Abstract
Rosario Hernandez
This article is dedicated to Rosario Hernandez for her warm support of my own work and her genuine enthusiasm for the work of her colleagues throughout her career. I first met Rosario as a research fellow in Harry Quigley’s laboratory between 1991 and 1993. Along with Harry, John Morrison, Elaine Johnson, Abe Clark, Colm O’Brien and many others, Rosario’s work has provided lamina cribrosa astrocyte cellular mechanisms that are biomechanically plausible and in so doing provided credibility to early notions of the optic nerve head (ONH) as a biomechanical structure.
We owe a large intellectual debt to Rosario for her dogged persistence in the characterization of the ONH astrocyte and lamina cribrosacyte in age and disease. Two questions run through her work and remain of central importance today. First, how do astrocytes respond to and alter the biomechanical environment of the ONH and the physiologic stresses created therein? Second, how do these physiologic demands on the astrocyte influence their ability to deliver the support to retinal ganglion cell axon transport and flow against the translaminar pressure gradient?
The purpose of this article is to summarize what is known about the biomechanical determinants of retinal ganglion cell axon physiology within the ONH in the optic neuropathy of aging and Glaucoma. My goal is to provide a biomechanical framework for this discussion. This framework assumes that the ONH astrocytes and glia fundamentally support and influence both the lamina cribrosa extracellular matrix and retinal ganglion cell axon physiology. Rosario Hernandez was one of the first investigators to recognize the implications of this unique circumstance. Many of the ideas contained herein have been initially presented within or derived from her work (Hernandez, M.R., 2000. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res. 19, 297–321.; Hernandez, M.R., Pena, J.D., 1997. The optic nerve head in glaucomatous optic neuropathy. Arch Ophthalmol. 115, 389–395.).
Keywords: Glaucoma, Acute IOP elevation, Optic Nerve Head, Neural Canal, Lamina Cribrosa Position and Thickness, Peripapillary Scleral Position and Thickness, Post-NCO Total Prelaminar Volume
Glaucoma, Cupping and Axonal Insult Within the Optic Nerve Head (ONH)
While glaucomatous damage to the visual system likely includes important pathophysiologies within the retinal ganglion cell body (Asai et al., 1987; Garcia-Valenzuela et al., 1995; Quigley, 1995a; Quigley et al., 2000; Quigley et al., 1995; Weber et al., 1998) photoreceptors (Janssen et al., 1996; Kendell et al., 1995; Nork et al., 2000; Panda and Jonas, 1992; Wygnanski et al., 1995), lateral geniculate body (Yucel et al., 2000; Yucel et al., 2001, 2003) and visual cortex (Yucel et al., 2003), strong evidence suggests that damage to the retinal ganglion cell axons within the lamina cribrosa of the ONH (Bellezza et al., 2003b; Burgoyne et al., 2004; Gaasterland et al., 1978; Minckler et al., 1977; Quigley et al., 1981; Quigley and Green, 1979) is a central pathophysiology. Recent studies in the monkey (Bellezza et al., 2003b; Burgoyne et al., 2004; Downs et al., 2005; Downs et al., 2007; Yang et al., 2007a; Yang et al., 2007b), rat (Cepurna et al., 2005; Johnson et al., 2000; Johnson et al., 1996), and mouse (Danias et al., 2003; Filippopoulos et al., 2006; Howell et al., 2007; Jakobs et al., 2005; Schlamp et al., 2006) support the importance of the ONH, by describing profound alterations and axonal transport disruption within the prelaminar, laminar and retrolaminar tissues of the ONH at the earliest detectable stage of experimental glaucoma.
The ONH tissues make up a dynamic environment wherein 1.2 to 2.0 million RGC axons converge, turn, and exit the eye through the inner (Bruch’s Membrane opening) and outer (scleral) portions of the neural canal (Figure 1). Within the scleral portion of the canal, the bundled axons pass, through a 3-dimensional (3D) meshwork of astrocyte-covered, capillary containing, connective tissue beams known as the lamina cribrosa (Figure 1). Within the lamina, axonal nutrition is thought to depend upon the movement of oxygen and nutrients from the laminar capillaries, through the laminar beam extracellular matrix, across the astrocyte basement membrane into the astrocyte, finally reaching the peripheral and central axons of each bundle, via cell processes (Anderson, 1969).
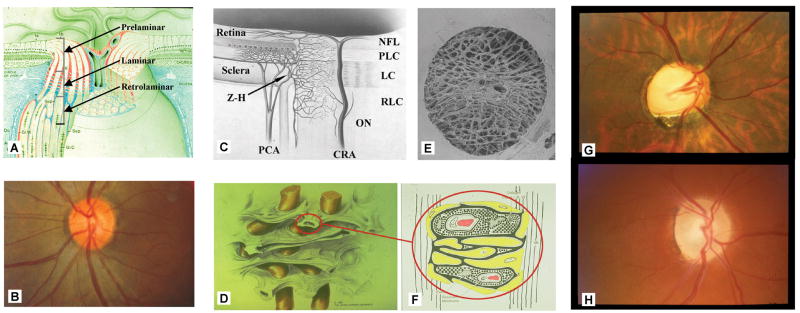
Figure 1. Glaucoma, cupping and axonal insult within the optic nerve head (ONH).
The ONH is made up of prelaminar, laminar and retrolaminar regions (A). Within the clinically visible surface of the Normal ONH (referred to as the optic disc) (B), central retinal vessels enter the eye and RGC axons appear pink due to their capillaries (which are principally supplied by branches from the posterior ciliary arteries (PCA) in (C). The primary site of RGC axon insult in glaucoma is within the lamina cribrosa (schematically depicted with axon bundles in (D), isolated by trypsin digest in a scanning electron micrograph in (E) and drawn with stippled extracellular matrix (ECM), central capillary (red) and surrounding astrocytes (yellow with basement membranes in black) (F). Blood flow within the ONH, while controlled by autoregulation, can be affected by non IOP-related effects such as systemic blood pressure fluctuation and vasospasm within the retrobulbar portion of the PCAs. Additional IOP-induced effects may include compression of PCA branches within the peripapillary sclera (due to scleral stress and strain) and compression of laminar beam capillaries reducing laminar capillary volume flow (C and F)(Langham, 1980). There is no direct blood supply to the axons within the laminar region. Axonal nutrition within the lamina (F) requires diffusion of nutrients from the laminar capillaries, across the endothelial and pericyte basement membranes, through the ECM of the laminar beam, across the basement membranes of the astrocytes, into the astrocytes, and across their processes to the adjacent axons (vertical lines). Chronic age-related changes in the endothelial cell and astrocyte basement membranes, as well as IOP-induced changes in the laminar ECM and astrocyte basement membranes may diminish nutrient diffusion to the axons in the presence of a stable level of laminar capillary volume flow. The clinical manifestation of IOP-induced ONH structural change is most commonly “deep cupping” (G) but in some eyes cupping can be shallower accompanied by pallor (H). Z-H = circle of Zinn-Haller; PCA= posterior ciliary arteries; NFL = nerve fiber layer; PLC = prelaminar region; LC = lamina cribrosa; RLC = retrolaminar region; ON = optic nerve; CRA = central retinal artery. (A) Reprinted with permission from Arch Ophthalmol (Anderson, 1969); (C) reprinted with permission from The Glaucomas. St. Louis: Mosby; 1996:177–97 (Cioffi and Van Buskirk, 1996); (D) reprinted with permission from Optic Nerve in Glaucoma. Amsterdam: Kugler Publications; 1995:15–36 (Quigley, 1995b); (E) reprinted with permission from Arch Ophthalmol (Quigley et al., 1990); (F) reprinted with permission from Arch Ophthalmol (Morrison et al., 1989b)
“Cupping” is a clinical term which is used to describe ONH structural change in all forms of optic neuropathy (Bianchi-Marzoli et al., 1995; Greenfield et al., 1998; Johns et al., 1989; Klein et al., 2006; Pederson and Anderson, 1980; Pederson and Gaasterland, 1984; Schwartz et al., 1975; Sponsel et al., 2001). However, “cupping” is also used as a synonym for the pathophysiology of glaucomatous damage to the ONH (Anderson and Cynader, 1997; Kalvin et al., 1966; Quigley and Green, 1979; Vrabec, 1976). Because the clinical and pathophysiologic contexts for “cupping” are seldom clarified there is a confusing literature regarding the presence, importance and meaning of “cupping” in a variety of disorders (Alward, 2004; Ambati and Rizzo, 2001; Bianchi-Marzoli et al., 1995; Danesh-Meyer et al., 2001; Greenfield, 1999; Greenfield et al., 1998; Hall et al., 2006; Hayreh and Jonas, 2001; Johns et al., 1989; Klein et al., 2006; Manor, 1995; Nicolela and Drance, 1996; Nicolela et al., 2003; Orgul et al., 1994; Pederson and Anderson, 1980; Pederson and Gaasterland, 1984; Piette and Sergott, 2006; Quigley and Anderson, 1977; Schwartz et al., 1975; Sharma et al., 1999; Sonty and Schwartz, 1983; Sponsel et al., 2001; Trobe et al., 1980; Votruba et al., 2003). Cupping in glaucoma is highly variable (Nicolela and Drance, 1996; Nicolela et al., 2003). We have previously proposed that only “laminar” or “deep” forms of “cupping” (those that include a connective tissue component) are pathognomonic for glaucoma (Burgoyne and Downs, 2008; Burgoyne et al., 2005; Yang et al., 2007a). We now further clarify that even a glaucomatous form of “cupping” is only one manifestation of the underlying pathophysiologic processes which drive the optic neuropathy of glaucoma. Cupping is therefore a manifestation of the neuropathy of glaucoma, not the optic neuropathy itself.
Damage to the retinal ganglion cell axon within the ONH is a second component of the optic neuropathy of glaucoma, but it also is not the optic neuropathy itself. While the pathophysiology of RGC axon damage is of fundamental importance in preserving vision, it may be only one component of, (or secondary to) the larger pathophysiologic events that drive the neuropathy. ONH biomechanics seeks to identify and explain the larger intraocular pressure (IOP)-related and non IOP-related determinants of glaucomatous damage so as to build a clinical science to predict eye-specific ONH behavior and susceptibility to a given level of IOP (Burgoyne and Downs, 2008; Burgoyne et al., 2005; Burgoyne and Morrison, 2001; Downs et al., 2009; Sigal and Ethier, 2009a; Sigal and Ethier, 2009b; Sigal et al., 2009a, b; Sigal et al., 2010b).
The Biomechanical Paradigm of Glaucomatous damage to the ONH
The biomechanical paradigm of glaucomatous ONH damage does not argue that the ONH is the earliest or only site of damage. ONH biomechanics provides a framework for explaining how IOP-related stress (force/cross-sectional area of the tissue experiencing that force) and strain (a measure of local deformation of a tissue induced by applied stress) within the load-bearing tissues of the ONH influence the physiology and pathophysiology of all three ONH tissue types. These include: 1) the connective tissues (load-bearing connective tissues of the peripapillary sclera, scleral canal wall, and lamina cribrosa), 2) the neural tissues (retinal ganglion cell axons), and 3) the cells which exist alone or in contact with both 1 and 2 (astrocytes, glial cells, endothelial cells, and pericytes and their basement membranes) (Burgoyne and Downs, 2008; Burgoyne et al., 2005; Burgoyne and Morrison, 2001; Downs et al., 2009; Sigal and Ethier, 2009a; Sigal and Ethier, 2009b; Sigal et al., 2009a, b; Sigal et al., 2010b).
ONH biomechanics provides a logic by which non-IOP-related risk factors such as ischemia, inflammation, auto-immunity, astrocyte and glial molecular biology are influenced by or interact with the effects of IOP. ONH biomechanics attempts to combine these ] “non IOP-related” factors with laminar and peripapillary scleral connective tissue geometry and material properties (strength, stiffness, structural rigidity, compliance and nutrient diffusion properties) to explain the physiology of normal ONH aging, ONH susceptibility to IOP, and the clinical manifestation of all forms of optic neuropathy. The engineering components of ONH biomechanics have been the focus of a series of recent reports (Burgoyne and Downs, 2008; Burgoyne et al., 2005; Downs et al., 2009; Sigal and Ethier, 2009a; Sigal and Ethier, 2009b; Sigal et al., 2009a, b; Sigal et al., 2010b). Figures 1 and 2 of this article explain a subset of these concepts in greater detail.
