Abstract
Two distinct types of Polycomb complexes have been identified in flies and in vertebrates, one containing ESC and one containing PC. Using LexA fusions, we show that PC and ESC can establish silencing of a reporter gene but that each requires the presence of the other. In early embryonic extracts, we find PC transiently associated with ESC in a complex that includes EZ, PHO, PH, GAGA, and RPD3 but not PSC. In older embryos, PC is found in a complex including PH, PSC, GAGA, and RPD3, whereas ESC is in a separate complex including EZ, PHO, and RPD3.
Keywords: Polycomb silencing, ESC/PHO, PcG complex
Polycomb group (PcG) proteins are required to maintain the repressed state of homeotic genes set by the products of the transiently expressed segmentation genes. Polycomb response elements (PREs) have been identified in the regulatory regions of several genes (for review, see Pirrotta 1997). Such elements, when inserted in a reporter gene construct, are specific targets for the PcG proteins and can maintain the repressed state of the reporter gene. The sequences that define the different PREs are not well characterized and the mechanisms that recruit PcG proteins and assemble the repressive complexes remain unclear. Among the 14 molecularly characterized PcG genes, the product of the pleiohomeotic gene (PHO) is the only one that binds directly to DNA. PHO binding sites have been identified in many PREs, and it has been proposed that PHO, the homolog of the mammalian YY1 factor, could be the initial recruiter of the PcG complex. However, although PHO binding sites are necessary for silencing in larvae (Fritsch et al. 1999), PHO protein is not sufficient to initiate a repressive complex by itself (Poux et al. 2001). Moreover, PHO does not coimmunoprecipitate with PC and the purification of a PcG complex, named PRC1, reveals the presence of various PcG proteins, including PC, PSC, PH, and SCM but not PHO (Shao et al. 1999).
Recent work has shown that the recruitment of PcG complexes is a more complicated process and is likely to involve other DNA-binding proteins. One is GAGA factor, which recognizes multiple sites in most known PREs. GAGA factor coprecipitates with PC-containing complexes and mediates their binding in vitro to PRE fragments, suggesting that it may contribute to their recruitment in vivo (Horard et al. 2000).
Biochemical purifications and coimmunoprecipitation experiments suggest that two other PcG proteins, ESC and EZ, interact directly together, forming a complex distinct from that containing PC (Jones et al. 1998; Tie et al. 1998). Similar results were obtained with the mammalian ESC and EZ homologs (Sewalt et al. 1998; van Lohuizen et al. 1998). In Drosophila, the existence of two distinct complexes was confirmed by the recent purification of the PRC1 and ESC–EZ complexes (Shao et al. 1999; Ng et al. 2000; Tie et al. 2001). The role of these two complexes in the establishment of the silenced state is not clear but both associate with PRE sequences, because PRE-containing transposons produce new binding sites for EZ and PC at the site of insertion on polytene chromosomes. Genetic analysis shows that PcG silencing by the PRE is absolutely dependent on both Pc and esc, suggesting that the two complexes cooperate to establish silencing at PRE sites (Simon et al. 1992).
The function of the EZ protein is not well understood. In embryos and in late larvae, EZ is necessary to maintain PcG repression (Jones and Gelbart 1990). In addition, EZ is probably involved in the maintenance of chromosome integrity during mitosis (Phillips and Shearn 1990). ESC has been thought to play a special role in PcG silencing, because it is required in the early embryo for the establishment of silencing but becomes unable to establish silencing if expressed shortly after the blastoderm stage (Struhl and Brower 1982; Simon et al. 1995). These properties suggested that it might be involved in the recruitment of the PcG complex. At the end of embryogenesis, the expression of esc fades and is not required for the production of viable adults (Ng et al. 2000). The sequence of the esc gene shows that the protein consists almost entirely of six WD40 domains, a motif thought to be involved in protein–protein interactions, arranged in a characteristic paddlewheel structure (Sathe and Harte 1995; Simon et al. 1995; Neer and Smith 1996). In addition, the Drosophila ESC–EZ complex contains the histone deacetylase RPD3 and the histone-binding protein p55 (Tie et al. 2001). The homologous complex in Xenopus includes YY1, the vertebrate homolog of PHO (Satijn et al. 2001).
