Abstract
Important basic science research is being conducted that has direct implications for the rehabilitation of patients, but the translation of this research to change clinical practice does not occur automatically. Advisory panels to the National Center for Medical Rehabilitation Research acknowledge a need for basic and applied research related to the factors underlying coordinated movements, such as the interactions of the neuromuscular and musculoskeletal systems. In this paper, we briefly describe recent studies that have examined the preceding interaction and discuss some basic issues related to the translation of these experiments to the clinic. More importantly, the main purpose of this paper is to discuss models/ways to translate basic science to clinical practice in a two-way and informed interaction between basic scientists and clinicians.
Key Words: Translation, Physical therapy, Rehabilitation, Peripheral nerve injury, Proprioception
Introduction
Translation of important basic science data into clinical practice is a critical mission of the National Institutes of Health [Zerhouni, 2005, 2006]. One part of this mission falls under the National Center for Medical Rehabilitation Research (part of the National Institute of Child Health and Human Development). We feel it is important to develop a working model of two-way and informed interaction between basic scientists and clinicians in practice to foster best translation to the clinical setting and help as many patients as possible. This article provides examples of how research on the interaction of spinal circuits and the musculoskeletal system, particularly related to peripheral nerve injury, can be translated to clinical populations.
Societal Responsibility
Clinicians have the responsibility to use available basic science information to guide evidence-based clinical services, while simultaneously alerting basic scientists to the critical gaps in clinical knowledge. At the same time, basic scientists have the responsibility to produce knowledge that is clinically useful while also increasing our basic science knowledge. For these responsibilities to be met, there should be a collaboration and two-way interaction between clinicians and basic scientists that facilitates the generation of new knowledge and the translation of pertinent knowledge to the clinic [Zerhouni, 2006].
Research Models
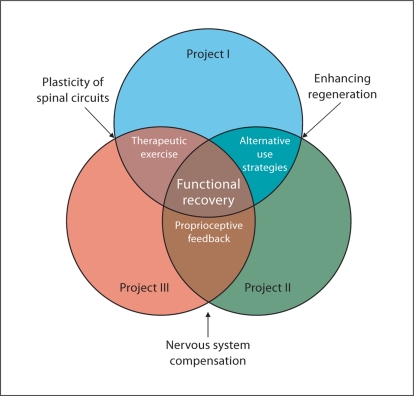
Research models should be developed with future translation kept in mind. This does not mean that models should be so contrived that important basic science information is not attained, but rather that investigators engage in dialogue with clinicians to determine models that best serve both purposes. One simple model that was adopted by our research group (fig. 1) indicates that planned overlap in multiple studies can help you refine your approach and increase the generalizability of your findings. Moreover, your research questions are designed to address issues in translatable terms. Figure 1 depicts different basic science projects that are inexorably tied together to address issues in clinically relevant ways. In a large part, this overlap between studies is formed by the comprehensive approach of the investigations. Importantly, the shared themes among the studies are all clinically germane. For example, one major theme is the manner in which the motor system adapts to the loss of a muscle or muscle group and its associated sensory feedback in the absence of therapeutic intervention, which is critical for guiding rehabilitation. Short-term adaptations to a loss of sensory feedback are likely to include alternative use strategies and enhanced utilization of feedback from neighboring muscles. The nature of these short-term adaptations includes changes to muscular and connective tissue elements in addition to changes in feedback gain and patterns of muscle activity. The role of feedback, in particular proprioceptive feedback, is also closely examined in the regulation of inter-joint coordination during locomotion. The differing roles of length and force feedback from muscles may constitute a major emphasis in the reinnervation process, whereas the role of cutaneous feedback as a source of proprioceptive information is being closely examined in intact animals. Clearly, all of these exemplary themes are germane to the many aspects of the practice of neuromuscular rehabilitation.
Fig. 1.
This figure demonstrates how a basic science project can overlap in the study of functional recovery in terms that are relevant to clinical practice.
