Abstract
With the increase in frequency of harmful algal blooms (HABs) worldwide, a better understanding of the mechanisms that influence toxin production is needed. Karenia brevis, the major HAB dinoflagellate in the Gulf of Mexico, produces potent neurotoxins, known as brevetoxins. Human health is directly impacted by blooms of K. brevis through consumption of shellfish contaminated by accumulated brevetoxins (neurotoxic shellfish poisoning) or from aerosolized brevetoxins in sea spray (reduced respiratory function); however, the reason for brevetoxin production has remained a mystery. Here we show that brevetoxin production increased dramatically in response to osmotic stress in three of the four K. brevis clones examined. By rapidly changing salinity to simulate a shift from oceanic conditions to a decreased salinity typical of coastal conditions, brevetoxin production was triggered. As a result, brevetoxin cell quota increased by >14-fold, while growth rate remained unchanged. Live images of K. brevis cells were also examined to assess changes in cell volume. In the K. brevis Wilson clone, cells responded quickly to hypoosmotic stress by increasing their brevetoxin cell quota from ∼10 to 160 pg of brevetoxin per cell, while cell volume remained stable. In contrast, the K. brevis SP1 clone, which has a consistently low brevetoxin cell quota (<1 pg per cell), was unable to balance the hypoosmotic stress, and although brevetoxin production remained low, average cell volume increased. Our findings close a critical gap in knowledge regarding mechanisms for toxin production in K. brevis by providing an explanation for toxin production in this harmful dinoflagellate.
Keywords: Imaging FlowCytobot, osmoregulation
Harmful algal blooms (HABs) are the proliferation of nuisance or toxic microalgae that can have devastating effects on coastal ecosystems, fisheries, and aquaculture (1). The majority of HABs are associated with dinoflagellates that produce a collection of diverse toxins (1). Karenia brevis, the major HAB dinoflagellate in the Gulf of Mexico, produces potent neurotoxins, known as brevetoxins (2). Brevetoxins can lead to human health concerns through the consumption of shellfish contaminated by accumulated brevetoxins, known as neurological shellfish poisoning, or through reduced respiratory function from aerosolized brevetoxins in sea spray (1, 3, 4). In addition, K. brevis blooms have led to massive fish kills and compromise sea turtle, sea bird, and marine mammal health, playing a major role in the ecology of the Gulf of Mexico (1, 5).
Brevetoxins are lipid-soluble polyether neurotoxins that are stored intracellularly and are only released during cell death. Once released from the K. brevis cell, brevetoxins have a high affinity for receptor sites in voltage-sensitive sodium channels, which cause the channel gates to remain open and inhibit fast inactivation (3). As K. brevis blooms age and enter a mature stage, the two parent brevetoxins, PbTx-1 and -2, begin to decline as brevetoxin derivatives (PbTx-3, -5, -6, -7, -8, -9, and -10) increase (6, 7). This change has been observed by comparing cultures of K. brevis containing healthy, intact cells with aged cultures containing dead cells. After cell death and lysis, a larger percentage of brevetoxin is extracellular (6, 7). In addition to brevetoxins, K. brevis produces a nontoxic polyether compound that inhibits brevetoxin from binding to sodium channels known as brevenal (8). Competition between brevetoxins and brevenal for sodium channels has been shown to increase fish survival (9). In addition, the unique ability of brevenal to compete with brevetoxin suggests that it may serve as a solution to aerosolized brevetoxins, and it has also been implicated as a therapy for cystic fibrosis (10). Unfortunately, K. brevis produces on average ∼1 pg per cell, which is only a fraction (∼6%) of the total brevetoxin concentration (11).
A functional role for the diverse collection of toxins produced by dinoflagellates is still unknown. In the mixotrophic dinoflagellate, Karlodinium veneficum, toxins have been linked to prey capture and relief from grazer pressure (12, 13). Although it has been speculated that toxins initially evolved to deter grazing pressure, this effect may be more of an indirect consequence of toxin production (14, 15). Others have suggested that toxins may play a role in allelopathy. For example, Alexandrium minutum has been shown to increase toxin concentration and inhibit the growth of a nontoxic dinoflagellate Prorocentrum micans under nutrient stress (16). Although K. brevis also produces allelopathic compounds (17, 18), brevetoxins do not inhibit the growth of competitor species, which suggests that other chemical compounds are responsible for the allelopathic response (17).
