Abstract
Objectives
The Sjögren’s International Collaborative Clinical Alliance (SICCA) is an ongoing NIH-funded registry whose cohort ranges from those with symptoms of possible Sjögren’s syndrome (SS) to those with obvious disease. Using this database we examined associations between labial salivary gland (LSG) histopathology and other phenotypic features of SS.
Methods
LSG biopsy specimens from SICCA participants underwent protocol-directed histopathological assessments. Among 1726 LSG specimens exhibiting any pattern of sialadenitis, we compared biopsy diagnoses against concurrent salivary, ocular and serological assessments.
Results
LSG specimens included 61% with focal lymphocytic sialadenitis, (FLS; 66% of which had focus scores [FS] ≥ 1 per 4 mm2) and 38% with non-specific or sclerosing chronic sialadenitis (NS/SCS). FS ≥ 1 was strongly associated with positive serum anti-SS-A/-B, rheumatoid factor and the ocular component of SS, but not with symptoms of dry mouth or eyes. Those with positive anti-SS-A/-B were 9 times more likely to have a FS ≥ 1 (95% CI: 7.4; 11.9) than FS<1 or another pattern, while those with unstimulated whole salivary flow < 0.1 ml/min were only 2 times more likely to have a FS ≥ 1 (95% CI:1.7; 2.8) than FS<1 or another pattern, while controlling for other phenotypic features of SS.
Conclusions
Distinguishing FLS from NS/SCS is essential in assessing LSG biopsies, before determining FS. A diagnosis of FLS with FS ≥ 1 per 4 mm2, as compared to FLS with FS< 1 or with NS/SCS, was strongly associated with the ocular and serological components of SS and reflects SS autoimmunity.
The Sjögren’s International Collaborative Clinical Alliance (SICCA) is a United States National Institutes of Health (NIH) supported international Sjögren’s syndrome registry, funded from 2003–2013. Through 2009, it comprised research groups in Buenos Aires, Argentina; Beijing, China; Copenhagen, Denmark; Kanazawa, Japan; London, UK, and San Francisco, USA where the SICCA data coordinating center and specimen repository are located at the University of California San Francisco (UCSF). The goals of SICCA include: 1) designing and implementing an international clinical data and biospecimen repository; 2) providing these resources for future studies of Sjögren’s syndrome (SS); and 3) developing standardized, universally acceptable classification criteria for SS. The SICCA registry prospectively enrolls individuals using broad eligibility criteria to establish a cohort ranging from participants with symptoms of possible SS to those with established disease. More information about the SICCA Registry is available in previously published articles (1, 2) and on its website http://sicca.ucsf.edu.
The observation and assessment of lymphocytic infiltration in minor salivary glands has long been associated with SS (3, 4). The first prospective study to include semi-quantitative histopathological examination of labial salivary glands (LSG) was from 40 patients diagnosed with SS, four different types of arthritis, or scleroderma and 60 postmortem specimens (5). Despite much study, the utility and application of focus scoring in the setting of focal lymphocytic sialadenitis (FLS) is still not universally accepted. The range of opinions include the view that it is a specific and accurate assessment of the salivary component of SS (6–11), that it is an alternative in the clinical assessment of SS (12–18), to the view that it is only a scientific assessment for research purposes (19).
In addition to FLS, other morphologic patterns of chronic inflammation occur commonly in LSG biopsy specimens: nonspecific chronic sialadenitis and sclerosing chronic sialadenitis (NS/SCS) (8). LSG biopsies with FLS and focus score (FS) >1 focus / 4mm2, rather than these other patterns, are associated with the diagnosis and severity of the ocular manifestations of SS (keratoconjunctivitis sicca) (11). However the specificity of FLS as compared to NS/SCS in relation to other phenotypic features of SS has not yet been established. Furthermore, a narrow range of FS values have been used as the significance threshold for diagnosing the salivary component of SS, including FS >1 (5, 6, 7, 8), FS≥ 1 (15, 16, 17, 18) and FS ≥ 2 (10) per 4 mm2, but FS < 1 have not been studied.
The specific aims of this study are to improve diagnostic applications of LSG biopsy by using data from the large, prospective SICCA cohort to: 1) distinguish FLS from NS/SCS in LSG biopsies from patients with suspected SS by analyzing their associations with specific ocular, serologic and salivary phenotypic features of SS; and 2) compare FLS focus score values < 1 to those ≥ 1 to assess the traditionally used threshold. These unique assessments are possible because in the SICCA registry a LSG biopsy is performed on all participants as part of their comprehensive baseline study visit.
Patients and Methods
Study Population
In the SICCA registry, examinations and specimen collections are performed by following standardized operating procedures (SOP) that are identical and consistently applied across all six research sites. Adherence to the SOP is ensured by ongoing specimen examination and quality assurance site visits. Eligibility criteria for enrollment require that a participant be at least 21 years of age and have at least one of the following: a complaint of dry eyes or dry mouth; a previous suspicion or diagnosis of SS; elevated serum ANA, RF, SS-A, or SS-B; bilateral parotid enlargement in the clinical setting of SS; a recent increase in dental caries; or a diagnosis of rheumatoid arthritis or systemic lupus erythematosus and possible secondary SS (1). The present analysis is based on a cohort of participants who had been enrolled in the SICCA registry and for whom biopsy results and all other data were available for analysis as of September 20, 2010. Informed consent was in compliance with the Helsinki Declaration and the study was approved by the University of California, San Francisco Committee on Human Research. Additional reviews and approvals were provided by local Institutional Review Boards at each of the institutions listed on the title page.
Variables and Measures
Participants undergo baseline evaluation starting with questionnaires that record among other information demographic data, oral and ocular symptoms and medical history. Three specialty examinations then follow: ocular (including lissamine green and fluorescein ocular surface staining to establish the presence or absence of keratoconjunctivitis sicca, the ocular component of SS, described in detail in reference 2), oral/salivary (including LSG biopsy), rheumatologic and serologic. In aggregate, nine types of biospecimens are collected from each participant, including formalin-fixed and frozen LSGs. Two year follow-up evaluations include the same clinical examinations and biospecimen collections; the results of these longitudinal analyses will be published in a separate manuscript. All SICCA questionnaires, data collection forms and clinical and specimen protocols are available for review on the SICCA website http://sicca.ucsf.edu.
