The term “biosimilars” (“follow-on biologics” in the USA) has been used by the European Medicines Agency (EMA) to describe officially-approved subsequent versions of innovator biotechnological products made by a different competitor after the patent and exclusivity rights have expired.1 Biosimilars pose a problem to the clinician who is bound to require guidance on how best to capitalize on these new pharmacological opportunities. In its mandate to promote the best hematologic care, the Italian Society of Hematology (SIE) and the affiliate societies SIES (Società Italiana di Ematologia Sperimentale) and GITMO (Gruppo Italiano Trapianto di Midollo Osseo) convened an expert panel to produce a position paper on the two marketed biosimilars in hematology, i.e. epoetin and filgrastim. The panel of 8 experts was selected according to the conceptual framework elements of the NIH Consensus Development Program.2 Panel bias was minimized by eliminating from consideration strong advocates for or against the use of biosimilars. Each panel member was asked to disclose any tie he or she may have had with pharmaceutical companies manufacturing the drugs considered in the paper in the last five years. The project was conducted independently from any pharmaceutical company which sold either conventional drugs or biosimilars.
Biotechnologicals (and biosimilars) as special drugs
Most traditional low molecular weight drugs are relatively simple molecules with well understood chemical structures that can be synthesized by standardized protocols and methods, starting from commercially available reagents. These features make these medicines relatively simple to produce and to analyze. Therefore, when the intellectual property exclusivity period of the original drug expires, competitors wishing to come on the market with the same product, i.e. “generics”, will only need to develop formulations to achieve similar (within ranges defined by authorities) pharmacokinetic and pharmacodynamic profiles. Such a concept is the basis of the bioequivalence studies required by European and American regulatory agencies to grant authorization of generic drugs. In contrast, biotechnological drugs are much larger (sometimes a thousand-fold) molecules with complex three-dimensional structures, whose integrity is critical for their biological actions. Moreover, biotechnological drugs are produced in industrial laboratories via highly complex processes starting from living prokaryotic or eukaryotic microorganisms that have first been ‘engineered’ to fulfill the best possible production and delivery characteristics. It is extremely important to note that such engineered cellular clones (the ‘production clones’) are not commercially available but that each company has to produce its own in-house, which per se represents an additional variability factor. Finally, differently to small molecular weight chemical drugs, depending upon specific conditions and on the nature of the production clone, biotechnological molecules can undergo variable post-translational modifications (e.g. glycosylation or sulphation), leading to the existence of several ‘isoforms’ with distinct biological properties, which further complicates their in vivo profile.3 As a consequence of the complexity of the structure and the process, both originator biotechnologicals and biosimilars are, at least at present, impossible to characterize fully from a physico-chemical viewpoint, as their size and tertiary structure are beyond the limits of modern equipment.
It has been shown that several product-related factors, such as amino-acid sequence variation, changes in the glycosylation state and production clone, presence of contaminants and process-related impurities, as well as changes in formulation, handling and storage, may significantly increase the probability of inducing immune effects.4,5 Importantly, it should be noted that there are no reliable methods to predict immunogenicity in humans and that pre-marketing clinical studies, which will be discussed below, do not have the statistical power to do so, as these events are usually extremely rare. In this respect, recombinant human erythropoietins represent an example of how even small biopharmaceutical differences can have clinical consequences.
The use of recombinant erythropoietin was associated with excellent tolerability until 1998, when an unexpected burst of pure red cell aplasia (PRCA) occurred in patients treated for anemia of renal failure. Complying with an EMA request to remove any proteins of human origin from the formulation in order to minimize the risk of serious infections induced by potentially lethal prions or viruses, the company replaced human albumin with polisorbate 80. The new formulation led to the development of neutralizing antibodies, resulting in neutralization of both the recombinant protein and the native hormone and, therefore, in PRCA.6 The thorough investigation carried out by the manufacturer on the immunogenic reaction led to further modification of the final product and to the solution of the problem. However, an important lesson was learned: any change in the formulation of a biopharmaceutical, even minimal, has the potential to induce an immune response.
