Abstract
Valvular heart disease is a major cause of mortality and morbidity. Revealing the cellular processes and molecules that regulate valve formation and remodeling is required to develop effective therapies. A key step in valve formation during heart development is the epithelial-mesenchymal transformation (EMT) of a subpopulation of endocardial cells in the atrioventricular cushion (AVC). The type III transforming growth factor-β receptor (TGFβR3) regulates AVC endocardial cell EMT in vitro and mesenchymal cell differentiation in vivo. Little is known concerning the signaling mechanisms downstream of TGFβR3. Here we use endocardial cell EMT in vitro to determine the role of 2 well-characterized downstream TGFβ signaling pathways in TGFβR3-dependent endocardial cell EMT. Targeting of Smad4, the common mediator Smad, demonstrated that Smad signaling is required for EMT in the AVC and TGFβR3-dependent EMT stimulated by TGFβ2 or BMP-2. Although we show that Smads 1, 2, 3, and 5 are required for AVC EMT, overexpression of Smad1 or Smad3 is not sufficient to induce EMT. Consistent with the activation of the Par6/Smurf1 pathway downstream of TGFβR3, targeting ALK5, Par6, or Smurf1 significantly inhibited EMT in response to either TGFβ2 or BMP-2. The requirement for ALK5 activity, Par6, and Smurf1 for TGFβR3-dependent endocardial cell EMT is consistent with the documented role of this pathway in the dissolution of tight junctions. Taken together, our data demonstrate that TGFβR3-dependent endocardial cell EMT stimulated by either TGFβ2 or BMP-2 requires Smad4 and the activation of the Par6/Smurf1 pathway.
Key Words: Transforming growth factor-β, Epithelial mesenchymal transformation, Atrioventricular cushion, Heart valve, Receptors
Introduction
Valve formation begins between the common atria and ventricle and in the distal outflow tract (OFT) when the heart is a simple tube comprised of 2 concentric cylinders of epithelia separated by a gel-like matrix termed the cardiac jelly. A critical step in valvulogenesis is the transformation of a subpopulation of endocardial cells from the inner cell layer into mesenchymal cells that invade the cardiac jelly and contribute to the valves and septa of the heart [Sadler, 1985]. Transformation occurs at the atrioventricular boundary to initiate formation of the mitral and tricuspid valves and in the OFT to initiate aortic and pulmonary valve formation. Cell transformation depends on factors derived from the myocardium [Bernanke and Markwald, 1982]. Although the later mechanisms of valve maturation are poorly understood it is clear that cushion mesenchyme is critical to the process of valve formation [Sadler, 1985; Keller and Markwald, 1998].
Atrioventricular cushion (AVC) transformation has been studied extensively in avian systems using an in vitro assay in which the AVC is excised and placed on a collagen gel [Barnett and Desgrosellier, 2003]. In this assay, transformation is divided into 3 steps based on cell morphology. Endocardial cells separate from the epithelial sheet and elongate in a step termed activation. Next, the elongated mesenchymal cells enter the matrix, a step termed invasion. Finally, cells migrate through the gel in the migration step. Epithelial-mesenchymal transformation (EMT) is tightly restricted so that endocardial cells in AVC explants undergo EMT whereas endocardial cells in the ventricle do not [Bernanke and Markwald, 1982]. Transforming cells downregulate the endothelial marker PECAM-1 and upregulate smooth muscle α-actin and procollagen type I [Brown et al., 1996; Sugi et al., 2004].
TGFβ is a key regulator of endocardial cell EMT [reviewed in Barnett and Desgrosellier, 2003]. TGFβ signals through 3 receptors: the type I (TGFβR1), type II (TGFβR2), and type III (TGFβR3) receptors. In the canonical pathway [Shi and Massague, 2003] ligand binding to TGFβR2 recruits TGFβR1 or activin receptor-like kinase (ALK) 5. The constitutively active kinase of TGFβR2 phosphorylates and activates the kinase domain of ALK5 which subsequently phosphorylates and activates downstream receptor-associated (R-) Smads 2 and 3 [Kretzschmar and Massague, 1998]. TGFβR3 or betaglycan has a highly conserved intracellular domain with no catalytic activity [Lopez-Casillas et al., 1991; Wang et al., 1991; Cheifetz et al., 1992]. TGFβR3 is required for AVC EMT in vitro [Brown et al., 1999] and mesenchymal cell maturation in vivo (unpublished data). Inactivation of Tgfbr3 in the mouse results in OFT and cushion defects with death at embryonic day 14.5 due to a failure of coronary vessel development [Compton et al., 2007]. In addition to Smads, TGFβ activates additional downstream effectors including RhoA [Bhowmick et al., 2001; Edlund et al., 2002; Bhowmick et al., 2003; Masszi et al., 2003; Deaton et al., 2005], Ras [Ward et al., 2002], MAP kinases [Bhowmick et al., 2001; Bakin et al., 2002; Xie et al., 2004; Deaton et al., 2005], and PI3K/AKt [Bakin et al., 2000] although the mechanisms by which TGFβ regulates these effectors are less well described. A signaling pathway has recently been described where Par6 acts downstream of TGFβ to control apical-basal polarity [Ozdamar et al., 2005]. Par6 is colocalized to the tight junctions with ALK5 by occludin. TGFβR2 is recruited to the tight junctions by TGFβ addition and phosphorylates Par6 resulting in the recruitment of Smurf1 [Wang et al., 2003]. Smurf1 ubiquitination of RhoA leads to the degradation of RhoA to promote tight junction dissolution and EMT [Bose and Wrana, 2006]. In addition to playing a critical role in heart valve development, endothelial cell EMT also has key roles in pathological processes in adults such as cardiac fibrosis [Zeisberg et al., 2007] and cancer progression [Potenta et al., 2008]. Here we use an in vitro model of endocardial cell transformation to test the hypothesis that Smad and Par6 signaling pathways regulate TGFβR3-dependent EMT in endocardial cells.
Materials and Methods
Construction of Adenoviral Constructs
Adenoviruses were generated [He et al., 1998] and titered as described [Townsend et al., 2008]. Viral titers ranged from 109 to 1014 pfu/ml. Injections were adjusted to achieve infection of 20–50% of endocardial cells.
Viral Injections and Collagen Gel Assays
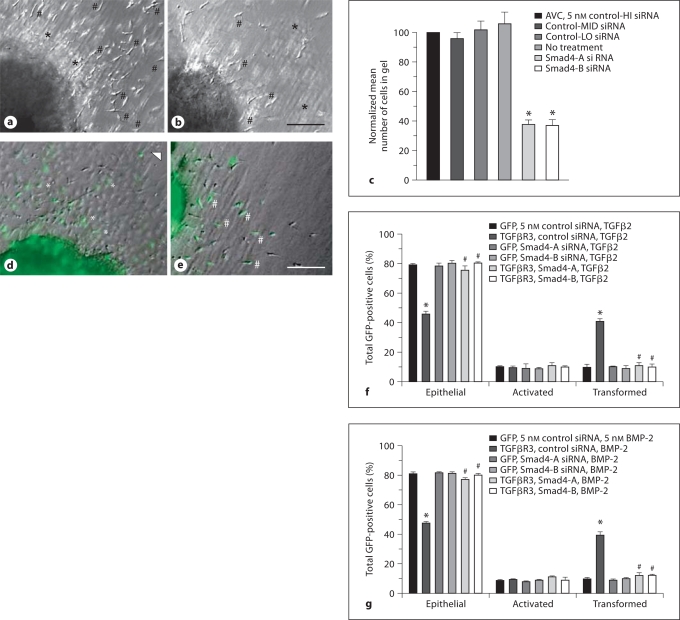
Stage 10–12 chick embryos were harvested, injected with adenovirus, and incubated, and ventricular or AVC explants were excised as described [Desgrosellier et al., 2005]. After 48 h, explants were fixed, and the phenotype of each GFP-expressing cell was scored as described [Desgrosellier et al., 2005; Townsend et al., 2008] (fig. 1d, e).
