIII in the liver was evaluated before we started the treatment (Fig. 4C).
Fig. 4.
Treatment with antisense to apoCIII delays onset of T1D and decreases apoCIII. (A) The median time to onset of T1D in the antisense treated group was 93 d (n = 10) versus 56 d in the control group (n = 6); P < 0.001. (B) Analysis of relative levels of apoCIII in blood samples taken at the last day of treatment (n = 5), when the rats were 40 d, reveals a significant lowering in the treated group given as area under the curve (AUC). A Kaplan and Meier curve was used to estimate diabetes incidence after antisense treatment, and the effect of treatment was evaluated with a log-rank (Mantel–Cox) test. P values < 0.05 were considered significant.***P < 0.001. (C) The mRNA expression of apoCIII in livers from rats treated with saline (gray bar), three different concentrations of the active apoCIII antisense oligonucleotide (ASO) (white bars), or control inactive ASO (black bar) (n = 6). The expressions are presented as the percentage of saline treated ± SEM.
Discussion
The aim of this study was to clarify the extent to which our previous findings—namely that apoCIII is increased in serum from T1D patients and that it affects the function and survival of pancreatic β cells (1, 2) in vitro—is true also in an in vivo model for T1D. Of the available animal models for T1D, the DPBB rat fulfills most of the criteria for the human form of the disease (5). In our strain, the time window, when the rats become diabetic, is narrow with an average time of around 60 d, and the incidence is 100%, which is important when investigating preventive measures.
To follow the development of diabetes, we studied three prediabetic age groups, namely 40, 50, and 60 d, with the first group relatively far from, and the last group close to, onset of disease. The glucose-stimulated insulin release data, with intermittent recovery of hormone secretion in 50-d-old prediabetic rats, were in agreement with the morphology of the pancreatic sections where there were a lower number of insulin-containing cells in islets from 40- and 60-d-old rats and no obvious difference in the 50-d-old rats, compared with age-matched controls. This phenomenon of a temporary improvement in islet function has been observed previously (6), and there are several studies showing increased cell replication before onset of T1D (7–10). The autoimmune attack with the inflammatory cell response has been suggested to be responsible for this stimulated cell proliferation (7–10).
When measuring changes in [Ca2+]i in islet cells cultured overnight in the presence of prediabetic sera, nearly 60% of the sera from 60-d-old DPBB rats induced a higher increase upon depolarization, as a result of activation of the voltage-gated Ca2+-channels. In our human material, including sera from both diabetic children and adults, ∼30% induced this effect on cellular Ca2+ handling (11). The human material had a heterogeneous genetic background whereas our BB rats are inbred. Hence, the most likely reason for not seeing the effect in all tested sera is that, although day 60 is close to onset of T1D, there is a variation of some days between the individual animals before they become diabetic. Our results in this study regarding [Ca2+]i changes and cell viability indicate that the detrimental effect of prediabetic sera comes very close to disease debut.
The amount of apoCIII was increased in DPBB rat sera, and to decrease the endogenous levels, we treated the prediabetic rats from an early age of 12 d until day 40 with an antisense against apoCIII. The amount of apoCIII in sera was measured at the last day of treatment and confirmed that the levels were significantly lower than in control animals and that this decrease resulted in a prolongation of the nondiabetic period. These results corroborate our in vitro findings, namely that apoCIII is involved in β-cell destruction in vivo and thereby may play an essential role in the development of T1D. The time to onset was increased on average by 40 d, which is highly significant considering the life span of a rat. This would be comparable to a delay of several years in a human being, which is important because every year without diabetes reduces the risk for developing complications. Why there was not a longer delay to onset or a total prevention of the disease could be due to several reasons, including whether the duration of treatment and the doses given were adequate. In a first treatment trial, the animals were injected with the same doses, but with the aim to continue until onset of diabetes. However, in some animals, we noted a deterioration of their conditions with an enlargement of the spleen as the most severe symptom after the prolonged treatment period. Interestingly, these side effects were seen in only DPBB and never in DRBB rats. We therefore chose to shorten the treatment period, and this eliminated all side effects in the animals. A way in which to decrease apoCIII levels after the regular treatment could be to give intermittent bolus doses, which will be the focus of future studies.
