Abstract
Objective
Rheumatoid arthritis culminates in joint destruction that in mouse models of disease, is supported by innate immune molecules including FcγRs and complement. However, the results may not predict outcomes in humans given the structural differences between murine and human activating FcγRs on neutrophils, a prominent component of joint exudates. In this study, we examined the role of the human neutrophil FcγRIIA in the development of arthritis and probed the underlying mechanism by which FcγRIIA initiated disease.
Methods
K/BxN serum transfer-induced arthritis was examined in mice that express FcγRIIA on neutrophils but lack their own activating FcγRs (γ-chain-deficient). The role of mast cells, complement (C3 and C5a) and CD18 integrins in FcγRIIA-initiated disease was examined using cell reconstitution approaches, inhibitors, and functional blocking antibodies respectively. Cross-talk between C5aR and FcγRIIA on neutrophils was evaluated in vitro.
Results
Neutrophil FcγRIIA expression was sufficient to restore susceptibility to K/BxN serum induced neutrophil recruitment, synovitis and bone destruction in γ-chain-deficient mice. Joint inflammation was robust and proceeded even in the absence of mast cells and vascular permeability, shown to contribute to disease in wild-type mice. Neutrophil recruitment was dependent on CD18 integrin LFA-1 and C5aR. C5aR in addition significantly enhanced FcγRIIA mediated phagocytosis and oxidative burst in vitro.
Conclusion
Human and murine activating FcγRs on neutrophils are not functionally equivalent, and in humans may play a primary role in arthritis. Cross-talk between neutrophil C5aR and FcγRIIA is essential for disease thus highlighting a new aspect of complement during the effector phase of inflammatory arthritis.
INTRODUCTION
Rheumatoid arthritis (RA) is an inflammatory disease of the joints with 1% prevalence in the industrialized world (1, 2). Neutrophils, lymphocytes, mast cells, macrophages, synovial tissue cells, and platelet microparticles present in the inflammed synovium have been implicated in the evolution of RA (3, 4). Circulating autoantibodies are present in a majority of patients and joint tissues are frequently covered with immune complexes (ICs) in RA (5, 6). Mechanisms of autoantibody driven disease have begun to be elucidated in mouse models such as the K/BxN arthritis model, which arises from the breakage of T cell tolerance followed by the generation of IgG autoantibodies to the glycolytic enzyme glucose-6-phosphate isomerase (GPI). K/BxN arthritis shares with RA certain clinical features including distal symmetric erosive polyarthritis and at the tissue level, pannus that is erosive into bone and cartilage, prominent vascular hyperplasia and neutrophil rich synovial fluid. The finding that disease can be passively transferred with autoantibodies from these animals to normal recipients together with results in knock-out mouse models support the view that although T and B cells are required for the initiation of disease, autoantibodies and innate immune mediators are the effector mechanisms that promote synovitis and joint destruction (7).
FcγRs for complexed IgG and the complement system play essential roles in disease progression. Polymorphisms in FcγRs are associated with RA (8, 9) and studies in mice suggest a significant role for these receptors in disease pathogenesis: mice deficient in the γ-chain, required for the expression and signaling of activating FcγRs, have reduced incidence and severity in models of arthritis, whereas a deficiency in the inhibitory receptor FcγRII exacerbates disease (10). Mast cells are among the FcγR-bearing cell types that contribute to articular inflammation by releasing mediators that induce vascular permeability, promote neutrophil recruitment, and activate resident synovial fibroblasts and macrophages (3). Generation of complement components is a key event in the effector phase of disease. Complement C5a levels correlate with neutrophil accumulation in patients (11) and in the K/BxN model, mice lacking C5 or C3 are resistant to disease (12). C5a exerts its effects primarily by binding C5a receptor (CD88) expressed on a wide variety of hematopoietic cells including neutrophils, mast cells and macrophages (13). Synergism between complement C3 receptors and FcγRs has been long recognized to enhance adherence and ingestion of complement fragment C3b and IgG opsonized targets (14, 15). However, only recently has cross-talk between C5aR and FcγRs been appreciated. A hierarchical relationship between the two receptors has been suggested. C5a attracts FcγR bearing leukocytes and C5aR transcriptionally regulates expression of inhibitory and activating FcγRs on macrophages to lower the threshold of IC activation (13). In other cases, complement and FcγRs clearly play redundant roles (16) or one pathway dominates over the other (17).
Despite the significant strides made in mice, the results may not predict outcomes in humans as the repertoire of activating FcγRs on neutrophils that bind ICs differ significantly between the two species. Human neutrophils express two activating FcγRs, which are uniquely human. Human FcγRIIA contains an immunotyrosine activating motif (ITAM) and ligand binding domain in a single polypeptide chain, and FcγRIIIB is glycophosphatidylinositol-linked. In contrast, murine neutrophils contain a transmembrane FcγRIII and a species-specific FcγRIV that complex with an ITAM-containing common γ-chain. Moreover, the contribution of FcγRs alone to disease pathogenesis remains unclear since deletion of theγ-chain in mice eliminates more than activating FcγRs (18). Previous work suggests that human FcγRIIA and FcγRIIIB expression on neutrophils of mice lacking their endogenous activating FcγRs (i.e. γ-chain−/−) restores susceptibility to nephrotoxic serum nephritis and the Reverse Passive Arthus reaction (induced by soluble ICs) (19).
Here we explored the role of human FcγRIIA on neutrophils in inflammatory arthritis and probed the underlying mechanisms by which this receptor mediates disease.
MATERIALS AND METHODS
Mice
Human FcγRIIA (FcγRIIA+/γ−/−) mice (19) and miR-CD18 mice (20) were previously described. Mice expressing human CD18 on neutrophils but lacking endogenous murine CD18 (hCD18+/mCD18−/−) were generated as follows. Human CD18 cDNA was subcloned into the BglII site of pUC18-hMRP8 vector (21). A HindIII-EcoRI fragment was released and injected into zygotes from C57Bl/6J mice. Transgenic mice were generated in the transgenic facility of Brigham & Women’s Hospital (Boston, MA). A high-expressing founder of hCD18 (hCD18+) was crossed with CD18 deficient mice (mCD18−/−) (22)on a C57Bl/6J-F12 background and bred to be hemizygous for the hCD18 transgene, and mCD18 deficient, as assessed by PCR of genomic DNA and flow cytometry analysis of cells.
All mice were maintained in a virus- and antibody-free facility at the NRB animal housing facility at Harvard Medical School. Mice used for each experiment were 8–10 weeks of age and sex matched. The Harvard Medical School Animal Care and Use Committee approved all procedures in this study.
Serum transfer induced arthritis and pharmacological treatments
25–150 μl of K/BxN serum was given intraperitoneally (i.p.) on day 0 and 2. Every second day up to day 14, clinical arthritis was graded in all four paws (clinical score) and ankle joint thickness was measured on both side using a micrometer (23, 24). Ankle joints were isolated and tissue specimens were stained with hematoxylin and eosin for histological evaluation (25) or rat monoclonal NIMP-R14 (Abcam Inc. Cambridge, MA) for neutrophil quantitation (26). The total neutrophil number in the closed articular cavity were counted and was divided by the total area (mm2) of the articular cavity in each specimen measured by Image J software (NIH). For quantification of mast cell degranulation, mice given 100μl of normal mouse serum or K/BxN serum were euthanized 18hrs later and toluidine blue stain was applied to tissue specimens (24).
