Abstract
Eukaryotic cells commit in G1 to a new mitotic cycle or to diverse differentiation processes. Here we show that Whi3 is a negative regulator of Cln3, a G1 cyclin that promotes transcription of many genes to trigger the G1/S transition in budding yeast. Whi3 contains an RNA-recognition motif that specifically binds the CLN3 mRNA, with no obvious effects on Cln3 levels, and localizes the CLN3 mRNA into discrete cytoplasmic foci. This is the first indication that G1 events may be regulated by locally restricting the synthesis of a cyclin. Moreover, Whi3 is also required for restraining Cln3 function in meiosis, filamentation, and mating, thus playing a key role in cell fate determination in budding yeast.
Keywords: Cell cycle, G1 regulation, differentiation, RNA-binding protein, cyclin, mRNA localization
Like most other eukaryotes, Saccharomyces cerevisiae cells monitor internal and external signals to choose between developmental options in the G1 phase of the cell cycle (Pringle and Hartwell 1981). Sexual pheromones arrest haploid cells in G1 transiently, whereas nutrient limitation causes a G1 arrest and a switch to either meiosis or pseudohyphal/invasive growth.
During mitotic proliferation, both external and internal signals are also integrated in G1 to coordinate cell growth in mass with cell cycle entry. Once a critical cell volume is attained in late G1, Cln3 bound to the cyclin-dependent kinase Cdc28 somehow activates the transcription factors SBF (Swi4/Swi6) and MBF (Mbp1/Swi6). These in turn induce the expression of a large set of genes in a process that leads to the G1/S transition (Tyers et al. 1993; Dirick et al. 1995; Stuart and Wittenberg 1995; Spellman et al. 1998). Cln3 is already present in early G1 cells, and Cln3 protein levels do not oscillate much throughout the cell cycle. However, a posttranslational mechanism that explains why SBF and MBF become active only in late G1 (or at a critical cell size) is yet to be found.
Here we show that Whi3, a recently identified protein that regulates cell size in budding yeast (Nash et al. 2001), exerts a negative role on Cln3 function in the G1 phase. Whi3 shows a C-terminal domain very similar to the RNA-recognition motif (RRM), and we show that it binds the CLN3 mRNA specifically. However, Whi3 does not obviously affect the abundance or translation of the CLN3 mRNA, or the overall Cln3 protein levels. Rather, Whi3 localizes the CLN3 mRNA to distinct cytoplasmic foci that may locally restrict Cln3 synthesis to modulate its activity. Importantly, we have found that Whi3, also through its RNA-recognition motif, is necessary for repressing Cln3 function when cells are challenged in G1 to decide among other developmental options such as meiosis or filamentation.
Results and Discussion
Whi3 negatively regulates Cln3 function in G1
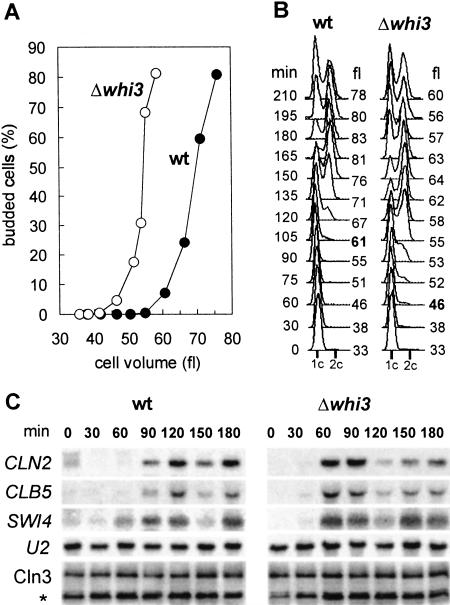
The WHI3 gene has been isolated as a regulator of cell size in budding yeast. Whi3-deficient cells show a 20% reduction in cell size with no changes in growth rate (Nash et al. 2001). To test a possible role of Whi3 in regulating the G1 phase, we analyzed small (early G1) cells obtained by elutriation from wild-type and whi3 mutant strains. Whi3-deficient cells budded and entered S phase with a volume ∼25% smaller than wild-type cells (Fig. 1A,B). These results suggest that Whi3 might be specifically involved in setting cell size at the G1/S transition, the point where Cln3 plays a rate-limiting role (Cross 1988; Nash et al. 1988). Indeed, transcriptional activation of G1/S genes such as CLN2, CLB5, and SWI4 also took place at a smaller cell size in whi3 cells (Fig. 1C). However, Cln3 protein and phosphorylation levels (Fig. 1C) were not affected.
Figure 1.