Guidelines for approval of biosimilars
The regulatory policy for biosimilars in Europe is governed by guidelines issued by the EMA that illustrate what requirements must be met to grant authorization.7 The number and extent of comparability studies required for granting a marketing authorization are detailed by EMA’s Committee for Medicinal Products for Human Use (CHMP). These guidelines cover a range of issues including manufacturing, demonstration of comparability for quality, non-clinical and clinical study reports, physico-chemical and biological analysis, and clinical trial requirements. The purpose is to demonstrate the similar nature of the biosimilar and the reference product in terms of quality, safety and efficacy. Quality must be shown by testing the new molecule by pharmacokinetic and pharmacodynamic studies, and it should be proven identical (in defined ranges) to the originator compound. Clinical efficacy and safety must be shown in general by testing the new molecule in clinical trials (phase III studies). Besides general guidelines addressing quality, non-clinical and clinical issues, additional product-class specific guidelines have been developed and are continuously revised. When a particular drug has more than one therapeutic indication, EMA requires that one indication be studied in depth, and if comparability is demonstrated, then the biosimilar will be able to acquire all the indications of the originator drug.
Current EMA guidelines for epoetin biosimilars, for example, state that comparability studies should be performed in patients with anemia due to chronic kidney disease (CKD) and that at least two randomized clinical trials are required.8 In addition, clinical comparability should be shown for both intravenous and subcutaneous routes of administration. In the case of filgrastim, current EMA guidelines state that the clinical model for the demonstration of comparability of biosimilars to the reference product is the prophylaxis of severe cytotoxic chemotherapy-induced neutropenia.9 Although in order to be approved biosimilars will have shown comparability in clinical trials, the EMA still requires that, from a safety perspective, active and passive pharmacovigilance should be put in place for the first five years of commercialization, as would be expected for a new biotechnological entity.
Epoetin and filgrastim biosimilars
The first biotechnological products in hematology were recombinant human erythropoietin (rhEPO, epoetin) and granulocyte-colony stimulating factor (rG-CSF; filgrastim). It is, therefore, not surprising that these are the first two classes of biosimilars that hematologists are having to deal with.
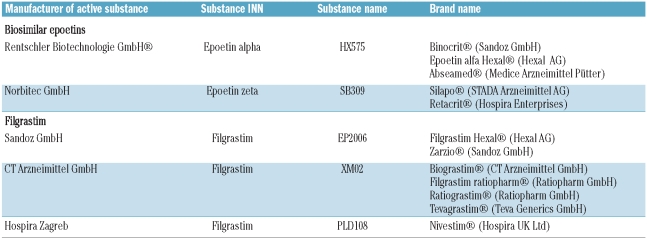
Two biosimilar epoetins, marketed under five separate names, have been approved by the EMA (Table 1) and have both used Eprex/Erypo as reference. One epoetin alfa biosimilar (substance HX575) has received EMA approval under the INN (International Non-proprietary Name) “epoietin alfa” while the other (substance SB309) has received EMA approval under the INN “epoetin zeta”. In 2009, the EMA granted a marketing authorization for a novel CHO cell-derived rhEPO, epoetin theta, which has been developed by Merckle Biotec (Ulm, Germany) using epoetin beta as a comparator. However, epoetin theta is not a biosimilar but has been developed as a stand-alone product.
Table 1.
Biosimilar epoetins and filgrastim which received EMA approval.
Biosimilar epoetins have received marketing authorization for intravenous (i.v.) administration in adult chronic kidney disease (CKD) patients on peritoneal dialysis or not yet undergoing dialysis, i.v. administration in pediatric CKD patients on hemodialysis, i.v. or subcutaneous (s.c.) administration in adult patients receiving chemotherapy for malignancies, and patients prior to major elective orthopedic surgery. Recently, epoetin zeta has also received marketing authorization for s.c. administration in adult CKD patients on peritoneal dialysis or not yet undergoing dialysis.
Both rG-CSFs from E. coli (filgrastim; Neupogen®, Amgen) and from CHO cells (lenograstim; Granocyte®, Chugai Pharma, Tokyo, Japan) are analogs of the 174 amino acid isoform of human G-CSF, noting that filgrastim has an additional N-terminal methionine with respect to endogenous G-CSF. Three biosimilar filgrastims have been launched in the EU, with Neupogen® as the reference product. They are all 175 amino acids non-glycosylated methionyl rG-CSFs expressed in E. coli, and are marketed by different companies under different brand names (Table 1). The biosimilar filgrastims are approved for the same indications as Neupogen®, which include myelosuppressive chemotherapy-induced neutropenia, mobilization of peripheral blood progenitor cells, severe chronic neutropenia (congenital, cyclic, or idiopathic) and persistent neutropenia associated with advanced HIV infection.
