Abstract
Objective
To determine the inter-relationships between MRI-defined lesion and atrophy measures of spinal cord involvement and brain involvement and their relationships to disability in a small cohort of patients with multiple sclerosis (MS).
Background
Although it is known that cervical spinal cord atrophy correlates with disability in MS, it is unknown whether it is the most important determinant when compared to other regions of the CNS. Furthermore, it is not clear to what extent brain and cord lesions and atrophy are related.
Design/methods
3T MRI of the whole brain and whole spinal cord was obtained in 21 patients with MS, including 18 with relapsing-remitting, one with secondary progressive, one with primary progressive, and one with a clinically isolated syndrome. Brain global gray and white matter volumes were segmented with SPM8. Spinal cord contour volume was segmented in whole by a semi-automated method with bins assigned to either the cervical or thoracic regions. All CNS volumes were normalized by the intracranial volume. Brain and cord T2 hyperintense lesions were segmented using a semi-automated edge finding tool.
Results
Among all MRI measures, only upper cervical spinal cord volume significantly correlated with Expanded Disability Status Scale score (r=−0.515, p=0.020). The brain-cord relationships between whole or regional spinal cord volume or lesions and gray matter, white matter, or whole brain volume or whole brain lesions were generally weak and all non-significant.
Conclusions/relevance
In this preliminary study of mildly disabled, treated MS patients, cervical spinal cord atrophy most strongly correlates with physical disability in MS when accounting for a wide range of other CNS measures of lesions and atrophy, including thoracic or whole spinal cord volume, and cerebral gray, white or whole brain volume. The weak relationship between spinal cord and brain lesions and atrophy may suggest that they progress rather independently in patients with MS.
Introduction
Multiple sclerosis (MS) produces both inflammatory and degenerative pathology of the brain and spinal cord. The degenerative process has been demonstrated by both histopathology and neuroimaging. Histologically, there is neuronal loss in the cortical and deep gray matter (GM), axonal loss in white matter (WM) and similar changes in the cervical and thoracic spinal cord.1–6 Neuroimaging shows CNS atrophy commonly affects the cerebral gray and WM and the cervical spinal cord.1,7–10 However, it is still unclear what is the relationship between the destructive processes in MS that affect the brain versus the spinal cord. A growing body of evidence indicates that CNS atrophy correlates with clinical disability in MS.11–12 This is in contrast to a relatively poor correlation between conventional lesion-based imaging measures of MS disease activity and clinical disability.13 MRI volumetric studies have demonstrated correlations between disability and atrophy of the whole brain, cerebral WM, cerebral GM, and cervical spinal cord.8,14–19
However, it is not yet clear which of these measures of lesions and atrophy, when compared side-by-side, are most closely linked to disability. Although atrophy has been detected throughout the CNS in MS patients, the relative clinical importance of atrophy in each CNS subregion has not been elucidated. In particular, comparison of subregions of spinal cord volume with brain volumes has yet to be performed. Thus, the relationship between clinical disability and compartment-specific atrophy (of the cerebral gray and WM and subregions of the spinal cord) is unknown.
The purpose of this study was to perform whole brain and spinal cord MRI at 3T to determine (1) which aspects of generalized brain or cord lesions/atrophy are most closely associated with physical disability and (2) the inter-relationships among brain and spinal cord compartmental lesions and atrophy.
Methods
Subjects
General patient demographics and clinical measures are listed in Table 1. In total, 21 patients were included: 18 with relapsing-remitting (RR), one with secondary progressive (SP), one with primary progressive (PP), and one with a clinically isolated syndrome (CIS) as defined by the International Panel criteria.20 Nineteen of the 21 patients (90%) were being treated with disease modifying agents at the time of enrollment. Of the treated patients, 18 (86%) were on glatiramer acetate or a beta-interferon agent - one of these 18 patients was on both glatiramer acetate and interferon agent; another two of these 18 patients were also on mycophenolate or daclizumab. One (5%) patient was on rituximab monotherapy alone.
Table 1.