Whi3-deficient cells enter the cell cycle at a smaller cell size. Small G1 wild-type (wt) or mutant (Δwhi3) cells were grown in YPD at 30°C, and samples were taken at different time points to plot (A) budding indexes versus cell volume mean values, and (B) DNA content distributions. (C) Northern analysis of G1/S genes, and Western analysis of Cln3 protein levels from samples taken at the specified time points. The U2 RNA and the αHA cross-reacting band (*) served as loading controls.
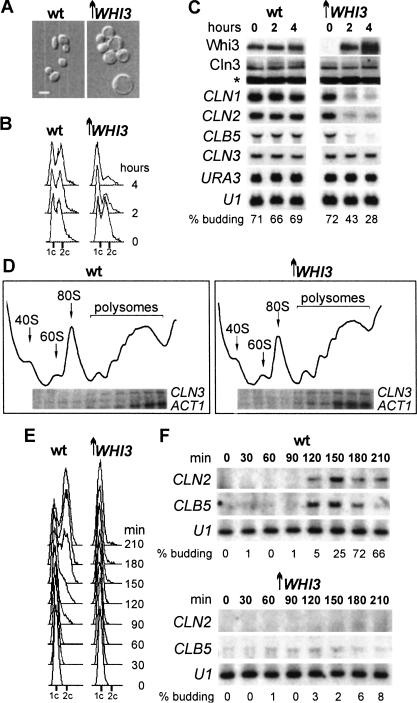
The effects of WHI3 on cell volume are dose-dependent, and WHI3 overexpression causes a lethal arrest in G1 (Fig. 2A,B; Nash et al. 2001). Moderate WHI3 overexpression also repressed transcription of CLN1, CLN2, and CLB5 (Fig. 2C) by preventing their activation in late G1 as deduced from elutriated cultures (Fig. 2E,F). Loss of Whi3 therefore accelerates the expression of genes controlled by SBF and MBF, and overproduction of Whi3 delays or represses expression of the same genes. The G1 arrest (but not the lethality) caused by WHI3 overexpression was suppressed by constitutive expression of CLN2 (data not shown), a condition able to compensate the major cell cycle deficiencies of a cln3 mutant (Stuart and Wittenberg 1995). Furthermore, a C-terminal truncated form of Swi4 that is largely independent of Swi6 or Cln3 (Baetz and Andrews 1999) was able to activate G1/S gene expression during WHI3 overexpression (data not shown). Again, however, lethality was not suppressed, suggesting that overexpressed WHI3 may interfere with additional targets. These observations are consistent with the idea that Whi3 inhibits the activation of SBF and MBF by Cln3. To cause a G1 arrest Whi3 should also down-regulate other activators of Start, perhaps Cln1 and Cln2 (and see below), for we have found that Cdc24, a protein that is retained in the nucleus by Far1 in haploid cells and is exported to the cytoplasm depending on Cdc28–Cln1,2 activity (Shimada et al. 2000), remains nuclear in the G1 arrest caused by Whi3 overexpression (data not shown). In any event, neither the G1 arrest nor the lethal effects caused by high doses of Whi3 can be solely explained by its effects on Cln3 function through SCB- and MCB-driven transcription, pointing to the existence of additional essential targets of Whi3.
Figure 2.
WHI3 overexpression prevents Cln3 from inducing G1/S gene expression and arrests cells in G1. Wild-type (wt) or GAL1p–WHI3 (↑WHI3) cells were added to 2% galactose to induce expression of WHI3, and samples were analyzed at 4 h or otherwise-specified time points. (A) Phase-contrast micrographs. (B) DNA content distributions. (C) Northern analysis of G1/S genes and Western analysis of Whi3 and Cln3 protein levels. (D) CLN3 mRNA distribution on polysomal profiles. Small G1 cells were elutriated 60 min after galactose addition and grown under inducing conditions to obtain (E) DNA content distributions and perform (F) Northern analysis of G1/S genes.
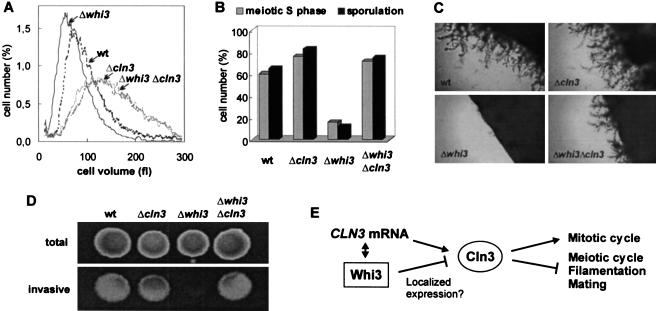
To ask whether Whi3 regulates G1/S gene expression via Cln3 we compared whi3, cln3, and double whi3 cln3 mutants. Deletion of WHI3 had no effect in a cln3 mutant strain (see Fig. 5A, below); that is, cln3 is epistatic to whi3 for cell size (and this is also true for other phenotypes of whi3; see below), suggesting that Whi3 acts via Cln3. In contrast, bck2, another mutation causing large cell size at Start (Di Como et al. 1995; Wijnen and Futcher 1999), was not epistatic to whi3 (data not shown). These genetic results support the idea that Whi3 is a negative regulator of Cln3 function.
