Abstract
The repair of cutaneous wounds in the postnatal animal is associated with the development of scar tissue. Directing cell activities to efficiently heal wounds while minimizing the development of scar tissue is a major goal of wound management and the focus of intensive research efforts. Type III collagen (Col3), expressed in early granulation tissue, has been proposed to play a prominent role in cutaneous wound repair, although little is known about its role in this process. To establish the role of Col3 in cutaneous wound repair, we examined the healing of excisional wounds in a previously described murine model of Col3 deficiency. Col3 deficiency (Col3+/–) in aged mice resulted in accelerated wound closure with increased wound contraction. In addition, Col3-deficient mice had increased myofibroblast density in the wound granulation tissue as evidenced by an increased expression of the myofibroblast marker, α-smooth muscle actin. In vitro, dermal fibroblasts obtained from Col3-deficient embryos (Col3+/– and –/–) were more efficient at collagen gel contraction and also displayed increased myofibroblast differentiation compared to those harvested from wild-type (Col3+/+) embryos. Finally, wounds from Col3-deficient mice also had significantly more scar tissue area on day 21 postwounding compared to wild-type mice. The effect of Col3 expression on myofibroblast differentiation and scar formation in this model suggests a previously undefined role for this ECM protein in tissue regeneration and repair.
Key Words: Type III collagen, Extracellular matrix, Myofibroblast, Wound healing, Scar, Mouse
Introduction
Cutaneous wound healing in the postnatal organism is a balance between rapid wound closure to reestablish the protective barrier of the skin and overexuberant wound healing resulting in excessive scar formation. Complications of either inadequate healing or pathologic dermal scarring represent significant clinical burdens that result in considerable morbidity and health care expenditure. Myofibroblasts play a key role in normal wound repair by facilitating wound closure through contraction [Serini and Gabbiani, 1999]. They modulate the behavior of other cells in the healing response directly through cell-cell interaction as well as indirectly via the secretion of extracellular matrix (ECM) proteins such as collagen, growth factors, and cytokines [Powell et al., 1999]. Their persistence in granulation tissue (GT), however, has been implicated in fibrotic lesions and pathologic scars [Desmouliere et al., 1995; Cass et al., 1997]. The elucidation of mechanisms which modulate the myofibroblast phenotype is critical to the development of strategies that optimize wound repair and promote regenerative healing.
The complex role of the ECM in tissue repair, regeneration, and maintenance is only beginning to be understood. Cells, ECM, and growth factors are all key components in directing the response to tissue injury. It is clear that the view of the ECM serving only as a scaffold to maintain tissue and organ structure is antiquated, and its roles have been dramatically expanded to include the regulation of many key aspects of cell behavior such as migration, proliferation, differentiation, and survival as well as the bioavailability of growth factors. Through effects on individual cell types in the wound, the composition of the ECM modulates reepithelialization (RE), GT formation, contraction, and remodeling. Delayed and pathologic healing of cutaneous wounds has been attributed to aberrant wound ECM composition and remodeling [Cook et al., 2000; Kyriakides and Bornstein, 2003; Järveläinen et al., 2006; Phillips et al., 2008; Liao et al., 2009].
Type III collagen (Col3) is a homotrimeric fibril-forming collagen found in many connective tissues, where it is believed to play a role in regulating collagen fibril diameter [Romanic et al., 1991; Liu et al., 1997], which in turn plays a role in regulating the physical properties of tissues. The expression of Col3 increases early in the healing processes of many tissues, including the skin [Merkel et al., 1988; Hurme et al., 1991; Liu et al., 1995]. This expression pattern suggests Col3 is a key mediator of tissue regeneration and may provide a target for therapeutic intervention for impaired wound healing. Increased expression of Col3 in fetal tissues relative to adult tissues has led some to hypothesize that Col3 may contribute to the scarless phenotype of midgestational cutaneous wounds [Cuttle et al., 2005; Goldberg et al., 2007]. Further supporting a role for Col3 in modulating scar formation is the fact that humans with Col3 mutations have impaired healing which may be associated with excessive scar formation [Burk et al., 2006; Germain, 2007; Sharma et al., 2009]. However, studies that have directly examined whether Col3 plays a role in regenerative tissue response by modulating scar tissue formation have yet to be performed.
Mutations in the Col3 gene lead to an inherited connective tissue disorder, vascular Ehlers-Danlos syndrome (EDS). Individuals with vascular EDS usually survive to the third or fourth decade of life but suffer morbidity associated with impaired wound healing before dying prematurely due to aortic or gastrointestinal rupture [Byers et al., 1979; Pepin et al., 2000; Burk et al., 2006]. Both individuals with Col3 mutations that act in a dominant negative fashion and individuals that are haploinsufficient for Col3 may exhibit similar phenotypes [Schwarze et al., 2001]. A mouse model displaying many of the features of vascular EDS was created by using homologous recombination to inactivate the Col3 gene [Liu et al., 1997]. Most homozygous mutant (Col3–/–) mice die in the early perinatal period, confirming a critical role for this protein in development but making them impractical for postnatal studies. The few mice that do survive develop great vessel and gastrointestinal rupture and display spontaneous, nonhealing cutaneous wounds [Liu et al., 1997]. Collagen fibrils in mutant mice are disorganized in the aorta and skin, two of the tissues most severely affected by Col3 mutations, confirming that Col3 plays structural roles in fibril architecture and tissue elasticity. However, the mechanisms by which Col3 contributes to tissue homeostasis and repair are poorly understood. The impaired healing of cutaneous wounds in vascular EDS patients and in Col3–/– mice indicates that this protein likely plays a significant role in wound healing, particularly in the dermis. Furthermore, the requirement for Col3 in tissue repair may increase with aging, as most people with vascular EDS do not present until the third decade of life [Pepin et al., 2000]. Heterozygous (Col3+/–) mice, shown to express half the amount of Col3 relative to wild-type (Col3+/+) mice, were initially described as phenotypically normal, although few tissues were examined and none were examined in the context of tissue repair [Liu et al., 1997]. However, two studies suggest that structural and functional defects develop in tissues and organs of Col3 haploinsufficient mice [Stevenson et al., 2006; Cooper et al., 2010].
In this study, we investigated the hypothesis that Col3 is an important mediator of regenerative tissue response by examining the effects of diminished Col3 on cutaneous wound repair using the murine model of Col3 deficiency described above [Liu et al., 1997]. We report herein that diminished Col3 expression in haploinsufficient (Col3+/–) mice results in both accelerated cutaneous wound closure by promoting myofibroblast differentiation and increased scar formation. Our data support a shift in the accepted paradigm of a role for Col3 in tissue maintenance and repair. We propose that, in addition to forming an extracellular scaffold which serves a structural purpose, Col3 also plays a critical role in the orchestration of cellular activities during tissue formation and regeneration.