Fig. 1.
Smad4 is required for AVC endocardial cell EMT. a, b Representative brightfield photomicrographs of AVC explants incubated with control (a) or Smad4-targeted (b) siRNA. Scale bar = 20 μm. Cells scored as transformed have elongated, separated from adjacent cells, and entered the collagen pad (#). Many activated or transformed cells are evident in control explants and are greatly reduced in Smad4 siRNA incubated explants. Rounded, often contiguous cells are scored as epithelial cells (*). c Quantification of the number of cells in the collagen gel. Data are derived from 3 independent experiments normalized to control-high (HI) siRNA. Endocardial cells from AVC explants incubated either with no addition or with any of 3 independent scrambled control siRNAs with varying GC content [HI 62%, medium (MID) 38%, and low (LO) 29%] transformed on collagen gels. However, 2 independent siRNAs targeted against Smad4 inhibited endocardial cell EMT. Control-HI siRNA: normalized to 100%, control-MID siRNA: 97 ± 4.3% (mean ± SEM), control-LO siRNA: 103 ± 6.0%, no addition: 106 ± 7.7%, Smad4-A siRNA: 39 ± 2.8%, and Smad4-B siRNA: 36 ± 3.7%. Two-tailed Student's t test (control-HI vs. specific siRNA) control-MID: p = 0.549; control-LO: p = 0.706; no addition: p = 0.528; Smad4-A: p = 0.002, (* p < 0.05), and Smad4-B: p = 0.003, (* p < 0.05). The number of AVC explants examined and the number of cells in each category were as follows: control-HI (n = 24; total number of cells in gel 3,676), n = number of explants; control-MED (n = 24; total number of cells in gel 3,557); control-LO (n = 24; total number of cells in gel 3,763); no addition (n = 24; total number of cells in gel 3,880); Smad4-A (n = 24; total number of cells in gel 1,415), and Smad4-B (n = 25; total number of cells in gel 1,391). d, e Representative merged brightfield and fluorescent photomicrographs of ventricular explants. Scale bar = 20 μm. d Adenoviral introduction of GFP-only with the plane of focus at the surface of the collagen pad. Rounded, adjacent cells scored as epithelial cells (asterisks) are seen adjacent to the cardiac muscle. Elongated cells in the plane of the surface of the explant are termed activated (arrowhead). e Adenoviral introduction of TGFβR3 and GFP with the plane of focus in the collagen pad. Cells scored as transformed are elongated, separated from adjacent cells, and have entered the collagen pad (#). f Smad4 is required for TGFβR3-dependent, TGFβ2-stimulated ventricular endocardial cell EMT. The average percent of total GFP-expressing cells scored as epithelial, activated, or transformed is derived from 3 separate experiments. Two independent Smad4 siRNA constructs blocked TGFβR3-dependent EMT whereas control siRNA had no effect. g Smad4 is required for TGFβR3-dependent, BMP-2-stimulated ventricular endocardial cell EMT. The average percent of total GFP-expressing cells scored as epithelial, activated, or transformed is derived from 3 separate experiments. Two independent Smad4 siRNA constructs blocked TGFβR3-dependent EMT whereas control siRNA had no effect. For f and g, * denotes significance versus ligand-incubated GFP-only-expressing explants whereas # denotes significance versus ligand-incubated TGFβR3 and GFP-expressing explants. For the actual counts and statistical analysis of f and g, see online supplementary table 1.
Ligand and Inhibitor Addition
Recombinant human TGFβ2 and BMP-2 (R&D Systems, Minneapolis, Minn., USA) addition occurred 12 h after the placement of explants on collagen pads. SB431542 (Sigma-Aldrich, St. Louis, Mo., USA) was added to conditioning media 12 h prior to the placement of explants on collagen pads.
siRNA Treatment of AVC and Ventricular Explants
AVC and ventricular explants were harvested and siRNA was introduced as described [Townsend et al., 2008]. Target sequences for Par6 and Smurf1 were as published [Townsend et al., 2008]. Target sequences for ALK5, TGFβR3, and the Smads (1, 2, 3, 5) are presented in online supplementary table 2 (for all online suppl. material, see www.karger.com/doi/10.1159/000322035). For control siRNA, 3 scrambled 21-oligonucleotide templates with varying GC content that did not blast to any gene in the chicken genome were designed (online suppl. table 2). The control with the GC content most similar to the target siRNA was used.
RNA Isolation and RT-PCR
Chick embryonic fibroblasts were used to confirm the knockdown of genes targeted with siRNA as described [Townsend et al., 2008] (online suppl. fig. S2). The primers used for RT-PCR of Smurf1 and Par6c were as described [Townsend et al., 2008]. Additional primers used for Smad4; ALK5; TGFβR3; Smads 1, 2, 3, 5, and GAPDH are presented in the online supplementary material and were used as described [Bushdid et al., 2001]. RT-PCR data was analyzed using the 2–ΔΔCT method [Livak and Schmittgen, 2001].
Results
Smads Are Required for Endocardial Cell EMT
TGFβ-stimulated EMT can be Smad-dependent or Smad-independent [reviewed in Lopez-Casillas et al., 1991]. To determine whether Smads are required for endocardial cell EMT, we used siRNA targeted to Smad4. Initially, we delivered siRNA constructs against Smad4 to AVC explants from stage 14 embryos. After incubation with siRNA [Townsend et al., 2008], explants were placed on a collagen gel and fixed and scored after 48 h. As compared to control siRNA, 2 independent siRNA constructs to Smad4 decreased the number of transformed cells by 60% (fig. 1a–c). These data demonstrate that endocardial cell EMT at least partially requires Smad4.
To address whether Smad4 is required for TGFβR3-dependent endocardial cell EMT we took advantage of our prior demonstration that TGFβR3 is sufficient and required for endocardial cell EMT in vitro. Overexpression of TGFβR3 in ventricular endocardial cells, which lack TGFβR3 expression, results in EMT after the addition of TGFβ2 [Brown et al., 1999] or BMP-2 [Kirkbride et al., 2008] (online suppl. fig. S1). To determine whether TGFβR3-dependent EMT requires Smad4, we overexpressed TGFβR3 in ventricular endothelial cells and incubated explants with either control siRNA or 1 of 2 siRNA constructs to Smad4. Stage 10–12 chick embryos were harvested and injected with adenovirus expressing either GFP alone or TGFβR3 and GFP. Embryos were cultured for 18–24 h, and ventricular explants from embryos between stages 13 and 15 were excised and placed on collagen gels preincubated with vehicle or siRNA. Twelve hours after explant placement, either vehicle or 200 pM TGFβ2 was added, and the incubation continued for 36 h. Explants were fixed and GFP-positive cells were scored as epithelial (round cells in a sheet on the surface of the gel), activated (elongated, individual cells on the surface of the gel), or transformed (elongated, individual cells in the collagen gel) (fig. 1d, e). Infection of cells with a virus that expressed GFP alone defined the basal distribution of the cells. The addition of TGFβ2 did not alter this distribution [Brown et al., 1999; Townsend et al., 2008] (online suppl. fig. S1). Cells infected with a virus that expressed TGFβR3 and GFP incubated with vehicle had a distribution comparable to that of cells infected with virus that expressed GFP alone. The addition of 200 pM TGFβ2 to cells expressing TGFβR3 resulted in a significant increase in the percent of transformed cells and a concomitant decrease in the percent of cells scored as epithelial. Incubation with siRNA to Smad4, but not control siRNA, inhibited the ability of TGFβ2 to stimulate EMT in TGFβR3-expressing cells (fig. 1f). Incubation with control siRNA did not alter the distribution of cells expressing TGFβR3 and incubated with vehicle.