Patient demographics and clinical measures (n=21)
| Variable | Mean ± SD (Range) |
|---|---|
| Age (years) | 40.9 ± 8 (28–55) |
| Height (cm) | 169.2 ± 12.7 (152.4–193.0) |
| Expanded Disability Status Scale score | 1.6 ± 1.6 (0–6.0) |
| Timed 25-foot walk (seconds) | 4.9 ± 1.0 (3.3–8.0) |
| Disease Duration (years) | 8.3 ± 7.5 (0.8–28.0) |
This Health Insurance Portability and Accountability Act (HIPAA)-compliant study included approval by an institutional review board (IRB) and informed consent. MS patients were enrolled consecutively from a community-based, university-affiliated MS clinic. Within 1 month of MRI, MS disease course and clinical measures including the Expanded Disability Status Scale (EDSS) score21 and timed 25 foot walk (T25FW)22 (Table 1) were assigned by a treating neurologist MS specialist at our institution. Different aspects of these patients are being reported separately.23
Image acquisition
Figure 1 shows brain and spinal cord from two representative patients. The patients underwent complete brain and spinal cord MRI on a 3T unit (GE 3T HDx Scanner, Milwaukee, WI) of the whole spinal cord with a multichannel phased array coil using axial fast spin-echo T2-weighted images (TR=6167 ms, TE=110 ms, 0.94 × 0.94 × 3 mm voxel size no inter-slice gaps). 150–200 axial slices were acquired on each subject to cover the whole spinal cord. Only the slices extending from the foramen magnum through the inferior extent of the conus medullaris were analyzed. For brain MRI, we obtained axial fast fluid-attenuated inversion recovery (FLAIR) sequences (TR=9000 ms, TE=90 ms, IR=2500 ms, 0.488 × 0.488 × 3 mm voxel size, no inter-slice gaps) to assess brain lesions. Images from part of these spinal and brain protocols have been previously published.24–25 Since 3D spoiled gradient recalled (SPGR) images produced inadequate gray-white differentiation at 3 T we used a 3D modified driven equilibrium Fourier transform (MDEFT) sequence26 which has been shown to produce superior gray-white segmentation relative to the similar MPRAGE sequence27.
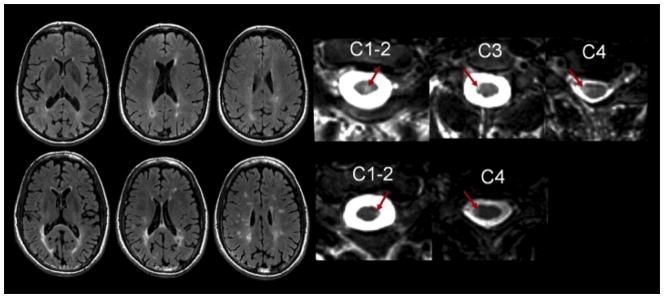
Figure 1.
MRI from two patients showing representative FLAIR brain and T2-weighted spinal cord images illustrating the unreliable correlation between brain and cord involvement. The top row is a 44 year-old woman with a history of relapsing-remitting MS with an EDSS score of 3. The patient had a relatively low total brain lesion volume (4015.2 mm3) and high total spinal cord lesion volume (558.9 mm3). The bottom row is 46 year-old man with a history of relapsing-remitting MS with an EDSS score of 0. The patient had a relatively high total brain lesion volume (26995.0 mm3) but low total spinal cord lesion load volume (134.8 mm3). Arrows represent spinal cord MS lesions.
Spinal cord image analysis
MRI post-processing and segmentation was performed using the Jim software package (v. 5.0, Xinapse Systems, Aldwincle, UK, http://www.xinapse.com). Scans were anonymized and randomized, and analysis was done in a blinded fashion by experienced observers.
Spinal cord atrophy measures
The whole spinal cord was defined using bony landmarks (foramen magnum rostrally to T12 vertebral body caudally). We stopped at the T12 level due to variability in cord anatomy below this level. The spinal cord was segmented into cervical and thoracolumbar regions, which were separated by the C7/T1 disc interspace, to derive whole cervical and whole thoracic spinal cord volume. We additionally measured an upper cervical cord volume from C2 to C3 by first measuring the cross-sectional area of the cord at the level of the C2/C3 intervertebral disc. Cross-sectional area was then assessed at the slice above and below the C2/C3 section. The mean volume of the three contiguous slices was calculated for each subject. This was assessed as a variable because it has been accepted and is a commonly used surrogate of spinal cord atrophy in MS.12,28 An edge-finding tool based on local thresholding was applied to each axial slice to identify the spinal cord contour. Manual adjustments were applied where necessary. Segmentation was performed by an experienced observer (A.C.). Consistency and accuracy of measurements were confirmed by independent and blinded review of the regions of interest by a second experienced observer (A.A.). Differences between the two observers were resolved by a senior observer (M.N.). Spinal cord volume in each region was normalized by dividing by the number of slices and intracranial volume (ICV) (see brain image analysis).
Spinal cord lesion measures
Image analysis for spinal cord T2 hyperintense lesions, as described in detail previously,24 was the consensus of experienced observers (J.S., M.N.) and confirmed by a senior observer (R.B.) to resolve any discrepancies. Image analysis was meant to replicate the clinical setting. Thus, images were optimized for clinical review by adjusting window width and level but not normalized. The total volume of lesions was assessed using an edge-finding tool based on local thresholding. Manual adjustments were applied where necessary.
