Abstract
In many species that form socially monogamous pair bonds, a considerable proportion of the offspring is sired by extrapair males. This observation has remained a puzzle for evolutionary biologists: although mating outside the pair bond can obviously increase the offspring production of males, the benefits of such behavior to females are less clear, yet females are known to actively solicit extrapair copulations. For more than two decades adaptionist explanations have dominated the discussions, yet remain controversial, and genetic constraint arguments have been dismissed without much consideration. An intriguing but still untested hypothesis states that extrapair mating behavior by females may be affected by the same genetic variants (alleles) as extrapair mating behavior by males, such that the female behavior could evolve through indirect selection on the male behavior. Here we show that in the socially monogamous zebra finch, individual differences in extrapair mating behavior have a hereditary component. Intriguingly, this genetic basis is shared between the sexes, as shown by a strong genetic correlation between male and female measurements of extrapair mating behavior. Hence, positive selection on males to sire extrapair young will lead to increased extrapair mating by females as a correlated evolutionary response. This behavior leads to a fundamentally different view of female extrapair mating: it may exist even if females obtain no net benefit from it, simply because the corresponding alleles were positively selected in the male ancestors.
Keywords: behavioral genetics, mating systems, promiscuity, quantitative genetics, sexual behavior
A contentious question in evolutionary biology is why females in most socially monogamous species mate with males other than their social mates (1–6). The latest reviews of the field (1, 2, 6) suggest that extrapair mating might sometimes even be maladaptive to females, because females may suffer higher costs (e.g., withdrawal of paternal care, sexually transmitted diseases, and predation risk) than they benefit from fertility insurance or from improved genetic quality of extrapair offspring. Given that in many species females actively seek extrapair copulations (4, 7), this poses the question of how female extrapair mating could have evolved. Two hypotheses based on genetic constraints have been proposed to resolve this evolutionary puzzle: the “between-sex” and the “within-sex” genetic correlation hypotheses.
The between-sex genetic correlation hypothesis states that male and female extrapair mating behaviors might be affected by the same set of genetic variants (8). Alleles that enhance promiscuous behavior in males are likely to be under strong positive selection (9), and those sexually selected alleles could also cause promiscuous behavior in females (because of pleiotropic effects). This hypothesis assumes promiscuous behavior to be heritable (10, 11) and to be positively genetically correlated between the sexes. Sexually antagonistic selection on male versus female promiscuity would promote the evolution of mechanisms that weaken this genetic correlation (12, 13), but any remaining correlation would represent a genetic constraint that prevents the sexes from reaching their respective behavioral optima. Under this scenario, female extrapair mating behavior would evolve through indirect selection on male extrapair mating behavior (14), even when extrapair mating is maladaptive from the female perspective.
The within-sex genetic correlation hypothesis states that a female's response to being courted by her partner might be affected by the same alleles that affect her response to courtship by extrapair males (2, 7). If this would be the case, female resistance to extrapair courtship might be selected against, because resistance alleles would also convey resistance to within-pair copulations and would therefore lead to infertility. As a consequence, resistance to extrapair courtship would not evolve because of its nonindependence from within-pair responsiveness.
Surprisingly, these important hypotheses, which appear to be the most viable explanations for maladaptive female promiscuity (2), have never been tested empirically in a species with socially monogamous pair bonds (where extrapair mating is to be explained). However, empirical testing requires extensive data on extrapair mating behavior, which cannot easily be gathered in the wild. Hence, we here use a captive population of zebra finches, where we can supplement data on realized levels of extrapair paternity with direct behavioral measurements that closely reflect individual differences in the readiness to engage in within- and extrapair copulations. We expected a higher heritability of such behavioral predispositions, as opposed to measurements of levels of extrapair paternity, because the latter will additionally depend on a multitude of confounding factors (10, 11). The proportion of extrapair offspring produced by a female will, for example, depend on her partner's mate-guarding effort and on the competitiveness of his sperm. Likewise, a male's success in siring extrapair offspring might not just depend on his mating effort but also on his attractiveness to extrapair females. In contrast, behavioral measures of female and male propensity to engage in extrapair matings should more directly reflect the underlying genetic predisposition for promiscuity. Therefore, the inclusion of behavioral data should facilitate establishing the magnitude and sign of genetic correlations.
Individual male zebra finches are known to differ markedly in their readiness to court unfamiliar females (15). These consistent individual differences in courtship rate are partly genetic (16) and affect male success in obtaining extrapair copulations (7), not because these males differ in their attractiveness to females, but because of individual differences in the number of extrapair copulation attempts (i.e., mating effort) (7). Our aim was to establish whether a female's sexual responsiveness to an extrapair male's courtship is genetically linked to the genetic differences in male propensity to seek extrapair copulations, as suggested by the between-sex genetic correlation hypothesis.
We studied a captive population of zebra finches that comprises over 1,500 individuals from five consecutive generations. Using pedigree information and experimental cross-fostering, we statistically separate genetic from environmental factors that contribute to individual differences in mating behavior. We used “animal models” (17) to estimate heritability and genetic correlations.
To measure individual differences in male courtship rate and in female sexual responsiveness, we first staged brief encounters between bachelor males and bachelor females. For 800 males and 754 females, we repeatedly measured how much males displayed by singing to females (“courtship rate”), and how positively (e.g., solicitation) or negatively (e.g., aggression) females responded to such courtship (“unpaired response”). Previous work (7) suggested that these standardized and highly repeatable measurements partly reflect a male's ability in obtaining extrapair copulations and a female's readiness to accept extrapair copulations, respectively.
