Abstract
Activation of polymorphonuclear leukocytes (PMN) can be modulated to intermediate ‘primed’ states characterized by enhanced responsiveness to subsequent stimuli. We studied priming in response to TNF-α in human PMN and PLB-985 cells, a myeloid cell line differentiated to a neutrophilic phenotype (PLB-D). PMN generated reactive oxygen species (ROS) in response to TNF-α alone, and NADPH oxidase activity increased in response to stimulation with formyl-Met-Leu-Phe after priming. PLB-D cells also demonstrated priming of NADPH oxidase activity. Similar to priming by endotoxin, priming of the respiratory burst by TNF-α was predominantly oxygen dependent, with marked attenuation of ROS generation if primed anaerobically. Both PMN and PLB-D cells displayed significant increases in cell surface CD11b and gp91phox expression after TNF-α priming and PMN displayed activation of MAPK. In response to TNF-α priming, neither mobilization of intracellular proteins nor activation of MAPK pathways was NADPH oxidase dependent. Priming of PMN and PLB-D cells by low TNF-α concentrations enhanced chemotaxis. These data demonstrate that pathophysiological concentrations of TNF-α elicit NADPH oxidase-derived ROS and prime cells for enhanced surface protein expression, activation of p38 and ERK1/2 MAPK pathways, and increased chemotaxis. Furthermore, PLB-D cells undergo TNF-α priming and provide a genetically modifiable model to study priming mechanisms.
Key Words: Chemotaxis, Cytokines, Endotoxin, Oxidative burst, Sepsis, Reactive oxygen species, NADPH oxidase
Introduction
The incidence of sepsis continues to increase [1,2] and, although it is clear that there are detrimental consequences of excessive polymorphonuclear leukocyte (PMN) activation, the requirement for intact neutrophil function during bacterial sepsis has been demonstrated unequivocally. PMN priming induces enhanced readiness for rapid response to a subsequent stimulus and facilitates progression to full activation. A critical aspect of PMN priming as an intermediate level of activation is that primed PMN do not release proteolytic granular contents in response to the priming stimulus. Thus, priming plays a potentially protective role through the prevention of secondary damage to surrounding host cells. Primed PMN have been demonstrated in the circulation of patients during sepsis [3,4] and other inflammatory disease processes [5], and have been extensively studied in vitro. Although the classical definition of priming suggests that there is not ‘full activation’ of the NADPH oxidase in response to a priming stimulus [6], we have recently demonstrated that the priming stimulus endotoxin elicits low-level reactive oxygen species (ROS) generation, in addition to priming PMN for an enhanced respiratory burst in response to subsequent stimuli [7].
A number of diverse agents have priming effects on PMN, and both the underlying mechanisms of priming and the primed PMN phenotype are variable and dependent on the stimulus (reviewed by Condliffe et al. [8]). TNF-α, an inflammatory cytokine released during sepsis, has been extensively studied as a priming agent for PMN functions. The clinical relevance for better understanding the phenomenon of priming by TNF-α is underscored by clear evidence that low levels of TNF-α are present in the circulation of patients with sepsis and, in some cases, increasing TNF-α levels inversely correlated with survival [9,10,11].
The phenotype of TNF-α-primed cells includes an enhanced oxidative burst in response to future stimuli [12], increased surface levels of the β2-integrins [13] and improved cell migration [14]. Review of this literature is complicated by the wide variation in TNF-α concentrations used to ‘prime cells, ranging from 1 to 200 ng/ml. However, the majority of studies employed at least 10 ng/ml [15,16,17], a concentration that is significantly in excess of the range commonly seen in patient serum samples (30–1,300 pg/ml) [9,10,11]. Similarly, investigations of the mechanisms and signaling pathways responsible for these changes in PMN function used greater than physiologic concentrations, with the rare study employing a range of TNF-α concentrations that includes pathophysiologically relevant levels [18].
We recently reported our studies of endotoxin-mediated priming of human PMN and demonstrated that the development of the primed state requires both NADPH oxidase-generated ROS and the anion transporter chloride channel-3 (CIC-3) [7]. The phagocyte NADPH oxidase is a multicomponent enzyme complex that produces substantial quantities of ROS necessary for optimal microbicidal activity against many pathogens. In addition, low levels of ROS are generated by PMN for signaling purposes and have been demonstrated to be involved in the regulation of integrin activation [19] and apoptosis [20]. Our recent investigation provides additional evidence that NADPH oxidase-generated ROS signaling is required for PMN priming.
Further dissection of the mechanism of CIC-3 interaction with NADPH oxidase function in human PMN priming has been hampered by a lack of selective inhibitors for anion transporters and the inability to transfect human neutrophils. In the current study, we focused on priming by the cytokine TNF-α and studied both primary human PMN and the myeloid cell line PLB-985 differentiated to a neutrophilic phenotype. The goals of the current study included: (1) investigation of the primed PMN phenotype in response to TNF-α using a range of concentrations of this cytokine, consistent with levels detected in human serum during sepsis; (2) determination of the oxygen dependence of the TNF-α priming process; (3) characterization of priming in a genetically modifiable cell line for future use. Herein, we show that both PMN and PLB-D cells undergo priming by TNF-α for enhanced NADPH oxidase activity, increased cell surface receptor expression and improved chemotaxis. These events occur with priming concentrations of TNF-α as low as 1 pg/ml. Alterations in directional motility as a result of TNF-α priming vary based on the concentration of the cytokine presented. Furthermore, TNF-α-mediated priming of the respiratory burst is partially oxygen dependent in both cell types, as significantly reduced priming occurs under anaerobic conditions. The mechanism of NADPH oxidase involvement appears distinct from that seen in endotoxin priming, as upregulation of CD11b and activation of p38 MAPK occur in an NADPH oxidase-independent manner.
Materials and Methods
Materials
Hank's balanced salt solution (HBSS) was obtained from BioWhittaker (Walkersville, Md., USA). Fetal bovine serum (FBS) was obtained from HyClone (Logan, Utah, USA). RPMI 1640, L-glutamine, penicillin, streptomycin and HEPES were purchased from Cellgro (Manassas, Va., USA). Dimethyl formamide (DMF) and formyl-Met-Leu-Phe (fMLF) were purchased from Fisher Scientific (Pittsburgh, Pa., USA). Nutridoma was purchased from Roche (Madison, Wisc., USA). Murine anti-human CD11b was purchased from Pharmingen (San Diego, Calif., USA). Fluorescently conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, Pa., USA). Mouse IgG1 was purchased from Sigma (St. Louis, Mo., USA). Antibody (clone 7D5) to the gp91phox component of the flavocytochrome b558, the membrane-bound subunit of NADPH oxidase, was purchased from Medical and Biological Laboratories (Nagoya, Japan). Recombinant TNF-α was purchased from R&D Systems (Minneapolis, Minn., USA). Paraformaldehyde was purchased from Electron Microscopy Sciences (Hatfield, Pa., USA). The fluorescein derivative formyl-Nle-Leu-Nle-Tyr-Lys (F-N) was purchased from Invitrogen (Carlsbad, Calif., USA). Additional reagents were all obtained from Sigma.
Human PMN Purification
Human PMN were isolated according to standard techniques from heparin anti-coagulated venous blood from healthy, consenting adults in accordance with a protocol approved by the Institutional Review Board for Human Subjects at the University of Iowa. PMN were isolated using dextran sedimentation and Hypaque-Ficoll density-gradient separation, followed by hypotonic lysis of erythrocytes as previously described [21]. PMN purity using this method of isolation was greater than 95%. PMN were maintained in HBSS without calcium and magnesium until used experimentally, always within 10 min of completion of the isolation process. Unless otherwise specified, 1% human serum albumin and 0.1% dextrose were added to commercial HBSS with calcium and magnesium as the working buffer solution for all assays of PMN function. In whole blood flow cytometry assays, heparinized whole blood was obtained and used within 10 min of phlebotomy.
Cell Culture and Differentiation of PLB-985 Cells
The human myeloid leukemia cell line PLB-985 (a generous gift from Dr. William M. Nauseef, Iowa City, Iowa, USA) was cultured in RPMI 1640 medium supplemented with L-glutamine, penicillin, streptomycin, HEPES and 10% heat-inactivated FBS, and cells were maintained at 37°C with 5% CO2 and passaged twice weekly. Granulocytic differentiation of cells was accomplished according to previously published methods [22]. Briefly, the cell culture medium was replaced with RPMI 1640 medium supplemented with L-glutamine, penicillin, streptomycin, HEPES, 0.5% heat-inactivated FBS, 0.5% DMF and 1% Nutridoma, and cells were maintained at 37°C with 5% CO2. The differentiation medium was changed after 3 days in culture. Differentiated cells were used for experiments after 6 days in differentiation culture medium. In one series of experiments, a stable line of PLB-985 cells with targeted disruption of gp91phox (a gift from Mary Dinauer), X-CGD cells, were used to discern the role of the NADPH oxidase in p38 MAPK activation elicited by TNF-α priming.