Brain image analysis
Brain volume measure
MDEFT scans were deskulled using a semi-automated tool in the Jim software package. Values of ICV were used to normalize all CNS volume measurements.
Tissue segmentation and volume determination
Manually skull stripped MDEFT images were first brought into approximate alignment with the ICBM template, then bias-field corrected, spatially normalized and automatically segmented into GM, WM and CSF probability maps using the unified segmentation model implemented in the segmentation routine of SPM829 running in Matlab (version 2009a, The MathWorks – Inc., Natick, Massachusetts). Within manual outlines of the intracranial cavities, mutually exclusive masks for each tissue were derived from SPM8 tissue probability maps as described previously.30 Estimates of white-matter volume (WMV), gray-matter volume (GMV), CSF volume (CSFV) and brain parenchymal volume (BPV = WMV+GMV) were automatically derived from SPM8 generated segmentations after corrections for T1-hypointensities misclassifications and deep gray matter underestimation (see corrections for misclassifications). These values were used to compute normalized compartment volumes: white-matter fraction (WMF = WMV/ICV), gray-matter fraction (GMF = GMV/ICV), CSF fraction (CSFF = CSFV/ICV) and brain parenchymal fraction (BPF = (WMV+GMV)/ICV).
Correction for misclassifications
MS hypointense white matter lesions on T1-WI are often misclassified as GM in SPM segmentation as previously reported.18,30 Classification errors can also occur in the tissue surrounding T1 hypointensities due to partial volume effects. T1 hypointensities within the WM compartment were segmented from MDEFT images using a semi-automated edge-finding tool with manual corrections applied as needed in Jim software. A mask of T1 hypointensities was created on MDEFT images including hypointense lesions that were identified and manually contoured on MDEFT images using cross-referencing with hyperintense lesions on FLAIR images in the same anatomic plane. Consensus was reached by two expert readers. The derived masks were used to correct SPM8-derived segmentations.
Segmentations were also manually corrected for underestimations of deep gray matter nuclei. Corrections were performed by a single expert rater using a drawing tool in 3D Slicer (version 3.4, http://www.slicer.org). Pixels that were reassigned to GM were automatically set to background in WM final masks. All the aforementioned steps were implemented in Matlab.
FLAIR hyperintense brain lesions
For analysis of hyperintense brain lesions, the software package Jim was again employed. FLAIR hyperintense lesions, as described in detail previously,25 were identified by the consensus of 2 experienced observers (J.S., M.N.) and differences were resolved by a senior observer (R.B.). Whole brain FLAIR lesion volume (FLLV) was then obtained by a semi-automated edge-finding tool based on local thresholding on each axial slice with manual adjustments as necessary.
Statistical analysis
All spinal cord volume measurements were normalized by dividing by ICV and the number of axial slices covering that region. Volumes were further adjusted for age by linear regression. Partial correlations assessed associations between MRI, and both EDSS score and T25FW while controlling for age. The magnitude of correlation was examined using Cohen’s method for comparing r values within subjects with the appropriate t-test with n-3 degrees of freedom.31 A p value less than 0.05 was considered significant. All statistical analyses were performed with SPSS for Windows (v. 17, SPSS Inc., Chicago, Illinois).
Results
Brain and spinal cord MRI lesion and volume (atrophy) data are summarized in Table 2. Sixteen of 21 patients had spinal cord lesions (76%).
Table 2.
Central nervous system volume and lesion data (n=21)
| Variable | Mean ± SD (Range) |
|---|---|
| Normalized whole cord volume (x10−4)* | 9.28 ± 1.71 (7.46–13.55) |
| Normalized cervical cord volume (x10−4)* | 14.09 ± 0.22 (1.03–1.80) |
| Normalized thoracic cord volume (x10−4)* | 7.51 ± 0.15 (6.04–11.42) |
| Normalized C2–C3 cervical cord volume (x10−4)* | 1.49 ± (1.04–2.00) |
| Total cord T2 hyperintense lesion volume (mm3) | 214.9 ± 195.9 (0.0–624.2) |
| Cervical cord T2 hyperintense lesion volume (mm3) | 159.7 ± 179.1 (0.0–624.2) |
| Thoracic cord T2 hyperintense lesion volume (mm3) | 55.2 ± 99.5 (0.0–374.2) |
| BPF | 0.83 ± 0.05 (0.70–0.89) |
| Brain gray matter fraction | 0.49 ± 0.04 (0.41–0.55) |
| Brain white matter fraction | 0.34 ± 0.02 (0.293–0.40) |
| Brain FLLV (mm3) | 15221.0 ± 16586.8 (867.8–56749.6) |
Note: BPF=global brain parenchymal fraction; FLLV=fluid-attenuated inversion-recovery hyperintense lesion volume;
spinal cord volumes were normalized by number of slices and intracranial volume (see Methods section).