Materials and Methods
Production and Genotyping of Col3-Deficient Mice
Animal utilization and care were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania and followed guidelines set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All mice for this study were generated in a colony established at the University of Pennsylvania from breeder pairs of Col3A1 heterozygous (Col3+/–) mice originally purchased from Jackson Laboratories (Bar Harbor, Me., USA). These mice had been generated by homologous recombination by replacement of the promoter region and first exon of the Col3 gene with a 1.8-kb PGKneo cassette [Liu et al., 1997]. Animals were genotyped for Col3 by PCR analysis of DNA extracted from tail biopsies as described previously [Stevenson et al., 2006]. At the time of genotyping, mice were microchipped for identification (Allflex FDX-B transponders; Allflex USA, Inc., Dallas, Tex., USA). All mice were identified based upon the last 4 digits of the implanted microchip so that wounding, tissue harvesting, and wound analysis were performed in a blinded fashion with respect to the genotype of the individual.
Wounding
Both young (6–11 weeks old) and aged mice were used in wounding experiments. Aged Col3+/+ mice were 57.0 ± 9.5 weeks old and Col3+/– mice were 56.6 ± 9.3 weeks of age. For day 7 wound area (WA) measurements, 9 young mice were used for each genotype. To examine Col3's effects on the wound healing of aged mice, 10 mice for each genotype at day 7, 5 Col3+/+ mice and 4 Col3+/– mice for examination of the scar area at day 21, and 4 mice for each genotype for detailed examination of the WA throughout the first 2 weeks postwounding were used. Wounding experiments were carried out under general anesthesia via inhaled isoflurane. After induction, all mice received preemptive analgesia (buprenorphine, 0.1 mg/kg subcutaneously) and were prepared using a sterile surgical technique. The dorsum was shaved using electric clippers and the clipped area scrubbed with 4% w/v chlorhexidine gluconate scrub. Two full-thickness wounds (through the panniculus carnosus) were created on either side of the spine on the midback of mice using a 6-mm dermal punch (Miltex) and then covered with a sterile occlusive dressing (Tegaderm; 3M, St. Paul, Minn., USA). Full-thickness wounds were also created over the crown of the skull using a 6-mm punch in 4 of the 10 aged mice of each genotype and covered with Tegaderm as described above. Wounds were photographed with an in-picture ruler for scale using a digital camera at the times indicated. The images were imported into an image analysis software (SigmaScan Pro; SPSS Science, Chicago, Ill., USA) and wound outlines were manually traced for calculation of the WA. The average WA for each mouse was utilized for statistical analysis.
Tissue Processing and Histomorphometry
Wound specimens, including a margin of nonwounded skin and the entire wound bed, were collected and fixed in buffered 4% paraformaldehyde. Bisected wounds were paraffin embedded and processed as described previously [Volk et al., 2007]. Serial 4-μm sections were obtained from the bisected wound edge and stained with hematoxylin and eosin (H&E) or Masson's trichrome stain (Accustain Masson's trichrome stain; Sigma Aldrich, St. Louis, Mo., USA) according to the manufacturer's protocol. To assess wound healing quantitatively, the epithelial gap (EG), RE, and GT area were measured at 7 days using computer-assisted morphometric analysis (SpotAdvanced Image Analysis Software 4.6; Diagnostic Imaging, Plainfield, Wisc., USA). Two observers (S.W.V. and E.A.M.), blinded to the genotype of the mouse, performed the measurements. As previously described [Badillo et al., 2007], the EG was defined as the distance between the encroaching epidermal elements. RE was calculated by adding together the lengths of the newly formed epithelium from the lateral edge of the hyperproliferative epithelium lacking hair follicles and the epithelial tongue. GT area was defined as the cellular area from the lateral transition zone from the normal epidermis to the hypertrophic epidermis, inferior to the level of the panniculus carnosus and superior to the epithelial basement membrane or open wound surface. The percentage of wound closure due to contraction was calculated as follows: % wound contraction = [original wound size – (RE + EG)]/original wound size × 100%. Scar tissue area, defined as the area of disorganized collagen deposition which was devoid of adnexal structures, was quantitated in Masson's trichrome-stained sections of bisected 21-day wounds.
Immunohistochemistry
For indirect immunohistochemistry, evaluation of 5-μm serial sections from formalin-fixed paraffin-embedded tissues were mounted on negatively charged glass slides (Superfrost Plus; Fisher Scientific, Pittsburgh, Pa., USA). Slides with tissue sections were heated in a 60°C oven for 1 h to help the adherence of paraffin-embedded tissue sections to slides. Deparaffinization and rehydration of the slides was obtained in several changes of PRO PAR Clearant (Anatech Ltd., Battle Creek, Mich., USA) and decreasing grades of ethanol (from 100 to 95%) and water. Antigen retrieval was performed by immersing slides in a citrate buffer (pH ∼6.0) for 7 min in a 200-watt microwave oven. Endogenous peroxidase activity was inactivated by the application of hydrogen peroxide to tissue sections for 10 min at room temperature. The slides were incubated with the primary antibody directed against alpha-smooth muscle actin (α-SMA; 1:500 dilution; Abcam) for 30 min at room temperature or the universal negative control antibody FLEX (Dako North America, Inc., Carpinteria, Calif., USA) to ascertain nonspecific staining. Detection of the antibody complex was achieved by use of a DAKO ENVISION+ kit containing an ENVpoly-HRP enzyme-labeled polymer-conjugated rabbit secondary antibody (Dako North America) applied for 30 min followed by a 5-min buffer wash. Visualization of antibody binding was obtained via a DAB+ chromogen (3 min), 3,3′-diaminobenzidine solution. The tissue sections were counterstained with hematoxylin for 1 min followed by progressive alcohol dehydration and coverslipping.
Fibroblast Isolation and Culture
The utilization of dermal fibroblasts obtained from fetal mice (E18.5) allowed the comparison of contractile properties of fibroblasts from all 3 genotypes (Col3+/+, +/–, and –/–). Skin from freshly harvested embryos was placed dermal side down and allowed to adhere to culture wells, allowing fibroblast outgrowth. The genotype from harvested embryos was determined as described above. Dermal fibroblasts were cultured in Dulbecco's Modified Eagle Medium (DMEM; Glutamax; Gibco, Grand Island, N.Y., USA) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, Ga., USA) and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin). Cultures were maintained in a humidified incubator in an atmosphere of 5% CO2 and 95% air. Fibroblasts were harvested from cultures using 0.25% trypsin containing 0.05% ethylenediaminetetraacetic acid in Hanks’ solution (Gibco) and passaged prior to 70–80% confluence. Cells were replated at a density of 0.86 × 104/cm2. Cells were used prior to passage 8 for all experiments.
Stressed Collagen Lattice Contraction Assay
To correlate the effect of Col3 expression with the contractile potential of fibroblasts, dermal fibroblasts were grown in attached collagen type I lattices [Grinnell et al., 1999a]. Briefly, fibroblast-populated collagen lattices (FPCLs) were created by suspending fibroblasts in a neutralized type I collagen solution (BD Biosciences; Bedford, Mass., USA) as previously described [Badillo et al., 2007]. The final concentration of the collagen was 1.2 mg/ml. The resuspended fibroblasts, at a final concentration of 0.25 × 106 cells/ml in the collagen solution, were added to each well and incubated at 37°C for 1 h for polymerization. The complete medium was carefully added to wells and replaced on day 2 or 3 in a manner so as to avoid gel detachment. All experiments were performed in triplicate using cells isolated from at least 3 individual embryos of each genotype. On day 5, gels were released and photographed after 30, 60, 120, and 240 min. The percent of gel contraction was determined by calculating the lattice area at the specified time relative to that prior to release.
