Abstract
Brassinosteroids are plant-derived polyhydroxylated derivatives of 5α-cholestane, structurally similar to cholesterol-derived animal steroid hormones and insect ecdysteroids. In this study, we synthesized a set of brassinosteroid analogues of a natural brassinosteroid (22S,23S)-homobrassinolide (HB, 1) including (22S,23S)-homocastasterone (2), (22S,23S)-3α-fluoro-homobrasinolide (3), (22S,23S)-3α-fluoro-homocastasterone (4), (22S,23S)-7v-aza-homobrassinolide (5), (22S,23S)-6-aza-homobrassinolide (6), and studied their anabolic efficacy in the L6 rat skeletal muscle cells in comparison to other synthetic and naturally occurring brassinosteroids (22R,23R)-homobrassinolide (7), (22S,23S)-epibrassinolide (8), and (22R,23R)-epibrassinolide (9). Presence of the 6-keto group in the B ring and stereochemistry of 22α,23α-vicinal hydroxyl groups in the side chain were critical for the anabolic activity, possibly due to higher cytotoxicity of the 22β, 23β-hydroxylated brassinosteroids. All anabolic brassinosteroids tested in this study selectively activated PI3K/Akt signaling pathway as evident by increased Akt phosphorylation in vitro. Plant brassinosteroids and their synthetic derivatives may offer a novel therapeutic strategy for promoting growth, repair, and maintenance of skeletal muscles.
Keywords: brassinosteroid, protein synthesis, protein degradation, anabolic steroids, muscle mass, muscle loss
Introduction
Brassinosteroids are plant-specific polyhydroxylated derivatives of 5α-cholestane, structurally similar to cholesterol-derived animal steroid hormones and ecdysteroids from insects. They are found at low levels in pollen, seeds, leaves, and young vegetative tissues throughout the plant kingdom 1. Similar to animal steroid hormones 2, brassinosteroids regulate the expression of specific plant genes and complex physiological responses involved in growth 3, partly via interactions with other hormones setting the frame for brassinosteroid responses 4. While animal steroid hormones are perceived by nuclear receptor family of transcription factors, brassinosteroids signal through a cell surface receptor kinase-mediated signal transduction pathway 5, 6.
The (22S,23S)-28-homobrassinolide (HB, Figure 1) is one of the most active brassinosteroids in inducing plant growth in various plant bioassay systems 7. Growth promoting effect of HB in plants is associated with the increased synthesis of nucleic acids and proteins 8, 9, and activation of total protein synthesis in plants subjected to heat shock 10. Due to the practical use of HB for increasing yield production, efforts are being made towards its synthesis 11, 12. Brassinosteroids have a favorable safety profile, since no treatment-related effect was observed at doses up to 4000 mg/kg when applied orally for epibrassinolide 13 or HB 14. Natural brassinosteroids also inhibited growth of several human cancer cell lines without affecting the growth of normal cells 15.
Figure 1.
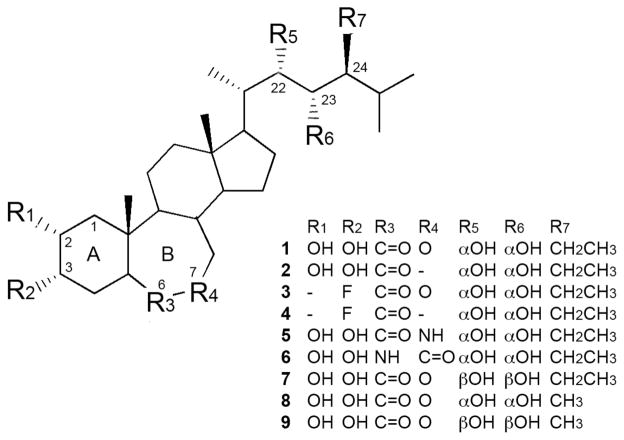
Chemical structure of homobrassinolide (1) and its analogues (2–9) investigated in this study.
The natural brassinosteroid and their synthetic analogues that have been identified so far have a common 5-α-cholestane skeleton and their structural variations come from the type and position of functional groups on the skeleton and the stereochemistry present in the A and B rings and the side chain 16. Recent structure-activity studies of brassinosteroids in the rice leaf lamina inclination bioassay have revealed that 5α-configuration is required for optimum activity, but the B-ring tolerates considerable variation, providing that the presence of a polar functional group is maintained 17. Structure-activity studies have also demonstrated that the (2α, 3α)- and (22R, 23R)-vicinal diol moieties are required for optimum bioactivity in plants 18. Although the structural requirements for biological activity in plants have been clearly recognized, a number of compounds bearing minor to major structural modifications retained potent plant growth promoter activity 19.
In the previous study we found that orally applied HB produced significant anabolic effects and improved physical fitness in healthy animals with minimal androgenic effects 20. Importance of identifying novel agents that influence muscle growth, development, or regeneration with possible therapeutic application for the age or disease-related skeletal muscle atrophy prompted us in this study to explore, for the first time, the structure-activity relationship between HB and its natural and synthetic analogues in their effects on protein synthesis and degradation in rat skeletal muscle cells.
Results
Synthesis of brassinosteroid analogues
To investigate the structure-activity relationship between position or stereochemistry of functional groups of HB and its anabolic activity, we synthesized a series of HB analogues 2–6 and compared them to other synthetic and naturally occurring brassinosteroids 7–9 in their ability to stimulate protein synthesis or inhibit protein degradation in the L6 rat skeletal muscle cells. (22S,23S)-Homocastasterone (2) was synthesized to evaluate the influence of C-6 lactone group on the anabolic activity of brassinosteroids. By synthesizing 3–4 that lack hydroxyl functional group at C-2 and are fluorinated at C-3, we tested the requirement for (2α, 3α)-vicinal diol moieties in the ability of brassinosteroids to promote protein accumulation in muscle cells. Finally, 5–6 were synthesized to contain 7-aza and 6-aza substitutions in the B ring of the brassinosteroid molecule, therefore evaluating the requirement for the 6-keto group for their biological activity. Additionally, we compared the anabolic activity of HB to other naturally occurring brassinosteroids that differ in the stereochemistry of the (22R, 23R)-vicinal diol moieties (7) or bearing a methyl group at C-24 in the side chain of its 5α-ergostane structure (8–9). Structures of HB and its analogues used in this study are shown in Figure 1, while detailed synthetic routes for each compound are summarized in Supporting Figures 1–4.
Protein synthesis
The bioactivity of HB and its analogues was evaluated by measuring increase in protein synthesis in the L6 rat skeletal muscle cells in vitro. Cells were incubated for 4 h with [3H]-phenylalanine and treated in triplicate with vehicle (0.1% ethanol) or test compound (10 μM), and protein synthesis was measured as incorporation of [3H]-phenylalanine into protein normalized by total protein (Table 1). Under these conditions, both HB and (22S,23S)-homocastasterone increased protein synthesis by 37.2 ± 5.9% (p < 0.001) and 41.0 ± 2.7% (p < 0.001), respectively. This compared favorably to the biological activity of IGF-1 at 6.5 nM (42.5 ± 4.5%, p<0.001) that served as a positive control in this assay. Removal of 2α-hydroxyl group and fluorination at C-3 in the A ring (3–4) led to a 50% decrease in bioactivity (24.6 ± 5.3%, p<0.01 and 22.5 ± 2.7%, p<0.01, respectively). Replacement of 7-oxalactone group with amine in the B ring of 6 reduced biological activity by half, while a similar replacement of the 6-carbonyl group with amine in 5 resulted in a complete loss of activation of protein synthesis. Modifications in the side chain (7–9) also abolished the activity.
Table 1. Effect of HB (1) and its analogues (2–9) on protein accumulation in the L6 rat skeletal muscle cells.