Although a primary cellular function for toxins has remained elusive, responses to environmental conditions, such as salinity, have been shown to influence the growth potential and toxin production of harmful dinoflagellates (11, 19–23). These studies have examined the role of salinity in growth and toxin production on cells preacclimated to new environmental conditions (11, 19–23). Although these studies have identified the acclimated response to salinity, they do not evaluate differences in growth potential and toxin production in response to rapid shifts in salinity, as would occur when blooms move from oceanic to coastal conditions. For example, optimal growth of K. brevis occurs at oceanic conditions where salinity is ∼35 (2); however, cells frequently come in contact with estuarine environments, and presumably lower salinities, where they are still able to form blooms and flourish (20, 24). Little information is available on the effects of advection and associated environmental influence on the growth and brevetoxin production of K. brevis based on a shift in environmental conditions.
To understand how movement onshore and subsequent osmotic stress impact growth and brevetoxin production, we exposed four clones of K. brevis acclimated to oceanic conditions (salinity of 35) to a rapid decrease in salinity to approximate coastal conditions (salinity of 27; refs. 25 and 26). We show that under hypoosmotic stress, brevetoxin and brevenal cell quotas increased by >10-fold compared with controls (cells acclimated to oceanic or coastal conditions). Time course experiments demonstrate that the response to hypoosmotic stress occurs quickly (<3 h) and enables cells to respond to changes in salinity. Cells that are unable to trigger brevetoxin production (i.e., cultures that contain low brevetoxin cell quotas) under hypoosmotic stress increase their cell volume and are unable to respond quickly. Our experiments identify osmotic stress as a trigger for brevetoxin and brevenal production in K. brevis and provide evidence for a cellular function for brevetoxins.†
Results
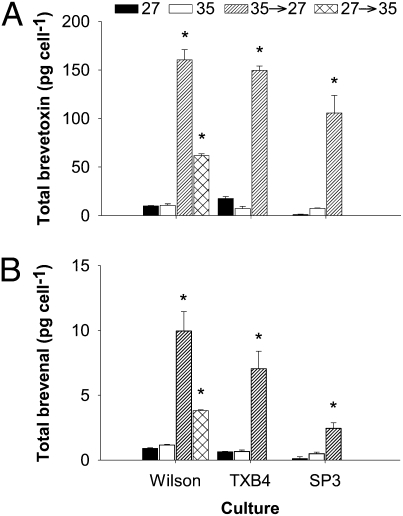
Under hypoosmotic stress, brevetoxin cell quotas for three K. brevis clonal cultures (Wilson, TXB4, and SP3; Table S1) increased by >10-fold compared with controls maintained at a salinity of 35 (P < 0.001). These observed cell quotas after hypoosmotic stress were also significantly higher than toxin content of controls maintained at a salinity of 27 (P < 0.001; Fig. 1). Hypoosmotic stress produced a much larger increase in toxin cell quota than observed previously (Fig. 1A). For the Wilson clone, which has been in culture since 1953 and consistently produces brevetoxin at levels similar to other clones (11), toxin content increased 16-fold from 9.97 ± 0.43 to 160.40 ± 10.50 pg per cell (SD). The TXB4 clone increased 20-fold from 7.33 ± 2.28 to 149.70 ± 4.40 pg per cell (SD), and the SP3 clone toxin content increased 14-fold from 7.50 ± 0.32 to 105.8 ± 18 pg per cell (SD; Fig. 1A). The reverse experiment, in which the Wilson clone was exposed to hyperosmotic stress, also produced an increase in toxin content, to 61.81 ± 1.84 pg per cell (SD). After hypoosmotic and hyperosmotic stress, the production of brevenal also increased by >10-fold and 4-fold, respectively (Fig. 1B).
Fig. 1.
Brevetoxin and brevenal cell quota for control and hypoosmotic stressed K. brevis cultures. (A) Mean total brevetoxin cell quota for three clones (Table S1) under control [acclimated to salinities of 27 (black) and 35 (white); redrawn from ref. 11] and after hypoosmotic stress from a rapid decrease in salinity from 35 to 27 (hatched) and hyperosmotic stress (crossed). (B) Mean total brevenal cell quota for control cultures and after hypoosmotic stress; legend as in A. Brevetoxins and brevenal were extracted during late logarithmic growth phase. Error bars represent 1 SD (n = 6, acclimated treatments, n = 4, hypoosmotic stress treatments, n = 2, hyperosmotic stress treatment). *P < 0.001 as determined by a paired t test.