Immunocytochemistry
Cells grown in collagen lattices were fixed with 3% paraformaldehyde in phosphate-buffered saline (PBS) containing 1 mM CaCl2 for 1 h at 37°C [Hinz et al., 2001]. Cells were washed in PBS followed by 10 min in ice-cold methanol and then washed in PBS. To examine α-SMA-positive stress fibers, fixed cells within the collagen lattices were incubated overnight at room temperature with a polyclonal antibody directed against α-SMA (Abcam; 1:150) on a rocking platform. To permit visualization of the antibody-antigen complex, FPCLs were incubated with an Alexa Fluor 488 donkey anti-rabbit antibody (Invitrogen, Carlsbad, Calif., USA) and 4′, 6′-diamidino-2-phenylindole (DAPI) to identify nuclei. Stained FPCLs were placed on glass slides and a coverslip was placed on top. All slides were viewed with an Olympus Fluorescent microscope with appropriate filters and digital photographs obtained using a constant exposure threshold. The percentage of α-SMA-positive cells within gels was quantitated as previously described [Vaughan et al., 2000] by counting at least 500 cells per gel from randomly selected 200× fields.
Data Analysis
Values are expressed as means ± standard deviation (SD) in the text and figures unless otherwise stated. Unpaired Student's t tests were used to determine the significance of differences between genotypes. Study groups were compared utilizing SigmaPlot software (Systat, Inc., Chicago, Ill., USA). Nonparametric analyses utilized the Shapiro-Wilk test for normality and the Mann-Whitney rank sum test. p < 0.05 was considered statistically significant.
Results
Wound Healing Assessment in Young and Aged Col3 Wild-Type and Col3 Heterozygous Mice
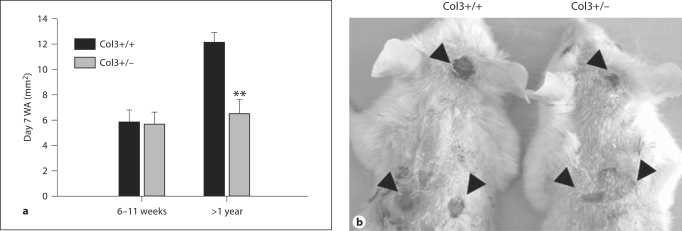
To investigate the effects of diminished Col3 production on cutaneous wound repair, 6-mm full-thickness wounds were created on the dorsum of young (n = 9) and aged (n = 10) mice (aged 6–11 weeks and >1 year, respectively, for each genotype). The aged group of mice was examined due to the fact that human vascular EDS patients are often not symptomatic and remain undiagnosed until the mean age of 28 years [Oderich et al., 2005]. Because Col3–/– mice rarely survive to weaning, heterozygotes were selected to examine the effect of Col3 deficiency in vivo. It has been previously established that tissue Col3 expression in heterozygotes is ∼50% relative to wild-type levels for multiple tissues, including the skin [Liu et al., 1997; Stevenson et al., 2006; Cooper et al., 2010]. Wound healing was examined both grossly and histologically on day 7 postwounding. Digital photographs of the wounds were taken prior to tissue harvesting and the WA was calculated. As expected, the day 7 WA in aged wild-type (Col3+/+) mice was greater than that in young mice, indicating a decreased efficiency of wound closure with age (fig. 1a). Although Col3 deficiency did not affect wound closure in young mice, aged Col3 heterozygous mice had a significant reduction in WA compared to age-matched wild-type mice 7 days after wounding (p < 0.001). In a subset of aged mice (n = 4 of each genotype), 6-mm wounds were also created on the dorsal surface of the skull (fig. 1b) to assess healing in an area where contraction is more limited due to the splinting effect of the bone [Reid et al., 2004]. The day 7 head WA in Col3-deficient heterozygous mice was also decreased compared to that in wild-type mice (10.0 ± 4.67 vs. 23.1 ± 2.9; p < 0.005).
Fig. 1.
Wound size is reduced in aged Col3-deficient (Col3+/–) mice relative to wild-type (Col3+/+) mice at day 7 post-excisional wounding. a Graph showing gross WA measurements of skin wounds 7 days postoperatively in young (6–11-week) and aged (>1-year) mice of both genotypes. Each bar represents the mean ± SEM. ** p < 0.001. b Gross appearance of excisional wounds on the dorsum and head of aged Col3+/+ (left) and Col3+/– (right) mice at 7 days. Wounds of a representative pair of mice from each genotype are shown.
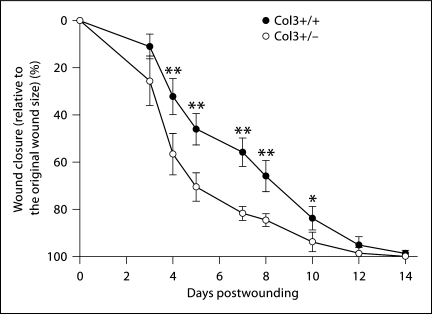
To further assess differences in the rate of wound closure in aged Col3+/– and Col3+/+ mice, the WA was assessed over a 2-week period following excisional wounding. The wounds were photographed over time and the percentage of wound closure was calculated [% wound closure = (the area of the remaining open wound/original WA) × 100] (fig. 2). The original wound size was not significantly different between mice of the 2 genotypes (data not shown). Col3-deficient mice (Col3+/–) demonstrated an accelerated rate of wound closure over time compared to wild-type mice. Significant differences in the percentage of wound closure were found between the 2 genotypes from 4 to 10 days postwounding, with the greatest differences between days 4 and 8 (fig. 2). After day 7, the differential between the 2 groups began to decrease and by day 12 no significant difference between Col3+/+ and Col3+/– wounds was present. By day 10, 25% of the wounds of Col3+/– mice were noted to be macroscopically closed compared to none of the wounds in wild-type mice. All mice in both groups had complete closure of 1 or both wounds by day 14.
Fig. 2.
Col3-deficient mice exhibit accelerated wound closure. Punch biopsy wounds (6 mm in diameter) were created on the dorsum of wild-type (Col3+/+) or Col3-deficient (Col3+/–) mice. Wound healing was expressed as a percent change in the WA relative to the original wound size (mean ± SD). Col3+/– values are significantly different from Col3+/+ values at corresponding time points at * p < 0.05 and ** p < 0.01.