Translation between Animal Models and Humans
The studies discussed in this special issue use animal models. It goes without saying that there are numerous important benefits to the use of animal models. Out of necessity, researchers use animal models for the study of questions that cannot be addressed ethically with use of human subjects. This allows well-controlled studies that ask precise questions and provide valuable information. The use of animal studies has been tremendously helpful in filling holes in our knowledge base. Yet, we still need to discuss the ‘elephant in the room’. Namely, cats and rats are not people.
Translation Requires Use of the Most Appropriate Animal Models
Despite the tremendous benefits from the use of animal models, there are some important differences between the animals that are tested and human patients. For instance, we need to be conscious of the potential difference in stability between bipedal and quadrupedal locomotor patterns. Removing proprioceptive feedback from one of a cat's four legs is potentially less destabilizing and may more easily allow for alternate adaptive strategies than the removal of proprioceptive feedback from one of a bipedal human's legs; however, see Zehr et al. [2009] who provide evidence of the quadrupedal nature of human locomotor control.
In addition, the complexity of the ‘lesion’ is often considerably different in the animal model compared to what we may see clinically. Scientists using animal models can produce deficits of a specific muscle or pair of muscles, whereas our patients often have a variety of lesions. In some cat/rat studies, this precise ablation results in the maintenance of intact synergists or unaffected joints within the same limb, perhaps giving the animals greater ability than many of our patients to compensate for the injury. This has been well recognized in spinal cord injury research, in which experimental devices have been developed to mimic human contusion injuries in animal models [Somerson and Stokes, 1987]. Finally, animal models differ from humans with regard to size. Because humans are substantially larger than rats or cats, studies of adaptation after peripheral nerve injury may be affected by substantial differences in the distances required for nerve regeneration. Indeed, Brushart et al. [2010] have recently demonstrated that preferential motor reinnervation is time and distance dependent, and thus, is unlikely to be the same for rats and humans. Alternative models such as larger primates, although quite expensive, may be necessary to truly address recovery from nerve injury and how pharmacological, trophic factors or similar substances and rehabilitation work together in human subjects.
Translation Is a Two-Way Street
As Allen Jette [2005] stated: ‘Advancement of practice requires an understanding of innovation and how it spreads. Let's not make the mistake I made as a student by assuming that the problem is simply a lack of knowledge on the part of clinicians.’ Nor is it simply a lack of understanding on the part of basic scientists. What is needed is a two-way communication between these groups to reduce such misunderstanding.
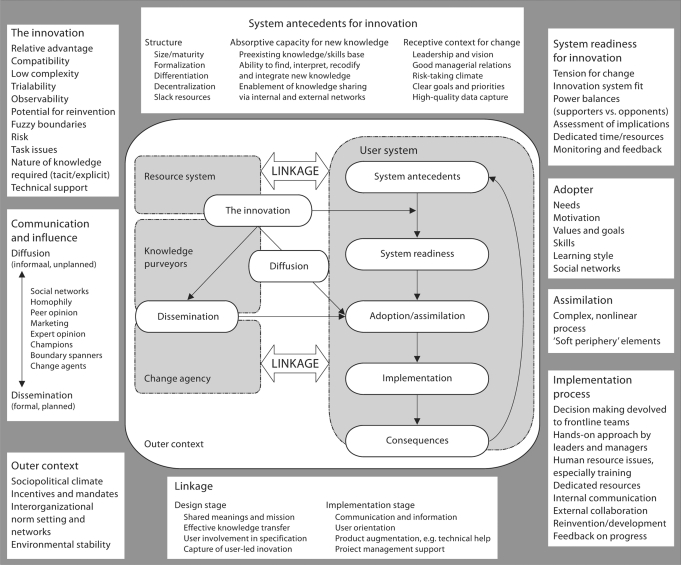
The challenge of translating innovations into common use requires a diffusion of innovation, in which new ideas become integrated over time through existing social systems [Rogers, 2003; Greenhalgh et al., 2004]. The timing of diffusion is unpredictable, because there is no clear plan or structure to assure that new ideas are adopted, and the complexities of existing social systems are vast. In contrast, a plan to disseminate new ideas typically involves a more active interchange between designers of innovation (e.g., basic scientists) and possible end-users of new ideas (e.g., rehabilitation therapists), whereby active efforts are made to design innovations for practice (fig. 2). In the early design stages, planned dissemination requires careful consideration of who will apply an innovation, as well as where and how it will be applied. This process has been extensively examined in health promotion studies using Glasgow's RE-AIM model [Glasgow et al., 1999].
