Abstract
Toxoplasma gondii is a member of the phylum Apicomplexa that includes several important human pathogens, such as Cryptosporidium and Plasmodium falciparum, the causative agent of human malaria. It is an obligate intracellular parasite that can cause severe disease in congenitally infected neonates and immunocompromised individuals. Despite the importance of attachment and invasion to the success of the parasite, little is known about the underlying mechanisms that drive these processes. Here we describe a screen to identify small molecules that block the process of host cell invasion by the T. gondii parasite. We identified a small molecule that specifically and irreversibly blocks parasite attachment and subsequent invasion of host cells. Using tandem orthogonal proteolysis–activity-based protein profiling, we determined that this compound covalently modifies a single cysteine residue in a poorly characterized protein homologous to the human protein DJ-1. Mutation of this key cysteine residue in the native gene sequence resulted in parasites that were resistant to inhibition of host cell attachment and invasion by the compound. Further analysis of the invasion phenotype confirmed that modification of Cys127 on TgDJ-1 resulted in a block of microneme secretion and motility, even in the presence of direct stimulators of calcium release. Together, our results suggest that TgDJ-1 plays an important role that is likely downstream of the calcium flux required for microneme secretion, parasite motility, and subsequent invasion of host cells.
The obligate intracellular protozoan parasite, Toxoplasma gondii, is capable of infecting almost any nucleated cell within an exceptionally broad host range. It is an opportunistic pathogen that asymptomatically infects 10–20% of the world population and can cause severe disease in congenitally infected neonates and immunocompromised individuals, in particular HIV patients (1). Although Toxoplasma rarely causes acute infection in healthy individuals, reactivation of latent infections can lead to toxoplasmic encephalitis, the pathology of which is associated with the parasite-mediated lytic destruction of infected host cells (2). Propagation of an infection by Toxoplasma gondii is dependent on the ability of the parasite to invade host cells. Although it is clear that this process requires the timed release of specific secretory organelles termed micronemes and rhoptries, relatively little is known about specific regulators of the invasion process. Because of the haploid nature of the Toxoplasma genome, classic forward genetic screens have proven difficult (reviewed in ref. 3). In addition, gene knockouts can result in up-regulation of related proteins or signaling pathways that can make it difficult to interpret the true function of gene products using this technology.
The use of pharmacological compounds in pathogens to perturb protein function is a viable alternative to classic genetic methods (4–8). In addition to identifying new tools for studying mechanisms of parasite invasion, small molecule screens can identify new drug targets and lead compounds for downstream development of chemotherapies. Recently a high-throughput screening effort was undertaken to identify novel inhibitors of Toxoplasma invasion (5). Using a large library of unbiased, small molecules in a microscopy-based invasion assay, this screen identified a number of novel molecules that can be used to dissect the complex process of host cell invasion. However, the resulting hits from the screen provided no clues as to the identity of the target protein or proteins responsible for their inhibitory activity. Although long-term follow-up efforts have identified interesting mechanisms of actions of at least one hit (9), the identification of the direct target of this compound has remained elusive. To overcome some of the issues with unbiased screening, we assembled a highly focused library of small molecules that covalently modify their targets by way of reactive electrophilic traps on the compunds (described in ref. 4). The covalent nature of the compounds greatly facilitates downstream target identification. We recently used this library to successfully identify two mechanistically distinct proteases of Plasmodium falciparum that cooperatively regulate erythrocyte rupture and release of newly invasive merozoites (4).
Here we report the screening of the same library of covalent inhibitors and the subsequent identification of a compound, WRR-086, that blocks T. gondii attachment and invasion of host cells. By converting the lead compound to a suitably labeled analog and using tandem orthogonal proteolysis–activity-based protein profiling [TOP-ABPP (10)] we identified a single cysteine residue on a poorly characterized protein, T. gondii DJ-1 (TgDJ-1), that is modified by WRR-086. Using parasite genetics we were able to show that mutation of this critical cysteine residue resulted in parasites that were resistant to the effects of WRR-086, suggesting that TgDJ-1 is the primary target responsible for the phenotypic effects of the compound. Further functional studies using WRR-086 indicated that disruption of TgDJ-1 function results in a block of microneme secretion and parasite motility, even in the presence of specific agents that directly induce calcium release in the parasite. Therefore, our results identify TgDJ-1 as a unique regulator of microneme secretion and motility, and suggest that this protein is downstream of calcium signaling in the cascade of molecular events that lead to the invasion of host cells.
Results
Identification of Small Molecules That Block Host Cell Invasion.
