Abstract
Alcohol and tobacco use covary at multiple levels of analysis, and co-use of the two substances may have profound health consequences. In order to characterize the motivationally relevant processes contributing to co-use, the current study used Ecological Momentary Assessment (EMA) to examine the subjective consequences of naturally occurring simultaneous use of alcohol and tobacco. Current smokers who reported frequently drinking alcohol (N = 259) monitored their daily experiences for 21 days using electronic diaries. Participants responded to prompted assessments and also initiated recordings when they smoked a cigarette or completed the first drink in a drinking episode. Momentary reports of smoking and alcohol consumption were associated with one another, and these effects remained after adjustment for occasion- and person-level covariates. When participants consumed alcohol, they reported increased pleasure and decreased punishment from the last cigarette. Smoking was associated with small increases in pleasure from the last drink. Ratings of “buzzed” and “dizzy” were synergistically affected by co-use of alcohol and tobacco. Co-use was also followed by higher levels of craving for both alcohol and tobacco. Results point to the importance of reward and incentive processes in ongoing drug use and suggest that alcohol intensifies real-time reports of the motivational consequences of smoking more strongly than smoking affects corresponding appraisals of alcohol effects.
Keywords: alcohol, cigarettes, electronic diary, craving, reward, reinforcement
Alcohol drinkers are more likely to smoke cigarettes than teetotalers and smokers are more likely to report drinking alcohol than nonsmokers (Falk, Yi & Hiller-Sturmhofel, 2006; Shiffman & Balabanis, 1995). Moreover, heavy drinkers are likely to be heavy smokers and vice versa (Shiffman & Balabanis, 1995). In psychiatric epidemiology, alcohol use disorders show substantial comorbidity with tobacco dependence (Falk, et al., 2006; Jackson, Sher, Wood, & Bucholz, 2003). The probability of smoking is strongly related to the number of alcohol dependence criteria endorsed (Madden, Bucholz, Martin & Heath, 2000). Indeed, this overlap is so robust that it could be argued alcoholism is most often a disorder involving problematic use of alcohol and tobacco. The covariation of the two substances is practically and clinically important. For instance, abuse of both alcohol and tobacco confers synergistic risk for some forms of cancer (e.g., Castellsague, et al., 1999). Even though alcoholism is associated with devastating medical consequences, a follow-up study of treated alcoholics found they were even more likely to die of tobacco-related disease (Hurt, et al., 1996).
Alcohol and tobacco not only tend to be used by the same individuals, but also tend to be used at the same time. In the laboratory, alcohol administration spurs tobacco use (e.g., Mello, Mendelson, & Palmieri, 1987; Mintz, Boyd, Rose, Charuvastra, & Jarvik, 1985). Some evidence suggests nicotine administration increases alcohol consumption, at least in males (Acheson, Mahler, Chi & de Wit, 2006; Barrett, Tichauer, Leyton, & Pihl, 2006). Field investigations have demonstrated associations between use of alcohol and tobacco in daily diary entries (e.g., Dierker, et al. 2006) and in momentary reports (Piasecki, McCarthy, Fiore, & Baker, 2008; Shapiro, Jamner, Davydov & James, 2002; Shiffman, Fischer, Paty, Gnys, Hickcox & Kassel, 1994; Shiffman, et al., 2002). By serving as a strong trigger for smoking (Shiffman, et al., 1994) and smoking relapse (e.g., Shiffman, Paty, Gnys, Kassel & Hickox, 1996), alcohol use contributes to both the persistence of tobacco dependence and risk of tobacco-related morbidity and mortality. In this light, the need to develop a more sophisticated understanding of the processes that give rise to the covariation of drinking and smoking is apparent (Littleton, Barron, Prendergast, & Nixon, 2007).
Although diverse causal mechanisms may play a role, one prominent hypothesis has been that co-administration of alcohol and tobacco produces distinct subjective effects (e.g., Littleton, et al., 2007; Perkins, 1997; Rose, Brauer, Behm, Cramblett, Calkins, & Lahon, 2004). This notion is appealing because it may help to explain the situational co-use of both substances. Perhaps the most natural conjecture is that the positively rewarding effects of smoking or drinking are enhanced when the two substances are used together. Another possibility is that acute cross-tolerance between alcohol and tobacco could result in the attainment of diminished pleasant drug effects, which might lead users to increase their intake to overcome this effect. Subjective processes beyond reward are also relevant. Use of one drug might prime craving for the other substance, thereby stimulating co-use. Negative reinforcement might play a role; co-use might offer especially potent relief from naturally-occurring dysphoric states. Additionally, both alcohol and tobacco can produce unpleasant sensations that are difficult to regulate with each substance alone. If one drug can alleviate aversive effects of the other, negative reinforcement might promote simultaneous use as part of a self-regulation strategy for optimizing drug effects.
In surveys, smokers report believing that co-use will enhance the taste of cigarettes (McClernon, Westman, Rose & Lutz, 2007) and result in enhanced positive and negative reinforcing effects from both alcohol and tobacco (McKee, Hinson, Rounsaville, & Petrelli, 2004). Cigarettes smoked under the influence of alcohol are rated as more liked, satisfying, and calming (Glautier, Clements, White, Taylor & Stolerman, 1996; Rose, et al., 2004). Alcohol also acutely modulates expectations for positive reinforcement, such that smokers experience more pleasurable anticipation of smoking when drinking (Kirchner & Sayette, 2007; Sayette, Martin, Wertz, Perrott, & Peters, 2005). Although there have been suggestions that nicotine generally enhances the rewarding effects of other reinforcers (e.g., Donny, et al., 2003), alcohol may have a greater effect on smoking reward than vice versa. Two recent alcohol challenge studies found that pretreatment with transdermal nicotine increased subjective perceptions of alcohol intoxication and sedation, but did not affect ratings of reward or stimulation (Acheson, et al., 2006; Kouri, McCarthy, Faust & Lukas, 2004). Alcohol administration robustly increases craving for tobacco (e.g., Burton & Tiffany, 1997; King & Epstein, 2005; Sayette, et al., 2005) and these effects are partially mediated by the rewarding effects of alcohol (Epstein, Sher, Young, & King, 2007). Nicotine administration can also increase alcohol craving, though this has not been uniformly observed (cf. Acheson, et al., 2006; Kouri, et al., 2004).
Laboratory tests involving co-administration of nicotine/tobacco and alcohol permit strong causal inference. However, by their very nature such tests must be conducted under rarefied conditions, raising questions about the representativeness or generalizability of the resulting observations. Drinking and smoking behaviors vary as a function of time of day, day of week, and physical, social, and emotional contexts (Piasecki, et al., 2008; Shiffman & Paty, 2006). Moreover, the subjective consequences of drug may also vary across such contexts (Piasecki, et al., 2008; Shiffman & Kirchner, 2009). Indeed, it has been argued that context is the critical determinant of alcohol outcomes that can range from euphoriant effects to “crying in one’s beer” (Steele & Josephs, 1990), at least at low to moderate doses (Donohue, Curtin, Patrick, & Lang, 2007). Nicotine’s mood effects have also been shown to be moderated by context (e.g., Kassel & Shiffman, 1997; Kassel & Unrod, 2000). Such findings encourage the use of Ecological Momentary Assessment (Stone & Shiffman, 1994) as a complementary strategy to controlled laboratory investigation. EMA studies include frequent assessments of immediate states and behaviors that are collected while respondents inhabit their natural environments. Thus, EMA seeks to minimize retrospective bias or forgetting and to maximize ecological validity. Because EMA studies capture drug effects as they occur in the environments in which users typically experience them, they may make vital descriptive contributions to the investigation of co-use effects.
Although numerous EMA studies have examined contextual and subjective stimulus conditions preceding drug use, surprisingly little attention has been given to the subjective consequences of drug use in the natural environment. To our knowledge, only two studies have used EMA to investigate the subjective effects of alcohol-tobacco co-use. Piasecki, et al (2008) examined appraisals of the last cigarette smoked in 7,707 prompted diary records collected from 74 heavy smokers during the pre-cessation period of a smoking cessation study. As expected, alcohol consumption and smoking behaviors covaried in the diary records. Reports of alcohol use in the past hour were associated with the occurrence of strong urges, a decreased likelihood of acute urge satisfaction, and effects potentially indicative of reward (good taste, rush/buzz).
Shiffman & Kirchner (2009) assessed the degree of subjective satisfaction 394 heavy smokers obtained from smoking nearly 15,000 cigarettes during an 11-day baseline period prior to a cessation attempt. In this research, consumption of alcohol in the 15 minutes prior to the smoking episode was not significantly related to reported cigarette satisfaction, though there was a trend in that direction. Also, cigarettes consumed during states of more intense cigarette craving and greater positive affect were rated as more satisfying. Given the tendency for alcohol to produce positive hedonic effects and craving (e.g., Epstein, et al., 2007), the Shiffman & Kirchner (2009) study provides indirect evidence that alcohol could enhance tobacco reinforcement.
A third recent EMA investigation did not examine co-use per se, but examined momentary associations between smoking behavior and urge to drink alcohol (N.L. Cooney, Litt, Cooney, Pilkey, Steinberg, & Oncken, 2007). In this study, 102 smokers undergoing intensive outpatient treatment for alcohol dependence were randomized to one of two smoking cessation treatments. After completion of treatment, they carried electronic diaries for 2 weeks, responding to time-based prompts and initiating entries before and after any cigarettes. Among those who smoked during diary monitoring, the probability of experiencing an urge to drink was low overall (i.e., < 20%) but urge to drink was slightly more likely after smoking compared to pre-smoking moments.