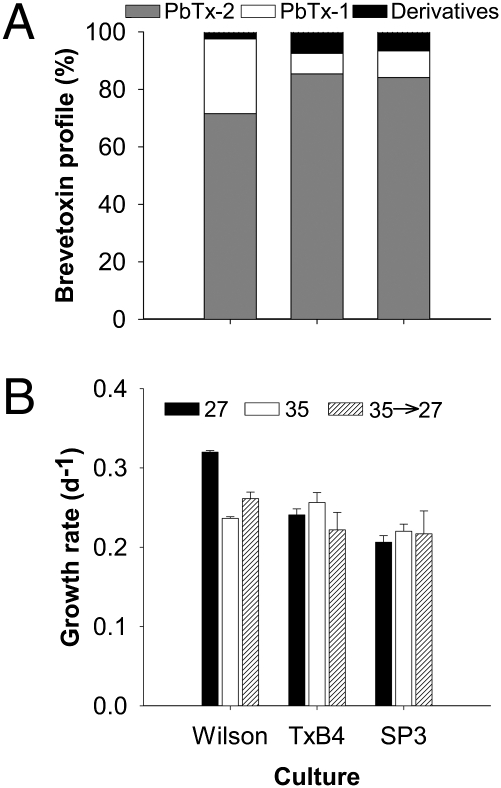
Our results show that after osmotic stress, brevetoxin profiles for all cultures remained at >94% PbTx-1 and -2, with little change in the derivatives (Fig. 2A). Growth rates of the cultures after hypoosmotic stress were also similar to acclimated cultures (Fig. 2B). Together, this evidence suggests that rapid shifts in salinity did not cause cell death or lysis (Fig. 2).
Fig. 2.
K. brevis cells remain viable after hypoosmotic stress. (A) Brevetoxin profiles for three clonal cultures (Table S1) after hypoosmotic stress. The two parent compounds, PbTx-1 (white) and PbTx-2 (gray), comprised >94% of the brevetoxin profile at day 6 after hypoosmotic stress. Derivatives (PbTx-3, -7, -9, and -cba), which would indicate cell lysis, were a small percentage of the total. (B) Comparison of growth rates for three clonal cultures acclimated to salinity treatments of 27 (black) and 35 (white) (redrawn from ref. 11) and after hypoosmotic stress produced by rapidly decreasing salinity from 35 to 27 (hatched, black and white). Error bars represent 1 SD (n = 8). Predominance of parent brevetoxin compounds and no significant decrease in growth rate indicate that cells remained intact during hypoosmotic stress.
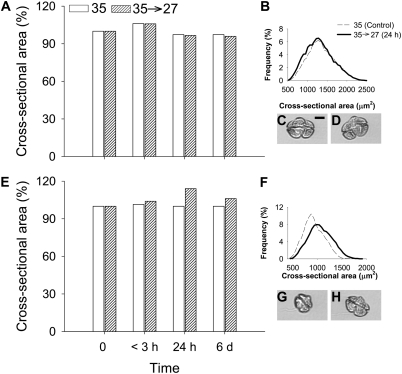
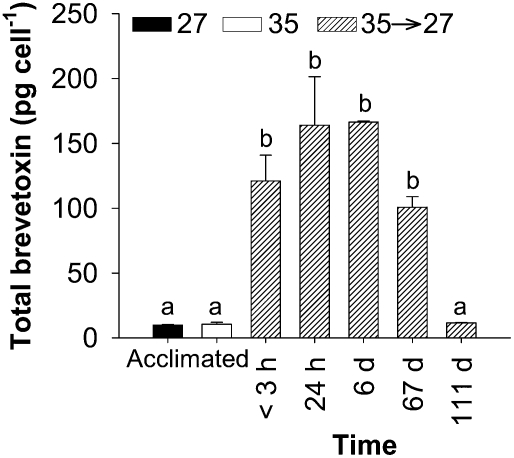
To examine the response of K. brevis to osmotic stress, we performed a time course experiment to measure changes in cell volume and brevetoxin production immediately (<3 h), 24 h, 6 d, 67 d, and 111 d after the rapid salinity shift. The cross-sectional area (CSA) of live cell images was used as a proxy for cell volume (refs. 27 and 28; Fig. 3). Experiments were initiated at 0800, when CSA was at the minimum for the light portion of the daily cycle for these synchronously dividing K. brevis cultures (Fig. S1). Within 3 h after hypoosmotic stress, the average CSA of K. brevis Wilson clone increased 5% from 1,349 ± 338 to 1,429 ± 355 μm2 (SD; Fig. 3A). This increase was comparable with the typical daily increase in CSA observed in a control culture between 0900 and 1200 (Fig. 3A and Fig. S1). By 24 h after the salinity shift, CSA had returned to a size equal to the control culture at 0800, which corresponded to the time period in which the images were collected (Fig. 3 A–D). Concurrently, brevetoxin per cell increased significantly immediately after the hypoosmotic stress and continued to increase through day 6 (Fig. 4).