We have shown that various PcG proteins fused to the LexA DNA-binding domain can silence the BHL4 reporter gene, which contains four LexA-binding sequences placed in front of the Ubx–lacZ gene (Poux et al. 2001). The reporter gene is driven by the embryonic BX enhancer and the H1 imaginal disc enhancer of Ubx to allow monitoring the repressed status at different developmental stages. With this system, we found that the core PcG proteins, PC, PSC, PH, and SUZ2, when expressed from an hsp70 promoter in preblastoderm embryos, can recruit silencing complexes. Like PRE-dependent silencing, the repression induced by our fusion proteins is sensitive to the state of activity of the target chromatin: Silencing is established only when the target is inactive, whereas expression is not affected in cells in which the target is transcriptionally active. In the work reported here, we use the same approach to test the effect of targeting ESC to the BHL4 reporter gene. We show that LexA–ESC can recruit a repressive complex if expressed before 2 h of development. Although ESC and PC are said to belong to two different, noncoprecipitating complexes, LexA–ESC silencing depends on PC, and LexA–PC silencing depends on ESC. We show that this apparent paradox is resolved by the existence of a transient interaction between an ESC-containing complex and a PC-containing complex. This interaction, observed in preblastoderm embryos but absent at later stages, provides a molecular link between ESC and PC function and reveals intermediate steps in the assembly of the silencing complex.
Results
Expression of LexA–ESC protein
The LexA–ESC gene, in which the ESC coding region is fused in frame to the LexA DNA-binding domain, was expressed either from the hsp70 promoter or from the α1-tubulin promoter (α1T; Fig. 1). Western analysis of extracts from corresponding transgenic lines showed that a protein of the correct size is expressed in both cases and is recognized by anti-LexA and anti-ESC antibodies. Heat shock induction of the hsp70 promoter produces abundant fusion protein, whereas the α1-tubulin promoter accumulates low amounts compared to the endogenous ESC protein. In 5- to 6-hour-old embryos, the transgenic fusion protein is present in two isoforms corresponding to a phosphorylated and an unphosphorylated form, as was shown for endogenous ESC (Ng et al. 2000).
Figure 1.
The Lex–ESC fusions. (A) The expression of the LexA–ESC protein is driven by the hsp70 promoter or by the constitutive α1-tubulin promoter (α1T). The arrows indicate the direction of transcription. (B) Western blots developed with anti-ESC antibodies, showing extracts from 5–6-h embryos (lane 1), extracts of pET–ESC bacteria (lane 2), extracts from hs-LexA–ESC adult flies after heat shock (lane 3). Extracts from α1T–LexA–ESC flies in esc+ background (lane 4) or esc6/esc6 background (lane 5) show that endogenous ESC is absent in extracts from esc6 flies and only LexA–ESC is detected. Note the doublet band of wild-type ESC in 5–6-h embryos, corresponding to phosphorylated and unphosphorylated protein, which is not seen when ESC is expressed in adult flies. When hs-LexA–ESC is induced at 2–3 h, a Western blot developed with anti-LexA detects a similar doublet in 5- to 6-hour-old embryos (lane 6) but not in overnight α1T–LexA–ESC embryos (lane 7). A doublet PC band is also observed. The upper band, relatively more abundant in early than late embryos (lanes 8 and 9, respectively), is converted to the lower band by phosphatase treatment in early and late (lanes 10 and 11, respectively) extracts.
Both transgenes are functional and able to rescue embryonic lethality in the progeny of esc10/esc2 females. When heat shock induced at 2–3 h of development, the hs-LexA–ESC gene rescues the lethality, consistent with the results of Simon et al. (1995). Homozygous esc6 flies carrying the α1T–LexA–ESC transgene produce viable progeny. The progeny males still display multiple sex combs if they carry only one copy of the transgene but are completely rescued by two copies of the transgene, and the transgenic flies can be maintained as a stable stock in an esc− background.