Figure 5.
Cell size and developmental defects of Whi3-deficient cells depend on Cln3. Wild-type (wt), Δcln3, Δwhi3, and double Δcln3 Δwhi3 mutant strains were used to analyze (A) cell volume distributions of haploid 1788 strains, (B) meiotic S-phase entry at 12 h and sporulation efficiencies at 24 h of diploid 1788 strains, (C) pseudohyphal growth of diploid Σ1278 strains in SLAD plates, and (D) invasive growth of haploid Σ1278 strains in YEPD plates. (E) Scheme to depict Whi3 and Cln3 functional interactions.
Whi3 binds the CLN3 mRNA specifically
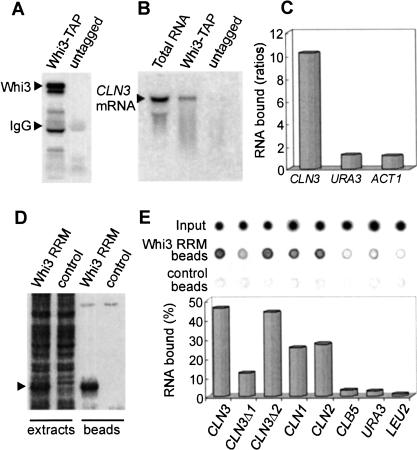
The Whi3 protein contains a domain similar to the RNA-recognition motif. Deletions and point mutations destroying the putative RRM yield null alleles of WHI3 with respect to both cell size (Nash et al. 2001) and developmental defects (see below), which suggests that the putative RRM is critical for the function of Whi3. To test whether Whi3 is in fact an RNA-binding protein and whether it binds the CLN3 mRNA specifically, we performed pull-down assays with a Whi3–TAP fusion expressed from the WHI3 promoter in yeast cells (Fig. 3A). The CLN3 mRNA was enriched more than 10-fold in Whi3–TAP beads compared to untagged controls as measured by dot-blot analysis, but neither URA3 nor ACT1 mRNA ratios differed significantly from 1 (Fig. 3C). In addition, despite the fact that the CLN3 mRNA is extremely unstable (Schneider et al. 1998), most CLN3 mRNA in the Whi3–TAP pull down was full length (Fig. 3B). Fusing the Whi3 RRM to the calmodulin-binding peptide (Fig. 3D) allowed us to perform in vitro binding assays and test a direct involvement of Whi3 in binding the CLN3 mRNA. Similarly to the in vivo assays, the Whi3 RRM was able to bind very efficiently and specifically to the CLN3 mRNA in vitro (Fig. 3E). Although the binding of CLN3 mRNA remained unaffected by the addition of cold yeast total RNA in a 10-fold excess, the binding of URA3 and LEU2 mRNAs showed a fivefold decrease (data not shown). Interestingly, the Whi3 RRM was also able to bind the mRNAs of the two other G1 cyclins, CLN1 and CLN2, albeit with reduced efficiency, whereas there was no binding to the mRNA of the S-phase cyclin CLB5. These results reinforce the idea that the G1 arrest caused by Whi3 overexpression may involve the functional inhibition of all Cln cyclins. A preliminary deletion analysis showed that the Whi3 RRM may bind the CLN3 mRNA at more than one region, although the 3′ region of the CLN3 open reading frame seems to contain the most important determinants for binding to Whi3 (Fig. 3E; see below). These observations support the idea that Whi3 acts as an RNA-binding protein, and that the CLN3 mRNA may be a direct target.
Figure 3.
Whi3 binds the CLN3 mRNA. (A) For in vivo analysis, WHI3–TAP and untagged yeast strains were used in pull-down assays with IgG beads. Bound proteins detected with a peroxidase anti-peroxidase antibody are shown. (B) Northern detection of bound CLN3 mRNA. Total RNA is shown as control. (C) Dot-blot detection of bound CLN3, URA3, and ACT1 mRNAs. Plotted are the values obtained from Whi3–TAP cells relative to untagged cells. (D) For in vitro analysis, the Whi3 RRM fused to the calmodulin-binding peptide (arrowhead) was bound to calmodulin beads to perform RNA binding assays. Shown are Coomassie-stained proteins in Whi3 RRM or control cell extracts and beads. (E) Dot-blot detection of input and bound RNAs to Whi3 RRM and control beads. Percentages of bound to input values are plotted.
