Abstract
Gout has not been described previously as a complication in cystic fibrosis (CF). Here we present data on nine CF patients who have presented with symptoms of acute gout. This gives an estimated prevalence of gout of around 2.5% in our adult CF population, compared to a previously described prevalence in the non-CF population of just over 1%. Serum urate is measured routinely at the annual review in our unit. Mean (SD) serum urate was 0.40 (0.09) mmol/L in male CF patients (n = 108) and 0.31 (0.08) mmol/L in female patients (n = 74). This was significantly greater than in historical controls. Thirty-seven percent of male CF patients and 36% of female patients had serum urate levels above the upper limit of normal.
Introduction
With improved CF survival, the management of other co-morbidities is becoming increasingly important, including complications that may not be directly related to the underlying defect in CFTR. Although gout has not previously been described in CF, it is a common disorder of uric acid metabolism.1 Elevated serum urate leads to deposition of monosodium urate crystals in tissues and joints, which triggers an intense inflammatory reaction in the joint. Gout typically presents with an acute monoarthritis, most commonly affecting the great toe, but it can also affect the foot, ankle, knee, wrist, fingers and elbow. It is more common in men and the elderly, with an overall population prevalence of just over 1%.2 Acute attacks are usually managed with NSAIDs, and typically resolve over 7–10 days. Other drugs that have been used include oral steroids and colchicine, though the latter is associated with a high incidence of toxicity.3
Gout is distinct from CF arthropathy, a complication that affects around 5–10% of CF adults.4 Unlike gout, the pathophysiology of classical CF arthropathy is poorly understood, but it is a non-erosive episodic arthritis characterized by painful swelling and stiffness of the knees, ankles, wrists and hands that may be due to immune complex deposition.5 Few reports of CF arthropathy have involved joint aspiration and microscopy, and no reports describe the presence of urate crystals.
We present here the details of CF patients who have presented with gout at our adult unit. We also present data on serum urate levels in asymptomatic patients, and speculate on the role of pancreatic enzyme supplements in hyperuricaemia.
Methods
Prevalence of gout
To derive an estimate of the prevalence of gout symptoms in the CF population, cases of gout encountered in the CF clinic were recorded over 2 years from the start of 2008. Case-notes were subsequently reviewed to extract information about presentation and serum urate. This cohort includes only those patients who self-presented with symptoms of gout, and patients were not systematically surveyed.
Urate levels in asymptomatic patients
Serum urate has been measured annually at the annual clinical review for the last 5 years in our unit. Most recent urate levels were reviewed from 200 randomly selected CF patients (every other name on patient list). The patients who had described gout symptoms were excluded, as were those who had undergone organ transplantation.
Serum urate values were compared to published data in healthy controls assessed as part of the US Framingham study.6 Upper limit of normal for controls was also derived from mean +1.65 × standard deviation (SD). A P value of <0.05 was considered statistically significant. Data were analysed using Prism (Graphpad Software, CA, USA). Population means compared by t-test and frequency of events by chi-squared.
Results
Prevalence of gout
Nine patients at our regional centre have been diagnosed with acute gout in the last 24 months (2.5% of total patients) (Table 1). The prevalence of gout was not significantly higher than that of 1.4% reported in the entire UK population (P = 0.07).2 However, if control subjects older than 54 years are excluded (in whom the population prevalence of gout is much higher), the prevalence of gout falls to 0.6%, and this difference is significant (P = 0.0005).
Table 1.
Characteristics of CF patients with clinical diagnosis of gout
| Patient number | Age (years) | Gender | Genotype | Joints affected | Highest serum urate (mmol/L) | Notes |
|---|---|---|---|---|---|---|
| 1 | 54 | M | ΔF508/3272-26A > G | 1st metatarsal | 0.57 | |
| 2 | 37 | M | ΔF508/ΔF508 | 1st metatarsal | 0.57 | |
| 3 | 42 | M | ΔF508/S945L | Ankle | 0.52 | |
| 4 | 36 | M | ΔF508/ΔF508 | 1st metatarsal | 0.5 | |
| 5 | 22 | M | ΔF508/621 + 1 | Knee and 1st metatarsal | 0.65 | |
| 6 | 41 | M | ΔF508/ΔF508 | 1st metatarsal | 0.54 | Confirmed by joint aspiration |
| 7 | 32 | M | ΔF508/ΔF508 | 1st metatarsal | 0.72 | Exacerbated by trial of allopurinol |
| 8 | 31 | M | ΔF508/ΔF508 | 1st Metatarsal | 0.57 | Settled with allopurinol |
| 9 | 32 | M | ΔF508/621 + 1 | Foot and 5th metatarsal | 0.64 |
All patients were Caucasian men, exocrine pancreatic insufficient and with normal renal function. Two patients were diabetic and five were ΔF508 homozygotes. The median age was 36 years (range 22–54 years). All but two presented with classical podagra (painful, hot swelling of the first metatarsal), though the knee was also involved in one case. Joint aspiration was only performed in one case, and confirmed the presence of uric acid crystals.