Fig. 3.
Time course of changes in CSA for K. brevis after hypoosmotic stress. (A) Percent increase in CSA for K. brevis Wilson clone from the control cultures at <3 h, 24 h, and 6 d after hypoosmotic stress. (B) CSA frequency for the Wilson clone before hypoosmotic stress and 24 h after hypoosmotic stress (control, n = 11,351; <3h, n = 15,825; 24 h, n = 20,856; 6 d, n = 29,107). (C) K. brevis Wilson clone cell before hypoosmotic stress. (D) K. brevis Wilson clone cell 24 h after hypoosmotic stress. (E) Percent increase in CSA for K. brevis SP1 clone, as in A. For the low brevetoxin-producing clone SP1, the increase in CSA after hypoosmotic stress was larger than daily variation in control culture. (F) Increase in CSA frequency for SP1 before hypoosmotic stress and 24 h after hypoosmotic stress (control, n = 22,296; <3h, n = 27,692; 24 h, n = 18,061; 6 d, n = 9,934). (G) K. brevis SP1 clone cell before hypoosmotic stress. (H) K. brevis SP1 clone cell 24 h after hypoosmotic stress. (Scale bar: 10 μm.)
Fig. 4.
Time course of brevetoxin cell quota for K. brevis (Wilson clone) after hypoosmotic stress. Mean total brevetoxin for Wilson clone acclimated to salinities of 27 (black) and 35 (white) (reproduced from ref. 11) and after hypoosmotic stress (hatched, black and white). Brevetoxin extractions were performed within <3 h, 24 h, 6 d, 67 d, and 111 d after the salinity decrease. Brevetoxin cell quota increased rapidly within <3 h after hypoosmotic stress and reached a plateau after 24 h that continued to day 6, with a decrease noted at day 67. Error bars represent 1 SD (n = 6 for acclimated treatments; n = 4 for <3 h, 24 h, and 6 d; n = 3 for 67 d and 111 d). Differences among brevetoxin cell quotas were determined by ANOVA (P < 0.001). Bonferroni's post hoc t test determined no significant differences between treatments denoted by either a or b.
Next, we repeated the experiment with a clone of K. brevis (SP1) that produces very low quantities of brevetoxin (11). After rapid hypoosmotic stress, the average CSA of K. brevis SP1 cells increased from 931 ± 205 to 968 ± 208 μm2 (SD; Fig. 3E). This result represented a 4% increase in CSA and was greater than the 1.5% increase observed in the control culture during the same time period. By 24 h after hypoosmotic stress, CSA increased to 1,062 ± 237 μm2 (SD), which was a 14% increase over initial CSA (Fig. 3 E–H). After 6 d, cell volume remained ∼6% larger than initial cell volume. The brevetoxin cell quota for SP1 remained low (slightly above the limit of detection) at <3 h and 24 h after hypoosmotic stress. After 6 d, brevetoxin cell quota for SP1 (1.24 ± 0.43 pg per cell; SD) was not significantly different from values reported for control cultures at 35 or 27 salinity growth conditions (P = 0.921 and 0.471, respectively).
Discussion
It has been assumed that brevetoxins have a functional role in K. brevis because they are produced and retained within the cell. Our experimental results demonstrate that a shift in the osmotic environment triggered rapid production of brevetoxins and brevenal (Figs. 1 and 4). The >10-fold increase in the cell quota under hypoosmotic stress for both these polyethers provides evidence to suggest that brevetoxins and brevenal do play a role in osmoregulation (Figs. 1 and 4) and thus do have a cellular function.
Marine algae acclimate to osmotic changes in a two-step process: changes in cell volume followed by osmolytic adjustment through regulation of organic osmolytes or modifications to ion concentrations (29, 30). The elastic properties of cell walls are important in osmotic adjustment and allow cells to handle short-term fluctuations in salinity on the order of minutes through precise changes in cell volume (29). Our experiments were able to identify small changes in the cell volume for both the Wilson and SP1 clones. The average cell volume of the Wilson clone did increase by 5% after the shift in salinity; however, the increase was indistinguishable from the diel change in volume (6%) over the same time period in control cultures (Fig. 3A). We suggest that Wilson was able to respond to the hypoosmotic stress by increasing its brevetoxin cell quota (Figs. 1 and 4). In comparison, the low brevetoxin cell quota of the SP1 clone appeared to prevent cells from responding efficiently to the change in salinity. The sustained increase in cell volume over a 24-h period after the shift in salinity illustrates the reduced capability of SP1 to quickly adjust to osmotic stress (Fig. 3 E and F). Eventually, SP1 was able produce a small quantity of brevetoxins (day 6), which may have assisted with the decrease in cell size in the following days.