We therefore examined the effects of Whi3 on CLN3 mRNA and protein. Neither overexpression nor deletion of WHI3 had any apparent effect on CLN3 mRNA levels, or on Cln3 protein levels (Figs. 1C, 2C). The polysome profile of CLN3 mRNA did not change during WHI3 overexpression (Fig. 2D), suggesting that translation of Cln3 was not affected. We could see no effect of Whi3 on levels of Cln3–Cdc28 histone H1 kinase activity (data not shown) or Cln3 protein phosphorylation levels (Figs. 1C, 2C), which suggest that Whi3 does not directly inhibit Cln3–Cdc28 complexes.
Whi3 colocalizes with the CLN3 mRNA into cytoplasmic foci
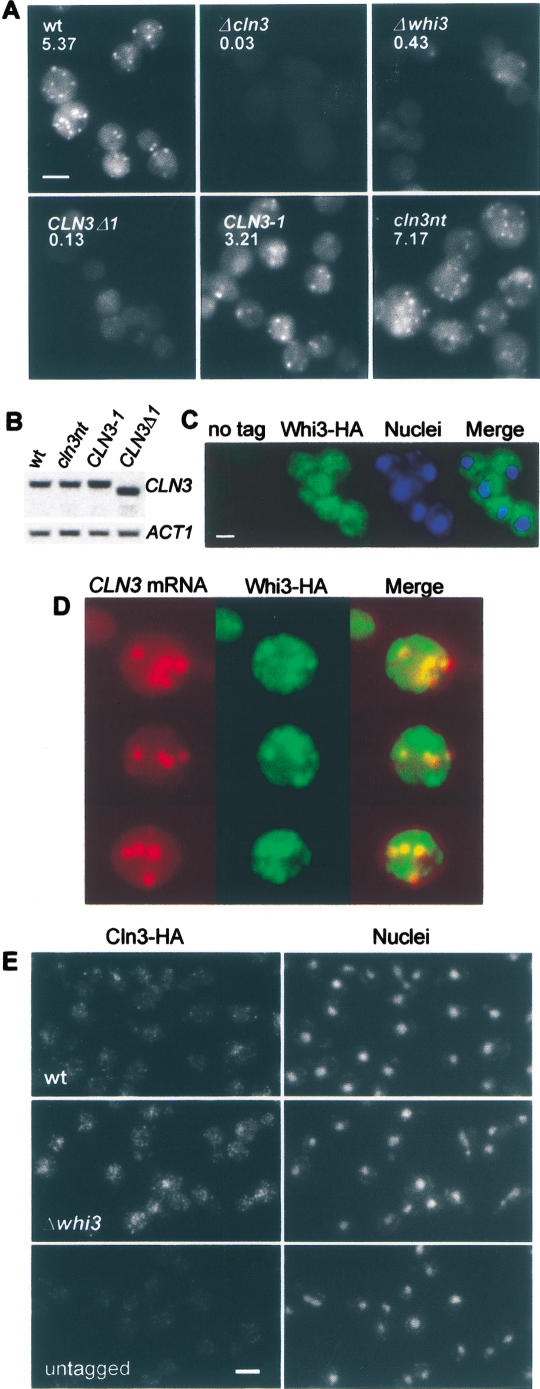
We next asked whether Whi3 could affect the localization of CLN3 mRNA. Analysis by FISH showed that the CLN3 mRNA forms discrete cytoplasmic foci in G2/M cells (Fig. 4A), a property found in mRNAs that are localized to direct the encoded protein to specific cytoplasmic compartments (Bashirullah et al. 1998). The brightness of these foci was greatly diminished in whi3 cells (Fig. 4A), whereas unrelated mRNAs such as URA3 or kanMX4 were unaffected (data not shown). In addition, a CLN3 mRNA deletion construct (CLN3Δ1) that shows reduced binding to the Whi3 RRM in vitro (see above) also produced much dimmer particles (Fig. 4A). Levels of CLN3 and CLN3Δ1 mRNAs were totally comparable when detected by Northern analysis in otherwise wild-type cells (Fig. 4B). Neither a CLN3 mRNA construct that codes for an identically C-terminal truncated product but retains the 3′ mRNA sequences (CLN3-1), nor a mutant that lacks the Start codon (cln3nt) affected particle appearance (Fig. 4A). Therefore, the localization of CLN3 mRNA into these foci requires Whi3 and certain CLN3 mRNA sequences, but does not require Cln3 protein or full translation of the CLN3 mRNA. Immunofluorescence showed that Whi3 was also present in cytoplasmic foci (Fig. 4C). A combination of FISH and immunofluorescence showed that the CLN3 mRNA foci colocalized with a large fraction of the Whi3 foci (Fig. 4D). The fact that we could only clearly detect the CLN3 mRNA foci in G2/M cells could be attributed to the transcriptional up-regulation of WHI3 during mitosis (Spellman et al. 1998; Gari et al., unpubl.). In addition, Whi3 overexpression increased the brightness of CLN3 mRNA foci in G1 cells (data not shown), suggesting that the Whi3/CLN3 mRNA ratio may also be crucial for foci formation and Cln3 function.