Since BMP-2 also binds TGFβR3 and stimulates endocardial cell EMT [Kirkbride et al., 2005] we performed similar experiments where 5nM BMP-2 was added instead of 200 pM TGFβ2. The addition of 5 nM BMP-2 to explants overexpressing TGFβR3 resulted in a significant increase in the percent of transformed cells and a concomitant decrease in the percent of cells scored as epithelial (fig. 1g). Incubation with siRNA against Smad4, but not control siRNA, prevented the ability of BMP-2 to maximally stimulate EMT in TGFβR3-expressing cells (fig. 1g). These data demonstrate that Smad4 is required for BMP-2-stimulated, TGFβR3-dependent EMT in endocardial cells.
Smads Are Required but Are Not Sufficient for Endocardial Cell EMT
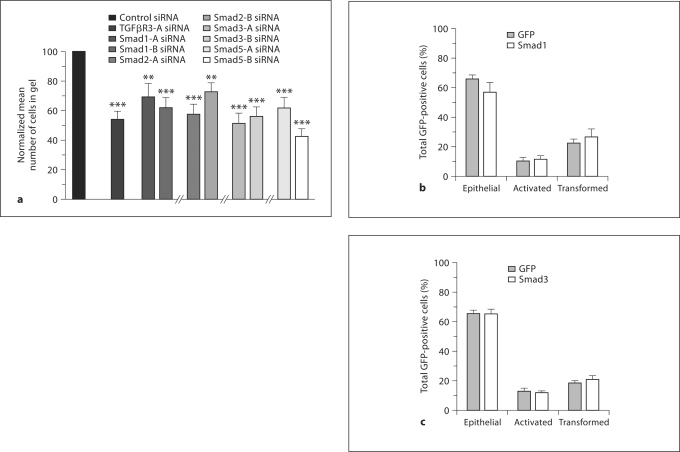
Since Smad4 is required for endocardial cell EMT, we investigated whether specific receptor-regulated Smads are required. Endocardial cell EMT is dependent on ALK5 kinase activity [Townsend et al., 2008]. In addition, both ALK2 and ALK3 have been implicated in endocardial cell EMT [Lai et al., 2000; Desgrosellier et al., 2005; Wang et al., 2005; Song et al., 2007]. Therefore, we targeted Smads downstream of both ALK5 (Smads 2, 3) and ALK2, 3 (Smads 1, 5) in AVC explants. We used 2 siRNA constructs targeted to either Smad 1, 2, 3, or 5, delivered these construct AVC explants from stage 14 embryos, and scored as described above. The targeting of each Smad resulted in a significant decrease in EMT, suggesting that each is required (fig. 2a). We next investigated whether Smad activation is sufficient to induce EMT in endocardial cells. Since overexpression of Smads results in constitutive activity [Edlund et al., 2002] we chose to overexpress Smad1 (downstream of ALK2, ALK3) and Smad3 (downstream of ALK5) independently in ventricular endocardial cells and score for EMT. To confirm that overexpression of Smad1 or Smad3 results in constitutive activity, we assessed the ability of each to activate specific downstream effectors (online suppl. fig. S3B). As expected, expression of Smad1 activated alkaline phosphatase production in C3H10T1/2 cells while Smad3 expression activated the p3TP-lux luciferase reporter. To determine the effects of Smad activation on endocardial cell EMT, embryos were injected, ventricular explants harvested, and cells scored as described above. Neither Smad1 nor Smad3 led to significant differences in the distribution of epithelial, activated, or transformed GFP-positive cells (fig. 2b, c). These data demonstrate that Smad overexpression is not sufficient to induce endocardial cell EMT.
Fig. 2.
Smads are required but not sufficient for EMT. a Targeting of Smads 1, 2, 3, and 5. AVC explants were incubated with control, TGFβR3, or Smad specific targeted siRNA. Cells were scored as in fig. 1a–c. Quantification of the number of cells in the collagen gel is depicted. Data are derived from 3 independent experiments normalized to control siRNA. siRNA to TGFβR3 is presented as a positive control. Representative controls are depicted and the complete cell counts and controls for each Smad examined are found below. Two-tailed Student's t test (control vs. specific siRNA) TGFβR3: p = 0.00003; Smad1-A: p = 0.006; Smad1-B: p = 0.0004; Smad2-A: p = 0.000005; Smad2-B: p = 0.002; Smad3-A: p = 0.0000006; Smad3-B: p = 0.0000008; Smad5-A: p = 0.0004, and Smad5-B: p = 0.0000008, (* p < 0.05). The number of AVC explants examined and cells in each category were as follows: control (n = 31; total number of cells in gel 4,588), n = number of explants; TGFβR3 (n = 29; total number of cells in gel 2,297); Smad1-A (n = 40; total number of cells in gel 4,216); Smad1-B (n = 47; total number of cells in gel 4,357); control (n = 29; total number of cells in gel 4,551); TGFβR3 (n = 32; total number of cells in gel 2,979); Smad2-A (n = 38; total number of cells in gel 3,407); Smad2-B (n = 42; total number of cells in gel 4,728); control (n = 38; total number of cells in gel 5,499); TGFβR3 (n = 39; total number of cells in gel 2,796); Smad3-A (n = 39; total number of cells in gel 3,002); Smad3-B (n = 36; total number of cells in gel 2,856); control (n = 25; total number of cells in gel 3,355); TGFβR3 (n = 23; total number of cells in gel 1,926); Smad5-A (n = 27; total number of cells in gel 2,198), and Smad5-B (n = 29; total number of cells in gel 1,686). b, c Average percent of total GFP-expressing cells scored as epithelial, activated, or transformed. Means are derived from 3 separate experiments. Neither Smad1 (b) nor Smad3 (c) caused statistically significant changes in transformed, activated, or epithelial cells. For the actual counts and statistical analysis, refer to online supplementary table 1.
Par6 Is Required for TGFβ2-Stimulated, TGFβR3-Dependent EMT
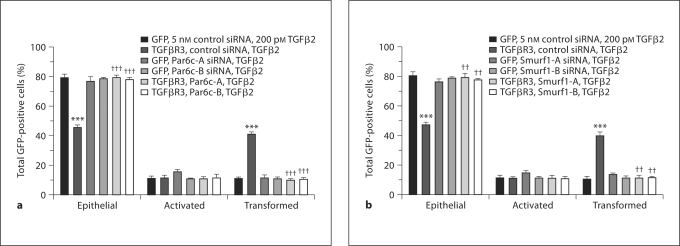
Par6 mediates the TGFβ-stimulated dissolution of tight junctions [Ozdamar et al., 2005]. Recently, we showed that the Par6/Smurf1/RhoA pathway is required for EMT in endocardial cells [Townsend et al., 2008]. To test the hypothesis that TGFβR3-dependent EMT requires Par6, we overexpressed TGFβR3 in ventricular endothelial cells and incubated explants with either control siRNA or siRNA constructs to Par6 as described [Townsend et al., 2008]. The addition of TGFβ2 to cells infected with virus expressing GFP alone did not alter the distribution of cells scored as epithelial, activated, or transformed (fig. 3a). Likewise, the cells infected with virus that expressed TGFβR3 and GFP incubated with vehicle had a distribution comparable to that of cells infected with virus that expressed GFP alone (data not shown). However, the addition of 200 pM TGFβ2 to cells infected with virus that expressed TGFβR3 and GFP resulted in a significant increase in the percent of transformed cells and a concomitant decrease in the percent of cells scored as epithelial. Two siRNA constructs targeted against Par6, but not control siRNA, inhibited the ability of TGFβ2 to stimulate EMT in TGFβR3-expressing cells (fig. 3a).
Fig. 3.