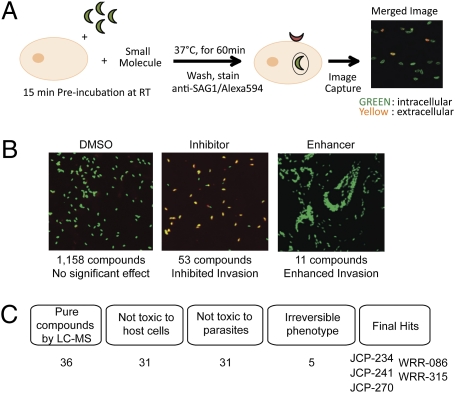
We recently described the assembly of a highly directed library of 1,222 small molecule inhibitors designed to covalently modify proteases in an activity-dependent manner (library described in ref. 4). The library includes compounds that were originally designed to target a variety of cysteine and serine proteases, as well as compounds that can act as general electrophiles for enzyme targets. The covalent binding nature of this library greatly facilitates downstream target identification (described in ref. 4). We screened the library using a previously described dual-fluorescence microscopy-based assay that allows direct monitoring of parasite attachment and entry into host cells (Fig. 1A) (5). Compounds that showed a gross qualitative change in either the number of intracellular parasites or in the overall number of parasites per field in two out of three assays were chosen as initial hits for further analysis (Fig. 1B). We identified compounds that block as well as enhance parasite invasion. However, for this study we focused on compounds that inhibited parasite attachment (decrease in number of parasites per field) or invasion (decrease in number of intracellular parasites). The initial inhibitor hits were further triaged using a series of secondary assays that included overall compound purity, general toxicity to host cells/parasites, and reversibility of the inhibitory phenotype (Fig. 1C). At the end of this process, only five compounds were sufficiently nontoxic, showed irreversible inhibitory effects on host cell invasion, and had no gross qualitative effect when used to pretreat host cells independent of parasites (Table 1). Further quantification of the inhibitory activities of these five compounds revealed that JCP-241 and WRR-086 had similar phenotypic profiles for both attachment and invasion. We chose to focus our efforts on WRR-086, because this compound could be more readily synthesized in tagged form and contained a clear electrophile that was likely to form covalent bonds with cysteine residues.
Fig. 1.
Small molecule screen to identify compounds that alter host cell attachment and invasion by T. gondii. (A) Diagram of the screen in which YFP-expressing parasites were pretreated with compounds, then allowed to invade host cells, followed by antibody staining of the SAG1 surface protein on parasites that remained outside host cells. Invaded parasites are green (YFP positive), whereas those that failed to invade are yellow (YFP positive/Alexa 594 positive). RT = room temperature. (B) Representative fluorescent images from the dual-fluorescence screen showing vehicle (DMSO), a compound that inhibited invasion, and a compound that enhanced invasion. Total numbers of compounds screened as well as inhibitors/enhancers identified are indicated. (C) Triage of preliminary hits based on compound purity by LC-MS, overall toxicity to host cells (24-h compound treatment) or parasites (1-h compound treatment of extracellular parasites), and reversibility of the phenotype. The final five hits are listed.
Table 1.
Phenotype summary for compounds that inhibit host cell invasion by T. gondii
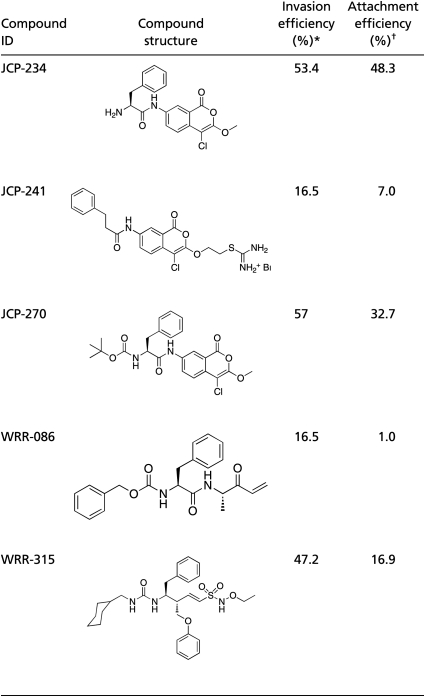 |
*Invasion efficiency is defined as the percentage of total parasites invaded relative to vehicle-treated controls.
†Attachment efficiency was determined by counting the average number of total parasites per field over 10 randomly selected fields and is expressed as percentage relative to vehicle-treated controls.
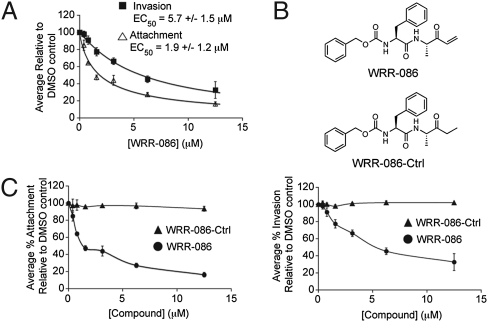
As a first step in characterization of the phenotypic effects of WRR-086, we measured the effector concentration for half-maximum response (EC50) of this compound in the attachment and invasion assay (Fig. 2A). These results confirmed that WRR-086 was maximally active in the low micromolar concentration range (EC50 = 1.9 ± 1.2 μM for attachment and EC50 = 5.7 ± 1.5 μM for invasion). To generate a negative control compound and determine whether the α.β-unsaturated ketone was responsible for the observed activity, we synthesized WRR-086-Ctrl, in which we reduced the double bond of the primary electrophile (Fig. 2B). The synthesis of this control as well as other analogs of WRR-086 was accomplished using a solid phase synthesis scheme that we developed (Fig. S1). As expected, WRR-086-Ctrl showed no inhibitory effects on either parasite attachment (Fig. 2C) or invasion (Fig. 2D), suggesting that the α.β-unsaturated ketone is required to react with the protein target(s).