Figure 4.
The CLN3 mRNA is localized to cytoplasmic foci that depend on and colocalize with Whi3. (A) CLN3 mRNA FISH analysis of wild-type (wt), Δcln3 as negative control, and Δwhi3 strains, as well as wild-type cells carrying a deletion in the 3′ region of the ORF (CLN3-Δ1), the ORF-equivalent point mutation (CLN3-1), or a nontranslatable CLN3 mRNA (cln3nt). (B) mRNA levels of the above CLN3 constructs detected by Northern using the ACT1 mRNA as loading control. (C) Immunofluorescence of Whi3-HA cells (untagged cells as negative control), showing also nuclei and merged images. (D) Combined CLN3 mRNA FISH and Whi3-HA immunofluorescence, showing also merged images. (E) Signal-amplification immunofluorescence of Cln3-HA in wild-type (wt), whi3 mutant (Δwhi3), and untagged cells as negative control, showing also nuclei stained with DAPI. Bars, 5 μm.
Localization of ASH1 mRNA particles into daughter cells depends on efficient transport via the actin cytoskeleton (Jansen et al. 1996; Long et al. 1997; Takizawa et al. 1997), whereas localization of other mRNAs in higher eukaryotes depends on their movement via microtubules (Arn and Macdonald 1998; Bashirullah et al. 1998). Latrunculin or nocodazole treatment of cells, which disrupts the actin or microtubule cytoskeletons, respectively, did not affect CLN3 mRNA foci appearance or number (data not shown), suggesting that transport may not be a key functional aspect of the CLN3 mRNA foci. Rather, as Cln3 is an extremely short-lived protein (Schneider et al. 1998), binding of Whi3 to the CLN3 mRNA could restrict both synthesis and presence of the Cln3 protein to a narrow molecular environment. If this environment were unfavorable for the activation of SBF and MBF, then Cln3 function would be inhibited, despite wild-type levels of Cln3 protein. Although Miller and Cross (2000) did not detect endogenous Cln3 protein in G1 cells or small-budded cells by immunofluoresence, they used several genetic approaches to propose that Cln3 should exert a rate-limiting function in the nucleus. By using a powerful signal-amplification method for immunofluorescence we have been able to detect endogenously expressed Cln3 in the cytoplasm of all wild-type cells (Fig. 4E), with some cells also showing a distinctive nuclear signal. In contrast, Whi3-deficient cells showed a more general and brighter nuclear pattern, including unbudded cells, suggesting that Whi3 regulates a cytoplasmic event that, directly or indirectly, affects the ability of Cln3 to accumulate in the nucleus.
Whi3 is required to repress Cln3 function during meiosis and filamentous growth
Down-regulation of Cln3 function seems to be a key condition for yeast cells to take other developmental options in G1 such as meiosis, filamentous growth, and mating. For example, Cln3 blocks the function of the meiotic inducer Ime1, thus making mitosis and meiosis incompatible (Colomina et al. 1999). Whi3 may be important for down-regulation of Cln3 in meiosis, because a homozygous whi3 strain entered meiotic S phase and sporulated very poorly, but a whi3 cln3 double mutant was as proficient as the parental strain (Fig. 5B). Similarly, Cln3 has an inhibitory effect on filamentous growth (Loeb et al. 1999), and whi3 has been independently identified in a search for mutants unable to undergo pseudohyphal differentiation (Mosch and Fink 1997). We therefore asked whether the failure of whi3 cells to filament could be alleviated by deletion of CLN3. Although deletion of whi3 blocked pseudohyphal differentiation in diploid strains and invasive growth in haploid strains, the corresponding double whi3 cln3 mutants showed similar pseudohyphal and invasive growth ability as parental strains (Fig. 5C,D). The behavior of a whi3ΔRRM mutant lacking the RNA-binding domain was indistinguishable from a complete deletion of the WHI3 gene in all these epistatic analyses (data not shown), which reinforces the notion that the observed direct interaction of Whi3 with the CLN3 mRNA plays an important role in regulating G1 events. Finally, whi3 cells mate with low efficiencies, and this defect shows strong genetic interactions with a hyperactive allele of CLN3 (Nash et al. 2001). In summary, the observed G1 cell cycle and developmental deficiencies of whi3 mutant cells can be all alleviated by loss of CLN3, which confirms both the negative effect of Whi3 on Cln3 function and the specificity of their functional relationship.