Aside from pancreatic enzyme supplements, no patients were on any medications known to increase uric acid or precipitate gout. Symptoms were managed with NSAIDs (6/9), allopurinol (3/9), colchicine (1/9) or codeine (1/9). Mean (SD) highest serum urate in these patients was 0.59 (0.07) mmol/L.
Urate levels in asymptomatic patients
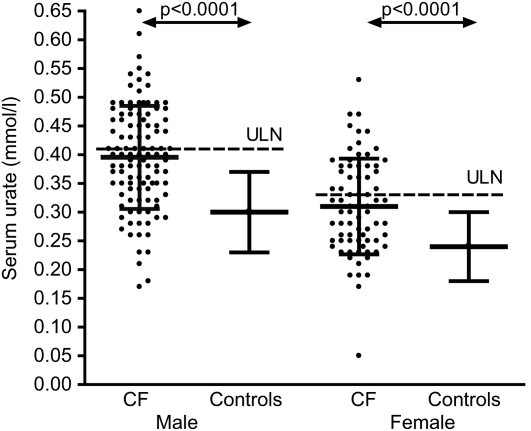
Serum urate levels were available in 182 patients (108 men). Mean (SD) serum urate was 0.40 (0.09) mmol/L in men and 0.31 (0.08) mmol/L in women. This is significantly greater than in historical controls: 0.30 (0.07) in men (n = 2283, P < 0.0001), 0.24 (0.06) mmol/L in women (n = 2844, P < 0.0001)6 (Table 2, Figure 1). All patients had normal renal function; mean serum creatinine was 65 µmol/L (max 108 µmol/L, normal range 60–120 µmol/L).
Table 2.
Serum urate in CF patients compared to historical controls
| Men |
Women |
|||
|---|---|---|---|---|
| CF | Controls* | CF | Controls* | |
| n | 108 | 2283 | 74 | 2844 |
| Mean age (range) yrs | 30 (18–54) | 44 (30–65) | 30 (17–62) | 44 (30–65) |
| Serum urate (mmol/L) | 0.40 (0.09) | 0.31 (0.08)† | 0.30 (0.07) ‡ | 0.24 (0.06)† |
Figure 1.
Serum urate levels in CF patients compared to those from historic controls.6 Individual patient data are shown for CF patients, together with mean and SD. Control data are presented as mean and SD. ULN = gender-specific upper limit of normal range, as derived from control data
Based upon the data in healthy controls, the upper limit of normal was calculated as 0.41 mmol/L (men) and 0.33 mmol/L (women). In the CF population therefore, 40 men (37%) and 27 women (36%) had elevated levels. The local laboratory quotes 0.42 mmol/L as the upper limit of normal for men and 0.38 mmol/L for women. Using these limits, 40 men (37%) and 19 women (26%) had elevated serum urate. This gives an overall prevalence of hyperuricaemia of 32%, significantly higher than even the highest estimates of population prevalence of hyperuricaemia (9.4% in New Zealand European men and 10.5% in women, P < 0.0001).7
The maximum recorded serum urate level was 0.65 mmol/L. Twenty-two patients (12%, 1 woman) had levels >0.48 mmol/L (8 mg/dl uric acid), historically associated with a high risk of developing gout (>33%).6
There was no significant association between urate levels and age (P = 0.92).
Discussion
We have estimated that around 2.5% of our adult CF patients have experienced gout symptoms in the last 2 years. This is significantly higher than in the age-adjusted non-CF population.2 This estimate of gout prevalence is likely to be an under-estimate, since this only includes patients who reported symptoms at the CF clinic. Patients whose symptoms spontaneously resolved, were self-managed, or handled by their general practitioner would not have been detected. The diagnosis of gout could be criticized because joint aspiration was only performed in a single case, but the combination of classical joint symptoms, elevated serum urate, and response to appropriate therapy rendered this procedure inappropriate in the majority of cases.1
We have demonstrated that elevated serum urate is common in patients with CF, and this may predispose CF patients to an increased risk of gout. Although asymptomatic hyperuricaemia is also common in the non-CF population, it appears to be at least three times more common in CF patients even before age and other risk factors are taken into account.7 Only a minority of people with hyperuricaemia go on to develop gout and asymptomatic hyperuricaemia does not usually require treatment.