Appropriateness of use of biosimilar epoetins in practice
Evaluation of the appropriate use of a biosimilar in clinical practice, as for any new drug, should be based on a critical appraisal of the benefit/cost ratio, grounded on the evidence of efficacy and tolerability, in particular on the documented equivalence between the biosimilar and the reference product. Since the experimentations presented to the EMA may be different for the different compounds, the decisions should be based on the careful scrutiny of the individual studies and on the specific European Public Assessment Reports (EPARs) published by the EMA.
As far as epoetin biosimilars are concerned, comparable efficacy and safety between intravenous administration of HX575 and Eprex® was demonstrated in a double blind randomized, parallel-group, multicenter phase III trial involving 479 hemodialysis patients with renal anemia.10 Comparable efficacy and safety between the SB309 and Eprex® was demonstrated in two randomized, double-blind phase III clinical trials with intravenous administration, a correction phase and a maintenance phase study, involving 922 hemodialysis patients with renal anemia.11,12 An additional maintenance treatment follow-up study was performed to obtain long-term safety data.13 For both epoetins, there have been no reports of immunogenicity with the intravenous use of the drug. A recent third trial with SB309 (epoetin zeta) has documented the therapeutic equivalence of epoetin zeta to epoetin alfa for the s.c. route of administration in renal anemia.14 However, a clinical study into the efficacy and safety of s.c. application of the biosimilar HX575 (epoetin alpha) had to be suspended due to adverse events.15 The study was conducted because in the initial approval procedure of epoetin biosimilars, comparative studies were not possible in the s.c. route as Eprex had shown immunogenicity problems and had been suspended for the s.c. route in nephrology. The German Federal Institute for Drugs and Medical Devices (BfArM) reported on its website that the immediate cause for the suspension of the clinical trial with subcutaneous HX575 was the occurrence of pure red cell aplasia in a patient as well as proof of neutralizing antibodies against erythropoietin in another study participant. After suspension of the clinical study, the BfArM stressed that epoetin alpha Hexal, Binocrit and Abseamed for renal anemia were only approved for endovenous application.
In summary, the head-to-head trials of epoetin biosimilars with the reference product have documented that there are no differences in terms of efficacy between the products in CKD. However, having an identical amino acid sequence to that seen in humans does not guarantee that an epoetin biosimilar will not have immunogenic effects. In the setting of CKD, the regulatory approval has respected the evidence on safety derived from intravenous administration. Thus, in this setting, the least costly product among that approved could be chosen to benefit the patients, and to offer cost savings to the health service. Namely, the use of biosimilar epoetins in epoetin-naïve patients with CKD needing correction of anemia has been almost unanimously accepted by scientific societies and health organizations.16,17 The recommendation on post-marketing monitoring agrees on the crucial issue that patient-years under treatment with these agents would need to be orders of magnitude higher than they were in the studies requested for regulatory approval to exclude serious immune effects.
Data from a study involving 114 cancer patients receiving chemotherapy were also submitted for approval of HX575, but this study was not adequately powered to demonstrate therapeutic equivalence to the reference product.18 Data were also presented from a study with SB309 involving 261 cancer patients receiving chemotherapy, but this study was not designed to demonstrate therapeutic equivalence between products in this patient population.19 In an uncontrolled study, the efficacy and safety of the biosimilar epoetin in the treatment of chemotherapy associated anemia was evaluated in 216 patients, of which 208 were available for complete evaluation.20 However, the study was not aimed at investigating bioequivalence. Thus, the approval of epoetin biosimilars for the indication of anemia of cancer has been made mainly via data extrapolation. As a matter of fact, the EMA’s guideline on the non-clinical and clinical development of biosimilar epoetins indicated that “since the mechanism of action of epoetin is the same for all currently approved indications and there is only one known epoetin receptor, demonstration of efficacy and safety in renal anemia will allow extrapolation to other indications of the reference medicinal product with the same route of administration”.8 However, there are concerns against the extrapolation of the results obtained in renal anemia to other therapeutic indications of the reference product.