Our results show that Whi3 inhibits Cln3 function, and suggest that the direct binding of Whi3 to CLN3 mRNA in cytoplasmic foci may be important for this inhibition. As Cln3 is an extremely short-lived protein, localizing its translation to particular cytoplasmic regions, or molecular environments, could modulate the ability of Cln3 to reach its presumptive nuclear targets and, hence, to induce G1/S gene expression at a proper cell size during the budding mitotic cycle. As a possibility, the ratio of unstable activators of Start (e.g., CLN3 mRNA and/or Cln3) to stable inhibitors (e.g., Whi3) may promptly reflect changes in growth rate versus mass or volume, which could determine cell size at Start for a particular set of external conditions. Whatever the precise molecular mechanism of inhibition, Whi3 is also very important for repressing Cln3 function in other developmental options that yeast cells take in G1: meiosis, filamentation, and mating (Fig. 5E). D-type cyclin hyperactivity also perturbs cell size control in G1 (Ohtsubo and Roberts 1993; Quelle et al. 1993) and inhibits differentiation (Skapek et al. 1995; Zhang et al. 1999) in mammalian cells. Although an specific RNA-binding protein has not yet been identified, localization of cyclin B1 mRNA by CPEB and maskin to the spindle and centrosomes in Xenopus oocytes is important for spindle dynamics (Groisman et al. 2000). The further functional and molecular characterization of the Whi3 device may therefore help us understand how eukaryotic cells integrate and centralize molecular information to regulate their cycle and take different cell fate options.
Materials and methods
Strains, plasmids, and growth conditions
Yeast parental strains (1788, W303, and Σ1278), standard growing conditions, and special media to induce sporulation, filamentation, or invasive growth were as described (Roberts and Fink 1994; Gallego et al. 1997; Colomina et al. 1999). Chromosomal gene disruptions, C-terminal fusions to a triple HA epitope or the TAP tag, as well as gene fusions to the GAL1 promoter were performed by gene transplacement methods as described (Gallego et al. 1997). CLN3-Δ1 carries a deletion from nucleotide +1213 to the end of the CLN3 ORF. CLN3-Δ2 lacks the first 1212 nucleotides of the CLN3 ORF. CLN3-1 has a nonsense mutation at +1210 (Nash et al. 1988), and cln3nt carries a point mutation that eliminates the Start codon. Details of strain and plasmid constructions are available upon request.
RNA-binding assays
Yeast cells expressing the Whi3–TAP protein were used in pull-down assays (Rigaut et al. 1999). Washed IgG beads were eluted with 0.5% SDS, 10 mM EDTA, 10 mM HCl-Tris at pH 8 for 5 min at RT, and RNA was isolated (Ausubel et al. 1999) to detect various mRNAs (CLN3, URA3, and LEU2) by dot or Northern blot analysis (see below). As deduced from Northern analysis, CLN3 mRNA stability was not particularly affected during extract preparation when compared to URA3 and ACT1 mRNAs used as internal controls. For in vitro binding assays, the Whi3RRM was fused to the calmodulin-binding peptide (CBP) in pCAL-n and affinity-purified on calmodulin beads (Stratagene), which were then used to test binding of 32P-labeled RNAs synthesized from T7/T3-expression vectors (Ausubel et al. 1999). Binding reactions contained 0.3 μg of 32P-labeled RNA and 1 μg of either CBP–Whi3RRM or CBP bound to calmodulin beads in cold CBB buffer (Stratagene). Binding was performed at 4°C for 1 h, beads were washed four times with cold CBB buffer, and bound RNAs were eluted as described above and spotted on Nylon+ membranes to quantify radioactivity with a Fuji BAS-1000 reader.
FISH and immunofluorescence
Cells were treated for FISH with a digoxigenin-labeled CLN3 riboprobe essentially as described (Takizawa et al. 1997), using a detection method based on development of a HNPP-fluorescent precipitate (Roche). As the CLN3 mRNA signal was significantly stronger during mitosis, cells were treated with 10 μg/mL nocodazole for 90 min before fixation. Foci per cell were counted in images all treated in such a way that negative control cells (Δcln3) were already not distinguishable against the field background, and mean values were obtained by counting a minimum of 300 cells per sample. The localization of Whi3-HA was done by immunofluoresence (Colomina et al. 1999) with a rat anti-HA antibody (clone 3F10, Roche), followed by incubation with Alexa488-labeled anti-rat antibody (Molecular Probes). To combine FISH and immunofluoresence, rat anti-HA and sheep alkaline-phosphatase anti-digoxigenin (Roche) antibodies were added together after washing the probe, followed by incubation with Alexa488-labeled anti-rat antibody, and HNPP precipitate development as above. Cln3-HA was detected by a signal-amplification method based on regular immunofluorescence procedures (Colomina et al. 1999). Briefly, after incubation with rat anti-HA antibody, slides were sequentially incubated with goat anti-rat, rabbit anti-goat, and goat anti-rabbit antibodies labeled with Alexa488 (Molecular Probes).