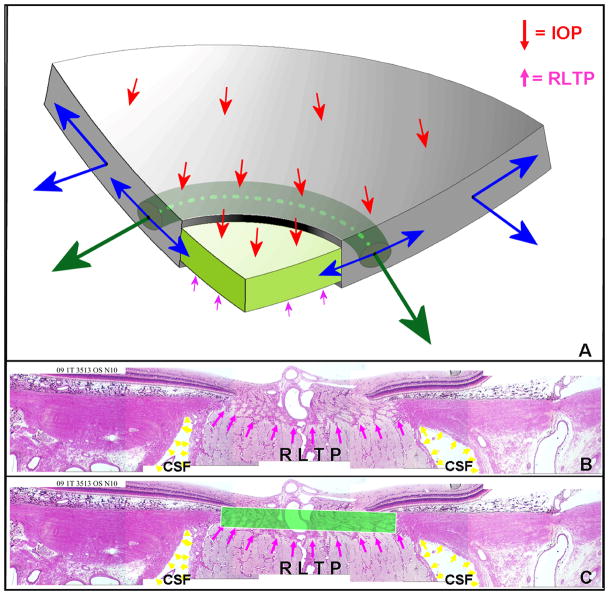
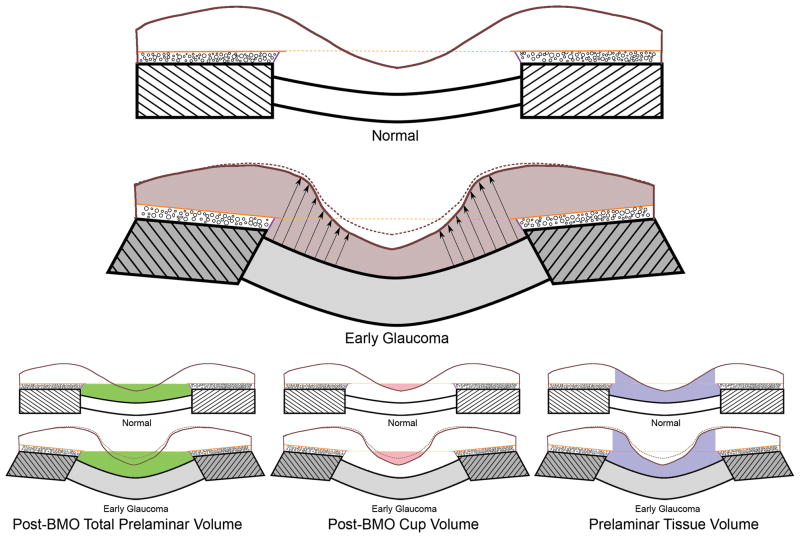
Figure 2. Principle distribution of forces, pressures and the translaminar pressure gradient within the optic nerve head (ONH).
A. Cut-away diagram of intraocular pressure (IOP)-induced mechanical stress in an idealized spherical scleral shell with a circular scleral canal spanned by a more compliant lamina cribrosa. In this case, the majority of the stress generated by IOP/orbital pressure difference (red arrows on the inner surface of the sclera) is transferred into a hoop stress borne within the thickness of the sclera and lamina (blue arrows) that is concentrated circumferentially around the scleral canal (green arrows). B. Note that the pressure behind the lamina is not simply CSF pressure but is retrolaminar tissue pressure (RLTP) which has been demonstrated to be approximately 0.82 × CSF + 2.9 mm Hg by Morgan, et al in dogs (Morgan et al., 1998) C. The difference between IOP and the retrolaminar tissue pressure is the translaminar pressure difference which generates both a net posterior (outward) force on the surface of the lamina (the red arrows over the lamina) and a hydrostatic pressure gradient (the translaminar pressure gradient - schematically shown in green) within the neural and connective tissues of the pre-laminar and laminar regions. Note that the in-plane hoop stress transferred to the lamina from the sclera is much larger than the stresses induced by the translaminar pressure difference. CSF directly influences laminar position through its effect on the translaminar pressure difference. CSF may also effect scleral flange position within the region it projects to the sclera (Figure 2), but in most eyes, because the projection of the CSF space is minimal this is not likely important (the CSF space within Panels B and C in this figure is greatly expanded due to perfusion fixation). IOP has a similar direct effect on laminar position, but has an additional (and potentially more important) effect on laminar position through the peripapillary sclera. However, while the magnitude of the translaminar pressure difference may be small relative to the stresses within the sclera and lamina, the axons experience it as the translaminar pressure gradient the steepness of which is influenced by the thickness of the tissues over which it is experienced. The translaminar pressure gradient, as such, may serve as a primary barrier to axon transport and flow within this region and an important physiologic determinant for the ONH axons and cells. (Panel A is modified from Downs et al, 2009).
ONH Connective Tissue Biomechanics and the Translaminar Pressure Gradient
The goal of this section is to elucidate the biomechanical determinants of the translaminar pressure gradient or the transition from intraocular pressure to retrolaminar tissue pressure experienced by the axons as they pass through the lamina cribrosa to exit the eye are illustrated and explained in Figure 2. The importance of this gradient to axonal physiology is separately discussed below. The key messages of this and the following sections are five-fold. First, energy is required for axon transport and the translaminar pressure gradient may increase the energy requirements of the RGC axons within the lamina cribrosa. Second, IOP-related stresses and strains within the ONH connective tissues are complicated and do not necessarily lead to deformation of the lamina out of the plane of the sclera. Third, scleral canal expansion that tightens the lamina within the canal and lessens posterior laminar deformation, still increases strain within the lamina. Fourth, posterior deformation of the lamina is likely not required for axon transport compromise. Fifth, IOP-related stress and strain within the ONH connective tissues may independently affect the delivery of nutrients to the RGC axons (and therefore affect axon transport) in the presence or absence of frank laminar deformation.
The difference between Intraocular and orbital pressure establishes a set of principal “engineering” or “mechanical” stresses (force/cross-sectional area of the tissue bearing the load) within the ONH neural and connective tissues, the magnitude of which are determined by the level of IOP and the 3D geometry or architecture of the tissues that carry them (Burgoyne et al., 2005; Downs et al., 2009; Sigal and Ethier, 2009b). These mechanical stresses are separate from physiologic stress which we define as physical and metabolic changes within a cell in response to alterations in its environment. The direct (outward) effect of intraocular pressure on the internal limiting membrane of the ONH prelaminar tissues is resisted by the pressure within the retrolaminar optic nerve tissues (retrolaminar tissue pressure) and the outward expansion of the scleral canal which pulls the lamina cribrosa “tight” within the canal, effectively increasing its resistance to outward deformation (Bellezza et al., 2003a; Downs et al., 2009).
The important concept for this discussion is that engineering models suggest that the stresses generated by the IOP/orbital pressure difference within the scleral connective tissues are far higher than the direct (outward) stresses on the neural and connective tissues of the lamina (figure 2) (Collins, 1967; Downs et al., 2009; Sigal et al., 2005; Sigal et al., 2009b). How the ONH connective tissues respond to a given distribution of mechanical stress is determined by their material properties. A growing body of experimental (Agoumi et al., 2010; Bellezza et al., 2003a) and theoretical work (Sigal, 2009; Sigal et al., 2005; Sigal et al., 2009b) supports the concept of a laminar-scleral dynamic in which the net compliance or rigidity of the sclera exerts a large influence over the magnitude of lamina cribrosa deformation at all levels of IOP. While a previous study of ONH surface deformation in dogs suggested substantial ONH surface movement with acute IOP elevation (Morgan et al., 2002), two recent studies in which laminar deformation was measured directly, suggest little posterior laminar deformation follows acute IOP elevation in normal monkey (Bellezza et al., 2003b) (Burgoyne et al., 2009. Deformation of the Normal Monkey Optic Nerve Head (ONH) Connective Tissues Following Acute IOP Elevation Within 3D Histomorphometric Reconstructions. Invest Ophthalmol Vis Sci. 50, ARVO Abstract# 4897) and human eyes (Agoumi et al., 2010).
How the axons respond to laminar deformation, (when present) can not be separated from other axonal effects of the constituent neural and connective tissue stresses and strains. Said in another way, the components of IOP-related stress and strain that drive ONH connective tissue deformation may not be the components that influence axon transport. Glaucomatous damage to the RGC axon within the ONH may not simply occur at locations with the highest levels of IOP-related connective tissue strain or stress. Rather as neural and connective tissue stress and strain increase, axon physiology may become compromised at those locations where the translaminar tissue pressure gradient is steepest (Figure 2) and/or where the axon’s energy supply is or otherwise becomes most vulnerable.
In these regards, it is important to clarify the separate concepts of the translaminar pressure difference (the difference between IOP and the retrolaminar tissue pressure) - which generates a net posterior (outward) force on the surface of the lamina and the translaminar pressure gradient (schematically shown in green in Figure 2) which is the hydrostatic pressure gradient within the neural and connective tissues of the pre-laminar and laminar regions created by the translaminar pressure difference. Note that the in-plane hoop stress transferred to the lamina from the sclera is much larger than the stresses induced by the translaminar pressure difference (Figure 2). CSF directly influences laminar position through its effect on the translaminar pressure difference. CSF may also effect scleral flange position within the region it projects to the sclera (Figure 2), but in most eyes, because the projection of the CSF space is minimal this is not likely important (Figure 2).
IOP has a similar direct effect on laminar position, but has an additional (and potentially more important) effect through the peripapillary sclera (Figure 2). However, while the magnitude of the translaminar pressure difference may be small relative to the stresses within the connective tissues of the sclera and lamina, the axons separately experience it as the translaminar pressure gradient, the steepness of which is influenced by the thickness of the tissues over which it is experienced. In eyes with thin laminas or in which the lamina becomes thin through the course of the neuropathy,(Jonas, 2007; Jonas et al., 2003, 2004; Morgan et al., 1998; Morgan et al., 2008) the translaminar pressure gradient may serve as a primary barrier to axon transport and flow within this region and an important physiologic determinant for the ONH axons and cells.
The fact that axon transport blockade within the “scleral” region of the mouse and rat ONH has been reported in multiples studies following acute and chronic IOP elevation (Danias et al., 2003; Filippopoulos et al., 2006; Howell et al., 2007; Jakobs et al., 2005; Johansson, 1983; Martin et al., 2006; Schlamp et al., 2006) is important to this discussion for the following reasons. First, it suggests that deformation of connective tissue lamina cribrosa beams is not necessary for axonal transport to be challenged in this location. Second, it suggests that the translaminar pressure gradient, alone, or in combination with the astrocyte and vascular response to it may provide an important challenge to axon transport when IOP is elevated.
Retinal Ganglion Cell Axon Transport Within the ONH
The purpose of this section is to identify the principal components of RGC axon transport within the ONH with emphasis on the delivery of nutrients required to meet its energy demands. Reviews of this subject (Anderson and Hendrickson, 1974; Barkus et al., 2008; Duncan and Goldstein, 2006; Gross et al., 2007; Minckler, 1994; Minckler et al., 1977; Morgan, 2000, 2004; Quigley and Anderson, 1976) should be consulted for greater detail.
Axon transport is energy dependant
In addition to the conduction of axon potentials, the retinal ganglion cell axons transport molecules, vesicles and organelles in both the anterograde (orthograde – away from the cell body, toward the brain) and retrograde (toward the cell body, away from the brain) directions. The axon cytoskeleton forms the molecular tracks for both anterograde and retrograde movement of motor proteins and their associated cargo in addition to maintaining RGC and axon shape. Neurofilaments, microtubules, microtubule-associated proteins (MAPs) and actin are the major constituents of the axon cytoskeleton. Microtubules are highly polarized structures made up of alpha and beta tubulin that possess a stable “minus” end close to the cell body and a “positive” end that is thought to be more unstable (Barkus et al., 2008; Duncan and Goldstein, 2006; Gross et al., 2007; Vale and Milligan, 2000). Physical disruption of the axon cytoskeleton has been reported to occur within 3 hours of acute IOP elevations and precede axon transport abnormalities in the porcine eye (Balaratnasingam et al., 2008).
All of the molecular motors for axon transport hydrolyze Adenosine-5′-triphosphate (ATP) to generate movement along the microtubules. Anterograde transport occurs in fast (50–400 mm/day) and slow (slow transport components A (SCa.3–3mm/day) and B (SCb 2–8 mm.day) forms. The molecular motor for anterograde transport is provided by a family of kinesin molecules. The kinesins are synthesized in the cell body and are activated on binding a cargo molecule whereupon they bind to the microtubules and commence movement. Retrograde transport is fast (200–400 mm/day) and uses dynein as the molecular motor. Dynein is synthesized in the cell body, transported to the axon terminal by both fast and slow mechanisms. It is activated upon binding to its target molecule whereupon it commences retrograde transport along the microtubules. Recently, a second family of vesicular molecular tracks (actin filaments and myosin motors) have been proposed for both anterograde and retrograde transport (Barkus et al., 2008; Duncan and Goldstein, 2006; Gross et al., 2007).
Mitochondria move within axons using both the actin and microtubule tracks (Brown, 2003; Hollenbeck and Saxton, 2005) to achieve positions in the cell where metabolic support is required such as myelin boundaries (Bristow et al., 2002) and regions of demylenation (Mutsaers and Carroll, 1998). Mitochondrial accumulation within, and on both sides of the lamina has been reported to be a feature of normal monkey (Minckler et al., 1976), cat (Bristow et al., 2002), and human (Barron et al., 2004; Hollander et al., 1995) eyes and ascribed to the extraordinary energy requirements of this region (Andrews et al., 1999; Howell, 1997; Morgan, 2004).