LexA–ESC silences when expressed constitutively
The ability of the hs-LexA–ESC product to recruit a repressive complex was tested by crossing flies carrying the fusion gene with flies carrying the BHL4 reporter gene, as described by Poux et al. (2001). The resulting embryos were heat shocked at 1.5–2.5 h after deposition to express the chimeric protein and incubated overnight at room temperature before fixing and staining. Under these conditions, hs-LexA–PC, hs-LexA–PSC, hs-LexA–PH, and hs-LexA–SUZ2 are all able to recruit silencing complexes that maintain the correct pattern of expression of the reporter gene (Poux et al. 2001). In contrast, the hs-LexA–ESC transgene does not maintain repression in the anterior regions of the embryo, and strong ectopic expression appears in the thorax and head during germ-band retraction (Fig. 2A). We suppose that although the hs-LexA–ESC transgene can rescue esc mutants with this treatment, the protein is produced too late to establish silencing at LexA sites, where it must act as the sole PcG recruiter.
Figure 2.
Silencing by LexA–ESC. (A) The hs-LexA–ESC protein does not silence the BHL4 reporter gene, whose pattern of expression of the BHL4 reporter gene in embryos heat shocked at blastoderm (1–2 h) is the same as in non heat-shocked embryos (no hs). (B) When α1T–LexA–ESC females were crossed with BHL4 males, expression in the resulting embryos was restricted to the abdomen and repression was maintained in the thorax (wt). The repression is lost in a Pc3 mutant background (Pc). The repression induced by the hs-LexA–PC protein (wt) is abolished in esc6 embryos from esc6 mothers (esc).
The α1-tubulin promoter is active in all cells, including ovarian nurse cells, therefore supplying the oocyte with maternal transgene product (O'Donnell et al. 1994). When females carrying two copies of the α1T-LexA–ESC were crossed with males carrying the BHL4 transposon, the resulting embryos showed correct expression of the reporter gene and maintenance of the segmental boundary of expression (Fig. 2B). This indicates that LexA–ESC can recruit a repressive complex and maintain expression when expressed constitutively. The results are entirely similar to those observed with α1T–LexA–PC: Expression of the reporter gene is restricted to four stripes roughly corresponding to parasegments 6, 8, 10, and 12 and remains repressed anterior to PS6 (Fig. 2B). As we observed previously with LexA–PC, the maintenance induced by LexA–ESC in the embryo does not persist in the larva, and no repression is observed in the imaginal discs (data not shown).
LexA–ESC is necessary in the first two hours of development
The role of timing and protein concentration in the establishment of silencing is illustrated by comparing the effect of maternally and zygotically supplied α1T–LexA–ESC transgene. When the transgene is paternally contributed, no silencing is observed in the embryos, indicating that the zygotic expression is not sufficient to recruit a repressive complex. When females with a single copy of the transgene are crossed with BHL4 males, only one-half of the embryos maintain the repressed pattern, presumably those that inherited the transgene. This suggests that the maternally loaded product produced by one copy of the α1T–LexA–ESC gene is insufficient to induce a repressive complex in the absence of a zygotic contribution. In contrast, when females carrying two copies of the transgene located on different chromosomes are crossed with BHL4 males, repression is induced in all embryos, although one-fourth have no zygotic copy. Therefore, the maternal product produced by two copies of the transgene is sufficient even in the absence of a zygotic contribution. The hs-LexA–ESC product, induced at the age of 3 h, is much more abundant than the α1T–LexA–ESC product but is unable to silence the reporter gene. We conclude that it is the timing, rather than the quantity of protein produced, that is critical, and that LexA–ESC function is required in the first 2 h of development to successfully recruit silencing to the LexA site. Attempts to load the oocyte with LexA–ESC by heat shocking the hs-LexA–ESC mothers were unsuccessful, probably because the heat-shock promoter is not efficiently induced in the nurse cells.
Involvement of endogenous PcG genes
Although PC and ESC have been shown to be part of distinct complexes, evidence linking the two came first from Müller (1995), who observed that a GAL4–PC chimeric protein does not have an intrinsic silencing activity but needs to interact with a full complement of endogenous PcG proteins, including ESC. Similarly, our LexA–PC protein cannot silence in embryos mutant for Psc, Su(z)2, or esc. Conversely, the repression induced by our α1T–LexA–ESC is dependent on PcG proteins: The silencing induced by LexA–ESC is lost in a Pc3 or Psc1 mutant background (Fig. 2B). Because only the LexA fusion protein is recruited directly to the reporter gene, these results imply that PC and ESC must be able to recruit one another. Because ESC is necessary in the first 2 h of development, we concluded that the interactions between PC and ESC complexes, if they exist, should be found at that time.