In a second step we explored, for a subset of birds (152 males, 155 females), whether these standardized measurements on bachelor individuals actually reflect their sexual fidelity when in a monogamous pair bond in communal breeding aviaries (i.e., in a socially complex, and hence biologically relevant environment). Using extensive video-monitoring of color-banded birds, we observed how monogamously paired females responded to courting by their partner (within-pair response) and by extrapair males (extrapair response). Additionally, we applied genetic parentage analysis (using microsatellite markers) to determine for each male the total number of extrapair young sired (male extrapair paternity), and for each female the proportion of her eggs sired by extrapair males (female extrapair paternity).
Results
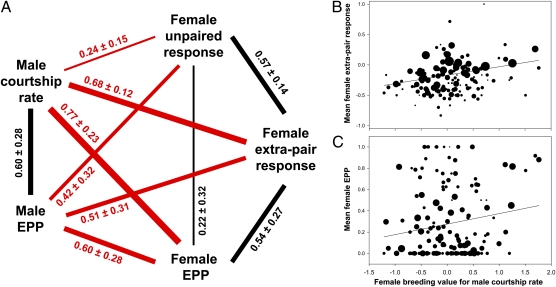
The sample sizes and distributions of all of the behavioral and paternity data are summarized in Table S1 (see Table S2 for parameter estimates of fixed effects). In males, we found that, at the genetic level, courtship rate measured in standardized tests was strongly positively related to male success in siring extrapair offspring (genetic correlation rA = 0.60 ± 0.28 SEM) (Fig. 1A and Tables S3–S6). This finding is in line with earlier findings at the phenotypic level showing that males with high courtship rate obtain more extrapair copulations because they make more attempts (7). In females, the responsiveness to males when socially unpaired (unpaired response) was not strongly correlated with extrapair paternity levels (rA = 0.22 ± 0.32), although the measurement of response to extrapair males in the communal aviaries (extrapair response) was a stronger genetic correlate of female extrapair paternity levels (rA = 0.54 ± 0.27). Hence, our measurements of male courtship rate and female response to extrapair courtship are closely related to patterns of paternity and could evolve through indirect selection on genetic parentage.
Fig. 1.
Genetic correlations between aspects of male and female extrapair mating behavior. (A) Average estimates of genetic correlations (± SEM) obtained from a series of five-trait animal models (Tables S3–S6). Between-sex genetic correlations (red) among traits related to extrapair mating are large and positive. For illustration, we show some of the data on which the most crucial estimates for between-sex correlations are based (B and C). (B) The average responsiveness score of 141 females when courted by an extrapair male (total n = 3,958 courtships) in relation to their estimated breeding value for male courtship rate. Breeding values are from a single-trait permanent environment model conducted in VCE (based on courtship rates from a total of 800 male relatives). Responsiveness scores vary from −1 (aggression) to +1 (solicitation). Dot size refers to the number of extrapair courtships observed for each female (range: 1–138, median: 19). A weighted regression line is shown (r = 0.33). (C) The average proportion of extrapair paternity among the eggs laid by 149 females (total n = 2,253 eggs) in relation to their estimated breeding value for male courtship rate. Dot size refers to the number of eggs laid by each female (range: 1–45, median: 14). A weighted regression line is shown (r = 0.19), suggesting a 2.9-fold increase in extrapair paternity levels over the observed range of female breeding values of male courtship rate. The large amount of scatter is because of the many other factors that influence paternity besides heritable differences in female extrapair mating behavior.
Between-Sex Genetic Correlation Hypothesis.
In strong support of the hypothesis that male and female extrapair mating are not genetically independent (8), we found large positive genetic correlations between the sexes in terms of levels of extrapair paternity and measurements of behavior (Fig. 1). Because, as expected, behavioral measurements generally showed a higher heritability than paternity measurements (Tables S5 and S6), estimates of the between-sex genetic correlations are more reliable for the former (as reflected in smaller SEMs). This overall pattern of strong positive between-sex genetic correlations is robust to changes in statistical methodology (Tables S3–S6).
Within-Sex Genetic Correlation Hypothesis.
In disagreement with the hypothesis that extrapair responsiveness cannot evolve independently of within-pair responsiveness (2, 7), our animal models suggest that extrapair and within-pair responsiveness are not positively genetically correlated (rA estimates vary from −0.19 ± 0.51 to 0.07 ± 0.28) (Tables S7 and S8). However, the large SEs currently preclude a decisive interpretation.
Discussion
Although our data do not confirm the within-sex genetic correlation hypothesis, the between-sex genetic correlation hypothesis is strongly supported. Male and female promiscuity cannot evolve independently from each other to the extent that the two traits are genetically correlated. Because the between-sex correlations were less than unity, there is a remaining fraction of additive genetic variance in at least one sex (but not necessarily in both sexes) that could evolve independently of selection on promiscuity in the other sex. Hence, female promiscuity may not entirely be attributable to a genetic constraint, but the constraint is large enough to be evolutionarily relevant.
Pleiotropy or Linkage Disequlibrium.