LSG biopsies are performed at the time of the SICCA baseline evaluation on all participants, or a previous LSG biopsy specimen is accepted if performed no more than three years previously and the microscopic slides are available for examination. LSG biopsies are performed after local anesthetic infiltration to harvest 5–10 glands (6, 8, 20), some of which are fixed in neutral buffered formalin; while others are quickly frozen in liquid nitrogen. Three to five formalin-fixed LSGs are processed by the local pathology departments (paraffin embedding, sectioning and hematoxylin and eosin [H&E] staining) and remaining glands are frozen and stored in liquid nitrogen. All biospecimens, including paraffin embedded and frozen salivary glands, are shipped quarterly to the SICCA Coordinating Center at UCSF.
H&E stained sections of each specimen are evaluated initially by one of three pathologists, who are blinded to the participants’ demographic, clinical or serological characteristics, and who assigns one of six possible diagnoses: FLS, non-specific chronic sialadenitis (NSCS), chronic sclerosing sialadenitis (SCS), granulomatous inflammation, MALT (marginal zone) lymphoma or within normal limits. All diagnoses are defined in the footnote to Table 1, and photomicrographs of FLS are provided as illustration (Figures 1, 2A, 2B and 2C). If a diagnosis of FLS is made in any specimen, the FS is then determined (20, 21). Specimens must have a glandular area of at least 4 mm2 (preferably 10–20 mm2 because smaller specimens can overestimate focus scores) and have lymphocytic foci ≥ 50 cells (Figure 2A), but most are larger (Fig 1, 2B, 2C). FLS may include hyperplasia and lymphocytic infiltration of ductal epithelium or lymphoid germinal centers (Fig 2C). A focus score of 12 foci/4 mm2 is usually the highest that can be counted; above that number of foci, infiltrates become confluent. Each specimen is then independently reevaluated by a second observer and any differences are resolved by consensus between the first two, or with a third observer. This approach provides an ongoing calibration of the examiners. We also conducted a formal assessment of the inter-examiner agreement rate on 56 biopsy specimens that had been read independently by the 2 main pathologists. We computed the Kappa statistics to assess agreement rate on their diagnoses, and the interclass correlation coefficient (ICC) to assess agreement rate for diagnosis, number of foci and FS.
Table 1.
Distribution of histopathological diagnoses and focus scores in labial salivary gland (LSG) biopsies collected from 1787 baseline participants in the SICCA Registry
| n | (%) | ||
|---|---|---|---|
| Histopathological diagnoses | |||
| Focal lymphocytic sialadenitis (FLS)1 | 1093 | (61) | |
| Non-specific chronic sialadenitis2 | 372 | (21) | |
| Sclerosing chronic sialadenitis3 | 296 | (17) | |
| Within normal limits (no lymphocytes)4 | 22 | (1) | |
| Granulomatous inflammation5 | 3 | (<1) | |
| Marginal zone (MALT) lymphoma6 | 1 | (<1) | |
| Focus scores (FS) among 1058 with FLS1 | |||
| Focus score > 1 foci per 4 mm2 | 693 | (66) | |
| Focus score = 1 foci per 4 mm2 | 37 | (3) | |
| Focus score < 1 foci per 4 mm2 | 328 | (31) | |
| Presence of germinal centers7 | 115 | (11) | |
| Focus score percentiles among 1058 with FLS (range: 0.1 – 13.5) | |||
| 1st percentile | 0.1 | ||
| 25th percentile | 0.8 | ||
| 50th percentile | 1.8 | ||
| 75th percentile | 3.7 | ||
| 99th percentile | 11.6 | ||
Focal lymphocytic sialadenitis (FLS) is the presence of one or more dense aggregates of 50 or more lymphocytes (usually several hundred or more) usually located in perivascular or periductal locations. The foci are located adjacent to normal-appearing mucous acini in gland lobes or lobules lacking duct dilation or interstitial fibrosis and contain no more than a minority proportion of plasma cells. This diagnosis is assigned when these foci are the only inflammation present in a specimen, or the most prominent feature. 1058 of these specimens were large enough (i.e. ≥ 4 mm2) for focus score assessment. Focus scores are then based on assessing the glandular area in each and calculating the number of lymphocytic foci present, per 4 mm2 of glandular area (described in detail in reference #20).
Non-Specific chronic sialadenitis (NSCS) is present when there are scattered or focal infiltrates of lymphocytes, macrophages and plasma cells that are not adjacent to normal-appearing acini and located in gland lobules that exhibit some combination of acinar atrophy, interstitial fibrosis, duct dilation and luminal inspissated mucus.
Sclerosing chronic sialadenitis (SCS) appears to be an advanced stage of NSCS in which interstitial fibrosis, various patterns of chronic inflammation and acinar atrophy predominate.
Within normal limits is diagnosed in minor salivary glands with normal appearing architecture and scattered plasma cells, but without acinar atrophy and few if any lymphocytes.
Granulomatous inflammation is present when there are clusters of CD-68 positive macrophages, with or without occasional multinucleated giant cells and absent necrosis.
Marginal zone (MALT) lymphoma is diagnosed in minor salivary glands exhibiting diffuse lymphocytic infiltration with loss of glandular architecture and composed of sheets of CD20 positive cells without follicular distribution, few scattered CD3 positive cells and few if any follicular dendritic (CD21 or CD 23 positive) cells.
Germinal center presence is estimated in H&E stained sections by the presence of a cluster of relatively clear staining cells within a lymphocytic focus. More specific identification of germinal centers requires immunohistochemical staining for follicular dendritic cells with anti-CD21 or CD23.
Figure 1.