Hyperuricaemia has been associated with certain drugs (e.g. thiazide diuretics) and diets (high fructose,8 purine or protein9 intake) or metabolic dysfunction. However, in CF patients there is another possible explanation for the high levels of serum urate. Pancreatic enzyme supplements derived from porcine pancreas are known to contain high levels of purines.10 This was first reported almost 40 years ago, when an association between the pancreatic enzyme dose and urate levels in the urine of children was first observed.10 Importantly, it was also shown that lowering the dose of enzymes could reduce the urinary excretion of urate.11 Hyperuricaemia was subsequently also reported in children and young adults with CF12, and this was more common in older subjects. This may have been due to the larger doses of enzymes used in adults or changes in the renal excretion of urate in the older subjects. The mean age of the subjects reported here was greater than that of the oldest subject in Davidson's report, and we saw no correlation with age, though we did not control for pancreatic function in this randomly selected sample.
That pancreatic supplements may be responsible for hyperuricaemia in a significant proportion of CF patients is a hypothesis that merits further investigation. Although as clinicians we are alert to the problems of under-dosing on pancreatic enzyme supplements, we may occasionally be guilty of paying less attention to excessive doses. Further studies are ongoing to explore more definitively the link between enzyme doses and serum urate, but it is notable that one of the patients with gout subsequently admitted to taking up to 80 tablets of Creon 25000 per day.
Although gout is relatively uncommon in CF, incidence is likely to increase as the adult CF population increases and grows older. Gout may be unrecognized in some and should be considered in all patients with single joint or recurrent arthritis, particularly if there is a history of elevated urate. Surveillance of serum urate is not routinely performed, but consideration should be given to adding this to annual review investigations, particularly in those with a history of rheumatological disorders. Clinical vigilance is required, since the management of recurrent attacks is different to other forms of arthropathy. In those with confirmed gout, or very extreme hyperuricaemia, a review of pancreatic enzyme intake is also indicated.
Conclusions
Hyperuricaemia is common in adult CF patients, and gout appears to occur with greater frequency than in the non-CF population. A possible cause of hyperuricaemia in CF is the use of pancreatic enzyme supplements, which contain high levels of purines, precursors of urate. The management of gout is different to that of CF arthropathy and it should be considered in those with recurrent or single joint arthritis. Review of pancreatic enzyme intake is indicated in those with confirmed gout or extreme hyperuricaemia.
DECLARATIONS
Competing interests
None declared
Funding
None
Ethical approval
Not applicable
Guarantor
AH
Contributorship
All authors contributed to the study and report; AH performed the analysis and wrote the report; JH collected the serum urate data; AB and RBT set up the database of gout patients; AKW conceived the idea for the study and initiated serum urate measurements at AR; AMJ oversaw the analysis and write-up; all authors reviewed and approved the final manuscript
Acknowledgements
None
References
- 1.Underwood M Diagnosis and management of gout. BMJ 2006;332:1315–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mikuls TR, Farrar JT, Bilker WB, Fernandes S, Schumacher HR Jr, Saag KG Gout epidemiology: results from the UK General Practice Research Database, 1990–1999. Ann Rheum Dis 2005;64:267–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahern MJ, Reid C, Gordon TP, McCredie M, Brooks PM, Jones M Does colchicine work? The results of the first controlled study in acute gout. Aust N Z J Med 1987;17:301–4 [DOI] [PubMed] [Google Scholar]
- 4.Dixey J, Redington AN, Butler RC, et al. The arthropathy of cystic fibrosis. Ann Rheum Dis 1988;47:218–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rush PJ, Shore A, Coblentz C, Wilmot D, Corey M, Levison H The musculoskeletal manifestations of cystic fibrosis. Semin Arthritis Rheum 1986;15:213–25 [DOI] [PubMed] [Google Scholar]
- 6.Hall AP, Barry PE, Dawber TR, McNamara PM Epidemiology of gout and hyperuricemia. A long-term population study. Am J Med 1967;42:27–37 [DOI] [PubMed] [Google Scholar]
- 7.Klemp P, Stansfield SA, Castle B, Robertson MC Gout is on the increase in New Zealand. Ann Rheum Dis 1997;56:22–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi JW, Ford ES, Gao X, Choi HK Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum 2008;59:109–16 [DOI] [PubMed] [Google Scholar]
- 9.Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 2004;350:1093–103 [DOI] [PubMed] [Google Scholar]
- 10.Stapleton FB, Kennedy J, Nousia-Arvanitakis S, Linshaw MA Hyperuricosuria due to high-dose pancreatic extract therapy in cystic fibrosis. N Engl J Med 1976;295:246–8 [DOI] [PubMed] [Google Scholar]
- 11.Nouisa-Arvanitakis S, Stapleton FB, Linshaw MA, Kennedy J Therapeutic approach to pancreatic extract-induced hyperuricosuria in cystic fibrosis. J Pediatr 1977;90:302–5 [DOI] [PubMed] [Google Scholar]
- 12.Davidson GP, Hassel FM, Crozier D, Corey M, Forstner GG Iatrogenic hyperuricemia in children with cystic fibrosis. J Pediatr 1978;93:976–8 [DOI] [PubMed] [Google Scholar]