Abstract
Proteolytic maturation of proBMP-4 is required to generate an active signaling molecule. We show that proBMP-4 is cleaved by furin in a sequential manner. Cleavage at a consensus furin site adjacent to the mature ligand domain allows for subsequent cleavage at an upstream nonconsensus furin site within the prodomain. BMP-4 synthesized from precursor in which the upstream site is noncleavable is less active, signals at a shorter range, and accumulates at lower levels than does BMP-4 cleaved from native precursor. Conversely, BMP-4 cleaved from precursor in which both sites are rapidly cleaved is more active and signals over a greater range. Differential use of the upstream cleavage site could provide for tissue-specific regulation of BMP-4 activity and signaling range.
Keywords: BMP, proteolytic maturation, furin, signaling range
During embryogenesis, a single family of cell–cell signaling molecules is often used to specify diverse cell fates. This is especially true for members of the transforming growth factor-β (TGF-β) family, such as bone morphogenetic protein-4 (BMP-4). BMP-4 participates in specification or patterning of virtually all organs and tissues (Hogan 1996).
Consistent with its multifunctional nature, the expression and activity of BMP-4 is regulated at multiple levels (Cho and Blitz 1998; Nakayama et al. 2000). At the transcriptional level, BMP-4 is expressed in a dynamically changing pattern throughout development. At the extracellular level, BMP-4 activity is regulated by secreted proteins (e.g., chordin, noggin, and DANs) that bind BMPs and block activation of cell-surface receptors, and by the protease Tolloid, which cleaves chordin to liberate active BMP-4. Inside the cell, BMP signaling is negatively regulated in responding cells by cytoplasmic inhibitors, Smad6 and Smad7, which function to block transmission of signals from the membrane to the nucleus (Christian and Nakayama 1999).
The bioactivity of BMP-4 may also be regulated posttranslationally, at the level of proteolytic activation. BMP-4 is synthesized as an inactive precursor that is cleaved following the multibasic motif -R-S-K-R- to yield the active, carboxy-terminal mature protein dimer (Aono et al. 1995). Proteolytic activation of BMP-4 is carried out by specific members of the proprotein convertase (PC) family of endoproteases (Cui et al. 1998; Constam and Robertson 1999). In mammals, seven members of this family have been characterized, and these exhibit overlapping but distinct substrate specificities (Steiner 1998). Furin, one of the best-characterized PCs, activates proproteins at the carboxy-terminal side of the preferred consensus sequence -R-X-R/K-R-, although it can also cleave following the minimal sequence -R-X-X-R- (Molloy et al. 1992). BMP-4 is an in vivo substrate of furin (Cui et al. 1998).
Intracellular processing of BMP-4 and other TGF-β family members may regulate the secretion, signaling range, and/or stability of the mature protein. BMP-4 and Xenopus nodal related-2 (Xnr-2), for example, normally act over a range of only one to two cells when expressed in Xenopus embryos, whereas the related TGF-β family member, activin, is freely diffusible (Jones et al. 1996). When the prodomain of activin is fused to the mature domain of either BMP-4 (Kessler and Melton 1995) or Xnr-2 (Jones et al. 1996), ligand cleaved from these precursors is more readily released from the cell and can signal over many cell diameters. Furthermore, mature Nodal cleaved from its native precursor protein appears to be highly unstable, whereas that cleaved from a chimeric precursor containing the BMP-4 prodomain is highly stable (Constam and Robertson 1999).
In the current study, we show that proBMP-4 is sequentially cleaved at two sites within the inactive prodomain. Furthermore, in vivo analyses show that differential use of the upstream cleavage site regulates the activity and signaling range of mature ligand, at least in part, by regulating protein stability.
Results and Discussion
The BMP-4 precursor undergoes ordered cleavage at two sites within the prodomain
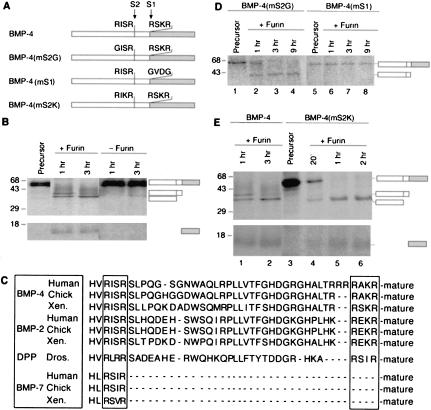
Our previous studies on proteolytic activation of BMP-4 suggested that proBMP-4 may be cleaved at more than one site within the prodomain (Cui et al. 1998). To test this possibility, [35S]proBMP-4 was incubated with 5 nm of recombinant furin in vitro and cleavage products were analyzed by SDS-PAGE and autoradiography at increasing time intervals (Fig. 1B). As expected, furin cleaved the 50-kD proBMP-4 at the previously identified consensus furin site (-RSKR285↓-, designated the S1 site in Fig. 1A) to yield the 15-kD mature BMP-4 peptide as well as the intact 35-kD prodomain. However, a third product of Mr 32 kD was also observed. With longer incubation times, the 35-kD prodomain was converted into the 32-kD form. The size of the smaller prodomain fragment is consistent with cleavage at a minimal furin consensus motif (-RISR250↓-; designated S2 in Fig. 1A) located 35 residues upstream of the S1 excision site.