Fig. 2.
In this figure from Greenhalgh et al. [2004], the complexity of diffusing innovation into practice is shown. A key feature of the figure is that the users and knowledge generators need to be linked. Without such a link, basic science innovation will not be translated into the clinic. Reproduced with permission.
The complexities of the process of dissemination and diffusion are highlighted in figure 2. Many factors may influence the adoption of new ideas. Greenhalgh et al. [2004] described several factors that are especially important in the adoption of innovative approaches including (1) the advantage of the innovation over existing approaches; (2) compatibility – an approach that is similar to current practice in terms of elements, intensity and duration may be easier to adopt; (3) complexity – a simpler approach will be easier to adopt; (4) ‘trial ability’ – an approach that is easier to experiment with and try out, without requiring extensive training or equipment may be easier to adopt, and (5) observability – an intervention that is easy for others to observe as ‘something new’ with clear benefits to the patient and therapist will logically have greater appeal for adoption. It may be simpler to implement the adoption of new concepts or skills, such as the expectation that task-specific practice will result in neuroplastic changes, than to adopt a specific intervention that requires specific protocols, staff training, specific equipment and resources.
Dissemination of innovations necessarily requires a readiness of existing systems with appropriate resources to adopt a new approach. There must be adopters who are motivated and skilled, with the coalescence of a ‘push’ of the innovation administratively from the ‘top’ (e.g., an administrator in a health care system advocates for the innovation as a new service) and a ‘pull’ for the innovation (patients demand the new service).
Dissemination can be engineered by ‘pushing’ new ideas into existing systems; these effects can be directly studied using models like the Glasgow's RE-AIM approach [Glasgow et al., 1999]. A critical feature of successful translation is that designers (basic scientists) and end-users (clinicians) have a two-way communication to influence developing innovations. This necessitates a shared mission of designers and end-users with open dialog to assure that knowledge is transferred between them. In applying a two-way linkage, experiments are designed with translation challenges in mind, where outcome measures in research and practice are identical, and the results of clinical application of the new knowledge is shared with the basic scientists to improve future experiments. This iterative process of communication results in a better end product that is based on strong science yet is realistic for the use in current clinical practice.
Clinical Implications of Basic Science Studies and Translation into Clinical Practice: Interaction of the Musculoskeletal System and Spinal Circuits
In the remainder of this paper, we will discuss the clinical implications of select recent relevant basic science studies involving the interaction of the musculoskeletal system with spinal circuits. The two main portions of this section are locomotion and posture/balance. We will also discuss in more detail ways/examples of translating basic science knowledge into clinical practice.
Spinal Circuits and Locomotion: Effects of Neurologic Lesions
Despite the differences between animal models and human patients, the use of animal models in research has provided clinicians with important information regarding sensorimotor control of limb movements during walking. We will now relate a few of these important findings to locomotion of patient populations, as well as address what may be some residual knowledge gaps that need to be filled.
