Abstract
Hormones play a major role in regulating behavior and physiology, and their efficacy is often dependent on the temporal pattern in which they are secreted. Significant insights into the mechanisms underlying rhythmic hormone secretion have been gained from transgenic rodent models, suggesting that many of the body's rhythmic functions are regulated by a coordinated network of central and peripheral circadian pacemakers. Some neuroendocrine rhythms are driven by transcriptional-posttranslational feedback circuits comprising ‘core clock genes’, while others represent a cyclic cascade of neuroendocrine events. This review focuses on recent data from the rhesus macaque, a non-human primate model with high clinical translation potential. With primary emphasis on adrenal and gonadal steroids, it illustrates the rhythmic nature of hormone secretion, and discusses the impact that fluctuating hormone levels have on the accuracy of clinical diagnoses and on the design of effective hormone replacement therapies in the elderly. In addition, this minireview raises awareness of the rhythmic expression patterns shown by many genes, and discusses how this could impact interpretation of data obtained from gene profiling studies, especially from nocturnal rodents.
Key Words: Cortisol, Dehydroepiandrosterone sulfate, Leptin, Luteinizing hormone, Testosterone
Biological Clocks Play an Important Role in the Regulation of Physiology
Most organisms live in an environment that changes rhythmically. Some of these changes occur with a period of approximately 1 day (circadian), while others (seasonal) are much slower and occur gradually. Examples include rhythmic alterations in available light, ambient temperature and food availability, and to survive and reproduce under such changing conditions organisms have evolved various physiological adaptations that rely on internal clocks for their coordination. Furthermore, rhythmic output from these biological clocks enables temporal compartmentalization of biochemical processes, which like spatial compartmentalization enables many proteins to perform their cellular functions more effectively.
In mammals, the central circadian clock, located in the suprachiasmatic nucleus (SCN) of the hypothalamus, receives direct neuronal signals from photoreceptors located in the retina, and under normal conditions it is synchronized to the light-dark cycle. GABA and vasoactive intestinal peptide are thought to coordinate the synchronized firing of SCN neurons, which are capable of relaying circadian signals to other tissues, either through direct autonomic innervations or through humoral pathways involving vasopressin as an intermediary [1,2,3,4,5]. Although the SCN clearly serves as a master circadian pacemaker, genetic components of the underlying clock mechanism have been detected in other brain regions as well as most peripheral organs. This raises the possibility that circadian physiology is ultimately controlled by a hierarchy of circadian oscillators synchronized by the SCN, rather than by a single central circadian clock [4,7].
Major advances in our knowledge about the circadian clock mechanism have been made through the use of transgenic knockout rodent models [7,8,9,10,11]. In its essence, this central clock consists of autoregulatory transcriptional-posttranslational feedback loops. Genes encoding Period (Per1 and Per2) and Cryptochrome (Cry1 and Cry2) are activated by a dimer comprising Bmal1 and other PAS-domain proteins Clock or Npas2. In turn, Per and Cry proteins enter the cell nucleus where they ultimately inhibit activation of their own genes. Ancillary feedback loops involving Rev-erbα and Rora, as well as casein kinases (CKI∊ and CKIδ), serve to stabilize the circadian rhythmicity.
In contrast to these well-documented advances in basic chronobiology, clinical applications have been slow. On the one hand, the existence of endogenous biological clocks has been known for many centuries [12]. In addition, knowledge of circadian rhythms has been used to develop therapies for jetlag and seasonal affective disorder [13,14,15]. On the other hand, because of difficulties in maintaining human subjects under carefully controlled environmental conditions and difficulty in performing human gene expression studies, it is unclear if perturbed biological rhythms have a much more widespread impact on human physiology, especially during aging. Moreover, general clinical practice rarely takes underlying biological rhythms into consideration. For example, the diagnosis of hormone levels is often based on infrequent blood samples collected at inappropriate times of day, and therapeutic administration of hormones often follows non-physiological paradigms.
Because endocrine rhythms play a major role in regulating behavioral and physiological functions, it is likely that their perturbation contributes to the etiology of various human pathologies [16,17,18,19,20,21]. However, progress in understanding the underlying causal mechanisms has been hampered by the lack of suitable experimental animal models. On the one hand, rodents have proven to be excellent models for systematically dissecting the biochemical components that constitute the central circadian clock and it primary outputs, but their clinical translational potential is limited. Not only are they nocturnal, but they do not show consolidated sleep-wake patterns, Furthermore, the patterns of hormone secretion from their adrenal glands and gonads differ significantly from those of humans. This review tries to fill the gap in our knowledge by focusing on rhesus macaques (Macaca mulatta), which like humans are large, long-lived diurnal species and show similar consolidated sleep-wake patterns. Importantly from a women's health perspective, female rhesus macaques have similar menstrual cycles and show similar age-related changes in their reproductive and adrenal neuroendocrine axes [22,23,24,25]. In this mini-review, recent data from rhesus macaques are used to illustrate the dynamic nature of hormone secretion, and the implication of these findings for clinical and basic research is discussed.
Circadian Hormone Rhythms Help with Adaptation to Daily Environmental Change
Rhesus macaques can be readily maintained under a tightly-controlled environment (e.g. photoperiod, temperature, diet, and medication), thereby eliminating the extraneous variables and selection bias that are unavoidable in human clinical trials. Furthermore, because they are large, they readily lend themselves to serial blood collection, using an implanted vascular catheter and a swivel-based remote sampling system [26,27]. Importantly, this enables blood samples to be repeatedly collected from minimally restrained, non-sedated animals across the day and night, even when they are asleep; this can then be used to establish detailed 24-hour plasma hormone profiles. By simultaneously monitoring motor activity in the animals (e.g. using Actiwatch recorders; Philips-Respironics, Bend, Oreg., USA), the phase of the underlying hormone rhythms can be readily linked to the animal's circadian activity-rest cycle.
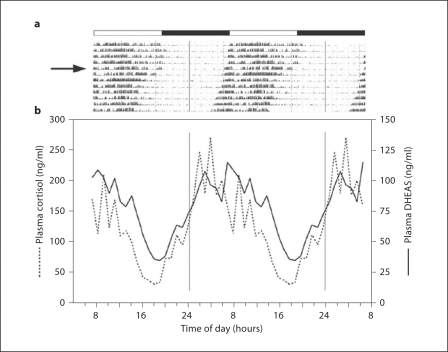
Circadian rhythms are defined as self-sustainable cyclic events that have a periodicity of approximately 24 h. Overt examples include daily cycles of activity-rest, alertness-sleep, and body temperature. Although these rhythms are usually entrained to an environmental cue (Zeitgeber), such as the daily light-dark cycle, many of them continue to be expressed even when environmental conditions are held constant [28,29]. In the rhesus macaque, like other mammals, many hormones have a 24-hour rhythm. However, not all of these rhythms are self-sustaining. For example, in humans, circulating growth hormone levels show a daily nocturnal peak, but this is not a true circadian rhythm because the peak stems directly from being asleep [30]. Similarly, the 24-hour prolactin rhythm is generally coupled to the sleep-wake cycle, although there is evidence for an underlying circadian rhythm component [30,31,32]. In contrast, adrenal steroids such as cortisol, dehydropiandrosterone (DHEA) and DHEA sulfate (DHEAS) not only have robust 24-hour patterns of release in rhesus macaques, but also they are self-sustaining, that is they continue to be clearly expressed even when the animals are maintained under continuous dim illumination. An example of this self-sustained rhythmicity is illustrated in figure 1. The motor activity data depicted in figure 1a were obtained from an adult rhesus macaque using an Actiwatch, and emphasize the highly entrained diurnal pattern of activity that occurs under fixed 12L:12D photoperiods [33,34,35,36]. In addition, the data show how the activity rhythm becomes free-running, with slight phase advancement, when the animal is exposed to continuous dim illumination (30 lx). Serial blood samples were remotely collected while the animals were maintained under the continuous dim illumination [26,27] and the plasma was assayed for cortisol and DHEAS; in both cases, 24-hour circadian rhythms were evident despite the absence of photoperiodic cues. Importantly, both of these hormones showed a peak that was associated with the onset of activity and a nadir that was associated with the onset of rest. The significance of the distinction between 24-hour and circadian hormone rhythms is that the latter are more likely to be regulated by a circadian molecular clock mechanisms and may act as auxiliary circadian pacemakers, helping to synchronize various physiological functions [37].