Figure 1.
Furin sequentially cleaves proBMP-4 at two sites within the prodomain. (A) Cleavage sites in native and mutant forms of Xenopus proBMP-4. White bar represents the prodomain, shaded bar represents the mature ligand domain. Radiolabeled native (B) or cleavage mutant forms (D,E) of proBMP-4 were incubated with furin for the times indicated. Bands corresponding to uncleaved precursor, intact prodomain following cleavage at the S1 site, amino-terminal prodomain fragment following cleavage at the S2 site, and mature BMP-4 are indicated to the right of each gel. Bands corresponding to mature BMP-4 in D were faint and did not reproduce well in print. (C) Alignment of sequences flanking the cleavage site(s) of BMP-2, BMP-4, and BMP-7 from human, chick, Xenopus (Xen), and DPP from Drosophila (Dros.) are shown.
The above results suggest that the BMP-4 precursor may be sequentially cleaved, first at an optimal furin motif (-R-X-R/K-R-; the S1 site), and subsequently at a minimal furin motif (-R-X-X-R-; the S2 site) within the prodomain. Consistent with the possibility that both of these sites are utilized in vivo, primary and upstream furin cleavage motifs are conserved in all known vertebrate BMP-2 and BMP-4 precursor proteins and in the Drosophila ortholog, decapentaplegic (DPP), but not in other BMP superfamily members, such as BMP-7 (examples shown in Fig. 1C).
To test whether both cleavage sites are recognized by furin, we assayed cleavage of mutant forms of proBMP-4, in which the furin consensus motif at either the S1 or the putative S2 site had been disrupted. ProBMP-4(mS2G), which lacks a furin motif at the S2 site (Fig. 1A), was cleaved to generate a single ∼35-kD prodomain fragment (Fig. 1D, lanes 1–4), indicating that the ∼32-kD proteolytic fragment requires the presence of the upstream -RISR- motif. In contrast, proBMP-4(mS1), in which the consensus furin motif at the S1 site had been disrupted (Fig. 1A), was completely resistant to cleavage by furin (Fig. 1D, lanes 5–8) despite containing the native S2 site. These studies show that cleavage of proBMP-4 by furin at the S1 site is required for subsequent cleavage at the S2 site.
To further test whether a minimal furin recognition sequence is required for sequential cleavage of BMP-4, we analyzed maturation of a mutant precursor protein [BMP-4(mS2K); Fig. 1A], in which the S2 site was converted to an optimal furin motif. When native BMP-4 was incubated with furin for 1 h, proteolytic fragments corresponding to the intact prodomain and to the amino-terminal prodomain fragment generated by cleavage at the S2 site were observed (Fig. 1E, lane 1) and by 3 h, cleavage at the S2 site was nearly complete (lane 2). In contrast, BMP-4(mS2K) was fully cleaved within 1 h to generate the amino-terminal prodomain fragment and a single mature fragment (Fig. 1E, lane 5) that comigrated with mature BMP-4 cleaved from the native precursor, suggesting that cleavage had occured at both the S1 and S2 sites. A fragment corresponding to the intact prodomain of BMP-4(mS2K), generated by cleavage of the S1 site alone, was barely detectable following a 20-min incubation with furin (Fig. 1E, lane 4). These data suggest that introduction of an optimal furin consensus motif at the S2 site disrupts sequential cleavage of the BMP-4 precursor and allows both sites to be cleaved simultaneously or nearly so. Further evidence that the minimal furin recognition sequence is required for sequential cleavage is provided by the finding that introduction of an optimal consensus motif into the S2 site of BMP-4(mS1) enables this site to be cleaved independent of cleavage at the S1 site (data not shown).