For complement depletion, 12.5 U/mouse CVF (Quidel, San Diego, California, USA) was injected i.p. on day -1, and 6.25 U/mouse on day 2, 5 and 10. C5a receptor antagonist (C5aRA, A8Δ71-73) (27) was injected intravenously (i.v.) 20 min before and 2 hrs after K/BxN serum injection, and then i.v. every 12 hrs up to day 4.
For blockade of LFA-1, 100μl of K/BxN serum was given at day 0 and 2 and 75μg functional blocking rat anti-CD11a IgG2a antibody (clone M17/4) (BD Pharmingen) or rat IgG isotype control were injected i.v. at day 0, 2 and 4 as previously described (22).
Analysis of vascular permeability in mouse limbs
Mouse heat-aggregated IgG (mHAGG) was generated essentially as previously described (28). 100μl of monomeric mouse IgG (5mg/ml), mHAGG, K/BxN serum or normal mouse serum was diluted with 150μl of 0.3% Evans blue and 50μl PBS, and injected i.v. All four limbs per mouse were harvested and incubated with 600μl dimethylformamide at RT for 4 days and Evans blue in the supernatant was quantified by measuring theabsorbance at 595nm.
Flow cytometry
Peripheral blood sampled from the retroorbital plexus of mice, human peripheral neutrophils, bone marrow derived macrophages, peritoneal cells were collected as described (19, 29). For analysis of mast cells, peritoneal cells were harvested and used immediately. All antibodies were from BD Biosciences-Pharmingen unless otherwise indicated. FITC or PE-mouse anti-human CD18 (clone 6.7, mouse IgG1κ), FITC-rat anti-mouse CD11a (clone M17/4, rat IgG2aκ) and APC-rat anti-mouse CD11b (clone M1/70, rat IgG2b, eBioscicence). Cell populations were identified as follows: PE or APC-rat anti-Gr-1 (clone RB6-8C5, APC-Gr-1; BioLegend) for neutrophils, PE-rat anti-CD115 (clone AFS98; eBioscience) for monocytes, PE-Cy7-hamster anti-mouse CD3ε (clone 145-2C11) for T cells, APC-rat anti-F4/80 (clone A3-1; Caltag Laboratories) for macrophages and FITC-rat anti-mouse CD19 (clone 1D3) for peripheral B cells.
Bone marrow neutrophil engraftment
Mature neutrophils (>95% pure) were isolated from the bone marrow of femurs and tibias of FcγRIIA+/γ−/−or γ−/− mice as described (30). 2.0×107 neutrophilswere administered i.v. into recipient W/Wv mice on days 0, 1, 2, 3, and 200 μl K/BxN serum was given on day 0 and 2.
In vitro neutrophil assays
Ligand binding assay
2×106 BMNs were stimulated with or without 100nM LTB4, 100nM human C5a, 100μg/ml fmlp (Sigma) for 20 min at 37°C, and washed twice at 4°C. Cells were incubated with mHAGG at 1:100 dilution on ice for 1hr, washed again, and stained with Alexa-conjugated donkey anti-mouse IgG (Invitrogen) and APC-anti Gr-1. Mean fluoroscent intensity (M.F.I.) in the Gr-1 positive cell population was evaluated by FACs to detect surface mHAGG.
Neutrophil phagocytosis
Sheep RBC (Lampire) were labeled with fluorescence dye PKH67 according to manufacturer’s instructions (Sigma). For IgG-RBC, RBC were incubated with anti-sheep RBC IgG (Cedarlane) for 1h. For C3b-RBC, RBC were incubated with anti-sheep RBC IgM (Cedarlane) for 1h followed by C5 deficient serum (Sigma) for 1h. RBCs were coincubated with neutrophils at a 10:1 ratio in the presence or absence of C5a (5μg/ml), PMA (100ng/ml) or DMSO at 37° for 30 mins. External RBCs were lysed in ice-cold water, followed by the addition of trypan blue to quench fluorescence from remaining external RBC. Cells were visualized under a fluorescence microscope to assess the percentage of neutrophils with internalized RBCs. Normalized phagocytosis percentage was calculated by subtracting phagocytosis of control RBC from phagocytosis of IgG or C3b-coated RBC.
FcγR cross-linking induced ROS generation
1×106 BMNs were suspended in PBS without Ca2+/Mg2+, and incubated with 10μg/ml mouse anti-hFcγRIIA (cloneIV.3, StemCell Technologies) on ice for 30 min. After washing in PBS twice, cells were incubated with or without 500ng/ml hC5a for 30 min. 50μM luminol in PBS with Ca2+/Mg2+ was added, followed immediately by goat anti-mouse F(ab′)2 (36μg/ml), (Jackson ImmunoResearch Laboratories). ROS generation was continuously monitored over time using a six-channel bioluminat LB-953 luminometer (Berthold).
Statistical analysis
All in vivo data are presented as mean±S.E.M. The in vitro data are presented as mean±S.D. Statistical differences were analyzed with the unpaired t-test. Significance was defined as p<0.05.
RESULTS
Neutrophil specfic expression of hFcγRIIA promotes K/BxN arthritis
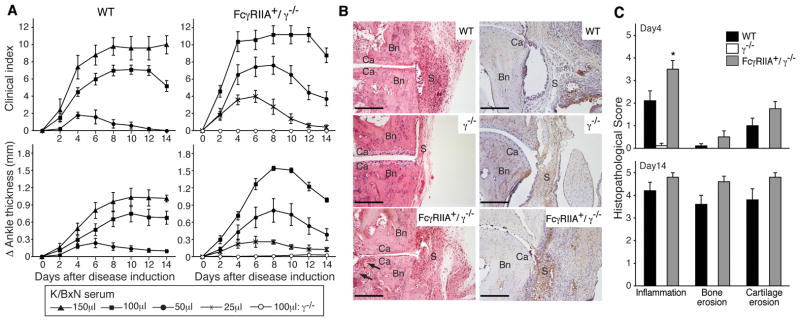
K/BxN serum-induced arthritis was evaluated in mice expressing the human FcγRIIA in γ−/−deficient mice (FcγRIIA+/γ−/−), mice lacking the common γ-chain (γ−/−) and wild-type mice. γ−/− mice transgenically expressing the other human neutrophil FcγR, FcγRIIIB+/γ−/− mice (19) were excluded from the analysis as hFcγRIIIB does not recognize mouse IgG (Supplemental S1). 100–150μl of K/BxN serum is the standard dose for inducing disease in wild-type mice. We used only 50μl of K/BxN as even this lower dose resulted in significant morbidity in FcγRIIA+/γ−/− mice as will become apparent below. As previously reported (12), γ−/− mice showed no evidence of disease as assessed by clinical and histological parameters. On the other hand, hFcγRIIA+/γ−/− mice demonstrated disease activity that significantly exceeded that observed in wild-type mice (Figure 1A) despite levels of FcγRIIA that are comparable to its murine counterpart FcγRIII on wild-type neutrophils (19). It is noteworthy that the inhibitory FcγRIIB is expressed in the FcγRIIA/γ−/− animals and is likely functional since administration of intravenous immunoglobulin (IVIG), which mediates protection in wild-type mice through its action on FcγRIIB (31), inhibits FcγRIIA mediated inflammation in the K/BxN model (data not shown). Histologically hFcγRIIA+/γ−/− mice exhibited severe synovial hyperplasia and bone erosion (Figure 1B) with robust neutrophil infiltration in the synovium and bone compared to γ−/− mice (Figure 1C,D). Thus activating human FcγRIIA on neutrophils is sufficient to restore neutrophil influx and susceptibility to arthritis in the absence of other activating FcγRs.