Fig. 1.
a Actogram from a representative female rhesus macaque, emphasizing diurnal activity and a consolidated nocturnal rest period; each row of data shows consecutive days of double-plotted activity. The first 5 days of the actogram show entrainment of the rhythm to a fixed 12L:12D light cycle, which is indicated by the white and black horizontal bars. The animal was then exposed to continuous dim illumination (DD, 30 lx), indicated by the arrow, and the rhythm began to free-run with a slight daily phase advancement. b Double-plotted plasma cortisol and DHEAS profiles, obtained by remote serial blood sampling after a week of exposure to DD. Note that both of these adrenal steroids show peaks that coincide with the animal's subjective dawn.
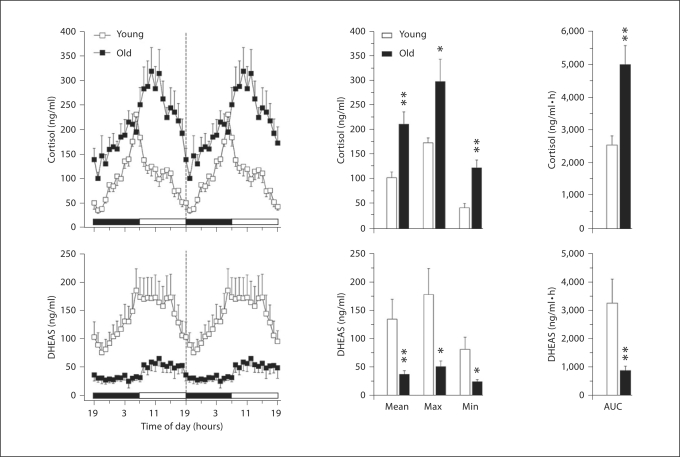
DHEAS and cortisol are both produced by the adrenal cortex and are two of the most abundant steroids in the circulation of adult humans and non-human primates. In both species, circulating DHEAS shows a profound age-related decrease [38,39,40,41,42]; this dramatic change is illustrated in figure 2, with respect to male rhesus macaques. In contrast, plasma cortisol levels do not show a decline and a clear 24-hour rhythm is still evident well into old age (fig. 2). Importantly, the age-related elevation of the cortisol baseline means that brain and peripheral organs, such as the liver, do not get a complete break from exposure to cortisol, which may predispose the elderly to insomnia and as well as to metabolic disorders. The exact physiological significance of an age-associated decrease in plasma DHEA and DHEAS levels is unclear. However, these steroids can attenuate the deleterious effects of cortisol and so the age-associated decline in DHEA:cortisol ratio is thought to underlie cognitive decline [43,44]. Additionally, lower levels of DHEA and DHEAS have been associated with cognitive disorders with a higher prevalence in the elderly, such as Alzheimer's disease [45] and depression [46]. In healthy old men [43] and postmenopausal women [47], elevated endogenous DHEAS levels have been linked to better cognitive performance. While studies of the frail elderly showed an inverse relationship between DHEAS and cognitive ability [48,49], a comparable study in non-human primates failed to disclose a similar association [50]. This difference in response could be due to the fact that frail non-human primates are usually not included in experiments, or that the timing of the single daily blood samples did not correspond to the animals’ circadian hormonal peak. In addition to direct actions within the central nervous system, DHEA and DHEAS may exert some of their beneficial effects indirectly, via intracrine conversion to sex steroids [51,52]. Many organs, including the brain, appear to express the enzymes necessary for this conversion, and it is well established that sex steroids can exert neuroprotective effects in brain areas such as the hippocampus [53]. Because estrogen can improve cognitive function and influence gene expression in various regions of the macaque brain [54,55,56,57,58], it is plausible that DHEA and DHEAS mediate some of their central actions via conversion to estrogen. It is also plausible that the age-related loss of humoral circadian signaling due to attenuated DHEA and DHEAS levels contributes to age-related desynchronization of peripheral oscillators and exacerbation of circadian dissonance in the elderly.
Fig. 2.
Effect of age on circulating 24-hour hormone patterns in male rhesus macaques. Left panels: Mean 24-hour plasma cortisol and DHEAS profiles from young (∼10 years old, n = 5) and old (∼26 years old, n = 6) males. Although the blood samples were collected over 24 h, from 19:00 to 19:00 h, the data have been double plotted (indicated by a vertical dashed line) to aid in the visualization of the night and day variations in hormone concentrations. The horizontal white and black bars on the abscissas correspond to the 12L:12D lighting schedule. Center panels: Analyses of age-related differences in mean, maximum, and minimum hormone values. Right panels: Analyses of age-related differences in the mean 24-hour area under the curve (AUC) of cortisol and DHEAS concentrations. Values are expressed as mean ± SEM. * p < 0.05, ** p < 0.01 [from [42], with permission].
The adipocyte-derived hormone, leptin, has been shown to have a 24-hour rhythm, with a nocturnal peak, in both lean and obese humans, as well as in individuals with type 2 diabetes [59,60,61]. Recent studies using rhesus macaques have shown that this rhythm is circadian, because it persists even under continuous dim illumination [62]. Interestingly, the rhythm is still evident in old male macaques but in peri- and postmenopausal females the difference between day- and nighttime plasma leptin levels becomes minimal. Because leptin is generally associated with suppression of appetite it makes biological sense for its peak to occur at night, which is when humans and rhesus macaques usually sleep. On the other hand, the physiological relevance of an age-related decline is unclear. One possibility is that disruption of the circadian leptin rhythm contributes to the development of metabolic disorders and obesity [63,64,65,66] and interferes with the maintenance of bone mass [67,68].