Priming of PMN or PLB-D Cells
In priming studies, isolated PMN or PLB-D cells were incubated with TNF-α at a range of concentrations as described in individual results. Incubations occurred at 37°C for all priming studies for 10–60 min, as specified. In order to minimize the effects of the PMN isolation process on measured functional endpoints, cells were used within 10 min of completion of isolation. In some assays, these results were compared directly with studies of PMN in whole blood.
Analysis of Oxygen Dependence of the Priming Process
To assess the requirement for oxygen during the priming process, some priming studies were conducted in an anaerobic chamber (Bactron II; Sheldon Manufacturing, Cornelius, Oreg., USA). PMN or PLB-D cells were brought into the anaerobic chamber and primed with TNF-α under anaerobic conditions. Cells were then removed from the chamber, and immediately placed on ice and assayed for NADPH oxidase activity in response to fMLF, or studied by flow cytometry for mobilization of intracellular stores of proteins, as described below. Conditions in the anaerobic chamber were assessed by analysis of PMA-induced NADPH oxidase activity in the chamber as measured by the reduction of ferricytochrome c (cyt c), see below. We have previously demonstrated that PMN pretreatment with 30 min of anaerobic conditions does not alter PMN cell viability or levels of NADPH oxidase activity in response to serum opsonized zymosan (OpZ) and PMA [7].
Measurement of NADPH Oxidase Activity
Chemiluminescence
Lucigenin-enhanced chemiluminescence (LUC-CL) assays of NADPH oxidase activity were performed in a 96-well plate using the Fluostar Omega (BMG Technologies, Troy, Mich., USA). Two hundred microliter of a PMN suspension containing 2.5 × 106 PMN/ml in HBSS with 1% HSA and 0.1% dextrose was added to each well with the final concentration of lucigenin (100 μM). PLB cells were initially stimulated by addition of either OpZ (5 particles per cell) or PMA 10 ng/ml final concentration. For priming experiments, cells were stimulated by addition of TNF-α (100 pg/ml to 100 ng/ml) ± fMLF, as specified. Chemiluminescence was quantitated as relative light units using a kinetic assay with readings every minute for 30–90 min.
Reduction of cyt c
Extracellular O2– generation was measured as the superoxide dismutase (SOD)-inhibitable reduction of cyt c in a 96-well microplate using the Fluostar Omega. PMN suspensions were diluted and added to the microplate as described above. cyt c (100 μM) was added to the suspension just prior to loading in the microplate. In duplicate wells, SOD was added at a final concentration of 50 μg/ml. The maximum rate (Vmax) of O2– generation and the total nanomoles O2–/min was calculated as the SOD-inhibitable reduction of cyt c, with readings at absorbance 550 nm every 15 s for 30 min following injection of the stimulus, as specified. For assessment of oxygen tension in the anaerobic chamber, paired groups of triplicate wells loaded with PMN and cyt c as described above were stimulated in the chamber with PMA (100 ng/ml) ± SOD. After 10 min, one set with PMA alone and one set with PMA + SOD were treated with diphenyleneiodonium (DPI), an inhibitor of flavoproteins, to inhibit further NADPH oxidase activity. The microplate was removed from the anaerobic chamber and incubated for an additional 10 min before endpoint readings were measured. The percent inhibition of NADPH oxidase activity in the anaerobic chamber was quantitated by comparing the SOD-inhibitable reduction of cyt c in the wells treated with DPI prior to removal from the chamber to those wells allowed to incubate an additional 10 min under normoxic conditions. By comparison to previous studies, we used 85% reduction of NADPH oxidase activity in response to PMA as the cutoff for adequacy of anaerobic chamber function, correlating to an oxygen level of <0.1% [23].
Analysis of Cell Surface Protein Expression by Flow Cytometry
PMN were analyzed using a FACScalibur flow cytometer (BD Biosciences, Franklin Lakes, N.J., USA). For assessment of surface expression of gp91phox and CD11b, PMN or PLB-985 cells were incubated in HBSS buffer ± TNF-α, as specified. Following incubation, cells were centrifuged and resuspended in blocking buffer containing PBS with 2% nonfat dry milk, and 4% normal goat serum for 20 min on ice. Primary antibodies, including murine IgG1 control, anti-CD11b or anti-gp91phox, all at final concentrations of 8.3 μg/ml, were added after blocking and incubated for 1 h on ice. Cells were centrifuged and resuspended in FITC-conjugated goat anti-mouse antibody at a 1:1,000 dilution and incubated for 30 min on ice. Cells were resuspended in buffer containing 5 μg/ml propidium iodide prior to analysis. In some experiments, DPI (50 μM) was used to inhibit the NADPH oxidase. This concentration of DPI was chosen after preliminary studies demonstrated >99% inhibition of superoxide generation in response to PMA as measured by reduction of cyt c, whereas 10 μM DPI inhibited approximately 95% of superoxide [7]. To analyze cell surface fMLF receptor expression, PMN or PLB-985 cells were incubated in buffer ± TNF-α, as specified. Following incubation, cells were fixed for 30 min on ice in 4% paraformaldehyde. Cells were centrifuged, washed with ice-cold buffer, and resuspended at 2 × 106 PMN/ml. F-N (10 nM final) was added in the absence or presence of an excess amount of fMLF (5 μM) and tumbled for 30 min at room temperature in the dark, as described [24,25]. For select assays, PMN surface expression of CD11b was analyzed by FACS using freshly obtained whole blood incubated with or without TNF-α and treated for 1 h on ice with primary antibodies: IgG1 control or anti-CD11b (6.25 μg/ml). Following incubation, RBCs were lysed using FACS lysis buffer (BD Biosciences) and PMN were washed, resuspended and incubated with FITC-conjugated goat anti-mouse secondary antibodies at 1:1,000 dilution for 30 min on ice, prior to washing and FACS analysis.
Analysis of PMN Chemotaxis by EZ-TAXIScan Assay
EZ-TAXIScan™ assays (Effector Cell Institute, Tokyo, Japan) were performed at 37°C using the assembled chemotaxis apparatus perfused in EZT buffer, as described [25,26]. Briefly, PMN at 1 × 107/ml were kept in HBSS without calcium and magnesium until ready for use. Cells were then diluted to 1 × 106/ml in HBSS with calcium and magnesium and treated with buffer (control) or with TNF-α (10 ng/ml) and incubated for 30 min at 37°C. In each of 6 separate channels in the apparatus, a PMN suspension (approx. 5 μl of 1 × 106/ml) was injected with a 10-μl microsyringe and cells were aligned along the edge of the PMN chambers using the fluid flow technique [26]. Chemoattractants (1 μl of fMLF or activated complement component 5, C5a, at specified concentrations) were then injected into the stimulus chambers (opposite from the PMN loading chambers) to form chemotactic spatial gradients. Data were recorded channel by channel at 1-second time-lapse intervals between channels, as described [25,26]. Chemotaxis assays were filmed for 60 min, with an image collection rate of 3 frames/min. Cells were manually tracked using Image J software and the percentage of motile cells from each experimental condition was calculated. Cells moving with an average instantaneous velocity (IV) less than 3 μm/min were considered nonmotile and were not included in the calculation of average chemotaxis parameters. Assessment of directionality, or chemotactic index (CI), was calculated as the ratio of net path length toward the chemoattractant to total path length of an individual cell. Chemotaxis parameters were computed using data obtained from individual cell tracks from at least 5 separate experiments, with at least 50 cells analyzed per condition.
Analysis of MAPK Phosphorylation by Immunoblotting
PMN (2 × 107) were treated with TNF-α for the specified time points. Some PMN were treated with DPI (50 μM) to inhibit the NADPH oxidase prior to incubation with TNF-α. Following incubation, cells were centrifuged and lysed in PMN lysis buffer (100 mM Tris, 150 mM NaCl, 2 mM MgCl2, 1% Triton, 1 mM PMSF, 2% leupeptin/pepstatin A) for 45 min at 4°C with tumbling. Lysates were centrifuged at 14,000 rpm for 7 min and removed to fresh tubes. Samples were heated to 103°C for 3 min prior to analysis by SDS-PAGE.
Protein Electrophoresis and Immunoblotting
Samples were resolved in an 11% gel by SDS-PAGE and then transferred to nitrocellulose. Blots were probed for phosphospecific p38 MAPK, ERK1/2 and JNK using the following antibodies: phospho-p38 MAP kinase (Thr180/Tyr182) antibody at 1:1,000 dilution; phospho-p44/42 MAPK (Thr202/Tyr204) rabbit monoclonal antibody at 1:2,000 dilution; phospho-SAPK/JNK (Thr183/Tyr185) rabbit monoclonal antibody at 1:1,000 dilution (Cell Signaling Technology, Danvers, Mass., USA). To quantify relative amounts between experiments, blots were stripped and reprobed with phosphorylation state-independent antibodies to p38, ERK1/2 and JNK using the following antibodies: p38 MAP kinase antibody; p44/42 MAP kinase (137F5) rabbit monoclonal antibody; SAPK/JNK (56G8) rabbit monoclonal antibody all at 1:1,000 dilution (Cell Signaling Technology). Immunoblots were processed using Alexa Fluor 680 goat anti-rabbit secondary antibody (Invitrogen) and detection was performed using the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, Nebr., USA).