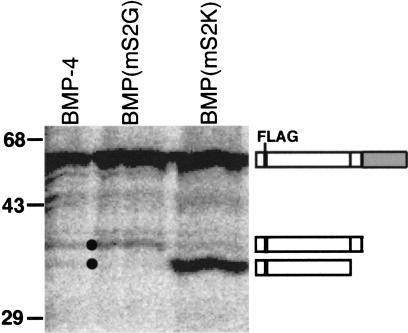
Analysis of proBMP-4 processing in vivo showed that, as observed in vitro, the prodomain is cleaved at the S1 and S2 sites. RNAs (25 ng) encoding epitope (FLAG)-tagged native or cleavage mutant BMP-4 precursors were injected into Xenopus oocytes, newly synthesized proteins radiolabeled by incubation in [35S]methionine for 48 h and FLAG-tagged proteins immunoprecipitated. Microinjection of RNA encoding native proBMP-4 produced both the 35- and 32-kD prodomain fragments (Fig. 2, black dots), showing cleavage at both the S1 and S2 sites. Further, in agreement with the in vitro studies, microinjection of proBMP-4(mS2G) produced only the 35-kD intact prodomain (corresponding to cleavage at only the S1 site), whereas expression of proBMP-4(mS2K) produced only the 32-kD prodomain fragment, consistent with rapid cleavage at the S1 and S2 sites.
Figure 2.
ProBMP-4 is cleaved at two sites within the prodomain in vivo. RNAs encoding FLAG-tagged BMP-4 precursors were injected into Xenopus oocytes that were metabolically labeled, and FLAG-tagged proteins immunoprecipitated. Bands corresponding to uncleaved proBMP-4, intact prodomain following cleavage at the S1 site, and amino-terminal prodomain fragment following cleavage at the S2 site are indicated. Differences in the relative level of prodomain cleaved from each precursor were not reproducible.
Ordered cleavages regulate the level of BMP signaling in vivo
The requisite order of processing of proBMP-4 at the S1 and S2 sites is contrary to the processing of many prohormones (e.g., POMC and proinsulin), in which mutation of one cleavage site does not affect processing at other sites (Zhou et al. 1999). The ordered processing of proBMP-4 is, however, reminiscent of the ordered autoproteolytic processing of profurin, in which initial cleavage at a consensus furin site adjacent to the mature enzyme domain allows a second cleavage to occur at an upstream, nonconsensus furin site in the proregion (Anderson et al. 1997). These ordered cleavages are required for transport of the proenzyme out of the endoplasmic reticulum and for generation of the active convertase.
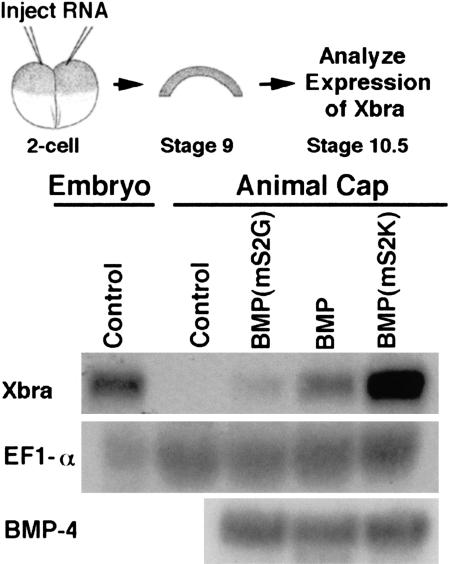
To test whether sequential cleavage of the BMP-4 proregion is required to generate a biologically active ligand, we asked whether mature BMP-4 generated from either of the S2 cleavage mutants was sufficient to activate the BMP-4 target gene, Xbra, in Xenopus animal pole explants. As shown in Figure 3, BMP activity was detected following injection of RNA encoding either S2 mutant precursor, but the level of activity varied dramatically, despite the fact that ligands cleaved from native and S2 mutant precursors are identical at the amino acid level. In multiple experiments, mature BMP-4 cleaved from pro-BMP-4(mS2G), in which the S2 site is not recognized, induced 60%–90% less Xbra expression than did BMP-4 cleaved from the native precursor. In contrast, mature ligand generated from proBMP-4(mS2K), in which the S1 and S2 sites are cleaved nearly simultaneously rather than sequentially, induced 150%–250% higher levels of Xbra than did ligand cleaved from native proBMP-4. Thus, cleavage at the S2 site, and the order of cleavage at this site, can regulate the level of BMP signaling in vivo.
Figure 3.