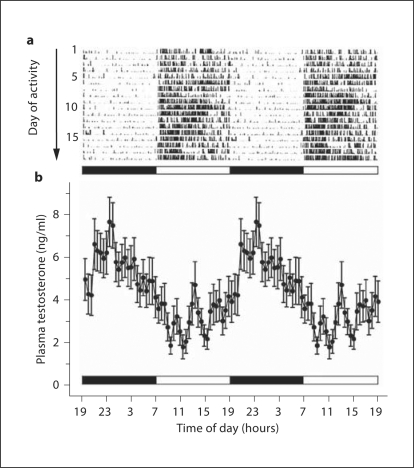
Another hormone that shows a pronounced 24-hour rhythm in both men [69,70,71] and adult male rhesus macaques [72,73,74] is testosterone. Like leptin, the plasma testosterone rhythm shows a nocturnal peak and is associated with sleep (fig. 3a, b); this contrasts with the timing of the daily cortisol and DHEAS peaks, which occur in the morning in association with onset of activity (fig. 1, 2). In this particular study the blood samples were remotely collected at 30-min intervals for 24 h, and the testosterone profiles depict mean values from 10 animals. Although the 24-hour rhythm is clearly evident there is much underlying fluctuation in the testosterone levels because this steroid is released in an episodic manner that corresponds to the underlying ultradian pattern of LH release [24]. Consequently, it is extremely difficult to accurately assess testicular endocrine function, or diagnose age-related changes, in individuals based on single time point testosterone measurements. Instead, multiple samples need to be collected, and ideally as close to the nocturnal peak as possible [72,73,74,75].
Fig. 3.
Characteristic plasma testosterone rhythm in adult male rhesus macaques, revealed by remote serial blood sampling every 30 min for 24 h. a Double-plotted actogram from a representative male rhesus macaque, showing diurnal activity that is entrained to the 12L:12D light cycle (indicated by the white and black horizontal bars). b Double-plotted mean plasma testosterone levels from 10 animals (±SEM). Note that the 24-hour rhythm is superimposed on ultradian testosterone level fluctuations that stem from an underlying pulsatile pattern of LH release. Note also that in contrast to the plasma cortisol and DHEAS rhythms, which show a peak in the early morning (cf. fig. 1), the testosterone peak occurs during the night when the animals are asleep.
Circadian Hormone Rhythms Are Influenced by Photoperiod and Season
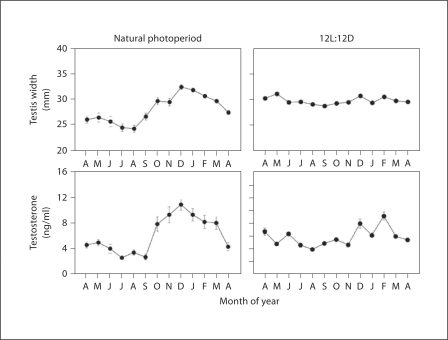
In humans, seasonal variations have been reported for blood pressure, immune response, birth rate, and sleep duration, as well as for behavioral traits associated with seasonal affective disorders, bulimia nervosa, anorexia, and suicide [76,77,78,79,80]. Although the underlying neuroendocrine mechanisms are unclear they are thought to be linked to seasonal circadian neuroendocrine changes. In this context, the pineal hormone, melatonin, has been the most widely studied, because its circadian pattern of release is markedly affected by the photoperiod [13]. As winter approaches, the duration of the night period becomes longer, causing more melatonin to be secreted. This provides a useful neuroendocrine cue that environmental conditions are changing, and this is exploited by temperate zone mammals to initiate various physiological adaptations. For example, long-day breeding species, such as hamsters and voles, rely on the short-day melatonin profile to terminate their breeding season. In contrast, larger species with a gestation periods of 5–6 months, such as sheep and deer, use it to initiate their breeding season. When maintained under natural photoperiods, rhesus macaques also show seasonal reproductive cycles with breeding confined to the autumn and winter [81,82,83]. In the males, testicular size and serum testosterone levels are markedly lower during the non-breeding season (fig. 4). Importantly, however, if the animals are housed indoors under fixed 12L:12D photoperiods they do not show an annual decrease in these reproductive parameters, instead they maintain large testes and elevated testosterone throughout the year, like men [84,85,86,87,88]. This suggests that some seasonal neuroendocrine rhythms might simply represent direct responses to changing environmental cues, or to a chain of neuroendocrine events that are analogous to those comprising the menstrual cycle, rather than being driven by a circannual intracellular molecular clock mechanism.
Fig. 4.
Seasonal reproductive rhythms in male rhesus macaques. Adult males were maintained either under natural Oregon day lengths (i.e. at latitude 45°N) (left panels) or were housed indoors under fixed 12L:12D photoperiods (right panels). A single serum sample was collected from each animal (n = 5 per group) in the early morning, once per month across the entire year, and subsequently assayed for testosterone. Annual changes in testis size were monitored using calipers to measure testicular width through the scrotal wall. The results (mean ± SEM) corroborate what is known about the reproductive physiology of rhesus macaques, namely that they are short-day seasonal breeders when maintained under natural photoperiods but not when maintained indoors under fixed 12L:12D photoperiods.
Because adrenal steroids play a major role in regulating behavior and physiology, their seasonal profiles have received much attention. However, the human data for cortisol are largely inconclusive. Some studies have reported seasonal differences [89,90,91,92,93], whereas others have failed to do so [94,95,96,97]. Similarly, some studies have reported seasonal differences in DHEAS levels [98,99,100], whereas one study found none [101]. In a recent examination of 24-hour plasma cortisol or DHEAS profiles of ovariectomized rhesus macaques, no effect of photoperiod was found on the mean or peak hormone levels. However, there was a marked phase advancement of both hormonal rhythms in short days, reflecting a similar phase advancement of the daily motor activity rhythm [35]. Furthermore, significant differences were detected in the gene expression profiles of the adrenal gland under different photoperiodic conditions. Together, these data reinforce the view that normal behavior and physiology is dependent on the maintenance of specific phase relationships between different neuroendocrine rhythms [20], and that these relationships may change under different environmental conditions, as well as during aging.
Many Genes Exhibit a 24-Hour Expression Pattern
Gene expression profiling in the rhesus macaque adrenal gland, using Affymetrix GeneChip arrays, has shown that many of the genes associated with rhythmic production of adrenal steroids have a rhythmic 24-hour expression profile, and that the adrenal gland itself expresses a circadian core-clock mechanism similar to that expressed in the SCN [33]. Equally important, a significant number of the genes showed a 24-hour expression pattern; some of these genes showed a peak of expression in the middle of the night while others showed a peak in the middle of the day (fig. 5). Subsequent studies showed that day length can also influence the expression of a wide variety of genes in the rhesus macaque adrenal gland [35] (fig. 6). Some of the main genes affected by a 4-hour photoperiodic change included those associated with development, metabolism and immune function (fig. 6, lower panels). Other rhesus macaque studies have shown that gene expression within the rhesus macaque brain can be significantly affected by ovarian steroids, and hence by the phase of the menstrual cycle [102]. Together, these findings emphasize the importance of collecting terminal tissue samples at the most appropriate time of the day, month, or season. This, however, can be problematic as some genes of interest may show a peak of expression in the middle of the night while others may show a peak in the middle of the day. Consequently, if necropsies are performed on experimental animals exclusively during the daytime, when some genes are exhibiting a nadir in their expression rhythm, important changes could be missed [102]. Furthermore, the situation is compounded when making inferences from rodent studies to those of humans, because daytime necropsies correspond to the subjective night of nocturnal rodents but to the subjective day of rhesus macaques and humans. Awareness of neuroendocrine rhythms, and the underlying rhythmic expression of many genes, represents a key aspect of effective experimental design.