Figure 1. K/BxN serum arthritis in FcγRIIA transgenic mice.
(A) Disease kinetics in wild type (WT) and human FcγRIIA (FcγRIIA+/γ−/−) mice. Arthritis was induced by intraperitoneal injection of the indicated dose of K/BxN serum on day 0 and 2. Total clinical score (upper panels) in four limbs, and ankle joint thickness (lower panels) in hind limbs were evaluated. Open circle shows γ-chain deficient mice (γ−/−) treated with 100μl of K/BxN serum. Data are mean ± SEM. N≥5 for each condition (B) Representative histology of ankle joints from wild type (WT, upper), γ-chain deficient (γ−/−, middle), and FcγRIIA+/γ−/− (lower) mice on day 14 after 100 μl K/BxN serum injection. Tissue is stained with hematoxylin and eosin (left panels) or NIMP-R14 for neutrophils (right panels). Bn, bone; Ca, cartilage; S, synovium. Arrow indicate bone erosion. Bar:200μm (C) Histological assessment on day 4 and day 14 in joint sections stained with H&E. All data are mean ± SEM of 10 joints from 5 mice per group. *p<0.05 vs WT mice.
Human FcγRIIA mediated arthritis is not associated with mast cell degranulation or vascular leakage
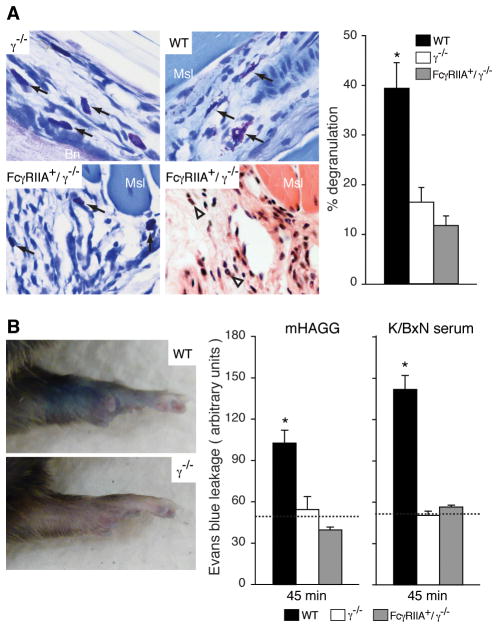
Mast cell deficient W/Wv and Sl/Sld mice fail to develop K/BxN serum-induced arthritis and degranulated mast cells are observed in joint tissue of wild-type animals within hours of K/BxN serum transfer (24). As reported, ankle specimens of wild-type mice after K/BxN serum-transfer showed significant mast cell degranulation while they were intact in γ−/− mice, which do not succumb to disease. However, ankle specimens of FcγRIIA+/γ−/− mice also exhibited virtually no mast cell degranulation despite significant synovitis (Figure 2A).
Figure 2. Contribution of mast cells to the development of K/BxN serum arthritis in FcγRIIA mice.
(A) Mast cell degranulation in ankle joints after K/BxN serum transfer. Representative pictures of ankle joint specimens taken from γ−/− (left upper), WT (right upper) and FcγRIIA+/γ−/− (left lower) 18 hrs after 100μl K/BxN serum injection and stained with toluidine-blue. Arrows indicate mast cells. A serial section from FcγRIIA+/γ−/− mice is stained with H&E (right lower) to document the inflammatory cell infiltration in the area. Bn, bone; Msl, muscle; Arrowhead, PMN. The number of degranulated mast cells was quantitated (right). All data are mean ± SEM of n=5 mice per group. *p<0.01 vs γ−/−. (B) Vascular permeability change in limbs. Representative pictures of Evans blue leakage are shown in limbs of WT (upper) and γ−/− (lower) mice 45 min after i.v. administration of l00μl of heat-aggregated IgG (mHAGG). Quantitation of Evans blue extracted from limbs 45 min after mHAGG (left), and 45 min after 100μl K/BxN serum injection (right). Data are mean ± SEM and n=5 per group. *p<0.005 vs γ−/−. Dotted lines indicate average value in wild-type animals treated with monomeric mIgG (left) or normal mouse serum (right) (n=3 per group)
K/BxN serum induces joint localized vascular permeability within 5 mins of administration that depends on mast cells, neutrophils and FcγRs in C57Bl/6 mice (32). Heat aggregated, non-specific IgG also induces leakage indicating that IgG-ICs rather than other components potentially present in K/BxN serum, are sufficient to promote permeability(32). ICs (mouse heat-aggregated IgG or K/BxN serum) did not induce vascular permeability in FcγRIIA+/γ−/− mice and indeed was similar to that observed in the γ−/−and wild type animals treated with (control) monomeric IgG (Figure 2B). Permeability was also evaluated 18hrs after introduction of K/BxN serum. At this later time point, Evans blue leakage significantly increased in the FcγRIIA/γ−/− mice (data not shown), which is likely secondary to the robust inflammation developing in these animals. In summary, in the presence of hFcγRIIA on neutrophils, disease proceeds through mechanisms that are independent of mast cell degranulation and is not associated with early changes in vascular permeability.
FcγRIIA mediated arthritis develops in the absence of mast cells
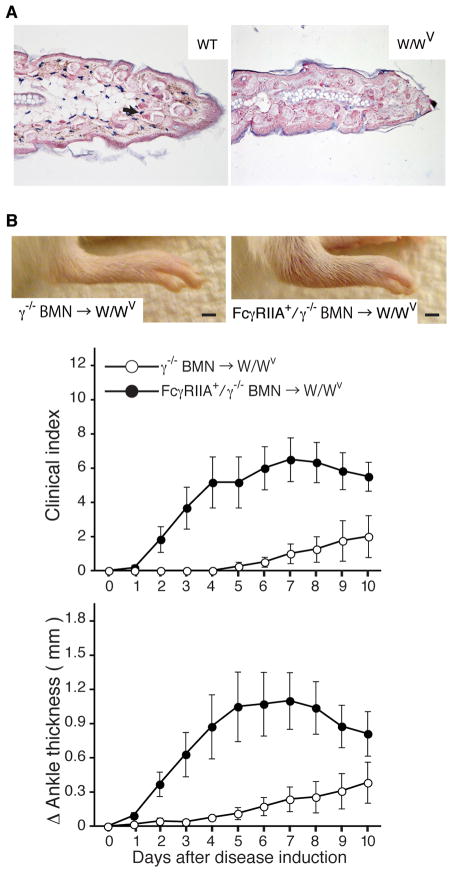
To directly evaluate the contribution of mast cells to FcγRIIA-induced arthritis, bone marrow neutrophils (BMN) isolated from FcγRIIA+/γ−/− or γ−/− mice were i.v. injected into the W/Wv strain of mice for 4 consecutive days and K/BxN serum was delivered into reconstituted W/Wv mice at day 0 and 2. Absence of mast cells in the W/Wv animals was confirmed by immunostaining of ear skin (Figure 3A). W/Wv mice reconstituted with FcγRIIA+/γ−/− BMNs exhibited significantly more disease activity than mice given γ−/−BMNs (Figure 3B). Thus, in the context of human neutrophil FcγRIIA, mast cells are not essential for development of arthritis.