Compounds were tested at 10 μM and results are expressed as the mean ± SEM of determinations performed in triplicate (* P<0.05, ** P<0.01, *** P<0.001 when compared with control by one-way ANOVA and Dunnett’s post-test).
| ID | Common name | Chemical name | Formula | MW | Protein syn- thesis, % increase over control | Protein deg- radation, % decrease over control |
|---|---|---|---|---|---|---|
| 1 | (22S,23S)-homobrassinolide | (22S,23S,24S)-2α,3α,22,23-tetrahydroxy- B- homo-7-oxa- 5α-cholestan-6-one | C29H50O6 | 494.70 | 37 ± 6*** | −24 ± 6* |
| 2 | (22S,23S)-homocastasterone | (22S,23S,24S)-2α,3α,22,23-tetrahydroxy-24- ethyl- 5α-cholestan-6-one | C29H50O5 | 478.70 | 41 ± 3*** | −23 ± 4* |
| 3 | (22S,23S)-3α-fluoro- homobrasinolide | (22S,23S,24R)-3α-fluoro-22,23-dihydroxy- 24-ethyl-B-homo-7-oxa- 5α-cholestan-6-one | C29H49FO4 | 480.70 | 25 ± 5** | −21 ± 1* |
| 4 | (22S,23S)-3α-fluoro- homocastasterone | (22S,23S,24S)-3α-fluoro-22,23-dihydroxy- 24-ethyl- 5α-cholestan-6-one | C29H49FO3 | 464.70 | 23 ± 3** | −16 ± 4 |
| 5 | (22S,23S)-7v-aza- homobrassinolide | (22S,23S,24S)-2α,3α,22,23-tetrahydroxy-24- ethyl- B-homo-7-aza- 5α-cholestan-6-one | C29H51NO5 | 493.72 | 21 ± 2** | −1 ± 3 |
| 6 | (22S,23S)-6-aza- homobrassinolide | (22S,23S,24S)-2α,3α,22,23-tetrahydroxy-24- ethyl- B-homo-6-aza- 5α-cholestan-7-one | C29H51NO5 | 493.72 | 3 ± 12 | −14 ± 4 |
| 7 | (22R,23R)-homobrassinolide | (22R,23R,24S)-2α,3α,22,23-tetrahydroxy- B- homo-7-oxa- 5α-cholestan-6-one | C29H50O6 | 494.70 | 13 ± 2 | −15 ± 4 |
| 8 | (22S,23S)-epibrassinolide | (22S,23S,24R)-2α,3α,22,23-tetrahydroxy-24- methyl-B-homo-7-oxa- 5α-cholestan-6-one | C28H48O6 | 480.68 | 4 ± 2 | −6 ± 8 |
| 9 | (22R,23R)-epibrassinolide | (22R,23R,24R)-2α,3α,22,23-tetrahydroxy-24- methyl-B-homo-7-oxa-5α-cholestan-6-one | C28H48O6 | 480.68 | 12 ± 4 | −7 ± 8 |
| Ref | IGF-1, 6.5 nM | Positive control | 43 ± 5*** | -- | ||
| Ref | Insulin, 10 nM | Positive control | -- | −20 ± 2* |
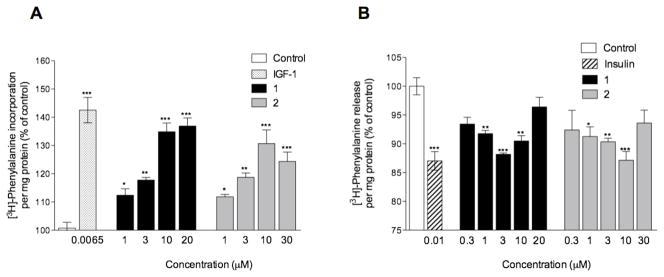
To investigate dose dependence effect of the most active brassinosteroids on protein synthesis, a study was performed with 0.3–30 μM of 1–2. Both responses approached saturation between 10 and 20 μM, with the maximum increases of 36.9 ± 2.9% and 40.7 ± 4.9% (p<0.01), respectively (Figure 2A).
Figure 2. Dose dependent effect of 1 and 2 on protein synthesis (A) and protein degradation (B) in L6 rat myotubes.
(A) Cells were incubated for 4 h with [3H]-phenylalanine and treated in triplicate with vehicle (0.1% ethanol), 6.5 nM of IGF-1 as a positive control, or test compound (0.3–30 μM), and protein synthesis was measured as incorporation of [3H]-phenylalanine into protein normalized by total protein. (B) Dose-dependent effect of HB on protein degradation was observed in cells labeled overnight with [3H]-phenylalanine and subsequently treated for 4 h with vehicle (0.1% ethanol), 10 nM of insulin as a positive control, or brassinosteroid analogues (0.3–30 μM); then protein degradation was measured as release of acid-soluble [3H]-phenylalanine into media. Results are expressed as the mean ± SEM of determinations performed in triplicate (* P<0.05, ** P<0.01, *** P<0.001 when compared with control by one-way ANOVA and Dunnett’s post-test).
Protein degradation
The bioactivity of HB and its analogues was also evaluated by measuring decrease in protein degradation in the L6 rat skeletal muscle cells in vitro. Cells were labeled overnight with [3H]-phenylalanine and subsequently treated for 4 h in triplicate with vehicle (0.1% ethanol) or test compound (10 μM), and protein degradation was assessed as release of acid-soluble [3H]-phenylalanine into the media normalized by total protein (Table 1). HB, homocastasterone (2), as well as brassinosteroid analogs 3–5 and 7 reduced protein degradation in vitro, but the potency of their activities differed according to their structure. 1–3 showed the strongest prevention of protein degradation, by more than 20%, that compared favorably with 10 nM insulin treatment that served as a positive control in this assay (20.2 ± 1.6%, p<0.05). Prevention of degradation was dependent on the presence of the ethyl group at the C-24 in the side chain (compare 1 and 7 versus 8–9) and partially dependent on the stereochemistry of the (22R, 23R)-vicinal diol moieties (compare 1 and 7). Interestingly, replacement of 7-oxalactone group with amine in the B ring of compound 6 completely abolished its effect on protein degradation, while a similar replacement of the 6-carbonyl group with amine in 5 had only a minor effect on its biological activity.
Among the most active compounds in this assay, HB at concentrations of 0.3–20 μM, inhibited protein degradation dose-dependently and its activity reached plateau between 3 and 10 μM (Figure 2B). At a lower concentration, 1 μM HB decreased protein degradation by 8.2 ± 0.6% above control levels (p < 0.05). 2 at concentrations of 0.3–30 μM inhibited protein degradation dose-dependently and its activity reached plateau at 10 μM. At the lower concentration, 2 at 1 μM suppressed protein degradation by 8.7 ± 1.7% above control levels (p < 0.05).
Cytotoxicity in L6 muscle and 3T3 fibroblast cells
All brassinosteroids and their analogues showed no toxicity in fully differentiated L6 rat skeletal myotubes up to 30 μM as established by the MTT assay and cytological observations (data not shown). We therefore tested all compounds in the standard test for basal cytotoxicity using 3T3/NIH murine fibroblast cell culture. 5 was the only brassinosteroid analogue that inhibited cell proliferation in a dose-dependent manner with IC50 of 12.5 μM. Fluorination at C-3 in the A ring (3–4) led to increased cytotoxicity as compared to the original brassinosteroids 1–2 (Figure 3).
Figure 3. Cell survival curves as measured by MTT assay for 1–9 against the murine fibroblast cell line NIH-3T3.
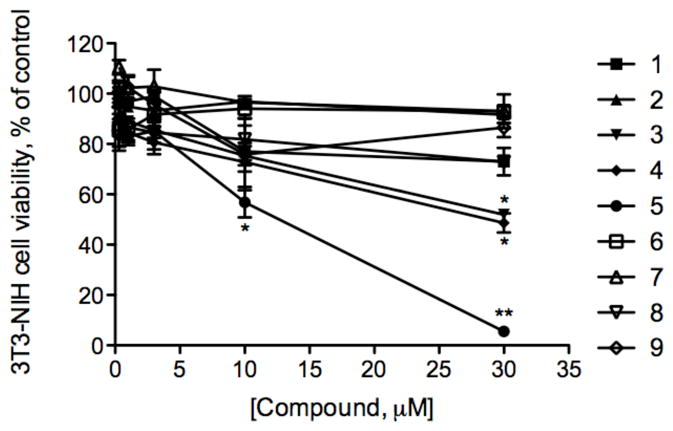
Cells were incubated with various concentrations of brassinosteroids (0.3–30 μM) for 24 h at 37 °C. The mean absorbance of the control cells represented 100% cell proliferation, and the mean absorbance of treated cells was related to control values to determine sensitivity. Error bars represent standard error (n=6) from mean cell proliferation as determined by repeated experiments.