Miscellaneous
Northern methods and probes used, as well as Western conditions to detect specific tagged proteins, were done as described previously (Gallego et al. 1997). TAP-tagged proteins were detected with a peroxidase-labeled rabbit anti-peroxidase antibody soluble complex (Sigma) as described (Rigaut et al. 1999). Cln3 immunoprecipitation and associated-kinase assays were also as described (Tyers et al. 1993). The distribution of the CLN3 mRNA in polysomes was analyzed in sucrose gradients (Cigan et al. 1991). Small G1 cells were obtained by elutriation (Tyers et al. 1993). Cell volume distributions were obtained in a Z2 Coulter Counter, and DNA content distributions and budding indexes were determined as described (Gallego et al. 1997).
Acknowledgments
We thank A. Bueno, C. Mann, S. Moreno, E. Schwob, and B. Séraphin for strains and helpful discussions, and J.X. Comella for comments on the manuscript. This work was supported by grants from the Spanish Ministry of Education and Culture, and La Paeria to E.G. and M.A., from AECI to H.W. and from the NIH to B.F. and T.V.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL marti.aldea@cmb.udl.es; FAX 34-973-702426.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.203501.
References
- Arn EA, Macdonald PM. Motors driving mRNA localization: New insights from in vivo imaging. Cell. 1998;95:151–154. doi: 10.1016/s0092-8674(00)81745-6. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. New York, NY: Wiley Interscience; 1999. [Google Scholar]
- Baetz K, Andrews B. Regulation of cell cycle transcription factor Swi4 through auto-inhibition of DNA binding. Mol Cell Biol. 1999;19:6729–6741. doi: 10.1128/mcb.19.10.6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashirullah A, Cooperstock RL, Lipshitz HD. RNA localization in development. Annu Rev Biochem. 1998;67:335–394. doi: 10.1146/annurev.biochem.67.1.335. [DOI] [PubMed] [Google Scholar]
- Cigan AM, Foiani M, Hannig EM, Hinnebusch AG. Complex formation by positive and negative translational regulators of GCN4. Mol Cell Biol. 1991;11:3217–3228. doi: 10.1128/mcb.11.6.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomina N, Gari E, Gallego C, Herrero E, Aldea M. G1 cyclins block the Ime1 pathway to make mitosis and meiosis incompatible in budding yeast. EMBO J. 1999;18:320–329. doi: 10.1093/emboj/18.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross FR. DAF1, a mutant gene affecting size control, pheromone arrest, and cell cycle kinetics of Saccharomyces cerevisiae. Mol Cell Biol. 1988;8:4675–4684. doi: 10.1128/mcb.8.11.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Como CJ, Chang H, Arndt KT. Activation of CLN1 and CLN2 G1 cyclin gene expression by BCK2. Mol Cell Biol. 1995;15:1835–1846. doi: 10.1128/mcb.15.4.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirick L, Böhm T, Nasmyth K. Roles and regulation of Cln-Cdc28 kinases at the start of the cell cycle of Saccharomyces cerevisiae. EMBO J. 1995;14:4803–4813. doi: 10.1002/j.1460-2075.1995.tb00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego C, Garí E, Colomina N, Herrero E, Aldea M. The Cln3 cyclin is down-regulated by translational repression and degradation during the G1 arrest caused by nitrogen deprivation in budding yeast. EMBO J. 1997;16:7196–7206. doi: 10.1093/emboj/16.23.7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman I, Huang YS, Mendez R, Cao Q, Theurkauf W, Richter JD. CPEB, maskin, and cyclin B1 mRNA at the mitotic apparatus: Implications for local translational control of cell division. Cell. 2000;103:435–447. doi: 10.1016/s0092-8674(00)00135-5. [DOI] [PubMed] [Google Scholar]
- Jansen RP, Dowzer C, Michaelis C, Galova M, Nasmyth K. Mother cell-specific HO expression in budding yeast depends on the unconventional myosin myo4p and other cytoplasmic proteins. Cell. 1996;84:687–697. doi: 10.1016/s0092-8674(00)81047-8. [DOI] [PubMed] [Google Scholar]
- Loeb JDJ, Kerentseva TA, Pan T, Sepulveda-Becerra M, Liu H. Saccharomyces cerevisiae G1 cyclins are differentially involved in invasive and pseudohyphal growth independent of the filamentation mitogen-activated protein kinase pathway. Genetics. 1999;153:1535–1546. doi: 10.1093/genetics/153.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long RM, Singer RH, Meng X, Gonzalez I, Nasmyth K, Jansen RP. Mating type switching in yeast controlled by asymmetric localization of ASH1 mRNA. Science. 1997;277:383–387. doi: 10.1126/science.277.5324.383. [DOI] [PubMed] [Google Scholar]