Mechanisms for controlling osmoregulation have not been identified in dinoflagellates, and it is unclear whether they use organic osmolytes or ion transport. In wallless cells, adjustment in cell volume for osmoregulation occurs continuously and is considered a basic biological function that allows for equilibrium between intracellular and extracellular solute concentrations (30, 31). In contrast, response to osmotic stress requires an additional energy expense, such as an increase in organic osmolytes or use of ion transport (30–32). Osmolytic metabolite adjustment may be accomplished by synthesis or elimination of osmolytes, such as glycerol (30, 32). During hyperosmotic stress, organic osmolytes are produced and retained inside the cell (32). K. brevis did increase brevetoxin cell quota and maintained the toxin within the cell when exposed to hyperosmotic stress (Fig. 1A). Under hypoosmotic stress, organic osmolytes are excreted from the cell or are degraded (32). Our experiments show that brevetoxins profiles were dominated by PbTx-1 and -2, which implies that K. brevis did not release or break down brevetoxins. Thus, brevetoxins likely do not act as an osmolytic metabolite.
The regulation of ions through the use of ion pumps has also been suggested as a mechanism for readjustment of ions for osmoregulation and after osmotic stress (29, 33). Recently, voltage-activated Na+/Ca2+ channels were detected in diatoms (34) and coccolithophores (35). Based on our results, we speculate that brevetoxins, which are produced constantly at low cell concentrations (1–20 pg per cell; refs. 6, 7, 11, 20, 21, 36, and 37), facilitate osmoregulation through interaction with Na+ channels and allow for ion adjustment. When K. brevis are exposed to osmotic stress, brevetoxin cell quotas increase as a response to the drastic changes in salinity. The presence of voltage-activated Na+ or Ca2+ channels could provide a link between ion transport and brevetoxin production; however, further research is needed to identify the presence of voltage-activated channels in dinoflagellates. It is important to note that similar studies with another toxic dinoflagellate, Alexandrium fundyense, found that osmotic stress did not influence toxin production (38), which suggests that osmoregulation may differ among dinoflagellate groups.
Brevenal, the recently discovered beneficial compound produced by K. brevis, acts as a brevetoxin antagonist by binding to the sodium channel receptor (9). In sheep, picomolar levels of brevenal have been shown to alleviate the bronchial constriction and wheezing response to brevetoxin (10). Because of the potential for clearing mucus from the lungs, brevenal has been suggested as a potential treatment for cystic fibrosis (10). By manipulating the growth conditions for this alga through relatively simple salinity changes, we have shown that the yield of brevenal can be greatly enhanced. Consequently, increasing the efficiency of production for this medically important compound can be greatly improved.
In conclusion, our study identifies a trigger for toxin production within a dinoflagellate HAB species. Our results suggest that as K. brevis blooms are transported onshore and subsequently experience rapid osmotic stress, brevetoxin concentrations could significantly increase. Further research is needed to verify this trigger in field populations. The identification of a direct link between toxin production and environmental conditions adds a unique dimension to our understanding of HAB dynamics. Furthermore, the role of salinity in activating brevenal production may benefit commercial production of medical therapies (9, 10).
Materials and Methods
Culture Conditions.
K. brevis clones (Table S1) were grown in L1-Si–enriched (39) natural seawater collected from the Gulf of Mexico. Seawater was filtered and sterilized before enrichment with sterile-filtered nutrients. All experiments were performed at 25 °C under irradiance levels of 70-μmol of photons·m−2·s−1 and a 12:12-h light:dark cycle using cool white bulbs. Control cultures grown at a salinity of 35 or 27 were acclimated slowly to growth conditions and maintained for a minimum of 10 transfers before experiments.
Osmotic Stress Treatment.
For each salinity treatment, 45-mL cultures were grown in 8-18 replicate 50-mL test tubes for 7–14 d, depending on individual growth rates. On the 4th day of growth, which was sufficient for a culture doubling (11), the salinity was reduced to 27 by adding MilliQ water (23% vol/vol). To examine hyperosmotic stress, salinity was increased by adding high-salinity seawater (salinity of 65; 23% vol/vol) to cultures acclimated to a salinity of 27. High-salinity seawater was produced through freezing filtered seawater and preserving the brine portion. This experiment was conducted with the Wilson clone only. Salinity changes were verified by using a VWR SympHony Multiparameter Research Meter (Model SB90M5) and a four-cell conductivity probe (Model 11388-382).