Akt phosphorylation
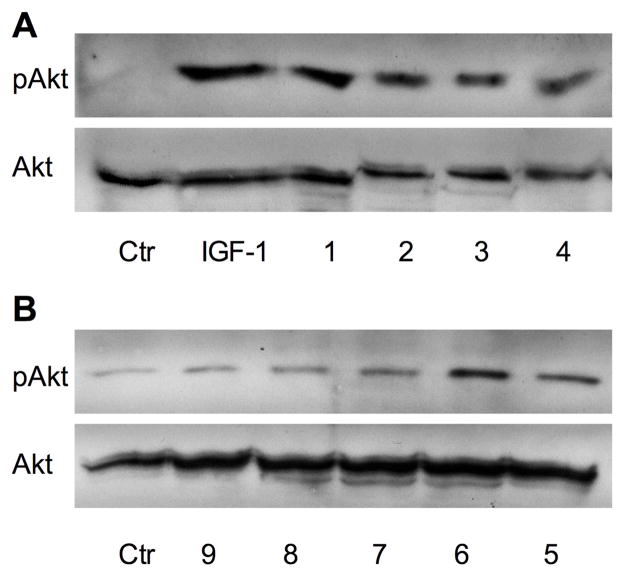
Akt is the key intermediate in the IGF-1 signaling pathway that modulates downstream targets known to regulate protein synthesis and degradation 21. Consistent with the results obtained with the [3H]-phenylalanine incorporation assay, bioactive brassinosteroids stimulated phosphorylation of Akt in rat skeletal muscle cell culture (Figure 4).
Figure 4. Effect of HB and its analogues on Akt (Ser473) phosphorylation in L6 myotubes.
Representative immunoblots of Akt phosphorylation stimulated with 10 μM 1–9 for 1 h or 6.5 nM IGF-1 for 10 min (positive control). Cells lysates normalized to contain 50 μg of total soluble protein were analyzed by immunobloting with phospho- and nonphospho-specific antibodies.
Pharmacogenomic effect of HB in vivo
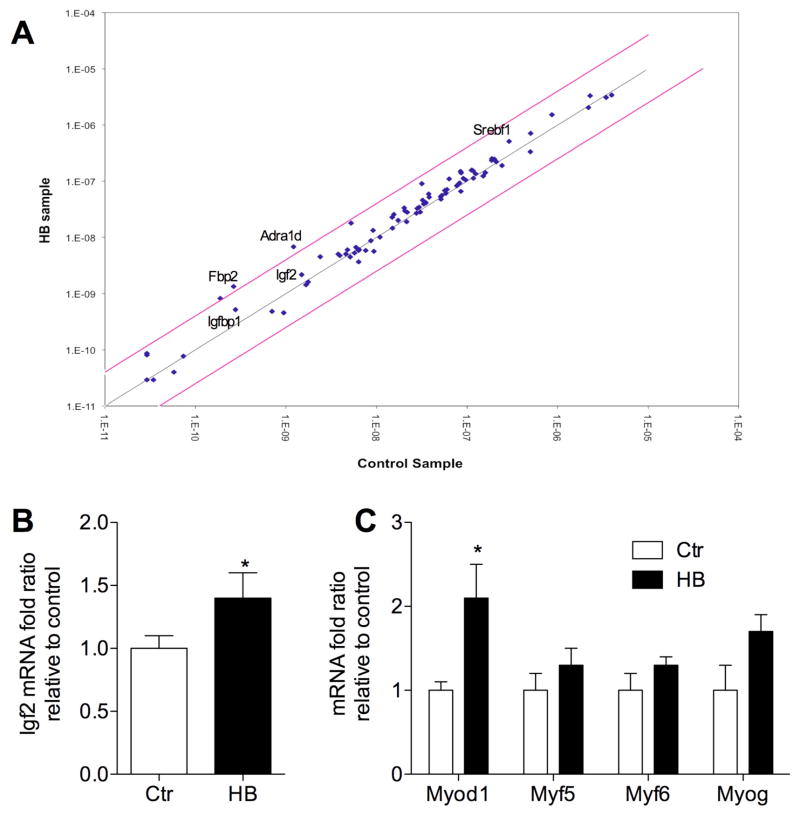
Earlier we studied the anabolic effects of biologically active brassinosteroid HB in animals. HB treatment (60 mg/kg body weight daily to healthy rats fed normal diet for 24 d) was associated with a 14.2% increase in the lean body mass and the improved physical fitness of untrained rats (limb grip strength was measured using digital force gauge) 20. Pooled RNA samples obtained from frozen gastrocnemius muscle biopsies of vehicle- (Ctr) and HB-treated animals were used for the rat insulin signaling PCR array. Of the 84 genes responsible for insulin signaling, PI3K and MAPK pathways, carbohydrate metabolism, and cell cycle regulation, two subsets of genes were higher expressed in HB-treated group than in the Ctr group, but the magnitude of the difference varied (Figure 5A). The first subset included a set of target genes upregulated through the PI3K/Akt signaling pathway: Adra1d (6.5 fold), Igfbp1 (2.5 fold), and Srebf1 (2.5 fold). The second subset contained genes that regulate muscle cell growth and carbohydrate metabolism: Fbp2 (4.5 fold) and Igf2 (1.4 fold). We next further verified these results for Igf2 gene by RT-PCR on individual muscle samples from Ctr and HD-treated animals (Figure 5B). No changes in expression of Eif2b1 were noted. Additionally, we analyzed expression levels of a series of the myogenic transcriptional factors that modulate muscle growth and differentiation, including positive regulators Myod1 (2.1 fold), Myf5 (1.3 fold), Myf6 (1.3 fold), Myog (1.7 fold) (Figure 5C).
Figure 5. Pharmacogenomic effect of HB in vivo.
(A) RNA was extracted from pooled (n=5) gastrocnemius muscle samples of control and HB-treated animals (60 mg/kg for 24 d) and analyzed using rat insulin signaling pathway PCR array to measure relative gene expression levels for 84 genes. Central black line indicates fold changes ((2^(-ΔCt)) of 1, while the pink lines indicate the 4 fold-change in gene expression threshold. (B) Results for Igf2 gene expression and (C) a set of the myogenic transcriptional factors that modulate muscle growth and differentiation were further confirmed by conventional RT-PCR on individual muscle samples (n=5) from control and HD-treated animals. Results are expressed as the mean ± SEM of determinations performed in duplicate (* P<0.05 when compared with control by Student t test).
Discussion
Brassinosteroids are a class of plant hormones with a polyoxygenated steroid structure showing pronounced plant growth regulatory activity 22. They also exhibit striking structural similarities with arthropod hormones of the ecdysteroid type such as 20-hydroxyecdysone 23 that have been reported to produce anabolic effects in mammals 24. Previously we reported that orally applied HB produced significant anabolic effects and improved physical fitness in healthy animals with minimal androgenic effects 20. In this study, a series of brassinosteroid analogues 2–6 related to HB were synthesized (Figure 1), and the structure activity relationships of these compounds were explored by carrying out protein synthesis and degradation assays in the L6 rat skeletal muscle cells. The results showed that (22S,23S)-homocastasterone could significantly increase protein accumulation in muscle cells similar to HB (Figure 2). Since the only difference between these compounds is additional 7-oxalactone group in the B ring of HB, these results indicated that 7-oxalactone moiety is not necessary for their anabolic properties. To the contrary, moving from the lactone to the 6-ketone in plants, it was observed that the brassinolide activity decreases by 50% between brassinolide and castasterone 7. Transformation of this moiety to either 6-oxo-7-aza (5) or 6-aza-7-oxalactone (6) groups dramatically reduced their ability to stimulate protein synthesis (Table 1). This is similar to plant brassinolide activity that was significantly reduced in 7-aza-homobrassinolide 25, while 6-aza-7-oxo-homobrassinolide was inactive 26.
The effect of ring A substituents on anabolic activity of brassinosteroids was less evident. Replacement of the two 2α, 3α-vicinal hydroxyl groups by α-fluoro group decreased but did not abolish bioactivity, however the cytotoxicity of these compounds against 3T3-NIH murine fibroblast cells was increased (Figure 3). Similarly, replacement of 3-hydroxy function by a 3-fluoro group yielded compounds active at the rice lamina inclination assay, but not as active as their parent compounds 27. Many papers have dealt with the relationship between the side chain structure and brassinolide activity in plants, suggesting that epibrassinolide is more active than homobrassinolide 22. On the contrary, our data indicated that the side chain at C-24 (methyl versus ethyl) is critically important for bioactivity in the mammalian system; therefore epibrassinolides 8–9 possess very low anabolic activity in skeletal muscle cells as compared to homobrassinolides 1 and 7. The reason for this is not clear, and may be a result of interaction of plant brassinosteroid with yet unknown nuclear or membrane receptor site through three structural motives: the B ring lactone and 22α,23α-hydroxyls, which are critical for anabolic activity, while 2α,3α-hydroxyls on the A ring have lesser receptor affinity. Several binding components on erythrocyte plasma membrane specific for ecdysteroids may indicate the existence of such receptor 28.