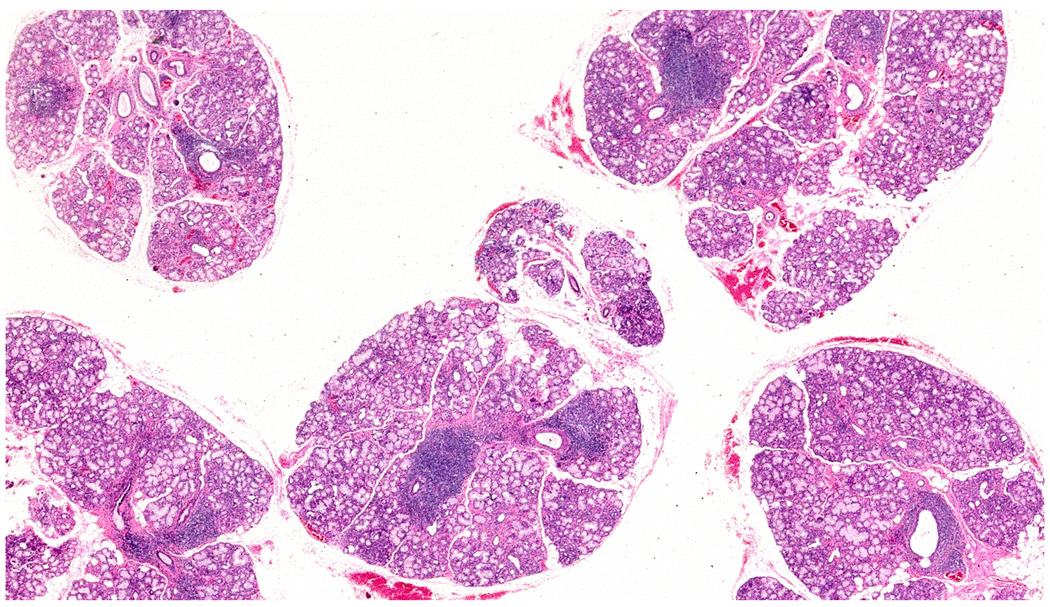
Five hematoxylin and eosin stained labial salivary glands exhibit focal lymphocytic sialadenitis in all glands. About 10 focal lymphocytic infiltrates can be seen in this image. In the microscope, there is a total glandular area of 24 mm2 giving a focus score of 2 foci per 4 mm2. Original magnification ×2.
Figure 2.
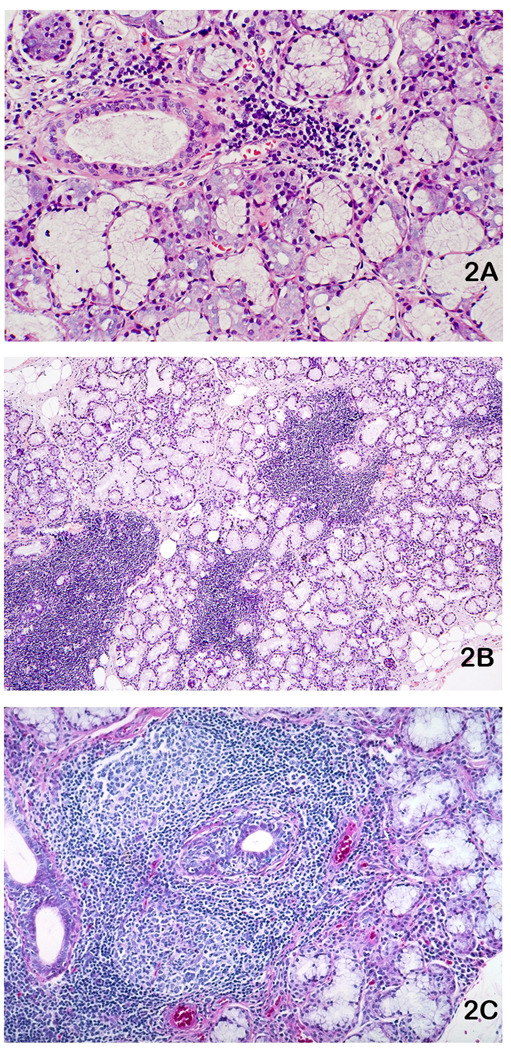
Labial salivary glands (LSG) stained with hematoxylin and eosin exhibiting focal lymphocytic sialadenitis (FLS): A. One LSG with a small lymphocytic aggregate that is minimally sized (> 50 cells) for inclusion in a focus score calculation. Original magnification X100. B. One LSG with four variously sized lymphocytic foci. Note normal appearing acini immediately adjacent to the lymphocyte aggregates, a characteristic feature of FLS. The entire specimen has a focus score of 3 foci per 4 mm2. Original magnification ×16. C. FLS with two prominent lymphocytic germinal centers and ductal hyperplasia with lymphocytic infiltration. Original magnification ×40.
Specimens exhibiting other patterns of chronic inflammation, as defined in Table 1, are classified as NSCS or SCS depending on the presence of interstitial fibrosis, atrophic or absent acini and scattered (Figure 3A) or focal chronic inflammation (Figures 3B and 3C). These aggregates are not counted for a focus score because of the absence of adjacent normal acini. Specimens containing epithelioid histiocytes and occasional Langhans-type giant cells forming non-caseating granulomas are further examined by immunohistochemistry to detect the pattern of CD68 antigen expression. In such cases, the absence of acid-fast bacilli in the specimen would lead to recommending that the participant be evaluated for sarcoidosis or other chronic granulomatous disease. Some specimens with no apparent lymphocytic infiltration or other inflammation are classified as “within normal limits”.
Figure 3.