Transient interaction of PC with the ESC complex
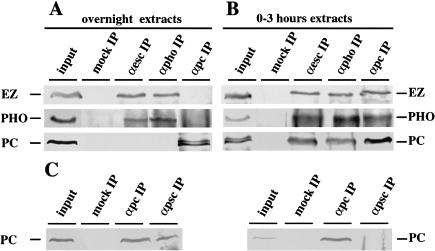
To determine the composition of the ESC/EZ complex during development, we prepared nuclear extracts from 0–3 h embryo collections as well as from the usual overnight collections. Coimmunoprecipitation experiments were performed using antibodies against various PcG proteins. In the overnight extracts, anti-ESC precipitated EZ and PHO, but not PC (Fig. 3A). Similarly, anti-PHO precipitated EZ but not PC. Conversely, anti-PC does not precipitate EZ or PHO, but it is associated with PH, PSC, and GAGA. Together with earlier observations (Horard et al. 2000; Ng et al. 2000; Poux et al. 2001; Tie et al. 2001; for the homologous vertebrate complexes, Satijn et al. 2001), these results indicate that in 10- to 14-hour-old embryos there exists a complex containing ESC, EZ, PHO, and RPD3, on the one hand, and a separate complex containing PC, PSC, PH, and GAGA factor on the other. We then performed parallel experiments using extracts from 0- to 3-hour-old embryos. Immunoprecipitation with anti-ESC showed that ESC is already associated with EZ and PHO before cellular blastoderm. Strikingly, PC also coprecipitates with ESC in this preblastoderm extract (Fig. 3B). The early association between the ESC/EZ/PHO complex and PC was confirmed when the coimmunoprecipitation was performed with anti-PHO or with anti-PC. These results imply the existence of a transient interaction in the preblastoderm embryo between PC and a complex that contains ESC, EZ, and PHO.
Figure 3.
Immmunoprecipitations using antibodies against ESC, PHO, or PC or without antibody (mock IP). Western blots of the immunoprecipitations were probed with antibodies against EZ, PHO, or PC. Whereas no interaction is detected between PC and the ESC/EZ/PHO complex in nuclear extracts from overnight embryos (A), PC coprecipitates with the ESC/EZ/PHO complex in nuclear extracts from 0–3-h embryos (B). (C) PC is coimmunoprecipitated by anti-PSC from overnight embryos but is not precipitated by anti-PSC in 0–3-h extracts.
We have described previously an alternative, more sensitive approach to detect interactions (Poux et al. 2001): In extracts containing, for example, LexA–ESC protein, not only the LexA fusion protein but any other protein associated with it should bind to a labeled DNA probe containing LexA binding sites. If, for example, EZ interacts with ESC, antibody against EZ should immunoprecipitate the LexA probe in the presence of LexA–ESC extracts but not in the presence of wild-type extracts. We used this approach with nuclear extracts containing different LexA-fusion proteins to confirm and explore further the associations among the different PcG proteins during early development.
With nuclear extracts from overnight embryos containing hs-LexA–PC (Poux et al. 2001), the LexA probe is precipitated by anti-PC, anti-PSC, anti-PH, and anti-GAGA factor and anti-RPD3 histone deacetylase but not by anti-ESC or anti-PHO antibodies, confirming that neither ESC nor PHO are associated with LexA–PC (Fig. 4A). Interestingly, RPD3 appears to be present also in the PC complex. Similarly, overnight nuclear extracts containing the LexA–PHO protein show that LexA–PHO is associated with ESC, but not with PC. Overnight extracts containing LexA–ESC show that this protein is associated with PHO and RPD3 but not with PC, PSC, PH, or GAGA factor (Fig. 4B). Overnight extracts containing LexA–EZ protein show that it recruits ESC and PHO but not PC (experiments not shown). However, when we used 0–3 h extracts containing LexA–ESC, we found that anti-PC, anti-PH, anti-GAGA, anti-RPD3, anti-PHO, and anti-ESC all precipitate the LexA probe, indicating that they are associated in a single complex. No precipitation of the LexA probe is obtained with any of these antibodies in the presence of wild-type 0–3 h embryonic extracts, showing that the binding is dependent on the presence of the LexA fusion protein. Finally, PSC does not bind the LexA probe in either early or late LexA–ESC extracts, implying that at early stages, PSC has not yet become part of the PC-containing complex. This surprising result was confirmed by direct immunoprecipitation experiments, which show that, while in the overnight extracts anti-PSC immunoprecipitates PC, in the 0–3 h extracts no detectable PC is precipitated (Fig. 3C).