Growth rates were determined from daily measurements of in vivo fluorescence (40). For osmotic stress conditions, growth rates were based on measurements taken after the salinity shift. Brevetoxins and brevenal were extracted during late logarithmic growth phase (11). For time course experiments, brevetoxins and brevenal were extracted at <3 h, 24 h, 6 d, 67 d, and 111 d after salinity shift. After the extraction taken at day 6 for the time course experiments, the culture was transferred into new medium, and growth rate was monitored. Once the culture reached late logarithmic growth phase, it was transferred again into new medium; this process continued for a total of 10 transfers or 111 d. Brevetoxins and brevenal extractions for days 6, 67, and 111 were conducted during late logarithmic growth phase.
Toxin extractions were performed during the morning or early afternoon to ensure the cells were not undergoing cytokinesis (41). Cell concentrations were determined for each treatment by using a Sedgwick-rafter counting chamber and an Olympus BX60 microscope (100×).
Brevetoxin Analysis: LC-MS.
Triplicate culture tubes were extracted for each treatment and stored at −20 °C until toxin analysis using established protocols (11). A semisynthetic PbTx standard was added as internal standard to determine recovery efficiency, which is typically >90%. Cell quotas of brevetoxins PbTx-1 and -2 (the polyether parent compounds found to be localized intracellularly in K. brevis), corresponding breakdown products (PbTx-7 and PbTx-3, -6, -9, and -cba, respectively), and brevenal were determined by using a LC-MS/MS multiple reaction monitoring method (11), and the percent toxin composition (profiles) was calculated.
Live Cell Imaging and Volume Estimates.
Images of live cells from the Imaging FlowCytobot were used to estimate cell volume (27). CSA was used as a proxy for cell volume. A red diode laser was used to measure the light scattering and chlorophyll fluorescence of each particle that passed through a flow cell and to trigger a flashlamp and CCD camera. The instrument used a 1,038× 1,378-pixel CCD camera and a 10× objective, resulting in 340-μm × 450-μm images with ∼1-μm resolution. The number of images analyzed ranged between 9,934 and 29,110 for each sampling hypoosmotic experiment. All images were collected between 0800 and 1200. To estimate the change in cell volume over a 24-h period, 5-mL subsamples were collected every 3 h, and the CSA was determined for both Wilson and SP1 clones (Fig. S1). Number of images collected ranged from 10,428 to 18,773 at each time point. Cultures were in midlogarithmic growth phase and subsampling volume did not exceed 10% of the total culture volume. During dark periods, culture vessels were wrapped in aluminum foil, and red light was used during sampling to limit the exposure of cultures to light.
The CSA for each image was obtained by summing the number of image pixels remaining after removing the background pixels surrounding the cell (28). Images of dividing cells were removed from the data set before analyses of CSA. Pixel size is based on imaging red florescent beads (Duke Scientific, XPR-1653) with a known diameter and applying the same method as explained above.
Statistics.
Differences in total brevetoxin and brevenal concentrations were tested for significance using a paired t test, with alpha values set at 0.05 (42). Shapiro-Wilk test was used to determine normality of the data sets (43). A paired t test was preferred because the objective of these experiments was to determine differences between acclimated and hypoosmotically stressed cultures. Differences among acclimated cultures were reported in Errera et al. (11). For the time course experiments ANOVA was used to determined differences in brevetoxin cell quota. Post hoc tests were performed by using a Bonferroni t test with an alpha value set at 0.05 (42).
Differences in the average CSA for each culture were calculated as a percent difference. Histograms of binned CSA areas were used to highlight the change in CSA for Wilson and SP1 clone before hypoosmotic stress and 24 h after the shift (Fig. 3 B and F). Because of the difference in number of images collected at each time point, the frequency of occurrence was transformed into a percentage.
Supplementary Material
Acknowledgments
We thank A. Bourdelais, D. Henrichs, and S. W. Lockless for help in methods development and discussion and J. Kessler for reading the manuscript. This work was supported by National Oceanic and Atmospheric Administration Ecology and Oceanography of Harmful Algal Blooms (ECOHAB) Award NA06NOS4780244 (to L.C. and J. R. Gold).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
†This paper is Ecology and Oceanography of Harmful Algal Blooms (ECOHAB) Contribution no. 658.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1104247108/-/DCSupplemental.
