The filamentous mould Rhizopus microsporus is a member of the zygomycetes (lower fungi). While some strains serve as food fermenting fungi, others represent infamous plant pathogens and opportunistic human pathogens. Recently, it was shown that some strains of R. microsporus are associated with symbiotic bacteria. Here, we outline why these organisms are important for human health and how they can be exploited for drug development. Furthermore, we illustrate what the investigation of bacterial–fungal symbiosis can teach us about the evolution of pathogenicity factors in general.
Rhizopus microsporus Harbors Bacterial Endosymbionts as Intracellular Toxin Factories
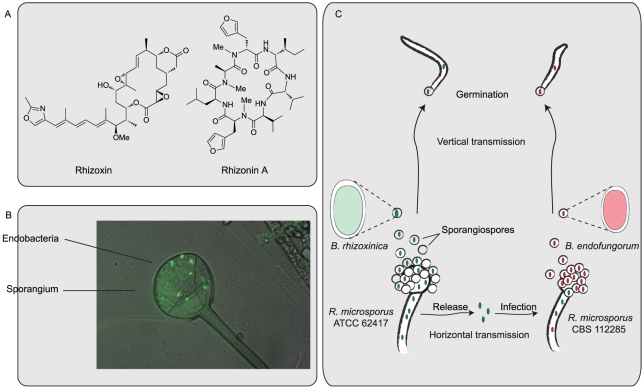
R. microsporus (ATCC 62417) attacks rice plants and illicits rice seedling blight, a severe crop disease affecting rice fields in Asia [1]. Symptoms of the infection include abnormal swelling of the roots and finally death of the affected tissue. Plant pathogenic R. microsporus strains live as necrotrophic pathogens, i.e., they derive energy from killed host cells. For that purpose they secrete rhizoxin (see Figure 1A), a toxin that blocks mitosis in eukarytotic cells by binding to β-tubulin [2]. Rhizopus itself is resistant to the toxin due to an amino acid exchange in the tubulin protein [3]. The production of toxins by plant pathogenic fungi is a widespread virulence mechanism [4]. In the case of R. microsporus, however, the search for biosynthetic genes coding for rhizoxin biosynthesis led to an unexpected discovery: it is not the fungus itself that produces the pathogenicity factor rhizoxin. Instead, the fungus harbors bacterial symbionts, which are the actual producers of the virulence factor [1]. Toxin formation by bacteria has been demonstrated in analogy with Koch's postulates in classical microbiology: it was discovered that rhizoxin-producing strains of R. microsporus contained symbionts, while nontoxinogenic strains did not. Removal of symbionts by antibiotics indeed abolished rhizoxin production. The bacteria could be isolated and grown in axenic (pure) culture. Finally, reintroduction into cured hosts clearly reestablished rhizoxin production [1]. Intriguingly, microscopic investigations revealed that the bacteria are true endosymbionts, i.e., they inhabit the intracellular space of fungal mycelium (see Figure 1B). The first endosymbiont of R. microsporus that could be isolated was named Burkholderia rhizoxinica for its capability to produce rhizoxin [5].
Figure 1. Chemical and biological features of endofungal bacteria.
(A) Chemical structures of rhizoxin, an antimitotic macrolide, and rhizonin A, a hepatotoxic cyclopeptide. (B) Light micrograph of a sporangium of R. microsporus (ATCC 62147) stained with a viability assay system (Invitrogen). Green spots are living cells of the bacterial endosymbiont B. rhizoxinica. (C) Life cycle of R. microsporus strains and their endosymbionts B. rhizoxinica and B. endofungorum. Endobacteria are propagated within fungal spores (vertical transmission). Under laboratory conditions, isolated bacteria can infect compatible host strains (horizontal transmission).