Figure 3. Human FcγRIIA on neutrophils is sufficient to induce K/BxN arthritis in mast cell deficient W/WV mouse.
Purified bone marrow neutrophils from FcγRIIA+/γ−/− or γ−/ −mice were delivered i.v. for 4 consecutive days into mast cell deficient W/Wv recipient mice. (A) Images of ear skin specimens of WT and W/Wv mice stained for mast cells (blue). Mast cells (arrow) were detected only in WT and not in the W/Wv mice as expected. (B) Representative pictures of hind paws and disease kinetics in W/Wv mice reconstituted with BMN from γ−/ − (γ−/ − BMN→W/WV, open circle) and FcγRIIA+/γ−/ − (FcγRIIA+/γ−/ − BMN→ W/Wv, closed circle) mice. Total clinical score in four limbs and ankle joint thickness were evaluated as in Fig. 1. n=4 mice/group/experiment. Bar: 2mm
Neutrophil CD18 is required for the development of FcγRIIA mediated rheumatoid arthritis
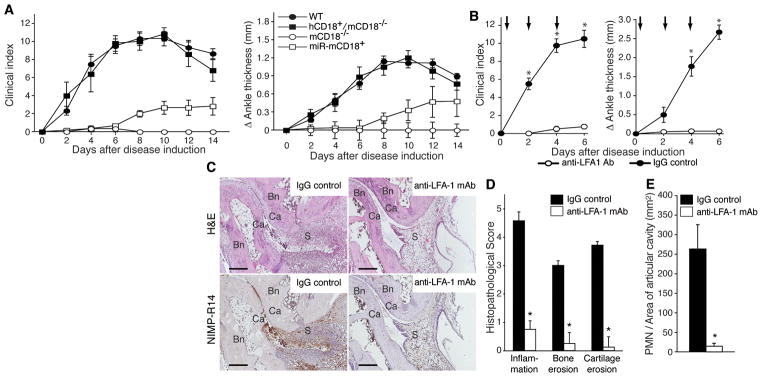
Mice lacking all CD18 integrins, or in which these integrins are functionally blocked, develop minimal arthritis (22). Here, the significance of CD18 integrins on neutrophils was evaluated by exploiting two complementary genetic approaches: The silencing of CD18 selectively in neutrophils of wild-type mice (miR-CD18) (20) and the expression of human CD18 selectively in neutrophils of mice lacking endogenous CD18 (hCD18+/mCD18−/−) (Supplemental S2). These two mouse models, together with wild-type mice and CD18-deficient mice were subjected to K/BxN serum arthritis. Neutrophil CD18 silencing impaired development of arthritis, CD18 deficient mice were resistant to disease, while hCD18+/mCD18−/− developed arthritis that was comparable to wild-type mice (Figure 4A). Together, these data provide compelling evidence that neutrophil CD18 is critical in the effector phase of arthritis.
Figure 4. Involvement of neutrophil CD18 in pathogenesis of K/BxN serum arthritis.
(A) Arthritis severity was evaluated in WT (n=6), miRCD18 mice with CD 18 silenced in neutrophils (miR-CD18, n=6), CD 18 deficient (mCD18−/−, n=5) and CD 18 deficient mice expressing human CD 18 in neutrophils (hCD18/mCD18−/−, n=5). 100 μl of K/BxN serum was administered and clinical score in limbs and ankle joint thickness were evaluated as in Fig. 1. (B) FcγRIIA+/γ−/− mice received 100 μl of K/BxN serum on day 0 and 2 and were treated with either anti-LFA-1 antibody (anti-LFA-1 mAb, open circle) or isotype-IgG (IgG control, closed circle) by tail vein injection at the indicated time points (arrow). Disease activity was evaluated as in Fig 1. *p<0.05 versus control IgG-treated group. (C) Representative pictures of H&E and NIMP-R14 stained joint samples in a control IgG (left) or anti-LFA-1 antibody-treated (right) FcγRlIA mouse are shown. Bar: 200μm. Bn, bone; Ca, cartilage; S, synovium. Histological assessment in arthritic ankle joints stained with H&E (D) and quantitation of neutrophil influx into the articular cavity in NlMP-R14-stained samples (E) on day 6 are shown. *p<0.005 vs control IgG-treated group.
Given the reliance of K/BxN arthritis on neutrophil CD18, we assessed the contribution of CD18 specifically in induction of arthritis in the hFcγRIIA mice. Functional blocking antibody to LFA-1 blocks K/BxN arthritis in wild-type mice (22). Here we show that this antibody completely suppressed clinical and histological features of arthritis in hFcγRIIA+/γ−/− mice (Figure 4C–D) that correlated with minimal joint neutrophil accumulation (Figure 4E).
Complement is essential for FcγRIIA mediated rheumatoid arthritis
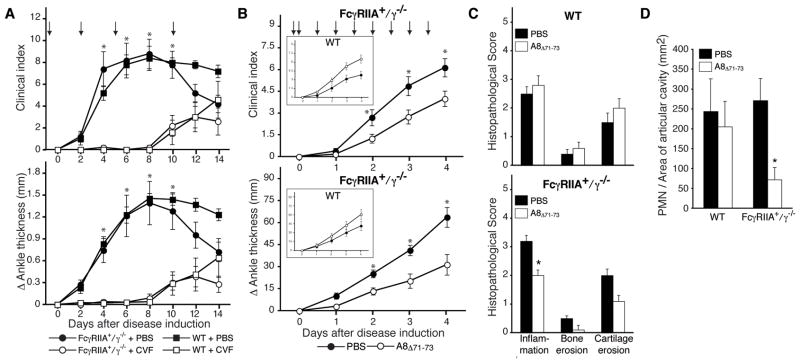
Wild-type and FcγRIIA+/γ−/− mice were evaluated following depletion of C3 by systemic administration of cobra venom factor (CVF) (Figure 5A) or blockade of C5a receptor using a specific antagonist of the two C5a receptors, CD88 and C5L2 (27) (Figure 5B). Both mouse strains treated with CVF failed to develop arthritis (Figure 5A). However, C5aR blockade attenuated arthritis only in FcγRIIA+/γ−/− mice while the C5aR antagonist in wild-type mice did not, and indeed had a trend towards greater disease for reasons that are unclear (Figure 5B,C). Consistent with these findings, a significant reduction in neutrophil influx was observed only in FcγRIIA+/γ−/− mice treated with the C5aR antagonist (Figure 5D). In conclusion, C5aR regulates hFcγRIIA dependent neutrophil recruitment and joint injury.
Figure 5. Role of complement C3 and C5a in FcγRIIA mediated arthritis.
