Abstract
Fitness costs of resistance to Bacillus thuringiensis (Bt) crops occur in the absence of Bt toxins, when individuals with resistance alleles are less fit than individuals without resistance alleles. As costs of Bt resistance are common, refuges of non-Bt host plants can delay resistance not only by providing susceptible individuals to mate with resistant individuals, but also by selecting against resistance. Because costs typically vary across host plants, refuges with host plants that magnify costs or make them less recessive could enhance resistance management. Limited understanding of the physiological mechanisms causing fitness costs, however, hampers attempts to increase costs. In several major cotton pests including pink bollworm (Pectinophora gossypiella), resistance to Cry1Ac cotton is associated with mutations altering cadherin proteins that bind this toxin in susceptible larvae. Here we report that the concentration of gossypol, a cotton defensive chemical, was higher in pink bollworm larvae with cadherin resistance alleles than in larvae lacking such alleles. Adding gossypol to the larval diet decreased larval weight and survival, and increased the fitness cost affecting larval growth, but not survival. Across cadherin genotypes, the cost affecting larval growth increased as the gossypol concentration of larvae increased. These results suggest that increased accumulation of plant defensive chemicals may contribute to fitness costs associated with resistance to Bt toxins.
Introduction
Corn and cotton engineered to produce insecticidal proteins from Bacillus thuringiensis (Bt) can increase agricultural profitability while reducing reliance on insecticide sprays [1], [2]. However, field-evolved resistance to toxins in Bt crops, which has been reported in several species of major insect pests, threatens these benefits [3]–[7]. Analysis of global monitoring data suggests that host plants that do not make Bt toxins and grow near Bt crops can reduce the risk of resistance [2]–[4]. Such non-Bt plant “refuges” can provide many susceptible individuals to mate with the rare resistant individuals surviving on Bt crops, yielding hybrid offspring. Refuges are expected to delay resistance most effectively when resistance is inherited as a recessive trait, so the hybrid offspring are killed on Bt crops [4], [8], [9].
Fitness costs of resistance occur in the absence of Bt toxins through pleiotropic effects that reduce fitness of individuals carrying resistance alleles relative to susceptible individuals that lack such alleles [10]. Because costs of Bt resistance are common, refuges not only delay resistance by providing susceptible individuals to mate with resistant individuals, but also by selecting against resistance alleles [10]–[14]. Costs are modulated by variation in environmental conditions, including host plants, competition, overwintering, and natural enemies [10]. Accordingly, refuges that magnify costs or make them less recessive could enhance resistance management [9], [10], [14]. Although dozens of studies have documented fitness costs of Bt resistance that affect many life history traits, little is known about the physiological mechanisms that cause such costs [10]. In particular, the mechanisms underlying variation in costs among host plants are not well understood, which hampers attempts to identify or create refuge plants that magnify costs.
Knowledge of the molecular and genetic basis of resistance to Bt toxins is essential for understanding what causes fitness costs and why such costs vary among host plants. In pink bollworm, Pectinophora gossypiella, and two other major lepidopteran pests of cotton, mutations in genes encoding cadherin proteins that bind Bt toxins are associated with resistance to Bt toxins [15]–[17]. In pink bollworm, three mutant alleles (r1, r2, and r3) linked with resistance to Bt toxin Cry1Ac encode incomplete versions of a cadherin protein that binds Cry1Ac in susceptible larvae [16], [18]. The normal role of Bt toxin-binding cadherin proteins, which occur in the larval midgut, remains unclear. They may affect the morphology of microvilli on the apical surface of midgut cells through several processes, including enhancement of cell adhesion and guidance of cell differentiation [19]–[22]. If cadherin mutations conferring resistance to Bt toxins interfere with midgut functional or structural integrity, such mutations could cause fitness costs by increasing the absorption and concentration of plant defensive chemicals in insect larvae.
Here we tested the hypothesis that fitness costs of resistance to Bt cotton in pink bollworm are associated with increased concentration in larvae of a plant defensive chemical, gossypol. Gossypol is a polyphenolic aldehyde from cotton (Gossypium spp.) that is toxic to many insects and plant pathogens [23]–[25]. Although the mechanisms responsible for gossypol toxicity to insects remain unknown, gossypol in the hemolymph may permeate cells and cause toxic effects on many life history traits. We reported previously that adding gossypol to the larval diet of pink bollworm reduced performance and increased fitness costs [23], but we did not measure the concentration of gossypol in larvae or identify their cadherin genotype. The results reported here confirm that gossypol reduces larval performance. We discovered that gossypol concentration was higher in larvae with cadherin resistance alleles than in larvae without such alleles. The results also show that across larval cadherin genotypes, increased gossypol concentration was associated with higher fitness costs.
Results
Effects of Cadherin Genotype on Larval Gossypol Concentration
As expected, gossypol was not detected in any larvae from control diet without gossypol, either from the related susceptible strain (MOV97H1-S) and resistant strain (MOV97-H1R) in experiment one, or from the hybrid strain (MOV97-H3) in experiment two (Table 1, n = 10). In contrast, across the two experiments, gossypol was detected in 93% of larvae from gossypol-treated diet (n = 175).