The positive genetic correlations between measures of male and female extrapair mating behavior may come about through three mechanisms. (i) Male and female extrapair mating propensities may be homologous traits, and alleles that enhance male promiscuity may also enhance the promiscuity of females carrying those alleles (pleiotropic effects) (8). (ii) Alleles that enhance male promiscuity may be in strong linkage disequilibrium with alleles that enhance female promiscuity; this might occur when the respective loci are strongly physically linked. Practically, the effect of two such closely linked alleles would be difficult to distinguish from the pleiotropic effect of a single allele. (iii) Linkage disequilibrium between physically unlinked alleles for male and female promiscuity because of assortative mating may also contribute to the genetic correlation between the sexes. Some linkage disequilibrium is expected to occur because extrapair young, as a rule, arise out of matings between a promiscuous male and a promiscuous female (hence, “assortative mating”). In our captive population, this assortative mating was partly prevented because many individuals in the population were not bred in communal aviaries, but rather in enforced cage pairings (see Methods). Thus, the genetic correlation between the sexes could even be higher in natural populations because of more opportunities for creating linkage disequilibrium. However, the rather low heritabilities of male and female promiscuity measures (Tables S5 and S6) imply that assortative mating at the phenotypic level will result in much weaker assortative mating at the genotypic level. Hence, the high observed between-sex genetic correlations cannot result from linkage disequilibrium because of assortative mating alone.
Pair-Bond Strength and Sexual Arousability.
The original proposition that female promiscuity might evolve as a genetic corollary of selection on male promiscuity (8, 18) has been criticized for its assumption that the same physiological mechanisms would underlie mating behavior in each sex (19). In apparent support of this criticism, a range of studies have failed to find a positive genetic correlation between male and female mating speed or mating frequency in chicken (20), Drosophila (21), stalk-eyed flies (22), and bean beetles (23). Only one study on mating frequency in burying beetles (24) has found a strong between-sex genetic correlation, although mating frequency was measured here with the same partner rather than with different partners.
The zebra finch differs from all these study systems in two important aspects. (i) Zebra finches form strong social pair bonds that usually last for a lifetime (7, 25). If social pair bonding evolved from a nonpair-bonding ancestral condition, then it likely did so by a genetic mechanism that was shared between the sexes (thus evolving in both sexes at the same time). Mutations affecting that mechanism might weaken or strengthen the pair bond and thereby increase or decrease promiscuity in both sexes in a correlated manner. More specific data (e.g., measures of attachment to the partner) would be required to examine whether variation in pair-bond strength lies at the heart of the between-sex genetic correlations we found. (ii) When female zebra finches are sexually motivated, they engage in a mutual courtship dance with a male and show a range of behaviors that characterize male courtship behavior, such as wiping the beak on the perch and hopping in a ritualized manner with the head and tail bent toward the partner. We interpret these apparently homologous behaviors as indicators of sexual motivation and arousal, because in the female they strongly predict the occurrence of copulation solicitation and in the male they precede most of the copulation attempts. Therefore, it seems possible that genetic variation in sexual arousability underlies the between-sex genetic correlations. To our knowledge, between-sex homology of courtship behavior is not apparent in any of the above-mentioned study organisms.
In humans, individual differences in attachment style, fidelity, and sociosexuality are known to have a hereditary basis (26–29). The degree to which variation in physiological mechanisms of attachment (30, 31) and of sexual arousal (32, 33) is shared between the sexes is not sufficiently known to predict whether between-sex correlations can be expected. At the phenotypic level, sexual fidelity correlates with several of the major axes of personality variation (extraversion, agreeableness, and conscientiousness) and, importantly, these correlations are largely consistent between the sexes (34, 35). The apparent multitude of aspects of personality that may influence sexual fidelity is in agreement with the hypothesis of Halliday and Arnold (8, 18): such a genetically complex trait would represent a large target for new mutations that would typically have similar (correlated) effects on both sexes.
Costs and Benefits of Extrapair Mating.
There is an ongoing debate about whether females obtain a net benefit from extrapair mating or not (2, 36–38). Our finding of a strong between-sex genetic correlation does not provide an answer to this question. However, the finding may serve as an explanation for the evolution of female extrapair mating in systems where the behavior appears maladaptive to females. Specifically, measurements of fitness consequences of the alleles affecting a female's extrapair mating behavior will be incomplete when not considering the effect these alleles have on the fitness of her male relatives.
The finding that promiscuous females will also produce promiscuous sons highlights the importance of considering indirect genetic benefits (i.e., a “promiscuous-son benefit,” similar to the “sexy-son benefit” in Fisher's runaway sexual selection) (39, 40). However, our results also call for caution to not overestimate the size of such an effect. As mentioned above, a male's extrapair siring success might not only depend on his courtship effort, but also on his attractiveness, which may be unrelated to courtship effort (7). In our aviary population, a male's total fitness (approximated as the total number of eggs sired) was closely related to the number of extrapair eggs sired (rp = 0.84, n = 152, P < 0.0001), which is a rather trivial part-whole correlation [sensu (41)]. In contrast, total fitness was only weakly positively correlated with courtship rate (a predictor of mating effort measured earlier in cages; rp = 0.18, n = 152, P = 0.023). The remarkably high genetic correlation (rA = 0.60) (Fig. 1A) between courtship rate and extrapair siring success implies that a high proportion of the additive genetic variation in extrapair siring success is because of genetic variation in courtship rate. Despite strong selection on extrapair siring success, the genetic variation in courtship rate may not be eroded because a high courtship effort may be expensive in terms of energy, time allocation, and vigilance costs. Furthermore, a high courtship effort will often not be rewarded in terms of obtaining copulatory access to fertile females, because this intrinsic variation in courtship effort occurs independently of variation in male attractiveness (7). Thus, alleles for high courtship rate will be positively selected in males, but only to the extent allowed by the costs (to males and females).
In our system, male extrapair siring success was under strong positive selection (see above), and this was not counteracted by a possible trade-off with the growth of within-pair chicks (correlation of extrapair siring success with offspring mass at day 8 after hatching: rp = 0.20, n = 114, P = 0.037; see ref. 42 for methods). In contrast, females with a high proportion of extrapair young did not lay more eggs (rp = 0.02, n = 140, P = 0.84) and tended to raise lighter offspring (rp = −0.13, n = 109, P = 0.16; all untransformed phenotypic correlations). Hence, extrapair mating was strongly related to aspects of fitness in males but not in females. However, it should be kept in mind that these estimates of fitness consequences are environment-specific and may be different in wild populations.