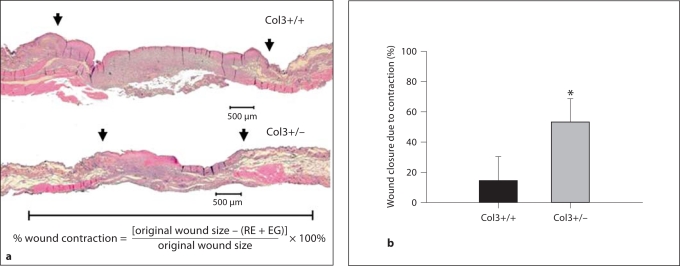
To assess the contribution of RE and wound contraction to wound closure in aged wild-type and Col3 heterozygous mice, histomorphometric analysis was performed. The percentage of wound closure due to contraction, assessed histologically, was found to be increased more than 3-fold in Col3 heterozygous mice compared to wild-type mice (fig. 3). Although the mean EG in the day 7 wounds of Col3-deficient heterozygotes was less than half of that found in control wounds, the difference between the 2 genotypes was not statistically significant (table 1). At that time, 4/10 wounds from wild-type mice and 5/10 wounds from heterozygous mice had completely reepithelialized (data not shown). In contrast, wounds of Col3-deficient mice were found to have a significant decrease in the amount of both neoepithelium and GT formed during the 7 days postwounding relative to wild-type mice (p < 0.001 and p < 0.01, respectively; table 1). The histomorphometric data show that increased wound closure in Col3-deficient mice is due to wound contraction rather than the formation of new tissue (neoepithelialization).
Fig. 3.
Wound contraction is accelerated in aged Col3-deficient (Col3+/–) mice compared to aged wild-type (Col3+/+) mice. a Representative H&E-stained sections through the central wounds from aged Col3+/+ (top) and Col3+/– (bottom) mice. Wounds were harvested at 7 days postoperatively. Arrows indicate the lateral margins of hyperproliferative epithelium (lateral edge of the reepithelialized wound). The scale bar beneath the histologic sections is equal to the original wound diameter of 6 mm. b Quantitative comparison of the percentage of wound closure due to contraction (mean ± SD). At 7 days postwounding, the Col3+/– wounds exhibited significantly more contraction than those in Col3+/+ mice (* p < 0.001, n = 10 mice per group).
Table 1.
Analysis of wound EG, RE, and GT area
| Col3+/+ | Col3+/− | |
|---|---|---|
| EG, μm | 1,373 ± 473 | 701 ± 247 |
| RE, μm | 3,853 ± 384 | 2,122 ± 249** |
| GT area, μm2 | 1,382,115 ± 158,491 | 814,048 ± 164,230* |
At 7 days postwounding, wounds were harvested and processed for histology and morphometric analysis. Data are presented as means ± SEM.
p ≤ 0.01 and
p ≤ 0.001 compared to the Col3 +/+ value.
Wounds in Col3-Deficient Mice Exhibit an Increase in GT α-SMA-Positive Myofibroblasts Compared to Wild-Type Mice
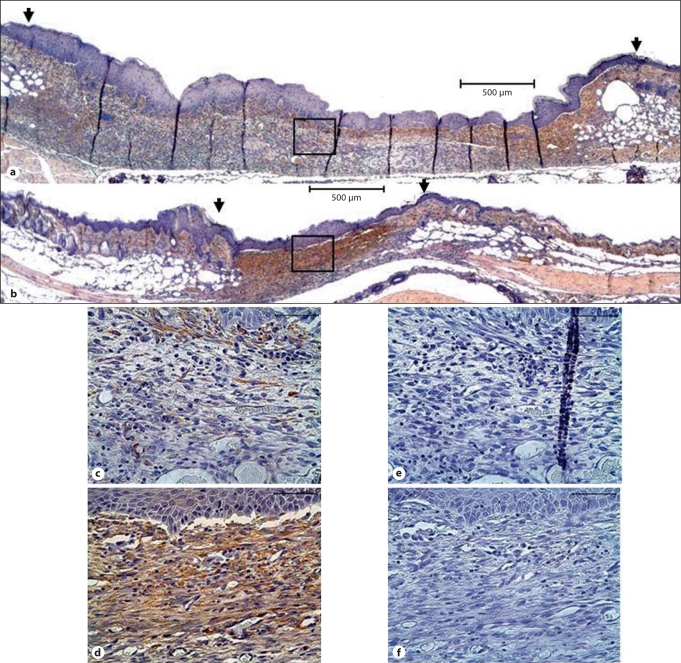
To elucidate a cellular mechanism by which Col3 deficiency leads to an increase in wound contraction, we investigated whether differences in Col3 expression in the wound bed environment modulated myofibroblast differentiation. Myofibroblasts are known to contribute to GT contraction and are identified by their expression of the procontractile protein α-SMA. To examine the effect of Col3 deficiency on α-SMA expression in vivo, immunostaining for α-SMA was performed in histological sections of day 7 wounds. α-SMA-positive myofibroblasts could be seen within the GT of all wounds at this time, with the greatest amount of staining in the upper layer of wounds from both Col3+/+ and +/– mice (fig. 4a–d). Increased staining within this region as well as throughout deeper regions of the granulation bed was observed in wounds of aged Col3+/– mice relative to Col3+/+ mice (fig. 4b, d vs. fig. 4a, c).
Fig. 4.
Wounds in aged Col3-deficient mice show increased wound contraction and GT α-SMA expression. Immunohistochemical staining for α-SMA (brown) in representative wounds from Col3+/+ (a) and Col3+/– (b) mice 7 days after wounding by excisional punch biopsy. Detailed views of the GT and overlying neoepithelium in wounds from Col3+/+ (c) and Col3+/– (d) mice reveal increased α-SMA immunoreactivity in wounds from Col3-deficient mice (Col3+/–) compared to wild-type mice. Corresponding negative controls from adjacent serial sections are shown (e and f, respectively). Scale bars = 500 μm (a, b) and 50 μm in high-magnification views (c–f).
In vitro Analyses of Fibroblast Contraction and Cellular α-SMA Immunostaining in Collagen Gels Reveals an Increase in Myofibroblast Differentiation in Col3-Deficient Fibroblasts Compared to Wild-Type Fibroblasts
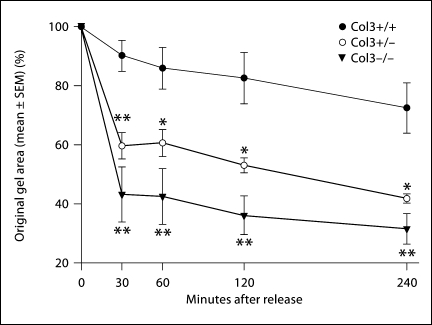
Dermal fibroblasts from Col3 mice were utilized in a stressed FPCL assay, an established model of in vitro wound contraction [Mochitate et al., 1991; Tomasek et al., 1992; Grinnell et al., 1999b]. In comparison to free-floating FPCL assays, stressed FPCL assays more specifically investigate the myofibroblast activity of the seeded cells [Mochitate et al., 1991; Grinnell et al., 1999a; Parizi et al., 2000]. Dermal fibroblasts were isolated from E18.5 fetuses to allow comparison of the contractile phenotype of fibroblasts obtained from all 3 genotypes: Col3+/+ (wild-type), Col3+/–, and Col3–/– (null) mice. Fibroblasts isolated from Col3–/– mice demonstrated a significantly increased contraction of type I collagen gels compared to wild-type fibroblasts (fig. 5; p < 0.01 for all time points examined after release, 30 min through 4 h). Fibroblasts isolated from Col3+/– fetuses exhibited intermediate contractility between those from wild-type and Col3-null mice.
Fig. 5.