Table 1. Mean larval weight (mg) and gossypol concentration (µg/g dry weight) on gossypol and control diet in cadherin genotypes from experiment one (MOV97-H1S and MOV97-H1R) and experiment two (MOV97-H3).
| Diet | Genotype | Larval weight1,3 | n2 | Gossypol concentration1,3 | n2 |
| Experiment 1: MOV97-H1S and MOV97-H1R | |||||
| Control | ss | 30.5 (0.3) | 311 | 0 | 1 |
| r3r3 | 28.6 (0.5) | 113 | 0 | 1 | |
| r1r3 | 27.9 (0.6) | 100 | 0 | 1 | |
| r1r1 | 28.6 (0.5) | 65 | 0 | 1 | |
| rr (averaged) | 28.3 (0.4) | 3 | 0 | 3 | |
| Gossypol | ss | 28.8 (0.3) | 232 | 0.56 (0.08) | 25 |
| r3r3 | 26.0 (0.5) | 85 | 1.06 (0.14) | 25 | |
| r1r3 | 25.1 (0.6) | 80 | 2.09 (0.32) | 23 | |
| r1r1 | 23.7 (0.7) | 50 | 4.87 (1.15) | 12 | |
| rr (averaged) | 24.9 (0.6) | 3 | 2.67 (1.14) | 3 | |
| Experiment 2: MOV97-H3 | |||||
| Control | ss | 29.9 (0.7) | 61 | 0 | 1 |
| r3s | 27.3 (0.6) | 86 | 0 | 1 | |
| r1s | 30.7 (0.8) | 39 | 0 | 1 | |
| r3r3 | 28.1 (1.0) | 33 | 0 | 1 | |
| r1r3 | 30.3 (0.9) | 32 | 0 | 1 | |
| r1r1 | 28.7 (1.0) | 24 | 0 | 1 | |
| rs (averaged) | 29.0 (0.7) | 2 | 0 | 2 | |
| rr (averaged) | 29.0 (0.9) | 3 | 0 | 3 | |
| Gossypol | ss | 28.8 (0.8) | 56 | 0.41 (0.08) | 22 |
| r3s | 25.7 (0.7) | 77 | 1.43 (0.08) | 31 | |
| r1s | 24.7 (1.1) | 33 | 2.17 (0.16) | 14 | |
| r3r3 | 25.4 (1.2) | 28 | 2.06 (0.24) | 12 | |
| r1r3 | 31.0 (1.5) | 21 | 3.52 (0.76) | 9 | |
| r1r1 | 20.2 (5.8) | 4 | 11.21 (3.80) | 2 | |
| rs (averaged) | 25.2 (0.9) | 2 | 1.80 (0.37) | 2 | |
| rr (averaged) | 25.5 (2.8) | 3 | 5.60 (2.84) | 3 | |
For each combination of genotype and diet, standard errors are reported in parentheses.
For each combination of genotype and diet, number of larvae (n) are reported.
The means for rr are the average across r3r3, r1r3, and r1r1; for rs these are the average across r3s and r1s.
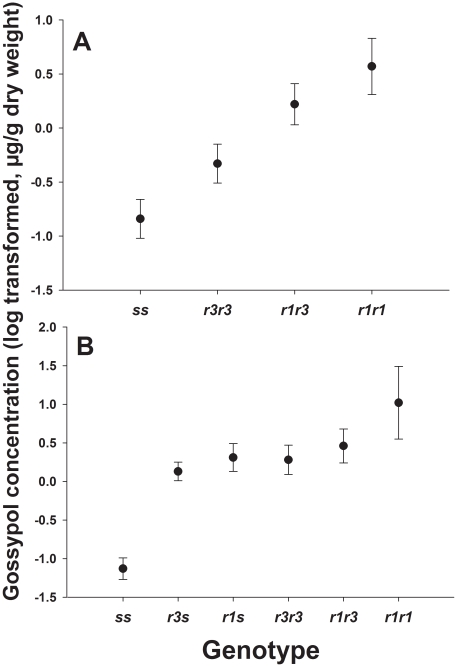
Across both experiments, the proportion of larvae from gossypol diet in which we detected no gossypol was significantly higher for susceptible (ss) larvae (10/47 = 0.21) than for larvae with either one r allele (rs) or two r alleles (rr) (3/128 = 0.02) (Fisher's exact test, P<0.0001). For larvae fed gossypol diet in both experiments, gossypol concentration was higher in rs and rr larvae than in ss larvae (Fig. 1 and Table 1). In experiment one, gossypol concentration was 4.8 times higher in rr (2.67 µg/g) than ss (0.56 µg/g) (t = 4.58, df = 81, P<0.0001). In experiment two, relative to the gossypol content of ss (0.41 µg/g), gossypol content was 13.6 times higher in rr (5.60 µg/g) and 4.4 times higher in rs (1.80 µg/g). Gossypol content was significantly higher in rr than ss (t = 6.35, df = 84, one-sided P<0.0001) and in rs than ss (t = 7.55, df = 84, one-sided P<0.0001), but did not differ significantly between rs and rr (t = 1.67, df = 84, P = 0.098). Because gossypol content of rs differed from ss but not rr, inheritance of this trait was not recessive. In both experiments, gossypol concentration differed between each rs or rr genotypes and ss (Fig. 1A, one-sided P values<0.024; Fig. 1B, one-sided P values<0.0001).
Figure 1. Gossypol concentration in pink bollworm cadherin genotypes.
Mean gossypol concentration (±SE, log [x+0.001] transformed) in larvae from (A) MOV97-H1S and MOV97-H1R and (B) MOV97-H3 fed on gossypol diet.
Effects of Gossypol and Cadherin Genotype on Larval Weight
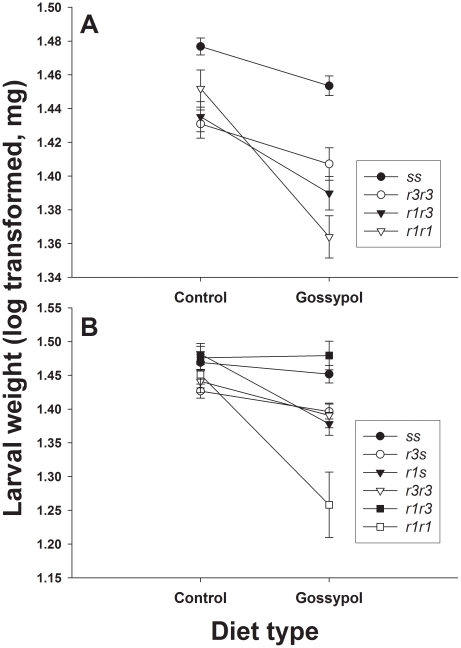
In both experiments, larval weight was lower on gossypol diet than on control diet. Also, in both experiments, the reduction in larval weight on gossypol diet relative to control diet was greater for rs and rr larvae than for ss larvae. In experiment one on control diet, weight was lower in rr (28.3 mg) than ss (30.5 mg) (t = 5.04, df = 1028, P<0.0001), showing a fitness cost without gossypol. On gossypol diet, weight was also lower in rr (24.9 mg) than ss (28.8 mg) (t = 7.81, df = 1028, one-sided P<0.0001). The coefficient of the linear contrast with associated 95% confidence interval was −0.037 (−0.49, −0.25) on control diet and −0.67 (−0.081, −0.053) on gossypol diet. As each coefficient lies outside the confidence interval for the other, costs were significantly higher on gossypol than control diet (P<0.05). Larval weight was generally lower on gossypol diet than control diet (Fig 2A, t = −6.17, one-sided P<0.0001). Relative to ss, weight differed more between gossypol diet and control diet in r1r1 (t = −3.52, one-sided P<0.00025), but not in the other genotypes (one-sided P values>0.08) (Fig. 1A).