Activity of BMP-4 generated by maturation of S2 cleavage mutants. Ectodermal cells were explanted from Xenopus embryos made to express native or S2 cleavage mutant forms of proBMP-4 and cultured to stage 10.5. Expression of the BMP-4 target gene Xbra was analyzed by Northern blot hybridization. The filter was rehybridized with a probe for EF-1α as a loading control. Levels of injected BMP-4 transcripts were analyzed by Northern blot hybridization of RNA extracted from sibling embryos at the 8-cell stage.
Differences in the level of BMP signaling following overexpression of native and S2 mutant precursors were also apparent in a whole-embryo ventralization assay. RNA (100 pg) encoding each precursor was injected near the animal pole of one-cell embryos. At the tailbud stage, embryos were scored for BMP-mediated loss of dorsal structures by use of the dorsoanterior index (DAI) scale (Kao and Elinson 1988), in which five signifies normal patterning and zero signifies complete loss of all dorsal and anterior structures. Consistent with the results of gene induction studies, rapid cleavage at the S1 and S2 sites led to a higher level of BMP-induced ventralization (average DAI of 1.8, n = 319), and cleavage at the S1 site alone generated a lower level of ventralization (average DAI of 3.0, n = 246), than did sequential cleavage of native proBMP-4 (average DAI of 2.6, n = 250).
Ordered cleavages within the prodomain regulate the signaling range of mature BMP-4
BMP-4 and DPP are morphogens that trigger distinct responses in target cells in a concentration-dependent manner. In some embryonic tissues, these molecules diffuse or are transported from a localized source to distal cells, whereas in other tissues they can signal only to adjacent cells (Neumann and Cohen 1997). Xenopus BMP-4, for example, acts over multiple cells within the embryonic mesoderm (Dosch et al. 1997), but acts only within the immediate environment of its synthesis in ectodermal explants (Jones et al. 1996). Similarly, DPP acts over a long range to specify cell fate in the wing disc, but signals at short range between germ layers of the gut (Neumann and Cohen 1997). The evolutionarily conserved correlation between the presence of two cleavage sites in the precursor (Fig. 1C) and regulated diffusibility of the cleaved morphogen led us to test the hypothesis that sequential cleavage of proBMP-4 regulates the range of action of the mature ligand.
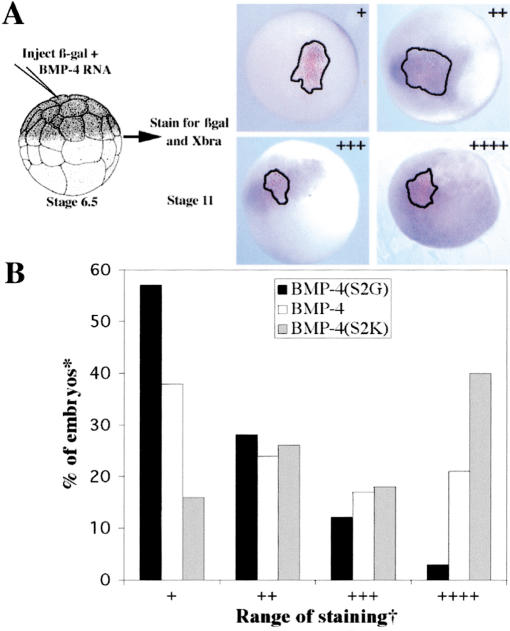
In embryonic ectoderm, BMP-4 generated from precursors in which sequential cleavage of the prodomain is disrupted showed dramatic differences in signaling range relative to BMP-4 cleaved from native precursor. Our ectodermal signaling assay (Fig. 4A) involved coinjecting RNAs encoding either native or mutant proBMP-4 (100–200 pg) together with β-galactosidase (100 pg, to mark the site of injection) into a single animal pole blastomere of 32- to 64-cell Xenopus embryos. Embryos were cultured to stage 11 (>20,000 cells) and stained for β-galactosidase (β-gal) activity (punctate red stain, outlined), and for expression of the BMP-4 target gene, Xbra (diffuse purple stain). Shown in Figure 4A are representative embryos in which Xbra staining was confined to the domain of β-gal expressing cells (+), extended 2–4 cells (++) or 10–20 cells (+++) beyond this domain, or was detected in all cells (++++) of the animal hemisphere. When individual embryos from multiple experiments (n = 95–140 for each group) were scored for spread of Xbra signal, we found that BMP-4 generated by cleavage at the S1 site alone [BMP-4(mS2G)] signaled primarily at short range, BMP-4 generated by ordered cleavage of the native precursor at intermediate range, and that generated from precursors in which S1 and S2 sites are cleaved simultaneously [BMP-4(mS2K)] at long range. Thus, failure to cleave at the S2 site restricts, whereas rapid cleavage at both sites enhances the range over which mature BMP-4 can signal in vivo.