A New Role for Endobacteria in Mycotoxin Research and Food Safety
The discovery of endobacteria as producers of “mycotoxins” was relevant in the areas of natural product research and microbiology, since similar symbionts in other antibiotic producing fungi might have been overlooked in the past [6]. Indeed, another dangerous “mycotoxin”, rhizonin (see Figure 1C), originally isolated from R. microsporus (CBS 112285) is produced by endobacteria as well [7]. These endobacteria are related to B. rhizoxinica, but represent another species, Burkholderia endofungorum [5]. Rhizonin is a cyclopeptide, which is highly toxic for mammals. Tested animals exhibited serious hepatic lesions and died from chronic failure of the liver [8]. The fact that the producing strain was found on ground nuts in Mozambique underlines the relevance of the Burkholderia–Rhizopus symbiosis for food safety and human health. Concerning food safety, it is even more distressing that another Burkholderia–Rhizopus association was isolated from a tempe/sufu starter culture in Vietnam [1]. Tempe and sufu are traditional soy preparations in Asia that are fermented with R. microsporus. It could be demonstrated that rhizoxin is indeed produced during sufu fermentation, thus revealing a potential threat to human health [9].
Endosymbionts of Rhizopus Were Detected in Human Pathogens but Are Not Essential for Zygomycoses
Rhizopus species including R. microsporus are regularly involved in zygomycoses (mucormycoses), disastrous fungal infections that affect immunocompromised patients. These diseases have high mortality rates [10] and are hard to treat by antifungal agents. Consequently, surgical debridement of infected tissue is often necessary [11]. After the discovery of B. rhizoxinica it was speculated whether toxin production by endosymbionts might enhance the virulence of fungal strains involved in human disease. The bacteria would then provide a promising target for the treatment of Rhizopus infections. Indeed, it has been shown that rhizoxin-producing strains are frequently involved in zygomycosis [11].
However, several lines of evidence suggest that rhizoxin production seems not to be essential for Rhizopus pathogenicity [11], [12]. First of all, clinical cases of zygomycosis are frequently caused by non-toxinogenic Rhizopus species [12]. Furthermore, when R. microsporus was cured from symbionts with antibiotics, it still retained its ability to infect mice under laboratory conditions [11]. Although these results show that endosymbionts are not the key players in zygomycosis, they might still raise the potential health threat caused by R. microsporus: it is well conceivable that endosymbionts that are released from fungal mycelium might cause sepsis or further complications. In particular, B. rhizoxinica and B. endofungorum could cause potential long-term damage by secretion of toxins into the human body. In fact, related Burkholderia strains have been isolated from clinical specimen [13]. Consequently, the existence of toxinogenic endosymbionts should be kept in mind when it comes to treatment of zygomycosis.
Investigation of Rhizoxin Biosynthesis Can Promote Antitumor Therapy
Despite all theses dangers emanating from toxin-producing Rhizopus/Burkholderia strains, rhizoxin could also assist in antitumor therapy: due to its ability to block mitosis via binding to β-tubulin, rhizoxin exhibits strong activity against tumor cell lines in vitro [14]. The substance has already tested in phase II clinical trials [15]. However, its in vivo activity was unsatisfactory, probably due to low activity and rapid elimination from plasma [15], and thus it would be desirable to produce more potent derivatives. Rhizoxin belongs to the family of macrolide antibiotics (as e.g., erythromycin) and is produced by a modular polyketide synthase. The enzymatic assembly line catalyzes repetitive condensation of activated acetate (acetyl-CoA and malonyl-CoA) units, in a similar way as in fatty acid biosynthesis [16]. In contrast to the latter, polyketide biosynthesis allows for variable degrees of chain processing, and the polyketide backbone is further modified by tailoring enzymes. Through genetic manipulation of the biosynthesis genes it is possible to engineer polyketide biosynthesis, and thus create new derivatives. Indeed, it was possible to identify the genes coding for rhizoxin biosynthesis [17] and to modify these genes in isolated symbionts. Thus, the function of some enzymes could be investigated in detail [18], [19]. Furthermore, it is promising that cultivation of B. rhizoxinica in pure culture could significantly increase the yield of rhizoxin production and lead to the isolation of new, significantly more active rhizoxin derivatives [20].
Endosymbionts of Rhizopus: Former Pathogens of Pathogens?