The PI3K/Akt/mTOR pathway is a crucial intercellular regulator of muscle hypertrophy 29. Activation of PI3K by upstream ligands such as IGF-1 or IGF-2 phosphorylates the membrane phospholipids and creates a lipid binding site for Akt, which in turn increases protein synthesis and suppresses proteolytic activity and gene expression of the proteolytic genes. Consistent with the results obtained with the [3H]-phenylalanine incorporation assays, bioactive brassinosteroids stimulated phosphorylation of Akt in rat skeletal muscle cell culture (Figure 4). Both HB and (22S,23S)-homocastasterone treatments resulted in significant activation of Akt after 1 h, a much slower response than that produced by IGF-1, which phosphorylates Akt within 10 min. A similar delayed Akt response has been reported for ecdysteroids 30.
The pharmacogenomic properties of HB were further characterized in healthy rats orally administered with 60 mg/kg HB for 24 d. The results indicated that HB potently stimulated two sets of genes involved in muscle cell growth and carbohydrate metabolism. Among those, adrenergic receptor alpha 1d (Adra1d) showed the most notable 6.5-fold induction (Figure 5A). The α1 adrenergic receptors mediate endogenous functions of catecholamines, which involve coupling to G proteins followed by activation of phospholipase Cβ and protein kinase C 31. Recently, it has also been suggested that Adra1d may potentially regulate muscle survival and differentiation 32. mRNA levels of insulin-like growth factor 2 (Igf2) and insulin-like growth factor binding protein 1 (Igfbp1) were also upregulated in skeletal muscle of rats administered with HB. IGF-2 expression during skeletal muscle differentiation is regulated at the transcriptional level 33, and signaling through the IGF-1 receptor by locally produced IGF-2 defines a pathway that is critical for normal muscle growth and regeneration 34. Igfbp1 has been proposed as an acute regulator of IGF-1 bioactivity 35 and its elevation in association with HB treatment is unclear. The physiological role of the observed fructose-1,6-bisphosphatase (Fbp2) upregulation in also unknown. A contribution of this enzyme to a de novo production of glucose from lactate within the muscle is unlikely due to the lack of glucose-6-phosphatase in this tissue. However it is possible that the substrate cycle formed by this enzyme and phosphofructokinase may provide the basis for amplification of flux regulation of glycolysis versus glyconeogenesis 36.
Activation of Akt in skeletal muscle leads to rapid muscle hypertrophy 37 accompanied by improved metabolism 38. Even though inducible activation of Akt is sufficient to increase skeletal muscle mass and force without satellite cell activation 39, we also evaluated effect of HB supplementation on expression of the genetically-determined transcriptional programs regulated by myogenic transcription factors Myod1, Myf5, Myf6, and Myog. Myogenic regulatory protein MyoD was the only transcriptional factor associated with greater than 2 fold upregulation following the HB treatment (Figure 5C). This factor induces cell differentiation by activating muscle specific genes and is important in the switch from cellular proliferation to differentiation 40.
In conclusion, this study provides evidence that 6-keto group and 22α,23α-hydroxyls are critical for anabolic activity of brassinosteroids in rat skeletal muscle cells. This may be useful for the design of novel therapeutic molecules possessing high anabolic selectivity. In addition, (22S,23S)-homobrassinolide and (22S,23S)-homocastasterone, which were confirmed to possess the greatest anabolic activity among the molecules analyzed, may be employed as pharmacological tools to investigate the biological functions of muscle growth and regeneration pathways.
Matherials and methods
Chemicals and analytical methods
Structures of HB and its analogues used in this study are shown in Figure 1. HB [(22R, 23R, 24S)-2α, 3α, 22,23-tetrahydroxy-24 ethyl-β-homo-7-oxo-5α-cholestane-6-one] (1) was purchased from Waterstone Technology (Carmel, IN). (22R,23R,24S)-2α,3α,22,23-tetrahydroxy-B-homo-7-oxa-5α-cholestan-6-one (7), (22S,23S,24R)-2α,3α,22,23-tetrahydroxy-24-methyl-B-homo-7-oxa-5α-cholestan-6-one (8), and (22R,23R,24R)-2α,3α,22,23-tetrahydroxy-24-methyl-B-homo-7-oxa-5α-cholestan-6-one (9) were purchased from SciTech (Praha, Czech Republic). Structures and purity (>95%) of all compounds were confirmed by Varian 1200 L (Varian, Palo Alto, CA) triple quadrupole mass detector with electrospray ionization (ESI) interface using a Dionex Acclaim RSLC 120 C18 reverse phase column (150 mm × 2.1 mm, 2.2 μm), 1H NMR, and 13C NMR spectra recorded on Varian 400, 500 MHz & Bruker Avance 950 MHz spectrophotometer.
L-[2,3,4,5,6-3H]-phenylalanine was obtained from GE Healthcare (Piscataway, NJ). Phospho-Akt and Akt mAbs were purchased from Cell Signaling Technology (Danvers, MA). All other chemicals and cell culture media were obtained from Invitrogen (Carlsbad, CA) and Sigma (Saint Louis, MO) unless specified otherwise.
Synthesis of brassinosteroid analogues
Steroidal-3α,5-cyclo-6-one and steroidal-2-en-6-one were synthesized from stigmasterol using a previously reported procedure 41 with modifications as shown on Supporting Figure 1. Stigmasterol (4.85 mmol, 2.0 g) was dissolved in methyl ethyl ketone (140 ml) and triethylamine (14.5 mmol, 1.47 g, 2.4 ml, 3 eq) was added and stirred for 10 min at room temperature; then the reaction mixture was cooled to 5 °C. Methanesulfonylchloride (1.112 g, 1.0 ml, 2 eq) was added dropwise to the mixture at 5 °C. After stirring for 1.5 h, 15% sodium chloride solution (20 ml) was added to the mixture, the organic layer was separated and washed with saturated sodium bicarbonate, brine solution and dried over anhydrous sodium sulphate and concentrated to dryness (2.80 g). This crude product 1a was dissolved in methyl ethyl ketone (50 ml) and stirred with water (16 ml); then potassium bicarbonate (9.7 mmol, 0.972 g, 2 eq) was added and refluxed at 120 °C for 5 h. The organic layer was separated and washed with brine, dried over anhydrous sodium sulphate, and concentrated (2.01 g). This crude product 1b was dissolved in methyl ethyl ketone (35 ml) and cooled to 0 °C. Jones reagent (1.05 ml) was added dropwise to the reaction mixture and stirred for 3 h at the same temperature. After adding 15% sodium chloride solution (17 ml), the organic layer was separated and washed with saturated aqueous sodium bicarbonate solution, brine solution, dried over anhydrous sodium sulphate, and concentrated in vacuo. The crude 3α, 5-cyclo-6-one (compound 1c) was chromatographed over silica gel with hexane/ethyl acetate (97:3) as eluent, yielding 54% from stigmasterol. The purity of the compound was confirmed by TLC using Hexane: EtOAc (80:20) as developing solvent (Rf = 0.7).
Compound 1c (5.6 mmol, 2.315 g) was dissolved in dimethyl formamide (23 ml) and stirred. P-toluenesulfonic acid (1.13 mmol, 0.214 g, 0.2 eq) and sodium bromide (2.82 mmol, 0.29 g, 0.5 eq) were added and heated under reflux for 3 h. The cooled reaction mixture was concentrated to dryness in vacuo and ethyl acetate (150 ml) was added to the reaction mixture, washed with water (100 ml), saturated sodium bicarbonate solution, brine solution, dried over anhydrous sodium sulphate, and concentrated. The crude compound 1d was chromatographed over silica gel with hexane/ethyl acetate (98:2) as eluent, yielding 81.6%. The purity of the compound was confirmed by TLC [Hexane: EtOAc (90:10); Rf = 0.68] and ESI-MS (m/z): 411.39 (M+1).