Collagen gel contraction by wild-type and Col3-deficient embryonic (∼E18.5) dermal fibroblasts. Wild-type and Col3-deficient fibroblasts were grown in attached collagen gels for 5 days. Stressed lattices were then released and the percentage of contraction calculated at 30, 60, 120, and 240 min post-release. Values represent means ± SEM for individual cell isolates from at least 3 embryos for each genotype with 3 lattices per individual cell isolate. Col3+/– and Col3–/– values were found to be significantly different from Col3+/+ values at all time points (** p < 0.01; * p < 0.05).
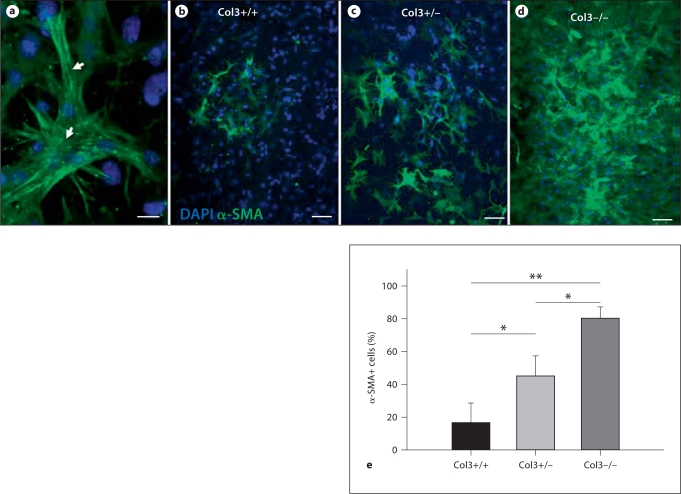
To examine whether the increased contraction in gels seeded with Col3-deficient fibroblasts was associated with increased myofibroblast differentiation, FPCLs were fixed and immunostaining for α-SMA was performed. Incorporation of α-SMA into stress fibers was identified within cells of all 3 genotypes (fig. 6a). Analysis of at least 3 cultures of fibroblasts harvested from 3 fetuses of each genotype demonstrated a dramatic increase in the number of α-SMA-positive cells in gels seeded with Col3-null and Col3+/– heterozygous fibroblasts compared to Col3+/+ fibroblasts (fig. 6b–e). Consistent with our observation that Col3–/– fibroblasts exhibited the most efficient contraction of gels compared to wild-type cells (fig. 5), Col3-null cells also had the highest percentage of α-SMA-positive cells (fig. 6d, e). Again, Col3+/– cells exhibited an intermediate phenotype compared to Col3+/+ and Col3–/– cells (fig. 6b–e); the percent of Col3+/– fibroblasts identified as myofibroblasts was significantly higher (p ≤ 0.05) than that in wild-type cells cultured under identical conditions. These cell culture data support our in vivo results described above (fig. 4), demonstrating increased myofibroblast density in GT in Col3+/– wounds and increased wound contraction.
Fig. 6.
Increased α-SMA expression by Col3-deficient (Col3+/– and Col3–/–) cells compared to Col3 wild-type (Col3+/+) cells. Cells from all 3 genotypes, utilized in the attached fibroblast-populated collagen gel assay, were immunostained for α-SMA (green) and counterstained with DAPI (blue). Collagen lattices seeded with cell isolates from at least 3 embryos per genotype were analyzed. a Myofibroblasts within collagen lattices were identified by α-SMA expression. α-SMA incorporation into stress fibers (arrows) could be visualized in cells from all 3 genotypes. b–d Representative images showing the percentage of myofibroblasts in cultures of Col3+/+ (b), Col3+/– (c), and Col3–/– (d) cells are presented. e Graphic representation of the percentage of α-SMA-positive cells quantitated as described in Materials and Methods, expressed as means ± SD. The percentages of α-SMA-positive Col3-deficient (Col3+/– and Col3–/–) fibroblasts were significantly increased relative to that of Col3+/+ fibroblasts. ** p < 0.001; * p < 0.05. Scale bars = 20 μm (a) and 100 μm (b–e).
The Scar Tissue Area Is Increased in Day 21 Wounds in Col3+/– Mice Relative to Col3+/+ Mice
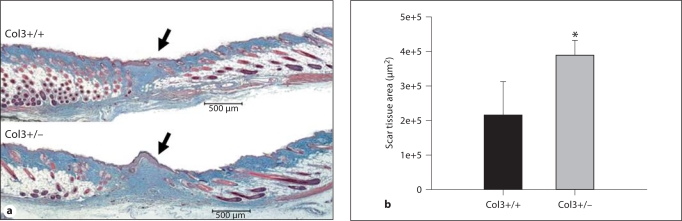
To assess whether an increase in myofibroblast numbers in Col3-deficient wounds resulted in increased scar formation, the area of scar tissue was quantitated in Masson's trichrome-stained sections of day 21 wounds of aged Col3+/– and Col3+/+ mice. The scar tissue area was found to be significantly increased in wounds of Col3+/– mice compared to those of Col3+/+ mice (fig. 7; p < 0.05).
Fig. 7.
Scar formation is increased in wounds of Col3-deficient (Col3+/–) mice relative to wild-type (Col3+/+) mice. a Representative Masson's trichrome-stained sections of bisected wounds from Col3+/+ (top) and Col3+/– (bottom) mice at 21 days postwounding. b Histomorphometric analysis reveals a significantly increased scar tissue area associated with Col3 deficiency (* p < 0.05). Scale bars = 500 μm.
Discussion
Col3 is often cited as an important ECM component of healing tissues, particularly cutaneous wounds, due to increased expression during the early repair phase of a variety of tissues [Merkel et al., 1988; Hurme et al., 1991; Liu et al., 1995]. However, its role in tissue repair has yet to be defined. Liu et al. [1997] previously defined a role for Col3 in regulating collagen fibril diameter, which in turn plays a role in regulating the physical properties of tissues. Analysis of surviving Col3-null mice suggests a critical role for Col3 in maintaining cutaneous integrity as it has previously been noted that ∼60% of Col3-null mice develop spontaneous skin wounds [Liu et al., 1997]. However, additional roles for Col3 in tissue maintenance and its specific role in repair have yet to be defined. We examined cutaneous wound repair in Col3-deficient (Col3+/–) and wild-type (Col3+/+) mice and identified a novel mechanism by which Col3 modulates tissue repair through the modulation of mesenchymal cell activities. Gross examination of healing wounds indicated that Col3 deficiency in aged mice accelerated wound closure (fig. 1, 2). Histomorphometric analysis confirmed that differences in wound closure between Col3-deficient mice and controls were due to an increase in wound contraction (fig. 3; table 1). Because differences in wound size and closure were found to be most significant between days 4 and 8, a time characterized by myofibroblast-mediated wound contraction, we examined the hypothesis that Col3 deficiency would promote myofibroblast differentiation. Both the in vitro and in vivo experiments presented here support this hypothesis (fig. 4, 5, 6). Furthermore, wounds in Col3+/– mice were found to heal with significantly more scar tissue compared to Col3+/+ mice (fig. 7).