WT and FcγRIIA+/γ−/ − mice, were given 100 μl and 50 μl K/BxN serum respectively on day 0 and 2 to insure similar severity of disease in both strains (refer to Fig 1). (A) Cobra venom factor (CVF) or PBS were i.v. injected on the indicated days (arrows). Total clinical score (upper) in limbs and ankle joint thickness (lower) were evaluated. N=5 per group. (B) C5a receptor antagonist (C5aRA, A8Δ71-73) was delivered i.v. into WT and FcγRIIA+/γ−/ − mice at the indicated times points (arrows). Total clinical score (upper) in limbs and ankle joint thickness (lower) in hind limbs were evaluated upto 4 days after induction of disease. N=9–10 per group. *p<0.05 vs CVF treated or C5aRA treated group for all points upto 8 days and 4 days, respectively. (C) Histological assessment on day4 in C5aRA and PBS treated animals. (D) Evaluation of neutrophil infiltration into the articular cavity of C5aRA and PBS treated animals. All data are mean ± SEM and n=5 mice per group. Consistent with results in (B) C5aRA blocked neutrophil influx only in FcγRIIA and not in WT treated mice. *p<0.05 vs PBS treated group.
C5a promotes hFcγRIIA dependent neutrophil cytotoxicity
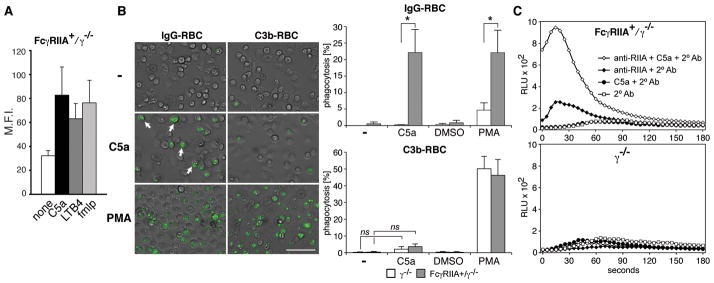
Activation of the recruited neutrophils via FcγRs and complement receptor 3 (CR3) is essential for tissue damage in vivo (30, 33). Here we conducted studies in vitro to explore the possibility that in addition to effects on neutrophil recruitment, C5a regulates FcγRIIA-arthritis by increasing FcγRIIA-dependent neutrophil cytotoxicity. The bacterial peptide fmlp increases FcγRIIA-dependent ligand binding (34). Here FcγRIIA+/γ−/− neutrophils treated with C5a or other G-protein coupled receptor agonists fmlp and LTB4 exhibited enhanced binding of ICs although this was not statisically significant (p=0.07) (Fig 6A). Next, FcγRIIA phagocytic functions in the presence of C5a was examined. Phagocytosis of IgG-opsonized RBCs was undetectable in FcγRIIA expressing neutrophils, while pretreatment of cells for 30 min with C5a induced significant phagocytosis (Figure 6B). The enhancement was specific for FcγRs as phagocytosis of complement C3b-opsonized RBC (C3-RBC), mediated by CR3 (33) was largely unaffected by C5a, but was robust in the presence of PMA (Figure 6B) as described (33). To determine if C5a can regulate FcγR signaling downstream of receptor engagement we evaluated whether it enhanced FcγRIIA cross-linking induced reactive oxygen species (ROS) generation (Figure 6C). ROS was not detected in FcγRIIA+/γ−/− neutrophils pretreated with C5a for 30 min (Figure 6C) as expected since ROS returns to baseline within 5 min of C5a treatment (data not shown). FcγRIIA cross-linking alone produced minimal ROS. On the other hand, FcγRIIA+/γ−/− neutrophils pretreated with C5a prior to FcγRIIA cross-linking exhibited a significant and rapid enhancement of ROS production. C5a, under treatment conditions used in the above two assays did not increase surface FcγRIIA levels (data not shown). In summary, C5a is essential for FcγRIIA dependent pro-inflammatory effector functions.
Figure 6. C5a promotes FcγRIIA mediated phagocytosis and reactive oxygen species generation.
BMNs were harvested from human FcγRIIA (FcγRIIA+/γ−/ − ) and γ−/ − mice. Binding of aggregated IgG (mHAGG) (A), phagocytosis of IgG- and C3b-opsonized fluorescently labelled-RBCs (B) and FcγRIIA cross-linking induced ROS generation (C) were analyzed. (A) BMNs were incubated with agonists and mHAGG, and bound IgG was detected by flow cytometry. Mean fluoroscent intensity (M.F.I.) ± SEM of 5 experiments is shown. (B) BMNs were incubated with C5a, PMA or negative controls (PBS or DMSO) for 30 mins and IgG- or C3b-coated fluorescently labelled sheep RBC. Representative pictures of phagocytosis (green fluorescence, arrow) by Fcγ RIIA+/γ−/ − neutrophils are shown. (Bar= 50μm). Normalized percentage of phagocytosis for each condition is given. Data are mean ± SD of three experiments; *p<0.05; ns, not significant. (C) BMNs were incubated with or without antibody to FcγRIIA (anti-RIIA) followed by PBS or C5a (+C5a) for 30 min. ROS was detected following the addition of secondary antibody against anti-FcγRIIA (2°Ab). ROS, detected following FcγRIIA cross-linking, was significantly enhanced by C5a. Minimal ROS generation was detected in control samples, FcγRIIA+/γ−/ − neutrophils without FcγRIIA antibody (C5a+2°Ab, 2°Ab), and γ−/ − neutrophils. One of four representative experiments is shown.
DISCUSSION
There is considerable evidence in humans and animal models that ICs and innate immune mediators promote inflammation in joints and other tissues. However, the hierarchy of events and the downstream cellular and molecular effector mechanisms remain largely unknown. Furthermore, since there exists significant divergence in FcγR structure and function between mouse and man, the relevance of results obtained only with murine FcγRs for human IC-driven diseases is debatable. Previous mechanistic studies in K/BxN arthritis have shown that antigen:antibody IC deposition in the articular cavity leads to activation of tissue-resident FcγR-expressing cells that feed back to initiate recruitment of circulating leukocytes (35). Importantly, since FcγRIIA is found only in higher primates, the role of neutrophils activated by this pathway remains unexplored both in this model and in human disease. Herein we demonstrate that expression of human FcγRIIA in neutrophils alone confers significant susceptibility to K/BxN induced arthritis that in fact exceeds that observed in wild-type mice that express their whole complement of endogenous FcγRs. We further show that FcγRIIA on neutrophils is acutely regulated by complement C5a. The cross-regulation of C5aR and FcγRIIA provides an important link between the FcγRs and the complement network, both of which connect upstream initiating events in RA to downstream effector responses (12). C5a also contributes to neutrophil recruitment, but it is the integrin LFA-1 that is critical in this respect as its blockade ameliorates FcγRIIA-dependent neutrophil influx and the associated joint destruction. Thus human FcγRIIA, C5aR and LFA-1 represent an important regulatory complex on neutrophils that can coordinate neutrophil influx and cytotoxicity in IC-driven inflammation.