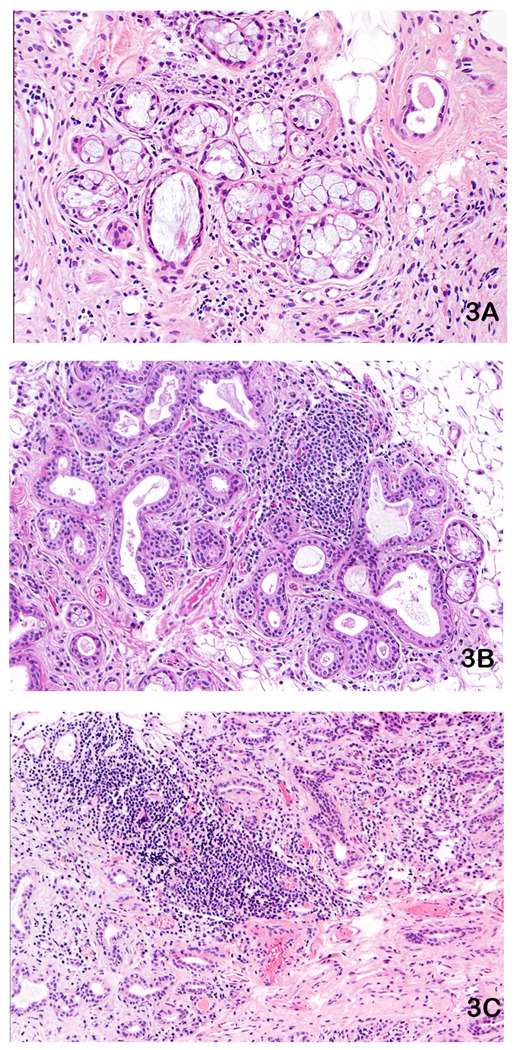
Labial salivary glands (LSG) stained with hematoxylin and eosin exhibiting non-specific chronic sialadenitis (NSCS) or sclerosing chronic sialadenitis (SCS). These patterns do not represent the salivary component of Sjögren’s syndrome and all of these specimens are from participants with negative: anti-SS-A, anti-SS-B antibodies and rheumatoid factor: A. SCS in a LSG exhibiting scattered lymphocytes and plasma cells with prominent interstitial fibrosis. Original magnification ×100. B. SCS in a LSG with duct dilation, interstitial fibrosis and a prominent lymphocytic infiltrate, but without adjacent normal-appearing acini. Original magnification ×50. C. SCS with severe interstitial fibrosis, a lymphocytic aggregate, many duct-like structures and no normal-appearing acini. Original magnification ×50.
Statistical Methods
We computed proportions to explore the distribution of histopathological diagnoses from the LSG biopsies and of the FS (among those with FLS), after categorizing the FS as > 1; = 1; or < 1 foci per 4 mm2, and the presence of germinal centers within specimens with FLS and an assessable FS. Among specimens found to exhibit any form of sialadenitis, we explored the associations between three categories of labial salivary gland diagnoses (FLS with FS ≥ 1, FLS with FS < 1, and NS/SCS) and other phenotypic characteristics of SS using a contingency table approach (with chi-square tests). We used a non-parametric approach (Wilcoxon rank-sum test) to compare FS as a continuous variable by presence/absence of each phenotypic feature of SS, and present FS median and range accordingly. We then fitted a logistic regression model to explore the explanatory role of various phenotypic features of SS in relation to the outcome “having FLS with FS ≥ 1 as compared to FS < 1 or NS/SCS”, among participants with sialadenitis. We thus present adjusted odds ratios (adjOR) with 95% confidence intervals (95%CI).
Results
We analyzed LSG biopsy specimens from 1787 participants enrolled in the SICCA Registry as of September 20, 2010. About one-quarter (26%) of the participants were enrolled from the US, 20% from Denmark, 17% from Argentina, 15% each from Japan and China, and 7% from the UK (since 2007). The majority (93%) was women with a median age of 54 years (range: 21–90). Eighty seven (5%) of the participants were classified as having secondary SS as they had confirmed diagnoses of rheumatoid arthritis, systemic lupus, and a few with scleroderma or mixed connective tissue disease. There was a mean of 4.7 (±1.6) minor glands per LSG specimen with a total mean glandular area of 14.4 mm2 ± 7.8 per specimen. Table 1 summarizes histopathological diagnoses from all baseline specimens. A total of 1093 (61%) specimens were diagnosed as FLS, 668 (36%) were diagnosed as one of two other forms of chronic sialadenitis and 26 (2%) were given other diagnoses including 22 within normal limits and one case of marginal zone (MALT) lymphoma. All histopathological grading criteria are included with Table 1. Among the 1093 specimens with FLS, 35 (3%) were too small to support calculating a focus score. Among the remaining 1058 specimens, 66% had a FS > 1, 3% had a FS = 1, 31% a FS < 1 and 11% included germinal centers. The FS ranged from 0.1 to 13.5 with a median of 1.8. Given the small proportion of FLS specimens with FS=1 (3%), we combined them with specimens with FS>1 in our analyses.
In a calibration exercise based on 56 slides reviewed independently by the 2 main pathologists, we found high agreement rates with respect to diagnosis (Kappa=0.98; 95%CI: 0.91; 1.00), number of foci (ICC=0.97; 95%CI: 0.96; 0.99), and FS (ICC=0.96; 95%CI: 0.94; 0.99).
Among the 1093 specimens diagnosed as FLS, 266 also included generally small areas of periductal sclerosis. Prior to combining this subset with the 827 specimens that did not exhibit such sclerosis, we ruled out any statistical differences between these two FLS subgroups with respect to serologic measures of autoimmunity (elevated serum ANA, RF, SS-A, or SS-B) controlling for FS. However, age was found to be associated with periductal sclerosis: the median age among the group with FLS and sclerosis was 61 years compared to 51 years in the FLS only group (P<0.001), both among those with FS ≥1 and among those with FS <1.
Among 1787 LSG biopsy specimens, 1726 had some form of sialadenitis (and an assessable FS), and among those we found a high proportion of participants with FS ≥ 1 (as compared to FS <1 or to NS/SCS) among those with positive serum SS-A/B (76%) and/or rheumatoid factor (RF) (72%), ANA titer >1:320 (72%), hypergammaglobulinemia (73%), ocular staining score ≥3 (50%), or unstimulated whole salivary flow rates less than 0.1 mL/min (53%) (Table 2). Strong statistical associations were observed between these four phenotypic features of SS and the pattern of sialadenitis (FS ≥1 versus FS<1 versus NS/SCS), all at P < .0001. There were no significant associations between any pattern and participants’ symptoms of dry mouth or dry eyes.
Table 2.