Fig. 2.
WRR-086 inhibits host cell attachment and invasion. (A) Structures of WRR-086 and the control analog WRR-086-Ctrl. (B) Dose–response of WRR-086 using an Endo-synchronized attachment/invasion assay. The average percentage of attached parasites relative to DMSO-treated control parasites and average percentage of invaded parasites relative to DMSO-treated control parasites is plotted relative to concentration of WRR-086. The EC50 of WRR-086 in attachment to and invasion of host cells as calculated from the curve fits is shown. (C) Dose–response of WRR-086 and WRR-086-Ctrl compound in attachment (Left) and invasion (Right) using the Endo-synchronized attachment/invasion assay described above.
To rule out the possibility that WRR-086 is a general inhibitor of the parasite lytic cycle we assayed overall inhibitory effects on intracellular growth using a FACS-based growth assay (Fig. S2). Parasite-infected host cells were treated with WRR-086 or vehicle control for 24 h, and then total parasite numbers per host cell were assayed by FACS. WRR-086 did not cause a significant decrease in the number of parasites per host cell at the concentrations at which we observe specific phenotypic affects on host cell invasion.
Identification of the Target of WRR-086 by TOP-ABPP.
To biochemically isolate and identify the target(s) of WRR-086, we used a recently described chemoproteomic method, TOP-ABPP (10). This allowed us to label target proteins in their native environment with an alkyne analog of WRR-086 (Alkyne-086; Fig. 3A) and then isolate them by click chemistry conjugation to an azide-biotin affinity tag containing a tobacco etch virus (TEV) protease cleavable linker. Probe-labeled proteins were isolated by affinity purification on streptavidin beads followed by on-bead trypsin digestion to release peptides for analysis by tandem LC-MS/MS. This process leaves any probe-modified peptides attached to the streptavidin beads. These peptides were then selectively released by the addition of the TEV protease, and the sites of probe modification were determined.
Fig. 3.
Identification of TgDJ-1 as the target of WRR-086. (A) Structure of Alkyne-086 for use in “click” chemistry applications. Alkyne-086 was synthesized as shown in Fig. S1. (B) Alignment of DJ-1 sequences from Toxoplasma (Tg), Neospora (Nc), Plasmodium (Pf), Cryptosporidium (Ch), Human (Hs), and E. coli YajL. Gene model in ToxoDB predicts a start codon 213 bp upstream from the beginning of the DJ-1 domain, which also contains a potential start Met. We used the downstream AUG to create the sequence shown. Arrowhead points to Cys127 that is modified by WRR-086. (C) Structural modeling of TgDJ-1 using structures of the dimeric E. coli YajL protein (Protein Data Bank ID: 2AB0). The highly conserved cysteine C104 and the Alkyne-086 binding residue C127 are indicated.
We ranked proteins that were identified in the trypsin digest in two out of three replicate runs according to the total number of spectral counts identified for each protein and average fold change between vehicle and WRR-086 pretreated samples. We eliminated proteins identified in two out of three runs that had less than an average of five spectral counts combined between both runs (a complete list of proteins meeting this cutoff can be found in Table S1). We also eliminated proteins that showed less than an average 1.6-fold change between vehicle and WRR-086 pretreated samples. Finally, we cross-referenced the remaining candidates with the list of proteins identified as having specific probe-modified peptides that appeared with three or more spectral counts (a complete list of probe-modified peptides can be found in Table S2). This narrowed down our hit list to six proteins (Table 2). Among the top six candidate proteins, most were not expected to play a specific role in host cell attachment or invasion on the basis of their documented biochemical activities (SI Discussion). In contrast, the second protein on our list of candidate targets, annotated as “intracellular protease” (TGME49_014290), was largely uncharacterized with respect to biochemical function. Sequence alignment revealed that this protein is a homolog of Escherichia coli YajL and human DJ-1 (hDJ-1), which is a member of the large multifunctional DJ-1 superfamily (Fig. 3B). We therefore refer to the T. gondii protein as TgDJ-1 going forward. We could also model the structure of the dimeric protein on the basis of the solved structure of E. coli YajL protein (Fig. 3C). This model confirms that the cysteine at position 127 (Fig. 3B; Toxoplasma numbering based on start at second methionine, Met71) that is modified by WRR-086 is in close proximity to the highly conserved Cys104 of the papain protease fold.
Table 2.