In the wild, zebra finches show much lower levels of extrapair paternity (2% of the offspring) (43, 44) than in captivity (28%) (45), although the extrapair behavior seemed remarkably similar between those two situations (7, 45, 46). Wild-caught birds studied in captivity showed intermediate levels of extrapair paternity (12–15%) (47), suggesting that both captivity and domestication may contribute to differences in extrapair paternity levels. However, this does not compromise the validity of our findings. We demonstrate that male and female mating behaviors are genetically correlated in a captive environment, and although genotype by environment interactions may occur, it is likely that this positive genetic correlation will also be manifested in at least some natural environments. Our study is a proof of principle that indirect selection on extrapair mating behavior can operate. Such indirect selection can work in several ways, of which two are particularly intriguing. (i) Positive selection on male promiscuity might lead to a correlated evolution of female promiscuity, despite possible net fitness costs to females. (ii) Conversely, strong selection against female promiscuity [e.g., because of male retaliation (48)], may lead to correlated effects on males, in the sense that males would forego opportunities for extrapair mating. Such maladaptive male behavior, however, was not observed in our study (SI Text), and we are not aware of other systems where this is a common phenomenon.
Methods
The study was approved by the Animal Care and Ethics Committee of the Max Planck Institute for Ornithology.
Subjects.
The origin of the birds, and rearing and housing conditions have been described in detail elsewhere (49–52). For the present study, we used 1,554 zebra finches from five consecutive generations (208 parental, 299 F1, 503 F2, 140 F3, and 404 F4), which is 97.3% of all of the birds in our captive population that survived to adulthood (> 100 d). In the first three generations, most of the birds (parental 72%, F1 99%, F2 100%) had been raised by foster parents. Initially (parental), cross-fostering involved swapping half clutches shortly after laying, but in the following two generations (F1 and F2) eggs were swapped individually, such that all foster-siblings were unrelated to each other (50). However, this practice of cross-fostering was abandoned from the F3 generation onwards, after recognizing that sexual behaviors were not influenced by the nest of rearing or by the identity of the foster parents (see below). Most of the birds (57%) originated from pairs breeding in separate individual cages; the rest came from breeding in large communal aviaries, where parentage was established using microsatellite markers (see Paternity Analysis). After reaching independence on day 35 of age, birds were either reared in mixed-sex peer groups of about equal sex ratio (53%) or in unisex peer groups (47%). Sometimes these different rearing conditions had a small effect on sexual behavior, which we accounted for statistically (see below). Given that those main effects were very small and often nonsignificant, despite large power, we conclude that these rearing conditions are insignificant for the development of sexual behavior. Hence, we consider significant G × E interactions related to these rearing conditions unlikely. A small number of individuals (3.5%) originated from cases of close inbreeding (up to F = 0.25), so we accounted for the coefficient of inbreeding (F, as calculated from a six-generation pedigree) as a covariate for all traits that showed a tendency toward inbreeding depression (53).
Behavioral Observations.
Traits related to extrapair mating were measured in the following two experimental set-ups that differ in social complexity, but also in measuring effort and precision. The high demands of quantitative genetic analyses on the data require the joint analysis of the biologically most relevant measures from complex environments (aviary observations: low number of individuals and low repeatabilities, but many observations per individual) with the related but more standardized measures (cage experiments: high number of individuals and high repeatabilities).
Cage Experiments on Bachelor Birds.
We launched a total of 3,776 male-female encounters (each lasting 5 min; for details see ref. 15), organized in 11 experimental batches conducted between July 2002 and May 2010, and involving 800 socially unpaired males and 754 socially unpaired females. In these tests, males encountered on average 4.7 ± 1.7 SD (range 2–8) different females, and females encountered on average 5.0 ± 2.2 SD (range 1–14) different males. For each trial we measured the total duration (in seconds) of male courtship: that is, song directed toward the female (referred to as “male courtship rate”). Female responsiveness was scored following ref. 7 on a five-point scale, where −1 represents a clear rejection (involving strong aggression, threat, or fleeing) and +1 a clear acceptance (involving copulation solicitation, beak wiping, and ritualized hopping). Responsiveness could be scored for 3,168 (84%) of all trials, and we refer to this measurement as “unpaired response” because it involves socially unpaired (bachelor) females.
Aviary Breeding Experiments.
For periods of 3 to 4 mo, groups of zebra finches were bred in aviaries (nine aviaries each in 2005 and 2006, and six aviaries in 2007, 2008, and 2009; 36 aviary seasons in total). Aviaries contained six males and six females, except in 2005 and 2006, when three aviaries held an additional three females (sex ratio 0.4), and another three held an additional three males (sex ratio 0.6) (54). Subsequent analyses control for this sex-ratio treatment. Birds in aviaries were always unfamiliar to each other (but not always unrelated), as familiarity (but not relatedness) has strong effects on mating behavior (52). For these aviary-breeding experiments, we selected a total of 176 males and 180 females (including a few replacements for birds that died) from the three central generations (F1–F3) of our pedigree. Individuals were selected so that all F3 birds would have two generations of ancestors with data from aviary experiments. Birds of the F1-generation were used in two consecutive breeding seasons (2005 and 2006), and F2- and F3-birds bred for only one season. In our final analyses we include behavioral and extrapair paternity data from 152 males and 155 females, which, based on daily observations, formed a socially monogamous pair bond (25).