Bivariate analysis exploring patterns of sialadenitis and focus scores by phenotypic features of Sjögren’s syndrome (SS) in 1726 SICCA participants with sialadenitis
| Sialadenitis Pattern1 | ||||
|---|---|---|---|---|
| FLS | FLS | NS/SCS | ||
| FS ≥1 | FS < 1 | no FS | ||
| n=730 | n=328 | n=668 | ||
| Phenotypic features of SS | n (%)2 | n (%)2 | n (%)2 | P (chi2) |
| Serum anti-SS-A/-B | ||||
| Positive | 487 (76) | 63 (10) | 91 (14) | |
| Negative | 243 (22) | 265 (24) | 575 (53) | < .0001 |
| Rheumatoid factor | ||||
| Positive | 458 (72) | 64 (10) | 113 (18) | |
| Negative | 270 (37) | 264 (24) | 555 (51) | < .0001 |
| Ocular Surface Staining | ||||
| ≥ 3 | 630 (50) | 206 (16) | 415 (33) | |
| < 3 | 99 (21) | 121 (26) | 253 (53) | < .0001 |
| ANA | ||||
| ≥ 1:320 | 477 (72) | 68 (10) | 115 (17) | |
| < 1:320 | 253 (24) | 260 (24) | 552 (52) | < .0001 |
| IgG | ||||
| > 1445 mg/dL | 424 (73) | 54 (9) | 104 (18) | |
| ≤ 1445 mg/dL | 305 (27) | 273 (24) | 561 (49) | < .0001 |
| UWS3 flow rate | ||||
| < 0.1 mL/min | 502 (53) | 148 (15) | 306 (32) | |
| ≥ 0.1 mL/min | 228 (30) | 179 (23) | 362 (47) | < .0001 |
| Dry mouth symptoms | ||||
| Present | 669 (43) | 292 (19) | 595 (38) | |
| Absent | 60 (36) | 35 (21) | 70 (42) | 0.3 |
| Dry eye symptoms | ||||
| Present | 624 (43) | 292 (20) | 549 (37) | |
| Absent | 105 (41) | 35 (14) | 117 (46) | 0.01 |
Among 1787 LSG biopsy specimens analyzed, 1726 had some form of sialadenitis as FLS: Focal lymphocytic sialadenitis, or NS/SCS: non specific or sclerosing chronic sialadenitis; FS=focus score (expressed in lymphocytic foci per 4 mm2 of glandular area)
Row percentages may not add to 100 due to rounding
Unstimulated whole salivary flow rate
A non-parametric analysis performed to explore FS as a continuous variable in relation to each phenotypic feature of SS, confirmed the associations displayed in Table 2 where FS was categorized as ≥ 1, < 1, and no FS. The median FS among those with positive anti-SS-A/B serology was 2.8 [range: 0.1 – 12.5] versus 0.9 [range: 0.1 – 13.5] among those with negative anti-SS-A/B (P < 0.0001). Participants with other abnormal serological tests such as positive RF, ANA ≥ 1:320, and hypergammaglobulinemia (IgG > 1445), also had a higher median FS (3, 2.8, and 3, respectively) than those with negative test results (1, 1.1, and 1, respectively). Similarly, a non parametric rank sum test (Wilcoxon) revealed statistically significant association between FS and each of these serological tests. The median FS was also elevated in those with abnormal OSS (≥ 3) and UWS < 0.5 mL/min (2.2 and 2.3, respectively) compared to 0.9 and 1.1 in those without abnormal OSS and UWS (P < 0.0001). Finally, the median FS among specimens with germinal centers was 4.3 [range: 0.8 – 13.5] and among those without germinal centers 1.5 [range: 0.1 – 12.5]. A non parametric test (Wilcoxon) performed to compare FS in the two groups revealed a statistically significant association between FS and the presence of germinal centers (P < 0.0001), with as described above, a higher median FS among those with germinal centers.
Regarding prescription drug use, when we stratified our contingency table analysis by participants using one or more of the many drugs that could reduce salivary secretion, we found the following: Among participants who were not taking such drugs, 50% of those reporting symptoms of dry mouth had a FS≥ 1 versus 17% with a FS<1 and 33% with NS/SCS (P=0.02). Among those who were taking such drugs, there was no association between the pattern of sialadenitis and symptoms of dry mouth (P=0.7), suggesting that anticholinergic drug use was an effect modifier. Similarly, responses to more specific questions such as “Do you need to sip liquids to swallow dry foods?” or “Does your mouth feel dry when eating a meal?” (22) were not associated with the pattern of sialadenitis among those taking these drugs, but an association was found among those not taking such drugs.
We found that those with positive anti-SS-A/B serology were 9 times more likely to have a FS ≥ 1 (95% CI: 7.4; 11.9) than those with negative serology, after controlling for abnormal ocular surface staining, abnormal UWS, and dry mouth/dry eyes symptoms. Similarly, those with either abnormal ocular surface staining (≥ 3) or abnormal UWS (< 0.1mL/min) were more than twice as likely to have FS ≥ 1 as those without these characteristics (Table 3).
Table 3.
Multivariate model exploring the explanatory role of various phenotypic features of Sjögren’s syndrome (SS) in relation to the outcome “having focal lymphocytic sialadenitis with focus score (FS) ≥ 1 as compared to FS < 1 or non-specific or sclerosing chronic sialadenitis” among 17161 SICCA participants with sialadenitis
| Phenotypic features of SS |
adjOR2 | 95%CI2 | P (chi2) |
|---|---|---|---|
| Positive anti-SS-A and/or –B serology | 9.4 | 7.4; 11.9 | < 0.0001 |
| SICCA dry eye score ≥ 3 | 2.2 | 1.6; 2.9 | < 0.0001 |
| UWS3 (ml/min) < 0.1 | 2.2 | 1.7; 2.8 | < 0.0001 |
| Reported dry mouth symptoms | 1.2 | 0.8; 1.8 | 0.5 |
| Reported dry eyes symptoms | 1.0 | 0.7; 1.4 | 0.9 |
10 participants among 1726 with sialadenitis had a missing observation on at least one of the independent variables
Adjusted odds ratios and 95% confidence intervals
Unstimulated whole salivary flow rate
Discussion
This is the first large scale, prospective cohort study to analyze and confirm the importance of distinguishing FLS from NS/SCS in LSG biopsies from patients with suspected SS and to demonstrate their associations with phenotypic features of the disease. We also compared FS thresholds of < 1 to ≥ 1 foci per 4 mm2 and found no basis to change the traditional threshold value of ≥1. This analysis of over 1,700 LSG biopsy specimens found that FLS with FS≥ 1 was strongly associated with the main phenotypic features of SS, including positive anti-SS-A/-B and RF serology, high ANA titers and IgG concentration, presence of keratoconjunctivitis sicca (ocular staining ≥3), and unstimulated whole salivary flow rates less than 0.1 mL/min). FLS with FS≥ 1 was not associated with the symptoms of dry mouth or dry eyes.