WRR-086–modified peptides identified by TOP-ABPP
| Gene ID | Protein | Molecular mass (kDa) | Modified peptide | Spectral count |
| TGME49_110640 | Uridine phosphorylase, putative | 33 | K.KGDLASLIVTVGC*EQE.A | 361 |
| TGME49_014290 | Intracellular protease, putative | 28 | K.AVAYPC*FMDQFPADMR.G | 26 |
| TGME49_090670 | Cytosol aminopeptidase | 60 | K.TVAVVLPTC*QK.V | 20 |
| TGME49_029000 | Kelch motif domain-containing protein | 68 | K.LAPVC*TTFSVLDVR.R | 8 |
| TGME49_089690 | Glyceraldehyde-3-phosphate dehydrogenase | 37 | K.GIISYTDEEVVSSDFVHC*K.F | 6 |
| TGME49_047600 | Conserved hypothetical protein | 73 | R.AVTALLDLQNFGSC*ASTAGEELVK.T | 3 |
Modified cysteine residues are indicated by an asterisk (*).
Genetic Validation of TgDJ-1 as the Relevant Target of WRR-086.
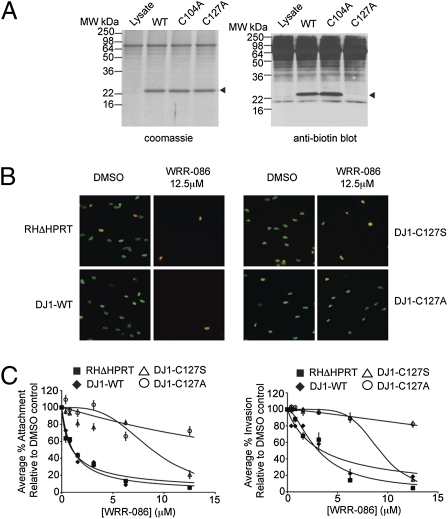
To further validate TgDJ-1 as the functionally relevant target of WRR-086 we expressed and purified the recombinant protein containing mutations according to our TOP-ABPP data. We recombinantly expressed both the WT and the C127A mutant TgDJ-1 proteins. Furthermore, because the DJ-1 protein has a highly conserved cysteine at position 104 (position 106 in the human protein) that is thought to be essential for function (11–13), we also generated the C104A mutant. Labeling of the purified proteins with a biotin-labeled version of WRR-086 indicated that the probe was able to efficiently label both the WT and the C104A mutant proteins but not the C127A mutant (Fig. 4A). This result confirmed that our compound is specifically reactive toward Cys127, even in the presence of the additional five cysteines in the protein (Fig. S3).
Fig. 4.
Validation of TgDJ-1 as the functionally relevant target of WRR-086. (A) Labeling of recombinantly expressed WT, C106A, and C127A TgDJ-1 proteins. Proteins were added to total cystosolic extracts from WT (RHΔHPRT) parasites and labeled with WRR-086-biotin. Protein loading was visualized by Coomassie staining (Left), followed by blotting using HRP-streptavidin (Right). (B) Representative images from the dual fluorescence Endo-synchronized invasion assays showing effects at the highest dose of WRR-086 tested. Extracellular parasites are dual-stained with a mouse anti-SAG1 antibody (without permeabilization) and a rabbit anti-SAG1 antibody (with permeabilization) and appear yellow, and intracellular parasites appear green. (C) Dose–response of WRR-086 against host cell attachment (Left) and invasion (Right) of WT (RHΔHPRT) parasites, DJ1-WT, DJ1-C127S, and DJ1-C127A mutants. Assays were performed as described in Fig. 2. The data represent mean values ± SEMs of three independent experiments and are expressed as percentages of solvent-treated controls.
We then generated parasites that express mutant TgDJ-1 proteins in place of the WT sequence. We replaced the endogenous, WT copy of TgDJ-1 with either a C127S (DJ1-C127S) or C127A (DJ1-C127A) mutation fused to the 3′ UTR of GRA2. We chose to also generate the more conservative serine mutation in case the Ala mutant perturbed the structure in vivo. Finally, we generated a control parasite line in which we replaced the endogenous copy of TgDJ-1 with a WT copy of the gene (DJ-1-WT) flanked by the 3′ UTR from GRA2. Correct integration of all constructs were verified by PCR and sequencing of the locus. In addition, we confirmed that the parasites expressing mutant DJ-1 sequences showed equivalent rates of attachment and invasion as the parent strain, suggesting that these mutations did not have any unexpected effects on DJ-1 function (Table S3).
We analyzed the ability of the genetically modified parasites to attach to and invade host cells in the presence of WRR-086 (Fig. 4 B and C). As expected, the original parasite line and parasites containing a copy of the WT TgDJ-1 sequence showed the expected sensitivity to WRR-086 for both attachment and invasion. Interestingly, parasites expressing the DJ1-C127S mutant only showed reduced sensitivity to the inhibitory effects of WRR-086. This finding is consistent with the reported reduced activity of the WRR-086 electrophile toward serine residues relative to cysteine (14). The DJ1-C127A mutant lacking a reactive nucleophile at position 127 was completely resistant to the attachment and invasion effects of WRR-086 at all of the concentrations tested. These results are in agreement with our initial biochemical findings and confirm that the effects of the WRR-086 on parasite attachment and invasion are mediated by its modification of Cys127 of TgDJ-1.