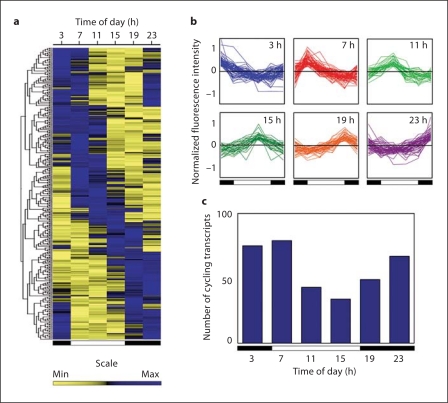
Fig. 5.
Temporal gene expression profiles in the rhesus macaque adrenal gland, emphasizing marked 24-hour differences. a Hierarchical clustering of the 335 oscillating transcripts. Each column represents a time point and each row represents a gene. Relationships between genes are depicted as a tree, with branch length reflecting the degree of similarity in time courses between the genes. b Gene expression profiles showing different phases across 24 h; the data have been normalized such that the medial signal intensity for each gene across all time points is 0. c Distribution of cycling transcripts across 24 h. In all panels the white and black bars represent day- and nighttime, respectively. Figure from Lemos et al. [33], with permission, The Endocrine Society©, 2006.
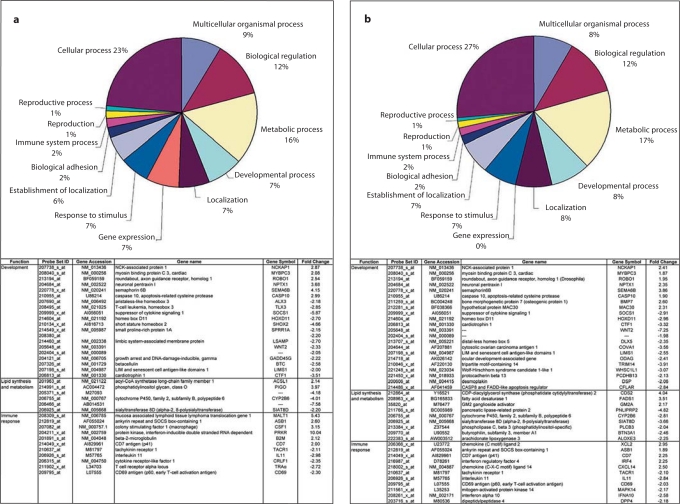
Fig. 6.
Effect of day length on adrenal gland gene expression in the rhesus macaque. Ovariectomized animals were maintained under short winter photoperiods (8L:16D), spring/summer photoperiods (12L:12D), or long summer photoperiods (16L:8D), for 10 weeks. Adrenal gland RNA was hybridized to the Affymetrix human HG_U133A GeneChip®, and the data were analyzed using the algorithm MAS 5.0. Upper panels: Functional clustering of genes found to be differentially expressed between a 8L:16D and 12L:12D and between b 12L:12D and 16L:8D photoperiodic exposures. Lower panels: Examples of genes involved in development, lipid synthesis and metabolism, and immune response that showed significant (p < 0.05) photoperiod-induced expression changes. Data adapted from Lemos et al. [35], with permission.
Clinical Implications and Future Perspectives
In humans, almost all behavioral and physiological functions occur on a rhythmic basis [103]. Given that hormones play a key role in the cross-talk between different systems, it is likely that perturbation of their rhythmic release contributes to the pathophysiology of a wide range of human disorders, especially during aging [40,103,104,105,106]. Before appropriate hormone supplementation or pharmaceutical intervention is prescribed, it is imperative that the underlying perturbed hormone levels are correctly identified. This may require collection of serial blood samples at specific times of the day, phase of menstrual cycle as well as time of year.
For example, with reference to testosterone rhythm depicted in figure 3, if blood samples are collected in the late afternoon they are likely to show lower testosterone concentrations than samples collected in the early morning. Furthermore, unless several blood samples are collected there is a high risk of missing one of the underlying ultradian testosterone pulses, thereby leading to further underestimation of testosterone production and release. The situation can be even more complicated if the individual has just returned from a long trip and is suffering from lag, or if he is a nightshift worker; in both cases the individual's testosterone rhythm could be significantly phase shifted and so the early morning may no longer be the most appropriate time of day for the collection of serial blood samples. This has important clinical implications, because in male rhesus macaques [72,73] and men [107,108,109,110,111,112] circulating testosterone levels decline during aging. Whether this decline represents a male andropause [113] is questionable because the age-related decline in testosterone levels is less abrupt or severe than the decline in ovarian steroids that occurs during female menopause [23,25], also the functional significance of a moderate age-related testosterone decline remains to be elucidated. Nevertheless, it is plausible that a well-defined nocturnal testosterone peak contributes to the overall maintenance of circadian physiology, including sleep patterns. Consequently, clinical treatment of low testosterone levels through androgen supplementation should ideally follow the underlying physiological 24-hour profile. For practical reasons, this is rarely the case, however. Common androgen supplementation paradigms typically involve long-term continuous release capsules, which have the advantage of low maintenance but which completely obliterate the 24-hour rhythm. Other paradigms involve cyclic transdermal delivery of testosterone, via the daily application of gels. However, these are generally applied in the morning rather than at night, to avoid accidental transfer of the steroid to a sleeping partner; unfortunately, this means that the daily plasma testosterone peak generated by the gel can be markedly out of phase with the endogenous testosterone rhythm. The long-term impact of these non-physiological androgen supplementation paradigms is unclear, and alternative approaches may prove to be more beneficial to overall physiology. An interesting novel approach has recently been demonstrated in men [114,115], which involves oral administration of micronized testosterone in sesame oil. Normally, orally administered testosterone has little physiological potency because after being taken up by the gut it is immediately transported to the liver via the hepatic portal vein and then passes back into the gut rather than into the general circulation. It appears that much of this enterohepatic circulation of testosterone can be bypassed if the steroid is mixed with sesame oil. Although the exact mechanism is unclear, it may involve preferential absorption by the lymphatics, bypassing the liver and reaching the circulation via the thoracic duct. Studies have shown that this administration paradigm can more closely mimic the natural 24-hour plasma testosterone rhythm [114,115].
Similarly, for DHEAS one would expect there to be an optimal time of day for supplementation of this hormone in the elderly. In the USA, DHEA is widely available to the public without prescription as a dietary supplement. DHEA is readily converted to DHEAS and vice versa by sulfyl transferase and steroid sulfatase enzymes, respectively, and because it has a much shorter half-life than DHEAS it shows a more pronounced 24-hour rhythm [116]. With reference to the DHEAS rhythm depicted in figures 1 and 2, it is clear that early morning supplementation with exogenous DHEA represents a more physiological paradigm than a similar DHEA dose in the early evening, and consequently morning DHEA supplementation is more likely to harmonize with the body's circadian physiology.
Acknowledgements
This work was supported by National Institutes of Health Grants AG-029612, AG-036670, HD-029186 and RR-000163.