Figure 2. Weight of pink bollworm cadherin genotypes on control and gossypol diet.
Mean weight (±SE, log transformed) of larvae from (A) MOV97-H1S and MOV97-H1R and (B) MOV97-H3.
In experiment two on control diet, weight of rr (29.0 mg) did not differ significantly from ss (29.9 mg) (t = 0.80, df = 482, P = 0.42), nor did weight of rs (29.0 mg) differ from ss (t = 0.96, df = 482, P = 0.34). Thus, no cost affecting weight was seen on control diet in this experiment. On gossypol diet, however, weight was lower for rr (25.5 mg) than ss (28.8 mg) (t = 3.33, df = 482, one-sided P = 0.00045), which indicates a cost. Furthermore, weight was lower for rs (25.2 mg) than ss (t = 3.93, df = 482, one-sided P<0.0001), but weight of rs and rr did not differ significantly (t = 0.53, df = 482, P = 0.59). Thus, gossypol induced a non-recessive cost affecting larval weight. Larval weight was lower on gossypol diet than control diet (Fig. 2B, t = −5.11, one-sided P<0.0001). Relative to ss, weight differed more between gossypol diet and control diet in r1r1 (t = −3.18, one-sided P = 0.008) and r1s (t = −2.96, one-sided P = 0.002), but not in the other genotypes (one-sided P values>0.14) (Fig. 1B).
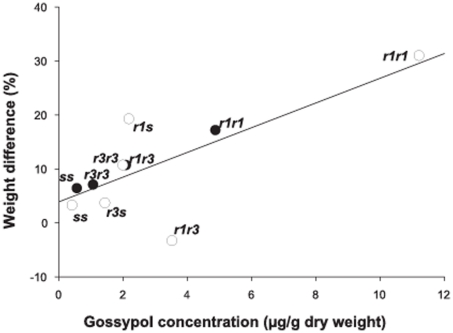
Pooling rr and rs genotypes across experiments, the mean cost (±SE) affecting weight was significantly different from zero both on control diet (5.3±1.7%; t = 3.04, df = 7, P = 0.019) and gossypol diet (12.9±3.7%; t = 3.52, df = 7, one-sided P = 0.0048). The increase in cost from control to gossypol diet was statistically significant (paired t-test, t = 1.98, df = 7, one-sided P = 0.044). Across the six larval genotypes tested in experiments one and two, a positive association occurred between gossypol content and the decrease in larval weight on gossypol diet compared to control diet (Fig. 3, t = 3.23, df = 8, one-sided P = 0.0060). The association between gossypol content and decreased weight was also significant when data were analyzed separately for experiment one (P = 0.0015) and two (P = 0.046). This suggests that increased gossypol concentration in larvae with one or two r alleles compared to ss increased the cost affecting larval growth.
Figure 3. Association between gossypol concentration and difference in weight on gossypol diet relative to control diet.
Closed circles show data from MOV97-H1S and MOV97-H1R; open circles show data from MOV97-H3.
Effects of Gossypol and Cadherin Genotype on Survival
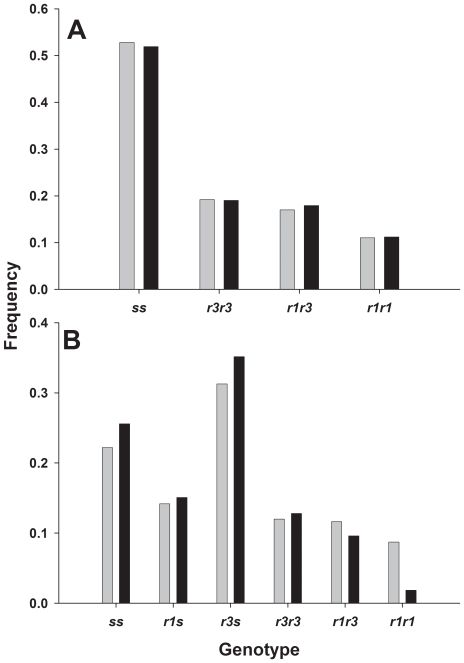
Similar to the results with larval weight, larval survival was lower on gossypol diet than on control diet in both experiments. In experiment one, survival was lower on gossypol diet (27.9%) than on control diet (36.8%) (one-sided P<0.0001). On control diet, survival of ss (38.9%) did not differ significantly from survival of rr (34.8%) (Table 1, P = 0.10). On gossypol diet, survival of ss (29.0%) also did not differ significantly from survival of rr (26.9%) (one-sided P = 0.18). Thus, gossypol did not increase the survival cost. The difference in genotype frequency on gossypol diet relative to control diet did not differ significantly between ss and r1r1, r1r3, or r3r3 (Fig. 4 A, one-sided P values>0.34), which suggests that gossypol did not reduce survival of any of the rr genotypes relative to ss.
Figure 4. Frequency of cadherin genotypes on control diet (grey bars) and gossypol diet (black bars).
(A) MOV97-H1S and MOV97-H1R and (B) MOV97-H3. For each type of diet, frequency for each genotype was calculated as the number of survivors of that genotype divided by total number of survivors.