In the current study, we sought to extend this line of inquiry in two ways. First, we recruited a sample of frequent drinkers who were not seeking smoking cessation treatment. Treatment-seeking is uncommon, and is concentrated in heavier and more dependent smokers (Shiffman, Brockwell, Pillitteri & Gitchell, 2008). Additionally, many of the participants in the current study were light, infrequent smokers. By focusing on heavy smokers interested in quitting, existing studies provide information that is clinically relevant but potentially limited in generalizability. For instance, if effects of co-use change with age or the development of dependence, such studies may not be able to capture the effects that explain initial acquisition of co-use behaviors and developmental aspects of the dependence process. Second, we used a diary protocol in which participants recorded both episodes of smoking and drinking, as well as being “beeped” at random for assessment when they were doing neither. Whereas existing diary studies have primarily examined the effects of alcohol consumption on tobacco effects (Piasecki, et al., 2008; Shiffman & Kirchner, 2009) or cigarette use on drinking motivation (N.L. Cooney, et al., 2007), the current design allowed prediction of subjective states from alcohol use, tobacco use, and their interaction, yielding a more informative and comprehensive assessment of the separate and joint effects of each substance. Additionally, we asked participants to complete simple one-item proxies for positive reinforcement, negative reinforcement, and punishment from the last cigarette and from the last drink. These explicit drug appraisals complement the analyses of mood and intoxication effects. This is important because ratings of particular states can be ambiguous with respect to their psychological significance. For instance, Shiffman & Kirchner (2009) questioned whether rush/buzz effects should be interpreted as indicators of positive reinforcement, noting head rush responses do not necessarily covary with ratings of pleasure or satisfaction in laboratory studies.
On the basis of existing laboratory and diary research, we expected that smoking and drinking behaviors would covary in momentary reports. Additionally, we hypothesized that co-use of alcohol and tobacco would be associated with a unique profile of subjective effects. Although existing diary data are somewhat inconsistent, we expected ratings of craving for alcohol and tobacco, head rush/buzz effects, positive affects, and ratings of drug pleasure to be especially responsive to co-use of alcohol and tobacco.
Method
Participants
Participants were current drinkers who reported consuming alcohol at least 4 times in the past 30 days; this criterion (i.e., approximately weekly drinking) was used to increase the likelihood that we would capture drinking experiences with electronic diaries (EDs) during a 3-week period. By design, we recruited a large number of current smokers (n=259, 64.1% of the sample) and recruited a smaller, non-equivalent comparison group of current drinkers who did not smoke (n=145; 35.9%) Because the current project examines the subjective effects of cigarette use, alcohol use, and their combination, we restricted analyses to the smokers. The selection criteria set a very low threshold for considering a participant to be a smoker (self-report of at least one cigarette per week) because subjective effects of co-use might vary as a function of smoking heaviness or experience and we sought to capture the full range of possible effects. Additional eligibility criteria were: (a) age 18 years or older, (b) ability to read and write English, (c) not currently trying to quit smoking or currently taking smoking cessation pharmacotherapy, (d) not planning to quit smoking during the next 30 days, (e) not regularly using non-cigarette tobacco products, (f) not interested in seeking treatment for drinking problems or an alcohol use disorder, (g) no report of unsuccessful attempts to cut down or quit drinking, (h) no history of arrests for alcohol-related offenses (excluding status offenses), and (i) if female, not currently pregnant or planning to become pregnant.
Participants were recruited via advertisements posted on public kiosks, published in a widely distributed commercial circular, and sent via mass email to employees and students at the University of Missouri. Participants could earn up to $150 by completing all study visits and returning the ED.
The analyzed sample included 121 women (46.7%) and was predominately White (84.6%). Participants ranged in age from 18 to 70, but young adults were overrepresented; the mean age was 25.1 (SD=8.5) and 73% of the analyzed sample were between the ages of 18 and 25. Seventy-four participants (28.6%) reported nondaily smoking. These individuals averaged 2.9 cigarettes per day (SD = 2.3, range = 1–12) on smoking days. A total of 184 smokers reported daily smoking and averaged 11.6 cigarettes per day (SD = 12.2, range = 1–80). Responses to the smoking history questionnaire were missing for one participant. Scores on the Fagerstrom Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) ranged from 0 to 8 with a mean of 2.2 (SD=2.2; n=249 due to missing data). The mean score on the Alcohol Use Disorders Identification Test (AUDIT; Babor, Higgins-Biddle, Saunders, & Monteiro, 2001) was 12.2 (SD = 5.7). Two participants were missing AUDIT data. In the remainder, 123 (47.4%) achieved scores (8–15) considered indicative of alcohol use exceeding low-risk guidelines, and another 73 (28.4%) achieved scores of 16 or more, a level indicating hazardous or harmful drinking (Babor, et al., 2001).
Procedure
Eligible individuals were scheduled in groups of 6 or fewer for a 2-hour orientation session during which informed consent was obtained and participants completed a battery of computerized questionnaires. Approximately 1–2 days later, participants were scheduled individually or in small groups (up to 10 participants) to complete a 45-minute ED training session. Participants were issued an ED and guided through a tutorial on the diary function and assessment protocol. After this session, participants carried the ED for 21 days. During the course of the study, participants returned to the lab for four drop-in sessions. At these sessions, staff uploaded data from the EDs to a central server, reviewed with participants interim summary reports generated from the ED data concerning compliance with the monitoring protocol, and answered any questions that participants had.
Electronic Diary Device and Protocol
EDs were implemented using palmtop computers (Palm m500, Palm Inc., Sunnyvale, CA) programmed with customized software designed by invivodata inc. (Pittsburgh, PA). The overall assessment scheme encompassed 5 types of ED entries: (1) time-based Random Prompts, (2) user-initiated Cigarette Reports in which participants logged instances of cigarette use, (3) user-initiated Drinking Reports, in which participants logged completion of the first drink in a drinking episode, (4) a series of automated, prompted Drinking Follow-Ups collected in the wake of the first drink log, and (5) Morning Reports made each day upon waking. The current analyses focus on data from 3 types of ED records (Random Prompts, Cigarette Reports, and Drinking Reports).
Random Prompts
The EDs were programmed to deliver audible signals up to 5 times per day, prompting participants to complete a diary entry. If participants were unable to complete a diary entry when the alarm sounded, they could use a delay function to reschedule the signal after a delay of as much as 20 minutes. Smokers used the delay after 7% of random prompts. Participants could also suspend delivery of audible prompts for up to 2 hours if they were entering a situation in which it could be unsafe or inappropriate to be prompted (e.g., driving, attending a religious service). Delivery of Random Prompts was pre-empted by scheduled assessments assessing the aftermath of drinking (described below).
Cigarette Reports
Participants were instructed to self-initiate a report after each cigarette they smoked. However, participants’ ability to initiate Cigarette Reports was preempted by scheduled assessments oversampling post-drinking moments (described below). Each calendar day was divided into four 6-hour blocks. The first cigarette logged in each block was followed by a detailed assessment similar to assessments delivered during Random Prompts. Subsequent cigarettes in each block of time were not followed by a series of questions. In these cases, once the cigarette was logged, the participant was simply thanked for making the recording and then the ED powered off. The goal was to obtain a representative sample of cigarette reports from smokers without overwhelming heavy smokers with recording burden. Overall, smokers logged a total of 16,670 smoking events via the Cigarette Report mechanism and 6,617 (39.7%) were followed by a full assessment. Shiffman (2009) reported that similar event-based reports of smoking corresponded with biochemical markers of smoking, indicating adequate compliance and validity.
Drinking Reports
Participants were instructed to initiate a report after finishing the first drink of a drinking episode. When these recordings were initiated, participants again completed questions concerning behaviors and subjective states that were similar to Random Prompt and Cigarette Reports. Smokers logged 1,423 first drinks during the study period. Twelve of these records (<1%) were abandoned before information concerning smoking behavior was recorded, leaving 1,411 reports for analysis.
Drinking Follow-Ups
The Drinking Follow-Up assessments are not included in the present analyses, but we describe them here to explain how they affect interpretation of the remaining data and to provide the rationale for their omission. Drinking Follow-Ups were designed to intensively oversample the post-drinking period. After a Drinking Report was made (or a drink was captured in another type of record), the ED delivered audible prompts at 30, 90, 150, and 210 minute latencies. The sequence of follow-ups was extended by one hour each time a new drink was reported in a follow-up. The sequence continued until 3 hours had elapsed without a drink report or until the participant indicated s/he was retiring for the evening. An important design feature was that initiation of the Drinking Follow-Up sequence pre-empted all other reporting modes. That is, no Random Prompts were delivered, and participants could not initiate a Cigarette Report or Drinking Report while the follow-up routine was running. Ongoing smoking and drinking behaviors were assessed in each Drinking Follow-Up using questions about the number of cigarettes and number of drinks since the last report. Thus, Cigarette Reports and Random Prompts were not sampled from periods of ongoing alcohol use. Moreover, effects of effects for alcohol use are limited to the effect of the first drink. The Drinking Follow-Up records contain information that is substantively interesting with respect to our core questions concerning subjective effects of alcohol-tobacco co-use and will be the focus of a separate report. However, because the follow-up sequence involved a unique sampling strategy (time-based vs. event-related) and strategy for assessing alcohol and tobacco use (quantity consumed since last report vs. log of single instance), a comprehensive analysis of extended drinking episodes requires will require separate treatment employing a different analytic strategy.
Compliance with Diary Recording
Random Prompts that were not answered within 3 minutes were logged as missed. The smokers completed 17,358 of 22,016 delivered prompts, representing a compliance rate of 78.8%. To generate an estimate of compliance with recording of cigarettes and drinking behaviors, we calculated an expected number of cigarettes and drinks for a 3-week period based on responses to a 30-day timeline follow-back measure (TLFB; Sobell & Sobell, 2000) completed by participants at baseline. We then compared these estimates to the total number of cigarettes and drinks captured across all diary record types during the study. The TLFB projections suggested we might expect smokers to consume 45,326 cigarettes during the course of the study. A total of 28,471 cigarettes were captured by the diary, representing 63% of the projection. In contrast, diary monitoring actually captured slightly more drinks (16,448) than projected based on the TLFB (15,813).
Measures
Alcohol and Cigarette Use
Recent drug use was indexed by explicit responses, not inferred from the record type. Questions specifically asking about recent tobacco and alcohol use were incorporated into each of the diary assessment types to ensure that moments were classified accurately1. Figure 1 summarizes how source records were reclassified for analysis based on responses to diary questions about smoking and drinking behaviors.
Figure 1.