Mutations in the Col3 gene lead to an inherited connective tissue disorder, the vascular form of EDS. The murine model of Col3 deficiency utilized here shares phenotypic overlap with vascular EDS patients with respect to the development of vascular lesions in aged haploinsufficient individuals [Cooper et al., 2010] and the catastrophic failure of vascular and intestinal tissues in homozygous-null individuals [Liu et al., 1997]. Cutaneous wounds in vascular EDS patients have been described to heal with increased scar formation [Burk et al., 2006; Germain, 2007; Sharma et al., 2009], which is evident in our Col3-deficient (+/–) mice. Homozygous-null (Col3–/–) mice have been noted to develop spontaneous nonhealing wounds [Liu et al., 1997], suggesting that the complete absence of Col3 leads to structural failure in the skin, similar to vascular and intestinal tissues. Interestingly, we observed significant differences in wound closure between aged Col3+/+ and +/– mice but did not detect any difference in young (6–8-week-old) mice. The synergistic detrimental effects of advancing age and Col3 deficiency appear to be important in the development of pathology, as vascular EDS in human patients is often not diagnosed until the third decade of life [Oderich et al., 2005]. The number and cumulative severity of vascular lesions in haploinsufficient mice has also been found to correlate with age [Cooper et al., 2010]. It may be that at a young age wounds heal with such efficiency that differences cannot be detected with the available methodology. Alternatively, the mechanism by which Col3 mediates its effect(s) may change during the aging process in such a manner that Col3 deficiency is compensated for in young individuals by differences in growth factors, ECM components, and/or other critical regulators but is not compensated under impaired healing conditions associated with aging.
Using an excisional model of wound repair, we found aged Col3+/– mice had accelerated wound closure compared to Col3+/+ mice. Histomorphometric analysis confirmed that this difference in healing was a result of increased wound contraction (fig. 3). The key cellular mediators of wound contraction are myofibroblasts, identified by their de novo expression of the procontractile protein α-SMA in stress fibers [Arora and McCulloch, 1994; Hinz et al., 2001; Hinz and Gabbiani, 2003]. The density of myofibroblasts in both GT and collagen gels has been associated with increased contraction in vivo and in vitro, respectively [McGrath and Hundah, 1982; Rungger-Brandle and Gabbiani, 1983; Hinz et al., 2001]. Our data confirm an increase in myofibroblast density in day 7 GT from Col3+/– mice compared to wild-type (Col3+/+) mice (fig. 4). A direct comparison of the differentiation capacity and activity of fibroblasts isolated from Col3-deficient mice (Col3+/– and –/–) versus those isolated from wild-type mice when placed in a uniform matrix (type I collagen) of the stressed fibroblast-populated collagen gel assay suggests that cells exposed to no or limited Col3 have an increased capacity for myofibroblast differentiation. These data may seem to be in conflict with data by Ehrlich et al. [1988] showing that the fibroblast contraction of Col3-rich lattices was superior to that of type I collagen. However, our studies have used the stressed FPLC model rather than the free-floating FPLC model used previously and therefore may highlight differences in the ability of Col3 to regulate cellular contraction versus fibroblast migration and reorganization. Secondly, differences in the biomechanical properties of lattices comprised of Col1 versus Col3, and therefore the ability of the fibroblasts to contract them, may further explain the differences seen in the contraction of matrices by fibroblasts in these 2 studies. The persistence of myofibroblasts within wound tissue has been associated with increased scar tissue formation [Desmouliere et al., 1995]. The fate of myofibroblasts in GT was not examined in this study; however, we did establish that wounds from Col3+/– mice, which had increased myofibroblasts on day 7, developed a significant increase in scar formation 2 weeks later (day 21 postwounding) compared with those of wild-type mice.
It is now widely accepted that ECM components contribute to tissue repair through the modulation of cell behavior beyond their ability to provide a structural framework for cells. Previous mechanisms by which ECM has been shown to alter cell activity and fate include their ability to modulate integrin expression, regulate growth factor expression and bioavailability, and control matrix metalloproteinase production and activation. Although the detailed mechanism by which Col3 modulates myofibroblast differentiation is currently under investigation, data by Zoppi et al. [2004] suggest that Col3 regulation of integrin expression may play a role in this process. Fibroblasts isolated from individuals with vascular EDS express increased αv and decreased α5β1 and α2β1 integrins compared to those isolated from individuals unaffected by EDS. Preliminary data from our laboratory support the conservation of this response in fibroblasts isolated from Col3–/– mice (data not shown). Both the αvβ3 receptor (vitronectin receptor) and the α5β1 receptor (fibronectin receptor) have been shown to be important in myofibroblast differentiation [Lygoe et al., 2007]. Differential integrin expression may alter the ability of fibroblasts to interact with the provisional matrix in an early wound and promote the differentiation of protomyofibroblasts to myofibroblasts.
Another potential mechanism currently under investigation is suggested by the phenotypic overlap of vascular EDS with Marfan syndrome, which has been shown to result from an increase in TGFβ bioavailability secondary to mutations in the ECM protein fibrillin [Neptune et al., 2003]. The N-propeptides of both type I and II collagen, which show close sequence homology with that of Col3, have previously been shown to modulate TGFβ family member signaling [Zhu et al., 1999; Oganesian et al., 2006], suggesting a potential mechanism for the regulation of TGFβ activity by Col3. Regulation of the TGFβ isoform's bioavailability is of particular interest due to its key role in the regulation of the myofibroblast phenotype and scar tissue formation [Desmouliere et al., 1993; Vaughan et al., 2000; Gabbiani, 2003]. Expression of TGFβ1 has been shown to correlate with a scarring phenotype [Lee et al., 1999]. Furthermore, the addition of TGFβ1 to fetal wounds induces scar formation in a typically scar-free environment and inhibition of TGFβ1 and 2 reduces scar formation [Lin et al., 1995; Shah et al., 1995]. Conversely, TGFβ3 is capable of diminishing scar formation [Shah et al., 1995]. The development of therapeutic strategies to prophylactically reduce scar formation, such as Avotermin (human recombinant TGFβ3), highlights the importance of elucidating the key players in the process of scar tissue formation [Occleston et al., 2010].
Due to the profound effect of Col3 on wound contraction, we focused our investigation on the ability of Col3 to regulate the myofibroblast phenotype. The effect of Col3 on other cell types within the wound is currently under investigation. In this study, we did note a significant decrease in the amount of neoepithelium formed in Col3-deficient wounds compared to controls (Col3+/+). Although this suggests that a Col3-rich environment positively influences RE and supports our hypothesis that Col3 provides an optimal regenerative niche for progenitor and other reparative cells, data must be interpreted cautiously in this model. Because wound closure was accelerated due to wound contraction in Col3+/– mice, it is unclear if the available WA (diminished compared to that in wild-type mice at specified time points) limited neoepithelial formation in these mice. Studies which limit wound contraction, by stenting the surrounding skin, are under investigation and should address this question.
Here we have described a role for Col3 in modulating myofibroblast differentiation and activities during cutaneous repair and have established a cellular mechanism for an increase in scar formation in wounded Col3-deficient skin. Although previous work has identified the role of Col3 in maintaining the structural integrity of tissues, we have shown a novel role for Col3 in the early reparative niche of healing tissues. Future studies will address molecular mechanisms by which Col3 directs the fate of fibroblasts/myofibroblasts and how it influences progenitor and other reparative cell populations in healing tissues. Our data support our overall hypothesis that healing in Col3-deficient environments occurs via a reduced regenerative response and provide support for the further investigation of Col3-rich biomaterials to improve tissue regeneration.