Aviary Observations.
Birds were equipped with colored leg bands for individual identification. Observations in 2005 showed that 35% of all courtships and 81% of all successful copulations occurred on an artificial tree structure present in each aviary and that courtship was most frequent in the early morning. Hence, during the following 4 y we used video cameras to monitor birds continuously in the artificial tree in each aviary, and we analyzed the first 30 min of every day, plus a random selection of 30-min intervals spread across other times of day. In 2007, we covered the complete first 3 wk of videos from dawn to dusk. This means that each aviary was monitored for 67 h in 2006, 309.5 h in 2007, 135 h in 2008, and 113 h in 2009. During a total of 3,948 h of video we observed 4,601 courtships with the social partner (within-pair, 143 different females), and 3,958 courtships with extrapair males (141 different females). For each courtship, we scored female responsiveness as follows (slightly modified from ref. 7): threat or aggression toward the male (−1), flying away (−0.5), mixed or ambiguous signs (0), courtship hopping and beak wiping (+0.5), and copulation solicitation (+1). These scores are referred to as “within-pair response” and “extra-pair response.” A description of the outcome of these courtships in terms of copulations is given in the SI Text.
Paternity Analysis.
Females in the aviary experiments laid a total of 3,406 eggs, of which 635 (18.6%) could not be sampled for DNA for a variety of reasons (infertile, dried out, broken, or disappeared), and 175 (5.1%) were used for hormone analysis for another study. The remaining 2,596 embryos or offspring were genotyped at 10 to 18 highly polymorphic microsatellite markers (34), allowing us to assign parentage by exclusion with high confidence (55). A subset of 540 offspring (21%) was additionally genotyped at 1,424 SNP markers (56), revealing 100% correct parentage assignment by the microsatellite markers alone. Monogamously paired males (n = 152, of which 50 bred in two seasons) sired a total of 865 eggs with females other than their social mate (including eggs laid by unpaired females). We refer to the number of eggs sired with females outside the own pair bond as “male extrapair paternity” (male EPP). Of the 2,253 eggs laid by socially monogamous (i.e., paired) females, 659 (29%) were sired by males other than the social partner (“female EPP”).
Statistical Approach to Fixed Effects.
The sample sizes and distributions of all of the behavioral and paternity data are summarized in Table S1. We used generalized linear mixed effect models [using the lmer function in R 2.10.1 (57)] to examine how each of the behavioral traits depended on a range of fixed effects (see Table S2 for parameter estimates).
Male courtship rate.
The duration of male courtship toward unfamiliar females (see Cage Experiments) was square-root transformed to approach normality (following refs. 7 and 15). Male courtship rate was not affected by female identity (see ref. 15). Hence, we used a model with male identity as the only random effect (explaining 57% of the variance after accounting for fixed effects). Male courtship rate differed significantly between the 11 batches of experiments, declined significantly over consecutive test days, declined with time of day, declined with male inbreeding coefficient, and was slightly higher for males reared in mixed-sex as opposed to unisex peer groups (Table S2).
Responsiveness of unpaired females to unfamiliar males.
Female responsiveness scores from cage experiments were not affected by male identity (see ref. 15). Hence, we used a model with female identity as the only random effect (accounting for 49% of the variance). Responsiveness differed significantly between the 11 batches of experiments, declined significantly over consecutive test days, and was slightly higher for females reared in mixed-sex as opposed to unisex peer groups (Table S2).
Female extrapair response.
In the aviary breeding experiments, females interacted with an average of 4.11 ± 1.46 SD (range 1–8; 96% with two or more) different extrapair males. Extrapair responsiveness scores were entered into a model with two random effects, where female identity (n = 141) accounted for 9.5% of the total variance, and the identity of the courting extrapair male (n = 164, including unpaired males) accounted for another 14.0% (i.e., 23.5% together). Replacing the two separate random effects with “female-male combination” (n = 579 levels) explained only little more of the total variance (25.8%), showing the weakness of idiosyncratic interactions. In other words, female extrapair responsiveness was affected by variation in male attractiveness (with high between-female agreement), but, importantly for the present study, also varied substantially between females (reflecting variation in female promiscuity). Extrapair responsiveness scores declined strongly with time after dawn and with duration of the pair bond (Table S2). In general, female responsiveness to courtship in the aviaries (within- and extrapair) varied markedly over the fertile cycle, with a peak 3 d before the start of egg laying (day 0) and with a continuous decline over the laying sequence. This general pattern was modeled by fitting two fixed effects (1 df each): (i) the number of days away from day −3 (≥5 coded as 5), and (ii) the number of eggs laid in the previous 5 d. We also controlled for year effects (3 df), and for observer differences (2 df), both of which had small effects (Table S2).
Female EPP.
We modeled the paternity of the 2,253 eggs using the lmer function as a binomial response (0 = within-pair, 1 = extrapair), with female identity (n = 149) as the only random effect. Extrapair paternity increased with the sex ratio in the aviary (2 df), strongly increased with the inbreeding coefficient of the female's social partner, and decreased with the duration of the pair bond (Table S2).
Male EPP.
The number of extrapair eggs sired per monogamously paired male per breeding season was square-root transformed to approach normality (Table S1). In a model with two random effects, male identity (n = 152) accounted for 22% of the total variance, but aviary identity (n = 36) accounted for only 6.7%. Extrapair siring success strongly increased with the number of days males were paired. This fixed effect accounts for variation in the length of the breeding seasons (range 84–113 d), for periods of absence because of death, and for periods of being unpaired. Hence, our measure effectively reflects the number of extrapair eggs sired by socially paired males per unit of time of being paired. Extrapair siring success declined with male inbreeding coefficient, and tended to be slightly lower in males from mixed-sex rearing (Table S2).