The first concern is on the therapeutic equivalence of the biosimilars in clinical settings, like in cancer, where, at variance from renal anemia, there is only a relative defect of erythropoietin production. This is due to the fact that sensitivity to epoetin is higher in erythropoietin-deficient than in non-erythropoietin-deficient conditions and is also dependent on the responsiveness of the bone marrow. The second concern is on safety, given the potential impact of the dose of the biosimilar on toxicity and on the immunogenicity risk. It should be noted that it is unlikely that immunogenicity induced by epoetins will arise in this setting, yet there are no sufficiently-powered studies available on the use of high dosage of epoetin biosimilars in cancer. Further studies are, therefore, required before therapeutic equivalence in this setting can be accepted unrestrictedly.
Epoetin is also licensed for the treatment of anemic patients who are at risk of perioperative blood loss from elective, non-cardiac, non-vascular surgery, to reduce the need for allogeneic blood transfusion. This use presents a clinical dilemma since the limited experience with epoetin biosimilars in this indication raises the concern that unexpected toxicity might have a detrimental effect in non-anemic individuals administered high doses of epoetin to support blood donation as a preventive measure. The use of biosimilar epoetins for this indication requires greater experience and adequate follow up.
Appropriateness of the use of filgrastim biosimilar in practice
The filgrastim biosimilars have been granted marketing authorization on the basis of clinical trials aimed at documenting the therapeutic equivalence with respect to the reference biotechnological in the prevention of neutropenia due to chemotherapy for cancer. XM02 has been tested for clinical equivalence in three clinical trials with approximately 900 patients. The first study was a multinational, multicenter, randomized, controlled study in 350 patients with breast cancer who received chemotherapy. Comparable safety and efficacy profiles for XM02 administered for up to a maximum of six chemotherapy cycles versus Neupogen was demonstrated.21 The incidence of observed or protocol defined febrile neutropenia (FN) in cycle 1 was 15.0% and 8.8% in the XM02 and Neupogen groups, respectively. The most often reported drug-related adverse effects in this study were myalgia, back pain, anemia, and headache, all known adverse drug reactions to G-CSFs. Of the observed deaths, none was related to the study drug but primarily to progression/refractoriness of the underlying disease and, to a smaller extent, to adverse events of chemotherapy.
The second study was in lung cancer patients.22 Equivalence of XM02 versus Neupogen and superiority versus placebo was clearly demonstrated with a duration of severe neutropenia (DSN) in cycle 1 of 1.1, 1.1, and 3.8 days in the XM02, Neupogen and placebo groups, respectively. In the same study, the incidence of FN in cycle 1 was 12.1%, 12.5%, and 38.1% under XM02, Neupogen, and placebo, respectively.
The third study in the same development program was conducted in non-Hodgkin’s lymphoma patients.23 In this study, there was a trend to better efficacy results in the XM02 group compared with the Neupogen group with a DSN of 0.5 days (XM02) and 0.9 days (Neupogen) and an incidence of FN of 11.1% (XM02) and 20.7% (Neupogen) in cycle 1.
The results on the incidence of FN in the three studies have been further investigated in a meta-analysis.24 Overall, 608 patients (363 under XM02 and 245 under Neupogen) were included in the meta-analysis. The incidence of FN in the first cycle of chemotherapy under primary G-CSF prophylaxis was low (in the range of 12–16%). XM02 was demonstrated to be non-inferior to Neupogen regarding the incidence of FN, irrespective of the myelotoxicity of the chemotherapy regimen.
Supportive evidence for Filgrastim Hexal was provided by a phase III study, the primary objective of which was the evaluation of the safety, tolerability and immmunogenicity of the biosimilar.25 The study was designed as an open, single arm, multicenter study in chemotherapy-naïve breast cancer patients receiving doxorubicin and docetaxel chemotherapy and filgrastim as primary prophylaxis of severe neutropenia. Thus, the comparable efficacy of filgrastim Hexal was established on the basis of a non-comparative study.