Ability of Spinal Circuits to Adapt
The first important point is that the spinal circuits adapt to the presence of altered feedback. Specifically, in the absence of localized muscle function, synergists have the potential to become upregulated to compensate for the loss [Pearson et al., 1999; Maas et al., 2010]. For example, denervation of the lateral gastrocnemius and/or medial gastrocnemius, which are major plantar flexors, results in increased activity of synergists (other plantar flexors) to maintain joint moments [Pearson et al., 1999; Maas et al., 2010]. The ability of synergists to compensate might have a particular influence on treatments applied to clinical populations. Clinically, we have to contend with altered muscle function from a variety of causes, whether through injury (resulting in paralysis or paresis), or through pharmacological interventions (such as botox injections), or surgery (such as tendon transfer surgery). Each of these ‘insults’ results in altered muscle function, ranging from a complete lack of muscle activation to a reduction in muscle force, to a change in the joint movement produced by activation of the muscle.
The flexibility in the muscles selected for a functional task allows for rapid compensations after a lesion and may result in considerable retention of function. This compensation may be controlled by spinal circuits. For many years, we did not have a great appreciation of the adaptability of spinal circuits in animal models or humans [Segal, 1997; Wolpaw and Tennissen, 2001]. Knowing that the spinal circuits have the ability to adapt provides clinicians with hope that patients can have improved functional recovery.
However, several questions remain. For example, what types of interventions are necessary to ensure that synergists are activated with the right timing and level of activation in the acute phase of recovery from a lesion? When should an intervention be applied? Answers to such questions will be informed by knowledge about whether the availability of specific chemical substrates affects the receptivity of spinal circuits to particular regenerated peripheral inputs. Finally, we need to be cautious of creating harmful compensations that we cannot eliminate if normal muscle function is later restored. Therefore, we will need to know how to return synergist activity to its previous or appropriate level if that is determined to be best for functional recovery. Ferris [Ferris and Lewis, 2009] is already beginning to address this issue in his lab using robotic exoskeletons. In these types of studies, the amount and timing of robotic assistance is titrated downward as the activity of impaired muscles recovers after a lesion.
Related to the adaptation of spinal circuits, coactivation of antagonists is evident after reinnervation [Gramsbergen et al., 2000; Sabatier et al., 2011]. Functionally, inappropriate muscle activity is quite common during gait cycle for many patients with neurologic injury. Therefore, it is feasible that the coactivation of functional antagonists or other ‘inappropriate’ muscle activity may be a natural adaptation that occurs within the denervation-reinnervation cycle. While physical therapists are often trained in musculoskeletal courses that coactivation may be beneficial for improving joint or limb stability, we also believe that coactivation may be detrimental to joint integrity due to increased joint compressive forces. Whether these cocontraction patterns persist in the presence of altered feedback, or whether patients with neurological injury can be taught to produce ‘normal’ movement patterns with reduced cocontraction remains to be seen. Moreover, if a coactivation pattern is the best strategy that the system can produce, should we try to reduce it?
Deficits Become Apparent with Functional Challenges
A second important point related to locomotor training is that in animals with disrupted sensory input, functional deficits may not be evident until a task is attempted that requires the deficient sensory input. This was observed when cats with self-reinnervated ankle extensor muscles adapted well to upslope and level walking, but exhibited prolonged deficits during downslope walking [Abelew et al., 2000; Maas et al., 2007]. These persistent deficits in movement during downslope walking were attributed to a misinterpretation of muscle length feedback at the spinal level. While the proprioceptive feedback from intact and reinnervated muscles reached the spinal cord, the spinal circuits did not respond appropriately to meet the mechanical demands imposed by walking downslope. Instead, the signal was inhibited from activating the α motor neurons, so that the cats were ambulating without active stretch reflexes in the triceps surae [Alvarez et al., 2010].
While muscle length feedback may not be needed to meet the demands of level and upslope walking in cats, it is critical to maintaining the stiffness of the muscles during downslope walking [Maas et al., 2010]. Ultimately, the animal's deficits only appear when the missing feedback is necessary for the functional movement being performed. Without the necessary proprioceptive feedback, the cats adopted a compensatory alternate strategy for downslope walking, which persisted after recovery. However, is it the downslope walking that is the critical challenge? This is clearly not the case, as shown in Sabatier et al. [2011]. In their study of recovery from hindlimb peripheral nerve injury in rats, both level and upslope walking, but not downslope walking, were impaired, depending on the site of the lesion. Uncovering the mechanisms of these locomotor deficits and the reasons why different mechanical demands (e.g., up- vs. downslope walking) might be affected with certain lesions is critical information for planning patient interventions.