The Par6c pathway is required for TGFβ2-stimulated, TGFβR3-dependent ventricular endocardial cell EMT. Average percent of total GFP-expressing cells scored as epithelial, activated, or transformed. Means are derived from 3 separate experiments. All explants were given 200 pM TGFβ2. GFP served as a negative control to determine the basal level of transformation. TGFβR3 induced statistically significant increases in transformed cells with a concomitant decrease in epithelial cells. a The addition of 2 independent siRNA constructs targeted against Par6c blocked EMT versus control siRNA. For the actual counts and statistical analysis, refer to online supplementary table 1. b The addition of 2 independent siRNA constructs targeted against Smurf1 blocked EMT versus control siRNA. For a and b, *** denotes significance versus ligand-incubated GFP-only-expressing explants whereas †† and ††† denote significance versus ligand-incubated TGFβR3- and GFP-expressing explants. For the actual counts and statistical analysis, refer to online supplementary table 1.
Since Par6 is required for TGFβR3-dependent EMT in endocardial cells, we sought to determine whether Smurf1, which is activated downstream of Par6 [Ozdamar et al., 2005], is required for transformation. We delivered siRNA constructs to Smurf1 [Townsend et al., 2008] to ventricular endocardial cells infected with adenovirus expressing either GFP alone or TGFβR3 and GFP. The addition of 200 pM TGFβ2 resulted in a significant increase in the percent of transformed cells and a concomitant decrease in the percent of cells scored as epithelial. Incubation with siRNA to Smurf1, but not control siRNA, inhibited the ability of TGFβ2 to stimulate EMT in TGFβR3-expressing cells (fig. 3b) Taken together, these data demonstrate that the Par6/Smurf1 pathway is required for TGFβR3 and TGFβ2-stimulated EMT.
Par6 is Required for BMP-2-Stimulated, TGFβR3-Dependent EMT
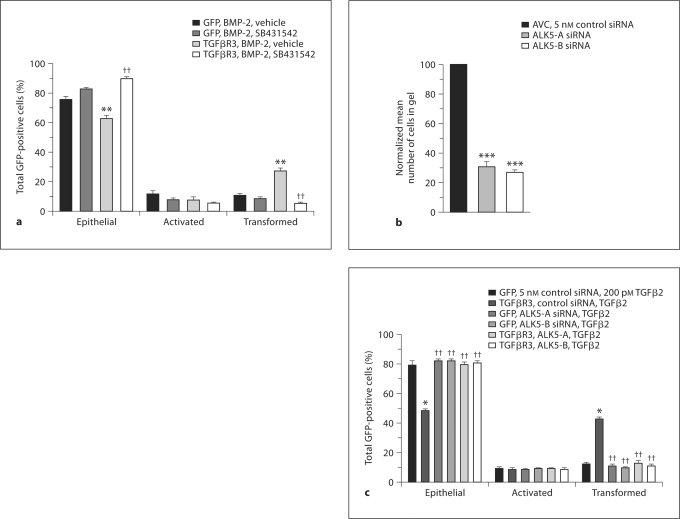
To determine whether BMP-2-stimulated, TGFβR3-dependent EMT requires the Par6 pathway, we initially wondered whether ALK5 kinase activity was required since ALK5 is known to interact with Par6 directly [Ozdamar et al., 2005]. We overexpressed TGFβR3 in ventricular endothelial cells and incubated explants in the presence or absence of 2.5 μM of the ALK5 kinase inhibitor, SB431542, plus or minus 5 nM BMP-2. The addition of BMP-2 to cells infected with virus expressing GFP alone did not alter the distribution of cells scored as epithelial, activated, or transformed (fig. 4a). Cells infected with virus that expressed TGFβR3 and GFP incubated with vehicle had a distribution comparable to that of cells infected with virus that expressed GFP alone (data not shown). In contrast, the addition of 5 nM BMP-2 resulted in a significant increase in the percent of transformed cells and a concomitant decrease in the percent of cells scored as epithelial. These data are consistent with our prior report that BMP-2 stimulates endocardial cell EMT in a TGFβR3-dependent manner [Kirkbride et al., 2008]. Incubation with SB431542 inhibited the ability of BMP-2 to stimulate EMT in TGFβR3-expressing cells, suggesting that ALK5 kinase activity is required for BMP-2-stimulated EMT. Incubation with SB431542 did not alter the distribution of cells expressing TGFβR3 and incubated with vehicle. These data are consistent with prior data using ALK5 kinase inhibitors in the AVC and in TGFβ2-stimulated, TGFβR3-dependent EMT [Townsend et al., 2008].
Fig. 4.
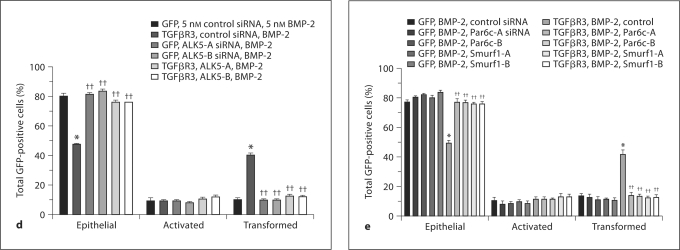
The Par6c pathway is required for BMP-2-stimulated, TGFβR3-dependent ventricular endocardial cell EMT. a–d ALK5 is required for AVC and TGFβR3-dependent ventricular endocardial cell EMT. a ALK5 inhibitor blocks BMP-2-stimulated, TGFβR3-dependent EMT. Average percent of total GFP-expressing cells scored as epithelial, activated or transformed. Means are derived from 3 separate experiments. All ventricular explants were given 5 nM BMP-2. GFP served as a negative control to determine the basal level of transformation. TGFβR3 induced statistically significant increases in transformed cells with a concomitant decrease in epithelial cells. This effect was abolished in the presence of ALK5 kinase inhibitor SB431542. b ALK5-targeted siRNA inhibits AVC endocardial cell EMT. Quantification of AVC endocardial cells migrated into collagen gel. Data are derived from 3 independent experiments normalized to control (LO) siRNA. Endocardial cells from AVC explants given control siRNA transform on collagen gels, whereas 2 independent siRNA constructs targeted to ALK5 inhibit transformation. Control siRNA: normalized to 100%, ALK5-A siRNA: 31 ± 1.2%, and ALK5-B siRNA: 27 ± 0.5%. Two-tailed Student's t test (control vs. treatment) ALK5-A: p = 0.0003 (*** p < 0.001) and ALK5-B: p = 0.000042 (*** p < 0.001). The number of AVC explants examined and cells in each category were as follows: control (n = 30; total number of cells in gel 4,694), n = number of explants; ALK5-A (n = 30; total number of cells in gel 1,463), and ALK5-B (n = 30; total number of cells in gel 1,284). c ALK5-targeted siRNA inhibits TGFβ2-stimulated, TGFβR3-dependent ventricular endocardial cell EMT. Average percent of total GFP-expressing cells scored as epithelial, activated, or transformed. Means are derived from 3 separate experiments. All ventricular explants were given 200 pM TGFβ2. GFP served as a negative control to determine the basal level of transformation. TGFβR3 induced statistically significant increases in transformed cells with a concomitant decrease in epithelial cells. This effect was abolished in the presence of 2 independent siRNAs targeted against ALK5.d, e BMP-2-stimulated, TGFβR3-dependent ventricular endocardial cell EMT requires intact ALK5/Par6c/Smurf1 pathway signaling. Average percent of total GFP-expressing cells scored as epithelial, activated or transformed. Means are derived from 3 separate experiments. All ventricular explants were given 5 nM BMP-2. GFP served as a negative control to determine the basal level of transformation. TGFβR3 induced statistically significant increases in transformed cells with a concomitant decrease in epithelial cells. This effect was abolished in the presence of 2 independent siRNAs targeted against ALK5 (d) or 2 independent siRNAs (e) targeted to either Par6c or Smurf1. Fora, c–e, * denotes significance versus ligand incubated GFP-only-expressing explants whereas †† denotes significance versus ligand-incubated TGFβR3- and GFP-expressing explants. For the actual counts and statistical analysis, refer to online supplementary table 1.