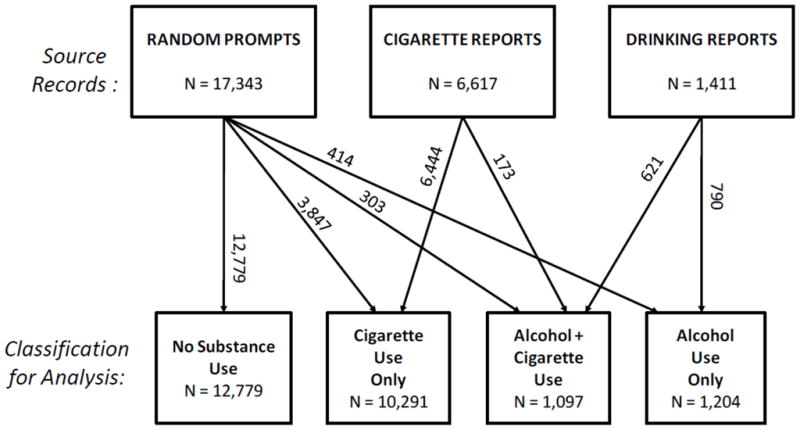
Summary of the origins and final substance use classifications of diary records included in the analyses.
Appraisals of Alcohol and Cigarette Effects
Each time a cigarette or alcoholic drink was reported, individual items, worded identically for cigarettes and drinks, served as probes for: (a) positive reinforcement (“Was the last [cigarette/drink] pleasurable?”), (b) negative reinforcement (“Did the last [cigarette/drink] relieve unpleasant feelings or symptoms?”), and (c) punishment (“Did the last [cigarette/drink] make you feel worse?”). These items were rated on 5 point scales (1 =” not at all” to 5 = “extremely”).
Current Subjective States
In each record, participants were asked to rate their experiences with 10 subjective states on a 5-point scale (1 = “not at all” to 5 = “extremely”). Each item stem asked participants to reflect on the past 15 minutes when making the ratings. Specific items tapped positive affects (enthusiastic, excited, happy), negative affects (distressed, sad), craving (crave a cigarette, crave a drink) and possible effects of alcohol intoxication and alcohol withdrawal/hangover (buzzed, dizzy, sluggish, headache, nauseous).
Occasion-Level Covariates
The ED automatically recorded a time and date stamp for each entry. Time of day was represented using 6 categories with 8pm to midnight (the period in which the most drinking was observed) as the reference category and the remaining categories representing exclusive 4-hour blocks. To be consistent with prior research (e.g., Piasecki, et al., 2008; Shiffman, et al., 1994), a record was counted as having been made on the weekend if the date/time stamp fell between 6:00 p.m. on Friday until 6:00 p.m. on Sunday.
In each diary record, participants responded to a question that asked “In the past 15 minutes, who have you been with? (check all that apply).” Following the presentation of this text, participants could check up to eight boxes (no one, partner/spouse, friend, co-worker, child, parent, other family, and other). For the current analyses, we classified responses as indicating the participant had been either alone (scored 0) or in presence of others (scored 1).
Participants were also asked “Where is your current location? (check all that apply).” Participants could check up to seven boxes (school, work, bar/restaurant, primary residence, outside, vehicle, and other). In the current analyses, separate binary variables indicated endorsement or non-endorsement of each category were constructed. These variables were entered as a set, with “work/school” serving as the reference category.
Participant-Level Covariates
At the orientation session, height and weight were measured for each participant using a physician’s scale. Body mass index (BMI) was calculated based upon the participant’s height and weight. Problem drinking was screened with the AUDIT (Babor, et al., 2001) and tobacco dependence was assessed using the FTND (Heatherton, et al., 1991). Participant sex and age were also recorded. In analyses, sex was coded so that male = 1 and female = 0. Age was represented using four categories comprising participants (a) younger than 21, (b) 21–30, (c) 30–40, and (d) older than 40. This categorical representation permits age effects to be nonlinear. Given the large number of young adults in the sample, we distinguished between participants older and younger than 21 because legal status with respect to purchasing alcohol could affect drinking behaviors and correlates.
Statistical Analysis
A generalized linear mixed modeling framework (Littell, Milliken, Stroup, Wolfinger & Schabenberger, 2006) was used to analyze diary data. These were 2-level models in which repeated assessments were nested within participants. The models assumed a normal link function when the dependent measure was continuous (subjective states, drug appraisals) and a logit link function when the outcome was binary (cigarette use, alcohol use). All models featured random intercepts, included random slopes for occasion-level cigarette and alcohol use variables, and assumed unstructured variance components in the random portion of the model.
First, for descriptive purposes, we tested bivariate momentary associations between drinking and smoking behaviors. One model treated alcohol use as the dependent measure and cigarette use as the predictor and another predicted cigarette use from alcohol reports. A second set of models expanded these bivariate analyses to determine whether momentary associations between drinking and smoking remained after accounting for the occasion- and person-level covariates. Continuously-scaled person-level covariates (BMI, AUDIT, FTND) were centered prior to model entry. These analyses also served to characterize the unique associations between the covariates and use of alcohol and tobacco, which is relevant for understanding the function of the covariates, which were included in all subsequent models. In a third set of models, dependent measures were the appraisals of alcohol and tobacco effects. Because these appraisals were only assessed in records reporting recent use of a given substance, these analyses were limited to records in which the appraised drug was used and the key predictor was the presence or absence of the other drug. For instance, ratings of how pleasurable the drink was were predicted in post-drinking reports from presence or absence of smoking. A supplementary set of analyses modeled appraisals of cigarettes and alcohol simultaneously as a function of (a) an indicator variable representing the substance being appraised, (b) an indicator variable representing co-use of alcohol and tobacco, and (c) the interaction of these two indicators. This permitted an evaluation of the relative magnitudes of motivational responses to alcohol and cigarettes and a determination of whether co-use more strongly affects responses to alcohol or tobacco. A final set of models evaluated whether momentary subjective states differed as a function of contemporaneous tobacco use, alcohol use, or their interaction.
Results
Momentary Associations between Alcohol and Tobacco Use
Momentary reports of tobacco use were significant in a model in which contemporaneous reports of alcohol use was the dependent measure, OR = 1.25, 95% CI = 1.06 – 1.46, p<.01. Similarly, alcohol use was significant when entered in a model with momentary tobacco use as the dependent measure, OR = 1.46, 95% CI = 1.25 – 1.72, p <.001. Both associations remained significant after covariate adjustments (Table 1). Additional predictors of alcohol use included time of day, weekend, presence of others, location, AUDIT scores, and age. Tobacco use was related to time of day, weekend, presence of others, location, and FTND scores. The significant effects involving occasion- and person-level covariates suggest apparent subjective effects of co-use could actually reflect contextual or individual differences rather than pharmacologic influences. This provides a rationale for covariate adjustment in subsequent analyses.
Table 1.
Results from multivariate models predicting momentary alcohol use or cigarette use from the other drug and occasion- and person-level covariates.
| Predictor | Dependent Measure |
|||||
|---|---|---|---|---|---|---|
| Alcohol Use |
Cigarette Use |
|||||
| OR | 95% CI | p | OR | 95% CI | p | |
| Cigarette Use | 1.22 | 1.04, 1.42 | .015 | -- | -- | -- |
| Alcohol Use | -- | -- | -- | 1.40 | 1.18, 1.67 | <.001 |
| Occasion-Level Covariates | ||||||
| Time of Day | ||||||
| Midnight–4 a.m. | 1.49 | 1.19, 1.86 | <.001 | 11.70 | 9.15, 14.96 | <.001 |
| 4 a.m. – 8 a.m. | 0.11 | 0.06, 0.20 | <.001 | 7.95 | 5.80, 10.91 | <.001 |
| 8-a.m. – 12 p.m. | 0.05 | 0.03, 0.07 | <.001 | 1.29 | 1.16, 1.44 | <.001 |
| 12.p. – 4 p.m. | 0.09 | 0.08, 0.11 | <.001 | 1.15 | 1.06, 1.26 | .001 |
| 4 p.m. – 8 p.m. | 0.32 | 0.29, 0.36 | <.001 | 1.13 | 1.04, 1.23 | .003 |
| 8 p.m–Midnight (Reference) | 1.00 | -- | -- | 1.00 | -- | -- |
| Weekend | 1.95 | 1.75, 2.17 | <.001 | 0.83 | 0.78, 0.89 | <.001 |
| Others Present | 2.23 | 1.91, 2.60 | <.001 | 1.20 | 1.11, 1.29 | <.001 |
| Location | ||||||
| Bar/Restaurant | 11.38 | 9.30, 13.92 | <.001 | 1.14 | 0.97, 1.34 | .113 |
| Home | 1.51 | 1.29, 1.77 | <.001 | 1.37 | 1.25, 1.49 | <.001 |
| Outside | 1.48 | 1.26, 1.75 | <.001 | 4.06 | 3.67, 4.49 | <.001 |
| Vehicle | 0.60 | 0.47, 0.76 | <.001 | 3.25 | 2.89, 3.64 | <.001 |
| Other Location | 2.76 | 2.29, 3.31 | <.001 | 1.20 | 1.07, 1.35 | .002 |
| Work/School (Reference) | 1.00 | -- | -- | 1.00 | -- | -- |
| Person-Level Covariates | ||||||
| Male | 1.07 | 0.86, 1.34 | .547 | 1.08 | 0.84, 1.39 | .536 |
| Body Mass Indexa | 1.01 | 0.99, 1.03 | .325 | 0.99 | 0.98, 1.02 | .808 |
| FTNDa | 0.96 | 0.91, 1.02 | .163 | 1.41 | 1.33, 1.50 | <.001 |
| AUDITa | 1.05 | 1.03, 1.07 | <.001 | 1.01 | 0.98, 1.03 | .675 |
| Age | ||||||
| 18–20 years | 0.29 | 0.19, 0.44 | <.001 | 0.89 | 0.55, 1.43 | .624 |
| 21–30 years | 0.49 | 0.33, 0.73 | <.001 | 1.26 | 0.81, 1.96 | .312 |
| 31–40 years | 0.56 | 0.33, 0.94 | .028 | 1.42 | 0.80, 2.51 | .231 |
| 41+ years (Reference) | 1.00 | -- | -- | 1.00 | -- | -- |
Note: OR = Odds ratio, CI = Confidence Interval, FTND = Fagerstrom Test for Nicotine Dependence, AUDIT= Alcohol Use Disorder Identification Test.
Predictor centered prior to model entry.