TgDJ-1 Has Diffuse Cytosolic Localization.
Interestingly, the native TgDJ-1 sequence contains an upstream start codon that, if used, could result in the generation of the TgDJ-1 protein with a signal sequence. However, this upstream start site is not conserved in other highly related species, such as Neospora, and the consensus sequence is also not optimal. Furthermore, we identified an N-terminal methionine in our MS analysis of the protein, suggesting that the protein is not synthesized as a secretory protein with a signal sequence. However, a signal sequence could direct TgDJ-1 into the secretory transport system and into direct contact with the micronemes, a potential site of action for TgDJ-1. To address this, we generated a parasite line in which we replaced the WT copy of the gene with the WT sequence in frame with a C-terminal HA tag. These parasites behaved similarly to WT parasites and importantly, showed the same sensitivity to WRR-086 in the invasion and secretion assays (Fig. S4 A–D). We then performed localization studies on both extracellular and intracellular tachyzoites using an anti-HA antibody (Fig. S4E). The results confirm that, as observed for homologs of DJ-1 in fungi and metazoa, TgDJ-1 shows a diffuse localization consistent with a general cytosolic disposition. This localization of the protein is consistent with TgDJ-1 not being targeted to the secretory pathway in any significant quantity.
TgDJ-1 Regulates Microneme Secretion and Parasite Motility.
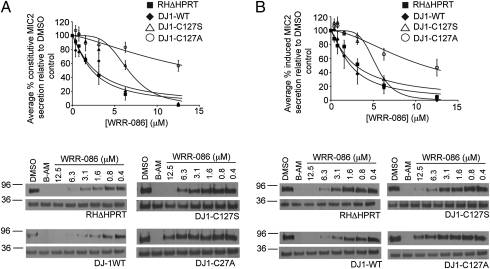
Parasite attachment to host cells is mediated by the coordinated release of microneme proteins onto the parasite surface (15). Microneme proteins are secreted constitutively at low levels, and secretion is dramatically up-regulated (induced) upon initial attachment to host cells. This up-regulation can be induced in vitro by the treatment of extracellular parasites with calcium ionophores (15, 16) or ethanol (17), which causes an increase in intracellular parasite calcium levels and subsequent release of microneme proteins. To gain insight into the mechanism by which TgDJ-1 functions, we measured the effects of WRR-086 on both constitutive secretion and calcium-mediated induced secretion. WRR-086, but not WRR-086-Ctrl, caused a dose-dependent inhibition of secretion of the microneme resident protein, MIC2, into the culture supernatant (EC50 for constitutive secretion = 5.2 ± 3.4 μM; EC50 for induced secretion = 6.0 ± 1.1 μM; Fig. S5). More importantly, the C127S mutant parasites were partially resistant to both induced and constitutive secretion, and the C127A mutant parasites were completely resistant to effect of WRR-086 on microneme secretion (Fig. 5). Thus, even when calcium release is directly enhanced by ethanol treatment, WRR-086 modification of TgDJ-1 blocks secretion, suggesting that it acts downstream of calcium signaling.
Fig. 5.
DJ1-C127A parasites are resistant to the effects of WRR-086 on constitutive and ethanol-induced microneme secretion. Dose–response of RHΔHPRT, DJ1-WT, DJ1-C127S, and DJ1-C127A parasites to WRR-086 in both constitutive (A) and ethanol-induced (B) microneme secretion. Assays were performed as described in Fig. 2. The data represent mean values ± SEMs of three independent experiments and are expressed as percentages of solvent-treated controls. Representative Western blots for MIC2 (Upper, each pair) and the loading control SAG1 (Lower, each pair) are shown below the graphs.
Secretion of microneme proteins is intimately coupled to parasite gliding motility (18). Parasites leave characteristic trails of highly abundant surface proteins and lipids while gliding. Therefore, overall gliding activity can be measured by visualizing trails produced as extracellular tachyzoites travel over coverslips coated with FBS. Treatment of parasites with WRR-086, but not WRR-086-Ctrl, leads to a dose-dependent reduction in the total number of trails observed (Fig. S6). Importantly, the concentrations of WRR-086 that induced inhibitory effects on motility closely matched the concentrations required for inhibition of invasion and microneme secretion.
Discussion
Despite the importance of attachment and invasion to the propagation of T. gondii, relatively little is known about the mechanisms and signaling pathways that drive these processes. There have been a few recent examples of studies that have identified both proteins (19) and natural small molecules (20) that, like TgDJ-1, regulate the process of microneme secretion. Here, using a dual-fluorescence microscopy-based assay, we identified WRR-086, a compound that inhibits parasite attachment and invasion of host cells. We were further able to show that WRR-086 modifies the parasite protein TgDJ-1 at a single cysteine residue (C127) and that it is this modification that mediates the effects of WRR-086. Finally, we show that TgDJ-1 has a general cystosolic localization and likely acts downstream of the calcium signaling that is required to initiate microneme secretion, parasite motility, and invasion of the host cell.