To further establish a role for ALK5 we used 2 independent siRNAs to ALK5 [Mercado-Pimentel et al., 2007]. Initially, we delivered these constructs to AVC explants from stage 14 embryos. Following incubation with siRNA [Townsend et al., 2008] explants were placed on a collagen gel and fixed and scored after 48 h. As compared to control siRNA, each siRNA construct to ALK5 decreased the number of transformed cells by 60% (fig. 4b). To address the role of ALK5 downstream of TGFβR3, we overexpressed TGFβR3 in ventricular endothelial cells and incubated explants with either control siRNA or 1 of 2 siRNA constructs to ALK5. The addition of TGFβ2 (fig. 4c) or BMP2 (fig. 4d) to cells infected with virus expressing GFP alone did not alter the distribution of cells scored as epithelial, activated, or transformed. Cells infected with virus that expressed TGFβR3 and GFP incubated with vehicle had a distribution comparable to that of cells infected with virus that expressed GFP alone (data not shown). However, the addition of 200 pM TGFβ2 (fig. 4c) or 5 nM BMP-2 (fig. 4d) to cells infected with virus that overexpressed TGFβR3 and GFP resulted in a significant increase in the percent of transformed cells and a concomitant decrease in the percent of cells scored as epithelial. This ability to undergo EMT is abolished by the addition of 2 independent siRNA constructs targeted against ALK5 (fig. 4c, d). Therefore, both TGFβ2- and BMP-2-stimulated, TGFβR3-dependent EMT require ALK5.
To further test the hypothesis that BMP-2-stimulated, TGFβR3-dependent EMT requires the activation of the Par6 pathway, we overexpressed TGFβR3 in ventricular endothelial cells and incubated explants with either control siRNA or siRNA constructs to Par6 as above. Incubation with siRNA against Par6, but not control siRNA, inhibited the ability of BMP-2 to stimulate EMT in TGFβR3-expressing cells (fig. 4e). Similarly, incubation with siRNA against Smurf1, but not control siRNA, inhibited the ability of BMP-2 to stimulate EMT in TGFβR3-expressing cells (fig. 4e). These data demonstrate that Par6 and Smurf1 are required for BMP-2-stimulated, TGFβR3-dependent EMT in endocardial cells.
Discussion
TGFβR3 facilitates signaling via TGFβR1/TGFβR2 but also appears to play a unique and nonredundant role in TGFβ signaling. Three lines of evidence support a unique role for TGFβR3 in mediating the actions of TGFβ. First, TGFβR3 is required for AVC endothelial cell transformation in vitro[Brown et al., 1999]. Second, the deletion of Tgfb2, which requires TGFβR3 for binding, results in embryos with a unique and nonoverlapping phenotype compared to Tgfβ1- and Tgfβ3-null animals [Shull et al., 1992]. Finally, the targeted deletion of Tgfbr3 results in embryos with defects distinct from those seen in Tgfbr1 and Tgfbr2 nulls [Stenvers et al., 2003; Compton et al., 2007]. Therefore, the determination of the downstream signaling mechanisms of TGFβR3 is likely to provide novel insight into TGFβ signaling in development and disease.
Here we show that TGFβR3-dependent endocardial cell EMT stimulated by either TGFβ2 or BMP-2 requires both Smad4 and the Par6/Smurf1 signaling pathway. We targeted Smad4, the common mediator Smad, and showed that Smad signaling is required for EMT in the AVC and TGFβR3-dependent EMT stimulated by TGFβ2 or BMP-2. We also found that the receptor-regulated Smads (1, 2, 3, and 5) are required for EMT, suggesting that both TGFβ and BMP pathways regulate EMT. However, overexpression of Smad1 or Smad3 does not induce EMT in ventricular endocardial cells. Although overexpression of Smad does not test for the ability of phosphorylated Smads to alter EMT and although Smad phosphorylation in domains outside of the receptor-regulated phosphorylation of the carboxy terminus domain may modulate Smad signaling [Hayashida et al., 2003], our data suggest that Smad signaling, although required for endocardial cell EMT, is not sufficient for EMT. Prior work examined the role of the inhibitory Smad, Smad6, in endocardial cell EMT and valve formation. Smad6-null mice have valvular hyperplasia suggesting either enhanced EMT or mesenchymal cell proliferation in the cushions [Galvin et al., 2000]. Overexpression of Smad6 in the AVC decreased EMT [Desgrosellier et al., 2005]. Since ALK2 activates Smad1 and Smad6 blocks Smad1 signaling [Hata et al., 1998], these data are consistent with the known role of ALK2 in endocardial cell EMT [Desgrosellier et al., 2005; Wang et al., 2005]. Our current data suggest that the ALK2 activation of Smad1 alone is not sufficient to induce EMT and that the activation of additional signaling pathways is required. In the canonical TGFβ signaling pathway, ligand binding activates ALK5 kinase activity followed by the phosphorylation and subsequent nuclear translocation of Smads [Wrana et al., 1994]. Our prior [Townsend et al., 2008] and current studies reveal a requirement for ALK5 activity downstream of TGFβR3 but suggest that Smad3 activation alone is not sufficient for endocardial cell transformation.
The TGFβ-dependent dissolution of tight junctions in NMuMG cells occurs via the Par6/Smurf1/RhoA pathway and is disassociated from Smad activation [Ozdamar et al., 2005]. We tested whether this pathway regulates TGFβR3-dependent EMT in endocardial cells. Overexpression of TGFβR3 in ventricular endocardial cells, followed by TGFβ2 addition, results in endocardial cell EMT. We previously demonstrated that ALK5 kinase activity is required for TGFβR3-dependent EMT [Townsend et al., 2008]. ALK5 activity is required for the activation of Par6 [Ozdamar et al., 2005]. To target the Par6/Smurf1/RhoA pathway we used siRNA against Par6 and Smurf1 [Townsend et al., 2008]. Consistent with the activation of this pathway downstream of TGFβR3, targeting either Par6 or Smurf1 significantly inhibited EMT. The demonstrated requirement for ALK5 activity, Par6, and Smurf1 for TGFβR3-dependent endocardial cell EMT is consistent with the documented role of this pathway in the dissolution of tight junctions associated with RhoA degradation [Ozdamar et al., 2005; Townsend et al., 2008]. The regulation of endothelial cell junctional complexes is important during embryonic development, angiogenesis, and leukocyte extravasation [Dejana et al., 2001; Wallez and Huber, 2007]. Further, the ubiquitination of RhoA by Smurf1 may play a role in regulating cell shape change and motility [Bryan et al., 2005; Sahai et al., 2007]. Therefore, TGFβR3 may access this pathway to regulate these processes in response to ligand in several tissues.
Recently, we showed that TGFβR3 binds several BMP ligands, including BMP-2, BMP-4, BMP-7, and GDF-5 [Kirkbride et al., 2005]. The BMP subfamily has 20 members with essential roles in development and bone formation [Zhao, 2003; Miyazono et al., 2005]. Specific BMPs elicit distinct effects, yet the mechanism by which a limited number of receptors mediate these effects is unknown. BMP-2 stimulates endocardial cell EMT in a TGFβR3-dependent fashion proving the functional significance of BMP-2 binding to TGFβR3 [Kirkbride et al., 2005]. Here we show that BMP-2-stimulated, TGFβR3-dependent EMT requires ALK5 and Smad4. As with TGFβ2 stimulation, BMP-2-stimulated endocardial cell EMT requires Par6 and Smurf1, suggesting that the Par6/Smur1/RhoA pathway is activated downstream of TGFβR3 via ALK5 in response to both ligands.