- Miller ME, Cross FR. Distinct subcellular localization patterns contribute to functional specificity of the Cln2 and Cln3 cyclins of Saccharomyces cerevisiae. Mol Cell Biol. 2000;20:542–555. doi: 10.1128/mcb.20.2.542-555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosch HU, Fink GR. Dissection of filamentous growth by transposon mutagenesis in Saccharomyces cerevisiae. Genetics. 1997;145:671–684. doi: 10.1093/genetics/145.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash R, Tokiwa G, Anand S, Erickson K, Futcher B. The WHI1+ gene of Saccharomyces cerevisiae tethers cell division to cell size and is a cyclin homolog. EMBO J. 1988;7:4335–4346. doi: 10.1002/j.1460-2075.1988.tb03332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash R, Volpe T, Futcher B. Isolation and characterization of WHI3, a size-control gene of Saccharomyces cerevisiae. Genetics. 2001;157:1469–1480. doi: 10.1093/genetics/157.4.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo M, Roberts JM. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science. 1993;259:1908–1912. doi: 10.1126/science.8384376. [DOI] [PubMed] [Google Scholar]
- Pringle JR, Hartwell LH. The Saccharomyces cerevisiae cell cycle. In: Strathern JN, et al., editors. The molecular biology of the yeast Saccharomyces cerevisiae: Life cycle and inheritance. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1981. pp. 97–142. [Google Scholar]
- Quelle DE, Ashmun RA, Shurtleff SA, Kato JY, Bar-Sagi D, Roussel MF, Sherr CJ. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes & Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- Rigaut G, Shevchenko A, Rutz B, Wilm M, Mann M, Seraphin B. A generic protein purification method for protein complex characterization and proteome exploration. Nat Biotechnol. 1999;10:1030–1032. doi: 10.1038/13732. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Fink GR. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: Mating and invasive. Genes & Dev. 1994;8:2974–2985. doi: 10.1101/gad.8.24.2974. [DOI] [PubMed] [Google Scholar]
- Schneider BL, Patton EE, Lanker S, Mendenhall MD, Wittenberg C, Futcher B, Tyers M. Yeast G1 cyclins are unstable in G1 phase. Nature. 1998;395:86–89. doi: 10.1038/25774. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Gulli MP, Peter M. Nuclear sequestration of the exchange factor Cdc24 by Far1 regulates cell polarity during yeast mating. Nat Cell Biol. 2000;2:117–124. doi: 10.1038/35000073. [DOI] [PubMed] [Google Scholar]
- Skapek SX, Rhee J, Spicer DB, Lassar AB. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science. 1995;267:1022–1024. doi: 10.1126/science.7863328. [DOI] [PubMed] [Google Scholar]
- Spellman PT, Sherlock G, Zhang MQ, Iyer VR, Anders K, Eisen MB, Brown PO, Botstein D, Futcher B. Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol Biol Cell. 1998;9:3273–3297. doi: 10.1091/mbc.9.12.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart D, Wittenberg C. CLN3, not positive feedback, determines the timing of CLN2 transcription in cycling cells. Genes & Dev. 1995;9:2780–2794. doi: 10.1101/gad.9.22.2780. [DOI] [PubMed] [Google Scholar]
- Takizawa PA, Sil A, Swedlow JR, Herskowitz I, Vale RD. Actin-dependent localization of an RNA encoding a cell-fate determinant in yeast. Nature. 1997;389:90–93. doi: 10.1038/38015. [DOI] [PubMed] [Google Scholar]
- Tyers M, Tokiwa G, Futcher B. Comparison of the Saccharomyces cerevisiae G1 cyclins: Cln3 may be an upstream activator of Cln1, Cln2 and other cyclins. EMBO J. 1993;12:1955–1968. doi: 10.1002/j.1460-2075.1993.tb05845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnen H, Futcher B. Genetic analysis of the shared role of CLN3 and BCK2 at the G1–S transition in Saccharomyces cerevisiae. Genetics. 1999;153:1131–1143. doi: 10.1093/genetics/153.3.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J-M, Wei Q, Zao X, Paterson BM. Coupling of the cell cycle and myogenesis through the cyclin D1-dependent interaction of MyoD with cdk4. EMBO J. 1999;18:926–933. doi: 10.1093/emboj/18.4.926. [DOI] [PMC free article] [PubMed] [Google Scholar]