This study confirms that our previous results demonstrating the harmful effects of apoCIII in β cells in vitro (2) are also valid for the in vivo situation as a lowering of the lipoprotein in prediabetic animals prolongs the time to onset of disease. These results are interesting because it has been shown that an apoCIII haplotype block in the promoter region of the apoCIII gene, leading to greater levels of the lipoprotein, is associated with TID (12). On the contrary, about 5% of the Lancaster (PA) Amish population are heterozygous carriers of a null mutation (R19X) in the gene encoding apoCIII, and this results in a lifelong 50% reduction of the apoCIII levels and an apparent cardioprotection (13). Furthermore, a group of Ashkenazi Jewish centenarians was found to have a higher prevalence of homozygosity for the-641C allele in the apoCIII promoter, a genotype associated with significantly lower levels of apoCIII, a favorable pattern of lipoproteins, increased insulin sensitivity, lower incidence of hypertension, and longevity (14). Therefore, it could be beneficial from multiple aspects to lower the apoCIII levels in individuals at risk for diabetes and/or cardiovascular disorders.
Materials and Methods
Animals.
BB rats were obtained from our breeding colony at Karolinska Institutet. The incidence of diabetes among our DPBB rats is 100%, and the mean age of onset is 60 d. The control rats, DRBB, do not develop diabetes. The animals were housed under specific pathogen-free conditions in a temperature- and humidity-controlled room with 12-h light:dark cycles. They were fed the R36 diet and water ad libitum. All experiments were carried out according to the Animal Experiment Ethics Committees at Karolinska Institutet.
The prediabetic rats were divided into three age groups: 40, 50, and 60 d old. Blood glucose was measured to ensure that the rats were still in the prediabetic stage before blood was withdrawn by cardiac puncture. Sera were collected and identically heat-inactivated for 30 min at 56 °C. Aliquots were frozen at –20 °C until use.
Media.
The medium used for isolation of pancreatic islets and in the experiments was a Hepes buffer (pH 7.4) containing (in mM) 125 NaCl, 5.9 KCl, 1.2 MgCl2, 1.28 CaCl2, and 3 glucose. BSA was added at a concentration of 1 mg/mL. For cell culture, S-MEM or RPMI 1640 culture medium supplemented with 100 μg/mL streptomycin, 100 μg/mL penicillin, 2 mmol/L glutamine, and 10% of the different sera were used.
Preparations of Islets and Cells.
Pancreatic islets from prediabetic and control BB rats were isolated by a collagenase technique (15) and, when islet cells were used, disrupted into cells by Cell Dissociating Buffer (Gibco). Islets were kept free-floating in petri dishes, and cells were seeded onto either coverslips or multiwell plates coated with poly-l-lysine (Sigma).
Histological and Immunohistochemical Techniques.
Pancreatic tissue were removed and fixed in 4% formaldehyde solution and embedded in paraffin. Sections (4 μm thick) were stained with hematoxylin and eosin and van Gieson's stain for collagen. The pancreatic β cells were detected by immunohistochemistry. The tissue sections were incubated with an insulin antibody from guinea pig (diluted 1:200) (Dako) overnight. The antibody binding in the sections was visualized using a standard avidin–biotin complex method (Vectastain, Vector Laboratories), using diaminobenzidin as chromogen.
Insulin Release.
Islets taken from control and prediabetic rats were incubated overnight in RPMI 1640 culture medium with 10% sera taken from the same animal as the islets. Dynamics of insulin release were studied by perifusing islets, mixed with Bio-Gel P4 polyacrylamide beads (Bio-Rad), in a 0.5-mL column at 37 °C (16). The flow rate was 0.2 mL/min, and 2-min fractions were collected and analyzed for insulin by RIA using a rat insulin standard (Novo Nordisk).
Measurements of [Ca2+]i.
Islet cells were isolated from control rats, attached to coverslips, and exposed to 10% prediabetic or age-matched control sera overnight. The cells were loaded with 2 μM fura-2/acetoxymethylester (Molecular Probes) for 30 min and mounted on an inverted Zeiss Axiovert epifluorescence microsope connected to a Spex Fluorolog-2 system for dual-wavelength excitation fluorimetry. The emissions due to the two excitation wavelengths of 340 and 380 nm were used to calculate the fluorescence ratio 340/380, reflecting changes in [Ca2+]i (17).
Determination of Cell Viability.