Engagement of human neutrophil FcγRIIA is well-known to trigger a cascade of signaling events leading to phagocytosis, ROS production, protease and leukotriene release and cytokine production in vitro (1). It is expressed on neutrophils, as well as a number of leukocyte subsets, platelets, mast cells, Langerhan and dendritic cells (36, 37). In previous studies, transgene expression of FcγRIIA primarily on platelets of wild-type mice increased susceptibility to collagen-induced arthritis (38). Here, the neutrophil-selective expression of FcγRIIA in the absence of other FcγRs demonstrates the potency of this molecular pathway in activating neutrophils in the context of inflammatory arthritis. We cannot formally rule out a role for the approximately 20% of monocytes expressing FcγRIIA (19) in development of arthritis. However, K/BxN induced RA proceeds normally in CSF-1 deficient mice (op/op) (31) which have a >90% reduction in monocytes and macrophages (39), and FcγRIIA is not detectable on macrophages of FcγRIIA/γ−/− mice (19). Therefore, the contribution of FcγRIIA on monocytes is likely not significant. Interestingly, the inflammatory pathway triggered by FcγRIIA does not proceed via increases in vascular permeability, a function previously ascribed to both neutrophils and mast cells (32). Further, the stimulation of neutrophils via FcγRIIA is not associated with mast cell degranulation and indeed proceeds in the absence of these cells. Thus human and murine activating FcγRs are not functionally equivalent, with neutrophil human FcγRIIA playing a primary and proximal role in the effector phase of arthritis. This might require a re-evaluation of what we have concluded about the pathogenesis of arthritis based on previous murine studies. Our work suggests that neutrophils were recruited through their own FcγRIIA. GPI expression has been observed on the surface of the synovial lining and on the endothelial cell surface (40). The latter observation suggests the possibility that FcγRIIA may directly interact with GPI/anti-GPI ICs within the joint vasculature in K/BxN serum transfer arthritis to promote neutrophil recruitment, similar to what we have shown by intravital microscopy to occur in the cremaster muscle following intravascular and tissue IC deposition (19).
Our analyses demonstrate a surprising requirement for the integrin LFA-1 in FcγRIIA-depenent neutrophil contributions to arthritis. Although neutrophil recruitment in joints of wild-type mice was previously reported to be dependent on neutrophil CD18 (22, 41), the LFA-1 requirement in our studies was somewhat unexpected as FcγRIIA can directly bind ICs both in vitro and in vivo (19). The absolute requirement for additional adhesion receptors was thus not apparent a priori. LFA-1 is known to co-cap with FcγRs (42) and cross-linking of FcγRs triggers proximity between the CD18 integrins and cortical microfilaments (43). We speculate that FcγRIIA engagement may lead to activation of LFA-1 and its subsequent interaction with its ligand ICAM-1 on the activated endothelium.
The role of complement in neutrophil FcγRIIA driven responses is also noteworthy. In wild-type mice, the alternate pathway of complement activation drives K/BxN arthritis (12). Here we show that C3 and C5a are required for FcγRIIA-induced disease pathogenesis. The effect of C5aR antagonism on neutrophil accumulation may have been expected as C5a is a powerful neutrophil chemoattractant. The additional information provided by our studies is that the effect of C5a appears to cooperate with FcγRIIA on neutrophils as wild-type mice were resistant to C5aR blockade with the antagonist. The reason for the discrepancy in results with the antagonist and previous work showing that C5aR (CD88) deficient are protected from K/BxN induced arthritis (12) is unclear. It is possible that antagonist penetration into joint tissues is inadequate to fully block C5aR. On the other hand, C5aRA also blocks the other C5aR, C5L2 which may act as a decoy receptor for C5a as has previously been demonstrated in some other models (13).
How does C5a regulate FcγRIIA dependent arthritis? Previous studies suggest that the regulatory impact of C5a on FcγRs on macrophages is mainly due to transcriptional downregulation of the α-chain of FcγRIIB (13) or upregulation of the γ-chain (44, 45), which is lacking in our system. Our studies in vitro suggest that C5a also has a more immediate effect by promoting FcγRIIA dependent phagocytosis and ROS generation in neutrophils. The absolute requirement for C5a in FcγRIIA-mediated phagocytosis of IgG-opsonized RBC suggests that FcγRIIA is relatively inactive in resting cells, which we anticipate may have evolved as a mechanism to maintain circulating neutrophils in a quiescent state much like leukocyte CD18 integrins that are activated to bind ligand only upon stimulation with inflammatory mediators (46). Consistent with this notion, under physiological flow conditions in vitro, FcγRIIA was insufficient to capture neutrophils on plate immobilized ICs (29) or IgG-coated endothelial cells (47) but in the presence of a GPCR agonist such as chemokines firmly adhered to ICs both in vitro (47, 48) and in vivo (19). We speculate that the C5a pathway may be particularly important under conditions of low avidity engagement of FcγRs where it may regulate the amplitude of the response. Interestingly, C5a appears to have some selectivity in its activity towards FcγR: The effect of C5a on complement C3/CR3 induced phagocytosis was marginal relative to its effects on FcγR mediated uptake. There is increasing appreciation of the cross-regulation between ITAM-associated FcγRs and Toll receptors (TLRs) and cytokine receptors (18, 49). However, unlike the TLRs and cytokine receptors, which indirectly link to ITAM-containing receptors, C5aR and FcγR cross-talk directly. The observation that C5a enhanced ROS generation upon direct clustering of FcγRIIA at the cell surface suggests a role for C5a/C5aR downstream of IgG-binding. C5aR may relocalize FcγRIIA to lipid rafts enriched in signaling molecules (50, 51) and/or engagement of C5aR and the ITAM-containing FcγRIIA may lead to convergent MAPK signaling (52).
In conclusion, our studies provide previously unappreciated insight into functional contributions from neutrophil FcγRIIA in joint inflammation in a humanized mouse model. Our studies focus attention on neutrophil FcγRIIA and by extension neutrophils, as critical links between antibody and immunological injury. This could not have been predicted from previous studies on the murine FcγRs. Translationally, since FcγRIIA bearing neutrophils comprise the predominant population in inflamed joint fluid in RA, with neutrophil influx estimated at greater than a billion cells/day in a single joint, it is likely that these insights bear significant relevance for our understanding of disease physiology and suggest that neutrophil FcγRIIA may represent a viable therapeutic target for treatment of RA. Further, there is growing evidence for the role of neutrophils and ICs in numerous other human diseases such as glomerulonephritis, IC-vasculitis, skin autoimmune diseases and others (1). Thus, it is highly likely that our finding of a FcγRIIA:C5aR:LFA-1 receptor triad that functions to provide tight regulation and fine tuning of neutrophil responses to ICs has relevance in other autoimmune diseases with evidence of IC pathophysiology.
Supplementary Material
Acknowledgments
The human MRP8 promoter construct was kindly provided by Dr. E. Lagasse (Univ. of Pittsburgh, PA), and I.L. Weissman (Stanford University, CA). We are grateful to Nick Calderone (Dept of Medicine, Brigham and Women’s Hospital. Boston, MA) for excellent technical assistance and Asuka Shimizu for generously helping N.T. with measurements in histological slides.