References
- 1.Landsberg JH. The effects of harmful algal blooms on aquatic organisms. Rev Fish Sci. 2002;10:113–390. [Google Scholar]
- 2.Steidinger KA, Vargo GA, Tester PA, Tomas CR. In: Physiological Ecology of Harmful Algal Blooms. Anderson DM, Cembella AD, Hallegraeff GM, editors. Berlin: Springer; 1998. pp. 133–153. [Google Scholar]
- 3.Baden DG, Bourdelais AJ, Jacocks H, Michelliza S, Naar J. Natural and derivative brevetoxins: historical background, multiplicity, and effects. Environ Health Perspect. 2005;113:621–625. doi: 10.1289/ehp.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirkpatrick B, et al. Literature review of Florida red tide: Implications for human health effects. Harmful Algae. 2004;3:99–115. doi: 10.1016/j.hal.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flewelling LJ, et al. Brevetoxicosis: Red tides and marine mammal mortalities. Nature. 2005;435:755–756. doi: 10.1038/nature435755a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roszell LE, Schulman LS, Baden DG. In: Toxic Marine Phytoplankton. Granéli E, Sundström B, Edler L, Anderson DM, editors. New York: Elsevier; 1989. pp. 403–406. [Google Scholar]
- 7.Pierce RH, Henry MS, Proffitt LS, Blum P, Payne S. In: Harmful Algal Blooms. Hallegraeff GM, Blackburn SI, Bolch CJ, Lewis RJ, editors. Paris: Intergovernmental Oceanographic Commission of UNESCO; 2001. pp. 421–424. [Google Scholar]
- 8.Bourdelais AJ, Jacocks HM, Wright JLC, Bigwarfe PM, Jr, Baden DG. A new polyether ladder compound produced by the dinoflagellate Karenia brevis. J Nat Prod. 2005;68:2–6. doi: 10.1021/np049797o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourdelais AJ, et al. Brevenal is a natural inhibitor of brevetoxin action in sodium channel receptor binding assays. Cell Mol Neurobiol. 2004;24:553–563. doi: 10.1023/B:CEMN.0000023629.81595.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abraham WM, et al. Airway responses to aerosolized brevetoxins in an animal model of asthma. Am J Respir Crit Care Med. 2005;171:26–34. doi: 10.1164/rccm.200406-735OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Errera RM, et al. Variation in brevetoxin and brevenal content among clonal cultures of Karenia brevis may influence bloom toxicity. Toxicon. 2010;55:195–203. doi: 10.1016/j.toxicon.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Adolf JE, Krupatkina D, Bachvaroff T, Place AR. Karlotoxin mediates grazing by Oxyrrhis marina on strains of Karlodinium veneficum. Harmful Algae. 2007;6:400–412. [Google Scholar]
- 13.Sheng J, Malkiel E, Katz J, Adolf JE, Place AR. A dinoflagellate exploits toxins to immobilize prey prior to ingestion. Proc Natl Acad Sci USA. 2010;107:2082–2087. doi: 10.1073/pnas.0912254107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner JT, Tester PA, Hansen PJ. In: Physiological Ecology of Harmful Algal Blooms. Anderson DM, Cembella AD, Hallegraeff GM, editors. Berlin: Springer; 1998. pp. 452–474. [Google Scholar]
- 15.Turner JT. In: Ecology of Harmful Algae. Granéli E, Turner JT, editors. Berlin: Springer; 2006. pp. 259–270. [Google Scholar]
- 16.Guisande C, et al. Fate of paralytic shellfish poisoning toxins ingested by the copepod Acartia clause. Mar Ecol Prog Ser. 2002;240:105–115. [Google Scholar]
- 17.Kubanek J, Hicks MK, Naar J, Villareal TA. Does the red tide dinoflagellate Karenia brevis use allelopathy to outcompete other phytoplankton? Limnol Oceanogr. 2005;50:883–895. [Google Scholar]
- 18.Prince EK, Myers TL, Kubanek J. Effects of harmful algal blooms on competitors: Allelopathic mechanisms of the red tide dinoflagellate Karenia brevis. Limnol Oceanogr. 2008;53:531–541. [Google Scholar]
- 19.Hamasaki K, Horie M, Tokimitsu S, Toda T, Taguchi S. Variability in toxicity of the dinoflagellate Alexandrium tamarense isolated from Hiroshima Bay, western Japan, as a reflection of changing environmental conditions. J Plankton Res. 2001;23:271–278. [Google Scholar]
- 20.Brown AFM, et al. Effect of salinity on the distribution, growth, and toxicity of Karenia spp. Harmful Algae. 2006;5:199–212. [Google Scholar]