Female within-pair response.
The analysis of this behavior was carried out in a different context (see below), so the results are presented only in Table S9. Female within-pair responsiveness scores from aviaries were fitted into a model with two random effects, where female identity (n = 143) accounted for 8.5% of the total variance, and the identity of the social partner (n = 138) accounted for 10.4%. However, these random effects were strongly aliased, because there were only 155 unique pair combinations (because of divorces being rare), which makes it hard to estimate the relative female vs. male contribution, and hence requires joint modeling of extrapair and within-pair response in a permanent-environment animal model (see the strong male-identity correlation given in SI Text, Model V, Table S7). Fixed-effect estimates (Table S9) for within-pair responsiveness were similar to those for extrapair responsiveness, except for one key difference. Within-pair responsiveness increased with the duration of the pair bond, whereas extrapair responsiveness decreased (Table S9).
Quantitative Genetic Analyses.
Performing quantitative genetic analyses on behavioral traits that have only moderate heritabilities and that can only be measured in one of the sexes is extremely demanding in terms of quality and quantity of data. As such, we extensively explored how robust the estimates of genetic correlations are across a wide range of methods of analysis. We therefore implemented both likelihood-based and Bayesian animal models, we varied the units of analysis (single observations vs. individual mean behavior), varied the number of traits included (ranging from two-trait to five-trait models), and varied the number of random effects estimated (e.g., including vs. excluding maternal effects). Although some parameter estimates (e.g., maternal-effect correlations) were rather sensitive to methodological changes, most of the essential between-sex genetic correlations were always large and positive. To illustrate the range of estimates obtained, we present four different versions of the five-trait model, which we used to estimate the between-sex genetic correlations (Models I to IV, described in SI Text, fixed-effect estimates shown in Table S2, and quantitative genetic parameter estimates shown in Tables S3–S6). Notably, these results also include the most conservative estimates we encountered; hence, we avoided presenting only the most convincing results. The values presented in Fig. 1A are the averages of the estimates from these four models. These average values serve the purpose of summarizing a range of results and thus should not be interpreted in the same way as the estimates that were directly obtained from an animal model. The two-trait model on female extrapair vs. within-pair responsiveness (test of the within-sex genetic correlation hypothesis) is also presented in two versions (Models V and VI, described in SI Text, fixed effect estimates shown in Table S9, and quantitative genetic parameter estimates shown in Tables S7 and S8).
For likelihood-based animal models, we used the software VCE 6.0.2 (58), a program for restricted maximum likelihood estimation of variance components. To verify our findings with an alternative, Bayesian, approach, we applied Monte Carlo-Markov Chain methods implemented by the software package MCMCglmm (59), which was run in R 2.10.1. All analyses were based on a six-generation pedigree including the focal birds and their direct ancestors (n = 1,995 individuals; number of founders = 198). The connectedness of the pedigree is high: for example, for each of the 155 females with data on EPP or extrapair response, there were on average 6.9 ± 3.8 SD (median = 7, range 1–19) closest male relatives (r = 0.5) with phenotypic data on courtship rate.
Supplementary Material
Acknowledgments
We thank S. Janker for help with data collection; M. Schneider for molecular work, E. Koch for technical support; and S. Bauer, E. Bodendorfer, A. Grötsch, J. Hacker, M. Halser, J. Minshull, P. Neubauer, F. Preininger, M. Ruhdofer, and A. Türk for animal care; T. R. Birkhead kindly providing the birds; L. Kruuk, J. Hadfield, J. Brommer, and E. Groeneveld for advice on quantitative genetic analyses; J. Brommer, J. Dale, N. Dingemanse, J. Reid, and three anonymous referees for their constructive comments on the manuscript; and P. Rohde for stimulating this study back in 2002. The study was funded by an Emmy-Noether Fellowship of the Deutsche Forschungsgemeinschaft FO 340/1-2 and 1-3 (to W.F.) and by The Max Planck Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103195108/-/DCSupplemental.