Descriptive Findings for Subjective States
Table 2 presents the means and standard deviations for each of the ED-measured subjective states as a function of reported momentary drug use. The table also documents the frequency with which each experience was denied (i.e., the “not at all” scale value of 1 was reported) across all records in which the item was administered. This column reveals that some states (e.g., pleasure from drinking or smoking, positive affects, cigarette craving) were very commonly experienced while other states (e.g., buzz, dizziness, nausea, punishment from smoking or drinking) were frequently absent.
Table 2.
Descriptive statistics for ED-measured subjective states.
| Variable | Momentary Drug Use |
Frequency of “Not at All” |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No Drug (N=12,779) |
Cigarette Only (N=10,291) |
Alcohol Only (N=1,204) |
Alcohol + Cigarette (N=1,097) |
|||||||
| M | (SD) | M | (SD) | M | (SD) | M | (SD) | N | (%) | |
| Drug Appraisals | ||||||||||
| Cigarette Pleasurable | -- | -- | 3.48 | (1.01) | -- | -- | 3.72 | (1.00) | 443 | (3.8) |
| Cigarette Relieved | -- | -- | 2.08 | (1.19) | -- | -- | 2.15 | (1.27) | 5,121 | (45.0) |
| Cigarette Felt Worse | -- | -- | 1.31 | (0.69) | -- | -- | 1.25 | (0.65) | 9,088 | (79.8) |
| Drink Pleasurable | -- | -- | -- | -- | 4.09 | (0.90) | 4.11 | (0.86) | 7 | (0.5) |
| Drink Relieved | -- | -- | -- | -- | 2.29 | (1.35) | 2.45 | (1.34) | 551 | (39.1) |
| Drink Felt Worse | -- | -- | -- | -- | 1.12 | (0.44) | 1.15 | (0.49) | 1,273 | (90.2) |
| Mood | ||||||||||
| Enthusiastic | 2.64 | (1.16) | 2.53 | (1.17) | 3.13 | (1.20) | 3.00 | (1.18) | 5,798 | (22.9) |
| Excited | 2.60 | (1.20) | 2.54 | (1.21) | 3.12 | (1.13) | 3.02 | (1.23) | 6,116 | (24.1) |
| Happy | 3.14 | (1.11) | 3.13 | (1.09) | 3.59 | (1.10) | 3.54 | (1.12) | 2,508 | (9.9) |
| Distressed | 1.89 | (1.14) | 1.83 | (1.14) | 1.66 | (1.05) | 1.69 | (1.07) | 14,025 | (55.3) |
| Sad | 1.50 | (0.94) | 1.47 | (0.93) | 1.40 | (0.89) | 1.47 | (0.96) | 18,559 | (73.2) |
| Craving | ||||||||||
| Crave a Cigarette | 2.16 | (1.32) | 2.86 | (1.28) | 2.60 | (1.39) | 3.10 | (1.28) | 9,076 | (35.8) |
| Crave a Drink | 1.57 | (1.08) | 1.57 | (1.06) | 2.93 | (1.43) | 2.88 | (1.41) | 17,411 | (68.6) |
| Intoxication, Withdrawal/Hangover | ||||||||||
| Sluggish | 1.84 | (1.17) | 1.73 | (1.06) | 1.57 | (0.98) | 1.59 | (0.98) | 15,068 | (59.4) |
| Buzzed | 1.20 | (0.63) | 1.30 | (0.77) | 1.76 | (1.08) | 1.95 | (1.18) | 21,149 | (83.4) |
| Dizzy | 1.15 | (0.52) | 1.15 | (0.52) | 1.18 | (0.56) | 1.28 | (0.71) | 22,912 | (90.3) |
| Headache | 1.33 | (0.80) | 1.30 | (0.74) | 1.25 | (0.74) | 1.27 | (0.70) | 20,955 | (82.6) |
| Nauseous | 1.17 | (0.59) | 1.16 | (0.54) | 1.13 | (0.51) | 1.16 | (0.56) | 22,904 | (90.3) |
Note: All diary variables were rated on a scale ranging from 1 (“not at all”) to 5 (“extremely”).
Appraisals of Cigarette and Alcohol Effects
Table 3 summarizes results of multilevel models predicting explicit appraisals of the last cigarette and the last drink. For purposes of brevity, in discussing these results and those contained in subsequent tables, we focus on the key effects of interest listed under “Drug Effects.” Findings for occasion- and person-level covariates are also tabled for each model for completeness. Consumption of alcohol significantly increased pleasure from smoking, was associated with a small reduction in punishment from smoking, and had no effect on self-reported negative reinforcement. Cigarette use was associated with modestly higher pleasure from drinking, but was not related to reports of drinking-contingent negative reinforcement or acute punishment from drinking.
Table 3.
Coefficients from multivariate models predicting appraisals of the last cigarette and the last drink from momentary alcohol or cigarette use and occasion- and person-level covariates.
| Predictor | Dependent Measure |
|||||
|---|---|---|---|---|---|---|
| Cigarette Pleasurable | Cigarette Relieved | Cigarette Felt Worse | Drink Pleasurable | Drink Relieved | Drink Felt Worse | |
| Intercepta | 3.57 | 2.49 | 1.37 | 3.87 | 2.83 | 1.37 |
| Drug Effects | ||||||
| Alcohol Use | 0.21*** | 0.04 | −0.09*** | -- | -- | -- |
| Cigarette Use | -- | -- | -- | 0.09* | 0.08 | 0.001 |
| Occasion-Level Covariates | ||||||
| Time of Day | ||||||
| Midnight–4 a.m. | 0.01 | −0.01 | −0.01 | −0.04 | −0.03 | 0.02 |
| 4 a.m. – 8 a.m. | −0.06 | −0.02 | 0.04 | −0.35 | 0.22 | 0.02 |
| 8 a.m. – Noon | −0.05 | −0.03 | −0.01 | −0.33 | 0.01 | 0.16 |
| Noon – 4 p.m. | −0.05* | −0.03 | −0.02 | −0.03 | −0.01 | −0.05 |
| 4 p.m. – 8 p.m. | −0.02 | −0.01 | −0.01 | 0.08 | −0.04 | −0.10* |
| 8 p.m–Midnight (Reference) | -- | -- | -- | -- | -- | -- |
| Weekend | 0.01 | −0.05** | −0.0002 | −0.06 | −0.11* | −0.004 |
| Others Present | 0.004 | −0.02 | −0.01 | 0.07 | −0.11 | −0.06 |
| Location | ||||||
| Bar/Restaurant | 0.04 | 0.08 | 0.01 | 0.24** | 0.05 | −0.03 |
| Home | −0.09*** | −0.04 | 0.04* | 0.06 | −0.05 | −0.07 |
| Outside | 0.03 | 0.01 | 0.01 | 0.02 | −0.14 | −0.01 |
| Vehicle | −0.02 | −0.002 | 0.04* | 0.12 | 0.09 | 0.02 |
| Other Location | 0.02 | −0.06 | 0.02 | 0.06 | −0.08 | 0.03 |
| Work/School (Reference) | -- | -- | -- | -- | -- | -- |
| Person-Level Covariates | ||||||
| Male | −0.11 | −0.24* | −0.03 | −0.07 | −0.24 | −0.01 |
| Body Mass Indexb | 0.01 | −0.01 | −0.01* | −0.002 | −0.03 | 0.002 |
| FTNDb | 0.001 | −0.01 | −0.02 | −0.02 | 0.03 | 0.01 |
| AUDITb | 0.004 | 0.03* | 0.01 | 0.01 | 0.05*** | 0.003 |
| Age | ||||||
| 18–20 years | 0.19 | −0.01 | −0.04 | 0.15 | −0.14 | −0.14* |
| 21–30 years | 0.05 | −0.26 | −0.03 | 0.04 | −0.22 | −0.15* |
| 31–40 years | −0.17 | −0.11 | 0.06 | 0.003 | −0.06 | −0.13 |
| 41+ years (Reference) | -- | -- | -- | -- | -- | -- |
p<.05,
p<.01,
p<.001.
Significance of intercept term not reported because test evaluates difference from zero, but subjective states were rated on 1–5 scale (i.e., no zero point).
Predictor centered prior to model entry. FTND = Fagerstrom Test for Nicotine Dependence, AUDIT= Alcohol Use Disorder Identification Test.
When drug pleasure ratings were modeled for both substances simultaneously, co-use was associated with significantly increased pleasure (b = .21, p < .001), there was a significant main effect for the substance being rated, indicating that the first drinks were rated as more pleasurable than cigarettes (b = .51, p <.001) and there was a significant interaction between co-use and the substance rated, indicating that co-use had a smaller influence when pleasure from drinking was being rated (b = −.15, p< .01). For negative reinforcement ratings, there was no main effect for co-use (b = .04, p = .30), but there was a main effect for substance rated, indicating that first drinks were appraised as more negatively reinforcing than cigarettes (b = .21, p<.001). The interaction term was marginally significant, suggesting that co-use had a modestly larger effect on ratings of negative reinforcement from drinking compared to cigarettes (b = .11, p =.053). The joint model of punishment ratings revealed that co-use was associated with decreased punishment (b = −.09, p < .001), and a main effect indicating that alcohol was rated as less punishing than cigarettes (b = −.24, p<.001). The interaction term was also significant and indicated that co-use tended to increase punishment ratings when alcohol was the appraised substance (b = .18, p <.001).
Mood States
Consumption of the first drink in an episode was associated with higher positive affects (enthusiasm, excitement, happiness) and decreased negative affects (distress, sadness; Table 4). Cigarette consumption was associated with small elevations in two positive affects (excited and happy). A modest Alcohol x Cigarette interaction was found for sadness, suggesting alcohol use was associated with a modest decrease in sadness, but only in the absence of smoking.
Table 4.