Age-related alterations in the retinal ganglion cell axon
Vrabec reported the presence of swollen axons within the nerve head at the level of the lamina in cadaver eye specimen from individuals ranging in age from 46 to 80 years, with the number of swollen axons increasing with age (Vrabec, 1977). Dolman, et al (Dolman et al., 1980) confirmed this finding in patients above the age of 65, in a separate study of 300 optic nerves from humans spanning the ages of birth to 96 years. In nerves from patients in their seventh decade, three fifteenths demonstrated this finding. In nerves from patients in their eighth decade it was five fifteenths, and from those in their ninth and tenth decades, eight fifteenths. Dolman ascribed the age-related swelling of axons in their study to the combination of age related alterations in astrocytes, blood vessels and axons they described (Dolman et al., 1980).Johnson found similar swellings in the optic nerves of aged mice (Johnson et al., 1978).
The nutrient supply of the RGC axons within the ONH has not been determined
Little is known regarding the actual delivery of nutrients to the retinal ganglion cell axon within the ONH. In the retina (Wang et al., 2002; Yu et al., 2010), prelaminar (Haefliger and Anderson, 1996), laminar and retrolaminar ONH (Haefliger and Anderson, 1996) astrocyte processes invest the retinal ganglion cell axons and are presumed to be responsible for maintaining the extracellular environment - possessing uptake mechanisms for the removal of potassium and glutamate from the extracellular space as well as providing neurotrophic support. How the retinal ganglion cell axons receive the nutrients necessary to power their mitochondria has not been rigorously studied. In the retina (Wang et al., 2002; Yu et al., 2010), prelaminar ONH (Haefliger and Anderson, 1996) and brain (Gordon et al., 2007; Mulligan and MacVicar, 2004) astrocytes have direct contact with the endothelial cells of capillaries and it is presumed there is direct transfer in addition to diffusion of nutrients (Haefliger and Anderson, 1996).
Within the lamina cribrosa of the monkey and human, where each capillary is embedded within a connective tissue sheath (Figure 1), the astrocyte that envelops the beam (and is also responsible for the maintenance of its extracellular matrix), is separated from the laminar beam capillary by its basement membrane, the laminar beam extracellular matrix, the pericytes, endothelial cells and the endothelial cell basement membranes. To date, there have been no definitive studies to determine if the laminar beam astrocyte sends a process to the laminar beam capillary. If, in fact, the laminar beam astrocyte does not have a direct connection to the laminar beam capillary, this is the only place in the central nervous system where that is the case. Several important implications follow. First IOP-related stress and/or strain may act as a hydrostatic and or compressive pressure and influence the volume flow of blood within the capillaries and/or the diffusion properties of these tissues. Second, age and/or disease related alterations in the beam and basement membrane connective tissues may enhance these effects.
IOP-related connective tissue stress and strain should affect laminar capillary blood flow
Retrobulbar blood flow within the posterior ciliary arteries is principally influenced by systemic blood pressure and vasospasm. However, IOP-related strain within the peripapillary sclera may significantly affect volume flow through the scleral branches of the short posterior ciliary arteries (Langham, 1980) (Figures 3 and 4). Thus, volume flow through the circle of Zinn Haller and the small penetrating vessels that pass anteriorly to the prelaminar nerve, transversely into the laminar insertion sites, and posteriorly to the pial branches supplying the retrolaminar optic nerve can be theoretically diminished by elevated levels of IOP-related strain (Langham, 1980). Separate from these retrolaminar effects, frank occlusion of the capillaries running within each laminar beam may occur due to tensile, compressive, or shear strains within the beam that are still within the beam’s elastic limits (Figures 4 and 5). This may be more likely to occur when capillary perfusion pressure has been adversely affected by retrolaminar effects of IOP-related strain or non-IOP related factors such as nocturnal hypotension, diabetes, and retrolaminar vasospasm (Hayreh et al., 1994; Langham, 1980; Spaeth, 1977).
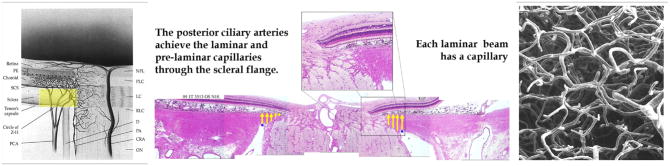
Figure 3. The volume flow of blood within the posterior ciliary arteries should be affected by IOP-related stress and strain within the peripapillary sclera and scleral flange.
The posterior ciliary arteries pass through the peripapillary sclera (yellow, left and center panel) immediately adjacent to the scleral portion of the neural canal. We refer to this portion of the sclera as the scleral flange (yellow, middle panel) (Yang et al., 2005. Neural Canal and Peripapillary Scleral Alterations Within Three-Dimensional Reconstructions of Early Glaucoma Monkey Optic Nerve Heads. Invest. Ophthalmol. Vis. Sci. 46, ARVO Abstract# 3511). The sclera thins here to accommodate an expansion of the neural tissues that occurs in a highly eye-specific fashion. While the large penetrating vessels to the choroid are outside of the flange, the circle of Zinn-Haller and the penetrating branches that pass to the pre-laminar, laminar and retrolaminar nerve pass directly through these tissues and are therefore subject to the compressive effects of their contained mechanical stress and strain. Within the lamina cribrosa, there is no direct blood supply to the axons or the axon bundles. Each laminar beam contains a capillary (Panel F, Figure 1) which are here shown in a vascular casting of a monkey eye (Cioffi and Van Buskirk, 1996). (Left and right panels reprinted with permission from The Glaucomas. St. Louis: Mosby; 1996:177–97.(Cioffi and Van Buskirk, 1996)
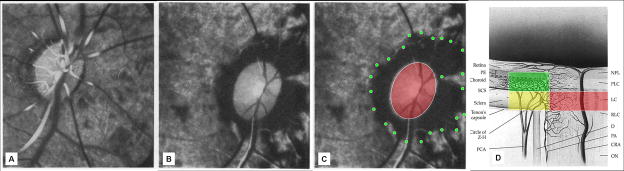
Figure 4. Hayreh (Hayreh et al., 1970) demonstrated sensitivity of the peripapillary choroidal circulation (green) to acute IOP elevation in the monkey ONH.
Fluorescence fundus angiogram of the right eye of a cynomologus monkey after experimental central retinal artery occlusion at normal (A) and 70 mm Hg IOP (B and C). The non perfused region of the peripapillary choroid is highlighted in green in (C). We and others have hypothesized that IOP-related stress and strain within the scleral flange (Figure 3) may contribute to this phenomenon and that similar effects may occur within the laminar capillary beds (red) (C and D). Panels A and B are reproduced with permission from the British Journal of Ophthalmology (Hayreh et al., 1970). (Panel D reprinted with permission from The Glaucomas. St. Louis: Mosby; 1996:177–97. (Cioffi and Van Buskirk, 1996)
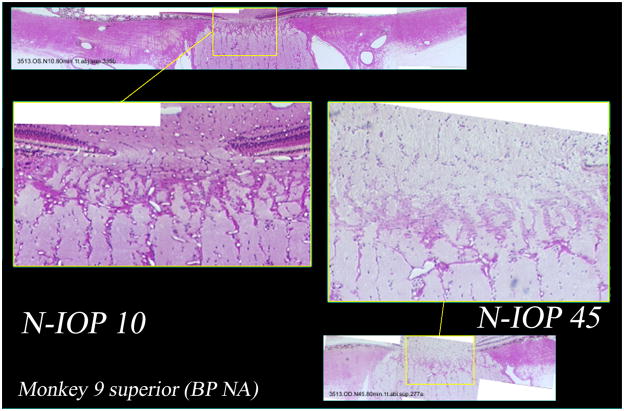
Figure 5. Histologic sections from the superior scleral canal of both eyes of a normal monkey perfusion fixed with one eye at IOP 10 mm Hg (middle left and above) and one eye at IOP 45 mm Hg (middle right and below).
Higher magnification demonstrates patent prelaminar, laminar, and retrolaminar capillaries in the IOP 10 eye (middle left). However in the IOP 45 eye (middle right), the prelaminar and anterior laminar capillaries do not appear patent, with only the posterior laminar and retrolaminar capillaries open.
The effects of IOP-related connective tissue stress and strain on nutrient transport from the laminar beam capillary to the astrocyte have not been determined
In the absence of a laminar beam astrocyte footplate on the laminar beam capillary, axonal nutrition within the lamina likely requires diffusion of nutrients from the laminar capillaries, across the endothelial and pericyte basement membranes, through the extracellular matrix of the laminar beam, across the basement membranes of the astrocytes, into the astrocytes, and across their processes to the adjacent axons (Panel F, Figure 1). Chronic age-related changes in the endothelial cell and astrocyte basement membranes, as well as IOP-induced changes in the laminar extracellular matrix and astrocyte basement membranes, may diminish nutrient diffusion to the axons even in the presence of a stable level of laminar capillary volume flow.
Age related alterations in the laminar astrocyte/laminar capillary dynamic may contribute to age-related and glaucomatous axon loss
The aged ONH is more likely to have stiff connective tissues (Albon et al., 2007; Albon et al., 1995; Albon et al., 2000a; Albon et al., 2000b; Bailey et al., 1998; Brown et al., 1994; Friedenwald, 1937; Hernandez et al., 1989; Jeffery et al., 1995; Kotecha et al., 2006; Morrison et al., 1989a; Pena et al., 1996; Quigley, 1982) and a compromised blood supply (Grunwald et al., 1993; Harris et al., 2000). However, age-related increases in laminar beam thickness(Albon et al., 2000a; Hernandez et al., 1989; Hernandez et al., 1991; Kotecha et al., 2006; Morrison et al., 1989a), laminar astrocyte basement membrane thickness(Hernandez et al., 1989; Hernandez et al., 1991) and laminar extracellular matrix (ECM) stiffening(Albon et al., 2000a; Hernandez et al., 1989; Hernandez et al., 1991; Morrison et al., 1989a) should not only increase laminar beam stiffness, but also diminish nutrient diffusion from the laminar capillaries into the adjacent axons. While the concepts of age-related optic nerve axon loss(Balazsi et al., 1984; Cepurna et al., 2005; Cull et al., 2003; Morrison et al., 1990; Repka and Quigley, 1989; Sandell and Peters, 2001, 2002) and an optic neuropathy of aging (Frisen, 1991; Jonas and Grundler, 1996; Klein et al., 2006; McKendrick et al., 2007; Sandell and Peters, 2002; See et al., 2009) remain controversial, we believe that the range of physiologic stress and strain within the ONH connective tissues experienced over a life-time are likely to be of central importance to both. Interestingly, Hogan and Zimmerman (Hogan and Zimmerman, 1969) proposed that thickening of the retrolaminar septa caused a reduction in the diffusion of nutrients from the contained capillaries to the axons, contributing to age-related loss.
Both normal and elevated levels of IOP-related strain can be expected to induce synthesis of extracellular matrix by the laminar astrocytes (Agapova et al., 2003; Agapova et al., 2001; Pena et al., 2001), which alters the material properties (and likely the diffusion properties) of the adjacent laminar trabeculae. Additionally, the laminar astrocytes and laminar capillary endothelial cells may thicken their basement membranes as a secondary response to the physical distortion induced by laminar deformation. Thickening of the basement membranes of the laminar capillary endothelial cells in response to elevated levels of IOP-related stress was reported in a poster by Hernandez’s group at the 1997 ARVO meeting (Pena et al., 1997). Basement membranes of the laminar trabeculae have also been noted to thicken with age (Hernandez et al., 1989).
Disease-related alterations in the laminar astrocyte/laminar capillary/retinal ganglion cell axon dynamic may increase susceptibility to axon loss
A review of the literature supports the notion that the relationships between the laminar astrocytes, beams, beam capillaries and retinal ganglion cell axons are profoundly altered throughout the neuropathy (Downs et al., 2009; Morgan, 2000, 2004; Quigley, 1999). Until 10 years ago, descriptions of very early changes in the neuropathy of glaucoma were only sporadically reported in the monkey and human literature (Agapova et al., 2003; Harwerth et al., 1999; Pena et al., 2001; Quigley et al., 1983; Tezel et al., 1997). In recent years, however, studies of early experimental glaucoma in the mouse, rat and monkey ONH have confirmed that the 3D histomorphometric (Burgoyne et al., 2004; Downs et al., 2007; Yang et al., 2007a; Yang et al., 2009; Yang et al., 2007b) and molecular changes (Du et al., 2007; Fu and Sretavan, 2010; Fu et al., 2010; Howell et al., 2007; Mabuchi et al., 2004) within weeks of the onset of modest IOP elevations are already profound.
Figures 6, 7 and 8 summarize the ONH alterations we have histologically (Bellezza et al., 2003b; Downs et al., 2001) and histomorphometrically (Burgoyne et al., 2004; Downs et al., 2007; Yang et al., 2007a; Yang et al., 2009; Yang et al., 2007b) described in an initial group of 3 monkeys with early unilateral experimental glaucoma and recently extended to an additional group of 6 animals (Yang et al., 2010). The onset of “cupping” in monkey experimental glaucoma in the setting of modest to moderate chronic IOP elevations is defined by co-localized posterior laminar deformation and neural canal expansion that is maximum within the superior temporal and/or inferior-nasal quadrants. These phenomena are accompanied by laminar thickening, axial elongation, and outward peripapillary scleral bowing in the majority of eyes.