References
- 1.Akçay E, Roughgarden J. Extra-pair paternity in birds: Review of the genetic benefits. Evol Ecol Res. 2007;9:855–868. [Google Scholar]
- 2.Arnqvist G, Kirkpatrick M. The evolution of infidelity in socially monogamous passerines: The strength of direct and indirect selection on extrapair copulation behavior in females. Am Nat. 2005;165(Suppl 5):S26–S37. doi: 10.1086/429350. [DOI] [PubMed] [Google Scholar]
- 3.Black JM. In: Partnerships in Birds—The Study of Monogamy. Black JM, editor. Oxford: Oxford University Press; 1996. pp. 3–20. [Google Scholar]
- 4.Cockburn A, et al. Superb fairy-wren males aggregate into hidden leks to solicit extragroup fertilizations before dawn. Behav Ecol. 2009;20:501–510. [Google Scholar]
- 5.Petrie M, Kempenaers B. Extra-pair paternity in birds: Explaining variation between species and populations. Trends Ecol Evol. 1998;13:52–58. doi: 10.1016/s0169-5347(97)01232-9. [DOI] [PubMed] [Google Scholar]
- 6.Westneat DF, Stewart IRK. Extra-pair paternity in birds: Causes, correlates, and conflict. Annu Rev Ecol Evol Syst. 2003;34:365–396. [Google Scholar]
- 7.Forstmeier W. Do individual females differ intrinsically in their propensity to engage in extra-pair copulations? PLoS ONE. 2007;2:e952. doi: 10.1371/journal.pone.0000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halliday T, Arnold SJ. Multiple mating by females—A perspective from quantitative genetics. Anim Behav. 1987;35:939–941. [Google Scholar]
- 9.Albrecht T, et al. Extrapair paternity and the opportunity for sexual selection in long-distant migratory passerines. Behav Ecol. 2007;18:477–486. [Google Scholar]
- 10.Reid JM, Arcese P, Sardell RJ, Keller LF. Heritability of female extra-pair paternity rate in song sparrows (Melospiza melodia) Proc Biol Sci. 2011;278:1114–1120. doi: 10.1098/rspb.2010.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid JM, Arcese P, Sardell RJ, Keller LF. Additive genetic variance, heritability, and inbreeding depression in male extra-pair reproductive success. Am Nat. 2011;177:177–187. doi: 10.1086/657977. [DOI] [PubMed] [Google Scholar]
- 12.Bonduriansky R, Chenoweth SF. Intralocus sexual conflict. Trends Ecol Evol. 2009;24:280–288. doi: 10.1016/j.tree.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Rice WR. Sexually antagonistic genes: Experimental evidence. Science. 1992;256:1436–1439. doi: 10.1126/science.1604317. [DOI] [PubMed] [Google Scholar]
- 14.Kirkpatrick M, Barton NH. The strength of indirect selection on female mating preferences. Proc Natl Acad Sci USA. 1997;94:1282–1286. doi: 10.1073/pnas.94.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forstmeier W. Female resistance to male seduction in zebra finches. Anim Behav. 2004;68:1005–1015. [Google Scholar]
- 16.Forstmeier W, Mueller JC, Kempenaers B. A polymorphism in the oestrogen receptor gene explains covariance between digit ratio and mating behaviour. Proc Biol Sci. 2010;277:3353–3361. doi: 10.1098/rspb.2010.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kruuk LEB. Estimating genetic parameters in natural populations using the “animal model”. Phil Trans Roy Soc Lond Ser B. 2004;359:873–890. doi: 10.1098/rstb.2003.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold SJ, Halliday T. Multiple mating—Natural selection is not evolution. Anim Behav. 1988;36:1547–1548. [Google Scholar]
- 19.Sherman PW, Westneat DF. Multiple mating and quantitative genetics. Anim Behav. 1988;36:1545–1547. [Google Scholar]
- 20.Dunnington EA, Siegel PB. Mating frequency in male chickens—Long-term selection. Theor Appl Genet. 1983;64:317–323. doi: 10.1007/BF00274171. [DOI] [PubMed] [Google Scholar]
- 21.Sgrò CM, Chapman T, Partridge L. Sex-specific selection on time to remate in Drosophila melanogaster. Anim Behav. 1998;56:1267–1278. doi: 10.1006/anbe.1998.0900. [DOI] [PubMed] [Google Scholar]
- 22.Grant CA, Chapman T, Pomiankowski A, Fowler K. No detectable genetic correlation between male and female mating frequency in the stalk-eyed fly Cyrtodiopsis dalmanni. Heredity. 2005;95:444–448. doi: 10.1038/sj.hdy.6800733. [DOI] [PubMed] [Google Scholar]
- 23.Harano T, Miyatake T. No genetic correlation between the sexes in mating frequency in the bean beetle, Callosobruchus chinensis. Heredity. 2007;99:295–300. doi: 10.1038/sj.hdy.6800996. [DOI] [PubMed] [Google Scholar]
- 24.House CM, et al. The evolution of repeated mating in the burying beetle, Nicrophorus vespilloides. Evolution. 2008;62:2004–2014. doi: 10.1111/j.1558-5646.2008.00422.x. [DOI] [PubMed] [Google Scholar]
- 25.Zann R. The Zebra Finch. New York: Oxford University Press; 1996. [Google Scholar]
- 26.Bailey JM, Kirk KM, Zhu G, Dunne MP, Martin NG. Do individual differences in sociosexuality represent genetic or environmentally contingent strategies? Evidence from the Australian twin registry. J Pers Soc Psychol. 2000;78:537–545. doi: 10.1037//0022-3514.78.3.537. [DOI] [PubMed] [Google Scholar]
- 27.Cherkas LF, Oelsner EC, Mak YT, Valdes A, Spector TD. Genetic influences on female infidelity and number of sexual partners in humans: A linkage and association study of the role of the vasopressin receptor gene (AVPR1A) Twin Res. 2004;7:649–658. doi: 10.1375/1369052042663922. [DOI] [PubMed] [Google Scholar]