In experiment two, survival was lower on gossypol diet (27.4%) than on control diet (34.4%) (one-sided P = 0.0014). We could not test directly for a fitness cost in experiment two (see Methods), but we could determine if the frequency of rs and rr relative to ss was reduced more on gossypol diet than on control diet. Compared to control diet, the frequency of rr (vs. ss) was marginally reduced on gossypol diet (one-sided P = 0.057), although the frequency of rs remained similar on control and gossypol diet (one-sided P = 0.45). The reduction in frequency on gossypol diet relative to control diet was significantly greater in r1r1 than ss (Fig. 4 B, χ2 = 11.73, one-sided P = 0.0003), but not in other genotypes with r alleles (all one-sided P values>0.15). This suggests that gossypol reduced survival of r1r1 relative to ss.
Discussion
We focused here on the effects of the cotton defensive compound gossypol on fitness costs by feeding Bt-resistant and susceptible pink bollworm larvae on diet without Bt toxin that either contained or lacked gossypol. We found that when pink bollworm larvae ate diet containing gossypol, the concentration of gossypol was higher in larvae with cadherin alleles conferring resistance to Cry1Ac than in susceptible larvae lacking such alleles (Fig. 1). In both experiments, costs affecting larval growth rate, as indicated by weight of 14-day-old larvae, were magnified on gossypol diet (Table 1; Fig. 2). In experiment two, larval gossypol concentration was non-recessive; it was higher in rs larvae than in ss larvae (Fig. 1). In addition, in this experiment, costs affecting growth were absent on control diet but dominant on gossypol diet (Table 1). These results show that higher larval gossypol content was associated with a non-recessive cost affecting larval growth. This is potentially important because non-recessive costs select more strongly against resistance than recessive costs [9]–[14].
When results from both experiments were considered, costs affecting growth were significantly higher on gossypol diet (12.9%) than on control diet (5.3%). Furthermore, a significant, positive association across genotypes occurred between larval gossypol concentration and the decrease in weight on gossypol diet relative to control diet (Fig. 3). These results show that cadherin mutations conferring resistance to Cry1Ac cotton in pink bollworm were associated with higher larval gossypol concentrations and a higher fitness cost affecting larval growth.
The strains MOV97-H1S and MOV97-H1R and MOV97-H3 had a common origin and contained similar r and s alleles, but differed in their rearing history. Costs reducing larval growth on control diet were present in MOV97-H1S and MOV97-H1R but not in MOV97-H3 (Fig. 2). Also, relative to ss, the frequency of r1r1 decreased more on gossypol than control diet in MOV97-H3 but not in MOV97-H1S and MOV97-H1R (Fig. 4). Such differences in the response of the same genotypes from different strains indicate that variation in genetic background affected costs. The r1 allele has a deletion of 24 base pairs resulting in two amino acid substitutions and the elimination of eight amino acids. The r3 allele has a deletion of 126 base pairs resulting in the omission of 42 amino acids [16]. Across all of the strains studied, we found that effects of gossypol in diet on larval gossypol concentration and growth were greater for r1 than r3 (Fig. 1, 2 and 3).
The results reported here confirm results from a previous study of fitness costs in three strains of pink bollworm derived from MOV97: a susceptible strain, a Cry1Ac-resistant strain, and their F1 progeny [23]. In the previous study, larval gossypol concentration was not measured and cadherin genotypes were not identified. However, similar to the results reported here, gossypol in diet significantly increased the cost affecting larval developmental time, but did not increase the cost affecting survival [23].
In general, costs of Bt resistance are significantly higher on plants than on diet [10]. Compared with the mean costs from many studies [10], the cost affecting growth on control diet seen here (5.3%) is virtually identical to the mean cost affecting development time on diet (5.0%), while the cost affecting growth on gossypol diet seen here (12.9%) is somewhat higher than the mean cost affecting developmental time on plants (8.9%).
Although it was hypothesized that fitness costs are higher on host plants with low suitability [26], [27], available data show no consistent relationship between costs of Bt resistance and host plant suitability [10]. In Trichoplusia ni, costs were negatively associated with host plant suitability, supporting this hypothesis [26]. In Plutella xylostella, however, the cost affecting development time was greater on the least suitable host plant, but the survival cost was higher on the most suitable host in one of the two P. xylostella populations investigated [27]. Furthermore in H. armigera, cotton, pigeon pea, and sorghum were equally suitable to ss individuals, but costs were larger or less recessive on sorghum and cotton than pigeon pea [28]. In P. gossypiella, survival was significantly greater on the cotton cultivar DP50 with high gossypol content than on TX53 with low gossypol content, but the cost affecting survival was recessive and of similar magnitude on both cultivars [29]. High content of defensive chemicals other than gossypol could have suppressed survival on TX53, however, and these chemicals could have compensated for effects of high gossypol content in DP50.
In the studies cited above, among-host variation in nutrient availability and defensive chemicals was not well characterized, and the molecular basis of resistance was known only in pink bollworm. Resistance to Bt toxins is associated with mutations affecting ABC transporter, aminopeptidase-N, and cadherin proteins that act as toxin receptors in the midgut, as well as with proteases that convert Bt protoxins to activated toxins [30], [31]. Mutations affecting aminopeptidase-N and proteases, which are two major digestive proteases, could impair protein digestion [10]. Mutations altering ABC transporters could contribute in reducing export of toxic compounds by midgut epithelial cells to the gut lumen [31], while cadherin mutations could contribute in increasing concentrations of plant defensive chemicals in larvae, as suggested by the results here. Accordingly, low availability of nutrients could be the main factor increasing costs in insects with aminopeptidase-N- and protease-mediated resistance, as it would be harder to compensate for digestive deficiencies when nutrient availability is low rather than high. In contrast, high concentration of defensive chemicals could be the most important reason for an increase in costs in insects with ABC transporter- and cadherin-mediated resistance. Therefore, future studies on variation in costs across host plants may benefit from a more mechanistic approach.