Figure 6. ONH neural and connective tissue changes at the onset of CSLT-detected ONH surface change.
These findings were initially reported in n=3 monkeys (Yang et al., 2007a) and have recently been expanded to n=9 animals (Yang et al., 2010). Normal lamina cribrosa (unhatched), scleral flange (hatched), prelaminar tissue (beneath the internal limiting membrane - brown line), Bruch’s membrane (solid orange line), BMO zero reference plane (dotted orange line), Border tissue of Elschnig (purple line), choroid (black circles) are schematically represented in the upper illustration. Changes in EG are depicted below. We have previously reported the bowing of the lamina and peripapillary scleral flange and thickening of the lamina in these same EG eyes (grey shading) (Yang et al., 2007b). These connective tissue changes underlie posterior deformation of the ONH and peripapillary retinal surface (dotted brown to solid brown ILM) that occurs in the setting of thickening (arrows) not thinning of the prelaminar neural tissues (brown shading) in EG. EG eye expansion of all three volumetric parameters is depicted below. The interactions between these parameters are important. While expansion of the cup and deformation of the surface are clinically detectable at this early stage of the neuropathy, because they occur in the setting of prelaminar tissue thickening, (not thinning), we believe that expansion of Post-BMO Total Prelaminar Volume (due to ONH connective tissue deformation) drives these findings. Thus, cupping in these nine EG eyes is “laminar” in origin, without a significant “prelaminar” component (Yang et al., 2007a).
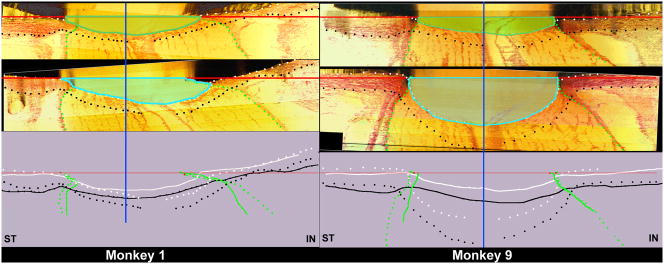
Figure 7. The range of ONH connective tissue deformation (cupping) at the onset of CSLT-detected ONH surface change in nine monkeys with unilateral experimental glaucoma (Yang et al., 2010).
Minimal (Monkey 1, left) to maximum deformation (Monkey 9, right) within the 9 animals is depicted. The following ONH landmarks are delineated within representative superior temporal (ST) to inferior nasal (IN) digital sections from the normal (upper panel) and early experimental glaucoma (EEG) (middle panel) eye of both animals: anterior scleral/laminar surface (white dots), posterior scleral/laminar surface (black dots), neural boundary (green dots), NCO reference plane (red line) and NCO centroid (vertical blue line). Delineated points for the normal (solid lines) and EEG eye of each eye are overlaid relative to the NCO centroid in the lower panel. For each animal, the area under the NCO reference plane and above the anterior laminar surface Post-NCO Total Prelaminar Volume is outlined in both the normal (darker green) and EEG (light blue) eye for qualitative comparison. The overlaid delineations for both eyes of each animal (lower panels) suggest that expansion of this area is due to posterior laminar deformation and neural canal expansion from its onset (Monkey 1) and that these two phenomena continue through more advanced stages of connective tissue deformation and remodeling (Monkey 9). As such,“glaucomatous” deformation of the ONH connective tissues is present early and progresses early in the neuropathy (Burgoyne and Downs, 2008; Yang et al., 2007a). These phenomena are accompanied by regional laminar thickening and outward bowing of the peripapillary sclera in both of these animals and most of the 9 EEG eyes. In addition, in both monkeys laminar migration to the point of partial pialization has occurred on the inferior side (right side of each schematic) of the canal (see Figure 8). (Yang et al., 2010. Optic Nerve Head Lamina Cribrosa Insertion Migration and Pialization in Early Non-Human Primate Experimental Glaucoma. Invest. Ophthalmol. Vis. Sci. 51, ARVO Abstract# 1631)
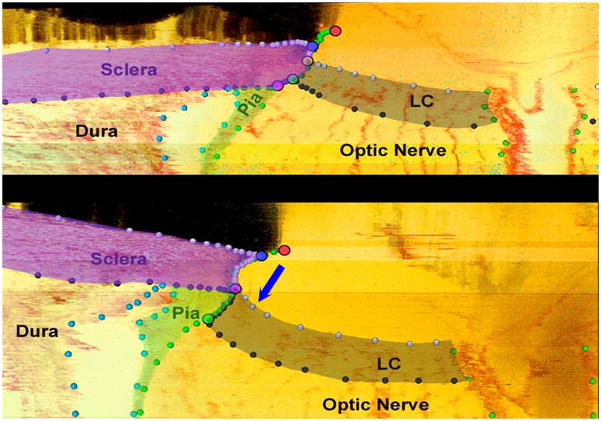
Figure 8. The pathophysiology of early experimental glaucomatous damage to the monkey ONH includes not only “thickening” but regional “migration” of the laminar insertion away from the sclera to the point that “complete pialization” of the laminar insertion is achieved in a subset of eyes.
Neural canal landmarks (Red – Neural Canal Opening (end of Bruch’s Membrane); Blue – Anterior Scleral Canal Opening; Yellow – Anterior Laminar Insertion; Green – Posterior Laminar Insertion; Purple – Posterior Scleral Canal Opening) and segmented connective tissue (dark grey - lamina cribrosa; purple - peripapillary sclera; light green - pial sheath) within digital section images from the inferior region of the normal (top) and the contralateral early experimental glaucoma (bottom) ONH of a representative monkey. Note that in most normal monkey eyes, the lamina inserts into the sclera as is demonstrated in this monkey’s normal eye (top). However at an identical location in the early experimental glaucoma eye of this animal (bottom) in addition to the lamina being thickened and posteriorly deformed, the laminar insertion has migrated outward such that both the anterior and posterior lamina effectively insert into the pial sheath. While regions of laminar insertion into the pia have been reported in normal human eyes (Sigal et al., 2010a), these findings are the first to suggest that active remodeling of the laminar insertion from the sclera into the pia is part of the pathophysiology of “glaucomatous” ONH damage. This phenomenon when present has important implications for the mechanism of axonal insult within these regions. (These data were reported by Hongli Yang, PhD at the 2010 ARVO meeting and are currently in preparation for publication, Yang et al., 2010. Optic Nerve Head Lamina Cribrosa Insertion Migration and Pialization in Early Non-Human Primate Experimental Glaucoma. Invest. Ophthalmol. Vis. Sci. 51, ARVO Abstract# 1631).
We have also reported preliminary evidence of laminar beam thickening (Grimm et al., 2007. 3D Quantification of Lamina Cribrosa Microarchitecture in Normal and Early Glaucomatous Monkey Eyes. Invest. Ophthalmol. Vis. Sci. 48, ARVO Abstract# 3295) at this early stage of damage. In addition, Roberts et al have reported preliminary evidence of retrolaminar septal recruitment (Roberts et al., 2009). However, separate from these phenomena, the lamina appears to actively migrate out of the sclera and into the pia within regions of the most affected eyes (Figure 8). In some instances complete “pialization” of the laminar insertion is achieved (Figure 8). Whether the driving event of this phenomenon is mechanical failure of the anterior laminar beams (pushing the lamina to seek additional connective tissue support by recruiting the retrolaminar septal tissues), loss of the anterior (scleral) blood supply or active recruitment of the retrolaminar tissues as part of “remodeling”, the implications of these alterations for the blood supply of the laminar beams, the steepness of the translaminar pressure gradient and astrocyte and axonal physiology are profound.
Laminar insertion into the pia has been observed in normal human cadaver eyes (Sigal et al., 2010a) (Girkin et al., 2010. Three-Dimensional Reconstruction of the Human Optic Nerve Head. Invest. Ophthalmol. Vis. Sci. 51, ARVO Abstract# 3854). However, laminar migration and pialization as an active component of the optic neuropathy of glaucoma (i.e. as an alteration from a previously normal state) has not been previously recognized. Our description of laminar migration and pialization within this group of 9 early experimental glaucoma monkeys as well as its contribution to the evolution of “glaucomatous cupping” in this species (Burgoyne et al., 2005; Downs et al., 2009) is in preparation and will be the subject of a future report.
Chauhan et al (Chauhan et al., 2002) described profound alterations of the ONH and scleral canal tissues as well as ERG changes in rats with 1 to 3 months exposure to chronic IOP elevation that were different from those following chronic exposure to endothelin (Chauhan et al., 2004) and appeared to relate to the magnitude of peak IOP elevation and cumulative IOP exposure of the treated eye. Johnson et al (Johnson et al., 2007) reported genomic alterations compatible with cell proliferation, immune response, lysozome, cytoskeleton, extracellular matrix and ribosome in a genomic analysis of the ONH tissues of rats after 5 weeks of chronic unilateral IOP elevation. A 2.7 fold increase in the number of cells in the ONHs of glaucoma eyes was separately confirmed by cell counts.
Howell, et al (Howell et al., 2007) reported axonal insults within the laminar regions of the DBA/2J mouse at the earliest detectable stages of optic neuropathy. This study confirmed previous work that suggested the “glial” lamina of the mouse eye was sufficient to be the site of axonal disruption (Danias et al., 2003; Filippopoulos et al., 2006; Howell et al., 2007; Jakobs et al., 2005; Schlamp et al., 2006) but did not elucidate the mechanism. Cytoskeletal, size and Glial Fibrillary Acidic Protein (GFAP) expression changes in astrocytes within regions of the porcine ONH that demonstrated axon transport abnormalities have been reported as early as 3 hours after acute IOP elevation (Balaratnasingam et al., 2008).
Taken together these data from monkey, rat and mouse eyes are compatible with the notion of a direct axonal insult by a variety of mechanisms coupled with astrocyte withdrawal of trophic support due to “preoccupation” with or “distraction” by a profoundly altered biomechanical environment (Hernandez, 2000; Hernandez and Pena, 1997; Jamara et al., 2000).
The mechanisms of retinal ganglion cell insult in glaucoma are likely multifactorial and different by region of the ONH and stage of disease
As of this writing, none of the classically proposed mechanisms of RGC axonal insult within the ONH can be discounted. These include: 1) ischemia through a variety of mechanisms (the traditional vascular hypothesis (Fechtner and Weinreb, 1994)), 2) physical compression of the axons secopndary to the deformation of intact laminar trabeculae (the traditional mechanical hypothesis (Fechtner and Weinreb, 1994)), and 3) spontaneous axonal compression secondary to tissue pressure differences across the intact lamina cribrosa (Yablonski and Asamoto, 1993). All remain plausible either acting individually or in combination within different regions of the nerve head at a given time and/or at different stages of the disease.
Recent work by a series of authors (Band et al., 2009; Berdahl et al., 2008a; Berdahl et al., 2008b; Morgan et al., 2008; Morgan et al., 1995; Ren et al., 2009a; Yablonski and Asamoto, 1993) strongly suggest that acquiring the energy necessary to maintain the physical integrity of the axon and its active transport mechanisms through the translaminar pressure gradient may represent the “final common pathway” of damage to the axon in the face of a variety of insults. Steepening this gradient by increasing its magnitude or “thinning” the region through which the axons experience it, may increase axonal susceptibility (Jonas, 2007; Jonas et al., 2004; Ren et al., 2009b).
However, there is also a growing realization that the optic neuropathy of glaucoma is an active process in which the scleral fibroblasts, laminar astrocytes and glia remodel their environment to better suit the altered biomechanical milieu in which they find themselves (Agapova et al., 2003; Hernandez, 2000; Hernandez et al., 2008; Hernandez and Pena, 1997; Johnson et al., 2007; Johnson and Morrison, 2009; Kirwan et al., 2004; Roberts et al., 2009; Varela and Hernandez, 1997; Vernon et al., 2008). In some ONHs, this may represent a small shift out of the biological continuum of aging (Burgoyne and Downs, 2008) that leads to an increase in the rate and magnitude of age-related axon loss such that they become clinically recognizable as glaucomatous (See et al., 2009). In others, this is a profound shift that may leave the astrocytes in a “better” biomechanical environment but distracted from their trophic task of retinal ganglion cell axon maintenance.
Future Directions
The concepts of the retinal ganglion cell axon being abandoned due to ONH astrocyte “distraction in addition to its being damaged by astrocyte “activation” should continue to be explored (Hernandez and Pena, 1997). Within this context, as well as within the larger discussion of retinal ganglion cell injury mechanisms - above, the following research questions are important.
Why do primates have a connective tissue lamina and does this influence axonal susceptibility? Is a connective tissue lamina “protective” of the axons; is it protective in bigger eyes or just longer living eyes, or both? If it is protective, what are the mechanisms and why are they important? Are rates of age-related axon loss in mice and rats higher than in tree shrew, pig and monkey? Are there detectable differences in the susceptibility of young and old mice and rats to acute and chronic IOP elevations and is this susceptibility more than in young and old tree shrews, pigs and monkeys?