Coefficients from multivariate models predicting mood states from momentary alcohol use, cigarette use, their interaction, and occasion- and person-level covariates.
| Predictor | Dependent Measure |
||||
|---|---|---|---|---|---|
| Enthusiastic | Excited | Happy | Distressed | Sad | |
| Intercepta | 2.16 | 2.00 | 2.64 | 2.37 | 2.01 |
| Drug Effects | |||||
| Alcohol Use | 0.41*** | 0.43*** | 0.37*** | −0.21*** | −0.08** |
| Cigarette Use | 0.01 | 0.06*** | 0.07*** | 0.00 | −0.02 |
| Alcohol x Cigarette | −0.06 | −0.07 | −0.04 | 0.08 | 0.07* |
| Occasion-Level Covariates | |||||
| Time of Day | |||||
| Midnight–4 a.m. | −0.09** | −0.07 | −0.04 | 0.03 | 0.03 |
| 4 a.m. – 8 a.m. | −0.20*** | −0.10** | −0.21*** | −0.02 | −0.04 |
| 8 a.m. – Noon | −0.06** | −0.20*** | −0.06** | 0.02 | −0.02 |
| Noon– 4 p.m. | −0.01 | −0.09*** | −0.03* | 0.04* | 0.00 |
| 4 p.m. – 8 p.m. | 0.02 | −0.01* | −0.01 | 0.03 | 0.01 |
| 8 p.m–Midnight (Reference) | -- | -- | -- | -- | |
| Weekend | 0.07*** | 0.11*** | 0.09*** | −0.11*** | −0.03** |
| Others Present | 0.16*** | 0.21*** | 0.21*** | −0.09*** | −0.10*** |
| Location | |||||
| Bar/Restaurant | 0.23*** | 0.22*** | 0.20*** | −0.07* | −0.04 |
| Home | −0.04** | −0.06*** | −0.03 | −0.04* | 0.05*** |
| Outside | 0.12*** | 0.10*** | 0.10*** | −0.05** | −0.01 |
| Vehicle | 0.13*** | 0.18*** | 0.14*** | 0.01 | 0.05** |
| Other Location | 0.14*** | 0.14*** | 0.14*** | −0.09*** | −0.001 |
| Work/School (Reference) | -- | -- | -- | -- | -- |
| Person-Level Covariates | |||||
| Male | 0.31** | 0.32*** | 0.10 | −0.32*** | −0.17* |
| Body Mass Indexb | 0.01 | 0.004 | 0.00 | −0.01 | −0.01 |
| FTNDb | −0.05* | −0.05* | −0.03 | −0.01 | 0.00 |
| AUDITb | −0.01 | −0.01 | −0.01 | 0.02* | 0.01 |
| Age | |||||
| 18–20 years | 0.30 | 0.49** | 0.43* | −0.26 | −0.38** |
| 21–30 years | 0.08 | 0.17 | 0.18 | −0.22 | −0.39** |
| 31–40 years | −0.23 | −0.18 | −0.06 | −0.22 | −0.52** |
| 41+ years (Reference) | -- | -- | -- | -- | -- |
p<.05,
p<.01,
p<.001.
Significance of intercept term not reported because test evaluates difference from zero, but subjective states were rated on 1–5 scale (i.e., no zero point).
Predictor centered prior to model entry. FTND = Fagerstrom Test for Nicotine Dependence, AUDIT= Alcohol Use Disorder Identification Test.
Craving
Both alcohol use and cigarette use were associated with increased craving for cigarettes (Table 5). There was also an Alcohol x Cigarette interaction. Cigarette craving was predicted to be highest during co-use moments, but the level of cigarette craving was lower than would be expected based on summing the main effects. Craving a drink was strongly elevated after consumption of the first drink. Cigarette use was also associated with increased craving to drink, although this effect was small in comparison to the effect of alcohol. No Alcohol x Cigarette effect was found for predicting craving a drink.
Table 5.
Coefficients from multivariate models predicting craving measures from momentary alcohol use, cigarette use, their interaction, and occasion- and person-level covariates.
| Predictor | Dependent Measure |
|
|---|---|---|
| Crave a Cigarette | Crave a Drink | |
| Intercepta | 2.48 | 1.45 |
| Drug Effects | ||
| Alcohol Use | 0.38*** | 1.06*** |
| Cigarette Use | 0.48*** | 0.06*** |
| Alcohol x Cigarette | −0.14** | −0.01 |
| Occasion-Level Covariates | ||
| Time of Day | ||
| Midnight–4 a.m. | 0.06 | −0.12*** |
| 4 a.m. – 8 a.m. | −0.06 | −0.47*** |
| 8 a.m. – Noon | −0.14*** | −0.42*** |
| Noon– 4 p.m. | −0.10*** | −0.28*** |
| 4 p.m. – 8 p.m. | −0.04* | −0.04* |
| 8 p.m–Midnight (Reference) | -- | -- |
| Weekend | 0.00 | 0.12*** |
| Others Present | 0.03 | 0.07*** |
| Location | ||
| Bar/Restaurant | 0.08* | 0.21*** |
| Home | −0.15*** | −0.06*** |
| Outside | 0.08*** | 0.05** |
| Vehicle | 0.01 | 0.07** |
| Other Location | −0.03 | 0.04* |
| Work/School (Reference) | -- | -- |
| Person-Level Covariates | ||
| Male | −0.15 | 0.16 |
| Body Mass Indexb | 0.02 | 0.01 |
| FTNDb | 0.12*** | 0.02 |
| AUDITb | 0.02** | 0.03** |
| Age | ||
| 18–20 years | 0.15 | 0.32* |
| 21–30 years | −0.03 | 0.09 |
| 31–40 years | −0.09 | 0.06 |
| 41+ years (Reference) | -- | -- |
p<.05,
p<.01,
p<.001.
Significance of intercept term not reported because test evaluates difference from zero, but subjective states were rated on 1–5 scale (i.e., no zero point).
Predictor centered prior to model entry. FTND = Fagerstrom Test for Nicotine Dependence, AUDIT= Alcohol Use Disorder Identification Test.
Intoxication and Withdrawal/Hangover Symptoms
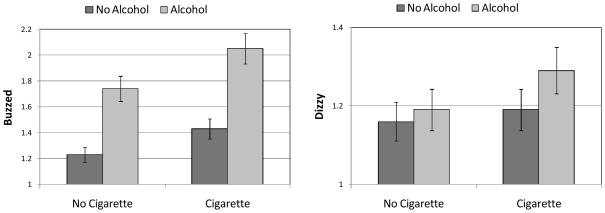
Consumption of the first drink was associated with lower sluggishness, headache, and nausea and increased buzz (Table 6). Smoking moments were associated with decreases in sluggishness and increases in buzz and dizziness. Four Alcohol x Cigarette interactions were significant. The effect for sluggishness indicated that the modest negative effect of smoking alone was absent when both tobacco and alcohol were used. The tendency for nausea to be modestly lower after alcohol was eliminated by co-use of tobacco. The effects for feeling buzzed and dizzy were synergistic, i.e., the effects of tobacco and alcohol together were greater than would be expected on the basis of the sum of the main effects (Figure 2).
Table 6.
Coefficients from multivariate models predicting intoxication and withdrawal/hangover symptoms from momentary alcohol use, cigarette use, their interaction, and occasion- and person-level covariates.
| Predictor | Dependent Measure |
||||
|---|---|---|---|---|---|
| Sluggish | Buzzed | Dizzy | Headache | Nauseous | |
| Intercepta | 1.79 | 1.07 | 1.08 | 1.44 | 1.10 |
| Drug Effects | |||||
| Alcohol Use | −0.24*** | 0.51*** | 0.03 | −0.08*** | −0.05* |
| Cigarette Use | −0.05** | 0.20*** | 0.03** | −0.01 | 0.00 |
| Alcohol x Cigarette | 0.09* | 0.11*** | 0.07*** | 0.05 | 0.05* |
| Occasion-Level Covariates | |||||
| Time of Day | |||||
| Midnight-4 a.m. | 0.14*** | 0.17*** | 0.01 | −0.01 | 0.02 |
| 4 a.m. – 8 a.m. | 0.32*** | −0.04 | 0.07** | 0.06 | −0.00 |
| 8 a.m. – Noon | 0.16*** | −0.06*** | 0.01 | −0.00 | 0.02 |
| Noon – 4 p.m. | 0.08*** | −0.06*** | 0.01 | 0.02 | 0.01 |
| 4 p.m. – 8 p.m. | 0.02 | −0.04*** | 0.00 | 0.01 | −0.01 |
| 8 p.m – Midnight (Reference) | -- | -- | -- | -- | -- |
| Weekend | 0.02 | 0.02** | 0.03*** | 0.03*** | 0.02*** |
| Others Present | −0.12*** | 0.01 | −0.01 | −0.01 | −0.00 |
| Location | |||||
| Bar/Restaurant | −0.06 | 0.04* | 0.04* | −0.02 | 0.05** |
| Home | 0.18*** | −0.01 | 0.02* | 0.02 | 0.02* |
| Outside | −0.10*** | 0.01 | 0.02* | −0.04** | 0.01 |
| Vehicle | 0.03 | 0.04** | 0.01 | 0.04** | 0.05*** |
| Other Location | −0.02 | 0.02 | 0.02 | 0.01 | 0.01 |
| Work/School (Reference) | -- | -- | -- | -- | -- |
| Person-Level Covariates | |||||
| Male | −0.17* | 0.14** | 0.02 | −0.15** | −0.01 |
| Body Mass Indexb | −0.00 | −0.00 | −0.00 | 0.00 | −0.00 |
| FTNDb | −0.01 | 0.01 | −0.01 | −0.00 | −0.01 |
| AUDITb | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| Age | |||||
| 18–20 years | 0.14 | 0.17 | 0.07 | −0.03 | 0.12 |
| 21–30 years | 0.09 | 0.07 | 0.01 | −0.07 | 0.03 |
| 31–40 years | −0.17 | 0.05 | 0.02 | −0.02 | −0.03 |
| 41+ years (Reference) | -- | -- | -- | -- | -- |
p<.05,
p<.01,
p<.001.
Significance of intercept term not reported because test evaluates difference from zero, but subjective states were rated on 1–5 scale (i.e., no zero point).
Predictor centered prior to model entry. FTND = Fagerstrom Test for Nicotine Dependence, AUDIT= Alcohol Use Disorder Identification Test.
Figure 2.
Marginal means and associated 95% confidence intervals depicting Alcohol x Cigarette interactions for momentary ratings of “buzzed” and “dizzy.” Note that the scales of the y-axes vary across the two panels to more clearly illustrate effects of different sizes. The interval between horizontal gridlines is the same in both panels to preserve a sense of scale.