References
- 1.Okamura H. Clock genes in cell clocks: roles, actions, and mysteries. J Biol Rhythms. 2004;19:388–399. doi: 10.1177/0748730404269169. [DOI] [PubMed] [Google Scholar]
- 2.Karatsoreos IN, Silver R. The neuroendocrinology of the suprachiasmatic nucleus as a conductor of body time in mammals. Endocrinology. 2007;148:5640–5647. doi: 10.1210/en.2007-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maywood ES, O'Neill JS, Chesham JE, Hastings MH. The circadian clockwork of the suprachiasmatic nuclei – analysis of a cellular oscillator that drives endocrine rhythms. Endocrinology. 2007;148:5624–5634. doi: 10.1210/en.2007-0660. [DOI] [PubMed] [Google Scholar]
- 4.Mendoza J, Challet E. Brain clocks: from the suprachiasmatic nuclei to a cerebral network. Neuroscientist. 2009;15:477–488. doi: 10.1177/1073858408327808. [DOI] [PubMed] [Google Scholar]
- 5.Bonnefont X. Circadian timekeeping and multiple timescale neuroendocrine rhythms. J Neuroendocrinol. 2010;22:209–216. doi: 10.1111/j.1365-2826.2010.01955.x. [DOI] [PubMed] [Google Scholar]
- 6.Kalsbeek A, Fliers E, Hoffman MA, Swaab DF, Buijs RM. Vasopressin and the output of the hypothalamic biological clock. J Neuroendocrinol. 2010;22:362–372. doi: 10.1111/j.1365-2826.2010.01956.x. [DOI] [PubMed] [Google Scholar]
- 7.Reppert S, Weaver D. Circadian timing. In: Squire LR, editor. Fundamental Neuroscience. San Diego: Academic Press; 2008. pp. 931–958. [Google Scholar]
- 8.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 9.Aton SJ, Herzog ED. Come together, right … now: synchronization of rhythms in a mammalian circadian clock. Neuron. 2005;48:531–534. doi: 10.1016/j.neuron.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hastings M, O'Neill JS, Maywood ES. Circadian clocks: regulators of endocrine and metabolic rhythms. J Endocrinol. 2007;195:187–198. doi: 10.1677/JOE-07-0378. [DOI] [PubMed] [Google Scholar]
- 11.Gan EH, Quinton R. Physiological significance of the rhythmic secretion of hypothalamic and pituitary hormones. Prog Brain Res. 2010;181:111–126. doi: 10.1016/S0079-6123(08)81007-2. [DOI] [PubMed] [Google Scholar]
- 12.Aschoff J. ‘Twenty years on’; In: Follett BK, Follett DE, editors. Biological Clocks in Seasonal Reproductive Cycles. Bristol: Scientechnica; 1981. pp. 277–288. [Google Scholar]
- 13.Urbanski HF. Influence of light and the pineal gland on biological rhythms. In: Conn PM, Freeman ME, editors. Neuroendocrinology in Physiology and Medicine. Totowa: Human Press; 1999. pp. 405–420. [Google Scholar]
- 14.Skene DJ, Arendt J. Human circadian rhythms: physiological and therapeutic relevance of light and melatonin. Ann Clin Biochem. 2006;43:344–353. doi: 10.1258/000456306778520142. [DOI] [PubMed] [Google Scholar]
- 15.Arendt J, Van Someren EJ, Appleton R, Skene DJ, Akerstedt T. Clinical update: melatonin and sleep disorders. Clin Med. 2008;8:381–383. doi: 10.7861/clinmedicine.8-4-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Cauter E, Shapiro ET, Tillil H, Polonsky KS. Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol. 1992;262:467–475. doi: 10.1152/ajpendo.1992.262.4.E467. [DOI] [PubMed] [Google Scholar]
- 17.Cardinali DP, Esquifino AI. Biological rhythms in neuroendocrinology. In: Cardinali DP, Pandi-Perumal SR, editors. Neuroendocrine Correlates of Sleep/Wakefulness. New York: Springer; 2005. pp. 59–86. chapt 4. [Google Scholar]
- 18.Cutolo M, Maestroni GJ, Otsa K, Aakre O, Villaggio B, Capellino S, Montagna P, Fazzuoli L, Veldi T, Peets T, Hertens E, Sulli A. Circadian melatonin and cortisol levels in rheumatoid arthritis patients in winter time: a north and south Europe comparison. Ann Rheum Dis. 2005;64:212–216. doi: 10.1136/ard.2004.023416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewy AJ. Melatonin and human chronobiology. Cold Spring Harb Symp Quant Biol. 2007;72:623–636. doi: 10.1101/sqb.2007.72.055. [DOI] [PubMed] [Google Scholar]
- 20.Phillips ML. Circadian rhythms: of owls, larks and alarm clocks: could out-of-sync body clocks be contributing to human disease? Nature. 2009;458:142–144. doi: 10.1038/458142a. [DOI] [PubMed] [Google Scholar]
- 21.Maury E, Ramsey KM, Bass J. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res. 2010;106:447–462. doi: 10.1161/CIRCRESAHA.109.208355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woller MJ, Everson-Binotto G, Nichols E, Acheson A, Keen KL, Bowers CY, Terasawa E. Aging-related changes in release of growth hormone and luteinizing hormone in female rhesus monkeys. J Clin Endocrinol Metab. 2002;87:5160–5167. doi: 10.1210/jc.2002-020659. [DOI] [PubMed] [Google Scholar]
- 23.Downs JL, Urbanski HF. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macaca mulatta) Biol Reprod. 2006;75:539–546. doi: 10.1095/biolreprod.106.051839. [DOI] [PubMed] [Google Scholar]
- 24.Plant TM, Witchel SF. Puberty in nonhuman primates and humans. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. ed 3. St Louis: Elsevier; 2006. pp. 2177–2230. [Google Scholar]
- 25.Zeleznik AJ, Pohl CR. Control of follicular development, corpus luteum function, the maternal recognition of pregnancy, and the neuroendocrine regulation of the menstrual cycle in higher primates. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. ed 3. St Louis: Elsevier; 2006. pp. 2449–2510. [Google Scholar]
- 26.Urbanski HF, Garyfallou VT, Kohama SG, Hess DL. Alpha-adrenergic receptor antagonism and N-methyl-D-aspartate-induced luteinizing hormone release in female rhesus macaques. Brain Res. 1997;744:96–104. doi: 10.1016/s0006-8993(96)01083-9. [DOI] [PubMed] [Google Scholar]
- 27.Urbanski HF. Circadian variation in the physiology and behavior of humans and nonhuman primates. In: Raber, editor. Animal Models of Behavioral Analysis. New York: Springer; 2011. pp. 217–235. chapt 9. [Google Scholar]
- 28.Turek FW, Van Cauter E. Rhythms in reproduction. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. ed 2. New York: Raven Press; 1994. pp. 487–540. [Google Scholar]
- 29.Wayne NL. The neuroendocrine control of circadian rhythms. In: Conn PM, Freeman ME, editors. Neuroendocrinology in Physiology and Medicine. Totowa: Human Press; 1999. pp. 421–433. [Google Scholar]
- 30.Van Cauter E. Diurnal and ultradian rhythms in human endocrine function: a minireview. Horm Res. 1990;34:45–53. doi: 10.1159/000181794. [DOI] [PubMed] [Google Scholar]