Ultimately, the cat's/rat's altered limb kinematics, kinetics and electromyographic activity during downslope [Abelew et al., 2000] or upslope [Sabatier et al., 2011] walking may be inconsequential, because the cats/rats are able to successfully walk down (or up) the ramp. Nevertheless, we propose that challenging the sensorimotor system to determine all deficits is important because it offers a window into the range of deficits that will appear with a lack of muscle length feedback (or other sensory feedback depending on the lesion).
Thinking more broadly about application to human patients, the flat, smooth hospital floor is very different from the outside world, in which patients have to contend with hills, ramps, curbs, cracks, potholes and other obstacles. Dealing with each of these challenges requires a greater reliance on sensory information, particularly proprioceptive feedback and multi-joint coordination. The evidence presented from the basic science animal models confirms the need for clinicians to practice community-based ambulation with a variety of sensory challenges for patients with proprioceptive and other sensory deficits. Such training provides a greater challenge, from a sensory stimulus perspective, which can reveal previously unseen movement deficits for appropriate clinical interventions. However, this rationale implies that challenging the system by providing exposure to repeated afferent feedback will encourage the beneficial adaptations that we expect and need to enhance function. Further evidence must be collected to confirm that this rationale has a foundation in humans (and in animal models), although early evidence from Lay et al. [2005] suggests that similar neuromuscular solutions to inclines are used by both humans and cats.
That deficits in certain locomotor conditions (e.g., downslope walking) persist, also suggests that the animal's afferent feedback system has not been sufficiently challenged during the recovery process. Laboratory animals [Abelew et al., 2000; Sabatier et al., 2011] may spend most of their recovery time housed in a ‘flat’ cage, and thus, may have been given insufficient opportunities to restore muscle length feedback to the spinal circuits. Evidence suggests that repeatedly challenging the system, by providing ample exposure to the misinterpreted afferent feedback, may serve to encourage motor adaptations that enhance function. For example, eccentric actions during locomotion may be particularly important in certain patient populations, not with regard to strengthening muscles, but for the specific muscle length feedback that these contractions supply the spinal cord to coordinate movement.
However, before clinicians start having all their patients walking up and down ramps, mounting their treadmills on wedged surfaces or augmenting feedback with ‘negative sole’ shoes, additional evidence is required to determine whether the motor system can adapt if the misinterpreted afferent information is repeatedly applied during an intervention. If it turns out that the provision of feedback is important for driving adaptations to spinal circuits for improving function, then clinicians are well equipped to provide appropriate feedback. Indeed, there are cases where we already work to emphasize limb position feedback during locomotor training. For example, the encouragement of full hip extension during walking retraining is thought to activate the hip flexor's group Ia (dynamic length-sensitive) afferents to facilitate subsequent hip flexion to help drive flexion during the swing phase [Edgerton et al., 2004]. Perhaps this repeated muscle lengthening is contributing to an adaptation of the spinal circuits.
Spinal Circuits and Balance: Effects of Neurologic Lesions
In addition to locomotor issues, patients with a loss of sensory information, particularly proprioceptive feedback from the lower extremities, often have difficulty maintaining balance [Diener et al., 1984]. Since coordination during tasks involving eccentric actions is apparently strongly affected by sensorimotor denervation and reinnervation, balance may be altered. Length feedback is certainly one of the sensory inputs necessary for normal balance, and thus, balance may be affected by peripheral nerve lesions when input is disrupted, or later, when reinnervation is misinterpreted. Thus, as clinicians, we may need to encourage compensation using other mechanisms while patients recover from the lesion. For example, we may need to encourage the use of other sensory inputs such as vision.