Functional studies of larvae with various cadherin genotypes are needed to determine the effects of cadherin mutations on midgut structure and permeability to plant defensive chemicals. To better understand how host plants affect costs, it will also be necessary to evaluate the relationships among defensive compounds, nutrient availability, and fitness when Bt-susceptible and -resistant insects develop on their natural host plants. Cotton was recently genetically engineered to produce plants with little gossypol in seeds but normal levels in stems and leaves [32]. As pink bollworm larvae primarily feed on seeds, these transgenic cultivars provide an ideal system to further assess the interaction between cadherin-based resistance to Bt cotton, absorption of defensive compounds and expression of fitness costs.
Materials and Methods
Insect Strains
We used three pink bollworm strains derived from strain MOV97, which was established from a single collection in Mohave Valley, Arizona in 1997 [33]. At the locus encoding a cadherin protein that binds Bt toxin Cry1Ac, MOV97 had two alleles (r1 and r3) that confer recessive resistance to Bt toxin Cry1Ac and transgenic cotton that produces Cry1Ac [16], [34]. At this locus, MOV97 also had s alleles that confer susceptibility to Cry1Ac [16], [34]. We conducted two independent experiments: In experiment one, we used two strains that had a similar genetic background, yet one was resistant (MOV97-H1R) and contained only the r1 and r3 alleles, while the other was susceptible (MOV97-H1S) and contained only s alleles. In experiment two, we used a hybrid strain (MOV97-H3) in which the r1, r3 and s alleles segregated at the cadherin locus. We refer to individuals with any two r alleles (r1r1, r3r3, or r1r3) as rr, and to individuals with one r allele (r1s and r3s) as rs.
MOV97-H1R, MOV97-H1S and MOV97-H3 were derived from the hybrid strain MOV97-H1. MOV97-H1 was produced by crossing a resistant strain (MOV97-R) and a susceptible strain (MOV97-S) that had been derived from MOV97 [34]. MOV97-H1R was produced by exposing larvae of the F18 generation of MOV97-H1 to a diagnostic concentration of Cry1Ac in synthetic diet (10 µg toxin per ml diet) that allows survival of only rr individuals [35], [36]. MOV97-H1S was initiated by screening mated pairs of MOV97-H1 (F17) with PCR to find pairs of ss individuals [36]. Ten mated pairs with either rr or ss individuals were caged individually to initiate MOV97-H1R and MOV97-H1S. From each of the 10 mated pairs, 12 pupae were collected (120 per strain). The adult population size was 378 per strain in the F2 generation and 1200 in the following generations [36]. MOV97-H3 was created by crossing insects from MOV97-H1 (F22), which were predominantly ss and rs, with a subset of insects from MOV97-H1 (F21) that had been selected with the diagnostic concentration of Cry1Ac and thus were resistant [34]. Adult population size in MOV97-H1 and MOV97-H3 was>1000 in every generation.
Experiment one was conducted in September 2005 with F4 larvae of MOV97-H1S and MOV97-H1R. Experiment two was done in November 2006 with F5 larvae of MOV97-H3. The parental strain MOV97-H1 had been reared for 17–18 generations on non-Bt diet before MOV97-H1S and MOV97-H1R were created, and 21–22 generations before MOV97-H3 was created. Also, before experiments were done, each of these three strains had been reared for either four or five generations on non-Bt diet. This rearing reduced linkage disequilibrium between cadherin alleles and other alleles that may affect fitness [37].
Insect Diet
To investigate the effects of gossypol, newly hatched larvae were placed on wheat germ diet [38] with gossypol (gossypol diet) or without gossypol (control diet). For gossypol diet, we added gossypol (95% in acetic acid crystal, Sigma-Aldrich) in 1 ml of hexane to achieve a concentration of 0.1% fresh weight (0.1 g gossypol/100g fresh weight) [29]. This is equivalent to 0.6% dry weight, a concentration within the range that occurs naturally in seeds of Gossypium spp. [29], [39], [40]. For control diet, we added the same amount of hexane without gossypol. We also made red diet without gossypol by adding red calco dye (Bio-Serv, Frenchtown, NJ, USA) to control diet at a concentration of 0.010% [41]. We used the red diet to monitor voiding of gossypol diet from the larval gut (see below).
Effect of Gossypol Diet on Costs
We randomly assigned neonate larvae of the susceptible strain (MOV97-H1S, n = 800), the resistant strain (MOV97-H1R, n = 800) and the hybrid strain (MOV97-H3, n = 400) to diet with or without gossypol. We reared larvae in trays with 16 wells (15 mm deep, 3 ml in volume). We put 1.5 g of diet and one neonate in each well. Each tray was sealed with a transparent cover with holes for ventilation. Trays containing one of the two diet types were randomly allocated to shelves in a growth chamber maintained at 27±2°C with ambient relative humidity and a photoperiod of 14∶10 (L∶D) h.
After feeding for 14 days, survivors from both diet types were placed on red diet for 1.5 h to void their gut of the undyed diet. Preliminary work showed that 1.5 h was sufficient to void the gut of diet. After 1.5 h on red diet, each larva was scored for mortality and weighed. The top half of the head capsule of each larva was excised with a razor blade such that no hemolymph was lost, and preserved in 100% EtOH for subsequent genotyping. Larvae were then freeze dried with liquid nitrogen, placed in glass extraction vials (KIMAX brand sample vials, borosilicate glass, with PTFE-lined screw cap 4 ml), and stored at −80 C until gossypol was extracted and quantified.
Insect Genotyping
We extracted DNA from individual larvae using the protocol from Morin et al. [16] with slight modifications [42]. We determined larval cadherin genotype using PCR with primer sets that selectively amplify each of the four types of cadherin alleles (r1, r2, r3, and s) [43]. As expected, no r2 alleles were found in any of the larvae genotyped.