Associations between spinal cord and brain MRI measures
Figures 1–3 and Table 3 show the relationship between spinal cord and brain MRI data. None of the spinal cord lesion or volume data correlated with brain lesion or volume data (the correlation between thoracic spinal cord lesion volume and brain FLAIR lesion volume approached but did not reach significance). The strongest correlations between spinal cord and brain volume data were observed between cervical spinal cord and brain, but these were not significant. The analysis revealed similar results when controlling for age (data not shown). Representative scatterplots illustrating the relationships between GMF or BPF and cervical spinal cord volume are provided in Figures 2 and 3. Representative patients showing the discordance between brain and cord lesions are shown in Figure 1. Age did not correlate with any of the CNS volume measures (data not shown; all p-values >0.155).
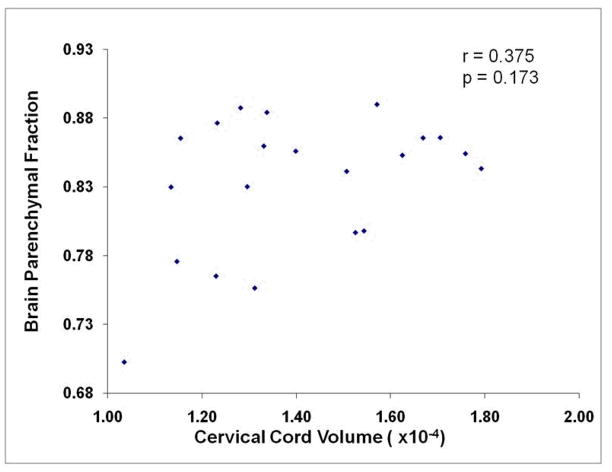
Figure 3.
The lack of a relationship between whole brain and global cervical spinal cord volume (r=0.375, p=0.103) is illustrated in this scatterplot.
Table 3.
Correlation between brain and spinal cord MRI data
| Spinal cord MRI measures: | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Volume measures* | Lesion measures | |||||||||||||
| C2–C3 | Cervical | Thoracic | Whole cord | Cervical | Thoracic | Whole cord | ||||||||
| Brain MRI measures: | r | p | r | p | r | p | r | p | r | p | r | p | r | p |
| GMF^ | 0.307 | 0.189 | 0.302 | 0.196 | 0.234 | 0.320 | 0.229 | 0.331 | −0.119 | 0.618 | 0.009 | 0.971 | −0.104 | 0.661 |
| WMF^ | 0.323 | 0.164 | 0.359 | 0.120 | 0.229 | 0.331 | 0.272 | 0.245 | 0.226 | 0.339 | 0.097 | 0.683 | 0.258 | 0.273 |
| BPF^ | 0.365 | 0.113 | 0.375 | 0.103 | 0.272 | 0.245 | 0.285 | 0.224 | −0.008 | 0.972 | 0.044 | 0.854 | 0.015 | 0.950 |
| FLLV | 0.092 | 0.699 | 0.062 | 0.794 | 0.035 | 0.883 | 0.098 | 0.682 | 0.085 | 0.720 | 0.443 | 0.050 | 0.307 | 0.188 |
Note: GMF=brain gray matter fraction; WMF= brain white matter fraction; BPF= global brain parenchymal fraction; FLLV=Brain fluid-attenuated inversion-recovery hyperintense lesion volume;
spinal cord volumes are normalized by number of slices and intracranial volume (see Methods section).
brain volumes are normalized by intracranial volume (see Methods section)
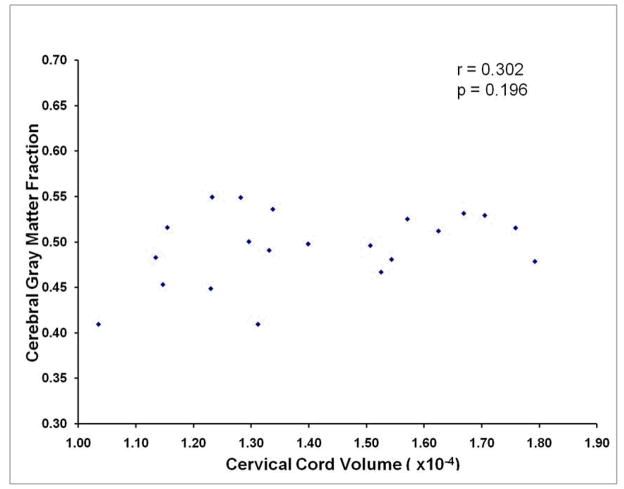
Figure 2.