Do astrocytes have processes on the laminar capillaries in species with connective tissue laminar beams? Hernandez suggested that there were laminar processes and that they were withdrawn in human glaucoma in a single article that did not employ electron microscopic characterization (Hama et al., 2004; Varela and Hernandez, 1997; Wilhelmsson et al., 2006). What is the relationship of the laminar astrocytes to the laminar capillaries in mice and rats compared to tree shrews pigs and monkeys? Does this change with age and early disease? Do astrocytes immediately withdraw their processes from the laminar beam capillaries and axon bundles as part of their initial response to insult? Can we intervene to stabilize these processes in a beneficial way?
What are the important components of axonal nutrition within the ONH? How are these altered by age and disease? Do the laminar beam connective tissues (including the beam and basement membrane ECM) influence nutrient diffusion and if so how? Can IOP-related stress and strain within the beams, (separate from their ECM) influence nutrient diffusion? If you make the laminar beam stiffer, is there a clinically important effect on diffusion? Are these relationships changed in age and disease?
Can IOP-related Stress and Strain within the ONH connective tissues directly diminish the volume flow of blood within the contained scleral branches of the posterior ciliary arteries and the laminar beam capillaries? What are the mechanisms of laminar beam capillary autoregulation? (Liang et al., 2010) Are they altered in aging and do they falter early in the optic neuropathy of glaucoma?
Do astrocytes migrate away from the beams early in the optic neuropathy of glaucoma? While Hernandez is often cited on this point, in fact the evidence for this is based on a single figure within a single article (Varela and Hernandez, 1997), is not quantitative, nor was electron microscopy employed (Hama et al., 2004; Wilhelmsson et al., 2006). A second assessment of astrocyte migration in cell culture was reported by Tezel (Tezel et al., 2001). This issue is important and needs to be rigorously revisited. If this is true, are the cells that migrate new cells (the result of astrocyte proliferation (see below) - leaving laminar beam astrocytes and their processes in place) or are these the cells that were previously anchored to the beam and have truly migrated away from it? If so what happens to their beam and axon processes? (Hernandez suggested they were withdrawn but the figure that supports this finding is based on immunohistochemistry and can not be interpreted (Varela and Hernandez, 1997))
Do astrocytes proliferate early in the neuropathy of glaucoma? A growing body of evidence supports the notion that astrocytes within the laminar region of mice, rats and monkeys with experimental glaucoma as well as human glaucoma eyes proliferate (Johnson et al., 2007; Prasanna et al., 2002). If this is true, is withdrawal of laminar beam and axon bundle processes part of this process and is axonal nutrition compromised as a result.
How many different cell types are there in the ONH and do they have functional roles that separately contribute to the optic neuropathy of glaucoma? In addition to oligodendrycytes and microglia, two types of ONH astrocytes have been identified (Ye and Hernandez, 1995) as well as a separate cell type known as the lamina cribrosacyte (Hernandez et al., 1988). Are there species differences in these subtypes? Are there regional distribution differences? Do these cells consistently demonstrate different patterns of cellular change early in the optic neuropathy of glaucoma?
Can we develop in vivo and/or clinical measures of the axon cytoskeleton and transport to assess acute and chronic perturbations of the RGC axon prior to frank structural loss? A series of investigators are working on in vivo assessments of the axon cytoskeleton (Fortune et al., 2008a; Fortune et al., 2008b; Huang and Knighton, 2005; Huang et al., 2006)(Fortune et al., 2009. The Effect of Acute Intraocular Pressure Elevation on Peripapillary Retinal Thickness, Retinal Nerve Fiber Layer Thickness and Retardance. Invest Ophthalmol Vis Sci. 50, ARVO Abstract# 5824) and transport (Kanamori et al., 2009) (Wang et al., 2010. Fluorescent Markers of Retinal Astrocytes, Ganglion Cells, Axons and Axonal Transport Visualized in vivo by Confocal Scanning Laser Ophthalmoscopy. Invest. Ophthalmol. Vis. Sci. 51, ARVO Abstract# 2144) that will provide important experimental and clinical tools to address the above questions.
Can a “glaucomatous” optic neuropathy be induced by a primary non-IOP-related insult such as ischemia, immune-mediation or experimental CSF-lowering alone? While endothelin has been proposed to be a model of ischemic optic neuropathy in a variety of species (Chauhan et al., 2004; Cioffi et al., 1995; Orgul et al., 1996a; Orgul et al., 1996b), a robust literature has called into question its actual mechanisms (He et al., 2007; Murphy et al., 2010; Rao et al., 2008; Stokely et al., 2002; Wang et al., 2008; Yorio et al., 2002)and there are no definitive studies that establish the “glaucomatous” nature of the neuropathy (Chauhan et al., 2004; Oku et al., 1999). One paper has reported the “glaucomatous” loss of retinal ganglion cells in rats following systemic exposure to retinal heat shock proteins, but documentation of the “glaucomatous” nature of the neuropathy has not been provided (Wax et al., 2008). Because axon loss alone and pre-laminar forms of cupping (Burgoyne and Downs, 2008; Burgoyne et al., 2005; Yang et al., 2007a), alone, are not pathognomonic for a “glaucomatous” optic neuropathy (see above), the following questions should apply to each of these models. What constitutes a “glaucomatous” optic neuropathy? To what experimental paradigm should these models be compared? What elements of the neuropathy need to be present to call it “glaucomatous”?
Acknowledgments
Portions of this article have appeared previously in three publications (Burgoyne, C.F., Downs, J.C., 2008. Premise and Prediction – How Optic Nerve Head Biomechanics Underlies the Susceptibility and Clinical Behavior of the Aged Optic Nerve Head. J Glaucoma. 17, 318–328.; Burgoyne, C.F., et al., 2005. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 24, 39–73.; Yang, H., et al., 2007. 3-D Histomorphometry of the Normal and Early Glaucomatous Monkey Optic Nerve Head: Prelaminar Neural Tissues and Cupping. Invest. Ophthalmol. Vis. Sci. 48, 5068–5084.).
Conversations with Lin Wang and Brad Fortune have greatly influenced my thinking on the astrocyte, blood vessel relationships in aging and glaucoma. Lin Wang is now NIH funded to explore the mechanisms of laminar capillary auto-regulation and their alterations in the monkey experimental glaucoma model. Email interactions with Doug Anderson and Harry Quigley regarding their interpretation of the literature on astrocyte processes to the beam capillary were very helpful.
Supported in part by USPHS grants R01EY011610 from the National Eye Institute, National Institutes of Health, Bethesda, Maryland; a grant from the American Health Assistance Foundation, Rockville, Maryland; a grant from The Whitaker Foundation, Arlington, Virginia; a Career Development Award; The Legacy Good Samaritan Foundation, Portland, Oregon; the Sears Trust for Biomedical Research, Mexico, Missouri; and the Alcon Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agapova OA, et al. Differential expression of matrix metalloproteinases in monkey eyes with experimental glaucoma or optic nerve transection. Brain Res. 2003;967:132–143. doi: 10.1016/s0006-8993(02)04234-8. [DOI] [PubMed] [Google Scholar]
- Agapova OA, et al. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human optic nerve head astrocytes. Glia. 2001;33:205–216. doi: 10.1002/1098-1136(200103)33:3<205::aid-glia1019>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Agoumi YS, et al. Laminar and Prelaminar Tissue Displacement During Intraocular Pressure Elevation in Glaucoma Patients and Healthy Controls. Ophthalmology. 2010 doi: 10.1016/j.ophtha.2010.05.016. In press. [DOI] [PubMed] [Google Scholar]
- Albon J, et al. Connective Tissue Structure of the Tree Shrew Optic Nerve and Associated Ageing Changes. Invest Ophthalmol Vis Sci. 2007;48:2134–2144. doi: 10.1167/iovs.06-0084. [DOI] [PubMed] [Google Scholar]
- Albon J, et al. Changes in the collagenous matrix of the aging human lamina cribrosa. Br J Ophthalmol. 1995;79:368–375. doi: 10.1136/bjo.79.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albon J, et al. Age related changes in the non-collagenous components of the extracellular matrix of the human lamina cribrosa. Br J Ophthalmol. 2000a;84:311–317. doi: 10.1136/bjo.84.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albon J, et al. Age related compliance of the lamina cribrosa in human eyes. Br J Ophthalmol. 2000b;84:318–323. doi: 10.1136/bjo.84.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alward WL. Macular degeneration and glaucoma-like optic nerve head cupping. Am J Ophthalmol. 2004;138:135–136. doi: 10.1016/j.ajo.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Ambati BK, Rizzo JF., 3rd Nonglaucomatous cupping of the optic disc. Int Ophthalmol Clin. 2001;41:139–149. doi: 10.1097/00004397-200101000-00013. [DOI] [PubMed] [Google Scholar]
- Anderson DR. Ultrastructure of human and monkey lamina cribrosa and optic nerve head. Arch Ophthalmol. 1969;82:800–814. doi: 10.1001/archopht.1969.00990020792015. [DOI] [PubMed] [Google Scholar]
- Anderson DR, Cynader MS. Glaucomatous optic nerve cupping as an optic neuropathy. Clin Neurosci. 1997;4:274–278. [PubMed] [Google Scholar]
- Anderson DR, Hendrickson A. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest Ophthalmol Vis Sci. 1974;13:771–783. [PubMed] [Google Scholar]
- Andrews RM, et al. Histochemical localisation of mitochondrial enzyme activity in human optic nerve and retina. Br J Ophthalmol. 1999;83:231–235. doi: 10.1136/bjo.83.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T, et al. [Retinal ganglion cell damage in human glaucoma. 2. Studies on damage pattern] Nippon Ganka Gakkai Zasshi. 1987;91:1204–1213. [PubMed] [Google Scholar]
- Bailey AJ, et al. Mechanisms of maturation and ageing of collagen. Mech Ageing Dev. 1998;106:1–56. doi: 10.1016/s0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- Balaratnasingam C, et al. Time-dependent effects of elevated intraocular pressure on optic nerve head axonal transport and cytoskeleton proteins. Invest Ophthalmol Vis Sci. 2008;49:986–999. doi: 10.1167/iovs.07-1090. [DOI] [PubMed] [Google Scholar]
- Balazsi AG, et al. The effect of age on the nerve fiber population of the human optic nerve. Am J Ophthalmol. 1984;97:760–766. doi: 10.1016/0002-9394(84)90509-9. [DOI] [PubMed] [Google Scholar]
- Band LR, et al. Intracellular Flow in Optic Nerve Axons: A Mechanism for Cell Death in Glaucoma. Invest Ophthalmol Vis Sci. 2009;50:3750–3758. doi: 10.1167/iovs.08-2396. [DOI] [PubMed] [Google Scholar]
- Barkus RV, et al. Identification of an axonal kinesin-3 motor for fast anterograde vesicle transport that facilitates retrograde transport of neuropeptides. Mol Biol Cell. 2008;19:274–283. doi: 10.1091/mbc.E07-03-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron MJ, et al. The distributions of mitochondria and sodium channels reflect the specific energy requirements and conduction properties of the human optic nerve head. Br J Ophthalmol. 2004;88:286–290. doi: 10.1136/bjo.2003.027664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellezza AJ, et al. Anterior scleral canal geometry in pressurised (IOP 10) and non-pressurised (IOP 0) normal monkey eyes. Br J Ophthalmol. 2003a;87:1284–1290. doi: 10.1136/bjo.87.10.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellezza AJ, et al. Deformation of the lamina cribrosa and anterior scleral canal wall in early experimental glaucoma. Invest Ophthalmol Vis Sci. 2003b;44:623–637. doi: 10.1167/iovs.01-1282. [DOI] [PubMed] [Google Scholar]