Gossypol Analyses
We measured gossypol concentration in individual larvae by creating an aniline Schiff's base and quantifying the resulting dianilino-gossypol complex with high pressure chromatography coupled with a triple quadrupole mass spectrometer [44]. We used an external calibration curve and an internal standard. We assumed extraction efficiency was 100%. The lowest detectable concentration was 0.025 µg/g. Values below this limit are reported as zero.
In experiment one, a total of 89 larvae from MOV97-H1S and MOV97-H1R were sent to Monsanto labs (Creve Coeur, MO) for analysis. These comprised a random sample of 25 MOV97-H1S larvae fed on gossypol diet, a random sample of 60 MOV97-H1R larvae fed on gossypol diet, one MOV97-H1S larva fed on control diet selected at random from ss larvae previously identified with PCR, and one of each of r1r1, r1r3 and r3r3 from MOV97-H1R fed on control diet and selected randomly from larvae previously identified with PCR. In the second experiment, a total of 96 MOV97-H3 larvae were sent to Monsanto labs. These comprised 90 randomly selected larvae that had fed on gossypol diet, and one larva from each genotype (i.e., ss, r1s, r3s, r1r1, r1r3 and r3r3) fed on control diet and selected randomly from larvae identified previously with PCR. Larvae were shipped overnight on dry ice and remained frozen for the duration of the shipment. Gossypol analyses were conducted without knowledge of genotype or experimental diet, as the shipped samples were identified only by a number.
Statistical Analyses
We used statistical analyses to test our main hypotheses: 1) for larvae fed gossypol diet, gossypol concentration is lower in ss larvae than in rr and rs larvae; 2) gossypol reduces performance of all larvae as indicated by lower larval weight or lower survival; 3) the extent of reduction in larval performance on gossypol diet relative to control diet is greater for rr and rs larvae than for ss larvae. As each of these a priori hypotheses specifies the direction of the difference between groups, we report one-tailed P values for all tests of these hypotheses.
In each experiment, one-way ANOVA followed by linear combinations of means (hereafter linear contrasts) were used to assess whether gossypol concentration (µg/g dry weight, transformed log x+0.001) differed among the ss, rs, and rr genotypes. Multiple regression with indicator variables for each genotype with r allele (s) was further used to compare gossypol concentration between r1r1, r1r3, r1r1 and ss in experiment one and between r1s, r3s, r1r1, r1r3, r3r3 and ss in experiment 2.
In each experiment, two-way ANOVA followed by linear contrasts was used to evaluate whether larval weight (fresh weight in mg, log transformed) differed among the ss, rs, and rr genotypes. In experiment one, costs on control and gossypol diet were compared by assessing whether each contrast coefficient (i.e., ss vs. rr) lied outside the 95% confidence interval for the other [45]. Multiple regression with indicator variables for each genotypes with r allele (s) and gossypol diet was further used to assess the effects of diet (gossypol versus control diet), genotype, and the interaction between these factors on larval weight (fresh weight in mg, log transformed) in each experiment. A significant negative coefficient associated with the interaction between a particular genotype and diet indicated that larval weight of this genotype was reduced more (relative to ss) on diet with gossypol than on control diet [45].
We measured costs affecting larval weight of a genotype with r alleles on a particular diet as: cost in % = 100%× ([mean weight (genotype) – mean weight ss] / mean weight ss). As costs did not differ between experiments on either diet (2-sample t-test, P values>0.15), we pooled genotypes from both experiment and used a one-sample t-test to assess whether costs were significantly different from zero on control and gossypol diet. We also used a paired t-test to evaluate whether costs were significantly greater on gossypol than control diet.
For each genotype, we calculated the percentage reduction in weight from control to gossypol diet as: 100%× ([mean weight on control diet – mean weight on gossypol diet]/mean weight on control diet). We used a covariance analysis to test for effects of experiment, gossypol concentration in a genotype, and the interaction between these factors on the change in weight from control to gossypol diet. Because the effects of experiment (P = 0.61) and interaction (P = 0.93) were not significant, we pooled data and used linear regression to assess the association between gossypol content and change in weight from control to gossypol diet. We also used linear regression to evaluate the association between gossypol content and change in weight from control to gossypol diet separately for experiment one and two.
In both experiments, we used a Fisher's exact test to evaluate whether the proportion of larval survival differed between gossypol and control diet. To test for fitness costs affecting survival in experiment one, we pooled the rr genotypes and used a Fisher's exact test to determine if the proportion of survival of ss and rr differed on gossypol or control diet. We could not measure survival of genotypes in experiment two because we did not know the initial number of individuals of each genotype, only the number of survivors of each genotype. Nevertheless, in both experiments, we assessed whether the frequency of rr or rs decline more from control to gossypol diet than the frequency of ss. In experiment two, we used a Fisher's exact test to compare the frequency of rr (three genotypes pooled) and rs (two genotypes pooled) to the frequency of ss on each diet (gossypol and control). In both experiments, we also used log-linear regression to assess whether frequency decreased more on gossypol than control diet in each genotype with r allele (s) than is ss. Explanatory variables in the regression model were indicator variables for diet, genotype, and the interaction between these factors, while the response variable was the number of individuals of each genotype surviving on the diet types. A significant negative coefficient associated with the interaction between a particular genotype and diet indicated that frequency of this genotype was reduced more (relative to ss) on diet with gossypol than on control diet [45]. Statistical analyses were performed in JMP [46].