The lack of a relationship between global cerebral gray matter and global cervical spinal cord volume (r=0.302, p=0.196) is illustrated in this scatterplot.
Clinical-MRI associations
Partial correlations, controlling for age, between MRI measures and disability (EDSS and T25FW) are shown in Table 4. Among all the MRI variables tested for an association with EDSS score, only C2–C3 cord volume showed a significant correlation (r=−0.515, p=0.020). Cervical spinal cord volume approached a significant correlation with EDSS score (r=−0.436, p=0.055). The correlation between cervical cord atrophy and disability was largely driven by the SP patients (data not shown). None of the MRI variables significantly correlated with T25FW. Similar results were seen in age-unadjusted analyses (data not shown)
Table 4.
Correlation between MRI measures and disability (n=21)
| Volume measures | Lesion measures | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spinal cord* | Brain^ | Brain | Spinal cord | |||||||||||||||||||
| C2–C3 cord volume* | Cervical cord volume | Thoracic cord volume | Whole cord volume | BPF | GMF | WMF | FLLV | Cervical cord T2LV | Thoracic cord T2LV | Whole cord T2LV | ||||||||||||
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| EDSS | −0.515 | 0.020** | −0.436 | 0.055 | −0.224 | 0.343 | −0.258 | 0.273 | 0.330 | 0.155 | −0.295 | 0.207 | 0.255 | 0.277 | −0.069 | 0.773 | −0.173 | 0.465 | −0.326 | 0.161 | 0.199 | 0.400 |
| T25FW | −0.257 | 0.274 | −0.180 | 0.449 | −0.23 | 0.924 | −0.094 | 0.694 | 0.116 | 0.626 | −0.012 | 0.960 | 0.282 | 0.229 | −0.440 | 0.052 | −0.048 | 0.840 | 0.164 | 0.488 | −0.019 | 0.937 |
Note: EDSS = Expanded Disability Status Scale; T25FW= timed 25-foot walk; FLLV=brain fluid-attenuated inversion-recovery hyperintense lesion volume; BPF=global brain parenchymal fraction; GMF=brain gray matter fraction; WMF=brain white matter fraction; T2LV=T2 hyperintense lesion volume;
spinal cord volumes are normalized by number of slices and intracranial volume (see Methods section);
brain volumes are normalized by intracranial volume (see Methods section);
p<0.05
Discussion
This is the first study to evaluate how lesions and volume in each subregion of the CNS differentially correlates with clinical disability in a small population primarily comprised of treated, mildly disabled RRMS patients. In our study, only upper cervical (C2–3) spinal cord volume significantly correlated with physical disability on the EDSS scale, indicating a relationship between cervical cord atrophy and clinical status. Other MRI measures of CNS lesions and volume did not show significant correlations with the EDSS score or T25FW, including whole brain lesions and volume, cerebral GM volume, cerebral WM volume, spinal cord lesion volume, or volume of the thoracic spinal cord.
As has been observed in prior studies, T2 lesion volume in the brain or spinal cord did not show significant correlations with EDSS score.13,24. Previous studies found correlations between CNS regional or global atrophy and disability, but did not specifically evaluate the relative importance of the array of measures pertaining to brain vs. cord pathology in RR patients.8,10,14–17,19,28 A study of SP patients found that all MRI compartmental volumes (cerebral GM, cerebral WM, and cervical cord) correlated with the multiple sclerosis functional composite score, but only cervical spinal cord cross-sectional area correlated with EDSS score.15 Full cervical/thoracic or whole spinal cord volumes were not assessed in that study.
The relative clinical importance of cervical spinal cord atrophy may reflect three anatomical considerations. First, the cervical volume may be an accurate correlate of upper motor neuron mediated limb and trunk motor function as it contains all descending corticospinal fibers destined for motor targets in the trunk, arms and legs. Second, the cervical cord is a crossroads for all cerebrospinal descending and spinocerebral ascending pathways. Thus, as compared to other brain and cord locations, damage to the cervical cord disproportionately affects myriad CNS functions. Third, the cervical cord volume also reflects remote volume by its reciprocal connectivity with distant brain and thoracolumbar cord axons and neurons. Thus, Wallerian degeneration in widespread CNS regions both rostral and caudal to the cervical cord will be reflected in spinal cord atrophy.