- Berdahl JP, et al. Cerebrospinal fluid pressure is decreased in primary open-angle glaucoma. Ophthalmology. 2008a;115:763–768. doi: 10.1016/j.ophtha.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Berdahl JP, et al. Intracranial pressure in primary open angle glaucoma, normal tension glaucoma, and ocular hypertension: a case-control study. Invest Ophthalmol Vis Sci. 2008b;49:5412–5418. doi: 10.1167/iovs.08-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi-Marzoli S, et al. Quantitative analysis of optic disc cupping in compressive optic neuropathy. Ophthalmology. 1995;102:436–440. doi: 10.1016/s0161-6420(95)31003-2. [DOI] [PubMed] [Google Scholar]
- Bristow EA, et al. The distribution of mitochondrial activity in relation to optic nerve structure. Arch Ophthalmol. 2002;120:791–796. doi: 10.1001/archopht.120.6.791. [DOI] [PubMed] [Google Scholar]
- Brown A. Axonal transport of membranous and nonmembranous cargoes: a unified perspective. J Cell Biol. 2003;160:817–821. doi: 10.1083/jcb.200212017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CT, et al. Age-related changes of scleral hydration and sulfated glycosaminoglycans. Mech Ageing Dev. 1994;77:97–107. doi: 10.1016/0047-6374(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Burgoyne CF, Downs JC. Premise and Prediction – How Optic Nerve Head Biomechanics Underlies the Susceptibility and Clinical Behavior of the Aged Optic Nerve Head. J Glaucoma. 2008;17:318–328. doi: 10.1097/IJG.0b013e31815a343b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne CF, et al. Three-dimensional reconstruction of normal and early glaucoma monkey optic nerve head connective tissues. Invest Ophthalmol Vis Sci. 2004;45:4388–4399. doi: 10.1167/iovs.04-0022. [DOI] [PubMed] [Google Scholar]
- Burgoyne CF, et al. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24:39–73. doi: 10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Burgoyne CF, Morrison JC. The Anatomy and Pathophysiology of the Optic Nerve Head in Glaucoma. J Glaucoma. 2001;10:S16–S18. doi: 10.1097/00061198-200110001-00007. [DOI] [PubMed] [Google Scholar]
- Cepurna WO, et al. Age related optic nerve axonal loss in adult Brown Norway rats. Exp Eye Res. 2005;80:877–884. doi: 10.1016/j.exer.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Chauhan BC, et al. Model of endothelin-1-induced chronic optic neuropathy in rat. Invest Ophthalmol Vis Sci. 2004;45:144–152. doi: 10.1167/iovs.03-0687. [DOI] [PubMed] [Google Scholar]
- Chauhan BC, et al. Effect of intraocular pressure on optic disc topography, electroretinography, and axonal loss in a chronic pressure-induced rat model of optic nerve damage. Invest Ophthalmol Vis Sci. 2002;43:2969–2976. [PubMed] [Google Scholar]
- Cioffi GA, et al. An in vivo model of chronic optic nerve ischemia: the dose-dependent effects of endothelin-1 on the optic nerve microvasculature. Curr Eye Res. 1995;14:1147–1153. doi: 10.3109/02713689508995821. [DOI] [PubMed] [Google Scholar]
- Cioffi GA, Van Buskirk EM. Vasculature of the anterior optic nerve and peripapillary choroid. 2. Mosby; St. Louis: 1996. [Google Scholar]
- Collins CC. Miniature passive pressure transensor for implanting in the eye. IEEE Trans Biomed Eng. 1967;14:74–83. doi: 10.1109/tbme.1967.4502474. [DOI] [PubMed] [Google Scholar]
- Cull G, et al. Estimating normal optic nerve axon numbers in non-human primate eyes. J Glaucoma. 2003;12:301–306. doi: 10.1097/00061198-200308000-00003. [DOI] [PubMed] [Google Scholar]
- Danesh-Meyer HV, et al. The prevalence of cupping in end-stage arteritic and nonarteritic anterior ischemic optic neuropathy. Ophthalmology. 2001;108:593–598. doi: 10.1016/s0161-6420(00)00602-3. [DOI] [PubMed] [Google Scholar]
- Danias J, et al. Quantitative analysis of retinal ganglion cell (RGC) loss in aging DBA/2NNia glaucomatous mice: comparison with RGC loss in aging C57/BL6 mice. Invest Ophthalmol Vis Sci. 2003;44:5151–5162. doi: 10.1167/iovs.02-1101. [DOI] [PubMed] [Google Scholar]
- Dolman CL, et al. Aging of the optic nerve. Arch Ophthalmol. 1980;98:2053–2058. doi: 10.1001/archopht.1980.01020040905024. [DOI] [PubMed] [Google Scholar]
- Downs JC, et al. Posterior Scleral Thickness in Perfusion-Fixed Normal and Early-Glaucoma Monkey Eyes. Invest Ophthalmol Vis Sci. 2001;42:3202–3208. [PubMed] [Google Scholar]
- Downs JC, et al. Mechanical Strain and Restructuring of the Optic Nerve Head. In: Shaarawy T, Sherwood MB, Hitchings RA, Crowston JG, editors. Glaucoma. 1. W. B. Saunders; London: 2009. [Google Scholar]
- Downs JC, et al. Viscoelastic material properties of the peripapillary sclera in normal and early-glaucoma monkey eyes. Invest Ophthalmol Vis Sci. 2005;46:540–546. doi: 10.1167/iovs.04-0114. [DOI] [PubMed] [Google Scholar]
- Downs JC, et al. 3-D Histomorphometry of the Normal and Early Glaucomatous Monkey Optic Nerve Head: Neural Canal and Subarachnoid Space Architecture. Invest Ophthalmol Vis Sci. 2007;48:3195–3208. doi: 10.1167/iovs.07-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, et al. Upregulation of EphB2 and ephrin-B2 at the Optic Nerve Head of DBA/2J Glaucomatous Mice Coincides with Axon Loss. Invest Ophthalmol Vis Sci. 2007;48:5567–5581. doi: 10.1167/iovs.07-0442. [DOI] [PubMed] [Google Scholar]
- Duncan JE, Goldstein LS. The genetics of axonal transport and axonal transport disorders. PLoS Genet. 2006;2:e124. doi: 10.1371/journal.pgen.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fechtner RD, Weinreb RN. Mechanisms of optic nerve damage in primary open angle glaucoma. Surv Ophthalmol. 1994;39:23–42. doi: 10.1016/s0039-6257(05)80042-6. [DOI] [PubMed] [Google Scholar]
- Filippopoulos T, et al. Topographic and morphologic analyses of retinal ganglion cell loss in old DBA/2NNia mice. Invest Ophthalmol Vis Sci. 2006;47:1968–1974. doi: 10.1167/iovs.05-0955. [DOI] [PubMed] [Google Scholar]
- Fortune B, et al. Relative course of retinal nerve fiber layer birefringence and thickness and retinal function changes after optic nerve transection. Invest Ophthalmol Vis Sci. 2008a;49:4444–4452. doi: 10.1167/iovs.08-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune B, et al. Intravitreal colchicine causes decreased RNFL birefringence without altering RNFL thickness. Invest Ophthalmol Vis Sci. 2008b;49:255–261. doi: 10.1167/iovs.07-0872. [DOI] [PubMed] [Google Scholar]
- Friedenwald J. Contribution to the Theory and Practice of Tonometry. AJO. 1937;20:985–1024. [Google Scholar]
- Frisen L. High-pass resolution perimetry and age-related loss of visual pathway neurons. Acta Ophthalmol (Copenh) 1991;69:511–515. doi: 10.1111/j.1755-3768.1991.tb02030.x. [DOI] [PubMed] [Google Scholar]
- Fu CT, Sretavan DW. Laser-Induced Ocular Hypertension in Albino CD-1 Mice. Invest Ophthalmol Vis Sci. 2010;51:980–990. doi: 10.1167/iovs.09-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CT, et al. Axonal/Glial Upregulation of EphB/ephrin-B Signaling in Mouse Experimental Ocular Hypertension. Invest Ophthalmol Vis Sci. 2010;51:991–1001. doi: 10.1167/iovs.09-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaasterland D, et al. Axoplasmic flow during chronic experimental glaucoma. 1. Light and electron microscopic studies of the monkey optic nervehead during development of glaucomatous cupping. Invest Ophthalmol Vis Sci. 1978;17:838–846. [PubMed] [Google Scholar]
- Garcia-Valenzuela E, et al. Programmed cell death of retinal ganglion cells during experimental glaucoma. Exp Eye Res. 1995;61:33–44. doi: 10.1016/s0014-4835(95)80056-5. [DOI] [PubMed] [Google Scholar]
- Gordon GR, et al. Astrocyte control of the cerebrovasculature. Glia. 2007;55:1214–1221. doi: 10.1002/glia.20543. [DOI] [PubMed] [Google Scholar]
- Greenfield DS. Glaucomatous versus nonglaucomatous optic disc cupping: clinical differentiation. Semin Ophthalmol. 1999;14:95–108. doi: 10.3109/08820539909056069. [DOI] [PubMed] [Google Scholar]
- Greenfield DS, et al. The cupped disc. Who needs neuroimaging? Ophthalmology. 1998;105:1866–1874. doi: 10.1016/S0161-6420(98)91031-4. [DOI] [PubMed] [Google Scholar]
- Gross SP, et al. Cargo transport: two motors are sometimes better than one. Curr Biol. 2007;17:R478–486. doi: 10.1016/j.cub.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Grunwald JE, et al. Effect of aging on retinal macular microcirculation: a blue field simulation study. Invest Ophthalmol Vis Sci. 1993;34:3609–3613. [PubMed] [Google Scholar]
- Haefliger IO, Anderson DR. Blood Flow Regulation in the Optic Nerve Head. In: Ritch R, Shields MB, Krupin T, editors. The Glaucomas. 2. Mosby; St Louis: 1996. [Google Scholar]
- Hall ER, et al. Age-related macular degeneration and optic disk cupping: the Beaver Dam Eye Study. Am J Ophthalmol. 2006;141:494–497. doi: 10.1016/j.ajo.2005.10.049. [DOI] [PubMed] [Google Scholar]
- Hama K, et al. Tri-dimensional morphometric analysis of astrocytic processes with high voltage electron microscopy of thick Golgi preparations. J Neurocytol. 2004;33:277–285. doi: 10.1023/B:NEUR.0000044189.08240.a2. [DOI] [PubMed] [Google Scholar]
- Harris A, et al. Aging affects the retrobulbar circulation differently in women and men. Arch Ophthalmol. 2000;118:1076–1080. doi: 10.1001/archopht.118.8.1076. [DOI] [PubMed] [Google Scholar]
- Harwerth RS, et al. Ganglion cell losses underlying visual field defects from experimental glaucoma. Invest Ophthalmol Vis Sci. 1999;40:2242–2250. [PubMed] [Google Scholar]
- Hayreh SS, Jonas JB. Optic disc morphology after arteritic anterior ischemic optic neuropathy. Ophthalmology. 2001;108:1586–1594. doi: 10.1016/s0161-6420(01)00649-2. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, et al. Vasogenic origin of visual field defects and optic nerve changes in glaucoma. Br J Ophthalmol. 1970;54:461–472. doi: 10.1136/bjo.54.7.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS, et al. Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am J Ophthalmol. 1994;117:603–624. doi: 10.1016/s0002-9394(14)70067-4. [DOI] [PubMed] [Google Scholar]
- He S, et al. Endothelin-1-Mediated Signaling in the Expression of Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in Astrocytes. Invest Ophthalmol Vis Sci. 2007;48:3737–3745. doi: 10.1167/iovs.06-1138. [DOI] [PubMed] [Google Scholar]
- Hernandez MR. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res. 2000;19:297–321. doi: 10.1016/s1350-9462(99)00017-8. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, et al. Cell culture of the human lamina cribrosa. Invest Ophthalmol Vis Sci. 1988;29:78–89. [PubMed] [Google Scholar]
- Hernandez MR, et al. Age-related changes in the extracellular matrix of the human optic nerve head. Am J Ophthalmol. 1989;107:476–484. doi: 10.1016/0002-9394(89)90491-1. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, et al. Astrocytes in glaucomatous optic neuropathy. Prog Brain Res. 2008;173:353–373. doi: 10.1016/S0079-6123(08)01125-4. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Pena JD. The optic nerve head in glaucomatous optic neuropathy. Arch Ophthalmol. 1997;115:389–395. doi: 10.1001/archopht.1997.01100150391013. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, et al. Localization of collagen types I and IV mRNAs in human optic nerve head by in situ hybridization. Invest Ophthalmol Vis Sci. 1991;32:2169–2177. [PubMed] [Google Scholar]
- Hogan MJ, Zimmerman LE. The Optic Nerve. In: Hogan MJ, Zimmerman LE, editors. Ophthalmic Pathology. W.B. Saunders Company; Philadelphia: 1969. pp. 577–590. [Google Scholar]
- Hollander H, et al. Evidence of constriction of optic nerve axons at the lamina cribrosa in the normotensive eye in humans and other mammals. Ophthalmic Res. 1995;27:296–309. doi: 10.1159/000267739. [DOI] [PubMed] [Google Scholar]
- Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J Cell Biol. 2007;179:1523–1537. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell N. Leber hereditary optic neuropathy: how do mitochondrial DNA mutations cause degeneration of the optic nerve? J Bioenerg Biomembr. 1997;29:165–173. doi: 10.1023/a:1022690030664. [DOI] [PubMed] [Google Scholar]
- Huang XR, Knighton RW. Microtubules contribute to the birefringence of the retinal nerve fiber layer. Invest Ophthalmol Vis Sci. 2005;46:4588–4593. doi: 10.1167/iovs.05-0532. [DOI] [PubMed] [Google Scholar]
- Huang XR, et al. Microtubule contribution to the reflectance of the retinal nerve fiber layer. Invest Ophthalmol Vis Sci. 2006;47:5363–5367. doi: 10.1167/iovs.06-0451. [DOI] [PubMed] [Google Scholar]
- Jakobs TC, et al. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J Cell Biol. 2005;171:313–325. doi: 10.1083/jcb.200506099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamara RJ, et al. Clinical quality assessment using computer monitor photoimages of optic nerve head cupping. Optom Vis Sci. 2000;77:433–436. doi: 10.1097/00006324-200008000-00011. [DOI] [PubMed] [Google Scholar]
- Janssen P, et al. Evidence for glaucoma-induced horizontal cell alterations in the human retina. Ger J Ophthalmol. 1996;5:378–385. [PubMed] [Google Scholar]
- Jeffery G, et al. The human optic nerve: fascicular organisation and connective tissue types along the extra-fascicular matrix. Anat Embryol (Berl) 1995;191:491–502. doi: 10.1007/BF00186739. [DOI] [PubMed] [Google Scholar]
- Johansson JO. Inhibition of retrograde axoplasmic transport in rat optic nerve by increased IOP in vitro. Invest Ophthalmol Vis Sci. 1983;24:1552–1558. [PubMed] [Google Scholar]
- Johns KJ, et al. The effect of panretinal photocoagulation on optic nerve cupping. Ophthalmology. 1989;96:211–216. doi: 10.1016/s0161-6420(89)32911-3. [DOI] [PubMed] [Google Scholar]
- Johnson EC, et al. Chronology of optic nerve head and retinal responses to elevated intraocular pressure. Invest Ophthalmol Vis Sci. 2000;41:431–442. [PubMed] [Google Scholar]
- Johnson EC, et al. Global Changes in Optic Nerve Head Gene Expression after Exposure to Elevated Intraocular Pressure in a Rat Glaucoma Model. Invest Ophthalmol Vis Sci. 2007;48:3161–3177. doi: 10.1167/iovs.06-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, Morrison JC. Friend or foe? Resolving the impact of glial responses in glaucoma. J Glaucoma. 2009;18:341–353. doi: 10.1097/IJG.0b013e31818c6ef6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, et al. The effect of chronically elevated intraocular pressure on the rat optic nerve head extracellular matrix. Exp Eye Res. 1996;62:663–674. doi: 10.1006/exer.1996.0077. [DOI] [PubMed] [Google Scholar]
- Johnson J, et al. A study of axonal degeneration in the optic nerves of aging mice. AGE. 1978;1:50–55. [Google Scholar]
- Jonas JB. Trans-lamina cribrosa pressure difference. Arch Ophthalmol. 2007;125:431. doi: 10.1001/archopht.125.3.431-a. author reply 431. [DOI] [PubMed] [Google Scholar]
- Jonas JB, et al. Anatomic relationship between lamina cribrosa, intraocular space, and cerebrospinal fluid space. Invest Ophthalmol Vis Sci. 2003;44:5189–5195. doi: 10.1167/iovs.03-0174. [DOI] [PubMed] [Google Scholar]
- Jonas JB, et al. Lamina cribrosa thickness and spatial relationships between intraocular space and cerebrospinal fluid space in highly myopic eyes. Invest Ophthalmol Vis Sci. 2004;45:2660–2665. doi: 10.1167/iovs.03-1363. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Grundler A. Optic disc morphology in “age-related atrophic glaucoma”. Graefes Arch Clin Exp Ophthalmol. 1996;234:744–749. doi: 10.1007/BF00189355. [DOI] [PubMed] [Google Scholar]
- Kalvin NH, et al. Experimental glaucoma in monkeys. I. Relationship between intraocular pressure and cupping of the optic disc and cavernous atrophy of the optic nerve. Arch Ophthalmol. 1966;76:82–93. doi: 10.1001/archopht.1966.03850010084017. [DOI] [PubMed] [Google Scholar]
- Kanamori A, et al. In Vivo Imaging Of Retinal Ganglion Cell Axons Within The Nerve Fiber Layer. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.09-4021. [DOI] [PubMed] [Google Scholar]
- Kendell KR, et al. Primary open-angle glaucoma is not associated with photoreceptor loss. Invest Ophthalmol Vis Sci. 1995;36:200–205. [PubMed] [Google Scholar]
- Kirwan RP, et al. Effect of Cyclical Mechanical Stretch and Exogenous Transforming Growth Factor-beta1 on Matrix Metalloproteinase-2 Activity in Lamina Cribrosa Cells from the Human Optic Nerve Head. J Glaucoma. 2004;13:327–334. doi: 10.1097/00061198-200408000-00011. [DOI] [PubMed] [Google Scholar]
- Klein BE, et al. Does the intraocular pressure effect on optic disc cupping differ by age? Trans Am Ophthalmol Soc. 2006;104:143–148. [PMC free article] [PubMed] [Google Scholar]
- Kotecha A, et al. Age related changes in the thickness of the human lamina cribrosa. Br J Ophthalmol. 2006;90:1531–1534. doi: 10.1136/bjo.2006.100388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langham M. The temporal relation between intraocular pressure and loss of vision in chronic simple glaucoma. Glaucoma. 1980;2:427–435. [Google Scholar]
- Liang Y, et al. Quantification of dynamic blood flow autoregulation in optic nerve head of rhesus monkeys. Exp Eye Res. 2010;90:203–209. doi: 10.1016/j.exer.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Mabuchi F, et al. Regional optic nerve damage in experimental mouse glaucoma. Invest Ophthalmol Vis Sci. 2004;45:4352–4358. doi: 10.1167/iovs.04-0355. [DOI] [PubMed] [Google Scholar]
- Manor RS. Documented optic disc cupping in compressive optic neuropathy. Ophthalmology. 1995;102:1577–1578. doi: 10.1016/s0161-6420(13)31810-7. [DOI] [PubMed] [Google Scholar]
- Martin KR, et al. Optic nerve dynein motor protein distribution changes with intraocular pressure elevation in a rat model of glaucoma. Exp Eye Res. 2006;83:255–262. doi: 10.1016/j.exer.2005.11.025. [DOI] [PubMed] [Google Scholar]
- McKendrick AM, et al. Contrast sensitivity changes due to glaucoma and normal aging: low-spatial-frequency losses in both magnocellular and parvocellular pathways. Invest Ophthalmol Vis Sci. 2007;48:2115–2122. doi: 10.1167/iovs.06-1208. [DOI] [PubMed] [Google Scholar]
- Minckler D. Posterior segment changes in glaucoma. In: Garner A, Klintworth K, editors. Pathobiology of Ocular Disease: A Dynamic Approach. 2. Marcel Dekker, Inc; New York: 1994. pp. 469–479. [Google Scholar]
- Minckler DS, et al. Orthograde and retrograde axoplasmic transport during acute ocular hypertension in the monkey. Invest Ophthalmol Vis Sci. 1977;16:426–441. [PubMed] [Google Scholar]
- Minckler DS, et al. Distribution of axonal and glial elements in the rhesus optic nerve head studied by electron microscopy. Am J Ophthalmol. 1976;82:179–187. doi: 10.1016/0002-9394(76)90416-5. [DOI] [PubMed] [Google Scholar]
- Morgan JE. Optic nerve head structure in glaucoma: astrocytes as mediators of axonal damage. Eye. 2000;14(Pt 3B):437–444. doi: 10.1038/eye.2000.128. [DOI] [PubMed] [Google Scholar]
- Morgan JE. Circulation and axonal transport in the optic nerve. Eye. 2004;18:1089–1095. doi: 10.1038/sj.eye.6701574. [DOI] [PubMed] [Google Scholar]
- Morgan WH, et al. Optic Disc Movement with Variations in Intraocular and Cerebrospinal Fluid Pressure. Invest Ophthalmol Vis Sci. 2002;43:3236–3242. [PubMed] [Google Scholar]
- Morgan WH, et al. The correlation between cerebrospinal fluid pressure and retrolaminar tissue pressure. Invest Ophthalmol Vis Sci. 1998;39:1419–1428. [PubMed] [Google Scholar]
- Morgan WH, et al. The role of cerebrospinal fluid pressure in glaucoma pathophysiology: the dark side of the optic disc. J Glaucoma. 2008;17:408–413. doi: 10.1097/IJG.0b013e31815c5f7c. [DOI] [PubMed] [Google Scholar]
- Morgan WH, et al. The influence of cerebrospinal fluid pressure on the lamina cribrosa tissue pressure gradient. Invest Ophthalmol Vis Sci. 1995;36:1163–1172. [PubMed] [Google Scholar]
- Morrison JC, et al. Aging changes of the rhesus monkey optic nerve. Invest. Ophthalmol. Vis. Sci. 1990;31:1623–1627. [PubMed] [Google Scholar]
- Morrison JC, et al. Structural proteins of the neonatal and adult lamina cribrosa. Arch Ophthalmol. 1989a;107:1220–1224. doi: 10.1001/archopht.1989.01070020286040. [DOI] [PubMed] [Google Scholar]
- Morrison JC, et al. Ultrastructural location of extracellular matrix components in the optic nerve head. Arch Ophthalmol. 1989b;107:123–129. doi: 10.1001/archopht.1989.01070010125040. [DOI] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Murphy JA, et al. The role of endothelin-1 and its receptors in optic nerve head astrocyte proliferation. Br J Ophthalmol. 2010 doi: 10.1136/bjo.2009.172098. [DOI] [PubMed] [Google Scholar]
- Mutsaers SE, Carroll WM. Focal accumulation of intra-axonal mitochondria in demyelination of the cat optic nerve. Acta Neuropathol. 1998;96:139–143. doi: 10.1007/s004010050873. [DOI] [PubMed] [Google Scholar]
- Nicolela MT, Drance SM. Various glaucomatous optic nerve appearances: clinical correlations. Ophthalmology. 1996;103:640–649. doi: 10.1016/s0161-6420(96)30640-4. [DOI] [PubMed] [Google Scholar]
- Nicolela MT, et al. Visual field and optic disc progression in patients with different types of optic disc damage: a longitudinal prospective study. Ophthalmology. 2003;110:2178–2184. doi: 10.1016/S0161-6420(03)00801-7. [DOI] [PubMed] [Google Scholar]
- Nork TM, et al. Swelling and loss of photoreceptors in chronic human and experimental glaucomas. Arch Ophthalmol. 2000;118:235–245. doi: 10.1001/archopht.118.2.235. [DOI] [PubMed] [Google Scholar]
- Oku H, et al. Experimental optic cup enlargement caused by endothelin-1-induced chronic optic nerve head ischemia. Surv Ophthalmol. 1999;44(Suppl 1):S74–84. doi: 10.1016/s0039-6257(99)00068-5. [DOI] [PubMed] [Google Scholar]
- Orgul S, et al. An endothelin-1-induced model of chronic optic nerve ischemia in rhesus monkeys. J Glaucoma. 1996a;5:135–138. [PubMed] [Google Scholar]
- Orgul S, et al. An endothelin-1 induced model of optic nerve ischemia in the rabbit. Invest Ophthalmol Vis Sci. 1996b;37:1860–1869. [PubMed] [Google Scholar]
- Orgul S, et al. Optic disc cupping in arteritic anterior ischemic optic neuropathy. Ophthalmologica. 1994;208:336–338. doi: 10.1159/000310534. [DOI] [PubMed] [Google Scholar]
- Panda S, Jonas JB. Decreased photoreceptor count in human eyes with secondary angle- closure glaucoma. Invest Ophthalmol Vis Sci. 1992;33:2532–2536. [PubMed] [Google Scholar]
- Pederson JE, Anderson DR. The mode of progressive disc cupping in ocular hypertension and glaucoma. Arch Ophthalmol. 1980;98:490–495. doi: 10.1001/archopht.1980.01020030486010. [DOI] [PubMed] [Google Scholar]
- Pederson JE, Gaasterland DE. Laser-induced primate glaucoma. I. Progression of cupping. Arch Ophthalmol. 1984;102:1689–1692. doi: 10.1001/archopht.1984.01040031373030. [DOI] [PubMed] [Google Scholar]
- Pena J, et al. Elastin associated microfibrils in human optic nerve heads in vivo and in vitro. Invest Ophthalmol Vis Sci. 1997;38:S162. [Google Scholar]
- Pena JD, et al. Increased elastin expression in astrocytes of the lamina cribrosa in response to elevated intraocular pressure. Invest Ophthalmol Vis Sci. 2001;42:2303–2314. [PubMed] [Google Scholar]
- Pena JD, et al. Tropoelastin gene expression in optic nerve heads of normal and glaucomatous subjects. Matrix Biol. 1996;15:323–330. doi: 10.1016/s0945-053x(96)90135-3. [DOI] [PubMed] [Google Scholar]
- Piette SD, Sergott RC. Pathological optic-disc cupping. Curr Opin Ophthalmol. 2006;17:1–6. doi: 10.1097/01.icu.0000193072.17122.f3. [DOI] [PubMed] [Google Scholar]
- Prasanna G, et al. Human optic nerve head astrocytes as a target for endothelin-1. Invest Ophthalmol Vis Sci. 2002;43:2704–2713. [PubMed] [Google Scholar]
- Quigley H, Anderson DR. The dynamics and location of axonal transport blockade by acute intraocular pressure elevation in primate optic nerve. Invest Ophthalmol Vis Sci. 1976;15:606–616. [PubMed] [Google Scholar]