- 21.Grzebyk D, et al. Effects of salinity and two coastal waters on the growth and toxin content of dinoflagellate Alexandrium minutum. J Plankton Res. 2003;25:1185–1199. [Google Scholar]
- 22.Lim P-T, Ogata T. Salinity effect on growth and toxin production of four tropical Alexandrium species (Dinophyceae) Toxicon. 2005;45:699–710. doi: 10.1016/j.toxicon.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Magana HA, Villareal TA. The effect of environmental factors on the growth rate of Karenia brevis (Davis) g. Hansen and Moestrup. Harmful Algae. 2006;5:192–198. [Google Scholar]
- 24.Dortch Q, et al. In: Harmful Algae. Reguera B, Blanco J, Fernandez ML, Wyatt T, editors. Santiago de Compostela: Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO, GRAFISANT; 1998. pp. 143–144. [Google Scholar]
- 25.Cochrane JD, Kelly FJ. Low-frequency circulation on the Texas-Louisiana Continental Shelf. Geophys Res Lett. 1986;91:10645–10659. [Google Scholar]
- 26.Weisberg RH, Zheng L. Circulation of Tampa Bay driven by buoyancy, tides, and wind simulated using a finite volume coastal ocean model. J Geophys Res. 1988;111:C01005. [Google Scholar]
- 27.Olson RJ, Sosik HM. A submersible imaging-in-flow instrument to analyze nano-and microplankton: Imaging FlowCytobot. Limnol Oceanogr Methods. 2007;5:195–203. [Google Scholar]
- 28.Henrichs DW, Sosik HM, Olson RJ, Campbell L. Phylogenetic analysis of Brachidinium capitatum (DINOPHYCEAE) from the Gulf of Mexico indicates membership in the Kareniaceae. J Phycol. 2011;47:366–374. doi: 10.1111/j.1529-8817.2011.00960.x. [DOI] [PubMed] [Google Scholar]
- 29.Kirst GO. Salinity tolerance of eukaryotic marine algae. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:21–53. [Google Scholar]
- 30.Wegmann K. Osmoregulation in eukaryotic algae. FEMS Microbiol Rev. 1986;39:37–43. [Google Scholar]
- 31.Mayfield AB, Gates RD. Osmoregulation in anthozoan-dinoflagellate symbiosis. Comp Biochem Physiol A Mol Integr Physiol. 2007;147:1–10. doi: 10.1016/j.cbpa.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 32.Bisson MA, Kirst GO. Osmotic acclimation and turgor pressure regulation in algae. Naturwissenschaften. 1995;82:461–471. [Google Scholar]
- 33.Glass DM. Regulation of ion transport. Annu Rev Plant Physiol. 1983;34:311–326. [Google Scholar]
- 34.Taylor AR. A fast Na+/Ca2+-based action potential in a marine diatom. PLoS ONE. 2009;4:e4966. doi: 10.1371/journal.pone.0004966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor AR, Brownlee C. A novel Cl- inward-rectifying current in the plasma membrane of the calcifying marine phytoplankton Coccolithus pelagicus. Plant Physiol. 2003;131:1391–1400. doi: 10.1104/pp.011791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baden DG, Tomas CR. Variations in major toxin composition for six clones of Ptychodiscus brevis. Toxicon. 1988;26:961–963. doi: 10.1016/0041-0101(88)90261-9. [DOI] [PubMed] [Google Scholar]
- 37.Loret P, et al. No difference found in ribosomal DNA sequences from physiologically diverse clones of Karenia brevis (Dinophyceae) from the Gulf of Mexico. J Plankton Res. 2002;24:735–739. [Google Scholar]
- 38.Anderson DM, Kulis DM, Sullivan JJ, Hall S, Lee C. Dynamics and physiology of saxitoxin production by the dinoflagellate Alexandrium spp. Mar Biol. 1990;104:511–524. [Google Scholar]
- 39.Guillard RRL, Hargraves PE. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia. 1993;32:234–236. [Google Scholar]
- 40.Brand LE, Murphy LS, Guillard RRL, Lee HT. Genetic variability and differentiation in the temperature niche component of the diatom Thalassiosira pseudonana. Mar Biol. 1981;62:103–110. [Google Scholar]
- 41.Van Dolah FM, Leighfield TA, Kamykowski D, Kirkpatrick GJ. Cell cycle behavior of laboratory and field populations of the Florida red tide dinoflagellate, Karenia brevis. Cont Shelf Res. 2008;28:11–13. [Google Scholar]
- 42.Dytham C. Choosing and Using Statistics: A Biologist's Guide. Boston: Blackwell; 2003. pp. 108–119. [Google Scholar]
- 43.Shapiro SS, Wilk MB. An analysis of variance test for normality. Biometrika. 1965;52:591–611. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.