Footnotes
Competing Interests: BE Tabashnik received support for research that is not related to this publication from the following sources: Cotton Foundation, Cotton Inc., National Cotton Council, Monsanto, and Dow AgroSciences. Tabashnik is also co-author of a patent application on engineering modified Bt toxins to counter pest resistance, which is related to research described by Soberon et al. (2007, Science 318: 1640-1642). Robert G. Orth and Graham Head are employed by a commercial company, Monsanto LLC, which provides transgenic crops for the control of pests. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This research was partly funded by USDA Biotechnology Risk Assessment Research Grant 2003-04371 (http://www.csrees.usda.gov/fo/biotechnologyriskassessment.cfm). No additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hutchison WD, Burkness EC, Moon RD, Leslie T, Fleischer SJ, et al. Areawide suppression of European corn borer with Bt maize reaps savings to non-Bt maize growers. Science. 2010;330:222–225. doi: 10.1126/science.1190242. [DOI] [PubMed] [Google Scholar]
- 2.Committee on the Impact of Biotechnology on Farm-Level Economics and Sustainability, National Research Council. Washington, DC: The National Academies Press; 2010. The impact of genetically engineered crops on farm sustainability in the United States.235 [Google Scholar]
- 3.Tabashnik BE, Gassmann AJ, Crowder DW, Carrière Y. Insect resistance to Bt crops: evidence versus theory. Nat Biotechnol. 2008;26:199–202. doi: 10.1038/nbt1382. [DOI] [PubMed] [Google Scholar]
- 4.Tabashnik BE, Van Rensburg JBJ, Carrière Y. Field-evolved insect resistance to Bt crops: definition, theory and data. J Econ Entomol. 2009;102:2011–2025. doi: 10.1603/029.102.0601. [DOI] [PubMed] [Google Scholar]
- 5.Bagla P. Hardy cotton-munching pests are latest blow to GM crops. Science. 2010;327:1439. doi: 10.1126/science.327.5972.1439. [DOI] [PubMed] [Google Scholar]
- 6.Downes S, Parker T, Mahon R. Incipient resistance of Helicoverpa punctigera to the Cry2Ab Bt toxin in Bollgard II cotton. e12567PloS ONE. 2010;5(9) doi: 10.1371/journal.pone.0012567. doi: 10.1371/journal.pone.0012567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Storer NP, Babcock JM, Schlenz M, Meade T, Thompson GD, et al. Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J Econ Entomol. 2010;103:1031–1038. doi: 10.1603/ec10040. [DOI] [PubMed] [Google Scholar]
- 8.Onstad DW. Insect resistance management: Biology, economics, and prediction San Diego: Elselvier. 2008. 305
- 9.Carrière Y, Crowder DW, Tabashnik BE. Evolutionary ecology of adaptation to Bt crops. Evol Appl. 2010;3:561–573. doi: 10.1111/j.1752-4571.2010.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gassmann AJ, Carrière Y, Tabashnik BE. Fitness costs of insect resistance to Bacillus thuringiensis. . Annu Rev Entomol. 2009;54:147–163. doi: 10.1146/annurev.ento.54.110807.090518. [DOI] [PubMed] [Google Scholar]
- 11.Carrière Y, Tabashnik BE. Reversing insect adaptation to transgenic insecticidal plants. Proc R Soc Lond B. 2001;268:1475–1480. doi: 10.1098/rspb.2001.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabashnik BE, Dennehey TJ, Carrière Y. Delayed resistance to trangenic cotton in pink bollworm. Proc Natl Acad Sci. 2005;43:15389–15393. doi: 10.1073/pnas.0507857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gould F, Cohen MB, Bentur JS, Kennedy GG, Van Duyn J. Impact of small fitness costs on pest adaptation to crop varieties with multiple toxins: A heuristic model. J Econ Entomol. 2006;99:2091–2099. doi: 10.1603/0022-0493-99.6.2091. [DOI] [PubMed] [Google Scholar]
- 14.Crowder DW, Carrière Y. Comparing the refuge strategy for managing the evolution of insect resistance under different reproductive strategies. J Theor Biol. 2009;261:423–430. doi: 10.1016/j.jtbi.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Gahan LJ, Gould F, Heckel DG. Identification of a gene associated with Bt resistance in Heliothis virescens. . Science. 2001;293:857–860. doi: 10.1126/science.1060949. [DOI] [PubMed] [Google Scholar]
- 16.Morin S, Biggs RW, Sisterson MS, Shriver L, Ellers-Kirk C, et al. Three cadherin alleles associated with resistance to Bacillus thuringiensis in pink bollworm. Proc Natl Acad Sci. 2003;100:5004–5009. doi: 10.1073/pnas.0831036100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu XJ, Yu LY, Wu YD. Disruption of a cadherin gene associated with resistance to Cry1Ac delta-endotoxin of Bacillus thuringiensis in Helicoverpa armigera. . Appl Environ Microbiol. 2005;71:948–954. doi: 10.1128/AEM.71.2.948-954.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fabrick JA, Tabashnik BE. Binding of Bacillus thuringiensis toxin Cry1Ac to multiple sites of cadherin in pink bollworm. Insect Biochem Mol Biol. 2007;37:97–106. doi: 10.1016/j.ibmb.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Schlichting K, Wilsch-Bräuninger, Demontis F, Dahmann C. Cadherin Cad99C is required for normal microvilli morphology in Drosophila follicle cells. J Cell Sci. 2006;119:1184–1195. doi: 10.1242/jcs.02831. [DOI] [PubMed] [Google Scholar]
- 20.Hill E, Broadbent ID, Chothia C, Pettitt J. Cadherin superfamily proteins in Caenorhabditis elegans and Drosophila melanogaster. . J Mol Biol. 2001;305:1011–1024. doi: 10.1006/jmbi.2000.4361. [DOI] [PubMed] [Google Scholar]
- 21.Midboe EG, Mehmet C, Bulla LA Expression of a midgut-specific cadherin BT-R1 during the development of Manduca sexta larva. Comp Biochem Physiol B. 2003;135:125–137. doi: 10.1016/s1096-4959(03)00054-x. [DOI] [PubMed] [Google Scholar]