Although our data clearly identify TgDJ-1 as a regulator of parasite attachment and invasion, the overall lack of understanding of the function of this protein in higher eukaryotes provides no clues as to the possible mechanistic roles in this process. All of the DJ-1 homologs including TgDJ-1 have a papain-like protease domain (hence the original designation as an intracellular protease in ToxoDB), although the potential proteolytic activity of this domain remains in question. The DJ-1 proteins of higher eukaryotes have lost this enzymatic activity and are likely to have evolved a different function (21). TgDJ-1 contains the predicted active site glutamic acid (E14) and cysteine (C104) but lacks the predicted catalytic histidine. Therefore, it is highly unlikely that TgDJ-1 possesses proteolytic activity. TgDJ-1 has eight cysteines, yet we only detected modification of Cys127, indicating that this residue is specifically reactive toward the probe. Interestingly, Plasmodium spp. have a highly homologus DJ-1 sequence, but the cysteine at position 127 is replaced by a serine. Because serine can also act as a nucelophile, this site may also be subject to targeting with small molecule probes. Molecular modeling of TgDJ-1 using the Pyrococcus horikoshii PH1704 protein (Fig. S3) suggests that Cys127 is not likely to be involved in disulfide bonds and may therefore play a role in protein function. The human DJ-1 protein (hDJ-1) has only three cysteines (12). Mutational analysis of the three cysteines in hDJ-1 revealed specific functions for each, including sensing of oxidative stress (C106, human numbering), S-nitrosylation (C46 and C53), and dimerization (C46), which are critical for proper function of this protein (11–13). The only cysteine residue conserved between hDJ-1 and TgDJ-1 is the redox sensitive C106 (C104 in TgDJ-1), and we show here that this residue is not directly involved in binding WRR-086. Using the modeled structure of TgDJ-1, we determined the location of Cys127 relative to the predicted redox sensitive Cys104 (Fig. 3C). The overall close proximity of these residues suggest that, if in fact the conserved Cys104 is required for function, WRR-086 could act by sterically blocking substrate/ligand binding or by changing the redox environment around this residue. Unfortunately, without any known interacting proteins, it will be difficult to test this hypothesis.
To further add to the complexity of DJ-1 function, mutations in the human protein have been linked to autosomal recessive, early-onset Parkinson's disease, possibly as a result of a loss of response to oxidative stress (22, 23). Further support for the proposed role of DJ-1 in the regulation of oxidative stress in higher eukaryotes comes from evidence that it interacts with the Nrf2 transcription factor, which itself is a master regulator of the oxidative stress response (24). Furthermore other molecules, such as the calcium-dependent protein kinases (19) and abscisic acid (20), participate in host cell invasion pathways and are also central players in stress responses in plants. Indeed, it has been hypothesized that egress from the host cell is the largest stress in the life cycle of Toxoplasma and other intracellular pathogens, and activation of stress responses involved in invasion has been shown to be critical for Toxoplasma extracellular viability (25). Therefore, if the function of a stress response protein such as DJ-1 is compromised, parasites may not be able to efficiently attach and invade into the host cell. Although this is an interesting hypothesis to consider, the lack of any real mechanistic insight into DJ-1 function, despite more than a decade of rigorous study, suggests that confirmation of this hypothesis is likely to take significant additional efforts. Regardless, our results clearly show that some aspect of TgDJ-1 function is critical for the productive invasion of host cells. This, coupled with the fact that small molecules can alter its function and it is found in all Plasmodium sp. and many other pathogens, suggests that DJ-1 may be a previously unappreciated regulator of multiple important human infections.
Materials and Methods
Reagents and Antibodies.
All chemicals and resins used for synthesis of WRR-086 and associated analogs were purchased from commercial suppliers and used without further purification. BAPTA-AM [1,2-bis-(o-Aminophenoxy)-ethane-N,N,N',N'-tetraacetic acid, tetraacetoxymethyl ester] was purchased from Molecular Probes. Ethanol was purchased from Sigma and was of reagent grade or better. Vectashield fluorescence mounting medium was purchased from Vector Labs. mAb 6D10 (MIC2) has been previously described (26) (kind gift from Vern Carruthers, University of Michigan, Ann Arbor, MI). mAb 11-132 (SAG1) was purchased from Argene. mAbDG52 (SAG1) and polyclonal rabbit anti-SAG1 antibodies were both kind gifts from John Boothroyd, Stanford University School of Medicine, Stanford, CA). Alexa 488-conjugated goat anti-rabbit and Alexa 594-conjugated goat anti-mouse secondary antibodies were purchased from Molecular Probes. ECL HRP-conjugated goat anti-mouse secondary antibody was purchased from Amersham, and the KPL HRP-conjugated goat anti-mouse secondary antibody was purchased from KPL.
Parasite and Host Cell Maintenance.