- 31.Désir D, Van Cauter E, L'Hermite M, Refetoff S, Jadot C, Caufriez A, Copinschi G, Robyn C. Effects of ‘jet lag’ on hormonal patterns. III. Demonstration of an intrinsic circadian rhythmicity in plasma prolactin. J Clin Endocrinol Metab. 1982;55:849–857. doi: 10.1210/jcem-55-5-849. [DOI] [PubMed] [Google Scholar]
- 32.Van Cauter E, Refetoff S. Multifactorial control of the 24-hour secretory profiles of pituitary hormones. J Endocrinol Invest. 1985;8:381–391. doi: 10.1007/BF03348519. [DOI] [PubMed] [Google Scholar]
- 33.Lemos DR, Downs JL, Urbanski HF. Twenty-four hour rhythmic gene expression in the rhesus macaque adrenal gland. Mol Endocrinol. 2006;20:1164–1176. doi: 10.1210/me.2005-0361. [DOI] [PubMed] [Google Scholar]
- 34.Downs JL, Dunn MR, Borok E, Shanabrough M, Horvath TL, Hohama SG, Urbanski HF. Orexin neuronal changes in the locus coeruleus of the aging rhesus macaque. Neurobiol Aging. 2007;28:1286–1295. doi: 10.1016/j.neurobiolaging.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 35.Lemos DR, Downs JL, Raitiere MN, Urbanski HF. Photoperiodic modulation of adrenal gland function in the rhesus macaque: effect on 24-hour plasma cortisol and dehydroepiandrosterone sulfate rhythms and adrenal gland gene expression. J Endocrinol. 2009;201:275–285. doi: 10.1677/JOE-08-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haley GE, Landauer N, Renner L, Weiss A, Hooper K, Urbanski HF, Kohama SG, Neuringer M, Raber J. Circadian activity associated with spatial learning and memory in aging rhesus monkeys. Exp Neurol. 2009;217:55–62. doi: 10.1016/j.expneurol.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- 38.Orentreich N, Brind JL, Vogelman JH, Andres R, Baldwin H. Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J Clin Endocrinol Metab. 1992;75:1002–1004. doi: 10.1210/jcem.75.4.1400863. [DOI] [PubMed] [Google Scholar]
- 39.Labrie F, Bélanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997;82:2396–2402. doi: 10.1210/jcem.82.8.4160. [DOI] [PubMed] [Google Scholar]
- 40.Wise PM. Neuroendocrine correlates of aging. In: Conn PM, Freeman ME, editors. Neuroendocrinology in Physiology and Medicine. Totowa: Human Press; 1999. pp. 371–387. [Google Scholar]
- 41.Urbanski HF, Downs JL, Garyfallou VT, Mattison JA, Lane MA, Roth GS, Ingram DK. Effect of caloric restriction on the 24-hour plasma DHEAS and cortisol profiles of young and old male rhesus macaques. Ann NY Acad Sci. 2004;1019:443–447. doi: 10.1196/annals.1297.081. [DOI] [PubMed] [Google Scholar]
- 42.Downs JL, Mattison JA, Ingram DK, Urbanski HF. Effect of age and caloric restriction on circadian adrenal steroid rhythms in rhesus macaques. Neurobiol Aging. 2008;29:1412–1422. doi: 10.1016/j.neurobiolaging.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Niekerk JK, Huppert JA, Herbert J. Salivary cortisol and DHEA: association with measures of cognition and well-being in normal older men, and effects of three months of DHEA supplementation. Psychoneuroendocrinology. 2001;26:591–612. doi: 10.1016/s0306-4530(01)00014-2. [DOI] [PubMed] [Google Scholar]
- 44.Karishma KK, Herbert J. Dehydroepiandrosterone stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons, and prevents corticosterone-induced suppression. Eur J Neurosci. 2002;16:445–453. doi: 10.1046/j.1460-9568.2002.02099.x. [DOI] [PubMed] [Google Scholar]
- 45.Weill-Engerer S, David JP, Sazdovitch V, Liere P, Eychenne B, Pianos A, Schumacher M, Delacourte A, Baulieu EE, Akwa Y. Neurosteroid quantification in human brain regions: comparison between Alzheimer's and nondemented patients. J Clin Endocrinol Metab. 2002;87:5138–5143. doi: 10.1210/jc.2002-020878. [DOI] [PubMed] [Google Scholar]
- 46.Michael A, Jenaway A, Paykel ES, Herbert J. Altered salivary dehydroepiandrosterone levels in major depression in adults. Biol Psychiatry. 2000;48:989–995. doi: 10.1016/s0006-3223(00)00955-0. [DOI] [PubMed] [Google Scholar]
- 47.Davis SR, Shah SM, McKenzie DP, Kulkarni J, Davison SL, Bell RJ. Dehydroepiandrosterone sulfate levels are associated with more favorable cognitive function in women. J Clin Endocrinol Metab. 2008;93:801–808. doi: 10.1210/jc.2007-2128. [DOI] [PubMed] [Google Scholar]
- 48.Morrison MF, Katz IR, Parmelee P, Boyce AA, TenHave T. Dehydroepiandrosterone sulfate and psychiatric and laboratory measures of frailty in a residential care population. Am J Geriatr Psychiatry. 1998;6:277–284. [PubMed] [Google Scholar]
- 49.Morrison MF, Redei E, TenHave T, Parmelee P, Boyce AA, Sinha PS, Katz IR. Dehydroepiandrosterone sulfate and psychiatric measures in a frail, elderly residential care population. Biol Psychiatry. 2000;47:144–150. doi: 10.1016/s0006-3223(99)00099-2. [DOI] [PubMed] [Google Scholar]
- 50.Herndon JG, Lacreuse A, Ladinsky E, Killiany RJ, Rosene DL, Moss MB. Age-related decline in DHEAS is not related to cognitive impairment in aged monkeys. Neuroreport. 1999;10:3507–3511. doi: 10.1097/00001756-199911260-00008. [DOI] [PubMed] [Google Scholar]
- 51.Labrie F. Intracrinology. Mol Cell Endocrinol. 1991;78:C113–C118. doi: 10.1016/0303-7207(91)90116-a. [DOI] [PubMed] [Google Scholar]
- 52.Sorwell K, Urbanski HF. Dehydroepiandrosterone and age-related cognitive decline. Age (Dordr) 2010;32:61–67. doi: 10.1007/s11357-009-9113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- 54.Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hao J, Rapp PR, Janssen WG, Lou W, Lasley BL, Hof PR, Morrison JH. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci USA. 2007;104:11465–11470. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eghlidi DH, Haley GE, Noriega NC, Kohama SG, Urbanski HF. Influence of age and 17β-estradiol on kisspeptin, neurokinin B, and prodynorphin gene expression in the arcuate-median eminence of female rhesus macaques. Endocrinology. 2010;151:3783–3794. doi: 10.1210/en.2010-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Noriega NC, Eghlidi DH, Garyfallou VT, Kohama SG, Kryger SG, Urbanski HF. Influence of 17β-estradiol and progesterone on GABAergic gene expression in the arcuate nucleus, amygdala and hippocampus of the rhesus macaque. Brain Res. 2010;1307:28–42. doi: 10.1016/j.brainres.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang AC, Hara Y, Janssen WG, Rapp PR, Morrison JH. Synaptic estrogen receptor-α levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. J Neurosci. 2010;30:12770–12776. doi: 10.1523/JNEUROSCI.3192-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sinha MK, Ohannesian JP, Heiman ML, Kriauciunas A, Stephens TW, Magosin S, Marco C, Caro JF. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97:1344–1347. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Langendonk JG, Pijl H, Toornvliet AC, Burggraaf J, Frölich M, Schoemaker RC, Doornbos J, Cohen AF, Meinders AE. Circadian rhythm of plasma leptin levels in upper and lower body obese women: influence of body fat distribution and weight loss. J Clin Endocrinol Metab. 1998;83:1706–1712. doi: 10.1210/jcem.83.5.4717. [DOI] [PubMed] [Google Scholar]