The therapeutic equivalence of Amgen filgrastim and the biosimilar filgrastim developed by Hospira26 was demonstrated in a phase III study in breast cancer patients treated with doxorubicin and docetaxel in the neoadjuvant/adjuvant or first-line metastatic setting, enrolled in 37 European centers. Patients were randomized (2:1) to receive Hospira filgrastim or Amgen filgrastim, after the end of chemotherapy. Filgrastim (5 μg/kg/day) was administered under double-blind conditions. Primary endpoint to demonstrate bioequivalence was DSN in cycle 1: 184 patients were randomized to Hospira filgrastim and 95 to Amgen filgrastim. Mean DSN in cycle 1 was similar with Hospira filgrastim (1.6 days) and Amgen filgrastim (1.3 days), meeting pre-defined criteria for bioequivalence. Secondary end points supporting bioequivalence included mean time to absolute neutrophil count recovery and incidence of FN. The most common treatment-related adverse event with Hospira filgrastim was grade 1–2 bone pain.
Overall, the results of the studies presented for marketing authorization indicate therapeutic equivalence and safety of the three filgrastim biosimilars in the prophylaxis of complications related to neutropenia caused by chemotherapy.
On the basis of the results in cancer neutropenia, the EMA extrapolated the therapeutic equivalence of the biosimilars also to the other indications of Neupogen, as for peripheral blood progenitor cell mobilization and transplantation, even though there are no data supporting clinical equivalence. The only evidence in this domain is the recovery of CD34 cells obtained in a phase I study in 144 healthy individuals, where XM02 was used at the dose of 5 ug/kg and 10 ug/kg, reporting that the recovery of hematopoietic stem cell is equivalent with the biosimilar and the originator.25 The European Group for Blood and Marrow Transplantation (EBMT)27 and the World Marrow Donor Association (WMDA)28 have stressed that the use of biosimilars for stem cell mobilization in healthy donors represents an ethical dilemma because the donors receive no therapeutic benefit from the use of these drugs. Considering the detrimental effect that unexpected toxicity by filgrastim might have in normal individuals donating their peripheral stem cells, careful long-term monitoring with biosimilars should be mandatory with regards to side effects such as immunogenicity and normal WBC function and leukemogenesis.29
According to the East Midlands G-CSF guideline,30 the same advice should also be considered for autologous stem cell mobilization. In this setting, a preliminary report comparing standard G-CSF versus biosimilar (ratiograstim) shows similar results in terms of days for collection and CD34+ yield.31 For the Austrian Society of Hematology Oncology32 the use of biosimilars cannot be recommended without concerns regarding stem cell mobilization with filgrastim for healthy persons, in the use of filgrastim for non-therapy related neutropenias, in therapeutical use of filgrastim for neutropenic fever and in the use of biosimilars for pediatric diseases. Finally, as regards the immunogenicity of biosimilar filgrastim, caution is needed in mobilization in autoimmune diseases because of the reported flares of the disease.33
Substitution, tracing and monitoring
Switching refers to the practice of rotating a single patient already undergoing treatment from one medicine to another that is considered equivalent while substitution refers to the practice whereby a health profession, other than the clinician, dispenses a drug which is considered equivalent to that prescribed.
Although, in the past, switching has been practised in hospital and community settings, we feel that a patient who is well treated with a particular epoetin or filgrastim (independently of cost and whether it is an originator or a biosimilar) should not undergo a change in treatment for purely economic reasons. Although there are no data on this, the main concern about switching from one epoetin to another is the issue of immunogenicity. In addition to the possibility of clinical consequences, multiple switchings also affect pharmacovigilance efforts, making it more difficult to trace back reported adverse events to the correct brand or manufacturer. It should be noted that there have been some studies which have investigated switching from originators to biosimilars34,35 and which have found no deleterious effects on efficacy and safety.
The standpoint against automatic substitution of biosimilars has been issued by authorities in most European countries.36 This is of particular importance in patients already well-treated with a particular biological drug, in whom therapeutic continuity should be guaranteed. On the other hand, automatic substitution should be allowed in drug-naïve patients for those indications for which the clinician accepts therapeutic equivalence (see above), as this will promote attractive hospital tenders. The clinician should have, nonetheless, the option of not choosing automatic substitution for individual patients.
Due to the limited clinical database on approval of biosimilars epoetin and filgrastim, collection of post-approval safety data for these drugs is required, and an important component of post-approval data collection is the ability to easily distinguish between different products.