African Green Monkey renal epithelial cells (BS-C-1; CCL 26, American Type Culture Collection) were used as host cells for the high-throughput invasion screen. Parasites were maintained by serial passage in human foreskin fibroblasts (HFF; kind gift from John Boothroyd, Stanford University School of Medicine, Stanford, CA) cultured in DMEM (Invitrogen) supplemented with 10% FBS, 2 mM l-glutamine, and 100 μg penicillin/100 μg streptomycin per milliliter maintained at 37 °C and 5% CO2. Parasites were harvested for use in assays by either syringe lysis of infected HFF monolayers or collection of parasites from culture supernatant after spontaneous lysis of the monolayer. Parasites were then centrifuged at 1,200 × g for 6 min and resuspended in HBSS (Invitrogen) supplemented with 10 mM Hepes, pH 7.0, and 1% (vol/vol) dialyzed FBS (Invitrogen) and filtered through 5-μm Millipore Syringe Filters.
Parasite Strains.
TgDJ-1 constructs were amplified from genomic Toxoplasma RH-strain DNA. All PCR was conducted using Phusion polymerase unless otherwise noted (NEB). To create the construct for endogenous tagging and allele replacement of TgDJ-1, the 5′ targeting sequence for TgDJ1 (including the coding sequence) was initially cloned into TOPO-blunt (Invitrogen) using the forward primer 5′- GCGCGGTACCGCAGTCAAAGTGCTTGTTCCCGTC-3′ and the reverse primer 5′-gcgcgatatc tcattatcaGTACGCGTAAAGGAGCTGTGCCG-3′ (in frame with three stop codons, in lowercase). The TOPO-TgDJ1 construct was used as a template to mutate C127S or C127A according to the quick change protocol using the primers 5′-CGGTTGCGTATCCAAGCTTCATGGACCA-3′ or 5′-CGGTTGCGTATCCCGCCTTCATGGACCA-3′, and their reverse complements, respectively. The WT or mutant 5′ targeting and the TgDJ1 3′ targeting sequences were then subcloned (using the same primers) into a vector such that they flank an HPRT cassette. Transgenic parasite strains were made by electroporating the RH-strain of T. gondii deleted for hypoxanthine-xanthine-guanine phosphoribosyl transferase [RH(ΔHPRT)] parasites with 15 μg of linearized plasmid encoding the construct of interest and selecting for HPRT-positive parasites, as previously described (27). Clonal parasites were grown from populations by limiting dilution. Integration was verified by PCR with a primer to the GRA2 3′ UTR of the vector (5′-TGGAACTACGGTGTTTGTTCCTTTCTGCG-3′) and a primer to genomic sequence upstream to the TgDJ-1 start (and therefore to sequence not included in the targeting sequence; 5′-GAAGTTCCTGTTCTAAGGGTGATCG-3′).
High-Throughput Invasion Assay.
The assembly of the directed protease inhibitor library was previously reported (4). The library was screened for compounds that block parasite invasion using a previously described high-throughput dual-fluorescence microscopy-based assay (5).
Details of the screening method as well as compound synthesis, target identification by TOP-ABPP, and secondary parasite assays are outlined in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank William Roush and James Powers for providing some of the irreversible inhibitors of cysteine and serine proteases making up our directed library; S. Verhelst for help and advice in designing the solid-phase synthetic strategy for WRR-086 and related analogs; A. Guzzetta for HR-MS analysis of WRR-086; and Vern Carruthers for providing antibodies, reagents, and advice on performing microneme secretion and motility assays. This work was funded by a Burroughs Wellcome Trust New Investigators in Pathogenesis Award (to M.B.) and by National Institutes of Health Grants R01-AI078947 and EB005011 (to M.B.), RO1 AI21423 (to J.C.B.), R01 AI054961 (to G.E.W.). C.I.H. was supported by an American Society for Microbiology Robert D. Watksins Fellowship. M.L.R. was supported, in part, by a fellowship from the American Cancer Society.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105622108/-/DCSupplemental.