Probing the Psychological Impact of Buzzed and Dizzy
“Buzzed” and “Dizzy” were the only two subjective states to show synergistic effects of co-use. Across all records, dizzy was significantly related to contemporaneous reports of buzzed (b = .51, p <.001). We performed supplementary analyses to investigate whether these effects should be interpreted as manifestations of drug reward. First, we expanded the model predicting cigarette pleasure (Table 3) to include momentary reports of buzz and dizziness as episode-level covariates. Current buzz was positively associated with pleasure from smoking (b = .10, p<.001), current dizziness was negatively related to smoking pleasure (b = −.11, p<.001), and alcohol use continued to be a significant predictor of pleasure from smoking when these two subjective states were covaried (b = .16, p < .001). We also performed a similar analysis predicting reports of pleasure from the last drink. Buzz was related to drinking pleasure (b = .12, p <.001), dizziness was unrelated to drinking pleasure (b = −.06, p = .17), and the effect of cigarette use on drinking pleasure was no longer significant (b = .08, p = .11).
Discussion
Alcohol consumption and cigarette smoking were associated with one another at the momentary level in this sample of smokers who reported frequent alcohol use. We investigated the subjective consequences of co-use because such effects may help to explain this tendency for both drugs to be co-administered. Notably, we gathered data from smokers who ranged widely in smoking habits, including many young, infrequent, and nondependent smokers. Thus, compared to prior research using clinical samples, the current study may better represent effects that are influential among less experienced tobacco users just beginning to associate these behaviors.
Responses to questions designed to serve as proxies for discrete motivational processes clearly highlighted the importance of positive reinforcement or reward in co-use. Alcohol use increased appraisals of pleasure from smoking and tobacco use increased reported pleasure from drinking. These effects were small in absolute terms. For example, the effect of alcohol on cigarette pleasure was estimated to be 0.21 points, or about 5% of the possible 1–5 scale range. It is difficult to evaluate the behavioral significance of the findings. It is possible that even small increases in hedonic reward, when experienced on a frequent basis, might have large cumulative effect on smoking or drinking behavior. Notably, there were significant random effects associated with co-use in all of the analyses of cigarette appraisals and the analysis of punishment from drinking (not tabled). Thus, although the overall estimates for co-use effects were small, there was also substantial between-person variation in the size of many responses. Accounting for these individual differences should be a focus of future investigations. It is also important to emphasize that additional analyses, beyond the scope of this paper, will be needed to assess the magnitude of effects during extended drinking bouts involving more alcohol and frank intoxication.
Joint analysis of drug appraisals revealed that, compared to cigarettes, first drinks had more potent, subjectively positive consequences – drinks were rated as more pleasurable, more negatively reinforcing, and less punishing. Co-use, however, more strongly modulated responses to tobacco. Alcohol had a significantly larger impact on cigarette pleasure than smoking had on appraisals of pleasure from the first drink. Similarly, co-use was more strongly associated with ratings of decreased punishment from smoking than from drinking. These effects may suggest that the robust covariation of alcohol and tobacco use at the occasion level is more likely to be driven by the reinforcement-enhancing effect of alcohol than by tobacco consumption’s effect on alcohol reinforcement. Smoking behavior is more contingent on alcohol use among light smokers and tobacco chippers compared to established smokers (Shiffman & Paty, 2006). If early tobacco use experiences are frequently accompanied by alcohol, then alcohol-augmented smoking reinforcement during this formative period could facilitate or accelerate the development of tobacco dependence.
Negative reinforcement accounts have been offered as prominent explanations for both tobacco dependence (Kassel, Stroud, & Paronis, 2003) and alcohol use disorders (e.g., Greely & Oei, 1999; Sher & Grekin, 2007). Most existing EMA research attempts to infer negative reinforcement from elevations in negative affect preceding substance use. Real-time ecological assessments gathered from periods of ongoing use frequently fail to identify negative moods as antecedents of tobacco use (e.g., Carter, et al., 2008; Cronk & Piasecki, 2010; Shiffman, et al, 2002; Shiffman, Paty, Gwaltney & Dang, 2004). The evidence linking diary-measured negative mood to later drinking is complex and somewhat inconsistent (e.g., Armeli, Conner, Cullum, & Tennen, 2010; Hussong, 2007; Hussong, Hicks, Levy, & Curran, 2001). In the current study, we used a different strategy, asking users to appraise the extent to which the drug they just consumed alleviated unpleasant feelings or symptoms. It was noteworthy that mean ratings for negative reinforcement from alcohol and tobacco were intermediate between pleasure (rated more strongly and nearly always present to some extent) and punishment (absent in 80–90% of use occasions; Table 2). Thus, endorsement of negative reinforcement is more common (and thus potentially more important) than drug-contingent punishment during ad lib use. However, the current findings suggest that negative reinforcement processes may not be important determinants of alcohol-tobacco co-use.
Only two subjective states – buzzed and dizzy – were found to be affected synergistically by co-use of alcohol and tobacco. Piasecki, et al. (2008) also found that reports of buzz from the last cigarette were more likely when alcohol was consumed. A small body of evidence examining retrospective reports of subjective reactions to cigarettes consumed at the outset of the smoking career suggests “buzz” and “dizzy” responses are unique predictors of progression to regular smoking (DiFranza, et al., 2004;O.F. Pomerleau, Pomerleau & Namenek, 1998; C.S. Pomerleau, Pomerleau, Namenek & Marks, 1999), leading to the suggestion that these adjectives capture rewarding effects of nicotine intoxication. Additionally, smokers who report buzz and dizziness responses to the first cigarette report qualitatively similar reactions to the first lifetime drink (C.S. Pomerleau, Marks, Pomerleau, & Snedecor, 2004).
Because the psychological impact of ecologically-assessed head rush responses such as buzz and dizziness is unclear, we explicitly tested whether these experiences were related to concurrent reports of drug pleasure. Results suggested that buzz covaries with reports of both alcohol and cigarette pleasure, and thus seem to index a rewarding drug effect, consistent with prior suggestions (e.g., DiFranza, et al., 2004;O.F. Pomerleau, et al, 1998;C.S. Pomerleau, et al., 1999). Buzz and dizziness were related to one another; the unique variance in dizziness was negatively related to cigarette pleasure and unrelated to alcohol-contingent pleasure. Including ratings of these states as occasion-level covariates did not eliminate the association between alcohol use and cigarette pleasure. This suggests perceived buzz is, at best, a fallible indicator of alcohol-induced smoking reward. In contrast, the modest effect of smoking on pleasure from drinking was no longer significant when buzz and dizziness were covaried. It is important to note that sensations of buzz and dizziness were absent most of the time (Table 2). The effects of co-use were also modest in magnitude. In sum, the current findings suggested that head rush effects (a) were synergistically affected by co-use, (b) were uncommon, and (c) showed discrepant relations with explicit ratings of drug pleasure when entered simultaneously. The hedonic experience of these effects and their relations to drug self-administration deserves further research. It is possible that states like buzz and dizziness have little direct behavioral influence, but are instead indirect markers of drug sensitivity or central drug action.
Craving for both alcohol and tobacco was significantly elevated after use compared to other moments. It is likely that even higher craving states preceded use and that self-administration acutely decreased craving, at least in the case of tobacco use. It is important to note, however, that we did not collect assessments immediately prior to use of either substance, so we cannot rule out the possibility that drug use acutely increased craving via priming (e.g., N.L. Cooney, et al., 2007; de Wit, 1996). A significant Alcohol x Cigarette interaction was observed in predicting cigarette craving. This effect was subadditive, but the raw and covariate-adjusted means still suggested the highest levels of cigarette craving occurred in the context of co-use. This finding of acute increases in tobacco craving under the influence of alcohol is congruent with evidence from prior diary (Piasecki, et al., 2008) and laboratory studies (e.g., Burton & Tiffany, 1997; King & Epstein, 2005, Sayette, et al., 2005). Several EMA investigations point to craving as the most robust antecedent of smoking behavior (e.g., Cronk & Piasecki, 2010; Piasecki, et al., 2007; Shiffman, et al., 2002; Shiffman et al., 2004). Indirectly, this suggests enhancement of tobacco craving by alcohol may be one mechanism driving co-use. Cravings have been construed as affects with varying valence (Baker, Morse, & Sherman, 1987). Craving experiences tend to be hedonically positive in the context of ongoing tobacco use (e.g., Sayette & Hufford, 1995; Zinser, Baker, Sherman & Cannon, 1992) and after alcohol administration (Sayette, et al., 2005). Moreover, cigarettes smoked under conditions of high craving are rated as more satisfying (Shiffman & Kirchner, 2009). These phenomena may explain why co-use is accompanied by reports of both enhanced drug pleasure and elevated craving.
Although not the main focus of this report, we note a number of significant effects were found for covariates in models predicting drug use behaviors and subjective states. At the person level, higher tobacco dependence predicted cigarette use and cigarette craving, but not appraisals of cigarette effects. Problem drinking (as indexed by the AUDIT) predicted alcohol use, craving for alcohol and tobacco, higher momentary distress, and reports of negative reinforcement from drinking and smoking. These findings are potentially congruent with the assertion that coping motives are especially important in problematic drinking (e.g., Cooper, Frone, Russell & Mudar, 1995). Relative to women, men reported more positive moods and buzz from drinking, lower negative moods and negative alcohol effects, and less relief from smoking. As would be expected, alcohol and tobacco use varied across time and locations. Mood states also varied with time and occasion-level measures, confirming the importance of adjusting for these variables when attempting to gauge the unique effects of co-use on such states.
Several limitations of this project must be acknowledged. To increase the likelihood of observing episodes of alcohol consumption, we recruited a sample who reported drinking on an approximately weekly basis. Effects of alcohol, tobacco, or their combination might differ in persons with less cumulative drinking experience or differing drinking patterns. Young adults were overrepresented in our sample. As noted earlier, however, this is valuable because existing EMA evidence is largely limited to data generated by older, chronic smokers. We attempted to assess reactions to a single drink, but there was almost certainly unmeasured variation in the amount of alcohol contained in these drinks. In some cases, alcohol use was captured in Random Prompts or Cigarette Reports. This could indicate noncompliance with the recording instructions. It seems more likely that these records caught alcohol use in progress before the completion of the first drink. More generally, the ethanol content of a “drink” can be quite variable, particularly when drinks are freely poured (e.g., Lemmens, 1994; Wansink & van Ittersum, 2005; White, Kraus, McCracken, & Swartzwelder, 2003). Owing to concerns about potential participant burden in a protocol involving frequent recording, the diary assessment was brief and covered a limited number of contextual features and subjective states. More comprehensive measurement of drug effects or covariates might reveal different results. We collected repeated measures during the drinking episode, but the current analyses were limited to the effects of the first drink. Future examinations of the intra-episode course of drug effects will be necessary. It is very plausible that as alcohol consumption increases to intoxicating levels, the nature of the relation between smoking and drinking could change. Indeed, it is very possible that for heavy drinking, infrequent smokers, the first cigarette in a drinking episode may not occur until late in the drinking bout. All our findings regarding drug effects were based on self-reports; some important reinforcement processes may not be accessible to introspection and self-report. Similarly, our single-item proxies for motivational effects of drug may not completely represent the targeted phenomena. For example, self-reported pleasure might reflect only one facet of a broader drug reward construct. We jointly modeled appraisals of cigarettes and alcohol. In these analyses, significant interactions between the substance being rated and an indicator of co-use could reflect psychometric differences in appraisals of alcohol vs. cigarettes rather than true cross-substance differences in the magnitude of co-use effects (e.g., Chapman & Chapman, 1978).