Moreover, based on recent animal model studies [Haftel et al., 2005; Alvarez et al., 2010], we know there is Ia input reaching the spinal cord with self-reinnervation, but that stretch reflexes are not occurring, probably due to at least some level of central suppression. Thus, it may not be enough to provide sensory input, but changes in central processing may need to be encouraged to have a positive therapeutic result. In addition, we will need to determine an appropriate time frame for initiating rehabilitation. For example, we may need to start rehabilitation once sensory input reaches the spinal cord, so that the spinal cord does not accommodate to not receiving this sensory input and learns to ignore it.
As was stated in the previous section, data from animal model studies strongly suggest that functional deficits are probably only detected when they are tightly related to the specific sensory deficit. Thus, when we try to translate these findings from animal models to patient care, we have to consider what types of muscle contraction may be impaired as we develop interventions. For example, some common postural control tasks involve eccentric contractions. Clearly, in addition to locomotion, there are a number of important tasks that may be influenced by loss of effective proprioceptive inputs. ‘Simple’, but important tasks such as quiet standing require feedback to properly control sway. Squatting is another task that requires effective proprioceptive input, which could be lost after reinnervation [Riemann and Lephart, 2002]. Problems with squatting could greatly limit the independence of an individual living alone. A final example of a task requiring eccentric contractions of leg muscles is going from sitting to standing, a key task for functional independence.
For patients with lower extremity sensory loss (e.g., proprioception), it is critical to perform a detailed initial assessment to include at least reflex testing, sensory testing, determination of muscle tendon unit extensibility and accurate force measures beyond the manual muscle test. Then, we can consider the functional tasks impacted by specific impairments and also consider compensatory mechanisms. This detailed assessment will allow clinicians to determine an effective treatment plan that will include the patient's present balance capabilities and weaknesses and may include the use of: (1) orthoses; (2) ↑ cocontraction; (3) ↑ sensitivity to sensory input other than that impaired; (4) noise-enhanced balance control, and (5) vibrotactile feedback.
Non-Neural Factors Affect Locomotion as well as Posture and Balance
Determining the contributions of passive mechanical structures for motor coordination is important for assessment and interventions in clinical practice, and changes to structures such as fascia, fat pads, tendons and ligaments may constrain degrees of freedom which can enhance the stability during various postural control tasks. Future and past animal studies, including recent work from the lab of Nichols [Stahl and Nichols, 2011], will give or have given clinicians insights into the importance of these ‘passive’ structures. For example, denervation of ankle muscles greatly increases ankle yield during stance while at the same time decreasing leg length during early stance [Stahl and Nichols, 2011]. Yielding is an abrupt decrease in the rate of rise of force or even an abrupt decrease in force when a muscle is under stretch [Nichols and Houk, 1976]. Disruption of the fascia changes limb function, but in a different manner than with neural disruption. In the case of crural fascia disruption in cats, there is no increase in yield but rather a decrease in propulsive force [Stahl and Nichols, 2011]. Moreover, other tissues, including adipose tissue, can alter the range of motion and increase energy expenditure [Falcon et al., 2011]. The researchers concluded that the popliteal fat may contribute to limiting angular acceleration of the knee which is beneficial unless there is too much fat. Perhaps, for patients with low extremity proprioceptive loss, we should consider allowing some muscle tendon unit shortening and joint capsule tightness to develop. Thus, we may want to avoid overstretching and use the soft tissue to help maintain the patients in relatively neutral alignment (medial-lateral), or possibly, use ankle supports or braces. However, at the same time, it will be important to maintain the range of motion for functional activities.