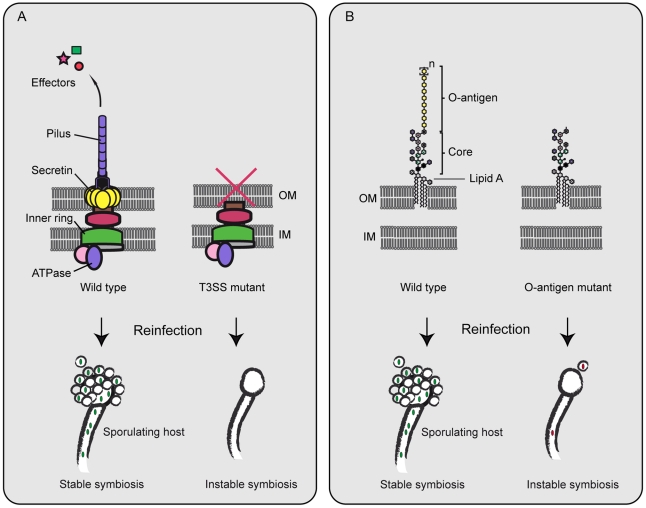
From a biologist's point of view, the Burkholderia–Rhizopus symbiosis has some intriguing aspects that go beyond zygomycosis and natural product research. For instance, the question was raised as to how the association between Rhizopus and Burkholderia has evolved and how it is maintained. Microscopic investigations with GFP-labeled endobacteria revealed that endosymbionts enter fungal spores to be “inherited” during vegetative reproduction [21]. Intriguingly, the reproduction of the host has been hijacked by the symbionts: the host is unable to sporulate when endosymbionts are removed [21]. Thus, they ensure their own propagation alongside the host lineage (vertical transmission, see Figure 1C). In addition, endobacteria are able to infect compatible host organisms in laboratory cultures [22]. Spread of symbionts by release and infection of another host is called horizontal transmission (see Figure 1C). Both vertical and horizontal transmission could be shown to be relevant during evolution of the Burkholderia–Rhizopus alliance: comparison of phylogenetic trees of host strains and the corresponding symbiont strains revealed that the topologies of these trees resemble each other [23]. This means that host fungi and symbionts have undergone “cospeciation”—symbiont lineages have been associated with their fungal host lineage over a long period of time. However, some host switching events could be detected, which must be due to horizontal transmission. These results suggest that endosymbionts have actually evolved from former parasites (pathogens) of Rhizopus. It looks like both partners benefit from their close relationship: while the fungus obtains powerful chemical weapons produced by B. rhizoxinica, the latter is supplied with nutrients and a safe niche. Any mutually beneficial symbiosis is called mutualism. However, the line between mutualism and parasitism is thin and shifts might occur during evolution. This parasitism–mutualism shift hypothesis is further supported by genome sequencing of B. rhizoxinica [24], [25]: while the genome retains only a reduced number of genes compared to free-living Burkholderia species, it encodes a large repertoire of typical virulence factors. It could be demonstrated that a type III secretion system (T3SS) [22] and the lipopolysaccharide O-antigen [26] are essential to maintain the symbiosis. Both factors are known pathogenicity factors of animal and plant pathogens. T3SSs are giant protein export machineries anchored in both membranes of Gram-negative bacteria (see Figure 2A). Usually, they accomplish export of effector proteins that manipulate cellular processes of the host [27]. Symbionts lacking these systems are unable to reinfect their host fungus and cannot illicit its sporulation like the wild type [22]. Similar results were obtained for mutants lacking the O-antigen—a long polysaccharide chain anchored in the outer membrane (see Figure 2B) [26]. It is plausible that the O-antigen is needed for protection against fungal defense mechanisms or recognition of the symbionts.
Figure 2. Symbiosis factors of B. rhizoxinica.
(A) The role of the type III secretion system (T3SS) in bacterial–fungal symbiosis. T3SSs consist of several ring-like structures anchored in both the outer (OM) and inner membrane (IM) of Gram-negative bacteria. Energy is derived from an ATPase component situated at the cytosolic side of the protein complex. Effector proteins are secreted through a pilus structure into host cells. When key components are inactivated, mutants fail to reinfect the host. (B) Role of the lipopolysaccharide (LPS) in bacterial–fungal symbiosis. LPS molecules are anchored in the outer membrane of Gram-negative bacteria by their lipid component (lipid A). The sugar components form a heterogeneous core oligosaccharide and a polymeric O-antigen. When the O-antigen is missing, mutants reinfect the host sporadically, but are incapable of establishing a stable symbiosis.