Labial salivary gland biopsy
The LSG biopsy has played an important role in SS because of its disease specificity, wide availability, minimal invasiveness, and opportunity to assess autoimmune disease-active cells within a SS target organ. This study has shown that the presence of FLS was highly associated with both the serological and ocular components of SS and was significantly more specific for the salivary component of SS than unstimulated salivary flow rate <0.1 ml/min. The LSG biopsy is generally a minimally invasive procedure that can yield histopathological information about the extent and nature of the disease process. The greatest weakness of LSG biopsy is inconsistent histopathological assessment, which can be overcome by following the protocol described in this study and on the SICCA website (20).
The FS threshold of >1 was first suggested in 1968 (5) and has since been applied in several large series (7, 8, 11). In 1993, a threshold of FS≥ 1 was proposed (15) and continued through 2002 (18). Among the participants in our cohort who had FLS, only 3% had FS=1. It is therefore somewhat arbitrary whether these specimens should be combined with the specimens with FS>1 or with those with FS<1 for classification purposes. To maintain consistency with more recent studies conducted by others, we decided to combine specimens with FS=1 with those with FS>1 for analyses. Table 2 shows that those participants with FLS and FS<1 had proportions of phenotypic features of SS that were significantly lower than among those with FS≥1. Thus this analysis confirms that FLS with FS ≥ 1 represents a distinct entity from FLS with FS < 1 or NS/SCS and is strongly associated with the phenotypic features of SS.
The salivary component of SS
In 1933 Henrik Sjögren first noted symptoms of hyposalivation in almost half of his19 study patients and observed significant lymphocytic infiltration of the parotid, sublingual and accessory salivary glands in examining one postmortem case (3). Thus began the uncertainty about the nature of the salivary component of SS. Is it a symptom of dryness, a secretory threshold value, results from salivary scintigraphy, a sialographic image, or a histopathological pattern? An early study defined SS as the presence of two out of three from “the triad of keratoconjunctivitis sicca [KCS] (‘dry eyes’), xerostomia (‘dry mouth’), and rheumatoid arthritis or other connective tissue disease” (23). Unfortunately, the term “xerostomia” was and continues to be applied, often indiscriminately, to either symptoms or signs of dry mouth with no consensus on how to assess either. This confusion 35 years ago led to defining the salivary component of SS as FLS (with a FS> 1) in an adequate LSG biopsy specimen, instead of “xerostomia” (7). Other methods to define the salivary component of SS have been introduced, including defined whole salivary or parotid flow rates, with or without stimulation, sialographic imaging of a major salivary gland, measuring technetium uptake and secretion with salivary scintigraphy, and ultrasound images of the glands. However, the specificity of these assessments to SS has not been clearly established. Meanwhile, a strong association was shown between the presence and severity of the ocular component of SS (keratoconjunctivitis sicca) and FLS in LSG biopsies (11), confirming the relevance of FLS as a disease-specific measure of the salivary component of SS.
Symptoms of dry mouth have been proposed as components of classification criteria for pSS since 1993 (15, 18). However, SICCA study participant responses to the questions “Does your mouth feel dry?” or “Do your eyes feel dry?” were not statistically associated with the presence of focal lymphocytic sialadenitis (focus score >1), serum anti-SS-A/B, or ocular staining ≥ 3 (indicating keratoconjunctivitis sicca-KCS) (Table 2) (1, 24). Furthermore, the presence of an association between the pattern of sialadenitis and symptoms of dry mouth among those not taking anticholinergic drugs, and absence of association among those taking these medication types, suggests the presence of a statistical interaction. Thus these findings confirm that symptoms of dry eyes or dry mouth may be non-specific and can be due to causes other than SS in a significant proportion of patients. We have also shown that unstimulated whole salivary flow rates were significantly associated with FLS and FS ≥1, but at a lower adjusted odds ratio than the other three phenotypic features of SS (Table 3).
Grading salivary lymphocytic infiltration
The Chisholm and Mason (C&M) grading scheme for assessing inflammation in LSG biopsies applied both qualitative and semi-quantitative assessments of lymphocytic infiltration to LSGs, while still embedded in mucosal epithelium and connective tissue (5). It introduced the useful SS-associated threshold value of “more than one focus of 50 or more lymphocytes per 4 mm2 of salivary tissue,” but its Grades 0 to 4 are now obsolete. It is a non-linear scale with C & M grades 0 and 1 assessed qualitatively, grade 2 assessed qualitatively or semi-quantitatively (focus scores < 1 per 4 mm2) and grades 3 and 4 semi-quantitatively (grade 3, focus scores of 1 per 4 mm2 and grade 4, focus scores >1 per 4 mm2). It can provide a useful severity threshold assessment, but does not further consider severity levels above focus scores of 1 (6) and, most importantly, does not distinguish between different patterns of chronic LSG inflammation (i.e. FLS vs NS/SCS), as reported in this study and previously (8, 11). Previous studies have not examined the associations of SS components to LSGs with FS < 1, which we report here to be very similar to the specimens with NS/SCS and significantly different from those with FS ≥ 1.
Based on reviews of previously diagnosed labial salivary gland biopsies, some pathologists do not perform the semi-quantitative part of assessing LSG biopsies to develop a focus score, or do so incorrectly (27). LSG biopsies must first be diagnosed qualitatively to assess the presence of FLS vs NS/SCS: if FLS is present then focus score assessment should follow, but if NS/SCS is present a focus score is unnecessary and would be misleading if performed.
We observed that FLS specimens exhibiting periductal sclerosis were from older participants (median 61 years) than those with FLS specimens without sclerosis (median 51 years). However, this age difference existed whether the FS is ≥ 1 or < 1, suggesting that while age is associated with periductal sclerosis, it is not a confounding variable in the FS analysis. The presence of sclerosis is consistent with an earlier observation that there is a proportional increase in salivary gland fibrous tissue with increasing age (25).