While we clearly have learned a lot from basic science about the way spinal circuits adapt to an altered sensorimotor state, we are still unsure about how to prepare the sensorimotor system for the time when successful motor reinnervation occurs. Furthermore, can sensorimotor deficits be overcome if therapists continuously challenge the nervous system by encouraging the sensory feedback that the central nervous system is missing and/or misinterpreting? If so, does this type of therapy need to happen acutely and/or can someone with chronic deficits experience the same positive motor adaptations? Finally, are there other sensory inputs that can be used by the central nervous system to substitute for the loss of length feedback or other proprioceptive feedback? Will inputs such as vibration, electrical stimulation or activation of tactile or cutaneous receptors be beneficial in overcoming these losses? Collaboration between basic science and clinical investigators to answer some of these questions and others will provide a fuller picture of how both the nervous system and musculoskeletal system adapt to injury for improvements in functional recovery.
Conclusions
The preceding insights from animal model studies provide a good start, but the next steps need to include clinician contribution to hypothesis generation regarding:
(1) effects of proprioceptive loss on balance and locomotion;
(2) effects of enhanced sensory input (natural or externally applied, e.g., electrical stimulation, vibration) on balance and locomotion;
(3) strategies for rehabilitation (e.g., increased cocontraction), interventions (e.g., multisensory training, enhanced sensory inputs or replacing sensory inputs), and external devices/supports (e.g., vibrating insoles) that may be effective for improving balance and locomotion;
(4) particular patient populations which are most similar to animal models;
(5) detailed analysis of non-neural factors that can enhance function or may in fact impede locomotion.
To accomplish the preceding, close communication must occur in a two-way manner between clinicians and basic scientists. More importantly, the communication cannot be of a casual nature. It must be formalized including having clinicians as investigators or consultants on basic science projects and having basic scientists formally part of clinical studies and participating in the education of rehabilitation clinicians.
Acknowledgements
This work was supported by grant HD032571 from the United States Public Health Service.
References
- Abelew T.A., Miller M.D., Cope T.C., Nichols T.R. Local loss of proprioception results in disruption of interjoint coordination during locomotion in the cat. J Neurophysiol. 2000;84:2709–2714. doi: 10.1152/jn.2000.84.5.2709. [DOI] [PubMed] [Google Scholar]
- Alvarez F.J., Bullinger K.L., Titus H.E., Nardelli P., Cope T.C. Permanent reorganization of Ia afferent synapses on motoneurons after peripheral nerve injuries. Ann NY Acad Sci. 2010;1198:231–241. doi: 10.1111/j.1749-6632.2010.05459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart T.M., Vyas A.A., O'Daly A., Abdullah M. Adult motor axons selectively reinnervate pre-degenerated adult muscle nerve (online abstract 541.19) Washington: Society for Neuroscience; 2010. [Google Scholar]
- Diener H.C., Dichgans J., Guschlbauer B., Mau H. The significance of proprioception on postural stabilization as assessed by ischemia. Brain Res. 1984;296:103–109. doi: 10.1016/0006-8993(84)90515-8. [DOI] [PubMed] [Google Scholar]
- Edgerton V.R., Tillakaratne N.J., Bigbee A.J., de Leon R.D., Roy R.R. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- Falcon I., Stahl V.A., Nichols T.R. Evidence that popliteal fat provides damping during locomotion in the cat. Cells Tissues Organs. 2011;193:336–341. doi: 10.1159/000323680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris D.P., Lewis C.L. Robotic lower limb exoskeletons using proportional myoelectric control. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:2119–2124. doi: 10.1109/IEMBS.2009.5333984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow R.E., Vogt T.M., Boles S.M. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramsbergen A., Ijkema-Paassen J., Meek M.F. Sciatic nerve transection in the adult rat: abnormal EMG patterns during locomotion by aberrant innervation of hindleg muscles. Exp Neurol. 2000;161:183–193. doi: 10.1006/exnr.1999.7233. [DOI] [PubMed] [Google Scholar]