These results teach us that bacterial symbionts of fungi employ similar factors as plant parasites or human pathogens. These mechanisms seem to be universal host control tools that can be adapted to highly versatile hosts and cellular targets.
Footnotes
The authors have declared that no competing interests exist.
The authors received no specific funding for this work.
References
- 1.Partida-Martinez LP, Hertweck C. Pathogenic fungus harbours endosymbiotic bacteria for toxin production. Nature. 2005;437:884–888. doi: 10.1038/nature03997. [DOI] [PubMed] [Google Scholar]
- 2.Sato Z, Noda T, Matsuda I, Iwasaki S, Kobayashi H, et al. Studies on rhizoxin, a phytotoxin produced by Rhizopus chinensis causing rice seedling blight. Annu Phytopathol Soc Japan. 1983;49:128. [Google Scholar]
- 3.Schmitt I, L.P. P-M, Winkler R, Voigt K, Einax E, et al. Evolution of host resistance in a toxin-producing bacterial-fungal alliance. ISME J. 2008;2:632–641. doi: 10.1038/ismej.2008.19. [DOI] [PubMed] [Google Scholar]
- 4.Moebius N, Hertweck C. Fungal phytotoxins as mediators of virulence. Current Opinion in Plant Biology. 2009;12:390–398. doi: 10.1016/j.pbi.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Partida-Martinez LP, Groth I, Schmitt I, Richter W, Roth M, et al. Burkholderia rhizoxinica sp. nov. and Burkholderia endofungorum sp. nov., bacterial endosymbionts of the plant-pathogenic fungus Rhizopus microsporus. Int J Syst Evol Microbiol. 2007;57:2583–2590. doi: 10.1099/ijs.0.64660-0. [DOI] [PubMed] [Google Scholar]
- 6.Lackner G, Partida-Martinez LP, Hertweck C. Endofungal bacteria as producers of mycotoxins. Trends Microbiol. 2009;17:570–576. doi: 10.1016/j.tim.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Partida-Martinez LP, de Looss CF, Ishida K, Ishida M, Roth M, et al. Rhizonin, the first mycotoxin isolated from the zygomycota, is not a fungal metabolite but is produced by bacterial endosymbionts. Appl Environ Microbiol. 2007;73:793–797. doi: 10.1128/AEM.01784-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson T, Rabie CJ, Fincham JE, Steyn PS, Schipper MA. Toxicity of rhizonin A, isolated from Rhizopus microsporus, in laboratory animals. Food Chem Toxicol. 1984;22:275–281. doi: 10.1016/0278-6915(84)90006-1. [DOI] [PubMed] [Google Scholar]
- 9.Rohm B, Scherlach K, Mobius N, Partida-Martinez LP, Hertweck C. Toxin production by bacterial endosymbionts of a Rhizopus microsporus strain used for tempe/sufu processing. Int J Food Microbiol. 2010;136:368–371. doi: 10.1016/j.ijfoodmicro.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Antoniadou A. Outbreaks of zygomycosis in hospitals. Clin Microbiol Infect. 2009;15(Suppl 5):55–59. doi: 10.1111/j.1469-0691.2009.02982.x. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim AS, Gebremariam T, Liu MF, Chamilos G, Kontoyiannis DP, et al. Bacterial endosymbiosis is widely present among zygomycetes but does not contribute to the pathogenesis of mucormycosis. J Infect Dis. 2008;198:1083–1090. doi: 10.1086/591461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Partida-Martinez LP, Bandemer S, Ruchel R, Dannaoui E, Hertweck C. Lack of evidence of endosymbiotic toxin-producing bacteria in clinical Rhizopus isolates. Mycoses. 2008;51:266–269. doi: 10.1111/j.1439-0507.2007.01477.x. [DOI] [PubMed] [Google Scholar]