The current International Non-proprietary Name (INN) system, administered by the World Health Organization (WHO), is an international mechanism essential to precisely identify each pharmaceutically active substance and ensure the sale prescription and dispensation of medicines.37 Currently, the naming of the epoetins and filgrastim biosimilars is based on the current INN system whereby drugs with the same active ingredient (irrespective of their productive process) are given the same name. In some countries, physicians are obliged or encouraged to prescribe by INN. We feel this should not apply to biologics.
In seeking traceability after the prescription of a biosimilar, it seems mandatory that epoetins and filgrastims are not prescribed by INN, but that they should be identified by brand names. This would allow pharmacovigilance and ensure that adverse events are assigned to the correct product. Furthermore, in case of an adverse event, it is necessary to identify the drug responsible by reporting the INN, the brand name and the relevant batch numbers.
Information needs
The EMA indicates that the final decision to treat a patient with a biosimilar medicine should be taken by a qualified health professional. Professionals can comply with this indication if they are fully aware of the elements of the decision. Entry of biosimilars onto the market will require transparent, unbiased dissemination of information to prescribers and other health care professionals. Thus, it is fundamental that hematologists be informed about the key concepts and critical issues concerning biosimilars. Since the decision on prescription and substitutability is complex, with unanimous opinion unlikely and sensitivity to numerous conflicts of interest common, the availability of appropriate information should be a prime duty of scientific societies. Interventions to help doctors make specific and deliberative choices among the options available should be made, such as organizing ad hoc meetings and producing guidelines on the use of biosimilars. Scientific societies should also help healthcare providers gain access to all data regarding biosimilars, so that they can make informed clinical decisions.
Footnotes
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
Editorial note: this article is a position paper on the use of epoetin and filgrastim biosimilars. As stated by Kassirer and Angell [Kassirer JP, Angell M. Financial conflicts of interest in biomedical research. N Engl J Med. 1993 Aug 19;329(8):570-1] “unlike reports of original research, these articles represent the judgment of their authors, based on their evaluation of the literature. What studies they select to discuss and their analysis of them are necessarily subjective”. In accordance with Haematologica policy, in the process of manuscript submission the authors of this paper have attested that it has not been sponsored and/or supported in any way by a company whose product (epoetin and/or filgrastim -either innovator products or biosimilars) is examined in the manuscript.
References
- 1.European Medicines Agency. Questions and Answers on biosimilar medicines (similar biological medicinal products) 2006. Available from: www.emea.europa.eu/pdfs/human/pcwp/7456206en.pdf.
- 2.Ferguson JH. The NIH Consensus Development Program. The evolution of guidelines. Int J Technol Assess Health Care. 1996;12(3):460–74. [PubMed] [Google Scholar]
- 3.Kuhlmann M, Covic A. The protein science of biosimilars. Nephrol Dial Transplant. 2006;21(Suppl 5):v4–8. doi: 10.1093/ndt/gfl474. [DOI] [PubMed] [Google Scholar]
- 4.Tamilvanan S, Raja NL, Sa B, Basu SK. Clinical concerns of immunogenicity produced at cellular levels by biopharmaceuticals following their parenteral administration into human body. J Drug Target. 2010;18(17):489–98. doi: 10.3109/10611861003649746. [DOI] [PubMed] [Google Scholar]
- 5.Schellekens H. Biosimilar therapeutics – what do we need to consider? NDT Plus. 2009;2(Suppl_1):i27–i36. doi: 10.1093/ndtplus/sfn177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadevall N, Eckardt KU, Rossert J. Epoetin-induced autoimmune pure red cell aplasia. J Am Soc Nephrol. 2005;16(Suppl 1):S67–9. doi: 10.1681/asn.2004110959. [DOI] [PubMed] [Google Scholar]
- 7.Looper YJ. Legislative initiatives in Europe, Canada and the US for market authorization of follow-on biologics. Curr Opin Drug Disc Dev. 2010;13(2):247–56. [PubMed] [Google Scholar]
- 8.European Medicines Agency. Guidance on similar medicinal products containing recombinant erythropoietins. 2006. Available from: http://www.emea.europa.eu/pdfs/human/biosimilar/9452605en.pdf.