- Greenhalgh T., Robert G., Macfarlane F., Bate P., Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Quarterly. 2004;82:581–629. doi: 10.1111/j.0887-378X.2004.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haftel V.K., Bichler E.K., Wang Q.B., Prather J.F., Pinter M.J., Cope T.C. Central suppression of regenerated proprioceptive afferents. J Neurosci. 2005;25:4733–4742. doi: 10.1523/JNEUROSCI.4895-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jette A.M. ‘Invention is hard, but dissemination is even harder’. Phys Ther. 2005;85:390–391. [PubMed] [Google Scholar]
- Lay A.N., Hass C.J., Smith D.W., Gregor R.J. Characterization of a system for studying human gait during slope walking. J Appl Biomech. 2005;21:153–166. doi: 10.1123/jab.21.2.153. [DOI] [PubMed] [Google Scholar]
- Maas H., Gregor R.J., Hodson-Tole E.F., Farrell B.J., English A.W., Prilutsky B.I. Locomotor changes in length and EMG activity of feline medial gastrocnemius muscle following paralysis of two synergists. Exp Brain Res. 2010;203:681–692. doi: 10.1007/s00221-010-2279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas H., Prilutsky B.I., Nichols T.R., Gregor R.J. The effects of self-reinnervation of cat medial and lateral gastrocnemius muscles on hindlimb kinematics in slope walking. Exp Brain Res. 2007;181:377–393. doi: 10.1007/s00221-007-0938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T.R., Houk J.C. Improvement in linearity and regulation of stiffness that results from actions of stretch reflex. J Neurophysiol. 1976;39:119–142. doi: 10.1152/jn.1976.39.1.119. [DOI] [PubMed] [Google Scholar]
- Pearson K.G., Fouad K., Misiaszek J.E. Adaptive changes in motor activity associated with functional recovery following muscle denervation in walking cats. J Neurophysiol. 1999;82:370–381. doi: 10.1152/jn.1999.82.1.370. [DOI] [PubMed] [Google Scholar]
- Riemann B.L., Lephart S.M. The sensorimotor system. 2. The role of proprioception in motor control and functional joint stability. J Athl Train. 2002;37:80–84. [PMC free article] [PubMed] [Google Scholar]
- Rogers E.M. Diffusion of Innovations. ed 5. New York: Free Press; 2003. [Google Scholar]
- Sabatier M.J., To B.N., Nicolini J., English A.W. Effect of axon misdirection on recovery of EMG activity and kinematics after peripheral nerve injury. Cells Tissues Organs. 2011;193:298–309. doi: 10.1159/000323677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal R.L. Plasticity in the nervous system: operant conditioning of the spinal stretch reflex. Top Stroke Rehabil. 1997;3:76–87. doi: 10.1080/10749357.1997.11754130. [DOI] [PubMed] [Google Scholar]
- Somerson S.K., Stokes B.T. Functional analysis of an electromechanical spinal cord injury device. Exp Neurol. 1987;96:82–96. doi: 10.1016/0014-4886(87)90170-1. [DOI] [PubMed] [Google Scholar]
- Stahl V.A., Nichols T.R. Short-term effects of muscular denervation and fasciotomy on global limb variables during locomotion in the decerebrate cat. Cells Tissues Organs. 2011;193:325–335. doi: 10.1159/000323679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw J.R., Tennissen A.M. Activity-dependent spinal cord plasticity in health and disease. Ann Rev Neurosci. 2001;24:807–843. doi: 10.1146/annurev.neuro.24.1.807. [DOI] [PubMed] [Google Scholar]
- Zehr E.P., Hundza S.R., Vasudevan E.V. The quadrupedal nature of human bipedal locomotion. Exerc Sport Sci Rev. 2009;37:102–108. doi: 10.1097/JES.0b013e31819c2ed6. [DOI] [PubMed] [Google Scholar]
- Zerhouni E.A. US biomedical research: basic, translational, and clinical sciences. JAMA. 2005;294:1352–1358. doi: 10.1001/jama.294.11.1352. [DOI] [PubMed] [Google Scholar]
- Zerhouni E.A. Clinical research at a crossroads: the NIH roadmap. J Investig Med. 2006;54:171–173. doi: 10.2310/6650.2006.X0016. [DOI] [PubMed] [Google Scholar]