- 22.Carrière Y, Showalter AM, Fabrick JA, Sollome J, Ellers-Kirk C, et al. Cadherin gene expression and effects of Bt resistance on sperm transfer in pink bollworm. J Insect Physiol. 2009;55:1058–1064. doi: 10.1016/j.jinsphys.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Carrière Y, Ellers-Kirk C, Biggs R, Dennehy TJ, Tabashnik BE. Effects of gossypol on fitness costs associated with resistance to Bt cotton in pink bollworm. J Econ Entomol. 2004;97:1710–1718. doi: 10.1603/0022-0493-97.5.1710. [DOI] [PubMed] [Google Scholar]
- 24.Mao YB, Cai WJ, Wang JW, Hong GJ, Tao XY, et al. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat Biotechnol. 2007;25:1307–1313. doi: 10.1038/nbt1352. [DOI] [PubMed] [Google Scholar]
- 25.Stipanovic RD, López JD, Dowd MK, Puckhaber LS, Duke SE. Effect of racemic (+)- and (–) –gossypol on survival and development of Heliothis virescens larvae. Physiol Ecol. 2008;37:1081–1085. doi: 10.1603/0046-225X(2008)37[1081:EORAGO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 26.Janmaat AF, Myers J. The cost of resistance to Bacillus thuringiensis varies with the host plant of Trichoplusia ni. . Proc Roy Soc B. 2005;272:1031–8. doi: 10.1098/rspb.2004.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raymond B, Sayyed AH, Wright DJ. Host plant and population determine the fitness cost to Bacillus thuringiensis. . Biol Lett. 2007;3:82–5. doi: 10.1098/rsbl.2006.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bird LJ, Akhurst RJ. Effects of host plant species on fitness costs of Bt resistance in Helicoverpa armigera (Lepidoptera:Noctuidae). Biol Contr. 2007;40:196–203. [Google Scholar]
- 29.Carrière Y, Ellers-Kirk C, Biggs R, Degain B, Holley D, et al. Effects of cotton cultivar on fitness costs associated with resistance of pink bollworm (Lepidopters: Gelechiidae) to Bt cotton. J Econ Entomol. 2005;98:947–54. doi: 10.1603/0022-0493-98.3.947. [DOI] [PubMed] [Google Scholar]
- 30.Bravo A, Soberón M. How to cope with insect resistance to Bt toxins? Trends Biotechnol. 2008;26:573–579. doi: 10.1016/j.tibtech.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Gahan LJ, Pauchet Y, Vogel H, Heckel DG. An ABC transporter mutation is correlated with insect resistance to Bacillus thuringiensis Cry1Ac toxin. PLoS Genet. 2010;6(12):e1001248. doi: 10.1371/journal.pgen.1001248. doi: 10.1371/journal.pgen.1001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sunilkumar G, Campbell LM, Puckhaber L, Stipanovic RD, Rathore KS. Engineering cottonseed for use in human nutrition by tissue-specific reduction of toxic gossypol. Proc Natl Acad Sci. 2006;103:18054–18059. doi: 10.1073/pnas.0605389103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabashnik BE, Patin AL, Dennehy TJ, Liu YB, Carrière Y, et al. Frequency of resistance to Bacillus thuringiensis in field populations of pink bollworm. Proc Natl Acad Sci. 2000;97:12980–12984. doi: 10.1073/pnas.97.24.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carrière Y, Ellers Kirk C, Biggs RW, Nyboer ME, Unnithan GC, et al. Cadherin-based resistance to Bt cotton in hybrid strains of pink bollworm: fitness costs and incomplete resistance. J Econ Entomol. 2006;99:1925–1935. doi: 10.1603/0022-0493-99.6.1925. [DOI] [PubMed] [Google Scholar]
- 35.Tabashnik BE, Biggs RW, Higginson DM, Unnithan DC, Unnithan GC, et al. Association between resistance to Bt cotton and cadherin genotype in pink bollworm. J Econ Entomol. 2005;98:635–644. doi: 10.1603/0022-0493-98.3.635. [DOI] [PubMed] [Google Scholar]
- 36.Gassmann AJ, Stock SP, Carrière Y, Tabashnik BE. Effect of entomopathogenic nematodes on the fitness cost of resistance to Bt toxin Cry1Ac in pink bollworm (Lepidoptera: Gelechiidae). J Econ Entomol. 2006;99:920–926. doi: 10.1603/0022-0493-99.3.920. [DOI] [PubMed] [Google Scholar]
- 37.Falconer DS. New York: Longman; 1981. Introduction to quantitative genetics.340 [Google Scholar]
- 38.Adkinsson PL, Vaderzant ES, Bull DL, Allision WE. A wheat germ medium for rearing the pink bollworm. J Econ Entomol. 1960;53:759–762. [Google Scholar]
- 39.Shaver TN, Parrott WL. Relationship of larval age to toxicity of gossypol to bollworms, tobacco budworms, and pink bollworms. J Econ Entomol. 1970;63:1802–1804. [Google Scholar]
- 40.Hron RJ, Kim HL, Calhoun MC, Fisher GS. Determination of (+)-, (–)-, and total gossypol in cottonseed by high-performance liquid chromatography. J Am Oil Chem Soc. 1999;76:1351–1355. [Google Scholar]
- 41.Graham HM, Mangum CL. Larval diets containing dyes for tagging pink bollworm moths internally. J Econ Entomol. 1971;64:376–379. [Google Scholar]
- 42.Higginson DM, Nyboer ME, Biggs RW, Morin S, Tabashnik BE, et al. Evolutionary trade-offs of insect resistance to Bacillus thuringiensis crops: fitness costs affecting paternity. Evolution. 2005;59:915–920. [PubMed] [Google Scholar]
- 43.Morin S, Carrière Y, Dennehy TJ, Brown JK, Tabashnik BE. DNA-based detection of Bt resistance alleles in pink bollworm. Insect Biochem Mol Biol. 2004;34:1225–1233. doi: 10.1016/j.ibmb.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Orth RG, Head G, Mierkowski M. Determining larval host plant use by a polyphagous lepidopteran through analysis of adult moths for plant secondary metabolites. J Chem Ecol. 2007;33:1131–1148. doi: 10.1007/s10886-007-9284-3. [DOI] [PubMed] [Google Scholar]
- 45.Ramsey FL, Schafer DW. Pacific Grove: Duxbury; 2002. The statistical sleuth: a course in methods of data analysis.742 [Google Scholar]
- 46.SAS. Cary: SAS Institute Inc.; 2008. JMP 8.0. [Google Scholar]