Of note, we found a lack of correlation between all brain and cord lesion/volume measures and T25FW, a clinical measure of ambulatory function. Other MRI measures have been shown to correlate with measures of ambulatory dysfunction, including cervical spinal cord volume, GM volume, brainstem volume, damage in cerebral normal appearing WM, and GM dentate nucleus T2 hypointensity.18,32–35 One potential explanation for the lack of correlation in our study and differences with previous studies is a restricted range in our sample. Our patients had generally well preserved ambulatory function and overall were mildly disabled. In addition, the sample size was small and thus, may not have been adequately powered to show such correlations between MRI measures and T25FW in the particular study population.
Our study also evaluated the relationship between brain and cord pathology as defined by MRI, including both compartment-specific and region specific atrophy of the brain and cord. We found no significant relationships between brain and cord lesions or volume. This is in line with previous studies that found no correlation between cervical cord and brain MRI metrics (T2 lesion volume, CNS volumes, and DTI measures).36,37 Both our findings and those from previous studies suggest a non-uniform disease process in MS. Thus, the degenerative and inflammatory processes may affect each region independently. Furthermore, there may exist phenotypes or HLA-related genotypes within MS cohorts who have selective brain or cord involvement.38 Additionally, cord volume measures are relatively insensitive to atrophy early in the disease course of MS, which is secondary to the offsetting effects on (expansion of) cord volume caused by edema and inflammation.23 Furthermore, brain volume measures, particularly the contribution from WM, suffer from similar transitory effects that can mask ongoing atrophy.11 Thus, tissue destruction in the brain and cord may occur in parallel, but may not be adequately reflected in global volume measures. These results need to be confirmed in a larger study group across a broader range of clinical disability and MS subtypes.
The strengths of this study include the novel means of volumetric analysis – full cord and brain segmentation and volume analysis at high resolution (3T) MRI. This improves upon our work and that done by others, using validated methods of volumetric analysis.28,39–42 Limitations include small study size and the predominance of mildly disabled RR patients. The study only offers a limited view into those severely impaired by MS, as the study population was treated and relatively high functioning, which limits the generalizability of these study results. Our results should be considered preliminary because of these limitations. The two main findings of this study should be confirmed in larger populations of RRMS, SPMS, and PPMS patients: (1) cervical cord atrophy is the best predictor of clinical disability in MS; (2) a weak relationship exists between MRI-defined spinal cord and brain pathology assessed by overt lesions and atrophy. Furthermore, studying other aspects of structural damage such as cerebral T1 hypointensities, cortical lesions, and diffuse damage assessed by advanced MRI techniques may provide additional insights into the effects and role of brain vs. cord pathology in MS.
Conclusions/relevance
In a small population of mildly disabled, treated MS patients, upper cervical spinal cord atrophy most strongly correlates with physical disability in MS when accounting for other CNS measures of lesions and atrophy, including thoracic or whole spinal cord volume, and cerebral gray, white or whole brain volume. The weak relationship between spinal cord and brain volume measures suggests that atrophy in these parts of the CNS may progress rather independently in patients with MS.
Acknowledgments
This study was supported by the National Institutes of Health (1R01NS055083-01) and National Multiple Sclerosis Society (RG3705A1; RG3798A2). These findings were presented in preliminary form at the 62nd annual meeting of the American Academy of Neurology, Toronto, Canada, April 10-17, 2010
References
- 1.Cifelli A, Arridge M, Jezzard P, et al. Thalamic neurodegeneration in multiple sclerosis. Ann Neurol. 2002;52:650–653. doi: 10.1002/ana.10326. [DOI] [PubMed] [Google Scholar]
- 2.DeLuca GC, Ebers GC, Esiri MM. Axonal loss in multiple sclerosis: a pathological survey of the corticospinal and sensory tracts. Brain. 2004;127:1009–1018. doi: 10.1093/brain/awh118. [DOI] [PubMed] [Google Scholar]