Analysis of MAPK Phosphorylation by Flow Cytometry
PLB-D or differentiated X-CGD PLB cells were incubated with or without TNF-α for the specified time points at 37°C, then placed immediately on ice. Cells were centrifuged and resuspended in 4% paraformaldehyde and fixed for 30 min on melting ice. Cells were spun and washed with ice-cold PBS and resuspended in ice-cold acetone to permeabilize for 5 min on ice. Following permeabilization, cells were washed twice and resuspended for blocking on ice for 20 min Primary antibodies were added on ice for 1 h (anti-phospho p38 1:100 or anti-total p38 1:25). Cells were washed and incubated with goat-anti rabbit FITC-conjugated secondary for 30 min on ice and washed and resuspended in ice-cold PBS for analysis.
Statistical Analysis
Results are expressed as means ± standard error of the mean. Statistical comparisons were performed by Student's t test, Mann-Whitney or two-way ANOVA with the Bonferroni test to analyze data with unequal variance between groups as appropriate. The percentage of motile PMN in individual PMN populations was assessed using χ2 analysis. A probability of p ≤ 0.05 was considered significant. N represents the number of experiments.
Results
Priming of the Respiratory Burst in Human PMN by TNF-α
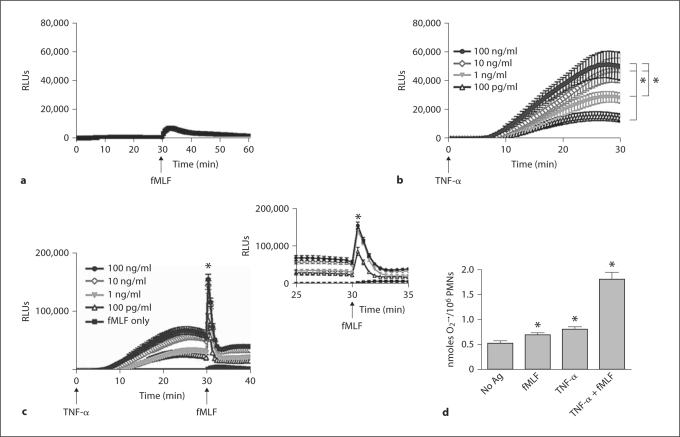
We initially focused on characterizing both the direct and priming responses of the NADPH oxidase after stimulation with pathophysiological concentrations of TNF-α in freshly isolated human PMN. We selected fMLF as the agonist for NADPH oxidase activity in the priming studies as it is well described to be a weak agonist of the respiratory burst in nonprimed cells, but elicits significantly enhanced ROS production after priming. As we have previously reported [7], stimulation of PMN with fMLF alone (1 μM) elicited very low levels of total cellular NADPH oxidase activity as measured by LUC-CL (fig. 1a). We tested a broad range of TNF-α concentrations for priming (100 pg/ml to 100 ng/ml), incubating cells with TNF-α for 30 min. There was dose-dependent generation of ROS in response to TNF-α alone as measured by LUC-CL (fig. 1b). PMN primed for 30 min with all concentrations of TNF-α displayed marked enhancement in NADPH oxidase activity in response to subsequent stimulation with fMLF (fig. 1c). A 44-fold increase in peak ROS level after fMLF was seen in cells primed with the 1 ng/ml TNF-α concentration, compared to ROS generated in response to fMLF alone (fig. 1c, inset). To localize the ROS generated in response to the TNF-α priming stimulus, we used an additional assay of NADPH oxidase activity, the reduction of cyt c,which measures only extracellular generation of superoxide anion. The TNF-α-primed burst in response to fMLF included significant extracellular generation of superoxide (3.4-fold increase in O2– in PMN stimulated with TNF-α and fMLF vs. unstimulated PMN at 30 min; fig. 1d). Low-level, but detectable, superoxide measured under unstimulated conditions represented NADPH oxidase activity occurring over time in our buffer conditions. Importantly, in contrast to the concentration-dependent total ROS stimulated by TNF-α alone as measured by LUC-CL (fig. 1b), the minimal increase in extracellular superoxide generation noted by reduction of cyt c in response to TNF-α alone, as compared to fMLF or unstimulated PMN, does not account for the total ROS generated in response to TNF-α. These data suggest that the ROS generated in response to the priming stimulus, TNF-α, was predominantly intracellular.
Fig. 1.
NADPH oxidase activity in PMN in response to priming by TNF-α. a Stimulation of untreated PMN with a weak agonist of NADPH oxidase activity, fMLF (1 μM), elicited minimal NADPH oxidase activity as measured by LUC-CL. b NADPH oxidase activity was stimulated by TNF-α alone in a concentration-dependent manner with peak total ROS generation at approximately 25 min. * p < 0.05 for TNF-α (100 pg/ml) versus 1, 10 and 100 ng/ml and for TNF-α (1 ng/ml) versus 10 and 100 ng/ml. c PMN primed with TNF-α (1 ng/ml) for 30 min prior to stimulation with fMLF (1 μM) demonstrated a 44-fold increase in ROS generation compared to PMN stimulated with fMLF alone. * p < 0.05. N = 18–21. d Measurement of extracellular superoxide generation by the SOD-inhibitable reduction of cyt c demonstrated a minimal increase in extracellular ROS generation at 30 min in response to fMLF or TNF-α alone. However, PMN primed with TNF-α (1 ng/ml) demonstrated a 3.4-fold increase in extracellular ROS generation following stimulation with fMLF (1 μM) compared to untreated control cells at 30 min, * p < 0.05. N = 15.
Induction of NADPH Oxidase Activity in Response to Differentiation of PLB-985 Cells
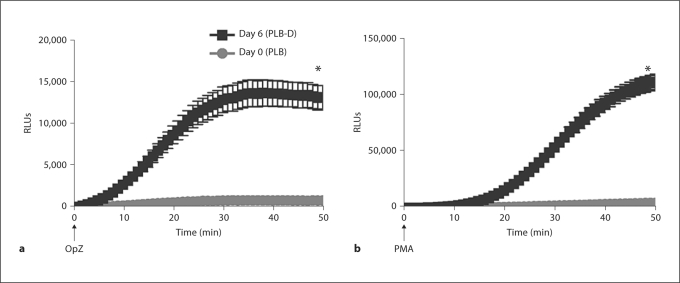
We next sought to investigate priming by TNF-α in a genetically modifiable cell line. We selected the myeloid cell line PLB-985, as there was previous evidence of these cells’ capacity to undergo priming by granulocyte macrophage colony-stimulating factor (GM-CSF). We used the differentiation protocol for PLB-985 cells described by Pedruzzi et al. [22] that demonstrates a greater percentage of cells differentiated into mature neutrophilic cells compared to previously reported methods. Using this protocol, we initially characterized the PLB cell cultures by microscopy according to the phenotypic characteristics initially described for these cells [27]. Following 6 days of differentiation, cells were 84 ± 1.7% differentiated, with 15.5 ± 1.7% in the undifferentiated category, 200 cells per experiment, N = 12. Consistent with previous reports [22], these differentiated PLB-D cells (day 6) displayed significant levels of NADPH oxidase activity as measured by LUC-CL, in response to both a particulate stimulus, OpZ (fig. 2a), and to the soluble agonist, PMA (fig. 2b). NADPH oxidase activity was not seen in the PLB-985 cells prior to the differentiation process (day 0). These data suggested that this differentiation approach was feasible for our studies of TNF-α priming.
Fig. 2.
NADPH oxidase activity in PLB-985 cells before and after differentiation. PLB-985 cells were kept undifferentiated in RPMI 1640 medium (day 0) or were differentiated for 6 days with RPMI 1640 with 0.5% FBS, 0.5% DMF and 1% Nutridoma (day 6). Using LUC-CL, PLB-D cells (day 6) demonstrated markedly enhanced NADPH oxidase activity in response to stimulation with the soluble agonist, PMA (10 ng/ml) (a), or in response to the particulate stimulus, OpZ, at a 5:1 particle:cell ratio as compared with undifferentiated PLB cells (day 0) (b). * p < 0.05. N = 15.
Priming of NADPH Oxidase Activity in PLB-D Cells
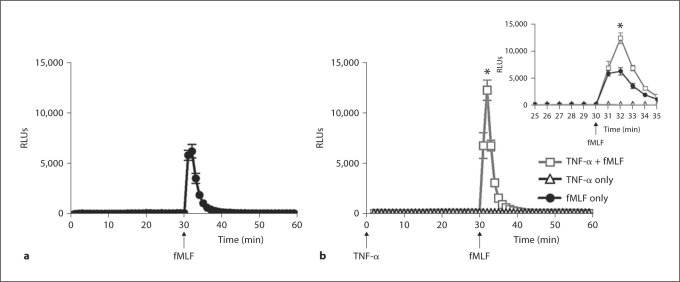
We further investigated NADPH oxidase activity in response to the priming agent TNF-α and in response to fMLF after TNF-α-mediated priming in PLB-D cells. As described above, fMLF was chosen as the postpriming stimulus because it is a weak agonist of the respiratory burst in resting PMN. However, the results of these studies in PLB-D cells were complicated because fMLF stimulated a much greater NADPH oxidase response in naïve (unprimed) PLB-D cells compared to unprimed PMN. A lower concentration of fMLF was studied to determine whether the direct NADPH oxidase response to fMLF might be reduced and priming effects more readily identified. Using 100 nM fMLF as the agonist, there was still significant generation of ROS in PLB-D cells (fig. 3a), but there was also evidence of priming of the respiratory burst in PLB-D cells primed by TNF-α (fig. 3b, inset). There was no increase in the primed burst using higher concentrations of TNF-α for priming. Interestingly, in contrast to the studies in PMN, only minimal levels of ROS were detected in response to the priming agent TNF-α alone (fig. 3b).