Noncompliance with diary recording might adversely affect the integrity of our conclusions. The response rate to signaled prompts (78%) was adequate, but lower than reported in some other investigations (e.g., Shiffman, et al. 2002 reported 91% compliance). The complexity of our diary protocol may have increased assessment burden. Another possibility is that the compliance rate reflects the relative youth and immaturity of our sample. In this regard, it is notable that we obtained a similar compliance rate with random prompts (81%) in another recent EMA study of involving a nonclinical sample of younger, light smokers (Piasecki, et al., 2007). We captured significantly fewer cigarettes via the diary than would be expected based upon the TLFB. However, drinks were not underreported compared TLFB projections. Discrepancies with TLB might reflect multiple influences, including recording noncompliance, inaccuracy or bias in TLFB estimates, and reactivity of behaviors to self-monitoring. Prior research suggests estimated of drinking tend to be comparable across EMA and TLFB (Hufford, Shields, Shiffman, Paty, & Balabanis, 2002) but smokers tend to report fewer cigarettes by diary than by retrospective means (Shiffman, 2009). Although we also found underreporting of cigarettes, the magnitude of the TLFB-diary discrepancy for cigarette recording (63% of TLFB captured by diary) in the current study was larger than observed in a recent report from a clinical sample (82%; Shiffman, 2009). This might be accounted for by the inclusion of many young, light smokers in our study. For these individuals, smoking patterns are likely to be variable over time and smoking behavior is often discretionary, meaning their smoking behaviors might be especially reactive to diary monitoring.
Bearing in mind these caveats, the current study extends the evidence for momentary covariation of drinking and smoking behaviors. Co-administration was followed by modestly enhanced drug reward, though alcohol more strongly augmented tobacco reward than vice versa. The findings attest to the importance of positive reinforcement, craving, and buzz in accounting for ongoing substance use. These mechanisms may help to account for the covariation of alcohol and tobacco, including comorbidity of alcohol use disorders and tobacco dependence. Thus, they deserve continuing scrutiny in research focused on co-administration.
Acknowledgments
Supported by National Institutes of Health grants P50AA011998 (Heath), K05AA017688 (Heath). K05AA017242 (Sher), and T32AA01352 (Sher). We thank Alison Richardson, Erin Hunt-Carter, Sarah Mitchell, and Courtney Bagge for assistance with data collection, Steven Richardson for programming assistance, and Margie Gurwit for assistance in data management.
Footnotes
Saul Shiffman is a cofounder of invivodata, inc., which provides electronic diary services for research, and was responsible for the software used in this study.
By design, Drinking Reports were to be logged after a single drink (the first in an episode). When alcohol use was captured in a Cigarette Report or a Random Prompt, the assessment items did not explicitly resolve the number of drinks or the exact amount of time elapsed since last drink. To assess whether uncertainty about timing of alcohol consumption affected the results, we first empirically determined the time since last report for all drinking reports captured by Cigarette Reports and Random Prompts (M= 4.07 hours, Mdn = 2.28 hours). All reported analyses were repeated after excluding such records when more than 1 hour had elapsed since last report. These analyses were very similar to the results using all records; the only difference was that the significant Alcohol x Cigarette interactions for sadness (Table 4) and nausea (Table 6) became only marginally significant (ps = .06). We elected to retain all the records because we suspect that, in most cases, they represent instances in which less than a full drink had been consumed. Participants were instructed to log a Drinking Report after finishing the first drink, but they could be signaled or complete a Cigarette Report before finishing the first drink and would answer “yes” to having consumed alcohol since the last report in such cases. The distribution of times of day for Cigarette Reports and Random Prompts containing reports of alcohol (e.g., 91% between noon and midnight; 49% between 8 p.m. and midnight) resembled the temporal distribution of Drinking Reports, consistent with the hypothesis that these records were typically made close in time to the initiation of drinking.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/abn
Contributor Information
Thomas M. Piasecki, Department of Psychological Sciences and Midwest Alcoholism Research Center, University of Missouri
Seungmin Jahng, Department of Psychological Sciences and Midwest Alcoholism Research Center, University of Missouri.
Phillip K. Wood, Department of Psychological Sciences and Midwest Alcoholism Research Center, University of Missouri
Brandon M. Robertson, Department of Psychological Sciences and Midwest Alcoholism Research Center, University of Missouri
Amee J. Epler, Department of Psychological Sciences and Midwest Alcoholism Research Center, University of Missouri
Nikole J. Cronk, Department of Family and Community Medicine, University of Missouri School of Medicine
John W. Rohrbaugh, Department of Psychiatry and Midwest Alcoholism Research Center, Washington University School of Medicine
Andrew C. Heath, Department of Psychiatry and Midwest Alcoholism Research Center, Washington University School of Medicine
Saul Shiffman, Smoking Research Group, University of Pittsburgh.
Kenneth J. Sher, Department of Psychological Sciences and Midwest Alcoholism Research Center, University of Missouri
References
- Acheson A, Mahler SV, Chi H, de Wit H. Differential effects of nicotine on alcohol consumption in men and women. Psychopharmacology. 2006;186:54–63. doi: 10.1007/s00213-006-0338-y. [DOI] [PubMed] [Google Scholar]
- Armeli S, Conner TS, Cullum J, Tennen H. A longitudinal analysis of drinking motives moderating the negative affect-drinking association among college students. Psychology of Addictive Behaviors. 2010;24:38–47. doi: 10.1037/a0017530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TR, Higgins-Biddle JC, Saunders JB, Monteiro MG. The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care. 2e. Geneva: World Health Organization; 2001. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Baker TB, Morse E, Sherman JE. The motivation to use drugs: A psychobiological analysis of urges. In: Rivers C, editor. Nebraska Symposium on Motivation. Vol. 34. Lincoln: University of Nebraska Press; 1987. pp. 257–323. [PubMed] [Google Scholar]
- Barrett SP, Tichauer M, Leyton M, Pihl RO. Nicotine increases alcohol self-administration in non-dependent male smokers. Drug and Alcohol Dependence. 2006;81:197–204. doi: 10.1016/j.drugalcdep.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Burton SM, Tiffany ST. The effect of alcohol consumption on craving to smoke. Addiction. 1997;92:15–26. [PubMed] [Google Scholar]
- Carter BL, Lam CY, Robinson JD, Paris MM, Waters AJ, Wetter DW, Cinciripini PM. Real-time craving and mood assessments before and after smoking. Nicotine & Tobacco Research. 2008;10:1165–1169. doi: 10.1080/14622200802163084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellsague X, Munoz N, DeStefani E, Victoria CG, Castelletto R, Rolon PA, Quintana MJ. Independent and joint effects of tobacco smoking and alcohol drinking on the risk of esophageal cancer in men and women. International Journal of Cancer. 1999;82:657–664. doi: 10.1002/(sici)1097-0215(19990827)82:5<657::aid-ijc7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of differential deficit. Journal of Psychiatric Research. 1978;14:303–311. doi: 10.1016/0022-3956(78)90034-1. [DOI] [PubMed] [Google Scholar]
- Cooney JL, Cooney NL, Pilkey DT, Kranzler HR, Oncken CA. Effects of nicotine deprivation on urges to drink and smoke in alcoholic smokers. Addiction. 2003;98:913–921. doi: 10.1046/j.1360-0443.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Cooney JL, Pilkey DT, Steinberg HR, Oncken CA. Alcohol and tobacco cessation in alcohol-dependent smokers: Analysis of real-time reports. Psychology of Addictive Behaviors. 2007;21:277–286. doi: 10.1037/0893-164X.21.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: A motivational model of alcohol use. Journal of Personality and Social Psychology. 1995;69:990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Cronk NJ, Piasecki TM. Contextual and subjective antecedents of smoking in a college sample. Nicotine & Tobacco Research. 2010;12:997–1004. doi: 10.1093/ntr/ntq136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Priming effects with drugs and other reinforcers. Experimental and Clinical Psychopharmacology. 1996;4:5–10. [Google Scholar]
- Dierker L, Lloyd-Richardson E, Stolar M, Flay B, Tiffany S, Collins L, Bailey S, Nichter M, Nichter M, Clayton R the Tobacco Etiology Research Network. The proximal association between smoking and alcohol use among first year college students. Drug and Alcohol Dependence. 2006;81:1–9. doi: 10.1016/j.drugalcdep.2005.05.012. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Fletcher K, Ockene JK, Rigotti NA, McNeill AD, Coleman M, Wood C. Recollections and repercussions of the first inhaled cigarette. Addictive Behaviors. 2004;29:261–272. doi: 10.1016/j.addbeh.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: Implications for nicotine self-administration and reinforcement. Psychopharmacology. 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Donohue KF, Curtin JJ, Patrick CJ, Lang AR. Intoxication level and emotional response. Emotion. 2007;7:103–112. doi: 10.1037/1528-3542.7.1.103. [DOI] [PubMed] [Google Scholar]
- Epstein AM, Sher TG, Young MA, King AC. Tobacco chippers show robust increases in smoking urge after alcohol consumption. Psychopharmacology. 2007;190:321–329. doi: 10.1007/s00213-006-0438-8. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi H, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use disorders: Findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Research & Health. 2006;29:162–171. [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Ostling EW, Martin CA, Kelly TH. Acute effects of alcohol on inhibitory control and information processing in high and low sensation-seekers. Drug and Alcohol Dependence. 2009;100:91–99. doi: 10.1016/j.drugalcdep.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glautier S, Clements K, White JAW, Taylor C, Stolerman IP. Alcohol and the reward value of cigarette smoking. Behavioural Pharmacology. 1996;7:144–154. [PubMed] [Google Scholar]