Islet cells were isolated from control rats. The cells were seeded into a 96-well plate (5,000 cells/well) and exposed to 10% pos or neg sera from 60-d-old prediabetic rats as well as neg sera from 40- and 50-d-old rats and age-matched control sera. After 24 h, viability was determined by MTT assay. The assay is based on conversion of the MTT tetrazolium salt to a colored formazan product by the mitochondrial enzyme succinate dehydrogenase and represents a measure of cellular oxidative capacity (18).
Quantification of apoCIII in Sera.
To evaluate the levels of apoCIII in pos, neg, and control sera, we used the Montage Albumin Deplete Kit (Millipore) or AlbuSorb (Biotech Support Group) that removes albumin from serum samples. The collected samples from the columns were freeze-dried overnight and run on sep-Pak C18. The eluted proteins were freeze-dried and thereafter dissolved in 100 μL 0.1% TFA and run on an ACE C18 10- × 0.21-cm column 20–60%, and the area under the curve, where apoCIII elutes, was evaluated. ApoCIII was identified by MALDI mass spectrometry.
Rat-Specific apoCIII Antisense Oligonucleotides.
A series of chimeric 20-mer phosphorothioate oligonucleotides containing 2′-O-methoxyethyl groups at positions 1–5 and 16–20 targeted to rat apoCIII were synthesized and purified as described (19) with an automated DNA synthesizer (380B; Perkin-Elmer Applied Biosystems). The antisense oligonucleotide (ASO), ISIS 353982 (5′-GAGAATATACTTTCCCCTTA-3′), effectively reduced mRNA expression of apoCIII in the liver, and was therefore used for all subsequent in vivo pharmacological assessments. ISIS 141923 (5′-CCTTCCCTGAAGGTTCCTCC-3′), which is in the same chemical and mechanistic class as the apoCIII compound but not complementary to any known gene sequences, was used as a control ASO.
In Vivo Treatment with Antisense to apoCIII.
DPBB rats were treated with apoCIII antisense (ISIS 353982) or an inactive control (ISIS 141923), n = 10 and 6, respectively. The substances were administrated i.p. twice per week between the ages of 12 and 40 d. The total dose given per week was 25 mg/kg bodyweight. Blood samples for analysis of apoCIII were taken at day 40.
Statistics.
All statistical significance, except the evaluation of the antisense treatments, was evaluated by Student's t test, and P values < 0.05 were considered significant. Data are presented as means ± SEM.
The product limit method of Kaplan and Meier was used to estimate diabetes incidence after antisense treatment, and the effect of the treatment was evaluated with a log-rank (Mantel–Cox) test. P values < 0.05 were considered significant.
Acknowledgments
We thank Kristina Edwardsson and Anna-Lena Gustafsson for their skills in caring for the animals involved in these studies. This work was supported by funds from Barndiabetesfonden, the Swedish Diabetes Association, Karolinska Institutet, the Swedish Research Council, the Swedish Society for Medical Research, the Novo Nordisk Foundation, The Erling-Persson Family Foundation, the Strategic Research Program in Diabetes at Karolinska Institutet, the Knut and Alice Wallenberg Foundation, the Skandia Insurance Company, Ltd., TONECA, Eurodia FP6-518153, VIBRANT (FP7-228933-2), and the Juvenile Diabetes Research Foundation International.
Footnotes
Conflict of interest statement: P.-O.B. is a co-founder of Biocrine, a company that is developing apoCIII as a target for the treatment of type 1 diabetes and its complications. R.M.C. and M.G. are affiliated with ISIS, a company whose main focus is to develop antisense treatment strategies for various diseases.
*This Direct Submission article had a prearranged editor.