Mechanistically, how might TGFβR3 signal through these diverse pathways to regulate both TGFβ2 and BMP-2-stimulated endocardial cell EMT? Signaling through Smad2/3, Smad1/5, and Smad4 is likely mediated, at least in part, by the traditional role of TGFβR3 in binding TGFβ superfamily ligands [Lopez-Casillas et al., 1993; Kirkbride et al., 2008] and presenting these ligands to their respective TGFβ superfamily receptors to enhance signaling [Lopez-Casillas et al., 1993; Kirkbride et al., 2008]. However, TGFβR3 forms complexes with TGFβ superfamily receptors and with the scaffolding molecules GIPC [Blobe et al., 2001a] and β-arrestin2 [Chen et al., 2003], and it has been demonstrated to regulate TGFβ superfamily receptors trafficking through these interactions to regulate TGFβ superfamily signaling through both Smad and non-Smad signaling pathways [Chen et al., 2003; You et al., 2007; Finger et al., 2008a; Lee et al., 2009]. In addition, TGFβR3, through its interaction with β-arrestin2, has been demonstrated to mediate signaling to non-Smad pathways, including the NF-kB pathway [You et al., 2009], and to Cdc42 [Mythreye and Blobe, 2009b], independently of other TGFβ superfamily receptors. Finally, as with other proteoglycan coreceptors [Mythreye and Blobe, 2009a], TGFβR3 might function as a structural/adaptor protein to regulate cell adhesion and migration during EMT. The relative contribution of these diverse roles of TGFβR3 to regulating endocardial cell EMT is currently being explored.
In addition to a prominent role during embryonic development, EMT also has a defined role in mediating several key steps in the metastatic cascade during cancer progression, including invasion through the basement membrane, intravasation, and extravasation [Thiery, 2002]. Accordingly, just as TGFβR3 has been demonstrated to have a role in developmental EMTs in the heart [Brown et al., 1999] and palate [Nakajima et al., 2007], TGFβR3 has been defined as having a role in EMTs during pancreatic cancer progression [Gordon et al., 2008, 2009] as well as being implicated in breast [Reeves et al., 2001] and skin [Levy and Hill, 2005] EMT models. Indeed, the physiological role of TGFβR3 in inhibiting migration and invasion associated with EMT may be a major mechanism by which TGFβR3 functions as a suppressor of cancer progression or as a metastasis suppressor in a broad spectrum of human cancers [Dong et al., 2007; Hempel et al., 2007; Turley et al., 2007; Finger et al., 2008b; Gordon et al., 2008]. These studies suggest that TGFβR3 is another common regulator shared during both developmental and cancer-associated EMT. The mechanism by which TGFβR3 expression is regulated during EMT and by which TGFβR3 functions to regulate EMT during both development and cancer progression is currently under investigation.
Our demonstration that both BMP-2 and TGFβ2 use TGFβR3 to access the pathway required to regulate endocardial cell EMT (online suppl. fig. S4) has several implications. First, these data force us to consider the role of BMP-2 signaling in the interpretation of the phenotype of the Tgfbr3-null mouse. Ablation of BMP-2 from the myocardium during development results in failure of the TGFβR3-expressing endothelial cells in the adjacent valve-forming region of the heart to undergo EMT [Ma et al., 2005; Rivera-Feliciano and Tabin, 2006] while the deletion of TGFβ2 allows EMT but results in inappropriate remodeling of the cushion associated with valvular hyperplasia [Sanford et al., 1997; Bartram et al., 2001]. These data suggest that a second TGFβR3-independent, BMP-2-stimulated pathway is present in endocardial cells to support EMT. However, after transformation, the resulting mesenchymal cells are dependent upon TGFβR3 signaling to support appropriate remodeling of the cushion. Second, given that endothelial cell EMT is recognized as a mechanism for cardiac fibrosis and cancer progression, TGFβR3 may be a potential therapeutic target for these processes. Lastly, TGFβR3 is widely expressed [Blobe et al., 2001b] and is a coreceptor for TGFβs, BMPs, and inhibin. Therefore, given the wide tissue distribution of TGFβR3 and its ability to bind and signal via several ligands in the TGFβ superfamily, TGFβR3 is poised to integrate TGFβ superfamily signaling at the level of the membrane.
Supplementary Material
Supplemental Figures
Supplemental Tables
Supplemental Methods
Acknowledgements
We would like to thank Dr. Andries Ziljstra and Tyson Foods, Inc., for the chicken eggs. The authors thank Dr. Christopher Brown for his critical review of the manuscript. This research was supported in part the by the National Institutes of Health, the Heart, Lung and Blood Institute (HL092551; J.V.B.), and the Institute of General Medical Sciences [GM007628 (T.A.T.) and GM062459 (J.Y.R.)]. C.R.D. was supported by the ASPET Summer Undergraduate Research Fellow Program.
Abbreviations used in this paper
- ALK
activin receptor-like kinase
- AVC
atrioventricular cushion
- BMP
bone morphogenic protein
- EMT
epithelial-mesenchymal transformation
- OFT
outflow tract
- TGFβ
transforming growth factor-β
- TGFβR1
type I transforming growth factor-β receptor
- TGFβR2
type II transforming growth factor-β receptor
- TGFβR3
type III transforming growth factor-β receptor
References
- Bakin A.V., Rinehart C., Tomlinson A.K., Arteaga C.L. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci. 2002;115:3193–3206. doi: 10.1242/jcs.115.15.3193. [DOI] [PubMed] [Google Scholar]
- Bakin A.V., Tomlinson A.K., Bhowmick N.A., Moses H.L., Arteaga C.L. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem. 2000;275:36803–36810. doi: 10.1074/jbc.M005912200. [DOI] [PubMed] [Google Scholar]
- Barnett J.V., Desgrosellier J.S. Early events in valvulogenesis: a signaling perspective. Birth Defects Res C Embryo Today. 2003;69:58–72. doi: 10.1002/bdrc.10006. [DOI] [PubMed] [Google Scholar]
- Bartram U., Molin D.G., Wisse L.J., Mohamad A., Sanford L.P., Doetschman T., Speer C.P., Poelmann R.E., Gittenberger-de Groot A.C. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-beta(2)-knockout mice. Circulation. 2001;103:2745–2752. doi: 10.1161/01.cir.103.22.2745. [DOI] [PubMed] [Google Scholar]
- Bernanke D.H., Markwald R.R. Migratory behavior of cardiac cushion tissue cells in a collagen-lattice culture system. Dev Biol. 1982;91:235–245. doi: 10.1016/0012-1606(82)90030-6. [DOI] [PubMed] [Google Scholar]
- Bhowmick N.A., Ghiassi M., Aakre M., Brown K., Singh V., Moses H.L. TGF-beta-induced RhoA and p160ROCK activation is involved in the inhibition of Cdc25A with resultant cell-cycle arrest. Proc Natl Acad Sci USA. 2003;100:15548–15553. doi: 10.1073/pnas.2536483100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick N.A., Ghiassi M., Bakin A., Aakre M., Lundquist C.A., Engel M.E., Arteaga C.L., Moses H.L. Transforming Growth Factor-beta1 Mediates Epithelial to Mesenchymal Transdifferentiation through a RhoA-dependent Mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobe G.C., Liu X., Fang S.J., How T., Lodish H.F. A novel mechanism for regulating transforming growth factor beta (TGF-beta) signaling: functional modulation of type III TGF-beta receptor expression through interaction with the PDZ domain protein, GIPC. J Biol Chem. 2001a;276:39608–39617. doi: 10.1074/jbc.M106831200. [DOI] [PubMed] [Google Scholar]
- Blobe G.C., Schiemann W.P., Pepin M.C., Beauchemin M., Moustakas A., Lodish H.F., O'Connor-McCourt M.D. Functional roles for the cytoplasmic domain of the type III transforming growth factor beta receptor in regulating transforming growth factor beta signaling. J Biol Chem. 2001b;276:24627–24637. doi: 10.1074/jbc.M100188200. [DOI] [PubMed] [Google Scholar]