Synthesis of (22S, 23S, 24S)- 2α, 3α, 22, 23-tetrahydroxy-24-ethyl-5α-cholestan-6-one
(22S, 23S)-homocastasterone (2) was synthesized according to the reported procedure 11 with following modifications. The solution of ruthenium tetroxide was prepared by adding a solution of sodium m-periodate (782 mg, 3.6 mmol) in water (2.9 ml) to ruthenium trichloride trihydrate (45 mg, 0.17 mmol). Half of the ruthenium tetroxide solution was added at once to a stirred solution of diene (0.5 g, 1.22 mmol) in ethyl acetate (17 ml), acetone (7.3 ml), and acetonitrile (7.3 ml) at 4 °C. After 5 min, the remaining half of the ruthenium tetroxide solution was added, and the reaction mixture was stirred at 5–6 °C for a further period of 5 min. Then, 20% sodium metabisulphite solution (12 ml) was added to the reaction mixture and stirred for 5 min. Solvents were evaporated and the residue was extracted with ethyl acetate (3 × 100 ml). The combined ethyl acetate layer was washed with water, brine solution and dried over anhydrous sodium sulphate and concentrated. The crude product was purified by column chromatography on silica gel using chloroform/methanol (95:5) as eluent, yielding 54.0%. The purity of the compound was confirmed by TLC using CHCl3: CH3OH (90:10); (Rf = 0.45), ESI-MS: (m/z) 479.35 (M+1) and 1H NMR (400MHz, CDCl3): δ = 0.70 (s, 3H), 0.76 (s, 3H), 0.88 (d, 3H, J = 6.9 Hz), 0.96 (m, 6H), 1.04 (d, 3H, J = 6.9 Hz), 2.30 (dd, 2H, J = 13.1, 4.5 Hz), 2.68 (dd, 1H, J = 12.1, 3.0 Hz), 3.60 (d, 2H, J = 7.3 Hz), 3.77 (dd, 1H, J = 6.9, 3.3 Hz), 4.05 (br, s, 1H). 13C NMR (100 MHz, CDCl3): δ = 11.9, 13.5, 13.9, 14.1, 14.5, 17.7, 18.5, 21.2, 21.7, 24.2, 26.3, 26.9, 27.8, 37.6, 39.3, 39.9, 42.3, 42.5, 43.4, 49.6, 50.7, 52.6, 53.7, 56.3, 68.3, 68.4, 70.6, 72.1, 211.9.
Synthesis of (22S, 23S, 24S)-3α-fluoro-22, 23-dihydroxy-24-ethyl-5α-cholestan-6-one and (22S, 23S, 24S)-3α-fluoro-22, 23-dihydroxy-7-oxo-24-ethyl-5α-cholestan-6-one
Two brassinosteroid analogues of containing a fluorine atom in the 3α position were prepared using standard operations 27, 42–43 with following modifications (Supporting Figure 2). The compound 1c (2.0 g, 4.88 mmol) was dissolved in 1,4-dioxane (42 ml). To this solution, 7.5 ml of 1M sulphuric acid was added and refluxed at 110 °C for 12 h. After cooling to room temperature, potassium carbonate (0.70 gm, 1.05 eq) was added and stirred for 30 min and most of the solvents was evaporated in vacuo. On dilution with brine solution (40 ml), the product formed white precipitate that was filtered off, washed with water and dried. The crude product 2a was purified by flash column chromatography using silica gel as adsorbent and eluting with hexane/ethyl acetate (75:25), yielding 59%. The purity of the compound was confirmed by TLC using Hexane: EtOAc (90:10) solvent (Rf = 0.28) and ESI-MS: (m/z) 429.32 (M+1).
Next, DAST (1.38 g, 1.12 ml, 8.55 mmol) was dissolved in dichloromethane (8.4 ml) and stirred at −78 °C. The solution of 2a (1.2 g, 2.8 mmol) in dichloromethane (50 ml) was added to DAST solution slowly. After 10 min, the reaction mixture was warmed to room temperature and maintained for 5 min. The reaction mixture was poured to water and extracted with dichloromethane (2 × 100 ml). The combined dichloromethane layer was washed with saturated sodium bicarbonate solution, brine solution, dried over anhydrous sodium sulphate, and concentrated. Purification of the crude product by flash column chromatography using silica gel and eluting with hexane/ethyl acetate (98.5:1.5) yielded 26.14% of 2b. The purity of the compound was confirmed by TLC using Hexane: EtOAc (80:20) solvent (Rf = 0.7) and ESI-MS (m/z): 431.56 (M+1).
Then, the solution of ruthenium tetroxide was prepared by adding a solution of sodium m-periodate (558 mg, 2.6 mmol) in water (2.1 ml) to ruthenium trichloride trihydrate (31 mg, 0.12 mmol). To a stirred solution of 2b (0.375 g, 0.87 mmol) in ethyl acetate (12.75 ml), acetone (5.5 ml), and acetonitrile (5.5 ml) at 4 °C was added half of the ruthenium tetroxide solution at once. After 5 minutes the remaining half of the ruthenium tetroxide solution was added and the reaction mixture was stirred at 5–6 °C for a further period of 5 min. 20% sodium metabisulphite solution (9 ml) was then added to the reaction mixture and continued the stirring for 5 min. All the solvents were evaporated and the residue was extracted with ethyl acetate (3 × 100 ml). The combined ethyl acetate layer was washed with water, brine solution and dried over anhydrous sodium sulphate and concentrated. The crude product was purified by column chromatography on silica gel using hexane/ethyl acetate (85:15) as eluent, yielding 45.0% of 4. The purity of the compound was confirmed by TLC using Hexane: EtOAc (2:1) solvent (Rf = 0.58) and ESI-MS (m/z): 465.34 (M+1) and 1H NMR (500 MHz, CDCl3): δ = 0.71 (s, 3H), 0.74 (s, 3H), 0.88 (d, 3H, J = Hz), 0.96 (m, 6H), 1.04 (d, 3H, J = 6.9 Hz), 2.32 (dd, 1H, J = 13.1, 4.5 Hz), 2.63 (dd, 1H, J = 12.6, 3.0 Hz), 3.61 (d, 2H, J = 5.0 Hz), 4.91 (d, 1H, J = 48.3). 13C NMR (125 MHz, CDCl3): δ = 11.9, 12.2, 12.3, 14.1, 14.5, 17.7, 18.5, 21.1, 21.7, 24.2, 26.9, 27.8, 31.9, 37.9, 39.4, 41.2, 42.3, 42.5, 43.4, 46.7, 49.6, 51.9, 52.6, 53.6, 56.3, 70.6, 72.1, 87.8, 211.7.
The solution of 4 (100 mg, 0.22 mmol) in dichloromethane (4 ml) was added dropwise to a stirred solution of trifluorooxyacetic acid (2.2 mmol, 10 eq, prepared from 30% aqueous hydrogen peroxide (0.25 ml) and trifluoroacetic anhydride (1.5 ml) in dichloromethane (4 ml) at 0°C. After 1 h, the reaction mixture was warmed to room temperature and maintained for 1 h. The reaction mixture was diluted with dichloromethane (10 ml) and the resulting solution was washed with saturated sodium bicarbonate solution, saturated sodium bisulphite, brine solution, dried over anhydrous sodium sulphate, and concentrated. The crude product was purified by column chromatography on silica gel using hexane/ethyl acetate (80:20) as eluent, yielding 19.0% of 3. The purity of the compound was confirmed by TLC using Hexane: EtOAc (2:1) solvent (Rf = 0.26) and ESI-MS (m/z): 481.30 (M+1).
Synthesis of (22S,23S,24S)-2α,3α,22,23-tetraacetoxy-24-ethyl-B-homo-6-aza-5α-cholestan-6-one
Two brassinosteroid analogues containing 6-aza and 7-aza substitutions in the B ring were prepared using previously published methods 43–44 with the following modifications (Supporting Figures 3–4). Acetic anhydride (2 ml) and DMAP (15 mg, 0.125 mmol) were added to the solution of 2 (600 mg, 1.25 mmol) in dry pyridine (4 ml). The reaction mixture was stirred at room temperature for 16 h, poured into ice water and extracted with ethyl acetate (50 ml X 2). The organic layer was washed with dilute HCl, saturated NaHCO3 solution, brine solution, and dried over anhydrous sodium sulphate. The crude 3a was purified by flash column chromatography using silica gel and hexane/ethyl acetate (90:10) to yield 700 mg. The purity of the compound was confirmed by TLC using Hexane: EtOAc (2:1) solvent (Rf = 0.3) and ESI-MS (m/z): 647.58 (M+1).