- 13.Gee JE, Glass MB, Lackner G, Helsel LO, Daneshvar M, et al. Characterization of Burkholderia rhizoxinica and B. endofungorum isolated from clinical specimens. PLoS ONE. 2011;6:e15731. doi: 10.1371/journal.pone.0015731. doi: 10.1371/journal.pone.0015731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuruo T, Oh-hara T, Lida H, Tsukagoshi S, Sato Z, et al. Rhizoxin, a macrocyclic lactone antibiotic, as a new antitumor agent against human and murine tumor cells and their vincristine-resistant sublines. Cancer Res. 1986;46:381–385. [PubMed] [Google Scholar]
- 15.McLeod HL, Murray LS, Wanders J, Setanoians A, Graham MA, et al. Multicentre phase II pharmacological evaluation of rhizoxin. Eortc early clinical studies (ECSG)/pharmacology and molecular mechanisms (PAMM) groups. Br J Cancer. 1996;74:1944–1948. doi: 10.1038/bjc.1996.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hertweck C. The biosynthetic logic of polyketide diversity. Angew Chem Int Ed Engl. 2009;48:4688–4716. doi: 10.1002/anie.200806121. [DOI] [PubMed] [Google Scholar]
- 17.Partida-Martinez LP, Hertweck C. A gene cluster encoding rhizoxin biosynthesis in “Burkholderia rhizoxina”, the bacterial endosymbiont of the fungus Rhizopus microsporus. Chembiochem. 2007;8:41–45. doi: 10.1002/cbic.200600393. [DOI] [PubMed] [Google Scholar]
- 18.Kusebauch B, Busch B, Scherlach K, Roth M, Hertweck C. Functionally distinct modules operate two consecutive alpha,beta→beta,gamma double-bond shifts in the rhizoxin polyketide assembly line. Angew Chem Int Ed Engl. 2009;49:1460–1464. doi: 10.1002/anie.200905467. [DOI] [PubMed] [Google Scholar]
- 19.Kusebauch B, Busch B, Scherlach K, Roth M, Hertweck C. Polyketide-chain branching by an enzymatic Michael addition. Angew Chem Int Ed Engl. 2009;48:5001–5004. doi: 10.1002/anie.200900277. [DOI] [PubMed] [Google Scholar]
- 20.Scherlach K, Partida-Martinez LP, Dahse H-M, Hertweck C. Antimitotic rhizoxin derivatives from a cultured bacterial endosymbiont of the rice pathogenic fungus Rhizopus microsporus. J Am Chem Soc. 2006;128:11529–11536. doi: 10.1021/ja062953o. [DOI] [PubMed] [Google Scholar]
- 21.Partida-Martinez LP, Monajembashi S, Greulich KO, Hertweck C. Endosymbiont-dependent host reproduction maintains bacterial-fungal mutualism. Curr Biol. 2007;17:773–777. doi: 10.1016/j.cub.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 22.Lackner G, Moebius N, Hertweck C. Endofungal bacterium controls its host by an hrp type III secretion system. ISME J. 2011;5:252–261. doi: 10.1038/ismej.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lackner G, Moebius N, Scherlach K, Partida-Martinez LP, Winkler R, et al. Global distribution and evolution of a toxinogenic Burkholderia-Rhizopus symbiosis. Appl Environ Microbiol. 2009;75:2982–2986. doi: 10.1128/AEM.01765-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lackner G, Moebius N, Partida-Martinez LP, Hertweck C. Complete genome sequence of Burkholderia rhizoxinica, the endosymbiont of Rhizopus microsporus. J Bacteriol. 2011;193:783–784. doi: 10.1128/JB.01318-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lackner G, Moebius N, Partida-Martinez LP, Boland S, Hertweck C. Evolution of an endofungal lifestyle: deductions from the Burkholderia rhizoxinica genome. BMC Genomics. 2011 doi: 10.1186/1471-2164-12-210. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leone MR, Lackner G, Silipo A, Lanzetta R, Molinaro A, et al. An unusual galactofuranose lipopolysaccharide warrants intracellular survival of toxin-producing bacteria in their fungal host. Angew Chem Int Ed Engl. 2010;49:7476–7480. doi: 10.1002/anie.201003301. [DOI] [PubMed] [Google Scholar]
- 27.Cornelis GR. The type III secretion injectisome. Nat Rev Microbiol. 2006;4:811–825. doi: 10.1038/nrmicro1526. [DOI] [PubMed] [Google Scholar]