- Bose R., Wrana J.L. Regulation of Par6 by extracellular signals. Curr Opin Cell Biol. 2006;18:206–212. doi: 10.1016/j.ceb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Brown C.B., Boyer A.S., Runyan R.B., Barnett J.V. Antibodies to the type II TGFbeta receptor block cell activation and migration during atrioventricular cushion transformation in the heart. Dev Biol. 1996;174:248–257. doi: 10.1006/dbio.1996.0070. [DOI] [PubMed] [Google Scholar]
- Brown C.B., Boyer A.S., Runyan R.B., Barnett J.V. Requirement of type III TGF-beta receptor for endocardial cell transformation in the heart. Science. 1999;283:2080–2082. doi: 10.1126/science.283.5410.2080. [DOI] [PubMed] [Google Scholar]
- Bryan B., Cai Y., Wrighton K., Wu G., Feng X.H., Liu M. Ubiquitination of RhoA by Smurf1 promotes neurite outgrowth. FEBS Lett. 2005;579:1015–1019. doi: 10.1016/j.febslet.2004.12.074. [DOI] [PubMed] [Google Scholar]
- Bushdid P.B., Chen C.L., Brantley D.M., Yull F., Raghow R., Kerr L.D., Barnett J.V. NF-kappaB mediates FGF signal regulation of msx-1 expression. Dev Biol. 2001;237:107–115. doi: 10.1006/dbio.2001.0356. [DOI] [PubMed] [Google Scholar]
- Cheifetz S., Bellon T., Cales C., Vera S., Bernabeu C., Massague J., Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992;267:19027–19030. [PubMed] [Google Scholar]
- Chen W., Kirkbride K.C., How T., Nelson C.D., Mo J., Frederick J.P., Wang X.F., Lefkowitz R.J., Blobe G.C. Beta-arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science. 2003;301:1394–1397. doi: 10.1126/science.1083195. [DOI] [PubMed] [Google Scholar]
- Compton L.A., Potash D.A., Brown C.B., Barnett J.V. Coronary vessel development is dependent on the type III transforming growth factor beta receptor. Circ Res. 2007;101:784–791. doi: 10.1161/CIRCRESAHA.107.152082. [DOI] [PubMed] [Google Scholar]
- Deaton R.A., Su C., Valencia T.G., Grant S.R. Transforming growth factor-beta1-induced expression of smooth muscle marker genes involves activation of PKN and p38 MAPK. J Biol Chem. 2005;280:31172–31181. doi: 10.1074/jbc.M504774200. [DOI] [PubMed] [Google Scholar]
- Dejana E., Spagnuolo R., Bazzoni G. Interendothelial junctions and their role in the control of angiogenesis, vascular permeability and leukocyte transmigration. Thromb Haemost. 2001;86:308–315. [PubMed] [Google Scholar]
- Desgrosellier J.S., Mundell N.A., McDonnell M.A., Moses H.L., Barnett J.V. Activin receptor-like kinase 2 and Smad6 regulate epithelial-mesenchymal transformation during cardiac valve formation. Dev Biol. 2005;280:201–210. doi: 10.1016/j.ydbio.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Dong M., How T., Kirkbride K.C., Gordon K.J., Lee J.D., Hempel N., Kelly P., Moeller B.J., Marks J.R., Blobe G.C. The type III TGF-beta receptor suppresses breast cancer progression. J Clin Invest. 2007;117:206–217. doi: 10.1172/JCI29293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund S., Landstrom M., Heldin C.-H., Aspenstrom P. Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell. 2002;13:902–914. doi: 10.1091/mbc.01-08-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger E.C., Lee N.Y., You H.J., Blobe G.C. Endocytosis of the type III TGF-beta receptor through the clathrin-independent/lipid raft pathway regulates TGF-beta signaling and receptor downregulation. J Biol Chem. 2008a;283:34808–34818. doi: 10.1074/jbc.M804741200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger E.C., Turley R.S., Dong M., How T., Fields T.A., Blobe G.C. TbetaRIII suppresses non-small cell lung cancer invasiveness and tumorigenicity. Carcinogenesis. 2008b;29:528–535. doi: 10.1093/carcin/bgm289. [DOI] [PubMed] [Google Scholar]
- Galvin K.M., Donovan M.J., Lynch C.A., Meyer R.I., Paul R.J., Lorenz J.N., Fairchild-Huntress V., Dixon K.L., Dunmore J.H., Gimbrone M.A., Jr, Falb D., Huszar D. A role for smad6 in development and homeostasis of the cardiovascular system. Nat Genet. 2000;24:171–174. doi: 10.1038/72835. [DOI] [PubMed] [Google Scholar]
- Gordon K.J., Dong M., Chislock E.M., Fields T.A., Blobe G.C. Loss of type III transforming growth factor beta receptor expression increases motility and invasiveness associated with epithelial to mesenchymal transition during pancreatic cancer progression. Carcinogenesis. 2008;29:252–262. doi: 10.1093/carcin/bgm249. [DOI] [PubMed] [Google Scholar]
- Gordon K.J., Kirkbride K.C., How T., Blobe G.C. Bone morphogenetic proteins induce pancreatic cancer cell invasiveness through a Smad1-dependent mechanism that involves matrix metalloproteinase-2. Carcinogenesis. 2009;30:238–248. doi: 10.1093/carcin/bgn274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A., Lagna G., Massague J., Hemmati-Brivanlou A. Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev. 1998;12:186–197. doi: 10.1101/gad.12.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida T., Decaestecker M., Schnaper H.W. Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. FASEB J. 2003;17:1576–1578. doi: 10.1096/fj.03-0037fje. [DOI] [PubMed] [Google Scholar]
- He T.C., Zhou S., da Costa L.T., Yu J., Kinzler K.W., Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel N., How T., Dong M., Murphy S.K., Fields T.A., Blobe G.C. Loss of betaglycan expression in ovarian cancer: role in motility and invasion. Cancer Res. 2007;67:5231–5238. doi: 10.1158/0008-5472.CAN-07-0035. [DOI] [PubMed] [Google Scholar]
- Keller B.B., Markwald R.R. Embryology of the heart. In: Alexander R.W., Schlant R.C., Fuster V., editors. Hurst's the Heart, Arteries and Veins. New York: McGraw-Hill; 1998. pp. 195–212. [Google Scholar]
- Kirkbride K.C., Ray B.N., Blobe G.C. Cell-surface co-receptors: emerging roles in signaling and human disease. Trends Biochem Sci. 2005;30:611–621. doi: 10.1016/j.tibs.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Kirkbride K.C., Townsend T.A., Bruinsma M.W., Barnett J.V., Blobe G.C. Bone morphogenetic proteins signal through the transforming growth factor-beta type III receptor. J Biol Chem. 2008;283:7628–7637. doi: 10.1074/jbc.M704883200. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M., Massague J. SMADs: mediators and regulators of TGF-beta signaling. Curr Opin Genet Dev. 1998;8:103–111. doi: 10.1016/s0959-437x(98)80069-5. [DOI] [PubMed] [Google Scholar]
- Lai Y.T., Beason K.B., Brames G.P., Desgrosellier J.S., Cleggett M.C., Shaw M.V., Brown C.B., Barnett J.V. Activin receptor-like kinase 2 can mediate atrioventricular cushion transformation. Dev Biol. 2000;222:1–11. doi: 10.1006/dbio.2000.9698. [DOI] [PubMed] [Google Scholar]
- Lee N.Y., Kirkbride K.C., Sheu R.D., Blobe G.C. The transforming growth factor-beta type III receptor mediates distinct subcellular trafficking and downstream signaling of activin-like kinase (ALK)3 and ALK6 receptors. Mol Biol Cell. 2009;20:4362–4370. doi: 10.1091/mbc.E09-07-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy L., Hill C.S. Smad4 dependency defines two classes of transforming growth factor beta (TGF-beta) target genes and distinguishes TGF-beta-induced epithelial-mesenchymal transition from its antiproliferative and migratory responses. Mol Cell Biol. 2005;25:8108–8125. doi: 10.1128/MCB.25.18.8108-8125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lopez-Casillas F., Cheifetz S., Doody J., Andres J.L., Lane W.S., Massague J. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-beta receptor system. Cell. 1991;67:785–795. doi: 10.1016/0092-8674(91)90073-8. [DOI] [PubMed] [Google Scholar]