- 9.European Medicines Agency. Guidance on similar medicinal products containing recombinant granulocyte-colony stimulating factor. 2006. Available from: http://www.emea.europa.eu/pdfs/human/biosimilar/3132905en.pdf.
- 10.Haag-Weber M, Vetter A Thyroff-Friesinger U; INJ-Study Group. Therapeutic equivalence, long-term efficacy and safety of HX575 in the treatment of anemia in chronic renal failure patients receiving hemodialysis. Clin Nephrol. 2009;72(5):380–90. [PubMed] [Google Scholar]
- 11.Baldamus C, Krivoshiev S, Wolf-Pflugmann M, Siebert-Weigel M, Koytchev R, Bronn A. Long-term safety and tolerability of epoetin zeta, administered intravenously, for maintenance treatment of renal anemia. Adv Ther. 2008;25(11):1215–28. doi: 10.1007/s12325-008-0111-1. [DOI] [PubMed] [Google Scholar]
- 12.Krivoshiev S, Todorov VV, Manitius J, Czekalski S, Scigalla P, Koytchev R. Comparison of the therapeutic effects of epoetin zeta and epoetin alpha in the correction of renal anaemia. Curr Med Res Opin. 2008;24(5):1407–15. doi: 10.1185/030079908x297402. [DOI] [PubMed] [Google Scholar]
- 13.Wizemann V, Rutkowski B, Baldamus C, Scigalla P, Koytchev R. Comparison of the therapeutic effects of epoetin zeta to epoetin alfa in the maintenance phase of renal anaemia treatment. Curr Med Res Opin. 2008;24(3):625–37. doi: 10.1185/030079908X273264. [DOI] [PubMed] [Google Scholar]
- 14.Krivoshiev S, Wizemann V, Czekalski S, Schiller A, Pljesa S, Wolf-Pflugmann M, et al. Therapeutic equivalence of epoetin zeta and alfa, administered subcutaneously, for maintenance treatment of renal anemia. Adv Ther. 2010;27:105–17. doi: 10.1007/s12325-010-0012-y. [DOI] [PubMed] [Google Scholar]
- 15.http://www.gabionline.net/Biosimilars/News/Safety-study-for-subcutaneous-epoetin-alfa-biosimilar-Binocrit-Epoetin-alfa-Hexal-Abseamed-suspended
- 16.Bouchet JL, Brunet P, Canaud B, Chanliau J, Combe C, Deray G, et al. Société de néphrologie, Société francophone de dialyse, Société de néphrologie pédiatrique; Société francophone de dialyse; Société de néphrologie pédiatrique. [Position statements regarding usage of biosimilars of Epoetins. Position paper of the Société de néphrologie, Société Francophone de dialyse, and Société de néphrologie pédiatrique] Nephrol Ther. 2009;5(1):61–6. doi: 10.1016/j.nephro.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Covic A, Cannata-Andia J, Cancarini G, Coppo R, Frazão JM, Goldsmith D, et al. Biosimilars and biopharmaceuticals: what the nephrologists need to know—a position paper by the ERA-EDTA Council. Nephrol Dial Transplant. 2008;23(12):3731–7. doi: 10.1093/ndt/gfn519. [DOI] [PubMed] [Google Scholar]
- 18.European Medicines Agency. Assessment Report for epoetin alfa Binocrit. Nonproprietary Name: epoetin alfa. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000725/WC500053615.pdf.