Figure 4.
Sequential cleavage of proBMP-4 regulates the signaling range of the mature ligand. (A) RNAs encoding proBMP-4 and β-gal were coinjected into a single blastomere of 32-cell embryos as illustrated. Embryos were stained for β-gal activity (red stain, outlined) and for Xbra (purple) at stage 11. Representative embryos showing short- (+), intermediate- (++ or +++), and long-range (++++) BMP signaling, as defined in the text, and in B are illustrated. (B) Graphic representation of the fraction of embryos made to express native or S2 mutant forms of proBMP-4 that show either short-, intermediate-, or long-range BMP-4 signaling. (✻) Only embryos positive for both Xbra and β-gal were scored. (†) Scored as Xbra staining that completely overlapped with β-gal (+), extended 2–4 (++) or 10–20 (+++) cells beyond β-gal domain, or extended over entire animal pole (++++).
Cleavage at the S2 site regulates the level of mature BMP-4 protein
To begin to ask how cleavages within the prodomain regulate the bioactivity and signaling range of the mature ligand, we analyzed expression of BMP-4 protein in embryos made to express myc-epitope tagged versions of native or S2 mutant proBMP-4. Introduction of this epitope tag does not affect the activity of BMP-4 in animal cap or whole-embryo ventralization assays (data not shown). RNA (1 ng) encoding native or mutant precursors was injected into zygotes and steady-state levels of pro- and mature BMP-4 were analyzed by probing Western blots of developmentally staged embryo extracts with anti-myc antibody (Fig. 5A). BMP precursor proteins were first detected at stage 6 (data not shown) or 7 (3–4 h after RNA was injected) and were robustly expressed by stage 8, whereas mature BMP-4 was barely detectable at stage 8 (5.5 h after RNA injection) and peaked at stage 9 (8 h after RNA injection). Interestingly, although endogenous BMP transcripts are present maternally, the BMP signaling pathway is first active at the mid-blastula transition (stage 8), and activation is independent of new gene transcription (Faure et al. 2000; Kurata et al. 2000). Our results raise the possibility that the timing of activation of the intracellular BMP-signaling pathway may be regulated by temporally restricted proteolytic activation of precursor protein.
Figure 5.
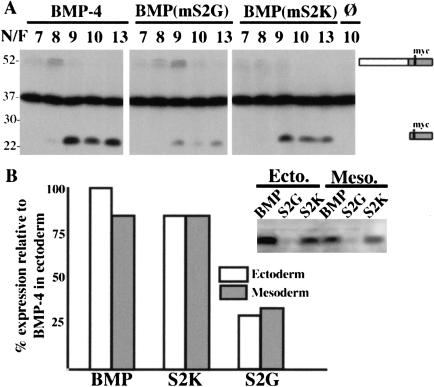
Failure to cleave at the S2 site leads to reduced levels of mature BMP-4. (A) One-cell embryos were injected with RNA encoding native or mutant myc-tagged proBMP-4, or with no RNA (ø) as indicated above each lane. Levels of precursor and ligand were examined by Western blot of embryonic extracts collected at the indicated Nieuwkoop and Faber (N/F) stages. Bands corresponding to uncleaved precursor and mature ligand are indicated to the right of the gel. The dark band in the center of the gel is a nonspecific signal that is present in uninjected embryos and serves as a loading control. (B) RNA encoding native or cleavage mutant forms of myc-tagged proBMP-4 were injected into a prospective ectodermal or mesodermal blastomere of 32-cell embryos and levels of mature BMP-4 examined by Western blot at stage 10 or 13. A representative blot with bands corresponding to mature ligand cleaved from the indicated precursors is shown. The level of expression of mature BMP-4 cleaved from native precursor in ectodermal cells (open bars) was arbitrarily set at 100% and expression of mature BMP-4 cleaved from native precursor in mesodermal cells, or from mutant precursors in either tissue was calculated relative to this value. The graph represents averaged values from three experiments.