Figure 4.
LexA immunoprecipitation assay. The labeled LexA probe consists of three copies of the LexA-binding site, and a control plasmid fragment is indicated by an asterisk. After incubation with nuclear extracts, the reactions were precipitated with no antibody (−), with anti-ESC (esc), anti-PC (pc), anti-PHO (pho), anti-PH (ph), anti-RPD3 (rpd3), anti-PSC (psc), or anti-GAGA antibodies (gaga). The precipitated fragments were analyzed on an acrylamide gel together with an aliquot of the input mixture (i). (A) No binding to the LexA probe is detected in extracts lacking an LexA fusion protein. In overnight nuclear extracts, LexA–PC interacts with PC, PSC, PH, RPD3, and GAGA but not with ESC or PHO. In overnight LexA–PHO extracts, ESC, but not PC, associates with the LexA–PHO protein. (B) In overnight extracts, LexA–ESC protein recruits PHO and RPD3 but not PC, PSC, PH, or GAGA, but it interacts with all of these proteins, except for PSC, in extracts from 0–3-h embryos.
Discussion
A transient interaction between ESC and PC
ESC, as first observed by Struhl and Brower (1982), plays a uniquely early role in PcG silencing. The experimentswith the LexA–ESC transgenes show that the chimeric protein must be present in the first 2 h of embryogenesis to recruit a repressive complex to the BHL4 target, and the much stronger expression of the hs-LexA–ESC induced in 2-hour-old embryos occurs too late to establish silencing at the LexA site. Because LexA–PC, LexA–PSC, or LexA–PH induced in exactly the same way can establish silencing, we conclude that LexA–ESC is required earlier than these products, or that lengthier processing is necessary before it becomes functional, by which time transcription of the reporter gene is too advanced to be repressible. An earlier heat-shock treatment is not possible, because it is lethal to embryos during the early phase of rapid nuclear divisions. The discrepancy between these experiments and the observation that a heat shock-induced ESC protein or a paternal esc+ allele can rescue homozygous esc embryos produced by esc females (Struhl and Brower 1982; Simon et al. 1995) suggests that the recruitment of a full-silencing complex by the LexA–ESC protein alone is a slow process. We suppose that at a PRE, other components can be independently recruited by other DNA-binding proteins.
Our results, together with those of Müller (1995), show that silencing by a targeted PC protein requires ESC. Conversely, LexA–ESC silencing is also abolished in Pc mutants. Assuming that both proteins act at the PRE, this implies that the two proteins or protein complexes must interact with one another at least transiently. We cannot exclude some complex mode of indirect interaction in which each of the two proteins produces a diffusible product that is recruited by the tethered component. Our discovery of a transient association between ESC and PC provides a simple explanation for the reciprocal requirement and implies that the two LexA fusion proteins recruit the same repressive complex. The fact that the interactions between PC and ESC complexes are very early and transient explains why they have not been detected in overnight nuclear extracts (Shao et al. 1999; Ng et al. 2000; Tie et al. 2001) but raises the questions of why this association is so brief and what role does it play in the establishment of silencing? One possibility is suggested by the finding that PHO, a DNA-binding protein, is a component of the ESC/EZ complex. PHO bound to the PRE might help recruit ESC/EZ and successively, through a transient interaction, PC and other core PcG proteins. However, LexA–PHO, although fully functional, is unable to establish silencing of the reporter gene, suggesting that additional functions are required to recruit ESC/EZ. Furthermore, the LexA–PC experiments imply that PC can recruit ESC directly, and that whether or not it contributes to recruitment, ESC must serve another essential role.