- 19.Weigang-Köhler K, Vetter A, Thyroff-Friesinger U. HX575, recombinant human epoetin alfa, for the treatment of chemotherapy-associated aymptomatic anaemia in patients with solid tumours. Onkologie. 2009;32(4):168–74. doi: 10.1159/000200783. [DOI] [PubMed] [Google Scholar]
- 20.Tzekova V, Mihaylov G, Elezovic I, Koytchev R Epoetin Zeta Oncology Study Group. Therapeutic effects of epoetin zeta in the treatment of chemotherapy-induced anaemia. Curr Med Res Opin. 2009;25(7):1689–97. doi: 10.1185/03007990903050876. [DOI] [PubMed] [Google Scholar]
- 21.del Giglio A, Eniu A, Ganea-Motan D, Topuzov E, Lubenau H. XM02 is superior to placebo and equivalent to Neupogen in reducing the duration of severe neutropenia and the incidence of febrile neutropenia in cycle 1 in breast cancer patients receiving docetaxel/doxorubicin chemotherapy. BMC Cancer. 2008;8:332. doi: 10.1186/1471-2407-8-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gatzemeier U, Ciuleanu T, Dediu M, Ganea-Motan E, Lubenau H, Del Giglio A. XM02, the first biosimilar G-CSF, is safe and effective in reducing the duration of severe neutropenia and incidence of febrile neutropenia in patients with small cell or non-small cell lung cancer receiving platinum-based chemotherapy. J Thorac Oncol. 2009;4(6):736–40. doi: 10.1097/JTO.0b013e3181a52964. [DOI] [PubMed] [Google Scholar]
- 23.Engert A, Griskevicius L, Zyuzgin Y, Lubenau H, del Giglio A. XM02, the first granulocyte colony-stimulating factor biosimilar, is safe and effective in reducing the duration of severe neutropenia and incidence of febrile neutropenia in patients with non-Hodgkin lymphoma receiving chemotherapy. Leuk Lymphoma. 2009;50(3):374–9. doi: 10.1080/10428190902756081. [DOI] [PubMed] [Google Scholar]
- 24.Engert A, del Giglio A, Bias P, Lubenau H, Gatzemeier U, Heigener D. Incidence of Febrile Neutropenia and Myelotoxicity of chemotherapy: A Meta-Analysis of Biosimilar G-CSF Studies in Breast Cancer, Lung Cancer, and Non-Hodgkin’s Lymphoma. Onkologie. 2009;32:599–604. doi: 10.1159/000232580. [DOI] [PubMed] [Google Scholar]
- 25.European Medicines Agency. Assessment report for biograstim. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000826/WC500053902.pdf.
- 26.Waller CF, Semiglazov VF, Tjulandin S, Bentsion D, Chan S, Challand R. A phase III randomized equivalence study of biosimilar filgrastim versus Amgen filgrastim in patients receiving myelosuppressive chemotherapy for breast cancer. Onkologie. 2010;33(10):504–11. doi: 10.1159/000319693. [DOI] [PubMed] [Google Scholar]
- 27.www.gitmo.net/Biosimilars_in%20mobilization%20of%20unrelat-ed%20and%20related%20donors.pdf.
- 28.ww.worldmarrow.org/fileadmin/.../CWG/.../CWG_Minutes_03-2009.pdf
- 29.Niederwieser D, Schmitz S. Biosimilar agents in oncology/haematology: from approval to practice. Eur J Haematol. 2011;86(4):277–88. doi: 10.1111/j.1600-0609.2010.01566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.East Midlands guidelines. www.inrcancernetwork.nhs.uk.
- 31.Publicover A, Richardson DS, Hill K, et al. Use of biosimilar granulocyte colony stimulating factor (GCSF) for peripheral blood progenitor cell mobilisation prior to autologous stem cell transplant: a single centre experience. Bone Marrow Transplant. 2010;45(suppl 2):S158. [Google Scholar]
- 32.Gastl G, Geissler D, Geissler K, Lang A, Ludwig H, et al. ASHO position paper on biosimilars. MEMO. 2009;2:232–233. [Google Scholar]
- 33.Atkins H. Hematopoietic SCT for the treatment of multiple sclerosis. Bone Marrow Transplant. 2010;45(12):1671–81. doi: 10.1038/bmt.2010.168. [DOI] [PubMed] [Google Scholar]
- 34.Wiȩcek A, Ahmed I, Scigalla P, Koytchev R. Switching epoetin alfa and epoetin zeta in patients with Renal Anemia on Dialysis: Posthoc analysis. Adv Ther. 2010;27(12):941–52. doi: 10.1007/s12325-010-0080-z. [DOI] [PubMed] [Google Scholar]
- 35.Lonnemann G, Wrenger E. Biosimilar epoetin zeta in nephrology - a single-dialysis center experience. Clin Nephrol. 2011;75(1):59–62. [PubMed] [Google Scholar]
- 36.Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars. Ann Oncol. 2008;19(3):411–9. doi: 10.1093/annonc/mdm345. [DOI] [PubMed] [Google Scholar]
- 37.WHO. Guidance on INN. Available from: http://www.who.int/medicines/services/inn/innquidance/en/index.htmlWHO.