Compound 3a (200 mg, 0.31 mmol) was dissolved in glacial acetic acid (5 ml) and methanesulphonic acid (298 mg, 0.24 ml, 3.1 mmol) and sodium azide (40 mg, 0.512 mmol) were added and stirred at room temperature. After 4 h, the reaction mixture was poured into saturated sodium bicarbonate solution and extracted with ethyl acetate (50 ml X 3). The combined ethyl acetate extracts were washed with brine solution and dried over anhydrous sodium sulphate. This crude compound was chromatographed over silica gel and eluted with dichloromethane/methanol (98:2) yielding 73% of 3b. The purity of the compound was confirmed by TLC using CHCl3: CH3OH (90:10) solvent (Rf = 0.57) and ESI-MS (m/z): 662.68 (M+1).
Next, the solution of 3b (150 mg, 0.23 mmol) and 40% sodium hydroxide solution (3 ml) in methanol (8 ml) was refluxed for 1 h and cooled to room temperature. The reaction mixture was neutralized with 6M HCl in aqueous methanol, followed by removal of methanol in vacuo. The aqueous extract was extracted with ethyl acetate (50 ml X 3), and combined ethyl acetate extracts were washed with water, brine solution, and dried over anhydrous sodium sulphate. The crude compound 5 was purified by flash column chromatography using silica gel with dichloromethane/methanol (98:2) as eluent, yielding 70%. The purity of the compound was confirmed by TLC using CHCl3: CH3OH (90:10) solvent (Rf = 0.2) and ESI-MS (m/z): 494.32 and 1H NMR (500MHz, CD3OD): δ = 0.76 (s, 3H), 0.86 (s, 3H), 0.96 (m, 6H), 1.03 (d, 3H, J = 6.9 Hz), 2.05 (m, 1H), 2.13 (dtd, 1H, J = 9.4, 6.9, 2.4 Hz), 2.21 (d, 1H, J = 13.3 Hz), 2.44 (m, 1H), 3.55 (dd, 2H, J = 8.5, 5.6 Hz), 3.60 (ddd, 1H, J = 7.0, 6.0, 3.3 Hz), 3.73 (dd, 1H, J = 12.2, 4.8 Hz), 3.92 (m, 1H). 13C NMR (125 MHz, CD3OD): δ = 10.8, 12.1, 13.3, 13.6, 14.4, 16.6, 17.3, 18.4, 21.1, 22.4, 25.6, 26.7, 27.4, 34.3, 34.4, 39.7, 39.9, 42.5, 42.9, 49.6, 52.5, 53.1, 55.2, 58.6, 67.8, 68.2, 70.0, 71.7, 178.2.
Synthesis of (22S,23S,24S)-2α,3α,22,23-tetrahydroxy-24-ethyl-B-homo-7-aza-5α-cholestan-6-one
NBS (207 mg, 1.16 mmol) and NaHSO4.SiO2 (100 mg, activated at 120 °C for 48 h) were added to a solution of 3a (500 mg, 0.77 mmol) in diethyl ether (15 ml), and stirred at room temperature. After 2.5 h, the reaction mixture was filtered to remove silica and diluted with diethyl ether (100 ml). The crude 4a was washed with saturated sodium bicarbonate solution, brine solution, and dried over anhydrous sodium sulphate.
Compound 4a (450 mg, 0.565 mmol) was dissolved in 75% pyridine (16 ml) and 1M NaOH (0.88 ml, 1.5 eq) was added slowly at room temperature. After 4 h, the reaction mixture was poured into ice cooled 1M HCl solution (200 ml) and extracted with ethyl acetate (100 ml X 2). The combined ethyl acetate extracts were washed with saturated sodium bicarbonate solution, brine solution and dried over anhydrous sodium sulphate. The crude 4b was chromatographed over silica gel and eluted with hexane/ethyl acetate (85:15) yielding 32%.
NaIO4 (100 mg, 0.453 mmol) was added to a solution of 4b (100 mg, 0.151 mmol) in acetic acid (4.5 ml) and stirred well at room temperature. After 18 h, the reaction mixture was diluted with diethyl ether (100 ml), washed with brine solution and dried over anhydrous sodium sulphate. The crude product was dissolved in methanol (4 ml), followed by addition of ammonium acetate (235 mg) and sodium cyanoborohydride (8 mg). The reaction mixture was stirred for 110 h at room temperature. Next, the mixture was diluted with water (25 ml), extracted with ethyl acetate (50 ml X 3), and combined ethyl acetate extracts were washed with water, brine solution and dried over anhydrous sodium sulphate. This crude 4c was chromatographed on silica gel and eluted with dichloromethane/methanol (85:15) yielding 20%. The purity of the compound was confirmed by TLC using CHCl3: EtOAc (2:1) solvent (Rf = 0.45) and ESI-MS (m/z): 662.68 (M+1).
Next, 40% NaOH solution (0.5 ml) was added to the solution of 4c (20 mg, 0.03 mmol) in methanol (5 ml) and refluxed for 30 min. After cooling, the mixture was neutralized by addition of ice-cold solution of 6M HCl in aqueous methanol and concentrated to remove methanol in vacuo. The remaining aqueous layer was diluted with water (10 ml), extracted with ethyl acetate (40 ml X 2), and combined ethyl acetate extracts were washed with water, brine solution and dried over anhydrous sodium sulphate. The resulting 6 was chromatographed on silica gel and eluted with dichloromethane/methanol (97:3) yielding 53%. The purity of the compound was confirmed by TLC using CHCl3: CH3OH (90:10) solvent (Rf = 0.22); ESI-MS (m/z): 494.30 (M+1) and 1H NMR (500MHz, CD3OD): δ = 0.76 (s, 3H), 0.86 (d, 3H, J = 6.9 Hz), 0.90 (s, 3H), 0.95 (dd, 3H, J = 14.8, 7.2 Hz), 1.03 (d, 3H, J = 6.9 Hz), 2.05 (m, 1H), 2.13 (dtd, 1H, J = 9.3, 6.8, 2.4 Hz), 3.01 (m, 1H), 3.08 (m, 2H), 3.56 (m, 1H), 3.92 (s, 1H). 13C NMR (125 MHz, CD3OD): δ = 10.8, 13.3, 13.6, 14.4, 16.6, 17.3, 18.4, 21.1, 22.4, 25.0, 26.7, 30.6, 37.3, 40.1, 41.2, 41.4, 42.5, 42.9, 48.6, 48.3, 49.6, 52.7, 52.9, 59.5, 68.1, 68.2, 70.0, 71.7, 178.8.
Cell culture
Rat L6 skeletal muscle cell line CRL-1458 was obtained from ATCC (Manassas, VA). Myoblasts were routinely maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS and 0.1% penicillin-streptomycin at 37°C and 5% CO2. Cells were subcultured into 24 well plates for protein synthesis, degradation, and cell viability studies and 6 well plates for Western blot analysis (Greiner Bio One, Monroe, NC). Once cells reached 90% confluence, differentiation was induced by lowering the serum concentration to 2%, and medium was changed every 2 days. After 7–9 days of culture the myoblasts had fused into multinucleated myotubes 41. NIH 3T3 murine embryonic fibroblast cell line (ATCC #CCL-92) was maintained in DMEM and 10% FBS at 37°C in 5% CO2, and passaged every 3–4 days.
Cell viability assay and dose range determination
Cell viability was measured by the MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay in triplicate essentially as described 42 and quantified spectrophotometrically at 550 nm using a microplate reader (Molecular Devices, Sunnyvale, CA). The concentrations of test reagents that showed no changes in cell viability compared with that of the vehicle (0.1% ethanol) were selected for further studies.
Measurement of protein synthesis
Fully differentiated myotubes were washed with serum-free DMEM and treated in triplicate with vehicle (0.1% ethanol), increasing concentrations of HB, or 6.5 nM of insulin-like growth factor-1 (IGF-1) as positive control. Compounds were added to serum-free medium containing 0.5 μCi/mL [3H]-phenylalanine and incubated for 4 h. The incubation was stopped by placing cells on ice, discarding the medium, and washing the cells extensively with ice cold PBS to remove the non-incorporated trace. Proteins were precipitated with 5% trichloroacetic acid and dissolved in 0.5N NaOH 43. Specific radioactivity of protein-bound phenylalanine was quantified using liquid scintillation counter LS 6500 (Beckman Coulter, Fullerton, CA) and normalized to mg of total protein determined by BCA protein assay (Pierce Biotechnology, Rockford, IL).