Our experiments show that in the early embryonic extracts, PC is already associated with PH and with GAGA factor, another DNA-binding protein that recognizes binding sites in the PRE (Horard et al. 2000), suggesting that PC is normally independently recruited to the PRE. We note, however, that the early complex does not yet include PSC, an essential PcG protein that is normally a component of the silencing complex seen at later stages (Kyba and Brock 1998; Shao et al. 1999; Horard et al. 2000). PSC is nevertheless required later, because repression induced by LexA–ESC is abolished in embryos mutant for Psc1. This suggests that the recruitment of PSC involves a slower or later step that follows the interaction between PC and the ESC/EZ complex. Evidence for a separate mechanism to bring PSC into play will be reported elsewhere (N. Hulo, R. Melfi, M. Pilyugin, and V. Pirrotta, in prep.). The transient nature of the interaction between PC and the ESC/EZ complex might be explained if in the subsequent steps, the ESC/EZ complex is displaced.
Role of ESC
Because of its structure, principally composed of WD40 domains, ESC has been suspected to be involved in chromatin regulation for many years (Sathe and Harte 1995; Simon et al. 1995). Many WD40 proteins interact with histones and histone deacetylase proteins (Chen et al. 1999; van der Vlag and Otte 1999; Guenther et al. 2000; Watson et al. 2000) and in some cases like Groucho, Tup-1, act as corepressors. The association of ESC/EZ with a histone deacetylase confirms this pattern. As proposed by Tie et al. (2001), a histone deacetylation mediated by the ESC/EZ/PHO complex might be necessary to deacetylate the nucleosomes and render them amenable to binding by a PC complex. Our finding of a more direct though transient interaction between ESC and PC complexes does not explain how LexA–ESC can recruit a Polycomb silencing complex to a LexA target if the PC/ESC interaction is so short lived. One possibility, suggested by the association of RPD3 with PC, is that after ESC separates from PC, the RPD3 associated with the PC complex would take over the function of preparing the chromatin substrate, rendering the participation of the ESC complex unnecessary. In fact, little or no ESC is normally expressed in late embryos or larvae. We favor an alternative model in which the ESC complex interacts with an independently recruited PC complex to mediate a transition, possibly involving the further recruitment of PSC, to establish the silencing complex. If this transition leads to the eventual dissociation of the ESC/EZ/PHO complex from the PC complex, it would explain why neither LexA–ESC nor LexA–PC can maintain a stable repressed state beyond the embryonic stage, as shown by the fact that repression is lost in larval imaginal discs (Poux et al. 2001). This implies that the RPD3 recruited by ESC or PC is not sufficient to maintain a stable PcG repressive complex at later stages. We interpreted this to mean that epigenetically stable repression requires additional functions that are recruited to a PRE but cannot be recruited by the LexA–PC or LexA–ESC proteins alone. One of these functions might involve an EZ/PHO complex. We speculate that this complex might have a histone methylase function analogous to that of mammalian SUV39, a SET domain protein like EZ, which stimulates the recruitment of HP1 to heterochromatin (Bannister et al. 2001; Lachner et al. 2001).
Materials and methods
Transposon constructs
The BHL4 target reporter gene was described in Poux et al. (2001). The LexA–ESC fusion contains the esc cDNA coding region from the BstBI site, fused in frame to the ClaI site at the 3′ end of the LexA coding region (Bunker and Kingston 1994). The fusion was inserted in the C4-Yellow hs vector (Poux et al. 2001). The α1T–LexA–ESC was made by replacing the PmeI–NotI fragment from α1T–LexA–PC with the corresponding LexA–ESC fragment. Details of the constructions are available on request.
Fly strains and mutants
All transgenic flies were produced using the Df(1)w67c23 host, which is y−w−. The PcG mutations used were Pc3, Psc1, esc2, esc10, and esc6. To test the effect of the mutations, the BHL4-reporter transposon and the LexA-fusions transposon were first recombined on the same chromosome, either the second or the third, according to the mutation to be tested. Mutations were balanced with a TM3 hb-lacZ or a Cyo hb-lacZ chromosome. Homozygous mutant embryos lack lacZ expression in the head region.
Antibodies
Rabbit polyclonal antibodies were raised using GST fusion proteins containing amino acids 93–276 of PHO, amino acids 191–354 of PC, amino acids 819–926 of PSC, amino acids 149–425 of GAGA, amino acids 87–431 of PH, or all but the first 10 amino acids of ESC. The production of the fusion proteins and the purification of the antibodies are described by Horard et al. (2000). For Western blots, anti-ESC antibodies were used at 1:1000, anti-PC at 1:1000, anti-LexA at 1:1500, and anti-EZ at 1:700.