- 3.Evangelou N, DeLuca GC, Owens T, et al. Pathological study of spinal cord atrophy in multiple sclerosis suggests limited role of local lesions. Brain. 2005;128:29–34. doi: 10.1093/brain/awh323. [DOI] [PubMed] [Google Scholar]
- 4.Gilmore CP, DeLuca GC, Bo L, et al. Spinal cord neuronal pathology in multiple clerosis. Brain Pathol. 2009;19:642–649. doi: 10.1111/j.1750-3639.2008.00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson JW, Bo L, Mork S, et al. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50:389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- 6.Vogt J, Paul F, Aktas O, et al. Lower motor neuron loss in multiple sclerosis and experimental autoimmune encephalomyelitis. Ann Neurol. 2009;66:310–322. doi: 10.1002/ana.21719. [DOI] [PubMed] [Google Scholar]
- 7.Brex PA, Leary SM, O’Riordan JIJ, et al. Measurement of spinal cord area in clinically isolated syndromes suggestive of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2001;70:544–547. doi: 10.1136/jnnp.70.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher E, Rudick RA, Simon JH, et al. Eight-year follow-up study of brain atrophy in patients with MS. Neurology. 2002;59:1412–1420. doi: 10.1212/01.wnl.0000036271.49066.06. [DOI] [PubMed] [Google Scholar]
- 9.Liu C, Edwards S, Gong Q, et al. Three dimensional MRI estimates of brain and spinal cord atrophy in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1999;66:323–330. doi: 10.1136/jnnp.66.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanfilipo MP, Benedict RH, Weinstock-Guttman B, et al. Gray and white matter brain atrophy and neuropsychological impairment in multiple sclerosis. Neurology. 2006;66:685–692. doi: 10.1212/01.wnl.0000201238.93586.d9. [DOI] [PubMed] [Google Scholar]
- 11.Bermel RA, Bakshi R. The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol. 2006;5:158–170. doi: 10.1016/S1474-4422(06)70349-0. [DOI] [PubMed] [Google Scholar]
- 12.Lin X, Tench CR, Evangelou N, et al. Measurement of spinal cord atrophy in multiple sclerosis. J Neuroimaging. 2004;14:20S–26S. doi: 10.1177/1051228404266265. [DOI] [PubMed] [Google Scholar]
- 13.Zivadinov R, Leist T. Clinical-magnetic resonance imaging correlations in multiple sclerosis. J Neuroimaging. 2005;15:10S–21S. doi: 10.1177/1051228405283291. [DOI] [PubMed] [Google Scholar]
- 14.Filippi M, Campi A, Colombo B, et al. A spinal cord MRI study of benign and secondary progressive multiple sclerosis. J Neurol. 1996;243:502–505. doi: 10.1007/BF00886870. [DOI] [PubMed] [Google Scholar]
- 15.Furby J, Hayton T, Anderson V, et al. Magnetic resonance imaging measures of brain and spinal cord atrophy correlate with clinical impairment in secondary progressive multiple sclerosis. Mult Scler. 2008;14:1068–1075. doi: 10.1177/1352458508093617. [DOI] [PubMed] [Google Scholar]
- 16.Lin X, Tench CR, Turner B, et al. Spinal cord atrophy and disability in multiple sclerosis over four years: application of a reproducible automated technique in monitoring disease progression in a cohort of the interferon beta-1a (Rebif) treatment trial. J Neurol Neurosurg Psychiatry. 2003;74:1090–1094. doi: 10.1136/jnnp.74.8.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin X, Blumhardt LD, Constantinescu CS. The relationship of brain and cervical cord volume to disability in clinical subtypes of multiple sclerosis: a three-dimensional MRI study. Acta Neurol Scand. 2003;108:401–406. doi: 10.1034/j.1600-0404.2003.00160.x. [DOI] [PubMed] [Google Scholar]
- 18.Sanfilipo MP, Benedict RH, Sharma J, et al. The relationship between whole brain volume and disability in multiple sclerosis: a comparison of normalized gray vs. white matter with misclassification correction. Neuroimage. 2005;26:1068–1077. doi: 10.1016/j.neuroimage.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson VL, Leary SM, Losseff NA, et al. Spinal cord atrophy and disability in MS: a longitudinal study. Neurology. 1998;51:234–238. doi: 10.1212/wnl.51.1.234. [DOI] [PubMed] [Google Scholar]
- 20.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 21.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 22.Fischer JS, Rudick RA, Cutter GR, et al. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler. 1999;5:244–250. doi: 10.1177/135245859900500409. [DOI] [PubMed] [Google Scholar]