The presence of germinal centers within lymphocytic infiltrates of LSGs was observed in 17% of a series of specimens from patients with SS, indicating lymphoid neogenesis within these target organs of SS (26). As noted in results from the present study, the median FS was higher in specimens with evidence of germinal center formation (4.3) compared with those without (1.5) and showing a strong association between higher focus scores and the presence of germinal centers. The small difference between the 17% prevalence of LSG germinal centers in the previous study and 11% in the present study is most likely a result of their using various immunohistochemical markers to identify germinal centers and our result based on their presence in H&E stained sections.
Misinterpretation of LSG biopsies
Assessing LSG biopsy specimens in the setting of SS can be subject to several types of misinterpretation. These include failing to perform a focus score on specimens exhibiting FLS and attempting to apply a focus score to specimens having non-specific patterns of inflammation (27). Based on the usual irregular distribution of lymphocytic foci in LSGs, another diagnostic pitfall is an assessment when too little tissue is present (e.g. only one gland or fragments of several glands are present), which can result in the focus score being overestimated. These can be avoided by following a protocol driven assessment of LSG that dictate minimum size of salivary gland tissue prior to focus scoring.
Conclusions
LSG biopsies with FS ≥ 1, as compared to FS < 1 or with NS/SCS, are strongly associated with phenotypic ocular and serological components of SS. LSG biopsy with FS ≥ 1 is not a “gold standard” for diagnosing SS, but remains the best method for diagnosing its salivary component and assessing an important site of autoimmune activity.
Acknowledgments
In addition to the listed authors, many other professional collaborators in the Sjögren’s International Collaborative Clinical Alliance have been essential to the conduct of this project. Their names and roles follow:
University of California, San Francisco, USA. Rheumatology K Sack, D Lee, Ophthalmology J Whitcher, N McNamara, E Strauss, Oral Medicine D Greenspan, Operations Director Y DeSouza, Clinical Coordinator / Phlebotomy D Drury, Clinical Coordinator A Do, Clinical Assistant L Scott, Data Manager J Nespeco, Finance Director J Whiteford, Administrative Assistant M Margaret
University of Buenos Aires and German Hospital, Buenos Aires, Argentina: Ophthalmology AM Heidenreich, Rheumatology C Vollenweider, Stomatology I Adler, AC Smith, Specimen processing S Daverio, Group Coordinator V Kambo
Peking Union Medical College Hospital, Beijing, China: Rheumatology W Zheng, Y Jiang, D Xu, J Su, Ophthalmology S Zhang, J Zhao, Stomatology/ Pathology D Du, Stomatology/ LSG biopsies H Wang, Z Li, J Xiao, Specimens / Rheumatology Q Wu, Phlebotomy C Zhang, W Meng, Project Assistant J Zhang, Founding Director Y Dong.
Copenhagen University Hospital Glostrup, Denmark: Ophthalmology S Johansen, S Hamann, Rheumatology P Helin, J Lindegaard, Group Coordinators/Specimen Handling AM Manniche, SP Kreutzmann.
Kanazawa Medical University, Ishikawa, Japan: Rheumatology Y Masaki, T Sakai, Ophthalmology K Kitagawa, N Shibata, Stomatology M Honjo, Specimen processing T Kawanami, Group Coordinator K Fujimoto, Founding Director S Sugai.
King’s College London, UK: Co-Director P Shirlaw, Rheumatology B Kirkham, Pathology P Morgan, Specimen processing L Fernandes-Naglik.
Aravind Eye Hospital, Madurai, India: Group Director M Srinivasan, co-Directors Jeena, M Das, A Kumar, Ophthalmology Pallavi, Physician R Banushree, Surgeon Dr. Jeena, Oral Medicine B Babu, Pathology R Shanthi, Administration A Ram, Saravanan, Kannappan, Group Coordinator N Kalyani
University of Pennsylvania, Philadelphia, USA: Group Director F Vivino, Rheumatology S Seghal, Ophthalmology V Bunya, M. Massaro-Giordano, Otolaryngology SK Abboud, Oral Medicine A Pinto, Group Coordinator L Fisher
Johns Hopkins University, Baltimore, Maryland, USA: Group Director A Baer, Ophthalmology E Akpek, Oral Medicine W Henderson, Otolaryngology C Gourin, Group Coordinator A Keyes
This project is supported by NIH contract “International Research Registry Network for Sjögren’s Syndrome” from the NIDCR, NEI and ORWH, 2003–2013. The project received an unrestricted gift from GlaxoSmithKline during the years 2004–2008.