Measurement of protein degradation
The effect of brassinosteroids on protein degradation was investigated in fully differentiated myotubes as described 44 with slight modifications. Fully differentiated myotubes were incubated for 16 h to allow labeling of cellular proteins with 1.5 μCi/mL [3H]-phenylalanine. Cells were washed twice with PBS to remove the non-incorporated trace and treated for 4 h with vehicle (0.1% ethanol), increasing concentrations of brassinosteroids, or 10 nM of insulin in serum-free medium. The incubation was stopped by placing the cells on ice, and protein in the medium was precipitated with 5% trichloroacetic acid. Specific radioactivity of protein-free phenylalanine was quantified using liquid scintillation counter LS 6500 (Beckman Coulter, Fullerton, CA) and normalized to mg of total cell protein determined by BCA protein assay (Pierce Biotechnology, Rockford, IL).
Western blot analysis
Fully differentiated L6 myotubes were cultured as described above, and whole cell extracts were prepared in ice-cold RIPA buffer supplemented with 10 mM sodium floride, 2mM sodium orthovanadate, 1 mM PMSF, and protease inhibitor cocktail (Sigma) and centrifuged at 12,000 g for 20 min at 4°C. Equal amounts of protein (50 μg) from the supernatants were separated on 10% SDS polyacrylamide gels and blotted onto the nitrocellulose membrane. Western blot detection was performed with monoclonal phospho-Akt (Ser473) antibodies according to the manufacturer’s instructions (Cell Signaling Technology, Danvers, MA). After being washed, the blots were incubated with an anti-rabbit peroxidase-labeled secondary antibody and visualized using ECL Western Blotting Detection Reagent (GE Healthcare, Piscataway, NJ). After being stripped, the same blots were probed with total Akt antibodies to serve as loading controls.
Animal study and gene expression studies
Animal experiment was performed according to procedures approved by the Rutgers Institutional Animal Care and Use Committee in the AAALAC accredited animal care facility as described previously 20. Briefly, six weeks old male Wistar rats (Charles River Laboratories, MA) fed normal diet (#5001 Rodent Chow diet, Purina, St. Louis, MO), were randomized into 2 groups (n=6) and gavaged daily for 24 d with either 1 ml of vehicle (5% DMSO in corn oil) or 60 mg/kg body weight of HB. At necropsy, tissue weights were recorded, then tissue samples were collected by snap-freezing in the liquid nitrogen and stored at −80°C for further studies. Total RNA was isolated using Trizol, its quantity and purity were determined using a NanoDrop (Nanodrop Technologies, Wilmington, DE). Pooled RNA samples were used for the rat insulin signaling PCR array (Qiagen, Valencia, CA) and analyzed according to the manufacturer’s protocol. cDNA synthesis and quantitative PCR analysis were performed essentially as described 45 with the following primers selected using the Primer Express version 2.0 software (Applied Biosystems, Foster City, CA) as follows: cyclophilin, forward primer 5′-AAT GCT GGA CCA AAC ACA AAT G-3′, reverse primer 5′-GCC ATC CAG CCA CTC AGT CT-3′; MyoD1, forward primer 5′-CAG AAC TGG GAC ATG GAG CTA CT-3′, reverse primer 5′-TGT CGC AAA GGA GCA GAG AGA-3′; Myf5, forward primer 5′-CTC GCC TTC CGA GTA CTT CTA TG-3′, reverse primer 5′-CAA ACT GGT CCC CAA ACT CAT C-3′; Myf6, forward primer 5′-GAG AAG TGC CAT CAA CTA CAT TGA G-3′, reverse primer 5′-CCC CAG CTC CTG CAT TTT C-3; myogenin, forward primer 5′-GGT ACC CAG TGA ATG CAA CTC-3′, reverse primer 5′-CAA TGC ACT GGA GTT TGG TCC-3′; IGF-2, forward primer 5′-TGT CTA CCT CTC AGG CCG TAC TT-3′, reverse primer 5′-TCC AGG TGT CGA ATT TGA AGA A-3′′ and actin, forward primer 5′-GGG AAA TCG TGC GTG ACA TT-3′, reverse primer 5′-GCG GCA GTG GCC ATC TC-3′.
Statistics
Statistical analyses were performed using Prism 4.0 (GraphPad Software, San Diego, CA). Unless otherwise noted, data were analyzed by one-way ANOVA with treatment as a factor. Post hoc analyses of differences between individual experimental groups were made using the Dunnett’s multiple comparison test. Significance was set at p < 0.05. Values are reported as means ± SEM. The 50% inhibitory concentration (IC50) was calculated by a nonlinear regression curve analysis.
Supplementary Material
Acknowledgments
The authors wish to acknowledge Rocky Graziose for NMR measurements, and Reneta Pouleva and Ruth Dorn for excellent technical assistance. This work was supported, in part, by utgers University, by the 5P50AT002776-05 grant from the National Center for Complementary and Alternative Medicine (NCCAM), and by Phytomedics Inc.
Abbreviations
- Akt
serine-threonine kinase PKB
- HB
homobrassinolide
- IGF-1
insulin-like growth factor 1
- MAPK
mitogen-activated protein kinase
- PI3K
phosphoinositide 3- kinase
Footnotes
Disclosure statement
I.R. served on the advisory board; A.P. and S.K. consulted for Phytomedics Inc.
Schematic synthesis routes for brassinosteroid analogues used in this study. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Bajguz A, Tretyn A. The chemical characteristic and distribution of brassinosteroids in plants. Phytochemistry. 2003;62:1027–1046. doi: 10.1016/s0031-9422(02)00656-8. [DOI] [PubMed] [Google Scholar]
- 2.Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4:46–56. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- 3.Clouse SD. Brassinosteroids. Plant counterparts to animal steroid hormones? Vitam Horm. 2002;65:195–223. doi: 10.1016/s0083-6729(02)65065-4. [DOI] [PubMed] [Google Scholar]
- 4.Mussig C. Brassinosteroid-promoted growth. Plant Biol (Stuttg) 2005;7:110–117. doi: 10.1055/s-2005-837493. [DOI] [PubMed] [Google Scholar]
- 5.Wang ZY, Seto H, Fujioka S, Yoshida S, Chory J. BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature. 2001;410:380–383. doi: 10.1038/35066597. [DOI] [PubMed] [Google Scholar]
- 6.Nam KH, Li J. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell. 2002;110:203–212. doi: 10.1016/s0092-8674(02)00814-0. [DOI] [PubMed] [Google Scholar]
- 7.Takatsuto S, Ikekawa N. Synthesis and activity of plant growth-promoting steroids, (22R,23R,24S)-28-homobrassinosteroids, with modifications in rings A and B. J Chem Soc Perkin Trans. 1984;1:439–447. [Google Scholar]
- 8.Bajguz A. Effect of brassinosteroids on nucleic acids and protein content in cultured cells of Chlorella vulgaris. Plant Physiology and Biochemistry. 2000;38:209–215. [Google Scholar]
- 9.Kartal G, Temel A, Arican E, Gozukirmizi N. Effects of brassinosteroids on barley root growth, antioxidant system and cell division. Plant Growth Regulation. 2009;58:261–267. [Google Scholar]
- 10.Kulaeva ON, Burkhanova EA, Fedina AB, Khokhlova VA, Bokebayeva GA, Vorbrodt HM, Adam Gn. Brassinosteroids. American Chemical Society; Washington, DC: 2009. Effect of Brassinosteroids on Protein Synthesis and Plant-Cell Ultrastructure under Stress Conditions; pp. 141–155. [Google Scholar]
- 11.Massey AP, Pore VS, Hazra BG. New route for the synthesis of (22S,23S)-28-homobrassinolide. Synthesis Stutt. 2003;3:426–430. [Google Scholar]