- 23.Klein J, Arora A, Neema M, Healy BC, Tauhud S, Godberg-Zimring D, Chavarro-Nieto C, Stankiewicz JM, Cohen AB, Buckle GJ, Houtchens MK, Ceccarelli A, Dell’Oglio E, Guttmann CRG, Alsop DC, Hackney DB, Bakshi R. A 3T MRI investigation of the topography of whole spinal cord atrophy in multiple sclerosis. AJNR Am J Neuroradiol. doi: 10.3174/ajnr.A2459. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stankiewicz JM, Neema M, Alsop DC, et al. Spinal cord lesions and clinical status in multiple sclerosis: A 1.5 T and 3 T MRI study. J Neurol Sci. 2009;279:99–105. doi: 10.1016/j.jns.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stankiewicz JM, Glanz BI, Healy BC, et al. Brain MRI lesion load at 1.5T and 3T versus clinical status in multiple sclerosis. J Neuroimaging. doi: 10.1111/j.1552-6569.2009.00449.x. [Epub ahead of print, Nov. 6, 2009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deichmann R, Schwarzbauer C, Turner R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging: technical implications at 1.5 and 3 T. Neuroimage. 2004;21:757–767. doi: 10.1016/j.neuroimage.2003.09.062. [DOI] [PubMed] [Google Scholar]
- 27.Tardif CL, Collins DL, Pike GB. Sensitivity of voxel-based morphometry analysis to choice of imaging protocol at 3 T. Neuroimage. 2009;44:827–838. doi: 10.1016/j.neuroimage.2008.09.053. [DOI] [PubMed] [Google Scholar]
- 28.Liptak Z, Berger AM, Sampat MP, et al. Medulla oblongata volume: a biomarker of spinal cord damage and disability in multiple sclerosis. AJNR Am J Neuroradiol. 2008;29:1465–1470. doi: 10.3174/ajnr.A1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Chard DT, Griffin CM, Parker GJ, et al. Brain atrophy in clinically early relapsing-remitting multiple sclerosis. Brain. 2002;125:327–337. doi: 10.1093/brain/awf025. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Assoc; 1988. [Google Scholar]
- 32.Jasperse B, Vrenken H, Sanz-Arigita E, et al. Regional brain atrophy development is related to specific aspects of clinical dysfunction in multiple sclerosis. Neuroimage. 2007;38:529–537. doi: 10.1016/j.neuroimage.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 33.Khaleeli Z, Sastre-Garriga J, Ciccarelli O, et al. Magnetisation transfer ratio in the normal appearing white matter predicts progression of disability over 1 year in early primary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 2007;78(10):1076–1082. doi: 10.1136/jnnp.2006.107565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tjoa CW, Benedict RH, Weinstock-Guttman B, et al. MRI T2 hypointensity of the dentate nucleus is related to ambulatory impairment in multiple sclerosis. J Neurol Sci. 2005;234:17–24. doi: 10.1016/j.jns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Vaithianathar L, Tench CR, Morgan PS, et al. Magnetic resonance imaging of the cervical spinal cord in multiple sclerosis--a quantitative T1 relaxation time mapping approach. J Neurol. 2003;250:307–315. doi: 10.1007/s00415-003-1001-8. [DOI] [PubMed] [Google Scholar]
- 36.Agosta F, Absinta M, Sormani MP, et al. In vivo assessment of cervical cord damage in MS patients: a longitudinal diffusion tensor MRI study. Brain. 2007;130:2211–2219. doi: 10.1093/brain/awm110. [DOI] [PubMed] [Google Scholar]
- 37.Rovaris M, Judica E, Ceccarelli A, et al. Absence of diffuse cervical cord tissue damage in early, non-disabling relapsing-remitting MS: a preliminary study. Mult Scler. 2008;14:853–856. doi: 10.1177/1352458507088103. [DOI] [PubMed] [Google Scholar]
- 38.Sombekke MH, Lukas C, Crusius JB, et al. HLA-DRB1*1501 and spinal cord magnetic resonance imaging lesions in multiple sclerosis. Arch Neurol. 2010;67:514. doi: 10.1001/archneurol.2009.278. [DOI] [PubMed] [Google Scholar]
- 39.Horsfield MA, Rovaris M, Rocca MA, et al. Whole-brain atrophy in multiple sclerosis measured by two segmentation processes from various MRI sequences. J Neurol Sci. 2003;216:169–177. doi: 10.1016/j.jns.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Losseff NA, Webb SL, O’Riordan JI, et al. Spinal cord atrophy and disability in multiple sclerosis. A new reproducible and sensitive MRI method with potential to monitor disease progression. Brain. 1996;119:701–708. doi: 10.1093/brain/119.3.701. [DOI] [PubMed] [Google Scholar]
- 41.Mann RS, Constantinescu CS, Tench CR. Upper cervical spinal cord cross-sectional area in relapsing remitting multiple sclerosis: application of a new technique for measuring cross-sectional area on magnetic resonance images. J Magn Reson Imaging. 2007;26:61–65. doi: 10.1002/jmri.20959. [DOI] [PubMed] [Google Scholar]
- 42.Zivadinov R, Banas AC, Yella V, et al. Comparison of three different methods for measurement of cervical cord atrophy in multiple sclerosis. AJNR Am J Neuroradiol. 2008;29:319–325. doi: 10.3174/ajnr.A0813. [DOI] [PMC free article] [PubMed] [Google Scholar]