Fig. 3.
NADPH oxidase activity in PLB-D cells in response to fMLF after priming by TNF-α. a PLB-D cells demonstrated ROS generation in response to fMLF (100 nM) as measured by LUC-CL. b PLB-D cells primed by 30 min incubation with TNF-α (1 ng/ml) demonstrated significant enhancement of the fMLF-stimulated respiratory burst. There were no detectable ROS generated in response to TNF-α alone. * p < 0.05 as compared to TNF-α only or fMLF. N = 18.
Cell Surface Expression of the fMLF Receptor in PMN and PLB-D Cells
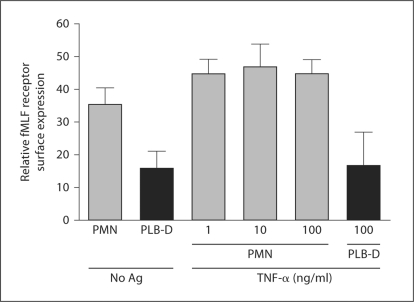
We reasoned that the enhanced NADPH oxidase activity seen in naïve PLB-D cells in response to fMLF might be secondary to greater fMLF receptor density on the surface of unstimulated PLB-D cells as compared with unstimulated PMN. To test this we used flow cytometry to measure binding of F-N, a fluorescent ligand which binds to formyl-peptide receptor 1 [24]. We have previously confirmed that this ligand binds the fMLF receptor by competition studies with unlabelled fMLF [25]. Both PMN and PLB-D cells had easily detectable surface levels of fMLF receptor using this method, however, PMN had greater cell surface expression of the receptor than did PLB-D cells at rest (fig. 4). In response to stimulation with TNF-α, PMN displayed slightly enhanced levels of fMLF receptor expression that were independent of the TNF-α concentration but not significantly increased over unstimulated PMN. There was no increase in F-N binding following TNF-α stimulation of PLB-D cells. Considered in combination with the significant direct NADPH oxidase response to fMLF alone, it appeared that alternate endpoints to assess the primed phenotype in PLB-D cells would be necessary as cellular responses to fMLF stimulation may not be equivalent in PMN and PLB-D cells.
Fig. 4.
Surface expression of fMLF receptor in PMN and PLB-D cells measured by flow cytometry. Cell surface levels of the fMLF receptor, as measured by binding of the fluorescent ligand, F-N, were higher in resting PMN than in unstimulated PLB-D cells. Incubation of PMN with TNF-α (1–100 ng/ml) for 30 min elicited a trend towards increased receptor density that was not significant, however, PLB-D cells displayed no increase in F-N binding following treatment with the highest concentration of TNF-α . N = 4–9.
Enhanced Cell Surface Protein Expression after TNF-α Priming in PMN and PLB-D Cells
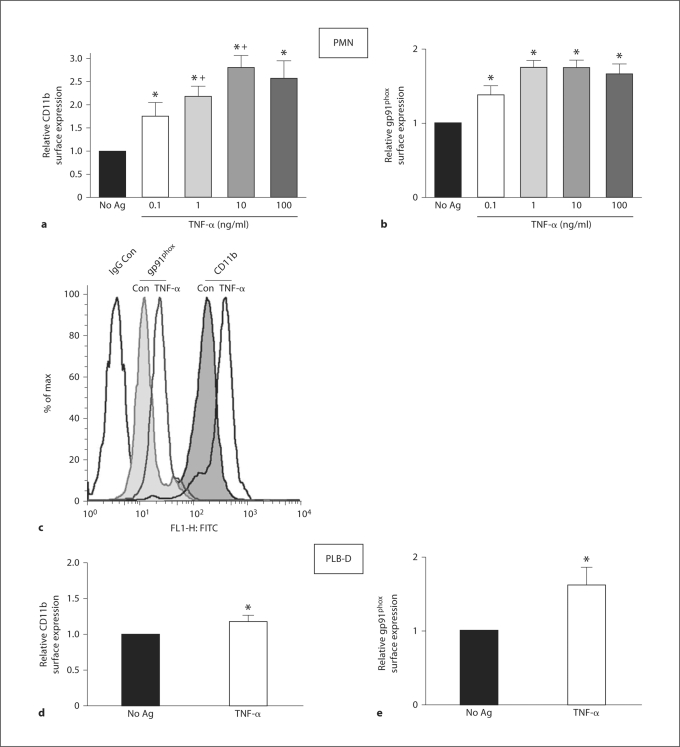
Mechanisms underlying the generation of the primed phenotype have been described to include cell surface mobilization of intracellular stores of certain proteins, including adhesion molecules and subunits of the NADPH oxidase, such that primed cells display enhanced readiness to respond to subsequent stimuli. In view of our previous data demonstrating enhanced PMN surface expression of gp91phox and the β2-integrin CD11b following endotoxin priming [7], we elected to study these additional endpoints of priming for comparison between PMN and PLB-D cells treated with TNF-α. It has been demonstrated previously that TNF-α priming elicits increased surface protein expression, although these studies employed significantly higher concentrations of TNF-α than are found in vivo[13]. Using flow cytometry to measure cell surface molecule expression, we found that TNF-α (100 pg/ml to 100 ng/ml) elicited significantly increased cell surface expression of both CD11b and gp91phox. Priming PMN with 100 pg/ml TNF-α, a concentration routinely found in the plasma of septic patients [9,10,11], increased cell surface levels of CD11b and gp91phox by 1.8- and 1.4-fold, respectively, as compared to unstimulated PMN (fig. 5a–c). Similarly, although the magnitude of change was not as great as that seen in PMN, TNF-α (10 ng/ml) elicited significant enhancement of these proteins on the PLB-D cell surface (fig. 5d–e). These data provided further evidence that PLB-D cells are a genetically modifiable cell line suitable to study priming in combination with studies on primarily isolated human PMN, and that physiologically relevant concentrations of TNF-α elicit changes in cell surface protein expression in PMN.
Fig. 5.
Surface expression of CD11b and gp91phox in PMN and PLB-D cells after priming by TNF-α . As measured by flow cytometry, PMN treated with 0.1–100 ng/ml TNF-α for 30 min demonstrated a concentration-dependent enhancement in cell surface expression of the β2-integrin, CD11b (a) and gp91phox levels (b). N = 5 (0.1 ng/ml), N = 8–12 (1–100 ng/ml). * p < 0.05 as compared to No Ag control value; + p < 0.05 as compared with TNF-α (0.1 ng/ml). c Representative histogram showing increase in CD11b and gp91phox surface expression after TNF-α (1 ng/ml) in PMN. PLB-D cells treated with TNF-α (10 ng/ml) also demonstrated significantly increased CD11b (d) and gp91phox (e) surface levels. * p < 0.05 as compared to No Ag control values. N = 6–8.
PMN and PLB-D Cell Chemotaxis Altered by TNF-α Priming in a Concentration-Dependent Manner
In view of the altered integrin levels observed in response to TNF-α-mediated priming in both PMN and PLB-D cells, we evaluated an additional endpoint of priming, PMN directional migration in response to chemoattractants. The literature on effects of priming on chemotaxis is not straightforward and has been hampered by analysis of TNF-α concentrations in excess of those typically seen during sepsis in vivo. Previous studies have shown that high concentrations of TNF-α inhibit PMN chemotaxis [28,29]. In studies using more physiologically relevant TNF-α concentrations, enhanced PMN migration was observed [30,31].