- Greeley J, Oei T. Alcohol and tension reduction. In: Leonard KF, Blane HT, editors. Psychological theories of drinking and alcoholism. New York: Guilford Press; 1999. pp. 14–53. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hufford MR, Shields AL, Shiffman S, Paty J, Balabanis M. Reactivity to ecological momentary assessment: An example using undergraduate problem drinkers. Psychology of Addictive Behaviors. 2002;16:205–211. [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton J. Mortality following inpatient addictions treatment: Role of tobacco use in a community-based cohort. JAMA. 1996;275:1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- Hussong AM. Predictors of drinking immediacy following daily sadness: An application of survival analysis to experience sampling data. Addictive Behaviors. 2007;32:1054–1065. doi: 10.1016/j.addbeh.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussong AM, Hicks RE, Levy SA, Curran PJ. Specifying the relation between affect and heavy alcohol use among young adults. Journal of Abnormal Psychology. 2001;110:449–461. doi: 10.1037//0021-843x.110.3.449. [DOI] [PubMed] [Google Scholar]
- Jackson KM, Sher KJ, Wood PK, Bucholz KK. Alcohol and tobacco use disorders in a general population: Short-term and long-term associations from the St. Louis Epidemilogical Catchment Area Study. Drug and Alcohol Dependence. 2003;71:239–253. doi: 10.1016/s0376-8716(03)00136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassel JD, Shiffman S. Attentional mediation of cigarette smoking’s effects on anxiety. Health Psychology. 1997;16:359–368. doi: 10.1037//0278-6133.16.4.359. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychological Bulletin. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Unrod M. Smoking, anxiety, and attention: Support for the role of nicotine in attentionally mediated anxiolysis. Journal of Abnormal Psychology. 2000;109:161–166. doi: 10.1037//0021-843x.109.1.161. [DOI] [PubMed] [Google Scholar]
- King AC, Epstein AM. Alcohol dose-dependent increases in smoking urge in light smokers. Alcoholism: Clinical and Experimental Research. 2005;29:547–552. doi: 10.1097/01.alc.0000158839.65251.fe. [DOI] [PubMed] [Google Scholar]
- Kirchner TR, Sayette MA. Effects of smoking abstinence and alcohol consumption on smoking-related outcome expectancies in heavy smokers and tobacco chippers. Nicotine & Tobacco Research. 2007;9:365–376. doi: 10.1080/14622200701188893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouri EM, McCarthy EM, Faust AH, Lukas SE. Pretreatment with transdermal nicotine enhances some of ethanol’s acute effects in men. Drug and Alcohol Dependence. 2004;75:55–65. doi: 10.1016/j.drugalcdep.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Lemmens PH. The alcohol content of self-report and “standard” drinks. Addiction. 1994;89:593–601. doi: 10.1111/j.1360-0443.1994.tb03336.x. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for mixed models. 2. Cary, NC: SAS Institute, Inc; 2006. [Google Scholar]
- Littleton J, Barron S, Prendergast M, Nixon SJ. Smoking kills (alcoholics)! Shouldn’t we do something about it? Alcohol and Alcoholism. 2007;42:167–173. doi: 10.1093/alcalc/agm019. [DOI] [PubMed] [Google Scholar]
- Madden PAF, Bucholz KK, Martin NG, Heath AC. Smoking and the genetic contribution to alcohol dependence risk. Alcohol Research & Health. 2000;24:209–214. [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Westman EC, Rose JE, Lutz AM. The effects of foods, beverages, and other factors on cigarette palatability. Nicotine & Tobacco Research. 2007;9:505–510. doi: 10.1080/14622200701243177. [DOI] [PubMed] [Google Scholar]
- McKee SA, Hinson R, Rounsaville D, Petrelli P. Survey of subjective effects of smoking while drinking among college students. Nicotine & Tobacco Research. 2004;6:111–117. doi: 10.1080/14622200310001656939. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Palmieri SL. Cigarette smoking by women: Interactions with alcohol use. Psychopharmacology. 1987;93:8–15. doi: 10.1007/BF02439579. [DOI] [PubMed] [Google Scholar]
- Mintz J, Boyd G, Rose JE, Charuvastra VC, Jarvik ME. Alcohol increases cigarette smoking: A laboratory demonstration. Addictive Behaviors. 1985;10:203–207. doi: 10.1016/0306-4603(85)90001-2. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Combined effects of nicotine and alcohol on subjective, behavioral, and physiological responses in humans. Addiction Biology. 1997;2:255–267. doi: 10.1080/13556219772552. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, McCarthy DE, Fiore MC, Baker TB. Alcohol consumption, smoking urge, and the reinforcing effects of cigarettes: An ecological study. Psychology of Addictive Behaviors. 2008;22:230–239. doi: 10.1037/0893-164X.22.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Richardson AE, Smith SM. Self-monitored motives for smoking among college students. Psychology of Addictive Behaviors. 2007;21:328–337. doi: 10.1037/0893-164X.21.3.328. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Marks JL, Pomerleau OF, Snedecor SM. Relationship between early experiences with tobacco and early experiences with alcohol. Addictive Behaviors. 2004;29:1245–1251. doi: 10.1016/j.addbeh.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF, Namenek RJ, Marks JL. Initial exposure to nicotine in college-age women smokers and never-smokers: A replication and extension. Journal of Addictive Diseases. 1999;18:13–19. doi: 10.1300/J069v18n03_02. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Namenek RJ. Early experiences with tobacco among women smokers, ex-smokers, and never-smokers. Addiction. 1998;93:595–599. doi: 10.1046/j.1360-0443.1998.93459515.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rose JE, Brauer LH, Behm FM, Cramblett M, Calkins K, Lawhon D. Psychopharmacologic interactions between nicotine and ethanol. Nicotine & Tobacco Research. 2004;6:133–144. doi: 10.1080/14622200310001656957. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Hufford MR. Urge and affect: A facial coding analysis of smokers. Experimental and Clinical Psychopharmacology. 1995;3:417–423. doi: 10.1037/1064-1297.11.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Perrott MA, Peters AR. The effects of alcohol consumption on cigarette craving in heavy smokers and tobacco chippers. Psychology of Addictive Behaviors. 2005;19:263–270. doi: 10.1037/0893-164X.19.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D, Jamner LD, Davydov DM, James P. Situations and moods associated with smoking in everyday life. Psychology of Addictive Behaviors. 2002;16:342–345. doi: 10.1037//0893-164x.16.4.342. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Grekin ER. Alcohol and affect regulation. In: Gross J, editor. Handbook of emotion regulation. New York: Guilford; 2007. pp. 560–580. [Google Scholar]
- Shiffman S. How many cigarettes did you smoke? Assessing cigarette consumption by global report, Time-Line Follow-Back, and ecological momentary assessment. Health Psychology. 2009;28:519–526. doi: 10.1037/a0015197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Balabanis M. Alcohol and tobacco: From basic science to clinical practice. 30. National Institute of Alcohol Abuse and Alcoholism Research Monograph; 1995. Associations between alcohol and tobacco; pp. 17–36. NIH Publication 95-3931. [Google Scholar]
- Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. American Journal of Preventive Medicine. 2008;34:102–111. doi: 10.1016/j.amepre.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Fischer LA, Paty JA, Gnys M, Hickcox M, Kassel JD. Drinking and smoking: A field study of their association. Annals of Behavioral Medicine. 1994;16:203–209. [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, Hickcox M, Gnys M. Immediate antecedents of cigarette smoking: An analysis from ecological momentary assessment. Journal of Abnormal Psychology. 2002;111:531–545. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Kirchner TR. Cigarette-by-cigarette satisfaction during ad libitum smoking. Journal of Abnormal Psychology. 2009;118:348–359. doi: 10.1037/a0015620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Paty J. Smoking patterns and dependence: Contrasting chippers and heavy smokers. Journal of Abnormal Psychology. 2006;115:509–523. doi: 10.1037/0021-843X.115.3.509. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JD, Hickcox M. First lapses to smoking: Within-subjects analysis of real time reports. Journal of Consulting and Clinical Psychology. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gwaltney CJ, Dang Q. Immediate antecedents of cigarette smoking: An analysis of unrestricted smoking patterns. Journal of Abnormal Psychology. 2004;113:166–171. doi: 10.1037/0021-843X.113.1.166. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. American Psychiatric Association. Handbook of psychiatric measures. Washington, DC: American Psychiatric Association; 2000. Alcohol Timeline Followback (TFLB) pp. 477–479. [Google Scholar]
- Steele CM, Josephs RA. Alcohol myopia: Its prized and dangerous effects. American Psychologist. 1990;45:921–933. doi: 10.1037//0003-066x.45.8.921. [DOI] [PubMed] [Google Scholar]
- Stone AA, Shiffman S. Ecological momentary assessment in behavioral medicine. Annals of Behavioral Medicine. 1994;16:199–202. [Google Scholar]
- Tiffany ST, Cox LS, Elash CA. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. Journal of Consulting and Clinical Psychology. 2000;68:233–240. doi: 10.1037//0022-006x.68.2.233. [DOI] [PubMed] [Google Scholar]
- Wansink B, van Ittersum K. Shape of glass and amount of alcohol poured: Comparative study of the effect of practice and concentration. BMJ. 2005;331:1512–1514. doi: 10.1136/bmj.331.7531.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Kraus CL, McCracken LA, Swartzwelder HS. Do college students drink more than they think? Use of a free-pour paradigm to determine how college students define standard drinks. Alcoholism: Clinical and Experimental Research. 2003;27:1750–1756. doi: 10.1097/01.ALC.0000095866.17973.AF. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychological Review. 1987;94:469–492. [PubMed] [Google Scholar]
- Zinser MC, Baker TB, Sherman JE, Cannon DS. Relation between self-reported affect and drug urges and cravings in continuing and withdrawing smokers. Journal of Abnormal Psychology. 1992;101:617–629. doi: 10.1037//0021-843x.101.4.617. [DOI] [PubMed] [Google Scholar]