References
- 1.Montoya JG, Liesenfeld O. Toxoplasmosis Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 2.Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 3.Mital J, Ward GE. Current and emerging approaches to studying invasion in apicomplexan parasites. Subcell Biochem. 2008;47:1–32. doi: 10.1007/978-0-387-78267-6_1. [DOI] [PubMed] [Google Scholar]
- 4.Arastu-Kapur S, et al. Identification of proteases that regulate erythrocyte rupture by the malaria parasite Plasmodium falciparum. Nat Chem Biol. 2008;4:203–213. doi: 10.1038/nchembio.70. [DOI] [PubMed] [Google Scholar]
- 5.Carey KL, Westwood NJ, Mitchison TJ, Ward GE. A small-molecule approach to studying invasive mechanisms of Toxoplasma gondii. Proc Natl Acad Sci USA. 2004;101:7433–7438. doi: 10.1073/pnas.0307769101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenbaum DC, et al. A role for the protease falcipain 1 in host cell invasion by the human malaria parasite. Science. 2002;298:2002–2006. doi: 10.1126/science.1077426. [DOI] [PubMed] [Google Scholar]
- 7.Phillips CI, Bogyo M. Proteomics meets microbiology: Technical advances in the global mapping of protein expression and function. Cell Microbiol. 2005;7:1061–1076. doi: 10.1111/j.1462-5822.2005.00554.x. [DOI] [PubMed] [Google Scholar]
- 8.Puri AW, Bogyo M. Using small molecules to dissect mechanisms of microbial pathogenesis. ACS Chem Biol. 2009;4:603–616. doi: 10.1021/cb9001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heaslip AT, et al. A small-molecule inhibitor of T. gondii motility induces the posttranslational modification of myosin light chain-1 and inhibits myosin motor activity. PLoS Pathog. 2010;6:e1000720. doi: 10.1371/journal.ppat.1000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weerapana E, Speers AE, Cravatt BF. Tandem orthogonal proteolysis-activity-based protein profiling (TOP-ABPP)—a general method for mapping sites of probe modification in proteomes. Nat Protoc. 2007;2:1414–1425. doi: 10.1038/nprot.2007.194. [DOI] [PubMed] [Google Scholar]
- 11.Anderson PC, Daggett V. Molecular basis for the structural instability of human DJ-1 induced by the L166P mutation associated with Parkinson's disease. Biochemistry. 2008;47:9380–9393. doi: 10.1021/bi800677k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito G, Ariga H, Nakagawa Y, Iwatsubo T. Roles of distinct cysteine residues in S-nitrosylation and dimerization of DJ-1. Biochem Biophys Res Commun. 2006;339:667–672. doi: 10.1016/j.bbrc.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 13.Moore DJ, Zhang L, Dawson TM, Dawson VL. A missense mutation (L166P) in DJ-1, linked to familial Parkinson's disease, confers reduced protein stability and impairs homo-oligomerization. J Neurochem. 2003;87:1558–1567. doi: 10.1111/j.1471-4159.2003.02265.x. [DOI] [PubMed] [Google Scholar]
- 14.Weerapana E, Simon GM, Cravatt BF. Disparate proteome reactivity profiles of carbon electrophiles. Nat Chem Biol. 2008;4:405–407. doi: 10.1038/nchembio.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carruthers VB, Giddings OK, Sibley LD. Secretion of micronemal proteins is associated with toxoplasma invasion of host cells. Cell Microbiol. 1999;1:225–235. doi: 10.1046/j.1462-5822.1999.00023.x. [DOI] [PubMed] [Google Scholar]
- 16.Arrizabalaga G, Boothroyd JC. Role of calcium during Toxoplasma gondii invasion and egress. Int J Parasitol. 2004;34:361–368. doi: 10.1016/j.ijpara.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Carruthers VB, Moreno SN, Sibley LD. Ethanol and acetaldehyde elevate intracellular [Ca2+] and stimulate microneme discharge in Toxoplasma gondii. Biochem J. 1999;342:379–386. [PMC free article] [PubMed] [Google Scholar]
- 18.Wetzel DM, Chen LA, Ruiz FA, Moreno SN, Sibley LD. Calcium-mediated protein secretion potentiates motility in Toxoplasma gondii. J Cell Sci. 2004;117:5739–5748. doi: 10.1242/jcs.01495. [DOI] [PubMed] [Google Scholar]
- 19.Billker O, Lourido S, Sibley LD. Calcium-dependent signaling and kinases in apicomplexan parasites. Cell Host Microbe. 2009;5:612–622. doi: 10.1016/j.chom.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagamune K, Moreno SN, Chini EN, Sibley LD. Calcium regulation and signaling in apicomplexan parasites. Subcell Biochem. 2008;47:70–81. doi: 10.1007/978-0-387-78267-6_5. [DOI] [PubMed] [Google Scholar]
- 21.Bandyopadhyay S, Cookson MR. Evolutionary and functional relationships within the DJ1 superfamily. BMC Evol Biol. 2004;4:6. doi: 10.1186/1471-2148-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonifati V, et al. DJ-1(PARK7), a novel gene for autosomal recessive, early onset parkinsonism. Neurol Sci. 2003;24:159–160. doi: 10.1007/s10072-003-0108-0. [DOI] [PubMed] [Google Scholar]
- 23.van Duijn CM, et al. Park7, a novel locus for autosomal recessive early-onset parkinsonism, on chromosome 1p36. Am J Hum Genet. 2001;69:629–634. doi: 10.1086/322996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. DJ-1, a cancer- and Parkinson's disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci USA. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joyce BR, Queener SF, Wek RC, Sullivan WJ., Jr Phosphorylation of eukaryotic initiation factor-2alpha promotes the extracellular survival of obligate intracellular parasite Toxoplasma gondii. Proc Natl Acad Sci USA. 2010;107:17200–17205. doi: 10.1073/pnas.1007610107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carruthers VB, Sibley LD. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. [PubMed] [Google Scholar]
- 27.Donald RG, Carter D, Ullman B, Roos DS. Insertional tagging, cloning, and expression of the Toxoplasma gondii hypoxanthine-xanthine-guanine phosphoribosyltransferase gene. Use as a selectable marker for stable transformation. J Biol Chem. 1996;271:14010–14019. doi: 10.1074/jbc.271.24.14010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.