Failure to cleave precursors at the S2 site led to a dramatic decrease, whereas rapid cleavage at both sites had no effect on steady-state levels of mature BMP-4. In multiple experiments, mature BMP-4 generated by cleavage of proBMP-4(mS2K) was present at equal or slightly lower levels (75%–90%) than that generated by cleavage of native proBMP-4 (Fig. 5A). Thus, differences in protein levels cannot explain the increase in bioactivity and signaling range of ligands cleaved from proBMP-4(mS2K). In contrast, at all stages examined, steady-state levels of mature BMP-4 in embryos made to express proBMP-4(mS2G) were 5%–25% of those in embryos made to express native precursor. These lower levels of mature BMP-4 might be due to inefficient cleavage of the precursor or to targeted degradation of the ligand. In the experiment shown in Figure 5A, levels of BMP-4(mS2G) precursor protein peaked at a later stage than did native proBMP-4, consistent with inefficient cleavage, but the levels and persistence of the three precursor proteins were identical in two other experiments. Furthermore, in all experiments, the amount of proBMP-4(mS2G) protein decreased dramatically after stage 9, yet levels of cleaved ligand did not increase over time as would be predicted if cleavage were merely delayed. Our data suggest that failure to cleave the upstream S2 site targets mature BMP-4 for rapid degradation, thereby leading to a reduction in bioactivity and signaling range.
To begin to assay for tissue-specific differences in cleavage of the S2 site, we compared levels of mature BMP-4 protein in embryos in which proBMP-4 was targeted to either ectodermal or mesodermal cells. RNAs (1 ng) encoding myc-tagged native or S2 cleavage mutant forms of proBMP-4 were injected into a single ectodermal or mesodermal progenitor of 32-cell embryos. At the gastrula stage, extracts isolated from sibling embryos made to express each precursor in either ectodermal or mesodermal cells were analyzed by Western blot. If the S2 site was recognized solely in ectodermal or mesodermal tissues, then levels of mature BMP would be lower in embryos expressing native proBMP-4 in one germ layer than in siblings expressing it in the other. Contrary to this prediction, relatively equivalent levels of mature ligand were detected in embryos made to express a given precursor in ectodermal or mesodermal cells (Fig. 4B). Steady-state levels of mature BMP-4 cleaved from proBMP-4(mS2G) were always lower than those cleaved from other precursors. These results argue against differential use of the S2 site in ectoderm versus mesoderm, at least prior to stage 9, when cleavage of ectopically introduced precursor is complete. A more complete analysis of potential tissue-specific differences in processing will require comparison of animals expressing endogenous levels of native or mutant precursors using an appropriate model system such as a knock-in mouse.
In the current study, we have shown that proBMP-4 is cleaved initially at a site adjacent to the mature ligand domain and then at a novel site within the prodomain. Our observation that cleavage at the upstream site can regulate the activity of BMP-4 after it has been excised from the prodomain is not unprecedented. Previous studies have shown that propeptides can influence the activity of their associated mature peptides even after they are proteolytically liberated and/or when added in trans. The propeptide of furin, for example, is excised at a consensus furin site but remains noncovalently bound and functions as an autoinhibitor to prevent premature activation of the zymogen. A secondary cleavage at an upstream nonconsensus furin site releases the active enzyme from the prodomain (Anderson et al., 1997). Furthermore, prodomains of a variety of precursor proteins, including members of the TGF-β family (Gray and Mason 1990), can function in trans to catalyze correct folding of the associated mature peptide (Shinde and Inouye 2000). Specific interactions between propeptides and their associated proteins have also been shown to modulate a variety of protein functions including substrate specificity, stability, protein–protein interactions, and the oligomerization status the protein (Shinde and Inouye 2000).
On the basis of the above studies, we propose the following model to explain how cleavage within the prodomain of BMP-4 might regulate the bioactivity of the mature ligand. In our model, the intact amino-terminal portion of the prodomain remains transiently and noncovalently associated with mature BMP-4 following cleavage at the S1 site. This interaction induces mature BMP-4 to adopt a conformation that targets it for rapid degradation either directly, or by promoting post-translational modifications and/or association with heterologous proteins. Subsequent cleavage at the S2 site triggers an additional conformational change and/or releases the prodomain fragments from the mature ligand, such that it is no longer targeted for degradation. Finally, rapid cleavage at both the S1 and S2 sites could induce premature release of the prodomain and/or induce mature BMP-4 to adopt a conformation that is hyperactive, possibly due to enhanced interactions with receptors or accessory proteins. We are currently using biochemical assays to test various aspects of this model.