Acknowledgements
This work is supported by a grant from NIAMS (S.W.V.; K08 AR053945). We thank Keith Alcorn and Jason Combs for mouse colony care and maintenance. We also thank Antoneta Radu, Patrice Costello, Juliana Burns, and Jackie Ferracone for their technical assistance with histopathology.
Abbreviations used in this paper
- α-SMA
alpha-smooth muscle actin
- Col3
type III collagen
- ECM
extracellular matrix
- EDS
Ehlers-Danlos syndrome
- EG
epithelial gap
- FPCL
fibroblast-populated collagen lattice
- GT
granulation tissue
- H&E
hematoxylin and eosin
- RE
reepithelialization
- WA
wound area
References
- Arora P.D., McCulloch C.A. Dependence of collagen remodelling on alpha-smooth muscle actin expression by fibroblasts. J Cell Physiol. 1994;159:161–175. doi: 10.1002/jcp.1041590120. [DOI] [PubMed] [Google Scholar]
- Badillo A.T., Redden R.A., Zhang L., Doolin E.J., Liechty K.W. Treatment of diabetic wounds with fetal murine mesenchymal stromal cells enhances wound closure. Cell Tissue Res. 2007;329:301–311. doi: 10.1007/s00441-007-0417-3. [DOI] [PubMed] [Google Scholar]
- Burk C.J., Aber C., Connelly E.A. Ehlers-Danlos syndrome type IV: keloidal plaques of the lower extemities, amniotic band limb deformity and a new mutation. J Am Acad Dermatol. 2006;56:S53–S54. doi: 10.1016/j.jaad.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Byers P.H., Holbrook K.A., McGillivray B., MacLeod P.M., Lowry R.B. Clinical and ultrastructural heterogeneity of type IV Ehlers-Danlos syndrome. Hum Genet. 1979;47:141–150. doi: 10.1007/BF00273196. [DOI] [PubMed] [Google Scholar]
- Cass D.L., Sylvester K.G., Yang E.Y., Crombleholme T.M., Adzick N.S. Myofibroblast persistence in fetal sheep wounds is associated with scar formation. J Pediatr Surg. 1997;32:1017–1021. doi: 10.1016/s0022-3468(97)90390-0. [DOI] [PubMed] [Google Scholar]
- Cook H., Davies K.J., Harding K.G., Thomas D.W. Defective extracellular matrix reorganization by chronic wound fibroblasts is associated with alterations in TIMP-1, TIMP-2, and MMP-2 activity. J Invest Dermatol. 2000;115:225–233. doi: 10.1046/j.1523-1747.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- Cooper T.K., Zhong Q., Krawczyk M., Tae H.J., Müller G.A., Schubert R., Myers L.A., Dietz H.C., Talan M.I., Briest W. The haploinsufficient Col3a1 mouse as a model for vascular Ehlers-Danlos syndrome. Vet Pathol. 2010;47:1028–1039. doi: 10.1177/0300985810374842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttle L., Nataatmadja M., Fraser J.F., Kempf M., Kimble R.M., Hayes M.T. Collagen in the scarless fetal skin wound: detection with Picrosirius-polarization. Wound Repair Regen. 2005;13:198–204. doi: 10.1111/j.1067-1927.2005.130211.x. [DOI] [PubMed] [Google Scholar]
- Desmouliere A., Geinoz A., Gabbiani F., Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in GT myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmouliere A., Redard M., Darby I., Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H.P. The modulation of contraction of fibroblast populated collagen lattices by types I, II, and III collagen. Tissue Cell. 1988;20:47–50. doi: 10.1016/0040-8166(88)90006-7. [DOI] [PubMed] [Google Scholar]
- Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- Germain D.P. Ehlers-Danlos syndrome type IV. Orphanet J Rare Dis. 2007;2:32–41. doi: 10.1186/1750-1172-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S.R., Quirk G.L., Sykes V.W., Kordula T., Lanning D.A. Altered procollagen gene expression in mid-gestational mouse embryonic wounds. J Surg Res. 2007;143:27–34. doi: 10.1016/j.jss.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Ho C.H., Lin Y.C., Skuta G. Differences in the regulation of fibroblast contraction of floating versus stressed collagen matrices. J Biol Chem. 1999a;2:918–923. doi: 10.1074/jbc.274.2.918. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Zhu M., Carlson M.A., Abrams J.M. Release of mechanical tension triggers apoptosis of human fibroblasts in a model of regressing granulation tissue. Exp Cell Res. 1999b;248:608–619. doi: 10.1006/excr.1999.4440. [DOI] [PubMed] [Google Scholar]
- Hinz B., Celetta G., Tomasek J.J., Gabbiani G., Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B., Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. Curr Opin Biotechnol. 2003;14:546. doi: 10.1016/j.copbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Hurme T., Kalimo H., Sandberg M., Lehto J.M., Vuorio E. Localization of type I and III collagen and fibronectin production in injured gastrocnemius muscle. Lab Invest. 1991;64:76–84. [PubMed] [Google Scholar]
- Järveläinen H., Puolakkainen P., Pakkanen S., Brown E.L., Höök M., Iozzo R.V., Sage E.H., Wight T.N. A role for decorin in cutaneous wound healing and angiogenesis. Wound Repair Regen. 2006;14:443–452. doi: 10.1111/j.1743-6109.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- Kyriakides T.R., Bornstein P. Matricellular proteins as modulators of wound healing and the foreign body response. Thromb Haemost. 2003;90:986–992. doi: 10.1160/TH03-06-0399. [DOI] [PubMed] [Google Scholar]
- Lee T.Y., Chin G.S., Kim W.J., Chau D., Gittes G.K., Longaker M.T. Expression of transforming growth factor beta 1, 2, and 3 proteins in keloids. Ann Plast Surg. 1999;43:179–184. [PubMed] [Google Scholar]
- Liao H., Zakhaleva J., Chen W. Cells and tissue interactions with glycated collagen and their relevance to delayed diabetic wound healing. Biomaterials. 2009;30:1689–1696. doi: 10.1016/j.biomaterials.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R.Y., Sullivan K.M., Argenta P.A., Meuli M., Lorenz H.P., Adzick N.S. Exogenous transforming growth factor-beta amplifies its own expression and induces scar formation in a model of human fetal skin repair. Ann Surg. 1995;222:146–154. doi: 10.1097/00000658-199508000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.H., Yang R.S., Al-Haikh R., Lane J.M. Collagen in tendon, ligament and bone healing: a current review. Clin Orthop Relat Res. 1995;318:265–278. [PubMed] [Google Scholar]
- Liu X., Wu H., Burne M., Krane S., Jaenisch R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc Natl Acad Sci USA. 1997;94:1852–1856. doi: 10.1073/pnas.94.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lygoe K.A., Wall I., Stephens P., Li C. Role of vitronectin and fibronectin receptors in oral mucosal and dermal myofibroblast differentiation. Biol Cell. 2007;99:601–614. doi: 10.1042/BC20070008. [DOI] [PubMed] [Google Scholar]