Grants and financial support: This work was supported by a Arthritis Foundation postdoctoral fellowship (N.T.), a scholarship from Novartis Foundation, (formerly Ciba-Geigy-Jubilee-Foundation) (T.E.), NIH RO1 HL065095 and AR050800 (T.N.M), KO1 AR054984 (X.C.), AI059745 (D.M.L) and AI059305 (J.K).
Footnotes
AUTHOR CONTRIBUTIONS
NT, TE, XL and HN designed and performed the experiments and analyzed and interpreted the data. MH aided in obtaining the data. NT also contributed to writing the paper. XC, DM, DML and JK provided critical reagents and experimental protocols and gave conceptual advice. TNM contributed to the conception and design of the research, had full access to all the data and data analysis, and wrote the paper. All authors revised the paper for important intellectual content.
References
- 1.Mayadas TN, Tsokos GC, Tsuboi N. Mechanisms of immune complex-mediated neutrophil recruitment and tissue injury. Circulation. 2009;120(20):2012–24. doi: 10.1161/CIRCULATIONAHA.108.771170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turesson C, O’Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis. 2003;62(8):722–7. doi: 10.1136/ard.62.8.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nigrovic PA, Lee DM. Synovial mast cells: role in acute and chronic arthritis. Immunol Rev. 2007;217:19–37. doi: 10.1111/j.1600-065X.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- 4.Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327(5965):580–3. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nigrovic PA, Lee DM. Innate Immunity and Immune complexes in rheumatoid arthritis. In: Firestein GS, Panayi G, Wollheim F, editors. Rheumatoid Arthritis. Oxford University Press; 2005. [Google Scholar]
- 6.Monach PA, Benoist C, Mathis D. The role of antibodies in mouse models of rheumatoid arthritis, and relevance to human disease. Adv Immunol. 2004;82:217–48. doi: 10.1016/S0065-2776(04)82005-4. [DOI] [PubMed] [Google Scholar]
- 7.Kyburz D, Corr M. The KRN mouse model of inflammatory arthritis. Springer Semin Immunopathol. 2003;25(1):79–90. doi: 10.1007/s00281-003-0131-5. [DOI] [PubMed] [Google Scholar]
- 8.Morgan AW, Griffiths B, Ponchel F, Montague BM, Ali M, Gardner PP, et al. Fcgamma receptor type IIIA is associated with rheumatoid arthritis in two distinct ethnic groups. Arthritis Rheum. 2000;43(10):2328–34. doi: 10.1002/1529-0131(200010)43:10<2328::AID-ANR21>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 9.Nieto A, Caliz R, Pascual M, Mataran L, Garcia S, Martin J. Involvement of Fcgamma receptor IIIA genotypes in susceptibility to rheumatoid arthritis. Arthritis Rheum. 2000;43(4):735–9. doi: 10.1002/1529-0131(200004)43:4<735::AID-ANR3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 10.Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- 11.Solomon S, Kassahn D, Illges H. The role of the complement and the Fc gamma R system in the pathogenesis of arthritis. Arthritis Res Ther. 2005;7(4):129–35. doi: 10.1186/ar1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji H, Ohmura K, Mahmood U, Lee DM, Hofhuis FM, Boackle SA, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16(2):157–68. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 13.Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46(14):2753–66. doi: 10.1016/j.molimm.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakus Z, Berton G, Ligeti E, Lowell CA, Mocsai A. Responses of neutrophils to anti-integrin antibodies depends on costimulation through low affinity Fc gamma Rs: full activation requires both integrin and nonintegrin signals. J Immunol. 2004;173(3):2068–77. doi: 10.4049/jimmunol.173.3.2068. [DOI] [PubMed] [Google Scholar]
- 15.Jones SL, Brown EJ. Functional cooperation between Fc-gamma receptors and complement receptors in phagocytes. R.G. Landes Company; 1996. [Google Scholar]
- 16.Trcka J, Moroi Y, Clynes RA, Goldberg SM, Bergtold A, Perales MA, et al. Redundant and alternative roles for activating Fc receptors and complement in an antibody-dependent model of autoimmune vitiligo. Immunity. 2002;16(6):861–8. doi: 10.1016/s1074-7613(02)00327-8. [DOI] [PubMed] [Google Scholar]
- 17.Binstadt BA, Hebert JL, Ortiz-Lopez A, Bronson R, Benoist C, Mathis D. The same systemic autoimmune disease provokes arthritis and endocarditis via distinct mechanisms. Proc Natl Acad Sci U S A. 2009;106(39):16758–63. doi: 10.1073/pnas.0909132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivashkiv LB. Cross-regulation of signaling by ITAM-associated receptors. Nat Immunol. 2009;10(4):340–7. doi: 10.1038/ni.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsuboi N, Asano K, Lauterbach M, Mayadas TN. Human neutrophil Fcgamma receptors initiate and play specialized nonredundant roles in antibody-mediated inflammatory diseases. Immunity. 2008;28(6):833–46. doi: 10.1016/j.immuni.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cullere X, Lauterbach M, Tsuboi N, Mayadas TN. Neutrophil-selective CD18 silencing using RNA interference in vivo. Blood. 2008;111(7):3591–8. doi: 10.1182/blood-2007-12-127837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagasse E, Weissman IL. bcl-2 inhibits apoptosis of neutrophils but not their engulfment by macrophages. J Exp Med. 1994;179(3):1047–52. doi: 10.1084/jem.179.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watts GM, Beurskens FJ, Martin-Padura I, Ballantyne CM, Klickstein LB, Brenner MB, et al. Manifestations of inflammatory arthritis are critically dependent on LFA-1. J Immunol. 2005;174(6):3668–75. doi: 10.4049/jimmunol.174.6.3668. [DOI] [PubMed] [Google Scholar]
- 23.Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10(4):451–61. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 24.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297(5587):1689–92. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 25.Chen M, Boilard E, Nigrovic PA, Clark P, Xu D, Fitzgerald GA, et al. Predominance of cyclooxygenase 1 over cyclooxygenase 2 in the generation of proinflammatory prostaglandins in autoantibody-driven K/BxN serum-transfer arthritis. Arthritis Rheum. 2008;58(5):1354–65. doi: 10.1002/art.23453. [DOI] [PubMed] [Google Scholar]
- 26.Van Lent PL, Blom A, Holthuysen AE, Jacobs CW, Van De Putte LB, Van Den Berg WB. Monocytes/macrophages rather than PMN are involved in early cartilage degradation in cationic immune complex arthritis in mice. J Leukoc Biol. 1997;61(3):267–78. doi: 10.1002/jlb.61.3.267. [DOI] [PubMed] [Google Scholar]
- 27.Otto M, Hawlisch H, Monk PN, Muller M, Klos A, Karp CL, et al. C5a mutants are potent antagonists of the C5a receptor (CD88) and of C5L2: position 69 is the locus that determines agonism or antagonism. J Biol Chem. 2004;279(1):142–51. doi: 10.1074/jbc.M310078200. [DOI] [PubMed] [Google Scholar]