Materials and methods
cDNA constructs
cDNAs encoding S2 cleavage mutant forms of proBMP-4 were generated by PCR-based amplification of FLAG-epitope-tagged BMP-4 or BMP-4(mS1) (Hawley et al. 1995) by use of primers carrying appropriate point mutations. Sequence encoding the myc epitope (Evan et al. 1985) was inserted in-frame following the codon for the eighth amino acid of mature BMP-4 (-RSKRSPKQ[myc]QR-) using the PCR-based splicing by the overlap extension technique (Horton et al. 1990). Regions of cDNAs generated by PCR were sequenced.
Embryo culture and manipulation
Xenopus embryos were obtained, microinjected, and cultured as described (Moon and Christian 1989). Embryonic stages are according to Nieuwkoop and Faber (1967). Ectodermal explants were isolated as described (Cui et al. 1998).
Oocyte injection, immunoprecipitation, and in vitro digestion
Oocytes were isolated, injected with RNAs encoding FLAG-tagged forms of proBMP-4, labeled with [35S]methionine and proteins immunoprecipitated from lysates as described (Cui et al. 1998) with the exception that monoclonal anti-FLAG antibody M2 (Sigma) was used. Radiolabeled FLAG-tagged proBMP-4 was isolated, digested in vitro with recombinant furin, and analyzed by SDS-PAGE as described (Cui et al. 1998). Radiolabeled proteins were visualized with a Molecular Dynamics PhosphorImager.
β-galactosidase staining, in situ hybridization, and Northern analysis
Embryos were stained for β-gal activity using Red-gal (Research Organics), and processed for in situ hybridization (Nakayama et al. 1998). Northern blot analyses were performed as described (Christian et al. 1990). Radiolabeled bands were visualized with a Molecular Dynamics PhosphorImager and quantified by use of the Macintosh IP lab gel program.
Western blot analysis
Frozen embryos were homogenized in 50 mM HEPES (pH 7.5), 2 mM EDTA, 2 mM EGTA, 0.5% NP-40, 2 mM benzamidine, and 200 mM PMSF on ice. Extracts were microcentrifuged for 8 min at 4°C and supernatant was added to sample buffer containing 5% BME. Proteins from three embryo equivalents were separated by electrophoresis on a 12% polyacrylamide gel and transferred to PVDF membrane (Christian et al. 1990). Blots were probed with anti-myc monoclonal 9E10 (1:200) followed by HRP-coupled secondary antibody (Zymed, 1:5000) that was visualized by chemiluminescence. Blots were blocked and washed in TBST with 5% nonfat dry milk with a final wash in TBST alone. Autoradiograms were scanned and bands quantified by use of the Macintosh IP lab gel program.
Acknowledgments
We thank K. Cho for flag-tagged BMP-4 plasmids; Dave Keller for generating mutant BMP constructs; T. O'Hare, and members of the Christian, Thomas, and Harland laboratories for comments on the manuscript; and F. Green for technical advice. This research was funded by grants from the NIH to J.L.C. (HD37976 and HD06711) and G.T. (DK37274).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL christia@ohsu.edu; FAX (503) 494-4253.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.940001.
References
- Anderson ED, VanSlyke JK, Thulin CD, Jean F, Thomas G. Activation of the furin endoprotease is a multiple-step process: Requirements for acidification and internal propeptide cleavage. EMBO J. 1997;16:1508–1518. doi: 10.1093/emboj/16.7.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aono A, Hazama M, Notoya K, Taketomi S, Yamasaki H, Tsukuda R, Sasaki S, Fujisawa Y. Potent ectopic bone-inducing activity of bone morphogenetic protein-4/7 heterodimer. Biochem Biophys Res Commun. 1995;210:670–677. doi: 10.1006/bbrc.1995.1712. [DOI] [PubMed] [Google Scholar]
- Cho KW, Blitz IL. BMPs, Smads and metalloproteases: Extracellular and intracellular modes of negative regulation. Curr Opin Genet Dev. 1998;8:443–449. doi: 10.1016/s0959-437x(98)80116-0. [DOI] [PubMed] [Google Scholar]
- Christian JL, Nakayama T. Can't get no SMADisfaction: Smad proteins as positive and negative regulators of TGF-β family signals. BioEssays. 1999;21:382–390. doi: 10.1002/(SICI)1521-1878(199905)21:5<382::AID-BIES5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Christian JL, Edelstein NG, Moon RT. Overexpression of wild-type and dominant negative mutant vimentin subunits in developing Xenopus embryos. New Biol. 1990;2:700–711. [PubMed] [Google Scholar]