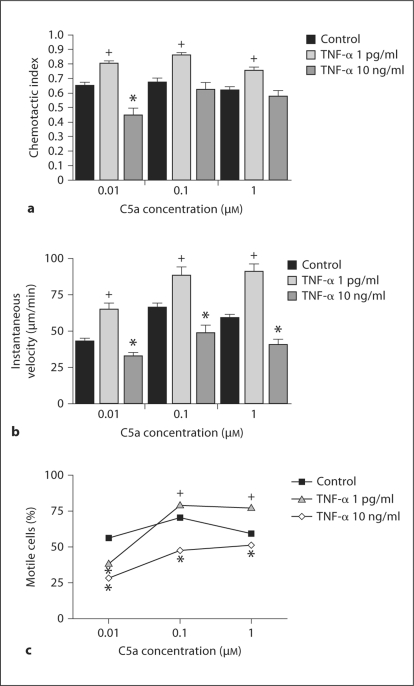
We explored potential alterations in chemotaxis in PMN and PLB-D cells primed with a range of TNF-α concentrations using the EZ-TAXIScan assay, which permits direct visualization of chemotaxis with measurement of individual cell directionality and velocity [25,26]. TNF-α has been shown to alter fMLF receptor-binding affinity [29], therefore, we assessed PMN chemotaxis in response to the anaphylatoxin, C5a, as well as to the classical chemoattractant, fMLF, used in our prior priming studies. We employed a range of chemoattractant concentrations to characterize PMN chemotaxis in response to increasing chemoattractant spatial gradients. PMN primed with a higher concentration of TNF-α (10 ng/ml) demonstrated impaired directional migration (or mean CI) to C5a, with significant impairment in response to the 0.01 μM C5a gradient compared to control PMN (fig. 6a). In contrast, PMN primed with low-level TNF-α (1 pg/ml) demonstrated significantly enhanced chemotaxis in all studied C5a gradients compared to control PMN. In addition, PMN average IV was significantly decreased following priming with 10 ng/ml TNF-α, but significantly increased after treatment with 1 pg/ml TNF-α in response to all studied C5a gradients (fig. 6b). These data were confirmed in our population analysis, demonstrating significantly altered motility curves for TNF-α primed PMN compared to control PMN (fig. 6c). No difference in CI or IV was noted between control and TNF-α (1 pg/ml or 10 ng/ml)-primed PMNs moving in the absence of a C5a gradient (data not shown). Importantly, when we tested the effect of TNF-α priming on chemotaxis in the PLB-985 cell line, PLB-D cells primed with 1 pg/ml TNF-α also demonstrated significantly enhanced chemotaxis compared to control cells in response to 1 μM C5a (control CI 0.45 ± 0.02 vs. TNF-α-primed CI 0.54 ± 0.02, p < 0.05) and 0.01 μM C5a (control CI 0.52 ± 0.02 vs. TNF-α-primed CI 0.69 ± 0.03, p < 0.05). Similar findings were seen in PMN responding to fMLF spatial gradients. PMN primed with 1 pg/ml TNF-α had increased average directional migration (control CI 0.74 ± 0.02 vs. TNF-α-primed CI 0.83 ± 0.01, p < 0.05) and velocity (control IV 60.4 ± 2.6 μm/min vs. TNF-α-primed IV 71.9 ± 3.5 μm/min, p < 0.05) compared to control PMN in response to 1 μM fMLF. In contrast, PMN primed with 10 ng/ml TNF-α had decreased average CI compared to controls in response to fMLF gradients (0.1 μM fMLF: control CI 0.75 ± 0.02 vs. TNF-α-primed CI 0.62 ± 0.04, p < 0.05; 10 μM fMLF: control CI 0.68 ± 0.02 vs. TNF-α-primed 0.53 ± 0.05, p < 0.05). These data suggest that TNF-α priming has concentration-dependent effects on PMN and PLB-D cell-directional motility and that PMN chemotaxis is enhanced by the pathophysiological TNF-α concentrations typically observed during sepsis in vivo.
Fig. 6.
PMN chemotaxis parameters in response to C5a spatial gradients after priming with TNF-α. a PMN primed with TNF-α (1 pg/ml) had significantly increased average CI in response to 0.01, 0.1 and 1 μM C5a spatial gradients compared to control PMN. PMN primed with a higher TNF-α concentration (10 ng/ml) had impaired average CI compared to control PMN, with significantly lower CI in response to 0.01 μM C5a. b PMN primed with TNF-α (1 pg/ml) had significantly increased average IV and PMN primed with TNF-α (10 ng/ml) had significantly decreased average IV compared to control PMN. N = 5–16. c By χ2 analysis, PMN primed with TNF-α (1 pg/ml) for 30 min demonstrated a reduced number of motile cells during the response to 0.01 μM C5a, but a significantly higher percentage of motile cells during the response to 0.1 and 1 μM C5a spatial gradients. PMN treated with TNF-α (10 ng/ml) demonstrated a significantly reduced number of motile cells during the response to C5a at all tested spatial gradient concentrations. Values are the percentage of motile cells in each treatment population with >150 total cells/population. * Denotes values significantly below control. + Denotes values significantly greater than control. p < 0.05. N = 5–16.
NADPH Oxidase Activity Not Required for TNF-α Activation of p38 and ERK1/2 MAPKs
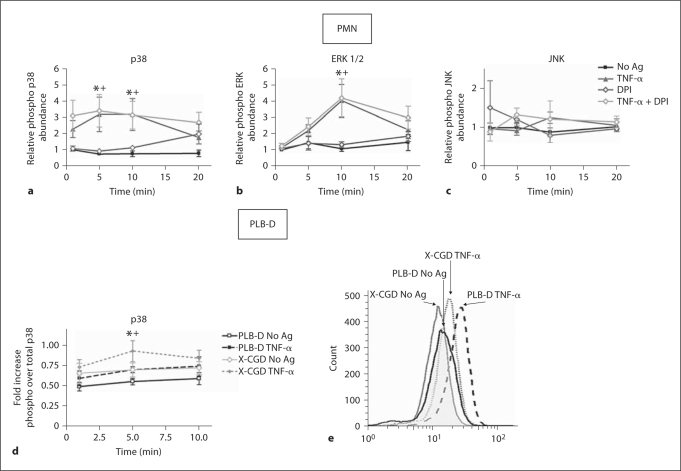
Intracellular signaling proteins involved in the prioritization of chemotactic responses, including the MAP kinases, are under investigation by multiple laboratories. We have previously demonstrated that priming of PMN by endotoxin elicits rapid phosphorylation of p38 MAPK that occurs in an NADPH oxidase-dependent manner [7]. Based on these data and the evidence that TNF-α generates a p38 MAPK-dependent stop signal involved in cell migration [31], we studied phosphorylation of the MAPK proteins in response to TNF-α under control conditions and in the setting of NADPH oxidase inhibition. Confirming the findings of others [18], with extended kinetic analysis, we demonstrated a time-dependent activation of p38 MAPK (fig. 7a) and ERK1/2 (fig. 7b) during incubation with TNF-α, but no alteration in phosphorylation of JNK (fig. 7c). In contrast to our findings with endotoxin priming [7], this enhanced MAPK phosphorylation was not inhibited by pretreatment of PMN with DPI, an inhibitor of flavoproteins that impairs NADPH oxidase function. In view of these data, we also performed analysis of p38 MAPK activation in differentiated PLB cells, as well as X-CGD PLB cells (lacking gp91phox). PLB-D cells demonstrated enhanced phosphorylation of p38 MAPK in response to TNF-α priming, although the magnitude of enhancement of activation was less than that seen in human PMN. Moreover, similar to our findings in DPI-treated human PMN, X-CGD PLB cells also displayed increased levels of phosphorylated p38 MAPK during TNF-α priming, with peak activation at 5 min after TNF-α. (fig. 7d, e).
Fig. 7.
NADPH oxidase activity not required for TNF-α activation of p38 and ERK 1/2 MAPKs. a–c In PMN, phosphorylation of p38, ERK1/2 and JNK was measured by protein isolation and immunoblotting following PMN stimulation with TNF-α (1 ng/ml) in the presence or absence of DPI (50 μM). a TNF-α priming of PMN elicited significantly higher amounts of phospho p38 than unstimulated (No Ag) control PMN at 5 and 10 min. * p ≤ 0.05. N = 7. PMN treated with DPI demonstrated a similar enhancement in phospho p38 levels following TNF-α priming compared to cells treated with DPI alone. + p ≤ 0.05. N = 7. b PMN primed with TNF-α demonstrated a significant increase in phospho ERK1/2 compared to unstimulated (No Ag) control at the 10 min time point. * p ≤ 0.05, N = 7. A significant increase in phospho ERK1/2 levels was also seen in primed PMN treated with DPI compared to PMN treated with DPI alone, + p ≤ 0.05. N = 7. c TNF-α elicited no increase in relative abundance of phospho JNK and DPI treatment had no effect on phospho JNK levels. N = 4. d, e In PLB-D cells, phosphorylation of p38 MAPK was assessed by flow cytometry (relative to total p38 MAPK levels) following stimulation with TNF-α (1 ng/ml). Both PLB-D cells and X-CGDPLB cells (lacking gp91phox) demonstrated a significant increase in phospho p38 at the 5-min time point of priming with TNF-α as compared to unstimulated (No Ag) control cells. *, + p < 0.05. N = 7. e Representative histogram demonstrating the 5 min time point in PLB-D and X-CGD cells ± TNF-α.