- McGrath M.H., Hundahl S.A. The spatial and temporal quantification of myofibroblasts. Plast Reconstr Surg. 1982;69:975–985. doi: 10.1097/00006534-198206000-00012. [DOI] [PubMed] [Google Scholar]
- Merkel J.R., DiPaolo B.R., Hallock G.G., Rice D.C. Type I and type III collagen content of healing wounds in fetal and adult rats. Proc Soc Exp Biol Med. 1988;187:493–497. doi: 10.3181/00379727-187-42694. [DOI] [PubMed] [Google Scholar]
- Mochitate K., Pawelek P., Grinnell F. Stress relaxation of contracted collagen gels: disruption of actin filament bundles, release of cell surface fibronectin, and down-regulation of DNA and protein synthesis. Exp Cell Res. 1991;193:198–207. doi: 10.1016/0014-4827(91)90556-a. [DOI] [PubMed] [Google Scholar]
- Neptune E.R., Frischmeyer P.A., Arking D.E., Myers L., Bunton T.E., Gayraud B., Ramirez F., Sakai L.Y., Dietz H.C. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- Occleston N.L., Metcalfe A.D., Boanas A., Burgoyne N.J., Nield K., O'Kane S., Ferguson M.W. Therapeutic improvement in scarring: mechanisms of scarless and scar-forming healing and approaches to the discovery of new treatments. Derm Res Pract. 2010;2010 doi: 10.1155/2010/405262. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oderich G.S., Panneton J.M., Bower T.C., Lindor N.M., Cherry K.J., Noel A.A., Kalra M., Sullivan T., Glowacki J. The spectrum, management and clinical outcome of Ehlers Danlos syndrome IV: a 30-year experience. J Vasc Surg. 2005;42:98–106. doi: 10.1016/j.jvs.2005.03.053. [DOI] [PubMed] [Google Scholar]
- Oganesian A., Au S., Horst J.A., Holzhausen L.C., Macy A.J., Pace J.M., Bornstein P. The NH2-terminal propeptide of type I procollagen acts intracellularly to modulate cell function. J Biol Chem. 2006;281:38507–38518. doi: 10.1074/jbc.M607536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parizi M., Howard E.W., Tomasek J.J. Regulation of LPA-promoted myofibroblast contraction: role of Rho, myosin light chain kinase, and myosin light chain phosphatase. Exp Cell Res. 2000;254:210–220. doi: 10.1006/excr.1999.4754. [DOI] [PubMed] [Google Scholar]
- Pepin M., Schwarze U., Superti-Furga A., Byers P.H. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular form. New Engl J Med. 2000;342:673–680. doi: 10.1056/NEJM200003093421001. [DOI] [PubMed] [Google Scholar]
- Phillips L.G., Seppinen L., Sormunen R., Soini Y., Elamaa H., Heljasvaara R., Phihlajaniemi T. Lack of collagen XVIII accelerates cutaneous wound healing, while overexpression of its endostatin domain leads to delayed healing. Matrix Biol. 2008;27:535–546. doi: 10.1016/j.matbio.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Powell D.W., Mifflin R.C., Valentich J.D., Crowe S.E., Saada J.I., West A.B. Myofibroblasts: paracrine cells important in health and disease. Am J Physiol. 1999;277:C1–9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- Reid R.R., Said H.K., Mogford J.E., Mustoe T.A. The future of wound healing: pursuing surgical models in transgenic and knockout mice. J Am Coll Surg. 2004;199:578–585. doi: 10.1016/j.jamcollsurg.2004.05.262. [DOI] [PubMed] [Google Scholar]
- Romanic A.M., Adachi E., Kadler K.E., Hojima Y., Prockop D.J. Copolymerization of pN collagen III and collagen I: pN collagen III decreases the rate of incorporation of collagen I into fibrils, the amount of collagen I incorporated and the diameter of the fibrils formed. J Biol Chem. 1991;266:12703–12709. [PubMed] [Google Scholar]
- Rungger-Brandle E., Gabbiani G. The role of the cytoskeletal and cytocontractile elements in pathologic processes. Am J Pathol. 1983;110:361–385. [PMC free article] [PubMed] [Google Scholar]
- Schwarze U., Schievink W.I., Petty E., Jaff M.R., Babovic-Vuksanovic D., Cherry K.J., Pepin M., Byers P.H. Haploinsufficiency for one Col3A1 allele of type III procollagen results in a phenotype similar to the vascular form of Ehlers-Danlos syndrome, Ehlers-Danlos syndrome type IV. Am J Hum Genet. 2001;69:989–1001. doi: 10.1086/324123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serini G., Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res. 1999;250:273–283. doi: 10.1006/excr.1999.4543. [DOI] [PubMed] [Google Scholar]
- Shah M., Foreman D.M., Ferguson M.W. Neutalisation of TGF-β1 and TGF-β2 or exogenous addition of TGF-β3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108:985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- Sharma N.L., Mahajan V.K., Gupta N., Ranjan N., Lath A. Ehlers-Danlos syndrome-vascular type (ecchymotic variant): cutaneous and dermatopathologic features. J Cutan Pathol. 2009;36:486–492. doi: 10.1111/j.1600-0560.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- Stevenson K., Kucich U., Whitbeck C., Levin R.M., Howard P.S. Functional changes in bladder tissue from type III collagen-deficient mice. Mol Cell Biochem. 2006;283:107–114. doi: 10.1007/s11010-006-2388-1. [DOI] [PubMed] [Google Scholar]
- Tomasek J.J., Haaksma C.J., Eddy R.J., Vaughan M.B. Fibroblast contraction occurs on release of tension in attached collagen lattices: dependency on an organized actin cytoskeleton and serum. Anat Rec. 1992;232:359–368. doi: 10.1002/ar.1092320305. [DOI] [PubMed] [Google Scholar]
- Vaughan M.B., Howard E.W., Tomasek J.J. Transforming growth factor-β1 promotes the morphological and functional differentiation of the myofibroblast. Exp Cell Res. 2000;257:180–189. doi: 10.1006/excr.2000.4869. [DOI] [PubMed] [Google Scholar]
- Volk S.W., Radu A., Zhang L., Liechty K.W. Stromal progenitor cell therapy corrects the wound healing defect in the ischemic rabbit ear model of chronic wound repair. Wound Repair Regen. 2007;15:736–747. doi: 10.1111/j.1524-475X.2007.00277.x. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Oganesian A., Keene D.R., Sandell L.J. Type IIA procollagen containing the cysteine-rich amino propeptide is deposited in the extracellular matrix of prechondrogenic tissue and binds to TGFβ1 and BMP2. J Cell Biol. 1999;144:1069–1080. doi: 10.1083/jcb.144.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoppi N., Gardella R., DePaepe A., Barlati S., Colombi M. Human fibroblasts with mutations in COL5A1 and COL3A1 genes do not organize collagens and fibronectin in the extracellular matrix, down-regulate α2β1 integrin, and recruit αvβ3 instead of α5β1 integrin. J Biol Chem. 2004;279:18157–18168. doi: 10.1074/jbc.M312609200. [DOI] [PubMed] [Google Scholar]