- Constam DB, Robertson EJ. Regulation of bone morphogenetic protein activity by pro domains and proprotein convertases. J Cell Biol. 1999;144:139–149. doi: 10.1083/jcb.144.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Jean F, Thomas G, Christian JL. BMP-4 is proteolytically activated by furin and/or PC6 during vertebrate embryonic development. EMBO J. 1998;17:4735–4743. doi: 10.1093/emboj/17.16.4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosch R, Gawantka V, Delius H, Blumenstock C, Niehrs C. Bmp-4 acts as a morphogen in dorsoventral mesoderm patterning in Xenopus. Development. 1997;124:2325–2334. doi: 10.1242/dev.124.12.2325. [DOI] [PubMed] [Google Scholar]
- Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure S, Lee MA, Keller T, ten Dijke P, Whitman M. Endogenous patterns of TGFβ superfamily signaling during early Xenopus development. Development. 2000;127:2917–2931. doi: 10.1242/dev.127.13.2917. [DOI] [PubMed] [Google Scholar]
- Gray AM, Mason AJ. Requirement for activin A and transforming growth factor-β 1 pro-regions in homodimer assembly. Science. 1990;247:1328–1330. doi: 10.1126/science.2315700. [DOI] [PubMed] [Google Scholar]
- Hawley SH, Wunnenberg-Stapleton K, Hashimoto C, Laurent MN, Watabe T, Blumberg BW, Cho KW. Disruption of BMP signals in embryonic Xenopus ectoderm leads to direct neural induction. Genes & Dev. 1995;9:2923–2935. doi: 10.1101/gad.9.23.2923. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins: Multifunctional regulators of vertebrate development. Genes & Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Horton RM, Cai Z, Ho ZN, Pease LR. Gene splicing by overlap extension: Tailor made genes using the polymerase chain reaction. BioTechniques. 1990;8:528–536. [PubMed] [Google Scholar]
- Jones CM, Armes N, Smith JC. Signalling by TGF-β family members: Short-range effects of Xnr-2 and BMP-4 contrast with the long-range effects of activin. Curr Biol. 1996;6:1468–1475. doi: 10.1016/s0960-9822(96)00751-8. [DOI] [PubMed] [Google Scholar]
- Kao KR, Elinson RP. The entire mesodermal mantle behaves as Spemann's organizer in dorsoanterior enhanced Xenopus laevis embryos. Dev Biol. 1988;127:64–77. doi: 10.1016/0012-1606(88)90189-3. [DOI] [PubMed] [Google Scholar]
- Kessler DS, Melton DA. Induction of dorsal mesoderm by soluble, mature Vg1 protein. Development. 1995;121:2155–2164. doi: 10.1242/dev.121.7.2155. [DOI] [PubMed] [Google Scholar]
- Kurata T, Nakabayashi J, Yamamoto T, Mochii M, Ueno N. Visualization of endogenous BMP signaling during Xenopus development. Differentiation. 2000;67:33–40. doi: 10.1046/j.1432-0436.2001.067001033.x. [DOI] [PubMed] [Google Scholar]
- Molloy SS, Bresnahan PA, Leppla SH, Klimpel KR, Thomas G. Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J Biol Chem. 1992;267:16396–16402. [PubMed] [Google Scholar]
- Moon RT, Christian JL. Microinjection and expression of synthetic mRNAs in Xenopus embryos. Technique. 1989;1:76–89. [Google Scholar]
- Nakayama T, Snyder MA, Grewal SS, Tsuneizumi K, Tabata T, Christian JL. Smad8 acts downstream of BMP-4 to modulate its activity during vertebrate embryonic patterning. Development. 1998;125:857–867. doi: 10.1242/dev.125.5.857. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Cui Y, Christian JL. Regulation of BMP/Dpp signaling during embryonic development. Cell Mol Life Sci. 2000;57:943–956. doi: 10.1007/PL00000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann C, Cohen S. Morphogens and pattern formation. BioEssays. 1997;19:721–729. doi: 10.1002/bies.950190813. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal lable of Xenopus laevis. Amsterdam, The Netherlands: North Holland Publishing; 1967. [Google Scholar]
- Shinde U, Inouye M. Intramolecular chaperones: Polypeptide extensions that modulate protein folding. Semin Cell Dev Biol. 2000;11:35–44. doi: 10.1006/scdb.1999.0349. [DOI] [PubMed] [Google Scholar]
- Steiner DF. The proprotein convertases. Curr Opin Chem Biol. 1998;2:31–39. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- Zhou A, Webb G, Zhu X, Steiner DF. Proteolytic processing in the secretory pathway. J Biol Chem. 1999;274:20745–20748. doi: 10.1074/jbc.274.30.20745. [DOI] [PubMed] [Google Scholar]