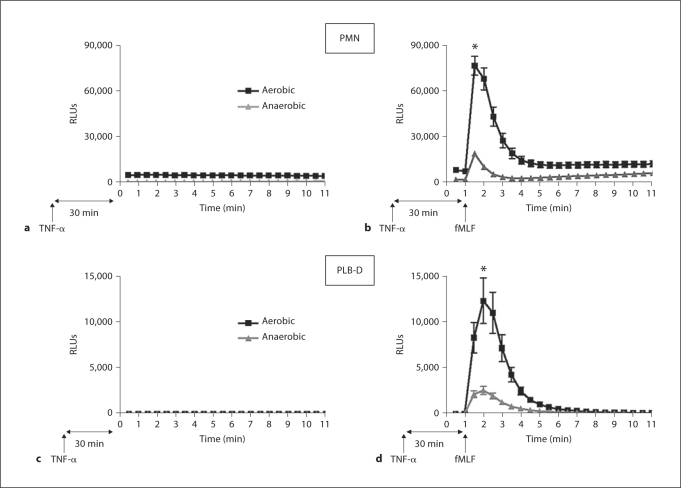
Priming of PMN and PLB-D Cells by TNF-α Is Oxygen Dependent
This contrast in the NADPH oxidase dependence of p38 MAPK activation in response to TNF-α versus endotoxin priming suggested that TNF-α priming might not be oxygen dependent. Moreover, the absence of any direct ROS generation in response to the priming stimulus, TNF-α, in PLB-D cells was notable in view of our previous data suggesting that endotoxin priming is both oxygen and NADPH oxidase dependent [7]. We reasoned that the mechanisms underlying the primed phenotype might be different in response to TNF-α, therefore, we investigated TNF-α-mediated priming under anaerobic conditions. Following priming in the anaerobic chamber, cells were removed to room air for stimulation with fMLF and measurement of NADPH oxidase activity by LUC-CL. This permitted direct comparison between cells primed under aerobic (room air) versus anaerobic, but otherwise identical, conditions. When primed under anaerobic conditions, PMN demonstrated a dramatic reduction in the primed respiratory burst (fig. 8a, b). PMN primed with TNF-α (1 ng/ml) for 30 min under anaerobic conditions had an 85.0% reductionin peak NADPH oxidase activity in response to fMLF, as compared with cells primed under aerobic conditions. For PLB-D cells, the primed burst was 79.0% reduced under anaerobic conditions (fig. 8c, d). This decrease in NADPH oxidase activity did not represent loss of cell viability based on prior controls demonstrating normal PMA- and OpZ-induced ROS generation after 30 min under anaerobic conditions [7]. Despite the generation of negligible levels of ROS in response to the priming stimulus alone in PLB-D cells, the inhibition of priming under anaerobic conditions was strikingly similar to the phenotype seen in PMN. Importantly, some degree of priming occurred even under anaerobic conditions in both PMN and PLB-D cells, suggesting both an oxygen-dependent and oxygen-independent pathway for PMN priming of the respiratory burst by TNF-α.
Fig. 8.
Priming of PMN and PLB-D cells by TNF-α under anaerobic conditions. a TNF-α (1 ng/ml) alone induced minimal NADPH oxidase activity under either aerobic or anaerobic conditions. b PMN primed with TNF-α (1 ng/ml) under anaerobic conditions and then brought to room air had significantly decreased NADPH oxidase activity in response to fMLF as compared to PMN primed under normoxic (aerobic) conditions, as measured by LUC-CL. * p < 0.05. N = 8. c TNF-α (1 ng/ml) alone induced minimal NADPH oxidase activity in PLB-D cells under aerobic or anaerobic conditions. d PLB-D cells primed with TNF-α (1 ng/ml) under anaerobic conditions and then brought to room air displayed a marked oxygen dependence for the primed respiratory burst in cells following 30 min TNF-α priming. * p < 0.05. N = 16.
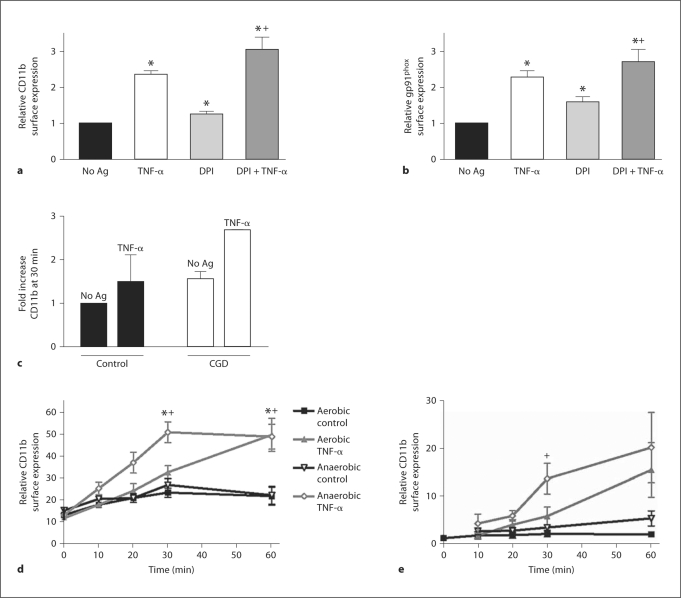
Role of NADPH Oxidase in PMN Priming by TNF-α
Based on the marked reduction in the primed respiratory burst seen under anaerobic conditions, we reasoned that NADPH oxidase might be required for the oxygen-dependent component of priming. We studied cell surface protein expression in control cells versus PMN treated with DPI. We found that unstimulated (no Ag) and DPI-treated PMN had a similar degree of enhancement in CD11b and gp91phox cell surface expression following priming with TNF-α (fig. 9a, b). These data suggest that surface protein mobilization in response to TNF-α-mediated priming is not NADPH oxidase dependent. We did see upregulation of cell surface protein expression in response to treatment by DPI alone, as we have previously described [7]. To further assess this phenotype, we studied the effect of TNF-α priming on PMN from two patients with chronic granulomatous disease who lack functional NADPH oxidase. Interestingly, human PMN lacking NADPH oxidase function also demonstrated increased CD11b surface expression compared to normal control PMN under both unstimulated and TNF-α-priming conditions (fig. 9c). Together, these data suggest that NADPH oxidase-dependent, basal oxidant signaling may be required to maintain cellular quiescence. We performed similar studies to determine whether priming-induced surface mobilization of intracellular protein stores was regulated in an oxygen-sensitive manner. Control PMN were primed aerobically or anaerobically with TNF-α (10 ng/ml) for 10–60 min. At the specified time points, cells were placed on ice and removed from the anaerobic chamber for processing for flow cytometry. Surprisingly, PMN primed with TNF-α under anaerobic conditions had more rapid enhancement of surface CD11b levels than those cells primed under identical, but normoxic, conditions. At the 30-min time point, anaerobically primed cells had a 1.9-fold enhancement in cell surface CD11b levels versus 1.4-fold in the aerobically primed cells compared with controls. By 60 min, both aerobic and anaerobically primed PMN had nearly identical levels of surface protein mobilization (fig. 9d). Based on our concerns that these data might be altered by the PMN isolation process and inadvertant priming during isolation, we repeated these analyses on whole blood under aerobic and anaerobic conditions. Kinetic analysis of PMN studied in whole blood demonstrated TNF-α-elicited changes in CD11b surface levels that were similar to our data from PMN isolated by standard techniques (fig. 9e). Taken together, these studies suggest that mobilization of intracellular stores of proteins occurs rapidly in response to TNF-α, does not require NADPH oxidase function and occurs in an oxygen-independent manner. Moreover, this phenotypic response is not an artifact of cell isolation and may represent a mechanism for enhanced cell motility under appropriate conditions.
Fig. 9.
PMN cell surface protein mobilization in response to TNF-α is not NADPH oxidase dependent. As measured by flow cytometry, PMN pretreated with the flavoprotein inhibitor DPI (50 μM) demonstrated no inhibition of CD11b (a) or gp91phox (b) surface expression in response to priming with TNF-α (10 ng/ml) for 30 min PMN treated with DPI alone displayed significant mobilization of both CD11b and gp91phox in the absence of any other stimulus. * p < 0.05 as compared to No Ag control value, + p < 0.05 as compared to DPI-treated cells in the absence of TNF-α. N = 5–6. c Surface mobilization of CD11b was studied in PMN from two patients with chronic granulomatous disease. PMN lacking functional NADPH oxidase demonstrated increased CD11b surface expression compared to normal control PMN at rest (No Ag) or in response to TNF-α (1 ng/ml). N = 2. Kinetic analysis of PMN CD11b surface mobilization under standard isolation conditions (d) or in whole blood (e). d Control PMN isolated by standard techniques were primed aerobically or anaerobically with TNF-α (1 ng/ml). PMN primed with TNF-α under anaerobic conditions had more rapid enhancement of surface CD11b levels than cells primed aerobically, with nearly identical levels of surface protein mobilization by 60 min. * p < 0.05 as compared to aerobic control; + p < 0.05 as compared to anaerobic control. N = 5–9. e PMN in whole blood stimulated with TNF-α (1 ng/ml) under aerobic and anaerobic conditions demonstrated TNF-α-elicited changes in CD11b surface levels similar to the changes seen in PMN isolated by standard techniques. N = 6.
Discussion
In vivo, TNF-α stimulation of human PMN occurs under a broad range of clinical conditions; however, the highest circulating levels of TNF-α have been documented early in the course of bacterial sepsis and during septic shock. In this setting, the requirement for PMN as an essential cellular component of the innate immune response is unequivocal, but the potential for host tissue damage by release of activated neutrophil products is significant. Host modulation of PMN activation states is accomplished by a number of mechanisms, including PMN priming.