- Quigley H, Anderson DR. Cupping of the optic disc in ischemic optic neuropathy. Trans Am Acad Ophthalmol Otolaryngol. 1977;83:755–762. [PubMed] [Google Scholar]
- Quigley HA. Childhood glaucoma: results with trabeculotomy and study of reversible cupping. Ophthalmology. 1982;89:219–226. doi: 10.1016/s0161-6420(82)34803-4. [DOI] [PubMed] [Google Scholar]
- Quigley HA. Ganglion cell death in glaucoma: pathology recapitulates ontogeny. Aust N Z J Ophthalmol. 1995a;23:85–91. doi: 10.1111/j.1442-9071.1995.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Quigley HA. Overview and introduction to session on connective tissue of the optic nerve in glaucoma. Chapter 2. In: Drance SM, Anderson DR, editors. Optic Nerve in Glaucoma. Kugler Publications; Amsterdam/New York: 1995b. pp. 15–36. [Google Scholar]
- Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999;18:39–57. doi: 10.1016/s1350-9462(98)00014-7. [DOI] [PubMed] [Google Scholar]
- Quigley HA, et al. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981;99:635–649. doi: 10.1001/archopht.1981.03930010635009. [DOI] [PubMed] [Google Scholar]
- Quigley HA, et al. The size and shape of the optic disc in normal human eyes. Arch Ophthalmol. 1990;108:51–57. doi: 10.1001/archopht.1990.01070030057028. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Green WR. The histology of human glaucoma cupping and optic nerve damage: clinicopathologic correlation in 21 eyes. Ophthalmology. 1979;86:1803–1830. doi: 10.1016/s0161-6420(79)35338-6. [DOI] [PubMed] [Google Scholar]
- Quigley HA, et al. Morphologic changes in the lamina cribrosa correlated with neural loss in open-angle glaucoma. Am J Ophthalmol. 1983;95:673–691. doi: 10.1016/0002-9394(83)90389-6. [DOI] [PubMed] [Google Scholar]
- Quigley HA, et al. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci. 2000;41:3460–3466. [PubMed] [Google Scholar]
- Quigley HA, et al. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36:774–786. [PubMed] [Google Scholar]
- Rao VR, et al. Endothelin-1 mediated regulation of extracellular matrix collagens in cells of human lamina cribrosa. Exp Eye Res. 2008;86:886–894. doi: 10.1016/j.exer.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R, et al. Cerebrospinal Fluid Pressure in Glaucoma. A Prospective Study. Ophthalmology. 2009a doi: 10.1016/j.ophtha.2009.06.058. Submitted. [DOI] [PubMed] [Google Scholar]
- Ren R, et al. Lamina cribrosa and peripapillary sclera histomorphometry in normal and advanced glaucomatous Chinese eyes with various axial length. Invest Ophthalmol Vis Sci. 2009b;50:2175–2184. doi: 10.1167/iovs.07-1429. [DOI] [PubMed] [Google Scholar]
- Repka MX, Quigley HA. The effect of age on normal human optic nerve fiber number and diameter. Ophthalmology. 1989;96:26–32. doi: 10.1016/s0161-6420(89)32928-9. [DOI] [PubMed] [Google Scholar]
- Roberts MD, et al. Remodeling of the Connective Tissue Microarchitecture of the Lamina Cribrosa in Early Experimental Glaucoma. Invest Ophthalmol Vis Sci. 2009;50:681–690. doi: 10.1167/iovs.08-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell JH, Peters A. Effects of age on nerve fibers in the rhesus monkey optic nerve. J Comp Neurol. 2001;429:541–553. doi: 10.1002/1096-9861(20010122)429:4<541::aid-cne3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Sandell JH, Peters A. Effects of age on the glial cells in the rhesus monkey optic nerve. J Comp Neurol. 2002;445:13–28. doi: 10.1002/cne.10162. [DOI] [PubMed] [Google Scholar]
- Schlamp CL, et al. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neurosci. 2006;7:66. doi: 10.1186/1471-2202-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JT, et al. Acquired cupping of the optic nerve head in normotensive eyes. Br J Ophthalmol. 1975;59:216–222. doi: 10.1136/bjo.59.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See JLS, et al. Rates of neuroretinal rim and peripapillary atrophy area change: a comparative study of glaucoma patients and normal controls. Ophthalmology. 2009;116:840–847. doi: 10.1016/j.ophtha.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Sharma M, et al. Methanol-induced optic nerve cupping. Arch Ophthalmol. 1999;117:286. doi: 10.1001/archopht.117.2.286. [DOI] [PubMed] [Google Scholar]
- Sigal IA. Interactions between geometry and mechanical properties on the optic nerve head. Invest Ophthalmol Vis Sci. 2009;50:2785–2795. doi: 10.1167/iovs.08-3095. [DOI] [PubMed] [Google Scholar]
- Sigal IA, Ethier C. DUPLICATE/INCORRECT ENTRY SEE RECORD #4395 Biomechanics of the Lamina Cribrosa. Exp Eye Res 2009a [Google Scholar]
- Sigal IA, Ethier CR. Biomechanics of the optic nerve head. Exp Eye Res. 2009b;88:799–807. doi: 10.1016/j.exer.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Sigal IA, et al. Factors influencing optic nerve head biomechanics. Invest Ophthalmol Vis Sci. 2005;46:4189–4199. doi: 10.1167/iovs.05-0541. [DOI] [PubMed] [Google Scholar]
- Sigal IA, et al. Modeling individual-specific human optic nerve head biomechanics. Part I: IOP-induced deformations and influence of geometry. Biomech Model Mechanobiol. 2009a;8:85–98. doi: 10.1007/s10237-008-0120-7. [DOI] [PubMed] [Google Scholar]
- Sigal IA, et al. Modeling individual-specific human optic nerve head biomechanics. Part II: influence of material properties. Biomech Model Mechanobiol. 2009b;8:99–109. doi: 10.1007/s10237-008-0119-0. [DOI] [PubMed] [Google Scholar]
- Sigal IA, et al. 3D Morphometry of the Human Optic Nerve Head. Exp Eye Res. 2010a;90:70–80. doi: 10.1016/j.exer.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Sigal IA, et al. Chapter 20: Biomechanical Changes of the Optic Disc. In: Levin LA, Albert DM, editors. Ocular Disease: Mechanisms and Management. Elsevier/Saunders; New York: 2010b. [Google Scholar]
- Sonty S, Schwartz B. Development of cupping and pallor in posterior ischemic optic neuropathy. Int Ophthalmol. 1983;6:213–220. doi: 10.1007/BF00141130. [DOI] [PubMed] [Google Scholar]
- Spaeth G. The pathogenesis of nerve damage in glaucoma: Contributions of fluorescein angiography. Grune & Stratton; London: 1977. pp. 123–132. [Google Scholar]
- Sponsel WE, et al. Frequency of sustained glaucomatous-type visual field loss and associated optic nerve cupping in Beaver Dam, Wisconsin. Clin Experiment Ophthalmol. 2001;29:352–358. doi: 10.1046/j.1442-9071.2001.d01-22.x. [DOI] [PubMed] [Google Scholar]
- Stokely ME, et al. Effects of Endothelin-1 on Components of Anterograde Axonal Transport in Optic Nerve. Invest Ophthalmol Vis Sci. 2002;43:3223–3230. [PubMed] [Google Scholar]
- Tezel G, et al. In vitro evaluation of reactive astrocyte migration, a component of tissue remodeling in glaucomatous optic nerve head. Glia. 2001;34:178–189. doi: 10.1002/glia.1052. [DOI] [PubMed] [Google Scholar]
- Tezel G, et al. Parapapillary chorioretinal atrophy in patients with ocular hypertension. II. An evaluation of progressive changes. Arch Ophthalmol. 1997;115:1509–1514. doi: 10.1001/archopht.1997.01100160679003. [DOI] [PubMed] [Google Scholar]
- Trobe JD, et al. Nonglaucomatous excavation of the optic disc. Arch Ophthalmol. 1980;98:1046–1050. doi: 10.1001/archopht.1980.01020031036004. [DOI] [PubMed] [Google Scholar]
- Vale RD, Milligan RA. The way things move: looking under the hood of molecular motor proteins. Science. 2000;288:88–95. doi: 10.1126/science.288.5463.88. [DOI] [PubMed] [Google Scholar]
- Varela HJ, Hernandez MR. Astrocyte responses in human optic nerve head with primary open-angle glaucoma. J Glaucoma. 1997;6:303–313. [PubMed] [Google Scholar]
- Vernon SA, et al. Peripapillary retinal nerve fibre layer thickness in highly myopic Caucasians as measured by Stratus optical coherence tomography. Br J Ophthalmol. 2008;92:1076–1080. doi: 10.1136/bjo.2007.127571. [DOI] [PubMed] [Google Scholar]
- Votruba M, et al. Optic disc morphology of patients with OPA1 autosomal dominant optic atrophy. Br J Ophthalmol. 2003;87:48–53. doi: 10.1136/bjo.87.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrabec F. Glaucomatous cupping of the human optic disk: a neuro-histologic study. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1976;198:223–234. doi: 10.1007/BF00410715. [DOI] [PubMed] [Google Scholar]
- Vrabec F. Age changes of the human optic nerve head. A neurohistologic study. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1977;202:231–236. doi: 10.1007/BF00407873. [DOI] [PubMed] [Google Scholar]
- Wang L, et al. Immunohistologic evidence for retinal glial cell changes in human glaucoma. Invest Ophthalmol Vis Sci. 2002;43:1088–1094. [PubMed] [Google Scholar]
- Wang X, et al. Acute endothelin-1 application induces reversible fast axonal transport blockade in adult rat optic nerve. Invest Ophthalmol Vis Sci. 2008;49:961–967. doi: 10.1167/iovs.07-1243. [DOI] [PubMed] [Google Scholar]
- Wax MB, et al. Induced autoimmunity to heat shock proteins elicits glaucomatous loss of retinal ganglion cell neurons via activated T-cell-derived fas-ligand. J Neurosci. 2008;28:12085–12096. doi: 10.1523/JNEUROSCI.3200-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber AJ, et al. Morphology of single ganglion cells in the glaucomatous primate retina. Invest Ophthalmol Vis Sci. 1998;39:2304–2320. [PubMed] [Google Scholar]
- Wilhelmsson U, et al. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci U S A. 2006;103:17513–17518. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wygnanski T, et al. Comparison of ganglion cell loss and cone loss in experimental glaucoma. Am J Ophthalmol. 1995;120:184–189. doi: 10.1016/s0002-9394(14)72606-6. [DOI] [PubMed] [Google Scholar]
- Yablonski M, Asamoto A. Basic sciences in clinical glaucoma: Hypothesis concerning the pathophysiology of optic nerve damage in open angle glaucoma. J Glaucoma. 1993;2:119–127. [PubMed] [Google Scholar]
- Yang H, et al. 3-D Histomorphometry of the Normal and Early Glaucomatous Monkey Optic Nerve Head: Prelaminar Neural Tissues and Cupping. Invest Ophthalmol Vis Sci. 2007a;48:5068–5084. doi: 10.1167/iovs.07-0790. [DOI] [PubMed] [Google Scholar]
- Yang H, et al. Physiologic intereye differences in monkey optic nerve head architecture and their relation to changes in early experimental glaucoma. Invest Ophthalmol Vis Sci. 2009;50:224–234. doi: 10.1167/iovs.08-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, et al. 3-D Histomorphometry of the Normal and Early Glaucomatous Monkey Optic Nerve Head: Lamina Cribrosa and Peripapillary Scleral Position and Thickness. Invest Ophthalmol Vis Sci. 2007b;48:4597–4607. doi: 10.1167/iovs.07-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, et al. Deformation of the Early Glaucomatous Monkey Optic Nerve Head Connective Tissue Following Acute IOP Elevation within 3-D Histomorphometric Reconstructions. Invest Ophthalmol Vis Sci. 2010:iovs.09–5122. doi: 10.1167/iovs.09-5122. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Hernandez MR. Heterogeneity of astrocytes in human optic nerve head. J Comp Neurol. 1995;362:441–452. doi: 10.1002/cne.903620402. [DOI] [PubMed] [Google Scholar]
- Yorio T, et al. Endothelin: is it a contributor to glaucoma pathophysiology? J Glaucoma. 2002;11:259–270. doi: 10.1097/00061198-200206000-00016. [DOI] [PubMed] [Google Scholar]
- Yu PK, et al. The Structural Relationship between the Microvasculature, Neurons and Glia in the Human Retina. Invest Ophthalmol Vis Sci. 2010;51:447–458. doi: 10.1167/iovs.09-3978. [DOI] [PubMed] [Google Scholar]
- Yucel YH, et al. Loss of neurons in magnocellular and parvocellular layers of the lateral geniculate nucleus in glaucoma. Arch Ophthalmol. 2000;118:378–384. doi: 10.1001/archopht.118.3.378. [DOI] [PubMed] [Google Scholar]
- Yucel YH, et al. Atrophy of relay neurons in magno- and parvocellular layers in the lateral geniculate nucleus in experimental glaucoma. Invest Ophthalmol Vis Sci. 2001;42:3216–3222. [PubMed] [Google Scholar]
- Yucel YH, et al. Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog Retin Eye Res. 2003;22:465–481. doi: 10.1016/s1350-9462(03)00026-0. [DOI] [PubMed] [Google Scholar]