- 12.Ramirez JA, Brosa C, Galagovsky LR. Synthesis and bioactivity of C-29 brassinosteroid analogues with different functional groups at C-6. Phytochemistry. 2005;66:581–587. doi: 10.1016/j.phytochem.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 13.Kuzmitsky BB, Mizulo NA. Technical report. Academy of Sciences of Belarus; Minsk: 1991. Study of acute toxicity of epibrassinolide and its preparative forms; pp. 1–44. [Google Scholar]
- 14.Murkunde YV, Balakrishna Murthy P. Developmental toxicity of homobrassinolide in wistar rats. Int J Toxicol. 2010;29:517–522. doi: 10.1177/1091581810375620. [DOI] [PubMed] [Google Scholar]
- 15.Malikova J, Swaczynova J, Kolar Z, Strnad M. Anticancer and antiproliferative activity of natural brassinosteroids. Phytochemistry. 2008;69:418–426. doi: 10.1016/j.phytochem.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 16.Wachsman MB, Ramírez JA, LBT, Galagovsky LR, Coto CE. Antiviral activity of natural and synthetic brassinosteroids. Curr Med Chem Anti Infect Agents. 2004;3:163–179. [Google Scholar]
- 17.Andersen DL, Back TG, Janzen L, Michalak K, Pharis RP, Sung GC. Design, synthesis, and bioactivity of the first nonsteroidal mimetics of brassinolide. J Org Chem. 2001;66:7129–7141. doi: 10.1021/jo015832+. [DOI] [PubMed] [Google Scholar]
- 18.Baron DL, Luo W, Janzen L, Pharis RP, Back TG. Structure-activity studies of brassinolide b-ring analogues. Phytochemistry. 1998;49:1849–1858. [Google Scholar]
- 19.Romero-Avila M, de Dios-Bravo G, Mendez-Stivalet JM, Rodriguez-Sotres R, Iglesias-Arteaga MA. Synthesis and biological activity of furostanic analogues of brassinosteroids bearing the 5alpha-hydroxy-6-oxo moiety. Steroids. 2007;72:955–959. doi: 10.1016/j.steroids.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Esposito D, Rathinasabapathy T, Poulev A, Komarnytsky S, Raskin I. Unpublished results. Anabolic effect of plant brassinosteroid. [Google Scholar]
- 21.Hajduch E, Alessi DR, Hemmings BA, Hundal HS. Constitutive activation of protein kinase B alpha by membrane targeting promotes glucose and system A amino acid transport, protein synthesis, and inactivation of glycogen synthase kinase 3 in L6 muscle cells. Diabetes. 1998;47:1006–1013. doi: 10.2337/diabetes.47.7.1006. [DOI] [PubMed] [Google Scholar]
- 22.Zullo MAT, Adam G. Brassinosteroid phytohormones - structure, bioactivity and applications. Braz J Plant Physiol. 2002;14:143–181. [Google Scholar]
- 23.Adler JH, Grebenok RJ. Biosynthesis and distribution of insect-molting hormones in plants--a review. Lipids. 1995;30:257–262. doi: 10.1007/BF02537830. [DOI] [PubMed] [Google Scholar]
- 24.Bathori M, Toth N, Hunyadi A, Marki A, Zador E. Phytoecdysteroids and anabolic-androgenic steroids--structure and effects on humans. Curr Med Chem. 2008;15:75–91. doi: 10.2174/092986708783330674. [DOI] [PubMed] [Google Scholar]
- 25.Takatsuto S, Ikekawa N, Morishita T, Abe H. Structure-activity relationship of brassinosteroids with respect to the A/B-ring functional groups. Chem Pharmaceutic Bulletin. 1987;35:211–216. [Google Scholar]
- 26.Anastasia M, Allevi P, Brasca MG, Ciuffreda P, Fiechi A. Synthesis of (2R,3S,22S,23S)-2,3,22,23-tetrahydroxy-B-homo-6-aza-5a-stigmastan-7-one, an aza analogue of brassinolide. Gazzeta Chimica Italiana. 1984;114:159–161. [Google Scholar]
- 27.Galagovsky LR, Gros EG, Ramirez JA. Synthesis and bioactivity of natural and C-3 fluorinated biosynthetic precursors of 28-homobrassinolide. Phytochemistry. 2001;58:973–980. doi: 10.1016/s0031-9422(01)00379-x. [DOI] [PubMed] [Google Scholar]
- 28.Mykhaylyk OM, Kotzuruba AV, Buchanevich OM, Korduban AM, Meged EF, Gulaya NM. Signal transduction of erythrocytes after specific binding of ecdysterone and cholesterol immobilized on nanodispersed magnetite. Journal of Magnetism and Magnetic Materials. 2001;225:226–234. [Google Scholar]
- 29.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 30.Gorelick-Feldman J, Maclean D, Ilic N, Poulev A, Lila MA, Cheng D, Raskin I. Phytoecdysteroids increase protein synthesis in skeletal muscle cells. J Agric Food Chem. 2008;56:3532–3537. doi: 10.1021/jf073059z. [DOI] [PubMed] [Google Scholar]
- 31.Strosberg AD. Structure, function, and regulation of the three beta-adrenergic receptors. Obes Res. 1995;3 (Suppl 4):501S–505S. doi: 10.1002/j.1550-8528.1995.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 32.Saini A, Al-Shanti N, Stewart C. C2 skeletal myoblast survival, death, proliferation and differentiation: regulation by Adra1d. Cell Physiol Biochem. 2010;25:253–262. doi: 10.1159/000276559. [DOI] [PubMed] [Google Scholar]
- 33.Kou K, Rotwein P. Transcriptional activation of the insulin-like growth factor-II gene during myoblast differentiation. Mol Endocrinol. 1993;7:291–302. doi: 10.1210/mend.7.2.8469241. [DOI] [PubMed] [Google Scholar]
- 34.Alzhanov DT, McInerney SF, Rotwein P. Long-range interactions regulate Igf2 gene transcription during skeletal muscle differentiation. J Biol Chem. 2010;285:38969–38977. doi: 10.1074/jbc.M110.160986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee PD, Giudice LC, Conover CA, Powell DR. Insulin-like growth factor binding protein-1: recent findings and new directions. Proc Soc Exp Biol Med. 1997;216:319–357. doi: 10.3181/00379727-216-44182. [DOI] [PubMed] [Google Scholar]
- 36.Challiss RA, Arch JR, Newsholme EA. The rate of substrate cycling between fructose 6-phosphate and fructose 1,6-bisphosphate in skeletal muscle. Biochem J. 1984;221:153–161. doi: 10.1042/bj2210153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai KM, Gonzalez M, Poueymirou WT, Kline WO, Na E, Zlotchenko E, Stitt TN, Economides AN, Yancopoulos GD, Glass DJ. Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol. 2004;24:9295–9304. doi: 10.1128/MCB.24.21.9295-9304.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Izumiya Y, Hopkins T, Morris C, Sato K, Zeng L, Viereck J, Hamilton JA, Ouchi N, LeBrasseur NK, Walsh K. Fast/Glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 2008;7:159–172. doi: 10.1016/j.cmet.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blaauw B, Canato M, Agatea L, Toniolo L, Mammucari C, Masiero E, Abraham R, Sandri M, Schiaffino S, Reggiani C. Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J. 2009;23:3896–3905. doi: 10.1096/fj.09-131870. [DOI] [PubMed] [Google Scholar]
- 40.Solomon AM, Bouloux PM. Modifying muscle mass - the endocrine perspective. J Endocrinol. 2006;191:349–360. doi: 10.1677/joe.1.06837. [DOI] [PubMed] [Google Scholar]
- 41.Mandel JL, Pearson ML. Insulin stimulates myogenesis in a rat myoblast line. Nature. 1974;251:618–620. doi: 10.1038/251618a0. [DOI] [PubMed] [Google Scholar]
- 42.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 43.Montgomery JL, Harper WM, Jr, Miller MF, Morrow KJ, Jr, Blanton JR., Jr Measurement of protein synthesis and degradation in C2C2) myoblasts using extracts of muscle from hormone treated bovine. Methods Cell Sci. 2002;24:123–129. doi: 10.1023/a:1024498316958. [DOI] [PubMed] [Google Scholar]
- 44.Fawcett J, Hamel FG, Duckworth WC. Characterization of the inhibition of protein degradation by insulin in L6 cells. Arch Biochem Biophys. 2001;385:357–363. doi: 10.1006/abbi.2000.2160. [DOI] [PubMed] [Google Scholar]
- 45.Komarnytsky S, Cook A, Raskin I. Potato protease inhibitors inhibit food intake and increase circulating cholecystokinin levels by a trypsin-dependent mechanism. Int J Obes (Lond) 2011;35:236–243. doi: 10.1038/ijo.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.