Staining of embryos and discs
The effect of the fusion proteins was tested by crossing flies carrying the target with flies carrying the fusion proteins. For the hs-LexA–ESC construct, the embryos were collected at 1-h intervals, aged for 30 min, and then heat shocked for 45 min at 37°C. After further incubation at room temperature, embryos were fixed and stained as described in Poux et al. (2001).
Embryonic extracts and immunoprecipitations assays
LexA–PC and LexA–PHO extracts are described in Poux et al. (2001). For α1T–LexA–ESC extracts, embryos were collected every 3 h or overnight. The preparation of the nuclear extracts, immunoprecipitations using the LexA probe, and the coimmunoprecipitation assays are described in Horard et al. (2000).
Acknowledgments
We thank R. Jones and P. Spierer for generous gifts of antibodies and B. Horard and J. Guiard for help in antibody purification. This research was supported by a grant to V.P. from the Swiss National Science Foundation and a contribution from the Georges and Antoine Claraz Donation.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL pirrotta@zoo.unige.ch; FAX 41-22-702-6776.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.208901.
References
- Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, Kouzarides T. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–124. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- Bunker CA, Kingston RE. Transcriptional repression by Drosophila and mammalian Polycomb group proteins in transfected mammalian cells. Mol Cell Biol. 1994;14:1721–1732. doi: 10.1128/mcb.14.3.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Fernandez J, Mische S, Courey AJ. A functional interaction between the histone deacetylase RPD3 and the corepressor groucho in Drosophila development. Genes & Dev. 1999;13:2218–2230. doi: 10.1101/gad.13.17.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch C, Brown JL, Kassis JA, Müller J. The DNA-binding polycomb group protein pleiohomeotic mediates silencing of a Drosophila homeotic gene. Development. 1999;126:3905–3913. doi: 10.1242/dev.126.17.3905. [DOI] [PubMed] [Google Scholar]
- Guenther MG, Lane WS, Fischle W, Verdin E, Lazar MA, Shiekhattar R. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes & Dev. 2000;14:1048–1057. [PMC free article] [PubMed] [Google Scholar]
- Horard B, Tatout C, Poux S, Pirrotta V. Structure of a polycomb response element and in vitro binding of polycomb group complexes containing GAGA factor. Mol Cell Biol. 2000;20:3187–3197. doi: 10.1128/mcb.20.9.3187-3197.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RS, Gelbart WM. Genetic analysis of the enhancer of zeste locus and its role in gene regulation in Drosophila melanogaster. Genetics. 1990;126:185–199. doi: 10.1093/genetics/126.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CA, Ng J, Peterson AJ, Morgan K, Simon J, Jones RS. The Drosophila esc and E(z) proteins are direct partners in polycomb group-mediated repression. Mol Cell Biol. 1998;18:2825–2834. doi: 10.1128/mcb.18.5.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyba M, Brock HW. The Drosophila polycomb group protein Psc contacts ph and Pc through specific conserved domains. Mol Cell Biol. 1998;18:2712–2720. doi: 10.1128/mcb.18.5.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachner M, O'Carroll D, Rea S, Mechtler K, Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410:116–120. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- Müller J. Transcriptional silencing by the Polycomb protein in Drosophila embryos. EMBO J. 1995;14:1209–1220. doi: 10.1002/j.1460-2075.1995.tb07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neer EJ, Smith TF. G protein heterodimers: New structures propel new questions. Cell. 1996;84:175–178. doi: 10.1016/s0092-8674(00)80969-1. [DOI] [PubMed] [Google Scholar]