- Lopez-Casillas F., Wrana J.L., Massague J. Betaglycan presents ligand to the TGF beta signaling receptor. Cell. 1993;73:1435–1444. doi: 10.1016/0092-8674(93)90368-z. [DOI] [PubMed] [Google Scholar]
- Ma L., Lu M.F., Schwartz R.J., Martin J.F. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- Masszi A., Di Ciano C., Sirokmany G., Arthur W.T., Rotstein O.D., Wang J., McCulloch C.A., Rosivall L., Mucsi I., Kapus A. Central role for Rho in TGF-beta1-induced alpha-smooth muscle actin expression during epithelial-mesenchymal transition. Am J Physiol Renal Physiol. 2003;284:F911–F924. doi: 10.1152/ajprenal.00183.2002. [DOI] [PubMed] [Google Scholar]
- Mercado-Pimentel M.E., Hubbard A.D., Runyan R.B. Endoglin and Alk5 regulate epithelial-mesenchymal transformation during cardiac valve formation. Dev Biol. 2007;304:420–432. doi: 10.1016/j.ydbio.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K., Maeda S., Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16:251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Mythreye K., Blobe G.C. Proteoglycan signaling co-receptors: roles in cell adhesion, migration and invasion. Cell Signal. 2009a;21:1548–1558. doi: 10.1016/j.cellsig.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mythreye K., Blobe G.C. The type III TGF-beta receptor regulates epithelial and cancer cell migration through beta-arrestin2-mediated activation of Cdc42. Proc Natl Acad Sci USA. 2009b;106:8221–8226. doi: 10.1073/pnas.0812879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima A., Ito Y., Asano M., Maeno M., Iwata K., Mitsui N., Shimizu N., Cui X.M., Shuler C.F. Functional role of transforming growth factor-beta type III receptor during palatal fusion. Dev Dyn. 2007;236:791–801. doi: 10.1002/dvdy.21090. [DOI] [PubMed] [Google Scholar]
- Ozdamar B., Bose R., Barrios-Rodiles M., Wang H.R., Zhang Y., Wrana J.L. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science. 2005;307:1603–1609. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- Potenta S., Zeisberg E., Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer. 2008;99:1375–1379. doi: 10.1038/sj.bjc.6604662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R., Edberg D.D., Li Y. Architectural transcription factor HMGI(Y) promotes tumor progression and mesenchymal transition of human epithelial cells. Mol Cell Biol. 2001;21:575–594. doi: 10.1128/MCB.21.2.575-594.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Feliciano J., Tabin C.J. Bmp2 instructs cardiac progenitors to form the heart-valve-inducing field. Dev Biol. 2006;295:580–588. doi: 10.1016/j.ydbio.2006.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler T. Cardiovascular system. In: Sadler T.W., editor. Langman's Medical Embryology. Baltimore: Williams and Wilkins; 1985. pp. 168–195. [Google Scholar]
- Sahai E., Garcia-Medina R., Pouyssegur J., Vial E. Smurf1 regulates tumor cell plasticity and motility through degradation of RhoA leading to localized inhibition of contractility. J Cell Biol. 2007;176:35–42. doi: 10.1083/jcb.200605135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford L.P., Ormsby I., Gittenberger-de Groot A.C., Sariola H., Friedman R., Boivin G.P., Cardell E.L., Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Shull M.M., Ormsby I., Kier A.B., Pawlowski S., Diebold R.J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D., Annunziata N., Doetschman T. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L., Fassler R., Mishina Y., Jiao K., Baldwin H.S. Essential functions of Alk3 during AV cushion morphogenesis in mouse embryonic hearts. Dev Biol. 2007;301:276–286. doi: 10.1016/j.ydbio.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Stenvers K.L., Tursky M.L., Harder K.W., Kountouri N., Amatayakul-Chantler S., Grail D., Small C., Weinberg R.A., Sizeland A.M., Zhu H.J. Heart and liver defects and reduced transforming growth factor beta2 sensitivity in transforming growth factor beta type III receptor-deficient embryos. Mol Cell Biol. 2003;23:4371–4385. doi: 10.1128/MCB.23.12.4371-4385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi Y., Yamamura H., Okagawa H., Markwald R.R. Bone morphogenetic protein-2 can mediate myocardial regulation of atrioventricular cushion mesenchymal cell formation in mice. Dev Biol. 2004;269:505–518. doi: 10.1016/j.ydbio.2004.01.045. [DOI] [PubMed] [Google Scholar]
- Thiery J.P. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Townsend T.A., Wrana J.L., Davis G.E., Barnett J.V. Transforming growth factor-beta-stimulated endocardial cell transformation is dependent on Par6c regulation of RhoA. J Biol Chem. 2008;283:13834–13841. doi: 10.1074/jbc.M710607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley R.S., Finger E.C., Hempel N., How T., Fields T.A., Blobe G.C. The type III transforming growth factor-beta receptor as a novel tumor suppressor gene in prostate cancer. Cancer Res. 2007;67:1090–1098. doi: 10.1158/0008-5472.CAN-06-3117. [DOI] [PubMed] [Google Scholar]
- Wallez Y., Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta. 2007;1778:794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Wang H.R., Zhang Y., Ozdamar B., Ogunjimi A.A., Alexandrova E., Thomsen G.H., Wrana J.L. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science. 2003;302:1775–1779. doi: 10.1126/science.1090772. [DOI] [PubMed] [Google Scholar]
- Wang J., Sridurongrit S., Dudas M., Thomas P., Nagy A., Schneider M.D., Epstein J.A., Kaartinen V. Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart. Dev Biol. 2005;286:299–310. doi: 10.1016/j.ydbio.2005.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.F., Lin H.Y., Ng-Eaton E., Downward J., Lodish H.F., Weinberg R.A. Expression cloning and characterization of the TGF-beta type III receptor. Cell. 1991;67:797–805. doi: 10.1016/0092-8674(91)90074-9. [DOI] [PubMed] [Google Scholar]
- Ward S.M., Gadbut A.P., Tang D., Papageorge A.G., Wu L., Li G., Barnett J.V., Galper J.B. TGFbeta regulates the expression of G alpha(i2) via an effect on the localization of ras. J Mol Cell Cardiol. 2002;34:1217–1226. doi: 10.1006/jmcc.2002.2073. [DOI] [PubMed] [Google Scholar]
- Wrana J.L., Attisano L., Wieser R., Ventura F., Massague J. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- Xie L., Law B.K., Chytil A.M., Brown K.A., Aakre M.E., Moses H.L. Activation of the Erk pathway is required for TGF-beta1-induced EMT in vitro. Neoplasia. 2004;6:603–610. doi: 10.1593/neo.04241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H.J., Bruinsma M.W., How T., Ostrander J.H., Blobe G.C. The type III TGF-beta receptor signals through both Smad3 and the p38 MAP kinase pathways to contribute to inhibition of cell proliferation. Carcinogenesis. 2007;28:2491–2500. doi: 10.1093/carcin/bgm195. [DOI] [PubMed] [Google Scholar]
- You H.J., How T., Blobe G.C. The type III transforming growth factor-beta receptor negatively regulates nuclear factor kappa B signaling through its interaction with beta-arrestin2. Carcinogenesis. 2009;30:1281–1287. doi: 10.1093/carcin/bgp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg E.M., Tarnavski O., Zeisberg M., Dorfman A.L., McMullen J.R., Gustafsson E., Chandraker A., Yuan X., Pu W.T., Roberts A.B., Neilson E.G., Sayegh M.H., Izumo S., Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- Zhao G.Q. Consequences of knocking out BMP signaling in the mouse. Genesis. 2003;35:43–56. doi: 10.1002/gene.10167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figures
Supplemental Tables
Supplemental Methods