- 28.Booth JW, Kim MK, Jankowski A, Schreiber AD, Grinstein S. Contrasting requirements for ubiquitylation during Fc receptor-mediated endocytosis and phagocytosis. Embo J. 2002;21(3):251–8. doi: 10.1093/emboj/21.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coxon A, Cullere X, Knight S, Sethi S, Wakelin MW, Stavrakis G, et al. Fc gamma RIII mediates neutrophil recruitment to immune complexes. a mechanism for neutrophil accumulation in immune-mediated inflammation. Immunity. 2001;14(6):693–704. doi: 10.1016/s1074-7613(01)00150-9. [DOI] [PubMed] [Google Scholar]
- 30.Hirahashi J, Mekala D, Van Ziffle J, Xiao L, Saffaripour S, Wagner DD, et al. Mac-1 signaling via Src-family and Syk kinases results in elastase-dependent thrombohemorrhagic vasculopathy. Immunity. 2006;25(2):271–83. doi: 10.1016/j.immuni.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Bruhns P, Samuelsson A, Pollard JW, Ravetch JV. Colony-stimulating factor-1-dependent macrophages are responsible for IVIG protection in antibody-induced autoimmune disease. Immunity. 2003;18(4):573–81. doi: 10.1016/s1074-7613(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 32.Binstadt BA, Patel PR, Alencar H, Nigrovic PA, Lee DM, Mahmood U, et al. Particularities of the vasculature can promote the organ specificity of autoimmune attack. Nat Immunol. 2006;7(3):284–92. doi: 10.1038/ni1306. [DOI] [PubMed] [Google Scholar]
- 33.Utomo A, Hirahashi J, Mekala D, Asano K, Glogauer M, Cullere X, et al. Requirement for Vav proteins in post-recruitment neutrophil cytotoxicity in IgG but not complement C3-dependent injury. J Immunol. 2008;180(9):6279–87. doi: 10.4049/jimmunol.180.9.6279. [DOI] [PubMed] [Google Scholar]
- 34.Nagarajan S, Venkiteswaran K, Anderson M, Sayed U, Zhu C, Selvaraj P. Cell-specific, activation-dependent regulation of neutrophil CD32A ligand-binding function. Blood. 2000;95(3):1069–77. [PubMed] [Google Scholar]
- 35.Hirano T. Revival of the autoantibody model in rheumatoid arthritis. Nat Immunol. 2002;3(4):342–4. doi: 10.1038/ni0402-342. [DOI] [PubMed] [Google Scholar]
- 36.Zhao W, Kepley CL, Morel PA, Okumoto LM, Fukuoka Y, Schwartz LB. Fc gamma RIIa, not Fc gamma RIIb, is constitutively and functionally expressed on skin-derived human mast cells. J Immunol. 2006;177(1):694–701. doi: 10.4049/jimmunol.177.1.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ivan E, Colovai AI. Human Fc receptors: critical targets in the treatment of autoimmune diseases and transplant rejections. Hum Immunol. 2006;67(7):479–91. doi: 10.1016/j.humimm.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Pietersz GA, Mottram PL, van de Velde NC, Sardjono CT, Esparon S, Ramsland PA, et al. Inhibition of destructive autoimmune arthritis in FcgammaRIIa transgenic mice by small chemical entities. Immunol Cell Biol. 2009;87(1):3–12. doi: 10.1038/icb.2008.82. [DOI] [PubMed] [Google Scholar]
- 39.Wiktor-Jedrzejczak WW, Ahmed A, Szczylik C, Skelly RR. Hematological characterization of congenital osteopetrosis in op/op mouse. Possible mechanism for abnormal macrophage differentiation. J Exp Med. 1982;156(5):1516–27. doi: 10.1084/jem.156.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaller M, Burton DR, Ditzel HJ. Autoantibodies to GPI in rheumatoid arthritis: linkage between an animal model and human disease. Nat Immunol. 2001;2(8):746–53. doi: 10.1038/90696. [DOI] [PubMed] [Google Scholar]
- 41.Monach PA, Nigrovic PA, Chen M, Hock H, Lee DM, Benoist C, et al. Neutrophils in a mouse model of autoantibody-mediated arthritis: critical producers of Fc receptor gamma, the receptor for C5a, and lymphocyte function-associated antigen 1. Arthritis Rheum. 2010;62(3):753–64. doi: 10.1002/art.27238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou M, Todd RF, 3rd, van de Winkel JG, Petty HR. Cocapping of the leukoadhesin molecules complement receptor type 3 and lymphocyte function-associated antigen-1 with Fc gamma receptor III on human neutrophils. Possible role of lectin-like interactions. J Immunol. 1993;150(7):3030–41. [PubMed] [Google Scholar]
- 43.Zhou MJ, Poo H, Todd RF, 3rd, Petty HR. Surface-bound immune complexes trigger transmembrane proximity between complement receptor type 3 and the neutrophil’s cortical microfilaments. J Immunol. 1992;148(11):3550–3. [PubMed] [Google Scholar]
- 44.Kumar V, Ali SR, Konrad S, Zwirner J, Verbeek JS, Schmidt RE, et al. Cell-derived anaphylatoxins as key mediators of antibody-dependent type II autoimmunity in mice. J Clin Invest. 2006;116(2):512–20. doi: 10.1172/JCI25536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shushakova N, Skokowa J, Schulman J, Baumann U, Zwirner J, Schmidt RE, et al. C5a anaphylatoxin is a major regulator of activating versus inhibitory FcgammaRs in immune complex-induced lung disease. J Clin Invest. 2002;110(12):1823–30. doi: 10.1172/JCI200216577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giblin PA, Lemieux RM. LFA-1 as a key regulator of immune function: approaches toward the development of LFA-1-based therapeutics. Curr Pharm Des. 2006;12(22):2771–95. doi: 10.2174/138161206777947731. [DOI] [PubMed] [Google Scholar]
- 47.Florey OJ, Johns M, Esho OO, Mason JC, Haskard DO. Antiendothelial cell antibodies mediate enhanced leukocyte adhesion to cytokine-activated endothelial cells through a novel mechanism requiring cooperation between Fc{gamma}RIIa and CXCR1/2. Blood. 2007;109(9):3881–9. doi: 10.1182/blood-2006-08-044669. [DOI] [PubMed] [Google Scholar]
- 48.Florey O, Haskard DO. Sphingosine 1-phosphate enhances Fc gamma receptor-mediated neutrophil activation and recruitment under flow conditions. J Immunol. 2009;183(4):2330–6. doi: 10.4049/jimmunol.0901019. [DOI] [PubMed] [Google Scholar]
- 49.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7(12):1266–73. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 50.Mansfield PJ, Hinkovska-Galcheva V, Borofsky MS, Shayman JA, Boxer LA. Phagocytic signaling molecules in lipid rafts of COS-1 cells transfected with FcgammaRIIA. Biochem Biophys Res Commun. 2005;331(1):132–8. doi: 10.1016/j.bbrc.2005.02.191. [DOI] [PubMed] [Google Scholar]
- 51.Kwiatkowska K, Frey J, Sobota A. Phosphorylation of FcgammaRIIA is required for the receptor-induced actin rearrangement and capping: the role of membrane rafts. J Cell Sci. 2003;116(Pt 3):537–50. doi: 10.1242/jcs.00254. [DOI] [PubMed] [Google Scholar]
- 52.Dang PM, Stensballe A, Boussetta T, Raad H, Dewas C, Kroviarski Y, et al. A specific p47phox -serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J Clin Invest. 2006;116(7):2033–43. doi: 10.1172/JCI27544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.