- 61.Simon C, Gronfier C, Schlienger JL, Brandenberger G. Circadian and ultradian variations of leptin in normal man under continuous enteral nutrition: relationship to sleep and body temperature. J Clin Endocrinol Metab. 1998;83:1893–1899. doi: 10.1210/jcem.83.6.4864. [DOI] [PubMed] [Google Scholar]
- 62.Downs JL, Urbanski HF. Aging-related sex-dependent loss of the circulating leptin 24-hour rhythm in the rhesus monkey. J Endocrinol. 2006;190:117–127. doi: 10.1677/joe.1.06745. [DOI] [PubMed] [Google Scholar]
- 63.Laughlin GA, Yen SS. Hypoleptinemia in women athletes: absence of a diurnal rhythm with amenorrhea. J Clin Endocrinol Metab. 1997;82:318–321. doi: 10.1210/jcem.82.1.3840. [DOI] [PubMed] [Google Scholar]
- 64.Matkovic V, Ilich JZ, Badenhop NE, Skugor M, Clairmont A, Klisovic D, Landoll JD. Gain in body fat is inversely related to the nocturnal rise in serum leptin level in young females. J Clin Endocrinol Metab. 1997;82:1368–1372. doi: 10.1210/jcem.82.5.3917. [DOI] [PubMed] [Google Scholar]
- 65.Balligand JL, Brichard SM, Brichard V, Desager JP, Lambert M. Hypoleptinemia in patients with anorexia nervosa: loss of circadian rhythm and unresponsiveness to short-term refeeding. Eur J Endocrinol. 1998;138:415–420. doi: 10.1530/eje.0.1380415. [DOI] [PubMed] [Google Scholar]
- 66.Støving RK, Vinten J, Handberg A, Ebbesen EN, Hangaard J, Hansen-Nord M, Kristiansen J, Hagen C. Diurnal variation of the serum leptin concentration in patients with anorexia nervosa. Clin Endocrinol (Oxf) 1998;48:761–768. doi: 10.1046/j.1365-2265.1998.00434.x. [DOI] [PubMed] [Google Scholar]
- 67.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 68.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 69.Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278–1281. doi: 10.1210/jcem-56-6-1278. [DOI] [PubMed] [Google Scholar]
- 70.Tenover JS, Matsumoto AM, Clifton DK, Bremner WJ. Age-related alterations in the circadian rhythms of pulsatile luteinizing hormone and testosterone secretion in healthy men. J Gerontol. 1988;43:M163–M169. doi: 10.1093/geronj/43.6.m163. [DOI] [PubMed] [Google Scholar]
- 71.Cooke RR, McIntosh JE, McIntosh RP. Circadian variation in serum free and non-SHBG-bound testosterone in normal men: measurements, and simulation using a mass action model. Clin Endocrinol (Oxf) 1993;39:163–171. doi: 10.1111/j.1365-2265.1993.tb01769.x. [DOI] [PubMed] [Google Scholar]
- 72.Garyfallou VT, Brown DI, Downs JL, James LJ, Urbanski HF. Effect of aging on circulating testosterone levels and on the expression of genes associated with testosterone biosynthesis. Endocr Soc Abstr. 2005:OR4–6. [Google Scholar]
- 73.Schlatt S, Pohl CR, Ehmcke J, Ramaswamy S. Age-related changes in diurnal rhythms and levels of gonadotropins, testosterone, and inhibin B in male rhesus monkeys (Macaca mulatta) Biol Reprod. 2008;79:93–99. doi: 10.1095/biolreprod.107.066126. [DOI] [PubMed] [Google Scholar]
- 74.Sitzmann BD, Leone EH, Mattison JA, Ingram DK, Roth GS, Urbanski HF, Zelinski MB, Ottinger MA. Effects of moderate calorie restriction on testosterone production and semen characteristics in young rhesus macaques (Macaca mulatta) Biol Reprod. 2010;83:635–640. doi: 10.1095/biolreprod.110.084186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Urbanski HF, Pau KY. A biphasic developmental pattern of circulating leptin in the male rhesus macaque (Macaca mulatta) Endocrinology. 1998;139:2284–2286. doi: 10.1210/endo.139.5.5962. [DOI] [PubMed] [Google Scholar]
- 76.Bronson FH, Heideman PD. Seasonal regulation of reproduction in mammals. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. ed 2. New York: Raven Press; 1994. pp. 541–583. [Google Scholar]
- 77.Malpaux B. The neuroendocrine control of seasonal rhythms. In: Conn PM, Freeman ME, editors. Neuroendocrinology in Physiology and Medicine. Totowa: Human Press; 1999. pp. 435–451. [Google Scholar]
- 78.Wehr TA. Photoperiodism in humans and other primates: evidence and implications. J Biol Rhythms. 2001;16:348–364. doi: 10.1177/074873001129002060. [DOI] [PubMed] [Google Scholar]
- 79.Bronson FH. Are humans seasonally photoperiodic? J Biol Rhythms. 2004;19:180–192. doi: 10.1177/0748730404264658. [DOI] [PubMed] [Google Scholar]
- 80.Malpaux B. Seasonal regulation of reproduction in mammals. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. ed 3. St Louis: Elsevier; 2006. pp. 2231–2281. [Google Scholar]
- 81.Walker ML, Wilson ME, Gordon TP. Endocrine control of the seasonal occurrence of ovulation in rhesus monkeys housed outdoors. Endocrinology. 1984;114:1074–1081. doi: 10.1210/endo-114-4-1074. [DOI] [PubMed] [Google Scholar]
- 82.Chik CL, Almeida OF, Libré EA, Booth JD, Renquist D, Merriam GR. Photoperiod-driven changes in reproductive function in male rhesus monkeys. J Clin Endocrinol Metab. 1992;74:1068–1074. doi: 10.1210/jcem.74.5.1569154. [DOI] [PubMed] [Google Scholar]
- 83.Urbanski HF. Excitatory amino acids and the control of seasonal breeding. In: Brann DW, Mahesh VB, editors. Excitatory Amino Acids: Their Role in Neuroendocrine Function. Boca Raton: CRC Press; 1995. pp. 253–279. [Google Scholar]
- 84.Roenneberg T, Aschoff J. Annual rhythm of human reproduction. I. Biology, sociology, or both? J Biol Rhythms. 1990;5:195–216. doi: 10.1177/074873049000500303. [DOI] [PubMed] [Google Scholar]
- 85.Roenneberg T, Aschoff J. Annual rhythm of human reproduction. II. Environmental correlations. J Biol Rhythms. 1990;5:217–239. doi: 10.1177/074873049000500304. [DOI] [PubMed] [Google Scholar]
- 86.Meriggiola MC, Noonan ES, Paulsen CA, Bremner WJ. Annual patterns of luteinizing hormone, follicle-stimulating hormone, testosterone and inhibin in normal men. Hum Reprod. 1996;11:248–252. doi: 10.1093/humrep/11.2.248. [DOI] [PubMed] [Google Scholar]