- Ng J, Hart CM, Morgan K, Simon JA. A Drosophila ESC–E(Z) protein complex is distinct from other polycomb group complexes and contains covalently modified ESC. Mol Cell Biol. 2000;20:3069–3078. doi: 10.1128/mcb.20.9.3069-3078.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell KH, Chen CT, Wensink PC. Insulating DNA directs ubiquitous transcription of the Drosophila melanogaster alpha 1-tubulin gene. Mol Cell Biol. 1994;14:6398–6408. doi: 10.1128/mcb.14.9.6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MD, Shearn A. Mutations in polycombeotic, a Drosophila polycomb-group gene, cause a wide range of maternal and zygotic phenotypes. Genetics. 1990;125:91–101. doi: 10.1093/genetics/125.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrotta V. PcG complexes and chromatin silencing. Curr Opin Genet Dev. 1997;7:249–258. doi: 10.1016/s0959-437x(97)80135-9. [DOI] [PubMed] [Google Scholar]
- Poux S, McCabe D, Pirrotta V. Recruitment of components of Polycomb group chromatin complexes in Drosophila. Development. 2001;128:75–85. doi: 10.1242/dev.128.1.75. [DOI] [PubMed] [Google Scholar]
- Sathe SS, Harte PJ. The Drosophila extra sex combs protein contains WD motifs essential for its function as a repressor of homeotic genes. Mech Dev. 1995;52:77–87. doi: 10.1016/0925-4773(95)00392-e. [DOI] [PubMed] [Google Scholar]
- Satijn DP, Hamer KM, den Blaauwen J, Otte AP. The polycomb group protein EED interacts with YY1, and both proteins induce neural tissue in Xenopus embryos. Mol Cell Biol. 2001;21:1360–1369. doi: 10.1128/MCB.21.4.1360-1369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewalt RG, van der Vlag J, Gunster MJ, Hamer KM, den Blaauwen JL, Satijn DP, Hendrix T, van Driel R, Otte AP. Characterization of interactions between the mammalian polycomb-group proteins Enx1/EZH2 and EED suggests the existence of different mammalian polycomb-group protein complexes. Mol Cell Biol. 1998;18:3586–3595. doi: 10.1128/mcb.18.6.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Raible F, Mollaaghababa R, Guyon JR, Wu CT, Bender W, Kingston RE. Stabilization of chromatin structure by PRC1, a Polycomb complex. Cell. 1999;98:37–46. doi: 10.1016/S0092-8674(00)80604-2. [DOI] [PubMed] [Google Scholar]
- Simon J, Chiang A, Bender W. Ten different Polycomb group genes are required for spatial control of the abdA and AbdB homeotic products. Development. 1992;114:493–505. doi: 10.1242/dev.114.2.493. [DOI] [PubMed] [Google Scholar]
- Simon J, Bornemann D, Lunde K, Schwartz C. The extra sex combs product contains WD40 repeats and its time of action implies a role distinct from other Polycomb group products. Mech Dev. 1995;53:197–208. doi: 10.1016/0925-4773(95)00434-3. [DOI] [PubMed] [Google Scholar]
- Struhl G, Brower D. Early role of the esc+ gene product in the determination of segments in Drosophila. Cell. 1982;31:285–292. doi: 10.1016/0092-8674(82)90428-7. [DOI] [PubMed] [Google Scholar]
- Tie F, Furuyama T, Harte PJ. The Drosophila Polycomb Group proteins ESC and E(Z) bind directly to each other and co-localize at multiple chromosomal sites. Development. 1998;125:3483–3496. doi: 10.1242/dev.125.17.3483. [DOI] [PubMed] [Google Scholar]
- Tie F, Furuyama T, Prasad-Sinha J, Jane E, Harte PJ. The Drosophila Polycomb Group proteins ESC and E(Z) are present in a complex containing the histone-binding protein p55 and the histone deacetylase RPD3. Development. 2001;128:275–286. doi: 10.1242/dev.128.2.275. [DOI] [PubMed] [Google Scholar]
- van der Vlag J, Otte AP. Transcriptional repression mediated by the human polycomb-group protein EED involves histone deacetylation. Nat Genet. 1999;23:474–478. doi: 10.1038/70602. [DOI] [PubMed] [Google Scholar]
- van Lohuizen M, Tijms M, Voncken JW, Schumacher A, Magnuson T, Wientjens E. Interaction of mouse polycomb-group (Pc-G) proteins Enx1 and Enx2 with Eed: Indication for separate Pc-G complexes. Mol Cell Biol. 1998;18:3572–3579. doi: 10.1128/mcb.18.6.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AD, Edmondson DG, Bone JR, Mukai Y, Yu Y, Du W, Stillman DJ, Roth SY. Ssn6-Tup1 interacts with class I histone deacetylases required for repression. Genes & Dev. 2000;14:2737–2744. doi: 10.1101/gad.829100. [DOI] [PMC free article] [PubMed] [Google Scholar]