In the current study we explored in vitro neutrophil priming by the cytokine TNF-α. Although PMN priming by TNF-α has been extensively studied, the literature is difficult to synthesize due to the broad range of protocols and concentrations of TNF-α used. The present work demonstrates two novel findings. First, TNF-α-mediated priming of the respiratory burst in response to fMLF is a partially oxygen-dependent process. However, TNF-α-elicited upregulation of CD11b and activation of p38 MAPK occurs in an NADPH oxidase-independent manner. These data are in direct contrast to our findings with endotoxin-elicited priming. Second, the myelocytic PLB cell line can be differentiated to behave in a fashion similar to PMN and can be used as a genetically modifiable adjunct in priming studies. Moreover, we sought to investigate priming by TNF-α at a range of concentrations for each endpoint to determine which elements of the primed phenotype were elicited at pathophysiological levels of TNF-α (that is, levels routinely demonstrated in the circulation of septic patients). Interestingly, priming of PMN occurred in response to picogram per milliliter TNF-α concentrations, which are substantially lower than most investigators have studied and well within the range found in plasma during sepsis [9,10,11]. The signaling mechanisms elicited by these pathophysiological TNF-α concentrations are currently under investigation in our laboratory.
Several signaling proteins required for priming of PMN by TNF-α have already been identified. A role for p38 MAPKs and ERKs, but not JNKs, was first described in priming of the PMN respiratory burst by TNF-α and GM-CSF more than 10 years ago [18]. It is now evident that p38 MAPK is specifically involved in the phosphorylation of serine345 on p47phox, a cytosolic component of the NADPH oxidase [15]. Furthermore, this phosphorylation event is required for TNF-α priming of the respiratory burst [16]. p47phox undergoes a series of phosphorylation events that are required for assembly of the multicomponent NADPH oxidase, but these data were the first evidence for a direct link between phosphorylation of a specific residue and a downstream functional endpoint [32].
Although we were the first to demonstrate an NADPH oxidase-dependent component of priming in neutrophil responses to endotoxin in vitro[7], the concept of ROS signaling involvement in priming has been suggested previously [33]. A role for NADPH oxidase-derived ROS as a signaling intermediary involved in the priming process challenges two fundamental concepts in neutrophil biology. First, the long-standing model of PMN NADPH oxidase activation suggests that nonphagocytic stimuli generate only extracellular ROS [32]. However, our previously published data exploring endotoxin priming [7] and results of the current investigation support our contention that oxidant generation into an intracellular compartment occurs in response to priming stimuli. Furthermore, a number of investigators have presented evidence that intracellular NADPH oxidase assembly occurs in the absence of a phagocytic stimulus with proposed generation of ROS into granular or vesicular structures [34,35,36]. The specific nature of these compartments has yet to be defined. Second, by the classical definition of priming, activation of the NADPH oxidase does not occur in response to the priming stimulus alone. We demonstrate here, and have previously published [7], that ROS are in fact generated in response to priming concentrations of TNF-α or endotoxin, although this is low-level ROS synthesis as compared to the primed burst in response to fMLF. Together, these data require an alteration in our understanding of the phenotypes of the primed cell and mechanisms of priming.
Although cellular responses to the inflammatory stimulus TNF-α and the bacterial product endotoxin are often grouped together based on the fact that both stimuli elicit NF-κB activation [37], the current investigation demonstrates that the mechanisms involved in TNF-α-mediated priming are distinct from the PMN priming response to endotoxin. The TNF-α priming process had both oxygen-dependent and oxygen-independent components. Specifically, the current study demonstrates that protein mobilization to the cell surface did not require NADPH oxidase-derived ROS, but rather occurred in an oxygen-independent manner. Moreover, activation of p38 MAPK occurred independently of NADPH oxidase activity. Bouaouina et al. [33] had previously suggested that p38 MAPK activation in PMN by TNF-α was redox regulated. In addition, Src family tyrosine kinases are involved in TNF-α-mediated β2-integrin activation, important for cell adhesion. Interpretation of these described investigations of TNF-α-elicited cell signaling is complicated by the use of supraphysiologic concentrations of TNF-α for cell stimulation and limited analysis of the specific role of NADPH oxidase derived ROS. Further work is necessary to determine if cellular responses to picogram per milliliter concentrations of TNF-α are qualitatively similar to those seen in response to concentrations >10 ng/ml.
These considerations regarding correlations between cellular responses and the concentration of cytokine studied seem particularly relevant in view of the chemotaxis data presented here. We found that 10 ng/ml TNF-α, a commonly employed concentration in priming studies, significantly inhibited PMN chemotaxis, while 1 pg/ml enhanced directional movement in both PMN and PLB-D cells. These findings may be of particular clinical relevance as we begin to better understand the balance between pro- and anti-inflammatory host responses to infection. Monocyte-derived TNF-α levels may vary widely during the host response to severe sepsis – from very high during the hyperinflammation phase to very low during the immunoparalysis phase [38] – and it appears that this may respectively impede or enhance PMN chemotaxis to sites of infection. Mortality rates associated with uncontrolled pro- or anti-inflammatory states are extremely high and understanding PMN function in vivoduring these clinical conditions remains an area of intense research.
Our characterization of priming responses in the differentiated PLB cell line is likely to facilitate many future studies, as these genetically modifiable cells will assist with manipulation of signaling proteins. A single previous investigation demonstrates priming in this cell type in response to GM-CSF [22], but our report is the first to study PLB 985 cell responses to inflammatory cytokines. There is a growing literature utilizing cell lines differentiated to neutrophil-like phenotypes for genetic manipulation, in combination with studies of primarily isolated PMN. In fact, mutagenesis was utilized in human promyelocytic leukemia HL-60 cells to define the critical serine residue of p47phox required for priming in response to TNF-α [16]. Although the limitations of these cell lines have been extensively documented by others, it is clear that when used in combination with PMN, PLB 985 cell studies will augment in-depth investigation of the signaling pathways involved in PMN function.
In conclusion, we have confirmed that several reported phenotypic endpoints of TNF-α-mediated priming occur in response to picogram per milliliter concentrations of TNF-α. We have demonstrated that differentiated PLB-985 cells undergo priming in response to these physiologically relevant concentrations of TNF-α, with increased NADPH oxidase activity, enhanced surface mobilization of the gp91phox subunit of the flavocytochrome b558 and the β2-integrin CD11b, and augmented chemotaxis, all important endpoints of PMN priming. This investigation has also demonstrated that while TNF-α-mediated priming of the respiratory burst is partially oxygen dependent, other priming endpoints are not similarly redox regulated. Understanding the oxidant-sensitive and oxygen-independent components of the TNF-α signaling pathways elicited in PMN is a current priority in our laboratory.
Acknowledgements
This work was supported by the following NIH-grants: AI079445, HD047349 and HD027748to A.P.D.V.; AI073872 to J.G.M.