- 87.Andersson AM, Carlsen E, Petersen JH, Skakkebæk NE. Variation in levels of serum inhibin B, testosterone, estradiol, luteinizing hormone, follicle-stimulating hormone, and sex hormone-binding globulin in monthly samples from healthy men during a 17-month period: possible effects of seasons. J Clin Endocrinol Metab. 2003;88:932–937. doi: 10.1210/jc.2002-020838. [DOI] [PubMed] [Google Scholar]
- 88.Brambilla DJ, O'Donnell AB, Matsumoto AM, McKinlay JB. Lack of seasonal variation in serum sex hormone levels in middle-aged to older men in the Boston area. J Clin Endocrinol Metab. 2007;92:4224–4229. doi: 10.1210/jc.2007-1303. [DOI] [PubMed] [Google Scholar]
- 89.Van Cauter EW, Virasoro E, Leclercq R, Copinschi G. Seasonal, circadian and episodic variations of human immunoreactive β-MSH, ACTH and cortisol. Int J Pept Protein Res. 1981;17:3–13. doi: 10.1111/j.1399-3011.1981.tb01962.x. [DOI] [PubMed] [Google Scholar]
- 90.Levine ME, Milliron AN, Duffy LK. Diurnal and seasonal rhythms of melatonin, cortisol and testosterone in interior Alaska. Arctic Med Res. 1994;53:25–34. [PubMed] [Google Scholar]
- 91.Walker BR, Best R, Noon JP, Watt GC, Webb DJ. Seasonal variation in glucocorticoid activity in healthy men. J Clin Endocrinol Metab. 1997;82:4015–4019. doi: 10.1210/jcem.82.12.4430. [DOI] [PubMed] [Google Scholar]
- 92.King JA, Rosal MC, Ma Y, Reed G, Kelly TA, Stanek EJ, 3rd, Ockene IS. Sequence and seasonal effects of salivary cortisol. Behav Med. 2000;26:67–73. doi: 10.1080/08964280009595753. [DOI] [PubMed] [Google Scholar]
- 93.Hansen AM, Garde AH, Skovgaard LT, Christensen JM. Seasonal and biological variation of urinary epinephrine, norepinephrine, and cortisol in healthy women. Clin Chim Acta. 2001;309:25–35. doi: 10.1016/s0009-8981(01)00493-4. [DOI] [PubMed] [Google Scholar]
- 94.Agrimonti F, Angeli A, Frairia R, Fazzari A, Tamagnone C, Fornaro D, Ceresa F. Circannual rhythmicities of cortisol levels in the peripheral plasma of healthy subjects. Chronobiologia. 1982;9:107–114. [PubMed] [Google Scholar]
- 95.Wehr TA, Moul DE, Barbato G, Giesen HA, Seidel JA, Barker C, Bender C. Conservation of photoperiod-responsive mechanisms in humans. Am J Physiol. 1993;265:R846–R857. doi: 10.1152/ajpregu.1993.265.4.R846. [DOI] [PubMed] [Google Scholar]
- 96.Van Dongen HP, Kerkhof GA, Souverijn JH. Absence of seasonal variation in the phase of the endogenous circadian rhythm in humans. Chronobiol Int. 1998;15:623–632. doi: 10.3109/07420529808993198. [DOI] [PubMed] [Google Scholar]
- 97.Lac G, Chamoux A. Do circannual rhythm of cortisol and testosterone interfere with variations induced by other events? Ann Endocrinol (Paris) 2006;67:60–63. doi: 10.1016/s0003-4266(06)72542-2. [DOI] [PubMed] [Google Scholar]
- 98.Deslypere JP, de Biscop G, Vermeulen A. Seasonal variation of plasma dehydroepiandrosterone sulphate and urinary androgen excretion in post-menopausal women. Clin Endocrinol (Oxf) 1983;18:25–30. doi: 10.1111/j.1365-2265.1983.tb03182.x. [DOI] [PubMed] [Google Scholar]
- 99.Nicolau GY, Lakatua D, Sackett-Lundeen L, Haus E. Circadian and circannual rhythms of hormonal variables in elderly men and women. Chronobiol Int. 1984;1:301–319. doi: 10.3109/07420528409063911. [DOI] [PubMed] [Google Scholar]
- 100.Garde AH, Hansen AM, Skovgaard LT, Christensen JM. Seasonal and biological variation of blood concentrations of total cholesterol, dehydroepiandrosterone sulfate, hemoglobin A1c, IgA, prolactin, and free testosterone in healthy women. Clin Chem. 2000;46:551–559. [PubMed] [Google Scholar]
- 101.Bjørnerem A, Straume B, Oian P, Berntsen GK. Seasonal variation of estradiol, follicle-stimulating hormone, and dehydroepiandrosterone sulfate in women and men. J Clin Endocrinol Metab. 2006;91:3798–3802. doi: 10.1210/jc.2006-0866. [DOI] [PubMed] [Google Scholar]
- 102.Urbanski HF, Noriega NC, Lemos DR, Kohama SG. Gene expression profiling in the rhesus macaque: experimental design considerations. Methods. 2009;49:26–31. doi: 10.1016/j.ymeth.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Monteleone P, Martiadis V, Maj M. Circadian rhythms and treatment implications in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010 doi: 10.1016/j.pnpbp.2010.07.028. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 104.Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci USA. 2002;99:10801–10806. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kalsbeek A, Palm IF, La Fleur SE, Scheer FA, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C, Buijs RM. SCN outputs and the hypothalamic balance of life. J Biol Rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- 106.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Plymate SR, Tenover JS, Bremner WJ. Circadian variation in testosterone, sex hormone-binding globulin, and calculated non-sex hormone-binding globulin bound testosterone in healthy young and elderly men. J Androl. 1989;10:366–371. doi: 10.1002/j.1939-4640.1989.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 108.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 109.Luboshitzky R, Shen-Orr Z, Herer P. Middle-aged men secrete less testosterone at night than young healthy men. J Clin Endocrinol Metab. 2003;88:3160–3166. doi: 10.1210/jc.2002-021920. [DOI] [PubMed] [Google Scholar]
- 110.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 111.Page ST, Matsumoto AM, Bremner WJ. DHEA and testosterone in the elderly. N Engl J Med. 2007;356:635–637. doi: 10.1056/NEJMc063190. [DOI] [PubMed] [Google Scholar]
- 112.Bremner WJ. Testosterone deficiency and replacement in older men. N Engl J Med. 2010;363:189–191. doi: 10.1056/NEJMe1006197. [DOI] [PubMed] [Google Scholar]
- 113.Handelsman DJ, Liu PY. Andropause: invention, prevention, rejuvenation. Trends Endocrinol Metab. 2005;16:39–45. doi: 10.1016/j.tem.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 114.Amory JK, Bremner WJ. Oral testosterone in oil plus dutasteride in men: a pharmacokinetic study. J Clin Endocrinol Metab. 2005;90:2610–2617. doi: 10.1210/jc.2004-1221. [DOI] [PubMed] [Google Scholar]
- 115.Amory JK, Page ST, Bremner WJ. Oral testosterone in oil: pharmacokinetic effects of 5α-reduction by finasteride or dutasteride and food intake in men. J Androl. 2006;27:72–78. doi: 10.2164/jandrol.05058. [DOI] [PubMed] [Google Scholar]
- 116.Wolf OT, Kirschbaum C. Actions of dehydroepiandrosterone and its sulfate in the central nervous system: effects on cognition and emotion in animals and humans. Brain Res Brain Res Rev. 1999;30:264–288. doi: 10.1016/s0165-0173(99)00021-1. [DOI] [PubMed] [Google Scholar]