- 28.Garcia JR, et al. Associations between dopamine D4 receptor gene variation with both infidelity and sexual promiscuity. PLoS ONE. 2010;5:e14162. doi: 10.1371/journal.pone.0014162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walum H, et al. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proc Natl Acad Sci USA. 2008;105:14153–14156. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher HE, Aron A, Mashek D, Li H, Brown LL. Defining the brain systems of lust, romantic attraction, and attachment. Arch Sex Behav. 2002;31:413–419. doi: 10.1023/a:1019888024255. [DOI] [PubMed] [Google Scholar]
- 31.Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 32.Melis MR, Argiolas A. Dopamine and sexual behavior. Neurosci Biobehav Rev. 1995;19:19–38. doi: 10.1016/0149-7634(94)00020-2. [DOI] [PubMed] [Google Scholar]
- 33.Stuckey BGA. Female sexual function and dysfunction in the reproductive years: the influence of endogenous and exogenous sex hormones. J Sex Med. 2008;5:2282–2290. doi: 10.1111/j.1743-6109.2008.00992.x. [DOI] [PubMed] [Google Scholar]
- 34.Nettle D. An evolutionary approach to the extraversion continuum. Evol Hum Behav. 2005;26:363–373. [Google Scholar]
- 35.Schmitt DP, Buss DM. Sexual dimensions of person description: Beyond or subsumed by the big five? J Res Pers. 2000;34:141–177. [Google Scholar]
- 36.Eliassen S, Kokko H. Current analyses do not resolve whether extra-pair paternity is male or female driven. Behav Ecol Sociobiol. 2008;62:1795–1804. [Google Scholar]
- 37.Griffith SC. The evolution of infidelity in socially monogamous passerines: Neglected components of direct and indirect selection. Am Nat. 2007;169:274–281. doi: 10.1086/510601. discussion 282–283. [DOI] [PubMed] [Google Scholar]
- 38.Hasson O, Stone L. Male infertility, female fertility and extrapair copulations. Biol Rev Camb Philos Soc. 2009;84:225–244. doi: 10.1111/j.1469-185X.2008.00068.x. [DOI] [PubMed] [Google Scholar]
- 39.Weatherhead PJ, Robertson RJ. Offspring quality and the polygyny threshold—Sexy son hypothesis. Am Nat. 1979;113:201–208. [Google Scholar]
- 40.Fisher RA. The Genetical Theory of Natural Selection. Oxford: Clarendon Press; 1930. [Google Scholar]
- 41.Sokal RR, Rohlf FJ. Biometry. 3rd Ed. New York: Freeman; 1995. [Google Scholar]
- 42.Schielzeth H, Burger C, Bolund E, Forstmeier W. Sexual imprinting on continuous variation: Do female zebra finches prefer or avoid unfamiliar sons of their foster parents? J Evol Biol. 2008;21:1274–1280. doi: 10.1111/j.1420-9101.2008.01568.x. [DOI] [PubMed] [Google Scholar]
- 43.Birkhead TR, Burke T, Zann R, Hunter FM, Krupa AP. Extra-pair paternity and intraspecific brood parasitism in wild zebra finches Taeniopygia guttata, revealed by DNA fingerprinting. Behav Ecol Sociobiol. 1990;27:315–324. [Google Scholar]
- 44.Griffith SC, Holleley CE, Mariette MM, Pryke SR, Svedin N. Low level of extrapair parentage in wild zebra finches. Anim Behav. 2010;79:261–264. [Google Scholar]
- 45.Burley NT, Parker PG, Lundy K. Sexual selection and extrapair fertilization in a socially monogamous passerine, the zebra finch (Taeniopygia guttata) Behav Ecol. 1996;7:218–226. [Google Scholar]
- 46.Birkhead TR, Clarkson K, Zann R. Extra-pair courtship, copulation and mate guarding in wild zebra finches Taeniopygia guttata. Anim Behav. 1988;36:1853–1855. [Google Scholar]
- 47.Tschirren B, Postma E. Quantitative genetics research in zebra finches: Where we are and where to go. Emu. 2010;110:268–278. [Google Scholar]
- 48.Albrecht T, Kreisinger J, Piálek J. The strength of direct selection against female promiscuity is associated with rates of extrapair fertilizations in socially monogamous songbirds. Am Nat. 2006;167:739–744. doi: 10.1086/502633. [DOI] [PubMed] [Google Scholar]
- 49.Bolund E, Schielzeth H, Forstmeier W. Intrasexual competition in zebra finches, the role of beak colour and body size. Anim Behav. 2007;74:715–724. [Google Scholar]
- 50.Forstmeier W. Quantitative genetics and behavioural correlates of digit ratio in the zebra finch. Proc Biol Sci. 2005;272:2641–2649. doi: 10.1098/rspb.2005.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forstmeier W, Schielzeth H, Schneider M, Kempenaers B. Development of polymorphic microsatellite markers for the zebra finch (Taeniopygia guttata) Mol Ecol Notes. 2007;7:1026–1028. [Google Scholar]
- 52.Schielzeth H, Burger C, Bolund E, Forstmeier W. Assortative versus disassortative mating preferences of female zebra finches based on self-referent phenotype matching. Anim Behav. 2008;76:1927–1934. [Google Scholar]
- 53.Bolund E, Martin K, Kempenaers B, Forstmeier W. Inbreeding depression of sexually selected traits and attractiveness in the zebra finch. Anim Behav. 2010;79:947–955. [Google Scholar]
- 54.Schielzeth H, Bolund E, Kempenaers B, Forstmeier W. Quantitative genetics and fitness consequences of neophilia in zebra finches. Behav Ecol. 2010;22:126–134. [Google Scholar]
- 55.Schielzeth H, Bolund E. Patterns of conspecific brood parasitism in zebra finches. Anim Behav. 2010;79:1329–1337. [Google Scholar]
- 56.Backström N, et al. The recombination landscape of the zebra finch Taeniopygia guttata genome. Genome Res. 2010;20:485–495. doi: 10.1101/gr.101410.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bates D, Maechler M. 2009. lme4: Linear mixed-effects models using S4 classes ( http://CRAN.R-project.org/package=lme4), R package version 0.999375-32.
- 58.Groeneveld E, Kovač M, Mielenz N. VCE User's Guide and Reference Manual. Mariensee, Germany: Institute of Farm Animal Genetics, Friedrich Loeffler Institute; 2008. Version 6.0. [Google Scholar]
- 59.Hadfield JD. MCMC methods for multi-response generalized linear mixed models: The MCMCglmm R package. J Stat Softw. 2010;33(2):1–22. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.