References
- 1.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Poulton B. Advances in the management of sepsis: the randomised controlled trials behind the Surviving Sepsis Campaign recommendations. Int J Antimicrob Agents. 2006;27:97–101. doi: 10.1016/j.ijantimicag.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Bass DA, Olbrantz P, Szejda P, Seeds MC, McCall CE. Subpopulations of neutrophils with increased oxidative product formation in blood of patients with infection. J Immunol. 1986;136:860–866. [PubMed] [Google Scholar]
- 4.Drost EM, Kassabian G, Meiselman HJ, Gelmont D, Fisher TC. Increased rigidity and priming of polymorphonuclear leukocytes in sepsis. Am J Respir Crit Care Med. 1999;159:1696–1702. doi: 10.1164/ajrccm.159.6.9803061. [DOI] [PubMed] [Google Scholar]
- 5.Chollet-Martin S, Montravers P, Gibert C, Elbim C, Desmonts JM, Fagon JY, Gougerot-Pocidalo MA. Subpopulation of hyperresponsive polymorphonuclear neutrophils in patients with adult respiratory distress syndrome. Role of cytokine production. Am Rev Respir Dis. 1992;146:990–996. doi: 10.1164/ajrccm/146.4.990. [DOI] [PubMed] [Google Scholar]
- 6.Sheppard FR, Kelher MR, Moore EE, McLaughlin NJ, Banerjee A, Silliman CC. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J Leukoc Biol. 2005;78:1025–1042. doi: 10.1189/jlb.0804442. [DOI] [PubMed] [Google Scholar]
- 7.Moreland JG, Davis AP, Matsuda JJ, Hook JS, Bailey G, Nauseef WM, Lamb FS. Endotoxin priming of neutrophils requires NADPH oxidase-generated oxidants and is regulated by the anion transporter clc-3. J Biol Chem. 2007;282:33958–33967. doi: 10.1074/jbc.M705289200. [DOI] [PubMed] [Google Scholar]
- 8.Condliffe AM, Kitchen E, Chilvers ER. Neutrophil priming: pathophysiological consequences and underlying mechanisms. Clin Sci (Lond) 1998;94:461–471. doi: 10.1042/cs0940461. [DOI] [PubMed] [Google Scholar]
- 9.Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771–778. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- 10.Calandra T, Baumgartner JD, Grau GE, Wu MM, Lambert PH, Schellekens J, Verhoef J, Glauser MP. Prognostic values of tumor necrosis factor/cachectin, interleukin-1, interferon-α, and interferon-γ in the serum of patients with septic shock. Swiss-Dutch j5 Immunoglobulin Study Group. J Infect Dis. 1990;161:982–987. doi: 10.1093/infdis/161.5.982. [DOI] [PubMed] [Google Scholar]
- 11.Heper Y, Akalin EH, Mistik R, Akgoz S, Tore O, Goral G, Oral B, Budak F, Helvaci S. Evaluation of serum c-reactive protein, procalcitonin, tumor necrosis factor α, and interleukin-10 levels as diagnostic and prognostic parameters in patients with community-acquired sepsis, severe sepsis, and septic shock. Eur J Clin Microbiol Infect Dis. 2006;25:481–491. doi: 10.1007/s10096-006-0168-1. [DOI] [PubMed] [Google Scholar]
- 12.Elbim C, Chollet-Martin S, Bailly S, Hakim J, Gougerot-Pocidalo MA. Priming of polymorphonuclear neutrophils by tumor necrosis factor-α in whole blood: identification of two polymorphonuclear neutrophil subpopulations in response to formyl-peptides. Blood. 1993;82:633–640. [PubMed] [Google Scholar]
- 13.Lauterbach M, O'Donnell P, Asano K, Mayadas TN. Role of TNF priming and adhesion molecules in neutrophil recruitment to intravascular immune complexes. J Leukoc Biol. 2008;83:1423–1430. doi: 10.1189/jlb.0607421. [DOI] [PubMed] [Google Scholar]
- 14.Montecucco F, Steffens S, Burger F, Da Costa A, Bianchi G, Bertolotto M, Mach F, Dallegri F, Ottonello L. Tumor necrosis factor-α (TNF-α) induces integrin CD11B/CD18 (Mac-1) up-regulation and migration to the CC chemokine CCL3 (MIP-1α) on human neutrophils through defined signalling pathways. Cell Signal. 2008;20:557–568. doi: 10.1016/j.cellsig.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Dewas C, Dang PM, Gougerot-Pocidalo MA, El-Benna J. TNF-α induces phosphorylation of p47(phox) in human neutrophils: partial phosphorylation of p47phox is a common event of priming of human neutrophils by TNF-α and granulocyte-macrophage colony-stimulating factor. J Immunol. 2003;171:4392–4398. doi: 10.4049/jimmunol.171.8.4392. [DOI] [PubMed] [Google Scholar]
- 16.Dang PM, Stensballe A, Boussetta T, Raad H, Dewas C, Kroviarski Y, Hayem G, Jensen ON, Gougerot-Pocidalo MA, El-Benna J. A specific p47phox-serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J Clin Invest. 2006;116:2033–2043. doi: 10.1172/JCI27544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Condliffe AM, Davidson K, Anderson KE, Ellson CD, Crabbe T, Okkenhaug K, Vanhaesebroeck B, Turner M, Webb L, Wymann MP, Hirsch E, Ruckle T, Camps M, Rommel C, Jackson SP, Chilvers ER, Stephens LR, Hawkins PT. Sequential activation of class IB and class IA PI3K is important for the primed respiratory burst of human but not murine neutrophils. Blood. 2005;106:1432–1440. doi: 10.1182/blood-2005-03-0944. [DOI] [PubMed] [Google Scholar]
- 18.McLeish KR, Knall C, Ward RA, Gerwins P, Coxon PY, Klein JB, Johnson GL. Activation of mitogen-activated protein kinase cascades during priming of human neutrophils by TNF-α and GM-CSF. J Leukoc Biol. 1998;64:537–545. [PubMed] [Google Scholar]
- 19.Blouin E, Halbwachs-Mecarelli L, Rieu P. Redox regulation of beta2-integrin cd11b/cd18 activation. Eur J Immunol. 1999;29:3419–3431. doi: 10.1002/(SICI)1521-4141(199911)29:11<3419::AID-IMMU3419>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Watson RW. Redox regulation of neutrophil apoptosis. Antioxid Redox Signal. 2002;4:97–104. doi: 10.1089/152308602753625898. [DOI] [PubMed] [Google Scholar]
- 21.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 22.Pedruzzi E, Fay M, Elbim C, Gaudry M, Gougerot-Pocidalo MA. Differentiation of PLB-985 myeloid cells into mature neutrophils, shown by degranulation of terminally differentiated compartments in response to N-formyl peptide and priming of superoxide anion production by granulocyte-macrophage colony-stimulating factor. Br J Haematol. 2002;117:719–726. doi: 10.1046/j.1365-2141.2002.03521.x. [DOI] [PubMed] [Google Scholar]
- 23.Gabig TG, Bearman SI, Babior BM. Effects of oxygen tension and pH on the respiratory burst of human neutrophils. Blood. 1979;53:1133–1139. [PubMed] [Google Scholar]
- 24.Liu L, Harbecke O, Elwing H, Follin P, Karlsson A, Dahlgren C. Desensitization of formyl peptide receptors is abolished in calcium ionophore-primed neutrophils: an association of the ligand-receptor complex to the cytoskeleton is not required for a rapid termination of the NADPH-oxidase response. J Immunol. 1998;160:2463–2468. [PubMed] [Google Scholar]
- 25.Volk AP, Heise CK, Hougen JL, Artman CM, Volk KA, Wessels D, Soll DR, Nauseef WM, Lamb FS, Moreland JG. CLC-3 and IClswell are required for normal neutrophil chemotaxis and shape change. J Biol Chem. 2008;283:34315–34326. doi: 10.1074/jbc.M803141200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanegasaki S, Nomura Y, Nitta N, Akiyama S, Tamatani T, Goshoh Y, Yoshida T, Sato T, Kikuchi Y. A novel optical assay system for the quantitative measurement of chemotaxis. J Immunol Methods. 2003;282:1–11. doi: 10.1016/j.jim.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Tucker KA, Lilly MB, Heck L, Jr, Rado TA. Characterization of a new human diploid myeloid leukemia cell line (PLB-985) with granulocytic and monocytic differentiating capacity. Blood. 1987;70:372–378. [PubMed] [Google Scholar]
- 28.Kharazmi A, Nielsen H, Bendtzen K. Modulation of human neutrophil and monocyte chemotaxis and superoxide responses by recombinant TNF-α and GM-CSF. Immunobiology. 1988;177:363–370. doi: 10.1016/S0171-2985(88)80004-4. [DOI] [PubMed] [Google Scholar]
- 29.Atkinson YH, Marasco WA, Lopez AF, Vadas MA. Recombinant human tumor necrosis factor-α. Regulation of N-formylmethionylleucylphenylalanine receptor affinity and function on human neutrophils. J Clin Invest. 1988;81:759–765. doi: 10.1172/JCI113381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bajaj MS, Kew RR, Webster RO, Hyers TM. Priming of human neutrophil functions by tumor necrosis factor: enhancement of superoxide anion generation, degranulation, and chemotaxis to chemoattractants C5a and F-Met-Leu-Phe. Inflammation. 1992;16:241–250. doi: 10.1007/BF00918813. [DOI] [PubMed] [Google Scholar]
- 31.Lokuta MA, Huttenlocher A. TNF-α promotes a stop signal that inhibits neutrophil polarization and migration via a p38 MAPK pathway. J Leukoc Biol. 2005;78:210–219. doi: 10.1189/jlb.0205067. [DOI] [PubMed] [Google Scholar]
- 32.Nauseef WM. Assembly of the phagocyte NADPH oxidase. Histochem Cell Biol. 2004;122:277–291. doi: 10.1007/s00418-004-0679-8. [DOI] [PubMed] [Google Scholar]
- 33.Bouaouina M, Blouin E, Halbwachs-Mecarelli L, Lesavre P, Rieu P. TNF-induced β2 integrin activation involves SRC kinases and a redox-regulated activation of p38 mapk. J Immunol. 2004;173:1313–1320. doi: 10.4049/jimmunol.173.2.1313. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi T, Robinson JM, Seguchi H. Identification of intracellular sites of superoxide production in stimulated neutrophils. J Cell Sci. 1998;111:81–91. doi: 10.1242/jcs.111.1.81. [DOI] [PubMed] [Google Scholar]
- 35.Karlsson A, Dahlgren C. Assembly and activation of the neutrophil NADPH oxidase in granule membranes. Antioxid Redox Signal. 2002;4:49–60. doi: 10.1089/152308602753625852. [DOI] [PubMed] [Google Scholar]
- 36.Ambruso DR, Cusack N, Thurman G. NADPH oxidase activity of neutrophil specific granules: requirements for cytosolic components and evidence of assembly during cell activation. Mol Genet Metab. 2004;81:313–321. doi: 10.1016/j.ymgme.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Muller JM, Ziegler-Heitbrock HW, Baeuerle PA. Nuclear factor κ B, a mediator of lipopolysaccharide effects. Immunobiology. 1993;187:233–256. doi: 10.1016/S0171-2985(11)80342-6. [DOI] [PubMed] [Google Scholar]
- 38.Frazier WJ, Hall MW. Immunoparalysis and adverse outcomes from critical illness. Pediatr Clin North Am. 2008;55:647–668. doi: 10.1016/j.pcl.2008.02.009. xi. [DOI] [PMC free article] [PubMed] [Google Scholar]