References
- 1.Juntti-Berggren L, et al. Increased activity of L-type Ca2+ channels exposed to serum from patients with type I diabetes. Science. 1993;261:86–90. doi: 10.1126/science.7686306. [DOI] [PubMed] [Google Scholar]
- 2.Juntti-Berggren L, et al. Apolipoprotein CIII promotes Ca2+-dependent beta cell death in type 1 diabetes. Proc Natl Acad Sci USA. 2004;101:10090–10094. doi: 10.1073/pnas.0403551101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakhooda AF, Like AA, Chappel CI, Murray FT, Marliss EB. The spontaneously diabetic Wistar rat. Metabolic and morphologic studies. Diabetes. 1977;26:100–112. doi: 10.2337/diab.26.2.100. [DOI] [PubMed] [Google Scholar]
- 4.Nakhooda AF, Like AA, Chappel CI, Wei CN, Marliss EB. The spontaneously diabetic Wistar rat (the “BB” rat). Studies prior to and during development of the overt syndrome. Diabetologia. 1978;14:199–207. doi: 10.1007/BF00429781. [DOI] [PubMed] [Google Scholar]
- 5.Mordes JP, Desemone J, Rossini AA. The BB rat. Diabetes Metab Rev. 1987;3:725–750. doi: 10.1002/dmr.5610030307. [DOI] [PubMed] [Google Scholar]
- 6.Reddy S, Bibby NJ, Fisher SL, Elliott RB. Longitudinal study of first phase insulin release in the BB rat. Diabetologia. 1986;29:802–807. doi: 10.1007/BF00873220. [DOI] [PubMed] [Google Scholar]
- 7.Bone AJ, Walker R, Dean BM, Baird JD, Cooke A. Pre-diabetes in the spontaneously diabetic BB/E rat: Pancreatic infiltration and islet cell proliferation. Acta Endocrinol (Copenh) 1987;115:447–454. doi: 10.1530/acta.0.1150447. [DOI] [PubMed] [Google Scholar]
- 8.Sherry NA, et al. Effects of autoimmunity and immune therapy on beta-cell turnover in type 1 diabetes. Diabetes. 2006;55:3238–3245. doi: 10.2337/db05-1034. [DOI] [PubMed] [Google Scholar]
- 9.Ablamunits V, Sherry NA, Kushner JA, Herold KC. Autoimmunity and beta cell regeneration in mouse and human type 1 diabetes: the peace is not enough. Ann N Y Acad Sci. 2007;1103:19–32. doi: 10.1196/annals.1394.006. [DOI] [PubMed] [Google Scholar]
- 10.Pechhold K, et al. Blood glucose levels regulate pancreatic beta-cell proliferation during experimentally-induced and spontaneous autoimmune diabetes in mice. PLoS ONE. 2009;4:e4827. doi: 10.1371/journal.pone.0004827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dekki N, et al. Type 1 diabetic serum interferes with pancreatic beta-cell Ca2+-handling. Biosci Rep. 2007;27:321–326. doi: 10.1007/s10540-007-9055-y. [DOI] [PubMed] [Google Scholar]
- 12.Hokanson JE, et al. Susceptibility to type 1 diabetes is associated with ApoCIII gene haplotypes. Diabetes. 2006;55:834–838. doi: 10.2337/diabetes.55.03.06.db05-1380. [DOI] [PubMed] [Google Scholar]
- 13.Pollin TI, et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322:1702–1705. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atzmon G, et al. Lipoprotein genotype and conserved pathway for exceptional longevity in humans. PLoS Biol. 2006;4:e113. doi: 10.1371/journal.pbio.0040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lernmark A. The preparation of, and studies on, free cell suspensions from mouse pancreatic islets. Diabetologia. 1974;10:431–438. doi: 10.1007/BF01221634. [DOI] [PubMed] [Google Scholar]
- 16.Kanatsuna T, Lernmark A, Rubenstein AH, Steiner DF. Block in insulin release from column-perifused pancreatic beta-cells induced by islet cell surface antibodies and complement. Diabetes. 1981;30:231–234. doi: 10.2337/diab.30.3.231. [DOI] [PubMed] [Google Scholar]
- 17.Kindmark H, et al. Protein kinase C activity affects glucose-induced oscillations in cytoplasmic free Ca2+ in the pancreatic B-cell. FEBS Lett. 1992;303:85–90. doi: 10.1016/0014-5793(92)80483-w. [DOI] [PubMed] [Google Scholar]
- 18.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 19.Baker BF, et al. 2′-O-(2-Methoxy)ethyl-modified anti-intercellular adhesion molecule 1 (ICAM-1) oligonucleotides selectively increase the ICAM-1 mRNA level and inhibit formation of the ICAM-1 translation initiation complex in human umbilical vein endothelial cells. J Biol Chem. 1997;272:11994–12000. doi: 10.1074/jbc.272.18.11994. [DOI] [PubMed] [Google Scholar]