Supported by NIH contract NOI-DE-32636
Abbreviations used
- C&M
Chisholm and Mason
- FLS
focal lymphocytic sialadenitis
- FS
focus score
- H&E
hematoxylin and eosin
- ICC
interclass correlation coefficient
- LSG
labial salivary gland
- MALT
mucosa-associated lymphoid tissue
- NSCS
non-specific chronic sialadenitis
- NS/SCS
non-specific or sclerosing chronic sialadenitis
- RF
rheumatoid factor
- SCS
sclerosing chronic sialadenitis
- SICCA
Sjögren’s International Collaborative Clinical Alliance
- SS
Sjögren’s syndrome
- UCSF
University of California, San Francisco
References
- 1.Daniels TE, Criswell LA, Shiboski C, Shiboski S, Lanfranchi H, Dong Y, Schiødt M, Umehara H, Sugai S, Challacombe S, Greenspan JS. An Early View of the International Sjögren’s Syndrome Registry. Arthritis Care Res. 2009;61:711–714. doi: 10.1002/art.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitcher JP, Shiboski CH, Shiboski SC, Heidenreich AM, Kitagawa K, Zhang S, et al. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjögren’s syndrome International Registry. Am J Ophthalmol. 2010;149:405–415. doi: 10.1016/j.ajo.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sjögren H. Zur kenntnis der keratoconjunctivitis sicca (Keratitis filiformis bei Hypofunktion der Tränendrüsen) Acta Ophthalmol (Copenh) 1933;11 Suppl 2:1–151. English translation by JB Hamilton: Sjögren H. A New Conception of Keratoconjunctivitis Sicca. Sydney, Australasian Medical Publishing Co. Ltd., 1943. [Google Scholar]
- 4.Waterhouse JP. Focal adenitis in salivary and lacrimal glands. Proc Roy Soc Med. 1963;56:911–918. doi: 10.1177/003591576305601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chisholm DM, Mason DK. Labial salivary gland biopsy in Sjögren’s disease. J Clin Path. 1968;21:656–660. doi: 10.1136/jcp.21.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenspan JS, Daniels TE, Talal N, Sylvester RA. The histopathology of Sjögren’s syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol. 1974;37:221–229. doi: 10.1016/0030-4220(74)90417-4. [DOI] [PubMed] [Google Scholar]
- 7.Daniels TE, Silverman S, Michalski JP, Greenspan JS, Sylvester RA, Talal N. The oral component of Sjögren's syndrome. Oral Surg Oral Med Oral Pathol. 1975;39:875–878. doi: 10.1016/0030-4220(75)90108-5. [DOI] [PubMed] [Google Scholar]
- 8.Daniels TE. Labial salivary gland biopsy in Sjögren's syndrome: Assessment as a diagnostic criterion in 362 suspected cases. Arthritis Rheum. 1984;27:147–156. doi: 10.1002/art.1780270205. [DOI] [PubMed] [Google Scholar]
- 9.Skopouli FN, Drosos AA, Papaioannou T, Moutsopoulos HM. Preliminary diagnostic criteria for Sjögren's syndrome. Scand J Rheumatol Suppl. 1986;61:22–25. [PubMed] [Google Scholar]
- 10.Fox RI, Robinson CA, Curd JG, Kozin F, Howell FV. Sjögren's syndrome. Proposed criteria for classification. Arthritis Rheum. 1986;29:577–585. doi: 10.1002/art.1780290501. [DOI] [PubMed] [Google Scholar]
- 11.Daniels TE, Whitcher JP. Association of patterns of labial salivary gland inflammation with keratoconjunctivitis sicca. Analysis of 618 patients with suspected Sjögren’s syndrome. Arthritis Rheum. 1994;37:869–877. doi: 10.1002/art.1780370615. [DOI] [PubMed] [Google Scholar]
- 12.Ohfuji T. Annual report of the ministry of Health and Welfare: Sjögren’s disease Research Committee. Japan: 1977. Review on research reports; pp. 3–8. (published in English by Homma et al in 1986) [Google Scholar]
- 13.Manthorpe R, Frost-Larsen K, Isager H, Prause JU. Sjögren's syndrome. A review with emphasis on immunological features. Allergy. 1981;36:139–153. doi: 10.1111/j.1398-9995.1981.tb01829.x. [DOI] [PubMed] [Google Scholar]
- 14.Homma M, Tojo T, Akizuki M, Yamagata H. Criteria for Sjögren's syndrome in Japan. Scand J Rheumatol Suppl. 1986;61:26–27. [PubMed] [Google Scholar]
- 15.Vitali C, Bombardieri S, Moutsopoulos HM, Balestrieri G, Bencivelli W, Bernstein RM, et al. Preliminary criteria for the classification of Sjögren's syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum. 1993;36:340–347. doi: 10.1002/art.1780360309. [DOI] [PubMed] [Google Scholar]
- 16.Vitali C, Bombardieri S, Moutsopoulos HM, Coll J, Gerli R, Hatron PY, et al. Assessment of European classification criteria for Sjögren’s syndrome in a series of clinically defined cases: results of a prospective multicentre study. Ann Rheum Dis. 1996;55:116–121. doi: 10.1136/ard.55.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujibayashi T. Revised diagnostic criteria for Sjögren’s syndrome. Rheumatology. 2000;24:421–428. (in Japanese) [Google Scholar]
- 18.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindahl G, Hedfors E. Labial salivary gland lymphocytic infiltration in Sjögren's syndrome (letter) Arthritis Rheum. 1991;34:1070–1071. doi: 10.1002/art.1780340822. [DOI] [PubMed] [Google Scholar]
- 20.SICCA website: http://sicca.ucsf.edu located in: “Questionnaires, Forms & Protocols;” “Labial Salivary Glands – Histopathological Assessment for General Application,” 6/1/10
- 21.Daniels TE. Benign lymphoepithelial lesion and Sjögren’s syndrome. In: Ellis GI, Auclair PL, Gnepp DR, editors. Surgical Pathology of the Salivary Glands. Philadelphia: W.B. Saunders; 1991. pp. 92–98. Ch. 6. [Google Scholar]
- 22.Fox PC, Busch KA, Baum BJ. Subjective reports of xerostomia and objective measures of salivary gland performance. JADA. 1987;115:581–584. doi: 10.1016/s0002-8177(87)54012-0. [DOI] [PubMed] [Google Scholar]
- 23.Bloch KJ, Buchanan WW, Wohl MJ, Bunim JJ. Sjögren’s syndrome: a clinical, pathological and serological study of sixty-two cases. Medicine. 1965;44:187–231. [PubMed] [Google Scholar]
- 24.Daniels T, Greenspan J, Cox D, Criswell L, DeSouza Y, Dong Y, et al. Objective measures in Sjögren’s syndrome are strongly associated with each other but not with sicca symptoms: analysis of 564 enrollees in the SICCA international registry and repository. Arthritis Rheumatism. 2007;56:S446. [Google Scholar]
- 25.Scott J. Qualitative and quantitative observations on the histology of human labial salivary glands obtained post mortem. Jour Biol Buccale. 1980;8:187–200. [PubMed] [Google Scholar]
- 26.Salomonsson S, Jonsson MV, Skarstein K, Brokstad KA, Hjelmström P, Wahren-Herlenius M, Jonsson R. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjögren’s syndrome. Arthritis Rheum. 2003;48:3187–3201. doi: 10.1002/art.11311. [DOI] [PubMed] [Google Scholar]
- 27.Vivino FB, Gala I, Hermann GA. Change in final diagnosis on second evaluation of labial minor salivary gland biopsies. J Rheumatology. 2002;29:938–944. [PubMed] [Google Scholar]


